Abstract
Background
In patients with covert cerebrovascular disease or proximal source of embolism, embolic silent brain infarction may precede major stroke events. Therefore, characterization of particularly cortical silent brain infarction is essential for identifying affected patients and commencing adequate secondary prevention. This study aimed to investigate differences in the distribution pattern of cortical ischemic stroke lesions to assess potential predilection sites of cortical silent brain infarction.
Methods and Results
We prospectively included all consecutive patients with stroke presenting from January 1 to December 31, 2018. Diffusion‐weighted imaging lesions were used to generate voxel‐based lesion maps and assigned to atlas‐based cortical regions of interest in middle cerebral artery territories. Each region‐of‐interest lesion frequency was related to the respective region‐of‐interest volume to identify frequently affected and underrepresented cerebral cortex areas. Diffusion‐weighted imaging data for voxel‐based lesion maps were available in 334 out of 633 patients. Primary analysis revealed that small‐ (<0.24 cc) and medium‐sized (0.24–2640 cc) lesions distributed predominantly along regions associated with sensorimotor or language function. Detailed analysis within middle cerebral artery territories showed an approximated frequency of missed cortical stroke lesions of up to 67% in the right and 69% in the left hemisphere. In particular, the frontal, temporal, and occipital cortices were underrepresented. Larger lesion size and areas associated with higher cortical function led to hospital admission.
Conclusions
Cortical brain infarcts in hospitalized patients are not dispersed equally but are predominantly located in brain structures associated with motor control and sensory and language function. Matching underrepresented cerebral cortex regions to symptoms not yet associated with stroke warrants further exploration.
Keywords: anticoagulants, atrial fibrillation, covert cerebrovascular disease, language, prevention and control, secondary prevention, silent brain infarction
Subject Categories: Risk Factors, Cerebrovascular Disease/Stroke, Ischemic Stroke, Epidemiology
Nonstandard Abbreviations and Acronyms
- DWI
diffusion‐weighted imaging
- MCA
middle cerebral artery
- PG
precentral gyrus
- ROI
region of interest
- SBI
silent brain infarction
Clinical Perspective.
What Is New?
This study demonstrated that cortical embolic stroke lesions do not disperse equally across the cerebral cortex in patients referred to the hospital for suspected ischemic stroke but are predominantly located in brain structures associated with sensorimotor control and language functions.
Detailed analysis of underrepresented cortical brain areas in hospitalized patients identified potential predilection sites of embolic silent brain infarction, which in particular included regions harboring higher cortical functions.
A disease model was developed to approximate the frequency of covert cortical silent brain infarction in the middle cerebral artery territories of both hemispheres.
What Are the Clinical Implications?
Many patients with underlying embolic cerebrovascular disease suffer covert cortical silent brain infarction but are not admitted to the hospital.
Improved identification of those patients may allow an earlier initiation of diagnostic workup and commencement of adequate secondary prevention before the occurrence of major ischemic stroke events of embolic origin.
Improved awareness of stroke syndromes that go beyond the impairment of speech and deficits in sensorimotor control is necessary to enable the clinical detection of patients affected by embolic silent brain infarction.
The clinical presentation of ischemic stroke largely depends on infarct location, infarct volume, and the lateralization between the hemispheres as well as global and regional brain connectivity. Specifically, infarct lesion location is fundamentally linked to stroke severity, 1 and the combination of acute stroke lesion topography and infarct volume better corresponds with National Institutes of Health Stroke Scale and functional outcome than the mere ischemic lesion volume alone. 2 , 3 In particular, lesion patterns affecting brain areas of motor control and lateralized brain functions were shown to largely influence the functional stroke outcome, 4 an association that was also demonstrated for large vessel occlusion ischemic stroke. 5 In contrary, some ischemic stroke lesions with a small infarct volume may lack clinically overt stroke‐like signs, present with National Institutes of Health Stroke Scale score of 0, and may remain completely unnoticed. 6 , 7 , 8 Those so‐called silent brain infarctions (SBIs) are present in ≈20% of stroke‐free older adults and are an independent risk factor for subsequent stroke and dementia, especially if underlying causes remain untreated. 6 , 9 , 10 The term covert brain infarction has been introduced synonymously to describe SBI in the context of covert cerebrovascular disease. 8 , 11 SBIs share vascular risk factors with clinical stroke, hence their detection warrants initiation of a thorough diagnostic workup and prompts more aggressive cerebrovascular risk factor control. 9 SBIs can occur in all parts of the brain and are frequently located in the basal ganglia, the cerebral white matter, and other subcortical areas, but also estimated to be in 10% to 15% of all cases in the cerebral cortex. 6 , 12 , 13 As cortical strokes are most likely of embolic—either cardioembolic or large artery disease—origin, their early detection is crucial for initiating secondary stroke prevention. 11 , 14 , 15 Especially patients with atrial fibrillation show a high prevalence of embolic SBI, and the presence of SBI is indicative of so‐far undescribed, incident atrial fibrillation. 16 , 17 Similarly, an association was demonstrated between downstream carotid artery atherosclerosis and SBIs of the cortical subtype, but not of the lacunar SBI subtype. 18 , 19 Without (pre‐)clinical recognition of cortical SBI secondary prophylactic measures may not—or not in time—be initiated, and subsequent embolization with large vessel occlusion and severe functional disability might be the first time that a patient with cortical SBI presents to the hospital. Therefore, the characterization of cortical SBI is of high interest to facilitate the timely detection of potential sources of proximal embolism. Understanding how symptomatic embolic strokes disperse over the cerebral cortex can be a first step to determine in which cortical regions ischemic strokes are frequently missed.
The aim of this study was to investigate differences in the distribution of cortical brain infarcts on diffusion‐weighted magnetic resonance imaging (MRI) in a 1‐year sample of consecutive patients with ischemic stroke to approximate the burden and topography of cortical SBI in the population.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
All consecutive patients who presented with acute ischemic stroke to the academic stroke center of the University Hospital Frankfurt from January 1 to December 31, 2018, were prospectively included in our vascular study database, which was reviewed for the purpose of this study. Ethical approval for the study was granted by the institutional review board of the ethics committee at the University Hospital Frankfurt (project number 187/19). No written informed consent was required. Sociodemographic baseline parameters, cardiovascular risk factors, and clinical parameters as well as final diagnoses were retrospectively recorded. Patients with a final diagnosis other than stroke, aged <18 years, or who had unavailable imaging data or missing clinical data were excluded from this study (Figure 1). Imaging modality was recorded in each patient and was dichotomized into either computed tomography (CT) only or availability of MRI. A baseline characterization of the patient sample comprised all patients for transparency and to minimize the possibility of a sample selection bias (Table 1). Afterward, patients without diffusion‐weighted imaging (DWI) magnetic resonance data, which were mandatory for the voxel‐based lesion mapping, had to be excluded from the lesion distribution analysis. MRI data were acquired on a 3 T whole‐body MRI scanner (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany) in the majority of patients using a standardized stroke imaging protocol. Patients admitted for acute stroke therapy from associated stroke centers received external MRI in various MRI scanners. At first, voxel‐based data on infarct location was retrieved for all consecutive patients to visualize the distribution pattern in the whole brain, including infratentorial, subcortical, and cortical areas. In a second step, closer investigation was focused on only the cortical brain infarcts in the territory of the middle cerebral artery (MCA), which will be outlined in Detailed Comparison of the Hemispheres.
Figure 1. Study flowchart.

Study flowchart outlining the enrollment of patients and stepwise patient exclusion. CT indicates computed tomography; DWI, diffusion‐weighted imaging; MCA, middle cerebral artery; MR, magnetic resonance; n. a., not available; and Tab., Table; and TIA, transient ischemic attack.
Table 1.
Patients With and Without MRI at Admission
| All, n=633 | MRI, n=434 | CT only, n=199 | P value* | |
|---|---|---|---|---|
| Baseline data | ||||
| Male sex, n (%) | 373 (58.9) | 268 (61.8) | 105 (52.8) | ns |
| Age, y, mean±SD | 70.12±14.6 | 68.38±14.7 | 73.9±13.7 | ns |
| Time window <4.5 h, n (%) | 250 (39.5) | 148 (34.1) | 102 (51.3) | <0.01 |
| Time window 4.5–24 h, n (%) | 147 (23.2) | 112 (25.8) | 35 (8.1) | ns |
| Time window >24 h, n (%) | 121 (19.1) | 92 (21.2) | 29 (14.6) | ns |
| Wake‐up stroke, n (%) | 56 (0.9) | 46 (10.6) | 10 (5.0) | <0.05 |
| NIHSS, median (IQR) | 4 (1–11) | 3 (1–8) | 8 (3–15) | <0.001 |
| mRS discharge, median (IQR) | 2 (1–4) | 2 (1–4) | 3 (1–5) | <0.001 |
| TIA, n (%) | 91 (14.4) | 66 (15.2) | 25 (12.5) | ns |
| Risk factors, n (%) | ||||
| Diabetes | 161 (25.4) | 111 (25.6) | 50 (25.1) | ns |
| Hypertension | 475 (75.0) | 317 (73.0) | 158 (79.4) | ns |
| Hypercholesterinemia | 119 (18.8) | 96 (22.1) | 23 (11.6) | <0.01 |
| Smoker | 113 (17.9) | 87 (20.0) | 26 (13.1) | <0.05 |
| Stroke pathogenesis, n (%) | ||||
| Thromboembolic | 146 (23.1) | 106 (24.4) | 40 (20.1) | ns |
| Small vessel disease | 90 (14.2) | 69 (15.9) | 21 (10.6) | ns |
| Cardioembolic | 185 (29.2) | 102 (23.5) | 83 (41.7) | <0.05 |
| ESUS | 129 (20.4) | 98 (22.6) | 31 (15.6) | ns |
| Other/unknown | 83 (13.1) | 59 (13.6) | 24 (12.1) | ns |
| Stroke therapy, n (%) | ||||
| Thrombolysis | 135 (21.3) | 68 (15.7) | 67 (33.7) | <0.001 |
| Endovascular therapy | 132 (20.9) | 60 (13.8) | 60 (30.2) | <0.001 |
| Stroke location, n (%) | ||||
| Right MCA | 200 (31.6) | 132 (30.4) | 68 (34.2) | ns |
| Left MCA | 223 (35.2) | 138 (31.8) | 85 (42.7) | <0.05 |
| Right PCA | 24 (3.8) | 19 (4.4) | 5 (2.5) | ns |
| Left PCA | 24 (3.8) | 21 (4.8) | 3 (1.5) | <0.05 |
| Right ACA | 3 (0.5) | 3 (0.7) | 0 | ns |
| Left ACA | 4 (0.6) | 2 (0.5) | 2 (1.0) | ns |
| Basilar artery | 100 (15.8) | 75 (17.3) | 24 (12.1) | ns |
| Multifocal | 52 (8.2) | 43 (9.9) | 9 (4.5) | <0.05 |
ACA indicates anterior cerebral artery; CT, computed tomography; ESUS, embolic stroke of unknown source; IQR, interquartile range; MCA, middle cerebral artery; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; ns, not significant; PCA, posterior cerebral artery; and TIA, transient ischemic attack.
Calculated between both categories using 2‐sided χ2 or Mann–Whitney U test, as applicable.
Voxel‐Based Lesion Mapping From DWI
Diffusion‐weighted images were automatically processed using a custom‐built shell script implementing tools from the FMRIB (Functional Magnetic Resonance Imaging of the Brain, Oxford University, UK) Software Library (FSL, version 5.0.7). For automatic segmentation of the ischemic lesion, an upper threshold of 620×10−6 mm2/s was applied to each individual apparent diffusion coefficient map, 20 retaining only voxels below this value. Because the thresholded apparent diffusion coefficient map commonly still contains voxels representing inner and outer cerebrospinal fluid as well as artifacts, an individual mask containing only voxels with apparent diffusion coefficient values <200×10−6 mm2/s and >1200×10−6 mm2/s was applied to remove cerebrospinal fluid and artifactual voxels. 21 The resulting segmented ischemic lesion was transformed into a binary lesion mask for further processing. For spatial normalization of each individual ischemic lesion mask, the following procedure was performed: the first diffusion‐weighted image (b=0, purely T2 weighted) was extracted, skull‐stripped with “BET” (FMRIB brain extraction tool), and linearly coregistered to the T1‐weighted Montreal Neurological Institute 152 (MNI152) standard space template (1‐mm isotropic resolution). This affine transformation was then used as an initialization for the nonlinear coregistration of the first diffusion‐weighted image to the MNI152 template. Finally, the warpfield coefficients obtained from this nonlinear coregistration were applied for normalization of the individual apparent diffusion coefficient map and the ischemic lesion mask to the MNI152 standard space. A lesion overlay was generated by summation of all individual ischemic lesion masks in MNI152 standard space using “FSLMATHS.” As dilated perivascular spaces may mimic lacunar infarction on the lower basal ganglia region, DWI lesions in this region <3 mm were eliminated from calculations by applying a custom‐built mask. Imaging data were qualitatively assessed for discrepancies in the distribution of stroke lesions between hemispheres and vascular territories.
All lesion masks were individually controlled and compared with the original corresponding DWI images and clinical data for quality control. If necessary, masks were corrected manually using MRIcron. The corrected masks of all patients were then used to create the overlay lesion heatmap (Figure 2) of the entire brain, including infratentorial, subcortical, and cortical brain regions. Moreover, lesion volumes of all corrected masks were assessed using “FSLMATHS,” and the sample was divided into tertials based on lesion volume. The 3 subgroups were labeled “small” (<0.240 cc), “medium” (0.240–2640 cc), and “large” (>2640 cc) depending on the ischemic core volume. Lesion overlay heatmaps were generated for each subgroup (Figure 3).
Figure 2. Overview of the magnetic resonance imaging analysis.

The figure outlines the processing pipeline of diffusion‐weighted imaging data. MCA indicates middle cerebral artery; MNI152, Montreal Neurological Institute 152; and ROI, region of interest.
Figure 3. Lesion overlays after stratification according to lesion volume (ischemic core volume tertials).

Lesion overlap based on magnetic resonance imaging data of 334 patients. Based on tertials of the ischemic core volume, the 3 groups are defined as small (A; <0.240 cc), medium (B; 0.240–2640 cc), or large (C; >2640 cc). Equidistant slices in Montreal Neurological Institute 152 standard space from z=32 to z=147.
Detailed Comparison of the Hemispheres
The region‐based detailed MRI analysis was restricted to the MCA territory of both hemispheres, as a plausible cause could be made for an inequity in the hemodynamic distribution of proximal emboli between the anterior cerebral artery and MCA territories and the posterior circulation. Hence, at first, masks for the MCA territories of the left and right hemispheres were drawn in MNI standard space according to the atlas of the cerebral vasculature by Tatu et al. 22 MCA masks were then multiplied with the Harvard‐Oxford cortical atlases implemented in FSL to create MCA‐specific regions of interest (MCA‐ROIs) for the cerebral cortex in each hemisphere in standard space. 23 , 24 Consequently, the following analysis only comprised ischemic lesions to the cerebral cortex, whereas subcortical lesions were deliberately not included in the detailed distribution pattern investigation. In addition, cortical lesions in the anterior cerebral artery territory and in the posterior circulation were excluded from further analysis as those regions were not part of the MCA territory mask, as stated previously. This resulted in a total number of 33 defined MCA‐ROIs on each side (66 MCA‐ROIs in total; Table 2). Finally, lesion overlay maps for “small” and “medium” infarct core size were multiplied with all 66 cortical MCA‐ROIs using “FSLSTATS,” yielding the individual total frequency of ischemic lesions in each cortical MCA‐ROI in both hemispheres as well as summated infarct core volumes for each of the 66 ROIs (data not shown). The number of times an MCA‐ROI was affected by an ischemic infarct (a “hit”) was determined for small, medium, and large stroke lesions separately throughout the entire patient collective. Involvement of a specific area by an ischemic lesion was counted as 1 hit, independently of the respective ischemic lesion volume.
Table 2.
MRI Cohort Analysis (n=334)*
| Ischemic core volume | P value† | |||
|---|---|---|---|---|
| Small (<0.240 cc), n=111 | Medium (0.240–2640 cc), n=112 | Large (>2640 cc), n=111 | ||
| Baseline data | ||||
| Age, y, mean (SD) | 69.6 (13.7) | 66.5 (15.2) | 67.6 (15.7) | ns |
| Male sex, % (n) | 64.9 (72) | 65.2 (73) | 63.1 (70) | ns |
| NIHSS at admission, median (IQR) | 2 (1–6) | 2 (1–7) | 9 (3–14) | <0.001 |
| mRS at discharge, median (IQR) | 1 (1–3) | 2 (1–3) | 3 (1–5) | <0.001 |
| Admission within 4.5‐h mark, % (n) | 27.9 (31) | 26.8 (30) | 31.5 (35) | ns |
| Thrombolysis, % (n) | 18.0 (20) | 17.0 (19) | 23.4 (26) | ns |
| Thrombectomy, % (n) | 10.8 (12) | 10.7 (12) | 31.5 (35) | <0.001 |
| Stroke pathogenesis (TOAST) | ||||
| Large‐artery atherosclerosis, % (n) | 24.3 (27) | 16.1 (18) | 31.5 (35) | ns |
| Cardioembolism, % (n) | 20.7 (23) | 25.0 (28) | 30.6 (34) | <0.05 |
| Small vessel occlusion, % (n) | 21.6 (24) | 23.2 (26) | 3.6 (4) | <0.01 |
| Stroke of other determined cause, % (n) | 6.3 (7) | 7.1 (8) | 12.6 (14) | ns |
| ESUS, % (n) | 26.1 (29) | 27.7 (31) | 20.7 (23) | ns |
| MRI | ||||
| Lesion volume, cc, median (IQR) | 0.053 (0.007–0.125) | 0.86 (0.46–1.269) | 13.255 (5.770–46.219) | <0.001 |
| Total number of MCA‐ROI affections | 121 | 526 | 2175 | <0.01 |
| Pure left hemisphere strokes, % (n) | 27.9 (31) | 27.7 (31) | 28.8 (32) | ns |
| Basilar artery, % (n) | 29.7 (33) | 17.0 (19) | 10.8 (12) | <0.01 |
| MCA right, % (n) | 20.7 (23) | 27.7 (31) | 45.9 (51) | <0.01 |
| MCA left, % (n) | 27.9 (31) | 26.8 (30) | 27.0 (30) | ns |
ESUS indicates embolic stroke of unknown source; IQR, interquartile range; MCA, middle cerebral artery; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; ns, not significant; and ROI, region of interest, and TOAST, Trial of Org 10172 in Acute Stroke Treatment stroke subtype classification system.
Data of n=15 patients unavailable for this MRI analysis.
Calculated between categories using 1‐way ANOVA.
Cortical Stroke Distribution Pattern Analysis
To establish a disease model for cortical stroke distribution and to determine the number of anticipated cortical infarcts in each MCA‐ROI, it was hypothesized that stroke lesions in the precentral gyri are generally symptomatic and affected patients are always referred to the hospital. Consequently, the number of anticipated infarctions equals the number of observed infarctions in the hospital. For all other MCA‐ROIs, the cortical volume of the MCA‐adjusted Harvard‐Oxford cortical atlas ROI mask was set in relation to the volume of the precentral gyrus (PG) MCA‐ROI. Based on the assumption that cortical infarctions in the MCA territory are hemodynamically divided up equally among the terminal MCA branches, the rate of anticipated infarctions based on cortical volume was calculated for each MCA‐ROI. For example, as the MCA‐ROI for the frontal pole has 143.7% the size (35.3 cc) of the PG (24.6 cc), a higher number of infarct lesions would be assumed for that ROI in comparison. In contrary, as Heschl's gyrus is considerably smaller (2.2 cc) than the PG, only 9.0% the number of stroke hits of the PG would be assumed in this MCA‐ROI. Dividing the number of observed infarctions from the number of anticipated infarctions provides an approximated range of cortical infarcts that are potentially missed in the respective functional cortical brain area. In addition, we calculated a “conservative” disease model in which—for example, because of a hemodynamic causality so far unknown—only 50% of the number of emboli affecting the PG are embolized into the other MCA‐ROI, hence lowering the number of anticipated stroke lesions in those brain areas considerably. An approximated percentage of potentially missed silent cortical stroke lesions was calculated for each stroke lesion size by dividing the cumulative number of observed lesions by the number of anticipated lesions for all MCA‐ROIs.
Statistical Analysis
Baseline differences between groups were tested by the independent‐samples t test for parametric data, Pearson χ2 test for categorical data, and Mann–Whitney U test for nonparametric data. Comparison of multiple categories was performed using 1‐way ANOVA on ranks. Descriptive statistics were used to present baseline characteristics, results of outcome measurements, and interquartile ranges. All patients with missing data were removed from the analysis. P values were considered significant at P<0.05.
RESULTS
Study Population
In total, 671 patients were prospectively included in the neurovascular research database. After the exclusion of 38 patients, 633 patients with a mean age of 70±14.7 years were eligible for analysis in this study, of whom 58.9% were men (Table 1). MRI data were available in 434 patients, whereas 199 patients had CT only. Patients with CT only had higher National Institutes of Health Stroke Scale values (P<0.001), presented more commonly within the 4.5‐hour time window (P<0.01), and received intravenous thrombolysis (P<0.001) and endovascular treatment (P<0.001) more frequently than patients with MRI. A preponderance was shown for patients with CT only for ischemic stroke of cardioembolic pathogenesis and left‐sided MCA or posterior cerebral artery stroke (P<0.05 each). Of 423 ischemic strokes (with either CT or MRI) in the MCA territories, 223 were left and 200 were right sided (not significant). Significantly more patients with transient ischemic attack on the left side (n=52) than with transient ischemic attack on the right side were admitted to the hospital (n=24; P<0.01). No other significant differences were found between left and right hemisphere stroke. Before performing voxel‐based lesion mapping from the DWI data, 66 patients with DWI‐negative ischemic stroke (eg, transient ischemic attack) and 34 patients with either incomplete or unretrievable DWI data were excluded. Ultimately, 334 DWI data sets of patients with ischemic stroke were available for MRI analysis.
Voxel‐Based Lesion Mapping From DWI
As the first step of the imaging analysis, the lesion distribution pattern across the entire brain was assessed for all consecutive patients with MRI. Voxel‐based lesion mapping performed on 334 DWI data sets revealed distinctive distribution patterns for the 3 subgroups of ischemic core volume tertials (Figure 3). Small‐sized lesions (<0.240 cc) were predominantly distributed among white matter tracts from the brainstem to the thalamus, inner and outer capsules, and the prefrontal and postcentral gyri. A similar predominance of sensorimotor function tracts and corresponding pre‐ and postcentral gyri was apparent in medium‐sized lesions (0.240–2640 cc). In addition, a left/right asymmetry between thalamic stroke lesions and parietotemporal brain areas was found. For large‐sized lesions (>2640 cc), we found an increased frequency and a broader distribution of right‐sided ischemic lesions compared with left‐sided large stroke lesions. This corresponded to a higher overall number of large infarct lesions in the right MCA territory (n=51 right‐sided versus n=30 left‐sided; P<0.01) in the subpopulation of 334 patients with MRI. The mean lesion volumes of large right‐sided lesions (n=51; 47.5±62.1 cc) in the MCA territory did not differ significantly from those of left‐sided lesions (n=30; 34.3±44.5 cc; P=0.48). Noteworthy, 12 bilateral strokes affecting both MCA territories were included and visualized in the overlay maps (Figure 3).
MCA‐ROI–Specific Lesion Distribution Frequency
The number of observed and anticipated cortical infarcts for each MCA‐ROI is outlined for small and medium volume stroke lesions in Figure 4 in detail (for large stroke volume lesions, see Figure 5). According to our theoretical considerations, anticipated and observed infarcts in the PG are identical.
Figure 4. Distribution of small‐ and medium‐sized cortical brain infarct lesions.

The data show a similar frequency and distribution pattern for small‐volume infarct lesions (<0.24 cc) between the left (A) and right (B) sides. In medium‐sized infarct lesions (0.24–2640 cc), right‐sided stroke lesions (D) show a higher overall frequency compared with the left side (C). Furthermore, medium‐sized lesions in the insular cortex; frontal, central, and temporal opercular cortices; Heschl's gyrus; and planum temporale were observed (blue) more frequently than anticipated from the number of ipsilateral infarctions in the precentral gyri (red). MCA indicates middle cerebral artery.
Figure 5. Distribution pattern of large‐sized cortical brain infarct lesions.

The data show a higher number of stroke lesions on the right (B) compared with the left (A) hemisphere for large infarct volumes (>2640 cc). This can likely be attributed to a selection error, as only patients with magnetic resonance imaging were considered. On both sides, observed lesions (blue) in the frontal and parietal operculum cortex, planum polare, Heschl's gyrus, and planum temporale much surpass the number of lesions anticipated from the number of ipsilateral infarctions in the precentral gyri (red). MCA indicates middle cerebral artery.
Cortical Brain Areas Associated With SBI
Based on the disease hypothesis of this study, we approximated that up to 67.2% of small‐sized infarcts on the right and up to 69.3% of small‐sized infarcts on the left side did not lead to a hospital admission (Figure 4). In medium‐size volume ischemic lesions, approximately only up to 48.4% of ischemic strokes on the right and up to 43.7% of infarcts on the left side did not lead to a stroke unit admission. For large‐size volume infarcts, the number of observed stroke lesions was higher than the number of anticipated lesions (as calculated from infarct lesions to the PG). In addition, a more conservative approach with a 50% reduced probability of stroke lesions in other MCA‐ROIs when compared with the PG was performed. With this reduced probability of infarction outside the PG, still approximately up to 34.6% of small‐sized infarcts on the right and up to 39.6% of small‐sized infarcts on the left did not lead to a hospital admission. In medium‐sized strokes, the number of observed lesions was higher than the anticipated number of strokes. Figure 6 illustrates the 5 cortical MCA‐ROIs for each individual side in which—based on the conservative disease model—most cortical infarct lesions were potentially missed and did not lead to a hospital admission. For each of the depicted MCA‐ROIs, at least 20% (range, 20.0%–100%; mean, 57.9%) of anticipated stroke lesions were missed based on the conservative model. The most underrepresented brain areas in relation to their volume in this disease model for small‐sized lesions were identical for right and left side and comprised the frontal pole (65.2% and 58.2% discrepancy between anticipated and observed lesions), middle frontal gyrus (43.8% and 77.5%), temporal pole (100% and 44.3%), middle temporal gyrus posterior division (100% and 100%), and lateral occipital cortex superior division (33.3% and 46.7%). For medium‐sized stroke lesions, only 3 brain areas were identical between the 2 sides and comprised the frontal pole (100% and 91.3% discrepancy), temporal pole (88.9% and 82.6%), and lateral occipital cortex superior division (36.0% and 33.3%). A major difference was observed for the angular gyrus, in which up to 83.1% of lesions (conservative model) were clinically silent in medium‐sized stroke lesions on the right, whereas on the left side even 31.8% more lesions led to a hospital admission than anticipated from the disease model (Figure 4). Furthermore, in the right‐sided frontal orbital cortex, up to 79.4% of stroke lesions were missed, whereas on the left side even 28.5% more lesions were observed than anticipated by the disease model. In contrary, on the left side, the superior parietal lobule (66.4%) and lateral occipital cortex inferior division (58.8%) cortical MCA‐ROIs were associated with the highest discrepancy of anticipated and observed stroke lesions.
Figure 6. Brain regions with the most underrepresented stroke frequency in relation to region size.
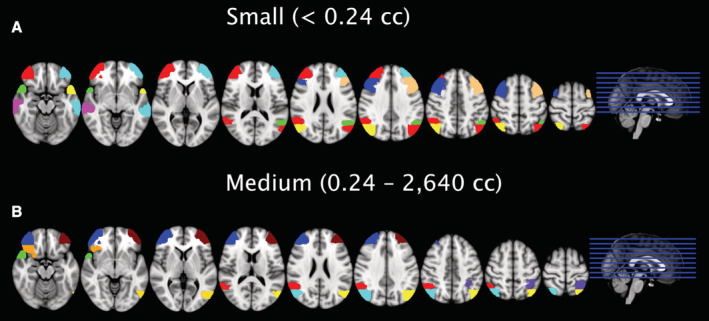
The figure demonstrates the top 5 regions of interest of the Harvard‐Oxford cortical atlas in the middle cerebral artery territory for small‐ (A) and medium‐sized (B) lesions for each side, which were most underrepresented in relation to their volume among hospitalized patients. In all presented regions of interest, a minimum of 50% of the stroke lesions were missed according to the distribution model. The calculation was based on the more conservative assumption that 50% of the number of emboli that end up in the precentral gyrus are as well embolized to other middle cerebral artery terminal cortical branches in the population.
DISCUSSION
This study sought to investigate the distribution pattern of ischemic lesions in a sample of consecutive patients with ischemic stroke admitted to our academic stroke center in a 1‐year period, with the ultimate objective to assess predilection sites of cortical SBIs and estimate their frequency from differences in the ischemic lesion distribution in patients who are symptomatic for stroke.
SBIs are common in the elderly population and take a hidden toll on the healthcare system by increasing the risk of dementia and recurrent stroke. 6 Consequently, a deeper understanding of the distribution of SBIs across the brain could improve stroke diagnosis, help identify stroke causes, and allow for the timely commencement of appropriate secondary prevention strategies. However, a particular interest lies also in the characterization of cortical SBIs, as cortical stroke is often caused by an undetected proximal source of embolism. However, because of the small absolute number of cortical SBIs in population‐based studies, the studies on asymptomatic, community‐dwelling individuals commonly lacked detailed information on the precise localization and distribution of SBIs in the cerebral cortex. 9 , 25 Therefore, the current hypothesis‐generating approach had the goal to demonstrate the inequity of the stroke lesion distribution in the cerebral cortex in more detail. The underrepresented brain cortex areas in this analysis would then be plausible predilection sites for SBIs, similar to a survivorship phenomenon. Previously, a comparable voxel‐based analyses on patients with cerebral small vessel disease demonstrated that cerebrovascular risk profiles promote T2‐weighted subcortical (white matter) lesion formation most severely at specific, well‐defined locations. 26 The current study based on diffusion‐weighted MRI data of a consecutive 1‐year sample of patients also demonstrated that symptomatic, small cortical infarcts are indeed not equally distributed across the brain. Rather, they are predominantly located along specific brain structures associated with motor control and sensory function (Figure 3A). Furthermore, a more detailed analysis of cortical ischemic stroke lesions in the MCA territory revealed a distinctive distribution pattern with an inequality between frequently affected and vastly underrepresented areas of the cerebral cortex (Figure 4A and 4B).
We hypothesized that in the population, cortical brain infarcts are hemodynamically evenly distributed in the MCA territories (with a variance allowance of up to 50%). Therefore, any observed inequity would be attributed to a previous selection process in which patients with a recognizable stroke syndrome were more frequently admitted to the hospital than patients without clinically overt stroke symptoms. This assumption allowed for approximating a frequency of up to 67% and 69% missed cortical stroke lesions in the right and left hemisphere, respectively, or according to a more conservative calculation, of up to 34% and 39% missed cortical stroke lesions on the right and on the left, respectively. Although those numbers are estimates drawn from a disease model based on the actual distribution of ischemic lesions in DWI data, these findings strongly support the notion that cortical SBIs represent a common phenomenon and that cortical infarcts are more likely to be recognized in certain cerebral cortex areas than in others. In earlier population‐based cohort studies, the mean prevalence of SBIs ranged from 5% to 62% and was strongly age dependent (range, 49–79 years), with higher frequencies observed in older patients. 9 , 25 As the current study comprised older patients with a mean age of 68.38±14.7 years, data on SBIs acquired in this hypothesis‐generating approach could well be within the expected range.
Because the main analysis of this study was based on DWI data sets, patients who received CT only could not be included in the lesion distribution analysis. Therefore, the study comprised a selected sample of patients who qualified for MRI in the clinical setting, which is a potential selection bias. To address this possibility of a sampling error, we performed a consecutive patient enrollment of 1 year (January 1 to December 31, 2018) and included all patients without regard of imaging modality in the baseline analysis. We then aimed to characterize the 2 patient subgroups based on the chosen imaging modality (MRI or CT) to demonstrate how they differed in regard to baseline and clinical parameters. Importantly, the majority of patients with stroke received MRI during their hospital stay (68.6%). The patients receiving CT only mainly comprised stroke syndromes of the left hemisphere with higher National Institutes of Health Stroke Scale scores that presented within the 4.5‐hour time window, hence directly qualifying for intravenous thrombolysis and/or mechanical thrombectomy without further delay by additional MRI. Therefore, when interpreting the data of this study, it must be considered that some “striking” stroke syndromes were not included in the MRI analysis.
The aim of this study was to describe how cortical brain infarcts in the MCA territory disperse in the cerebral cortex as accurately as possible. Prior studies already demonstrated that symptomatic lacunar white matter lesions typically involved the main motor and sensory fiber tracts. 27 , 28 It was therefore plausible that also small cortical cerebral infarcts would show a similar pattern in this study (Figures 3A and 4A, 4B). Interestingly, the cortex areas associated with higher language function were rarely affected in small‐sized infarcts (up to 0.24 cc), presumably because the lesion volume was too small to cause a symptomatic deficit (eg, aphasia). In relation to their volume, the frontal (frontal pole, middle frontal gyrus), temporal (temporal pole, middle temporal gyrus posterior division), and occipital (lateral occipital cortex superior division) cortices were underrepresented with regard to the presence of ischemic lesions. Hence, those brain areas may be especially prone to staying below the clinical detection threshold and frequently harbor cortical SBIs. With an increase in lesion size (0.24–2640 cc, medium), cortical lesions to areas associated with higher cortical functions (such as language), for example, in the angular gyrus, planum temporale, and operculum cortex, now became clinically overt, causing a hospital admission. In the other cortex areas, the distribution pattern was very similar between small‐ and medium‐sized stroke lesions.
It is well known that left hemisphere stroke lesions are overrepresented among hospitalized patients, although ischemic strokes disperse equally among both hemispheres in the general population. 29 , 30 This lateralization inequality is commonly attributed to linguistic, dominant‐hemisphere cortical symptoms in territorial MCA and thalamic stroke. 31 In the current study, no significant differences between the left and the right side were found in small infarctions. In all likelihood, lesions <0.24 cc are too small to cause higher function cortical symptoms and effectively intertwine with large language system processing tracts such as the arcuate fascicle. 32 Consequently, as lesion volume got large enough to interfere with linguistic connectivity, we encountered differences between medium‐sized infarct lesions on the left and right in the thalamus and angular gyri. However, the most underrepresented stroke regions in relation to their size were completely identical between the left and right hemispheres (Figure 4). This might be rather unexpected, as one could have argued that especially infarctions in the right hemisphere would go unnoticed, 33 whereas medium‐sized left hemisphere strokes would more commonly present in the hospital. As this was not the case, we conclude that the effect of a clinically covert frontal, temporal, and occipital brain in stroke care is stronger than the differences between the hemispheres concerning the respective higher cortical functions (eg, aphasia versus neglect). In large and very large infarct volumes (>2640 cc)—assumably associated with territorial infarction—lesion mapping showed a higher frequency and a broader dissemination of right‐sided stroke lesions. This could be explained by the sample selection bias of patients with MRI only, as patients with an easily recognizable left‐sided (dominant) hemisphere syndrome with leading symptoms of aphasia in addition to hemiparesis might have been more likely to receive CT only before recanalizing therapy. In contrary, right‐sided territorial stroke lesions may present with less obvious stroke symptoms such as neglect, dysarthria, or anosognosia and may have frequently needed MRI for confirmation of stroke. Interestingly, Wu et al presented a very similar stroke lesion distribution pattern based on MRI, although this effect was left undiscussed by the authors. 3 Because of this sampling error concerning large lesions and the improbability of clinically asymptomatic large or very large territorial stroke lesions, stroke lesions >2640 cc were not considered in the analysis of SBI distribution in this study.
The findings of this study demonstrate that brain infarctions in the cerebral cortex of hospitalized patients with stroke are clustered in certain brain regions to a higher degree than presumably explainable by hemodynamic inequality, and some brain areas are vastly underrepresented in relation to their size. We argue that this implies the presence of a clinical selection process of patients with more easily recognizable stroke symptoms, such as deficits in motor function, sensation, language, or speech. Knowledge of underrepresented, “silent” areas of the brain may help to determine more subtle symptoms of ischemia in the frontal, temporal, and occipital brain areas (see Figure 4) that are still insufficiently associated with the presence of stroke, such as avolition, change in character, acute psychosis, memory problems, or complex visual–spatial disorders.
It was already pointed out that the detection and subsequent initiation of prophylactic treatment of white matter SBIs are crucial to prevent stroke sequelae and vascular dementia. 6 , 9 , 10 An analysis of the Rotterdam study revealed a connection between markers of cerebral small vessel disease and asymptomatic DWI lesions, which also increased the risk of further strokes, although the true benefit of screening and treating people with DWI lesions was left to further investigation. 13 However, because of their embolic origin, early recognition of cortical SBI is of even greater diagnostic value and might precede prophylactic anticoagulant treatment or carotid artery interventions. 8 In contrast to small vessel disease, treating a proximal source of embolism may prevent high‐impact stroke syndromes because of large vessel (eg, M1 or basilar artery) occlusion. Consequently, the American Heart Association highlighted the categories of cardioembolism and large vessel disease as the major areas of interest in relation to SBIs because of the strong relationship between diagnostic risk stratification and treatment decisions. 11 For example, a patient with multiple cortical SBIs might benefit from long‐term ECG monitoring to detect subclinical atrial fibrillation, which could then be addressed with oral anticoagulation. 8 The application of intensified magnetic resonance screening programs for cortical SBIs in patients with a cardiovascular risk profile might help prevent subsequent symptomatic embolic stroke, including large vessel occlusion, thus reducing the burden of stroke considerably in the future population.
Limitations
The limitations of this study include the limited sample size of 671 patients with stroke in the study and only 334 data sets available for MRI analysis. A strength, however, is the recruitment of all consecutive patients in 1 year (2018), which did considerably reduce the risk of a sample selection error. Furthermore, because of the limited sample size, we were able to allow for an intricate analysis with manual, slice‐by‐slice correction of every DWI image set. Noteworthy, the findings of this study are limited by a selection bias as all patients with CT only could not be included in the voxel‐based MRI analysis. Therefore, a part of the patient sample could not be considered for in‐depth SBI analysis. However, this methodological limitation, which applies to most retrospective studies in the field, was specifically addressed in this study, and comprehensive information characterizing both subgroups was provided (Table 1). Furthermore, the core hypothesis of this study implicates a disease model by which the presence of evenly distributed cortical stroke lesions in the brain is postulated. Based on this presumption, the amount of “missed,” nonobserved lesions was approximated. This assumption of an anatomical and hemodynamic equivalence of cortical stroke lesions in the population cannot yet be backed by literature. However, we are not aware of data in the literature suggesting a preferred embolization pattern within the MCA territory. Furthermore, in a more conservative approach, we allowed for a hemodynamic variance of up to 50% in this study—with similar results.
CONCLUSIONS
This hypothesis‐generating study demonstrates that cortical brain infarcts are not distributed equally in hospitalized patients but, rather, are predominantly associated with structures responsible for sensorimotor or language function. Matching these underrepresented “silent” cerebral cortex areas to specific symptoms not associated with stroke warrants further exploration.
Sources of Funding
None.
Disclosures
Dr Schaller‐Paule, Dr Fritz, Dr Schaefer, Dr Hattingen, and Dr Seiler declare that there is no conflict of interest to report. Dr Foerch received speakers' honoraria from Boehringer Ingelheim and Bristol Myers Squibb and honoraria for participating in advisory boards from Boehringer Ingelheim and Prediction Bioscience.
For Sources of Funding and Disclosures, see page 13.
References
- 1. Payabvash S, Taleb S, Benson JC, McKinney AM. Acute ischemic stroke infarct topology: association with lesion volume and severity of symptoms at admission and discharge. AJNR Am J Neuroradiol. 2017;38:58–63. doi: 10.3174/ajnr.A4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menezes NM, Ay H, Wang Zhu M, Lopez CJ, Singhal AB, Karonen JO, Aronen HJ, Liu Y, Nuutinen J, Koroshetz WJ, et al. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke. 2007;38:194–197. doi: 10.1161/01.STR.0000251792.76080.45 [DOI] [PubMed] [Google Scholar]
- 3. Wu O, Cloonan L, Mocking SJ, Bouts MJ, Copen WA, Cougo‐Pinto PT, Fitzpatrick K, Kanakis A, Schaefer PW, Rosand J, et al. Role of acute lesion topography in initial ischemic stroke severity and long‐term functional outcomes. Stroke. 2015;46:2438–2444. doi: 10.1161/STROKEAHA.115.009643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng B, Forkert ND, Zavaglia M, Hilgetag CC, Golsari A, Siemonsen S, Fiehler J, Pedraza S, Puig J, Cho TH, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke. 2014;45:1695–1702. doi: 10.1161/STROKEAHA.114.005152 [DOI] [PubMed] [Google Scholar]
- 5. Ernst M, Boers AMM, Aigner A, Berkhemer OA, Yoo AJ, Roos YB, Dippel DWJ, van der Lugt A, van Oostenbrugge RJ, van Zwam WH, et al. Association of computed tomography ischemic lesion location with functional outcome in acute large vessel occlusion ischemic stroke. Stroke. 2017;48:2426–2433. doi: 10.1161/STROKEAHA.117.017513 [DOI] [PubMed] [Google Scholar]
- 6. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9 [DOI] [PubMed] [Google Scholar]
- 7. Eskioglou E, Huchmandzadeh Millotte M, Amiguet M, Michel P. National Institutes of Health Stroke Scale zero strokes. Stroke. 2018;49:3057–3059. doi: 10.1161/STROKEAHA.118.022517 [DOI] [PubMed] [Google Scholar]
- 8. Meinel TR, Kaesmacher J, Roten L, Fischer U. Covert brain infarction: towards precision medicine in research, diagnosis, and therapy for a silent pandemic. Stroke. 2020;51:2597–2606. doi: 10.1161/STROKEAHA.120.030686 [DOI] [PubMed] [Google Scholar]
- 9. Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, et al. Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke. 2016;47:719–725. doi: 10.1161/STROKEAHA.115.011889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population‐based study in 85‐year‐old subjects. Stroke. 2004;35:1816–1820. doi: 10.1161/01.STR.0000131928.47478.44 [DOI] [PubMed] [Google Scholar]
- 11. Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 12. Das RR, Seshadri S, Beiser AS, Kelly‐Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hilal S, Baaij LGA, de Groot M, Niessen WJ, Ikram MK, Ikram MA, Vernooij MW. Prevalence and clinical relevance of diffusion‐weighted imaging lesions: the Rotterdam study. Neurology. 2019;93:e1058–e1067. doi: 10.1212/WNL.0000000000008090 [DOI] [PubMed] [Google Scholar]
- 14. Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population‐based Rotterdam Scan Study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629 [DOI] [PubMed] [Google Scholar]
- 15. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1–5. doi: 10.1159/000352050 [DOI] [PubMed] [Google Scholar]
- 16. Hahne K, Monnig G, Samol A. Atrial fibrillation and silent stroke: links, risks, and challenges. Vasc Health Risk Manag. 2016;12:65–74. doi: 10.2147/VHRM.S81807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, Ruskin JN. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:650–658. doi: 10.7326/M14-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baradaran H, Gialdini G, Mtui E, Askin G, Kamel H, Gupta A. Silent brain infarction in patients with asymptomatic carotid artery atherosclerotic disease. Stroke. 2016;47:1368–1370. doi: 10.1161/STROKEAHA.116.013193 [DOI] [PubMed] [Google Scholar]
- 19. Finn C, Giambrone AE, Gialdini G, Delgado D, Baradaran H, Kamel H, Gupta A. The association between carotid artery atherosclerosis and silent brain infarction: a systematic review and meta‐analysis. J Stroke Cerebrovasc Dis. 2017;26:1594–1601. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purushotham A, Campbell BC, Straka M, Mlynash M, Olivot JM, Bammer R, Kemp SM, Albers GW, Lansberg MG. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. 2015;10:348–353. doi: 10.1111/ijs.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Gobin YP, Vinuela F, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380 [DOI] [PubMed] [Google Scholar]
- 22. Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699 [DOI] [PubMed] [Google Scholar]
- 23. Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 24. Mori S, Wakana S, Van Zijl PC, Nagae‐Poetscher LM. MRI Atlas of Human White Matter. 1st ed. ‐ May 11, 2005; eBook ISBN: 9780080456164. Elsevier Science; 2005. Available at: https://www.elsevier.com/books/mri‐atlas‐of‐human‐white‐matter/mori/978‐0‐444‐51741‐8. Accessed April 15, 2022. [Google Scholar]
- 25. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population‐based cohorts. BMC Med. 2014;12:119. doi: 10.1186/s12916-014-0119-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altermatt A, Gaetano L, Magon S, Bauer L, Feurer R, Gnahn H, Hartmann J, Seifert CL, Poppert H, Wuerfel J, et al. Clinical associations of T2‐weighted lesion load and lesion location in small vessel disease: insights from a large prospective cohort study. Neuroimage. 2019;189:727–733. doi: 10.1016/j.neuroimage.2019.01.052 [DOI] [PubMed] [Google Scholar]
- 27. Song YM. Distinct location of subcortical silent infarcts compared with symptomatic lacunar infarcts. J Neurol Sci. 2009;287:197–199. doi: 10.1016/j.jns.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 28. Valdes Hernandez Mdel C, Maconick LC, Munoz Maniega S, Wang X, Wiseman S, Armitage PA, Doubal FN, Makin S, Sudlow CL, Dennis MS, et al. A comparison of location of acute symptomatic vs. 'silent' small vessel lesions. Int J Stroke. 2015;10:1044–1050. doi: 10.1111/ijs.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Portegies ML, Selwaness M, Hofman A, Koudstaal PJ, Vernooij MW, Ikram MA. Left‐sided strokes are more often recognized than right‐sided strokes: the Rotterdam Study. Stroke. 2015;46:252–254. doi: 10.1161/STROKEAHA.114.007385 [DOI] [PubMed] [Google Scholar]
- 30. Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann‐Haefelin T, Arbeitsgruppe SH. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366:392–393. doi: 10.1016/S0140-6736(05)67024-9 [DOI] [PubMed] [Google Scholar]
- 31. Schaller‐Paule MA, Oeckel AM, Schure JR, Keil F, Hattingen E, Foerch C, Rauch M. Isolated thalamic stroke—analysis of clinical characteristics and asymmetry of lesion distribution in a retrospective cohort study. Neurol Res Pract. 2021;3:49. doi: 10.1186/s42466-021-00148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Group ES . Silent brain infarction in nonrheumatic atrial fibrillation. EAFT Study Group. European Atrial Fibrillation Trial. Neurology. 1996;46:159–165. doi: 10.1212/wnl.46.1.159 [DOI] [PubMed] [Google Scholar]


