Abstract
Background
Despite well‐recognized differences in the atherosclerotic cardiovascular disease risk between men and women, sex differences in risk factors and sex‐specific mechanisms in the pathophysiology of atherosclerotic cardiovascular disease remain poorly understood. Lipid metabolism plays a central role in the development of atherosclerotic cardiovascular disease. Understanding sex differences in lipids and their genetic determinants could provide mechanistic insights into sex differences in atherosclerotic cardiovascular disease and aid in precise risk assessment. Herein, we examined sex differences in plasma lipidome and heterogeneity in genetic influences on lipidome in men and women through sex‐stratified genome‐wide association analyses.
Methods and Results
We used data consisting of 179 lipid species measured by shotgun lipidomics in 7266 individuals from the Finnish GeneRISK cohort and sought for replication using independent data from 2045 participants. Significant sex differences in the levels of 141 lipid species were observed (P<7.0×10−4). Interestingly, 121 lipid species showed significant age‐sex interactions, with opposite age‐related changes in 39 lipid species. In general, most of the cholesteryl esters, ceramides, lysophospholipids, and glycerides were higher in 45‐ to 50‐year‐old men compared with women of same age, but the sex differences narrowed down or reversed with age. We did not observe any major differences in genetic effect in the sex‐stratified genome‐wide association analyses, which suggests that common genetic variants do not have a major role in sex differences in lipidome.
Conclusions
Our study provides a comprehensive view of sex differences in circulatory lipids pointing to potential sex differences in lipid metabolism and highlights the need for sex‐ and age‐specific prevention strategies.
Keywords: lipidome, sex differences, sex‐stratified genome‐wide association study
Subject Categories: Biomarkers; Basic Science Research; Genetic, Association Studies; Precision Medicine
Nonstandard Abbreviations and Acronyms
- TC
total cholesterol
Clinical Perspective.
What Is New?
Lipidomes exhibit significant sex differences that tend to narrow down or reverse with age because of distinct or opposite effect of age on lipidomes in men and women.
The study presents first comprehensive investigation of sexual heterogeneity in genetic influences on plasma lipidome and does not support a major role of common genetic variants in sex differences in lipidome.
What Are the Clinical Implications?
Sex differences in the lipidome may provide further insights into the mechanism in sex disparity in cardiovascular disease burden, with more studies needed in the future.
With lipidomic biomarkers entering clinic, it is important to understand age‐dependent sex differences in their concentrations for effective prediction and prevention strategies.
Although atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death among both men and women worldwide, 1 there are substantial sex differences in the prevalence and burden of its manifestations. 2 , 3 Despite many efforts, ASCVD remains understudied, underrecognized, underdiagnosed, and undertreated in women. 4 Limited understanding of sex differences in cause and clinical presentations of ASCVD often leads to misdiagnosis in women, resulting in higher disease burden and mortality among women compared with men. 4 This emphasizes an urgent need for unraveling underlying biological mechanisms that contribute to sex difference in ASCVD pathophysiology to develop sex‐specific strategies for early detection and prevention.
Plasma lipids are well‐established heritable risk factors for ASCVD 5 and are routinely monitored to assess its clinical risk. 6 , 7 Sex differences in plasma levels of total cholesterol (TC), triglycerides, high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) (referred henceforth as traditional lipids) have been recognized. 8 , 9 However, understanding of sex differences in detailed lipidome profiles is limited and less explored. 10 , 11 Several studies have demonstrated potential of lipidomics that allow simultaneous measurements of hundreds of lipid species or subspecies in understanding ASCVD and risk prediction beyond traditional lipids. 12 , 13 , 14 , 15 , 16 Integration of lipidomics with genomics has also provided new insights into genetic regulation of lipid metabolism and ASCVD pathophysiology. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Strong influence of genetic variants, such as FADS1‐2‐3, LDLR, and PCSK9, in maintenance of lipid homeostasis, calls for a comprehensive evaluation of potential role of genetic mechanisms in sex differences in lipid metabolism. Thus, stronger consideration should be given to understand sex differences in lipidome and their genetic determinants.
In this study, we examined sex differences in human plasma lipidome in a large data set comprising 179 lipid species measured by shotgun lipidomics from 7266 participants from the GeneRISK cohort. We further performed sex‐stratified genome‐wide association analyses for all the lipid species to evaluate contribution of genetic factors in sex differences in lipidomes. We sought for replication of the findings in an independent data set comprising 169 lipid species from 2045 participants measured by the same lipidomics platform. Our results show that lipidome exhibits significant age‐dependent differences between men and women, pointing to potential sex differences in lipid metabolism, and highlights the need for sex‐ and age‐stratified analyses in lipidome‐based studies. The sex‐stratified genome‐wide association analyses did not suggest a major role of common genetic variants in sex differences in lipidome.
METHODS
The summary‐level data supporting the findings of this study are available within the article. Full data are available through the Institute for Molecular Medicine Finland Data Access Committee for authorized researchers who have an institutional review board/ethics approval and an institutionally approved study plan.
Study Participants
The study included participants from the following cohorts.
GeneRISK Cohort
The study included 7292 participants, aged 45 to 66 years, from the ongoing prospective GeneRISK cohort, recruited during 2015 to 2017 from Southern Finland. The recruitment process and sample collection procedures are described in detail in another study. 25 Briefly, participants were instructed to fast overnight for 10 hours before the blood samples were collected for plasma, serum, and DNA extraction. Fasting serum lipids, including HDL‐C, LDL‐C, triglycerides, TC, apolipoprotein A1, and apolipoprotein B, were measured using standard enzymatic methods. The 10‐year ASCVD risk was calculated using the estimates given in Widén et al, 25 including age, sex, smoking status, TC, HDL‐C, systolic blood pressure, current use of antihypertensive medication, and family history of early‐onset coronary heart disease as risk factors. The 10‐year ASCVD estimates show the combined risk for events of coronary artery disease and stroke and were interpreted as outlined in the Finnish National Guidelines (ie, a 10‐year risk <2% is considered low, 2%–10% is intermediate, and >10% is considered high risk). GeneRISK study participants' DNA, blood, serum, and plasma samples, in addition to their demographic information and health data, have been stored in the THL Biobank (https://www.thl.fi/en/web/thlfien/topics/information‐packages/thl‐biobank).
Replication Cohorts
For the replication of the findings from the lipidome analyses, we included data from 2181 participants from the EUFAM (European Multicenter Study on Familial Dyslipidemias in Patients with Premature Coronary Heart Disease) and FINRISK cohorts with lipidomics and genetic data that we have reported previously. 21 Sample recruitment and study protocols for the EUFAM and FINRISK cohorts have been described previously. 26 , 27 Briefly, the EUFAM study cohort is composed of the Finnish familial combined hyperlipidemia families and composed of related individuals. The Finnish National FINRISK study is a population‐based survey conducted every 5 years since 1972, and thus far samples have been collected in 1992, 1997, 2002, 2007, and 2012, and are stored in the (National Institute for Health and Welfare/THL) Biobank.
To replicate the findings from the analyses of traditional lipids, we used lipid measurements (TC, LDL‐C, HDL‐C, triglycerides, apolipoprotein A1, and apolipoprotein B) for 24 614 individuals (11 238 women and 13 376 men) from the UK Biobank who had fasted between 7 and 15 hours before blood sample collection. We excluded individuals with reported fasting duration <7 or >15 hours to reduce potential bias that might arise because of difference in fasting duration. Information on participants' age, body mass index, diabetes, smoking status, medications, and hormone replacement therapy was also obtained to be used as covariates. Details on sample handling and assays used for lipid measurements in the UK Biobank cohort have been described previously 28 (https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf).
Ethical Statement
The study was performed according to the principles of the Declaration of Helsinki and the Council of Europe's Convention of Human Rights and Biomedicine. All study participants gave their informed consent to participate in the study. The study protocols were approved by the ethics committees of the participating centers (The Hospital District of Helsinki and Uusimaa Coordinating Ethics committees, approval Nos. 201/13/03/00/14 and 184/13/03/00/12).
Shotgun Lipidomics
Lipidomic measurements were performed using mass spectrometry–based shotgun lipidomic analysis at Lipotype GmbH (Dresden, Germany). Samples were analyzed by direct infusion in a QExactive mass spectrometer (Thermo Scientific) equipped with a TriVersa NanoMate ion source (Advion Biosciences). 29 Data were analyzed using in‐house developed lipid identification software and data management system. 30 , 31 Lipids with signal/noise ratio >5 and amounts >5‐fold higher than in corresponding blank samples were considered. Reproducibility was assessed by the inclusion of 8 reference plasma samples per 96‐well plate. Using 8 reference samples per 96‐well plate batch, lipid amounts were corrected for batch variations and for analytical drift if the P value of the slope was <0.05 with an R2 >0.75 and the relative drift was >5%. Five samples with low total lipids content and number of lipids detected were removed, and lipid species detected in <70% of the remaining samples were excluded. Furthermore, samples with >30% missingness for the quality control (QC) passed lipid species were also excluded. After QC, lipidomics data in GeneRISK cohort were composed of 179 lipid species from 13 lipid classes for 7266 individuals, including 2624 men and 4642 women. As expected, many lipid species are highly correlated, with 70 principal components explaining >90% of the variation in the data. After the same QC procedures in the replication cohorts, data for 169 lipid species matching with GeneRISK data for 2045 individuals were available.
Genotyping and Imputation
Genotyping for the GeneRISK study participants was performed using the HumanCoreExome BeadChip (Illumina Inc, San Diego, CA). The genotypes were called using GenomeStudio and zCall at the Institute for Molecular Medicine Finland. Genotyping data were lifted over to build version 38 (GRCh38/hg38) (as described in dx.doi.org/10.17504/protocols.io.nqtddwn). Preimputation QC included exclusion of individuals with <95% call rate, discrepancies between biological and reported sex, extreme heterozygosity (±4 SDs), and non‐Finnish ancestry, as well as of variants with <98% call rate, deviation from Hardy‐Weinberg equilibrium (P<1×10−6), and minor allele frequency <0.05. Prephasing of genotyped data was performed with Eagle 2.3.5 with the number of conditioning haplotypes set to 20 000. 32 Imputation was done with Beagle 4.1 33 (as described in https://doi.org/10.17504/protocols.io.nmndc5e) using population‐specific Sequencing Initiative Suomi v3 reference panel that was developed from high‐coverage (25×–30×) whole‐genome sequences for 3775 Finnish individuals. Postimputation QC included exclusion of variants with imputation information score <0.70 and minor allele frequency <0.01. Genotyping for both the EUFAM and FINRISK cohorts was performed using the HumanCoreExome BeadChip. Details about the quality control and imputation of the EUFAM and FINRISK cohorts have been described previously. 21
Statistical Analysis Investigating Sex Differences in Lipidome
All statistical analyses related to age and sex differences and data visualization were done using R 3.6.3. Associations with P<7.1×10−4 (P<0.05/70 principal components to adjust for multiple tests) were considered statistically significant. Lipid measurements were log10 transformed before the analyses. Age‐related trends in traditional lipids, apolipoprotein A1, and apolipoprotein B were determined by calculating mean levels at each 5‐year interval for age ranges of 45 to 50, 51 to 55, 56 to 60, and 61 to 66 years. To visualize changes in lipidome profiles with age in the heatmap, mean levels at each 1‐year interval for each lipid species were calculated separately in men and women and were normalized to the respective mean levels of those aged 45 to 50 years (used as reference groups). For normalization, mean value of each lipid species for the reference group was subtracted from each 1‐year interval mean level of that lipid and then divided by the SD of the lipid in the reference group for both sexes. Linear regression analyses were performed to statistically determine relationship between log10 lipid levels and age, separately in men and women, adjusted for body mass index, diabetes, cardiac disease, lipid‐lowering medication, and smoking; and additionally, for hormone replacement therapy for women. Interaction between age and sex was determined using age, sex, body mass index, diabetes, cardiac disease, lipid‐lowering medication, and smoking habits as covariates and interaction term for age and sex. Differences between the lipidome profiles of men and women were evaluated for each 5‐year interval by linear regression, adjusting for age, body mass index, diabetes, cardiac disease, lipid‐lowering medication, and smoking habits. Differences between the lipidome profiles of men and women in full data set (without age‐based stratification) were additionally adjusted for age.2 Menopause‐related analyses were performed in a subgroup of participants aged 45 to 55 years to assess age‐independent effect of menopause. Menopause status was assessed through questionnaire filled at the time of recruitment and confirmed by the status reported during the follow‐up visit in the GeneRISK study (≈2 years later). Only women with consistent reporting of menopause status (524 premenopausal and 663 postmenopausal women) at 2 visits were included. Heterogeneity in the effect sizes between 2 groups was estimated using the following equation:
P values for heterogeneity (P het) were obtained from Hetß under the null assumption of equal effect sizes in the 2 groups (referred to as a and b in the equation), from the standard χ2 distribution with 1 degree of freedom. For better visualization and interpretation, the effect sizes are presented as percentage differences (or changes) calculated from β coefficients obtained from linear regression analyses (difference [%]=100×[10β−1] as log10 lipid levels were used in linear regression models).
Sex‐Stratified Genome‐Wide Association Analyses
For sex‐stratified genome‐wide association study (GWAS) in the GeneRISK cohort, residuals obtained after regressing for age, age2, collection site, lipid medication, and first 10 genetic principal components, and additionally for menopause status and hormone replacement therapy in women, were inverse‐normal transformed separately in men and women and were used as outcome variables. Genetic relatedness between the participants was estimated using KING algorithm implemented in PLINK2.0 (http://pngu.mgh.harvard.edu/purcell/plink/ 34 ), and 585 pairs of individuals with second‐degree or closer relatedness in the GeneRISK cohort were found. GWASs were performed using linear regression model implemented in PLINK2.0. The effect estimates for genetic variants are presented as the change in lipid levels in standardized units per allele of a variant. Heterogeneity in the effect sizes between men and women was assessed using the equation provided above for Hetß. In the genome‐wide scans, associations with P<7.1×10−10 (5.0×10−8/70 principal components explaining >90% variance) were considered statistically significant. For the replication of the identified associations, association analyses in the replication cohort were performed using sex‐specific inverse‐normal transformed lipid levels adjusted for age, age2, cohort, lipid medication, familial hyperlipidemia, first 10 genetic principal components, and additionally for hormone replacement therapy in women using linear mixed model implemented in MMM. 35
RESULTS
After quality control, lipidomics data in GeneRISK cohort were composed of 179 lipid species from 13 lipid classes from 7266 individuals, including 2624 men and 4642 women. The basic clinical characteristics of the study population are provided in Table S1. The details of the lipid species included in the study and their mean plasma levels are provided in Figure S1 and Table S2. Men and women participants had similar age distributions, ranging from 45 to 66 years, with mean age of 55.9 (±5.9) and 55.7 (±5.7) years, respectively. Consistent with the known sex differences in ASCVD risk, women participants had relatively lower estimated 10‐year ASCVD risk and higher levels of HDL‐C and apolipoprotein A1 compared with men (Figure 1A and 1B). Men had significantly higher levels of LDL‐C, triglycerides, and apolipoprotein B compared with those of women (Table S3). However, levels of TC, LDL‐C, and triglycerides showed age‐dependent sex differences (age‐sex interaction term P<7.0×10−4) (Table S4), resulting in either reversed or abrogated differences in older age groups (Figure 1B). Similar age‐dependent sex differences in traditional lipids were observed in the UK Biobank data set (Figure S2 and Table S3).
Figure 1. Sex differences in atherosclerotic cardiovascular disease (ASCVD) risk and traditional plasma lipids.
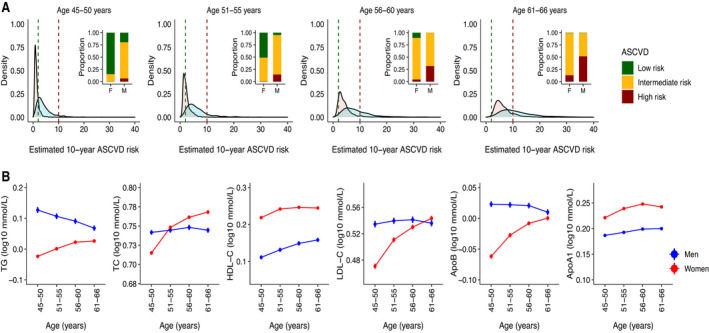
A, Distributions of 10‐year ASCVD risk estimated on the basis of classic risk factors in men (blue) and women (pink) at 5‐year interval. The vertical green and red lines on density plots mark the low (<2%) and high (>10%) 10‐year estimated risk for ASCVD, respectively. The bar plot inserts in each density plot show the proportion of men and women with low risk (<2%), intermediate risk (2%–10%), and high risk (>10%) for ASCVD in the respective age group. Individuals with preexisting medical conditions (N=795) were not included in data used for these plots. B, Age‐related trends for traditional lipids in the GeneRISK cohort. Mean levels after log10 transformation and SEs of total cholesterol (TC), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), apolipoprotein B (ApoB), and apolipoprotein A1 (ApoA1) at 5‐year intervals are plotted for men (blue) and women (red). Individuals with lipid‐lowering medications were excluded before calculating the mean levels of lipids for this analysis.
Age and Sex Interactions in Lipid Species Levels
Of 179 lipid species, significant differences in plasma levels of 141 lipid species between men and women were observed (Figure 2A). However, as descriptive analysis of traditional lipids suggested significant effect of age and sex interaction in circulating lipid levels, we further evaluated for age‐sex interactions in individual lipid species. Significant age‐sex interactions for 121 lipid species (interaction term P<7.0×10−4) were found in models including covariates and age‐sex interaction term (Table S4). A total of 116 of these 121 lipid species also showed significant sex heterogeneity in their effect sizes for association with age (P het<7.0×10−4) (Figure 2B and Table S5). Effect of age‐sex interactions on lipidome is also evident from the heatmaps depicting 1‐year change (in SD units adjusted for covariates) in plasma levels of each lipid species in men and women relative to 45‐ to 50‐year‐old men and women, respectively (Figure S3, Data Set 1). Among men, age was associated with decrease in levels of 72 lipid species and increase in levels of 10 lipid species (Table S5 and Figure S4). On the contrary, age was positively associated with 108 lipids and negatively associated only with 3 lipid species in women. Interestingly, of the 51 lipid species that were significantly associated with age in both men and women, 39 lipid species showed opposite age‐related trends in men and women. Sensitivity analyses after excluding individuals with lipid‐lowering medications provided similar results (Table S5).
Figure 2. Age and sex interactions in the levels of circulatory lipids.

A, Association of 179 lipid species with sex. The plot shows percentage differences in lipid levels between men and women in full data set on the x axis and the corresponding P values on the y axis. Positive difference means higher level in men, and negative difference means higher level in women. The effect sizes and P values were obtained using linear regression models, including log10 lipid levels as outcomes, sex as independent variable, with age, age2, body mass index (BMI), diabetes, cardiac disease, lipid‐lowering medication, and smoking as covariates. B, Heterogeneity in association of lipid species with age between men and women. Scatterplot shows percentage change in lipid levels per year in women on the x axis and percentage change in lipid levels per year in men on the y axis. The models estimating relationship between log10 lipid levels and age measured in years were adjusted for BMI, diabetes, cardiac disease, lipid‐lowering medication, and smoking (and additionally for hormone replacement therapy for women). Lipid species with significant heterogeneity (P het<7.0×10−4) are filled with white color and colored by lipid class. C, Age‐related trends for representative lipid species in men and women, illustrating effect of age on sex differences. Mean plasma levels with SE for 1‐year interval are plotted for men (blue) and women (red). Phosphatidylcholine (PC) 18:1;0_20:4;0: opposite trends in men and women leading to increased sex difference with age; triacylglyceride (TAG) 56:6;0: opposite trends in men and women resulting in narrowing of sex difference; ceramide (CER) 42:2;2: association with age only in women, resulting in narrowing of sex difference; PC 16:0;0_22:6;0: similar age‐related trends in men and women with no effect heterogeneity. CE indicates cholesteryl ester; DAG, diacylglyceride; LPC, lysophosphatidylcholine; LPE, lysophosphatidylamine; PCO, phosphatidylcholine‐ether; PE, phosphatidylamine; PEO, phosphatidylamine‐ether; PI, phosphatidylinositol; SM, sphingomyelin; ST, free cholesterol; and TAG, triacylglyceride.
Consistent age‐sex interactions were also observed in replication analysis that included 169 lipid species that matched with the GeneRISK lipidomics data set (Table S6). From 121 lipid species with significant age‐sex interactions in the GeneRISK cohort, 113 lipid species were available in replication cohort, of which 88 lipid species had significant age‐sex interactions (P<0.05) (Table S4). Moreover, of the 82 and 111 lipid species that were associated with age in men and women, respectively, in the GeneRISK, 67 and 52 lipid species, respectively (of 79 and 104, respectively, available in replication cohort), were validated in replication cohort (P<0.05) (Table S7 and Figure S5). Overall, most of the age‐associated lipid species in the GeneRISK cohort had similar effect sizes in the replication cohort (r 2 in men=0.84; r 2 in women=0.63) (Figure S6).
Age‐Dependent Sex Differences in Lipid Species Levels
As we observed significant age‐sex interactions in lipidome, we present sex differences in lipid species at 5‐year intervals (Table S8). Although levels of 81 lipid species, including triacylglycerides, diacylglycerides, cholesteryl esters, ceramides, and lysophospholipids, were higher among men aged 45 to 50 years, decline in their levels with age resulted in significantly lower levels of most lipid species (N=85) in 61‐ to 66‐year‐old men compared with the women of same age range. Effect of distinct age‐related changes on sex differences in lipid levels are depicted for representative lipid species in Figure 2C and for all lipids in Figure S7. Sex differences in triacylglycerides and diacylglycerides narrowed down with age, and finally resulted in similar triacylglyceride and diacylglyceride profiles in men and women in older age (Figure 3). On the other hand, sex differences in sphingomyelins continued to increase with age, whereas sex differences in most of the phospholipids and cholesteryl esters either abrogated or reversed in direction with age, resulting in lower phospholipids and cholesteryl esters in older men compared with women of the same age group. Altogether, from the 109 lipid species that had significant sex differences in 45‐ to 50‐year‐old participants, 55 lipid species, including triacylglycerides, diacylglycerides, and phospholipids with polyunsaturated fatty acids, did not remain significant in older participants, whereas 11 lipid species reversed the gaps. Analysis in the replication cohort provided similar trends in age‐dependent sex differences in most of the lipid species (Table S9 and Figure S8). Strong correlation between effect sizes for association with sex in the GeneRISK and replication cohort at different age groups was found (r 2 45‐50y=0.94, r 2 51‐55y=0.85, r 2 56‐60y=0.85, and r 2 61‐66y=0.75) (Figure S9).
Figure 3. Age‐dependent sex differences in plasma lipidome.
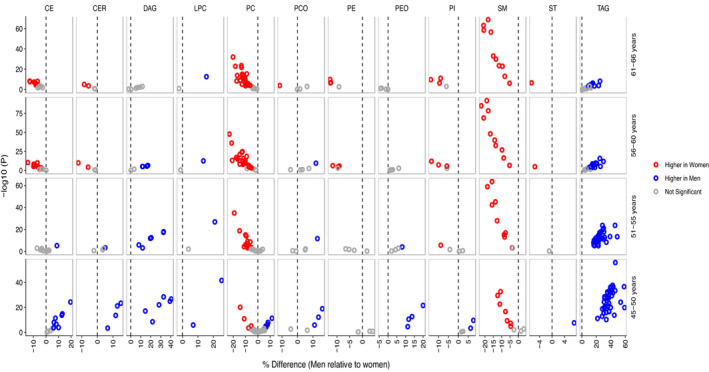
Association of lipid species with sex in different age groups is shown with lipid species being grouped by lipid classes. The x axes show percentage differences in men relative to women, and the corresponding P values are plotted on y axes as obtained by linear regression models, including log10 lipid levels as outcomes, sex as independent variable, and age, body mass index, diabetes, cardiac disease, lipid‐lowering medication, and smoking as covariates. Positive difference represents higher lipid level in men (blue), and negative difference represents higher lipid level in women (red), after multiple testing correction (P<7.0×10−4). Only the lipid species with significant age and sex interactions are plotted herein for clarity. CE indicates cholesteryl ester; CER, ceramide; DAG, diacylglyceride; LPC, lysophosphatidylcholine; LPE, lysophosphatidylamine; PC, phosphatidylcholine; PCO, phosphatidylcholine‐ether; PE, phosphatidylamine, PEO, phosphatidylamine‐ether; PI, phosphatidylinositol; SM, sphingomyelin; ST, free cholesterol; and TAG, triacylglyceride.
As menopause has previously been suggested to have effect on lipid levels, we performed exploratory analyses to investigate effect of menopause status on the observed age‐dependent sex differences in lipidome. Only 2 lipid species (ceramide 42:2;2 and sphingomyelin 38:2;2) showed significant association with menopause status (P<7.0×10−4) (Figure S10 and Table S10). No significant interaction between age and menopause was found for any of the lipid species (Table S11). Moreover, comparison of differences in lipid levels between men compared with premenopausal women and men compared with postmenopausal women revealed effect heterogeneity only in 17 lipid species (P het<7.0×10−4) (Table S12 and Figure S10). Overall, our results do not support a major effect of menopause on the plasma levels of lipid species analyzed herein and their sex differences.
Effect of Genetic Factors on Sex Differences in Lipidome
To evaluate if the observed sex differences in lipidome are attributable to differential effect of genetic variants in men and women, we conducted 2 types of genome‐wide searches: (1) genome‐wide heterogeneity scan and (2) sex‐specific GWAS scans. In the genome‐wide heterogeneity scan, variants with P he t<7.0×10−10 (5.0×10−8/70 principal components explaining >90% variance in lipidomes) were defined as sex‐dimorphic variants. On the basis of this criterion, sex heterogeneity was found at rs7760319 in the intron of SASH1 for PC 17:0;0_20:4;0 (P het=1.9×10−10) (Figure 4A), which was nominally associated with the lipid with opposite effects in men (ßmen=−0.13±0.03, Pmen=3.7×10−6) and women (ßwomen=0.10±0.02, Pwomen=1.2×10−5) in the sex‐stratified GWAS. However, the signal was not validated in the replication cohort (ßmen=−0.015±0.047, Pmen=0.75; ßwomen=0.01±0.045, Pwomen=0.84). List of all the associations with P het<5.0×10−8 is provided in Table S13.
Figure 4. Effect of genetic heterogeneity on sex differences in lipidome.
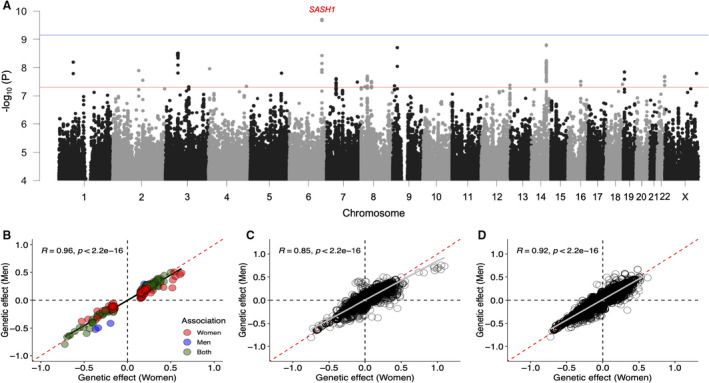
A, Genome‐wide heterogeneity in genetic effect on lipidome. Manhattan plot shows –log10 P het on the y axis for all the lipid species and genetic variants on the x axis arranged by chromosome position on different chromosomes. Red and blue horizontal lines represent P value threshold for genome‐wide significance (P<5.0×10−8) and after multiple testing correction (P<7.0×10−10). B, Heterogeneity in effect of variants identified in sex‐stratified genome‐wide association study (GWAS). Scatterplot shows comparison of β estimates in standardized units for 193 unique locus‐lipid associations with P<7.0×10−10 identified in sex‐stratified GWAS. Blue and red colored points represent associations reaching threshold for multiple testing correction (P<7.0×10−10) in GWASs for men and women, respectively, whereas green colored points represent associations reaching threshold for multiple testing correction in GWASs for both men and women. C, Heterogeneity in effect of variants identified in previous GWAS for traditional lipids. D, Heterogeneity in effect of variants identified in previous GWAS for lipidome. The scatterplots (C and D) show genetic effect of known lipid loci in men (y axis) and women (x axis) on lipidome, and only the associations with P<7.0×10−4 are plotted.
In the sex‐specific GWAS, variants with significant associations (P<7.0×10−10) in either men or women were selected and tested for heterogeneity in their effects between men and women. In male‐specific GWAS, 5828 variant‐lipid pair associations were found, whereas female‐specific GWAS identified 13 392 variant‐lipid pair associations (Data Sets 2 and 3). In total, 193 unique locus‐lipid associations involving 26 independent genomic loci and 127 lipid species in either men or women were identified and tested for differences in their effect estimates between men and women. Using P het<2.5×10−4 (0.05/193) as the threshold, none of the tested variants showed significant heterogeneity (Table S14); rather, the effect sizes for these associations were similar and highly correlated in men and women (Figure 4B). We further tested for heterogeneity in effects of the genetic variants identified in previous GWASs for traditional lipids and lipidome (Data Sets 4 and 5), which also suggested that effect estimates of the known lipid variants are similar in men and women, as shown in Figure 4C and 4D.
We further explored for the heterogeneity in the genomic regions with genes encoding enzymes with desaturase and elongase activities: FADS1‐2‐3, SCD1, ELOVL5, and ELOVL6 for association with lipid indexes representing efficiency of respective enzymes. The lipid indexes used in the analysis and their calculations are provided in Table S15. Such indexes and ratios have been applied previously to free fatty acids, but their validity for complex lipids is not known. Although no strong evidence for heterogeneity at these regions was found, nominal heterogeneity at FADS1‐2‐3 and SCD1 regions was observed and listed in Table S16. Notably, at FADS1‐2‐3 region, a nonsynonymous variant rs35723406 in TKFC gene showed effect heterogeneity between men and women for Δ6 desaturase in triacylglyceride (D6D in triacylglyceride) (P het=6.2×10−6) (Figure S11), which might be of interest for further investigation.
DISCUSSION
This study presents a comprehensive characterization of sex differences in circulatory lipids using high coverage lipidomics and effect of age and genetic factors on differences between lipidome profiles of men and women. The results show significant sex differences in the lipidome; and demonstrate that age has distinct effect on lipidome in men and women. In general, most of the cholesteryl esters, ceramides, lysophospholipids, triacylglycerides, and diacylglycerides were higher in men at 45 to 50 years of age, but these sex differences tend to narrow down or reverse with age. Our results did not provide support for major roles of menopause and common genetic factors in sex differences in plasma lipidome.
Age‐specific sex differences in circulatory lipids could have important implications in risk assessment and precision medicine for cardiometabolic diseases. Sphingolipids, including ceramides and sphingomyelins, have emerged as promising new diagnostic or prognostic markers for cardiovascular disease (CVD) with potential clinical utility. CERT score, based on ceramide(d18:1/16:0), ceramide(d18:1/18:0), and ceramide(d18:1/24:1) and their ratios with ceramide(d18:1/24:0), is shown to predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐C. 14 Another risk score termed as sphingolipid‐inclusive coronary artery disease risk score that includes sphingomyelins in addition to ceramides has been proposed as a strong predictor of CVD. 15 In this study, we found that ceramide(d18:1/24:0) (measured as ceramide 42:1;2) and ceramide(d18:1/24:1) (measured as ceramide 42:2;2) are significantly higher in 45‐ to 50‐year‐old men compared with women, but their plasma levels become similar in men and women in older age groups (>55 years) because of increase in their levels with age among women. A previous study also reported higher levels of ceramides in women aged >45 years compared with men. 36 However, we did not find significant increase in ceramide levels in men with age, as reported earlier. 10 , 36 Similarly, sphingomyelin levels were higher among women compared with men, and their levels increased with age in women, as also reported previously. 10 , 37 These observations of increased levels of ceramides and sphingomyelins in older women are consistent with the known increase in CVD risk with age in women. Our results also point out that sex differences in sphingolipids need to be considered in ceramide‐ and sphingomyelin‐based prediction scores.
In addition to sphingolipids, many lipid species, including phosphatidylcholines and triacylglycerides, have been identified as risk factors for CVD. We found significant differences in the levels of most of the phosphatidylcholines and triacylglycerides between men and women, which is consistent with the previous report. 10 It has been consistently demonstrated that phospholipids with saturated and monounsaturated fatty acyl chains are positively associated with CVD risk, whereas polyunsaturated phospholipids are inversely associated with CVD risk. 16 On similar lines, we found that although most of the phosphatidylcholines increase with age in women, among men, mainly phosphatidylcholines with polyunsaturated fatty acids change with age and those with saturated and monounsaturated fatty acids were not associated with age (P>0.05 for 10 of 14 principal components with saturated and monounsaturated fatty acids analyzed in the study). However, there have been inconsistent reports on association of phosphatidylcholines with age in men and women in longitudinal studies. 38 The KORA (Cooperative Health Research in the Augsburg Region) study by Chak et al showed decrease in phosphatidylcholine levels with age in women, 39 whereas Darst et al (2019) reported increase in several phosphatidylcholines with age in women. 40 Similarly, triacylglycerides have opposite age‐related trends in men and women, with significantly higher levels of most of triacylglycerides in men until 60 years of age. All these observations further emphasize the importance of considering sex differences in lipids in ASCVD risk assessment and sex‐specific prevention strategies.
Our findings also suggest potential sex differences in phospholipid and glycerolipid metabolism. Opposite age‐related trends for triacylglycerides in men and women point to sex‐specific regulation of triglyceride metabolism with age. Our study also suggests sex dimorphism in polyunsaturated fatty acid metabolic pathway. We found that cholesteryl esters and phosphatidylcholines with C20:3 and C20:4 fatty acids have opposite age‐related trends in men and women (increase in women and decrease in men), but those with C20:5 and C22:6 had similar age‐related trend (ie, they increase with age in both sexes). Our results also suggest that age and sex do not have substantial effect on lipid species with less unsaturated fatty acids and may have stronger regulation by other endogenous factors. These findings call for further studies to understand the underlying mechanisms.
Menopause has been reported to contribute to the increase in CVD risk with age in women and is accompanied by unfavorable changes in CVD risk factors, including lipid profiles. 41 , 42 , 43 However, the impact of menopause and depleted endogenous estrogen levels distinct from that of advancing age remains controversial. 44 , 45 An alternate hypothesis has emerged that proposes that increased premenopausal cardiovascular risk promotes early menopause and is supported by a few studies. 46 , 47 , 48 This implies that the direction of causality in the relationship between menopause and CVD is unclear. There have been inconsistencies in the association of lipids with menopause status also. Higher levels of TC, LDL‐C, triglycerides, and apolipoprotein B and subfraction of lipoproteins and their lipid contents have been associated with menopause. 49 Beyene et al also reported higher levels of phosphatidylcholines, phosphatidylinositols, sphingomyelins, phosphatidylamines, ceramides, and triacylglycerides in postmenopausal women. 10 Contrary to these observations, a recent study does not support a major role of menopause in lipid‐level changes. 50 Our study also did not find substantial effect of menopause status on sex differences in lipidome, although the reduced sample size in the menopause‐related analyses limits statistical power. Moreover, as age and menopause status are highly collinear variables, it is difficult to tease out the independent effect of age and menopause on outcome variables. Further investigation in this regard is important to understand the role of menopause in modulating lipid levels.
Contribution of genetic factors in endogenous regulation of lipid metabolism is well recognized. Despite the known and expected differences in the lipid levels, men and women are typically analyzed together using sex as a covariate to account for potential sex differences in the lipid levels. Sex‐combined analyses are underpowered to detect significant associations if the effects are in opposite directions. Although sex heterogeneity in effect of a few lipid loci, such as LPL, APOE, and KLF, on traditional lipids has been reported, 51 , 52 there has been no effort to systematically evaluate differential effects of genetic variants on plasma lipidome in men and women. To our knowledge, our study presents the first comprehensive investigation of sexual heterogeneity in genetic influences on plasma lipidome.
Our observation that genetic factors have no major impact on sex differences in plasma levels of lipid species is in accordance with the findings from metabolomics‐based study. 11 , 53 In a sex‐specific GWAS study by Mittelstrass et al that included many lipid species from phosphatidylcholines, lysophospholipids, and sphingomyelins, no genome‐wide significant difference in effect estimates for genetic variants for any of the lipid species was found. 11 Although we acknowledge the challenge of limited statistical power for sex‐stratified analyses, our finding of distinct age‐related changes in lipid levels for men and women underlines that these differential profiles need to be accounted for to detect sex‐dimorphic genetic influence. Given the sample size of the present study, we did not have sufficient statistical power to perform age‐stratified GWAS, but a recent study highlighted difference in the influence of polygenic risk score for coronary heart disease on apolipoprotein in different age tertiles, emphasizing the importance of age‐stratified analysis. 54 Our study is the first effort toward understanding genetic mechanisms in sex differences in lipidome, and we believe that it would pave the way for further studies in the direction.
The mass spectrometry–based shotgun lipidomics platform used in the study detected 179 lipid species across 13 lipid classes with acyl chain resolution for all lipid classes, except triacylglycerides. Comparison of the platform with other comparable commercial platforms used in large cohorts (Metabolon and Biocrates) was provided in our previous study. 21 Although Nightingale platform detects a wide range of metabolites, including total lipid contents in various lipoprotein subclasses, it does not provide resolution to lipid species. The more recent Lipidyzer platform provides expanded coverage, but larger studies/cohorts using the platform have not yet been reported.
Although the study is based on a large population‐based cohort with a broad lipidome coverage, it is not without limitations. This is a cross‐sectional study, and the findings of effect of age on lipidome need to be replicated in longitudinal studies with long follow‐up durations. Our study included participants aged 45 to 66 years. A wider range of age would be needed to provide more insights to the changes in lipidome profile in early adulthood and to get better picture of effect of menopause on lipidome. As suggested in our study, demographic and other cohort characteristics could affect lipidome profiles; it is not clear if findings of this study could be generalized to other populations. However, most of our findings are consistent or in accordance with the current understanding in the field. Furthermore, lipidome profiles were measured in whole plasma, which does not provide information at the level of individual lipoprotein subclasses and limits our ability to gain detailed mechanistic insights. Further advances in lipidomics platforms might help to capture more comprehensive and complete lipidome profiles, including the position of fatty acyl chains in the glycerol backbone of triacylglycerides and glycerophospholipids and detection of sphingosine‐1‐P species and several other species, which would allow us to overcome these limitations.
In conclusion, our study reports considerable insights into the sex differences and age‐related trends in circulatory lipids at molecular lipid species level. We show that men and women have distinct age‐related changes in lipidome profiles that result in variable sex differences in plasma lipid levels at different age. Our findings emphasize the importance of sex‐ and age‐stratified analyses in lipidome studies. The study paves the way for further evaluation in a sex‐specific manner toward precision medicine and sex‐specific genetic and lipid biomarker discovery and further highlights the need for sex‐specific prevention and management of ASCVD risk.
Sources of Funding
The GeneRISK study was funded by Business Finland through the Personalized Diagnostics and Care program coordinated by SalWe Ltd (grant No. 3986/31/2013). Dr Ripatti was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (grant No. 312062), the Finnish Foundation for Cardiovascular Research, the Sigrid Juselius Foundation, and University of Helsink HiLIFE Fellow and Grand Challenge grants. Dr Pirinen was supported by the Academy of Finland (grants 338507 and 336 825) and Sigrid Juselius Foundation. Dr Tukiainen was supported by the Academy of Finland (grants 315589 and 320129), Sigrid Juselius Foundation, and the University of Helsinki 3‐year research project grant.
Disclosures
Dr Gerl is an employee of Lipotype GmbH. Dr Simons is CEO of Lipotype GmbH. Drs Simons and Klose are shareholders of Lipotype GmbH. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S16
Figures S1–S11
Dataset 1
Dataset 2
Dataset 3
Dataset 4
Dataset 5
Acknowledgments
We would like to thank Johanna Aro, Sari Kivikko, and Ulla Tuomainen for management assistance in the project. We thank all study participants of the study for their participation. This research has been conducted using the UK Biobank Resource under application No. 22627.
Preprint posted on MedRxiv May 31, 2022. doi: https://doi.org/10.1101/2022.05.30.22275704.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027103
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Rubina Tabassum, Email: rubina.tabassum@helsinki.fi.
Samuli Ripatti, Email: samuli.ripatti@helsinki.fi.
References
- 1. Cardiovascular diseases (CVDs). WHO . Accessed March 31, 2022. https://https://www.who.int/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds)
- 2. Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17:47–66. doi: 10.1038/s41574-020-00431-8 [DOI] [PubMed] [Google Scholar]
- 3. DeFilippis EM, Collins BL, Singh A, Biery DW, Fatima A, Qamar A, Berman AN, Gupta A, Cawley M, Wood MJ, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the mass general Brigham YOUNG‐MI registry. Eur Heart J. 2020;41:4127–4137. doi: 10.1093/eurheartj/ehaa662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CS, Maas AH, et al. The lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X [DOI] [PubMed] [Google Scholar]
- 5. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis society consensus panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/S0195-668X(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 7. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 8. Balder JW, de Vries JK, Nolte IM, Lansberg PJ, Kuivenhoven JA, Kamphuisen PW. Lipid and lipoprotein reference values from 133,450 Dutch lifelines participants: age‐ and gender‐specific baseline lipid values and percentiles. J Clin Lipidol. 2017;11:1055–1064.e6. doi: 10.1016/j.jacl.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 9. Feng L, Nian S, Tong Z, Zhu Y, Li Y, Zhang C, Bai X, Luo X, Wu M, Yan Z. Age‐related trends in lipid levels: a large‐scale cross‐sectional study of the general Chinese population. BMJ Open. 2020;10:e034226. doi: 10.1136/bmjopen-2019-034226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beyene HB, Olshansky G, T Smith AA, Giles C, Huynh K, Cinel M, Mellett NA, Cadby G, Hung J, Hui J, et al. High‐coverage plasma lipidomics reveals novel sex‐specific lipidomic fingerprints of age and BMI: evidence from two large population cohort studies. PLoS Biol. 2020;18:e3000870. doi: 10.1371/journal.pbio.3000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch‐Margl W, Polonikov A, Peters A, Theis FJ, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide‐ and phospholipid‐based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 14. Laaksonen R, Ekroos K, Sysi‐Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐cholesterol. Eur Heart J. 2016;37:1967–1976. doi: 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, Hunt SC, Holland WL, Summers SA, Playdon MC. Machine learning reveals serum sphingolipids as cholesterol‐independent biomarkers of coronary artery disease. J Clin Invest. 2020;130:1363–1376. doi: 10.1172/JCI131838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabassum R, Ripatti S. Integrating lipidomics and genomics: emerging tools to understand cardiovascular diseases. Cell Mol Life Sci. 2021;78:2565–2584. doi: 10.1007/s00018-020-03715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gieger C, Geistlinger L, Altmaier E, Hrabé de Angelis M, Kronenberg F, Meitinger T, Mewes HW, Wichmann HE, Weinberger KM, Adamski J, et al. Genetics meets metabolomics: a genome‐wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4:e1000282. doi: 10.1371/journal.pgen.1000282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, Altmaier E, CARDIoGRAM , Deloukas P, Erdmann J, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demirkan A, van Duijn CM, Ugocsai P, Isaacs A, Pramstaller PP, Liebisch G, Wilson JF, Johansson Å, Rudan I, Aulchenko YS, et al. Genome‐wide association study identifies novel loci associated with circulating phospho‐ and sphingolipid concentrations. PLoS Genet. 2012;8:e1002490. doi: 10.1371/journal.pgen.1002490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhee EP, Ho JE, Chen MH, Shen D, Cheng S, Larson MG, Ghorbani A, Shi X, Helenius IT, O'Donnell CJ, et al. A genome‐wide association study of the human metabolome in a community‐based cohort. Cell Metab. 2013;18:130–143. doi: 10.1016/j.cmet.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabassum R, Rämö JT, Ripatti P, Koskela JT, Kurki M, Karjalainen J, Palta P, Hassan S, Nunez‐Fontarnau J, Kiiskinen TT, et al. Genetic architecture of human plasma lipidome and its link to cardiovascular disease. Nat Commun. 2019;10:4329. doi: 10.1038/s41467-019-11954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lotta LA, Pietzner M, Stewart ID, Wittemans LBL, Li C, Bonelli R, Raffler J, Biggs EK, Oliver‐Williams C, Auyeung VP, et al. A cross‐platform approach identifies genetic regulators of human metabolism and health. Nat Genet. 2021;53:54–64. doi: 10.1038/s41588-020-00751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin X, Chan LS, Bose D, Jackson AU, VandeHaar P, Locke AE, Fuchsberger C, Stringham HM, Welch R, Yu K, et al. Genome‐wide association studies of metabolites in Finnish men identify disease‐relevant loci. Nat Commun. 2022;13:1644. doi: 10.1038/s41467-022-29143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cadby G, Giles C, Melton PE, Huynh K, Mellett NA, Duong T, Nguyen A, Cinel M, Smith A, Olshansky G, et al. Comprehensive genetic analysis of the human lipidome identifies loci associated with lipid homeostasis with links to coronary artery disease. Nat Commun. 2022;13:3124. doi: 10.1038/s41467-022-30875-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Widén E, Junna N, Ruotsalainen S, Surakka I, Mars N, Ripatti P, Partanen JJ, Aro J, Mustonen P, Tuomi T, et al. How communicating polygenic and clinical risk for atherosclerotic cardiovascular disease impacts health behavior: an observational follow‐up study. Circ Genom Precis Med. 2022;15:e003459. doi: 10.1161/CIRCGEN.121.003459 [DOI] [PubMed] [Google Scholar]
- 26. Porkka KV, Nuotio I, Pajukanta P, Ehnholm C, Suurinkeroinen L, Syvänne M, Lehtimäki T, Lahdenkari AT, Lahdenperä S, Ylitalo K, et al. Phenotype expression in familial combined hyperlipidemia. Atherosclerosis. 1997;133:245–253. doi: 10.1016/S0021-9150(97)00134-2 [DOI] [PubMed] [Google Scholar]
- 27. Borodulin K, Vartiainen E, Peltonen M, Jousilahti P, Juolevi A, Laatikainen T, Männistö S, Salomaa V, Sundvall J, Puska P. Forty‐year trends in cardiovascular risk factors in Finland. Eur J Public Health. 2015;25:539–546. doi: 10.1093/eurpub/cku174 [DOI] [PubMed] [Google Scholar]
- 28. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Surma MA, Herzog R, Vasilj A, Klose C, Christinat N, Morin‐Rivron D, Simons K, Masoodi M, Sampaio JL. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids. Eur J Lipid Sci Technol. 2015;117:1540–1549. doi: 10.1002/ejlt.201500145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herzog R, Schwudke D, Schuhmann K, Sampaio JL, Bornstein SR, Schroeder M, Shevchenko A. A novel informatics concept for high‐throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011;12:R8. doi: 10.1186/gb-2011-12-1-r8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herzog R, Schuhmann K, Schwudke D, Sampaio JL, Bornstein SR, Schroeder M, Shevchenko A. Lipidxplorer: a software for consensual cross‐platform lipidomics. PLoS One. 2012;7:e29851. doi: 10.1371/journal.pone.0029851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loh PR, Danecek P, Palamara PF, Fuchsberger C, Reshef A Y, K Finucane H , Schoenherr S, Forer L, McCarthy S, Abecasis GR, et al. Reference‐based phasing using the haplotype reference consortium panel. Nat Genet. 2016; 48:1443–1448. 10.1038/ng.3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Browning BL, Browning SR. Genotype imputation with millions of reference samples. Am J Hum Genet. 2016;98:116–126. doi: 10.1016/j.ajhg.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Purcell S, Neale B, Todd‐Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole‐genome association and population‐based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pirinen M, Donnelly P, Spencer CCA. Efficient computation with a linear mixed model on large‐scale data sets with applications to genetic studies. Ann Appl Stat. 2012;7:369–390. [Google Scholar]
- 36. Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Demographic and clinical variables affecting mid‐ to late‐life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. 2015;14:1014–1023. doi: 10.1111/acel.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore longitudinal study of aging. Aging Cell. 2015;14:112–121. doi: 10.1111/acel.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohammadzadeh Honarvar N, Zarezadeh M, Molsberry SA, Ascherio A. Changes in plasma phospholipids and sphingomyelins with aging in men and women: a comprehensive systematic review of longitudinal cohort studies. Ageing Res Rev. 2021;68:101340. [DOI] [PubMed] [Google Scholar]
- 39. Chak CM, Lacruz ME, Adam J, Brandmaier S, Covic M, Huang J, Meisinger C, Tiller D, Prehn C, Adamski J, et al. Ageing investigation using two‐time‐point metabolomics data from KORA and CARLA studies. Metabolites. 2019;9:44. doi: 10.3390/metabo9030044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD. Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY). 2019;11:1262–1282. doi: 10.18632/aging.101837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 42. Hjortland MC, McNamara PM, Kannel WB. Some atherogenic concomitants of menopause: the Framingham study. Am J Epidemiol. 1976;103:304–311. doi: 10.1093/oxfordjournals.aje.a112228 [DOI] [PubMed] [Google Scholar]
- 43. Akahoshi M, Soda M, Nakashima E, Shimaoka K, Seto S, Yano K. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation. 1996;94:61–66. doi: 10.1161/01.CIR.94.1.61 [DOI] [PubMed] [Google Scholar]
- 44. Cauley JA, Gutai JP, Kuller LH, Powell JG. The relation of endogenous sex steroid hormone concentrations to serum lipid and lipoprotein levels in postmenopausal women. Am J Epidemiol. 1990;132:884–894. doi: 10.1093/oxfordjournals.aje.a115731 [DOI] [PubMed] [Google Scholar]
- 45. Bittner V. Menopause and cardiovascular risk cause or consequence? J Am Coll Cardiol. 2006;47:1984–1986. [DOI] [PubMed] [Google Scholar]
- 46. Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/j.jacc.2005.12.066 [DOI] [PubMed] [Google Scholar]
- 47. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi‐ethnic study of atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, Brunner EJ, Bruinsma F, Giles GG, Demakakos P, et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol. 2019;34:235–246. doi: 10.1007/s10654-019-00490-w [DOI] [PubMed] [Google Scholar]
- 49. Auro K, Joensuu A, Fischer K, Kettunen J, Salo P, Mattsson H, Niironen M, Kaprio J, Eriksson JG, Lehtimäki T, et al. A metabolic view on menopause and ageing. Nat Commun. 2014;5:4708. doi: 10.1038/ncomms5708 [DOI] [PubMed] [Google Scholar]
- 50. Muilwijk M, Callender N, Goorden S, Vaz FM, van Valkengoed IGM. Sex differences in the association of sphingolipids with age in Dutch and south‐Asian Surinamese living in Amsterdam, The Netherlands. Biol Sex Differ. 2021;12:13. doi: 10.1186/s13293-020-00353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kanoni S, Graham SE, Wang Y, Surakka I, Ramdas S, Zhu X, Clarke SL, Bhatti KF, Vedantam S, Winkler TW, et al. Implicating genes, pleiotropy and sexual dimorphism at blood lipid loci through multi‐ancestry meta‐analysis. medRxiv. 2021. doi: 10.1101/2021.12.15.21267852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krumsiek J, Mittelstrass K, Do KT, Stückler F, Ried J, Adamski J, Peters A, Illig T, Kronenberg F, Friedrich N, et al. Gender‐specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fang S, Holmes MV, Gaunt TR, Smith GD, Richardson TG. An atlas of associations between polygenic risk scores from across the human phenome and circulating metabolic biomarkers. medRxiv. 2021. doi: 10.1101/2021.10.14.21265005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S16
Figures S1–S11
Dataset 1
Dataset 2
Dataset 3
Dataset 4
Dataset 5


