Abstract
Background
The usefulness of preprocedural nutritional status to stratify prognosis after transcatheter aortic valve implantation has been evaluated; however, the studies conducted so far have been relatively small and/or focused on a single nutritional index. This study sought to assess the prevalence and prognostic impact of malnutrition in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation.
Methods and Results
We applied the Controlling Nutritional Status score, Geriatric Nutritional Risk Index, and Prognostic Nutritional Index to 1040 consecutive older Japanese patients at high surgical risk who underwent transcatheter aortic valve implantation. According to the Controlling Nutritional Status score, Geriatric Nutritional Risk Index, and Prognostic Nutritional Index, 16.6%, 60.5%, and 13.8% patients had moderate or severe malnutrition, respectively; 89.3% were at least mildly malnourished by at least 1 score. Worse nutritional status was associated with older age, lower body mass index, higher degree of frailty, worse symptoms and renal function, atrial fibrillation, and anemia. During a median follow‐up of 986 days (interquartile range, 556–1402 days), 273 (26.3%) patients died. Compared with normal nutrition, malnutrition was associated with an increased risk for all‐cause death (adjusted hazard ratio for moderate and severe malnutrition, respectively: 2.19 (95% CI, 1.45–3.31; P<0.001) and 6.13 (95% CI, 2.75–13.70; P<0.001) for the Controlling Nutritional Status score, 2.02 (95% CI, 1.36–3.02; P=0.001) and 3.24 (95% CI, 1.86–5.65; P<0.001) for the Geriatric Nutritional Risk Index, and 1.60 (95% CI, 1.06–2.39; P=0.024) and 2.32 (95% CI, 1.50–3.60; P<0.001) for the Prognostic Nutritional Index).
Conclusions
Malnutrition is common in patients undergoing transcatheter aortic valve implantation and is associated with increased mortality.
Keywords: body mass index, frailty, nutrition assessment, nutritional status, prognosis, transcatheter aortic valve replacement
Subject Categories: Clinical Studies, Diet and Nutrition, Heart Failure, Valvular Heart Disease, Aortic Valve Replacement/Transcather Aortic Valve Implantation
Nonstandard Abbreviations and Acronyms
- CFS
Clinical Frailty Scale
- CONUT
Controlling Nutritional Status
- GNRI
Geriatric Nutritional Risk Index
- PNI
Prognostic Nutritional Index
- TAVI
transcatheter aortic valve implantation
- THV
transcatheter heart valve
CLINICAL PERSPECTIVE.
What Is New?
The usefulness of preprocedural nutritional status to stratify prognosis after transcatheter aortic valve implantation has been evaluated; however, the studies conducted so far have been relatively small.
Malnutrition affects more than two‐thirds of the patients undergoing transcatheter aortic valve implantation and is associated with poor prognosis irrespective of body mass index, Society of Thoracic Surgeons Predicted Risk of Mortality score, Clinical Frailty Scale, left ventricular ejection fraction, or renal function.
What Are the Clinical Implications?
These data support the importance of evaluating nutritional status of all candidates for transcatheter aortic valve implantation.
Prospective multicenter studies are warranted to assess the impact of nutritional interventions on outcomes of patients undergoing transcatheter aortic valve implantation.
Transcatheter aortic valve implantation (TAVI) was established as a therapeutic alternative to surgical aortic valve replacement for inoperable or high‐risk patients with severe aortic stenosis. 1 , 2 , 3 Although indications for TAVI have been expanded to include patients who are at a lower surgical risk, 4 , 5 , 6 long‐term prognosis after TAVI remains poor. Thus, optimal patient risk stratification based on modifiable clinical characteristics is essential to improve prognosis after TAVI.
Malnutrition, a risk factor that is modifiable by providing intervention in terms of dietary patterns, is known as a driver of disease progression by causing cytokine activation 7 and is associated with poor prognosis in patients with various cardiovascular diseases, including heart failure, 8 acute coronary syndrome, 9 and peripheral artery disease. 10 The usefulness of the preprocedural nutritional status to stratify prognosis after TAVI, which is excluded from the classic surgical risk models such as the Society of Thoracic Surgeons score, has also been reported; however, a relatively small number of relevant studies have been conducted so far. 11 , 12
Therefore, we investigated the prevalence and prognostic impact of malnutrition in a large cohort of patients with severe aortic stenosis undergoing TAVI using 3 scoring systems (Controlling Nutritional Status [CONUT] score, Geriatric Nutritional Risk Index [GNRI], and Prognostic Nutritional Index [PNI]).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Procedure
From October 2013 to May 2020, 1046 consecutive patients with symptomatic severe aortic stenosis who underwent TAVI were prospectively included in our database. After excluding 6 patients (2 patients with no data about nutritional parameters including height, weight, serum albumin level, total cholesterol level, or lymphocyte count; 2 patients with a diagnosis of leukemia, lymphoma, or multiple myeloma; and 2 patients with previous transcatheter heart valves [THVs]), we analyzed a total of 1040 patients to assess the impact of malnutrition on clinical outcomes after TAVI (Figure 1).
Figure 1. Study workflow.
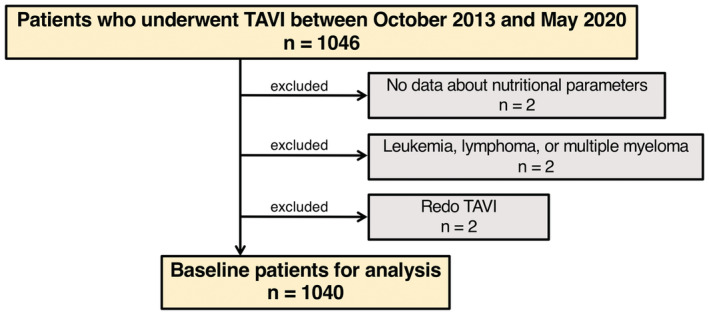
The flowchart providing information about the included and excluded patients. TAVI indicates transcatheter aortic valve implantation.
All patients were considered to be ineligible or high risk for surgical aortic valve replacement via consensus of the heart team. The sizes of THVs were mainly determined using multidetector computed tomography or echocardiography. Approach routes were chosen via the femoral artery first; if the femoral access was inappropriate, the iliac artery, apical, subclavian, or direct aortic routes were considered. Procedural outcomes and postprocedural THV function were assessed according to the Valve Academic Research Consortium–2 criteria. 13 Prosthesis–patient mismatch was classified based on prosthetic effective orifice area indexed to body surface area as severe (<0.65 cm2/m2) or moderate (0.65–0.85 cm2/m2) in the general population and as severe (<0.60 cm2/m2) or moderate (0.60 to 0.90 cm2/m2) in the obese population (body mass index [BMI] ≥30 kg/m2).
Computed Tomographic Data Analysis
All computed tomographic examinations were performed as previously described. 14 Images were reconstructed and assessed by using 3mensio Valves software version 7.0 or 8.0 (Pie Medical Imaging, Maastricht, The Netherlands). The aortic annulus and left ventricular outflow tract (LVOT) area were measured in the mid‐systole. The LVOT calcification was classified in a semiquantitative fashion as previously described 15 : mild calcification was recorded in the presence of 1 nodule of calcification extending <5 mm in any dimension and covering <10% of the perimeter of the LVOT, moderate calcification was documented in the presence of 2 nodules of calcification or 1 extending >5 mm in any direction or covering >10% of the perimeter of the LVOT, and severe calcification was considered in cases of multiple nodules of calcification of a single focus extending >10 mm in length or covering >20% of the perimeter of the LVOT.
Malnutrition Screening Tools
All patients were screened for malnutrition using 3 scoring systems (Figure 2A). The CONUT score was developed by Ulíbarri et al in 2005 as a screening tool for malnutrition among patients admitted in a hospital. 16 It takes into account serum albumin level, total cholesterol level, and lymphocyte count. A score of 0 to 1 is considered normal; scores of 2 to 4, 5 to 8, and 9 to 12 reflect mild, moderate, and severe malnutrition, respectively.
Figure 2. Prevalence of malnutrition according to the 3 scoring indexes.
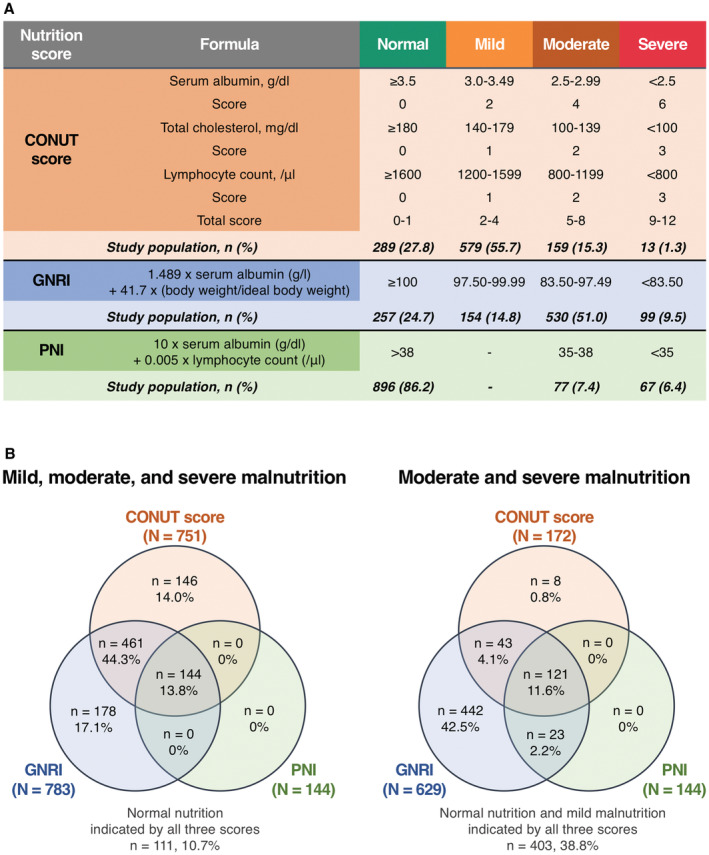
A, Formula and prevalence of malnutrition for each nutritional index. B, Venn diagrams demonstrating the frequency of malnutrition according to each nutritional index with the percentages of the total 1040 patients. The overlapping area shows the frequency with which the identification of malnutrition by an index overlaps with the others. CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; and PNI, Prognostic Nutritional Index.
The GNRI, which is also a widely used index for assessing nutritional status, is calculated using the following formula: 1.489×serum albumin (g/L) + 41.7×(current body weight [kg]/ideal body weight [kg]). 17 The ideal body weight is calculated as follows: body height (cm)−100−[(body height [cm]−150)/4] for male patients, and body height (cm)−100−[(body height [cm]−150)/2.5] for female patients. 18 As defined in previous studies, a score of ≥100 was considered normal; scores of 97.50 to 99.99, 83.50 to 97.49, and <83.50 reflected mild, moderate, and severe malnutrition, respectively.
The PNI was calculated using the following formula: 10×serum albumin (g/dL)+0.005×total lymphocyte count (/μL). 19 A score of >38 is considered normal; scores of 35 to 38 and<35 reflect moderate and severe malnutrition, respectively. Note that there is no “mild” category for the PNI.
Outcome Measures and Follow‐Up
The primary outcome measure of this study was all‐cause mortality after TAVI. The causes of death were categorized as cardiovascular and noncardiovascular deaths. The definition of cardiovascular mortality was also applied to the Valve Academic Research Consortium–2 criteria and included death attributed to cardiac causes and noncoronary vascular conditions, such as stroke associated with neurological events, procedure‐related aortic dissection, rupture, or other vascular diseases. All procedure‐ and valve‐associated deaths and sudden, unwitnessed, and unknown deaths were also classified as cardiovascular mortality. The secondary end point was heart failure hospitalization after TAVI. For patients with multiple hospitalizations, only the first episode was included in the analysis. Information on the occurrence of adverse events after discharge was obtained from follow‐up outpatient visits or telephone interviews conducted on the 30th day, the sixth month, and annually thereafter. The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from all patients before the TAVI procedure.
Statistical Analysis
Categorical variables were described as numbers and percentages and compared using the χ2 test. Continuous variables were described as mean±SD or median (interquartile range [IQR]) and were compared using the independent Student t test or Kruskal–Wallis test depending on their distributions. Venn diagrams were used to illustrate the relationship between the 3 nutritional scoring systems.
The cumulative event rates were analyzed using the Kaplan–Meier estimation. Poisson models were used to estimate the incidence rates. A Cox proportional hazards regression analysis was performed to identify predictors of mortality. To test the predictive ability of the nutritional status, multivariable Cox proportional hazard models were constructed, which comprised variables known to be associated with poor prognosis based on clinical plausibility 20 , 21 or P values <0.05 in the univariate analysis. Model 1 was adjusted for preprocedural variables, and model 2 was additionally adjusted for postprocedural variables including echocardiographic data and in‐hospital outcomes. For post hoc analyses, we dichotomized patients based on (1) age (at the median of 85 years), (2) sex, (3) BMI level (at the median of 22 kg/m2), (4) Clinical Frailty Scale (CFS) (at the median score of 3), (5) chronic renal failure status, and (6) left ventricular ejection fraction (LVEF) (“preserved [≥50%]” or not) to assess the interaction of nutritional status with these factors.
Receiver operating characteristic curves were used to illustrate and assess the predictive performance of the 3 nutritional indexes for mortality, and the best discriminatory thresholds were calculated by determining the Youden index. Moreover, the areas under the curve between the indexes were compared using the method of DeLong et al. 22
All statistical analyses were performed using JMP 14.2.0 (SAS Institute Inc., Cary, NC) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All reported P values were 2‐tailed, and P values <0.05 were considered statistically significant.
Results
Baseline Patient Characteristics
A total of 1040 patients (median age, 85 years; 32.9% men; and median Society of Thoracic Surgeons Predicted Risk of Mortality score of 6.0%) were included in the analysis. Of the patients, 142 (13.7%) patients were underweight (BMI<18.5 kg/m2), 679 (65.3%) patients had normal weight (18.5≤BMI≤24.9 kg/m2), 190 (18.3%) patients were overweight (25.0≤BMI≤29.9 kg/m2), and 29 (2.8%) patients were obese (BMI≥30.0 kg/m2). Common comorbidities were hypertension (85.4%), dyslipidemia (54.9%), coronary artery disease (33.7%), diabetes (22.0%), and atrial fibrillation (22.0%). Additional data on the baseline clinical characteristics are outlined in Table 1.
Table 1.
Baseline Characteristics of Patients
| Total (N=1040) | CONUT score | GNRI | PNI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal/mild, 0–4 (N=868) | Moderate/severe, 5–12 (N=172) | P value | Normal/mild, ≥97.5 (N=411) | Moderate/severe, <97.5 (N=629) | P value | Normal, >38 (N=896) | Moderate/severe, ≤38 (N=144) | P value | ||
| Demographics | ||||||||||
| Age, y | 85 (82–88) | 85 (82–88) | 87 (83–90) | 0.004 | 84 (80–87) | 86 (83–89) | <0.001 | 85 (82–88) | 87 (83–90) | 0.002 |
| Male sex | 342 (32.9) | 269 (31.0) | 73 (42.4) | 0.004 | 137 (33.3) | 205 (32.6) | 0.803 | 292 (32.6) | 50 (34.7) | 0.614 |
| Height, cm | 146.7 (142.9–156.2) | 148.5 (142.9–155.9) | 150.0 (142.9–159.0) | 0.142 | 149.0 (143.0–158.5) | 148.5 (142.5–155.5) | 0.066 | 148.6 (143.0–156.4) | 149.4 (142.1–155.7) | 0.810 |
| Weight, kg | 49.2 (42.2–56.8) | 49.8 (42.5–57.3) | 46.8 (40.6–55.1) | 0.032 | 53.8 (47.0–60.7) | 45.9 (40.0–53.8) | <0.001 | 49.9 (42.6–57.5) | 45.8 (40.0–54.0) | <0.001 |
| Body mass index, kg/m2 | 22.0 (19.7–24.4) | 22.3 (19.9–24.6) | 21.2 (18.8–23.6) | <0.001 | 23.6 (21.8–25.7) | 20.7 (18.7–23.2) | <0.001 | 22.3 (19.9–24.6) | 20.6 (18.6–23.2) | <0.001 |
| Clinical Frailty Scale | 3 (3–4) | 3 (3–4) | 4 (3–5) | <0.001 | 3 (3–4) | 4 (3–4) | <0.001 | 3 (3–4) | 4 (3–6) | <0.001 |
| NYHA functional class III/IV | 465 (44.7) | 358 (41.2) | 107 (62.2) | <0.001 | 148 (36.0) | 317 (50.4) | <0.001 | 375 (41.9) | 90 (62.5) | <0.001 |
| STS‐PROM score, % | 6.0 (4.1–8.9) | 5.6 (3.9–8.3) | 8.2 (5.4–12.9) | <0.001 | 5.0 (3.6–7.5) | 6.7 (4.5–9.9) | <0.001 | 5.6 (3.9–8.3) | 8.3 (5.8–13.8) | <0.001 |
| Comorbidities | ||||||||||
| Hypertension | 888 (85.4) | 744 (85.7) | 144 (83.7) | 0.504 | 370 (90.0) | 518 (82.4) | 0.001 | 771 (86.1) | 117 (81.3) | 0.142 |
| Dyslipidemia | 571 (54.9) | 495 (57.0) | 76 (44.2) | 0.002 | 275 (66.9) | 296 (47.1) | <0.001 | 508 (56.7) | 63 (43.8) | 0.004 |
| Diabetes | 229 (22.0) | 194 (22.4) | 35 (20.4) | 0.560 | 109 (26.5) | 120 (19.1) | 0.005 | 198 (22.1) | 31 (21.5) | 0.878 |
| Atrial fibrillation | 229 (22.0) | 164 (18.9) | 65 (37.8) | <0.001 | 70 (17.0) | 159 (25.3) | 0.002 | 173 (19.3) | 56 (38.9) | <0.001 |
| Coronary artery disease | 350 (33.7) | 279 (32.1) | 71 (41.3) | 0.022 | 150 (36.5) | 200 (31.8) | 0.118 | 290 (32.4) | 60 (41.7) | 0.031 |
| Previous coronary bypass | 53 (5.1) | 44 (5.1) | 9 (5.2) | 0.929 | 27 (6.6) | 26 (4.1) | 0.084 | 47 (5.3) | 6 (4.2) | 0.575 |
| Previous valve surgery | 26 (2.5) | 22 (2.5) | 4 (2.3) | 0.871 | 12 (2.9) | 14 (2.2) | 0.487 | 23 (2.6) | 3 (2.1) | 0.724 |
| Peripheral artery disease | 90 (8.7) | 70 (8.1) | 20 (11.6) | 0.143 | 30 (7.3) | 60 (9.5) | 0.205 | 75 (8.4) | 15 (10.4) | 0.429 |
| Chronic obstructive pulmonary disease | 107 (10.3) | 87 (10.0) | 20 (11.6) | 0.533 | 45 (11.0) | 62 (9.9) | 0.572 | 88 (9.8) | 19 (13.2) | 0.231 |
| Cerebrovascular disease | 120 (11.5) | 98 (11.3) | 22 (12.8) | 0.578 | 40 (9.7) | 80 (12.7) | 0.137 | 101 (11.3) | 19 (13.2) | 0.510 |
| Active cancer | 53 (5.1) | 43 (5.0) | 10 (5.8) | 0.645 | 15 (3.7) | 38 (6.0) | 0.080 | 48 (5.4) | 5 (3.5) | 0.316 |
| Blood tests | ||||||||||
| Hemoglobin, g/dL | 11.2 (10.1–12.3) | 11.4 (10.3–12.5) | 10.2 (9.2–11.2) | <0.001 | 11.8 (10.8–12.8) | 10.8 (9.7–11.9) | <0.001 | 11.4 (10.3–12.5) | 10.1 (9.1–11.1) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 51.2 (38.4–64.4) | 52.0 (39.2–65.6) | 47.6 (31.4–57.9) | <0.001 | 52.7 (39.3–65.0) | 49.5 (37.7–64.0) | 0.043 | 51.9 (39.2–65.0) | 43.3 (30.7–57.7) | <0.001 |
| Albumin, g/dL | 3.8 (3.4–4.1) | 3.9 (3.6–4.1) | 3.1 (2.9–3.4) | <0.001 | 4.1 (3.9–4.3) | 3.5 (3.3–3.7) | <0.001 | 3.8 (3.6–4.1) | 3.0 (2.8–3.2) | <0.001 |
| Total cholesterol, mg/dL | 168 (146–193) | 173 (153–197) | 142 (122–164) | <0.001 | 177 (155–199) | 164 (143–188) | <0.001 | 171 (150–195) | 152 (131–177) | <0.001 |
| Lymphocyte count, μL | 1254 (1019–1591) | 1314 (1062–1646) | 1030 (757–1168) | <0.001 | 1313 (1080–1643) | 1210 (986–1563) | <0.001 | 1305 (1056–1637) | 1013 (754–1192) | <0.001 |
| Brain natriuretic peptide, pg/mL | 183.6 (79.7–427.6) | 160.0 (71.2–352.2) | 384.4 (173.1–666.8) | <0.001 | 105.7 (59.5–233.5) | 266.3 (106.4–529.6) | <0.001 | 162.7 (72.7–353.0) | 430.2 (187.7–721.5) | <0.001 |
| Echocardiographic data | ||||||||||
| Aortic valve area, cm2 | 0.66 (0.55–0.76) | 0.66 (0.56–0.77) | 0.64 (0.52–0.72) | 0.002 | 0.69 (0.60–0.78) | 0.64 (0.53–0.75) | <0.001 | 0.66 (0.56–0.77) | 0.64 (0.53–0.75) | 0.071 |
| Indexed aortic valve area, cm2/m2 | 0.50 (0.42–0.57) | 0.50 (0.40–0.51) | 0.40 (0.40–0.50) | 0.006 | 0.50 (0.40–0.50) | 0.50 (0.40–0.50) | 0.305 | 0.50 (0.40–0.50) | 0.50 (0.40–0.56) | 0.726 |
| Mean aortic gradient, mm Hg | 46.0 (35.6–60.2) | 46.1 (36.3–60.2) | 44.5 (32.1–59.7) | 0.096 | 47.0 (37.7–60.2) | 45.3 (34.2–60.1) | 0.250 | 46.7 (36.5–60.5) | 43.0 (30.4–57.3) | 0.006 |
| Left ventricular ejection fraction, % | 62.3 (55.1–65.6) | 62.6 (56.5–65.9) | 58.1 (46.7–64.1) | <0.001 | 63.3 (58.0–66.3) | 61.5 (52.7–65.2) | <0.001 | 62.7 (56.4–65.9) | 58.0 (46.8–63.9) | <0.001 |
| Left ventricular end‐diastolic diameter, mm | 44.1 (40.2–48.0) | 44.0 (40.2–47.5) | 45.4 (40.6–50.3) | 0.012 | 44.4 (41.0–47.8) | 43.8 (40.0–48.0) | 0.045 | 44.0 (40.2–47.6) | 45.3 (40.8–50.0) | 0.0600 |
| Aortic regurgitation ≥moderate | 61 (5.9) | 45 (5.2) | 16 (9.3) | 0.048 | 21 (5.1) | 40 (6.4) | 0.398 | 45 (5.0) | 16 (11.1) | 0.008 |
| Mitral regurgitation ≥moderate | 48 (4.6) | 29 (3.3) | 19 (11.1) | <0.001 | 0 | 48 (7.6) | <0.001 | 29 (3.2) | 19 (13.2) | <0.001 |
| Tricuspid regurgitation ≥moderate | 36 (3.5) | 22 (2.5) | 14 (8.1) | 0.001 | 4 (1.0) | 32 (5.1) | <0.001 | 25 (2.8) | 11 (7.6) | 0.008 |
| Systolic pulmonary arterial pressure, mm Hg | 31.8 (26.0–38.0) | 31.0 (26.0–37.0) | 35.0 (28.0–44.0) | <0.001 | 30.0 (26.0–36.0) | 32.6 (27.0–40.0) | <0.001 | 31.0 (26.0–37.0) | 35.0 (29.0–45.0) | <0.001 |
| MDCT data | ||||||||||
| Annulus area, mm2 | 396.1 (351.4–453.8) | 392.7 (349.7–446.4) | 419.4 (366.4–493.0) | <0.001 | 394.0 (352.8–448.6) | 398.0 (350.9–455.7) | 0.497 | 393.1 (350.4–449.1) | 408.6 (369.2–466.8) | 0.007 |
| Annulus perimeter, mm | 71.4 (67.6–76.3) | 71.2 (67.4–75.8) | 73.0 (68.7–79.5) | <0.001 | 71.3 (67.8–75.9) | 71.5 (67.5–76.5) | 0.524 | 71.3 (67.4–76.2) | 72.2 (68.7–77.2) | 0.036 |
| LVOT area, mm2 | 390.4 (329.8–477.6) | 384.9 (325.6–467.3) | 422.9 (357.9–529.5) | <0.001 | 386.5 (324.0–468.4) | 392.1 (334.5–483.7) | 0.227 | 388.6 (326.7–475.2) | 407.2 (353.7–503.2) | 0.033 |
| STJ height, mm | 18.6 (16.8–20.6) | 18.6 (16.8–20.5) | 19.1 (16.8–21.3) | 0.102 | 18.7 (16.8–20.6) | 18.6 (16.8–20.7) | 0.723 | 18.6 (16.8–20.6) | 18.8 (16.7–21.2) | 0.731 |
| STJ diameter, mm | 24.6 (22.8–27.0) | 24.5 (22.6–26.8) | 25.7 (23.3–27.4) | 0.001 | 24.6 (22.6–26.9) | 24.8 (22.8–27.1) | 0.304 | 24.6 (22.7–26.9) | 25.2 (23.3–27.3) | 0.022 |
| Mean SOV diameter, mm | 29.5 (27.7–31.8) | 29.3 (27.7–31.5) | 30.4 (28.2–32.6) | 0.001 | 29.3 (27.7–31.8) | 29.6 (27.7–31.8) | 0.539 | 29.4 (27.7–31.7) | 29.8 (28.2–32.3) | 0.054 |
| Left coronary artery height, mm | 13.4 (11.9–14.8) | 13.4 (11.8–14.8) | 13.4 (12.1–14.6) | 0.928 | 13.4 (11.9–14.8) | 13.4 (11.9–14.8) | 0.997 | 13.4 (11.8–14.8) | 13.1 (12.3–14.3) | 0.389 |
| Right coronary artery height, mm | 15.0 (13.1–17.0) | 14.9 (13.0–16.9) | 15.3 (13.5–17.3) | 0.053 | 14.9 (13.0–17.0) | 15.0 (13.2–17.0) | 0.620 | 15.0 (13.1–17.1) | 15.0 (13.3–17.0) | 0.705 |
| LVOT calcification ≥moderate | 68 (6.5) | 47 (5.4) | 21 (12.2) | 0.002 | 18 (4.4) | 50 (8.0) | 0.020 | 50 (5.6) | 18 (12.5) | 0.004 |
Values are number (percentage) or median (interquartile range). CONUT indicates Controlling Nutritional Status; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; LVOT, left ventricular outflow tract; MDCT, multidetector computed tomography; NYHA, New York Heart Association; PNI, Prognostic Nutritional Index; SOV, sinus of Valsalva; STJ, sinotubular junction; and STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality.
Prevalence and Clinical Associations of Malnutrition
The prevalence of malnutrition differed according to the 3 nutritional indexes as follows: 72.2% with the CONUT score, 75.3% with the GNRI, and 13.8% with the PNI. Among them, by the CONUT score, GNRI, and PNI, 172 (16.6%), 629 (60.5%), and 144 (13.8%) patients had moderate to severe malnutrition, respectively (Figure 2A). Although the 3 nutritional indexes correlated with each other (CONUT score versus GNRI, r=−0.63, P<0.001; CONUT score versus PNI, r=−0.81, P<0.001; GNRI versus PNI, r=0.83, P<0.001), only 144 (13.8%) patients were categorized as undernourished (any degree of malnutrition) in all 3 indexes, and only 111 (10.7%) patients were categorized as normally nourished in any of the indexes (Figure 2B). The prevalence of malnutrition was higher in men than in women based on the CONUT score (79.0% versus 68.9%; P<0.001), whereas the prevalence of malnutrition did not differ significantly according to sex based on the GNRI (72.2% versus 76.8%; P=0.126) and NRI (14.6% versus 13.5%; P=0.633). The prevalence of malnutrition was higher in patients with a BMI≤22 kg/m2 than in those with a BMI>22 kg/m2 (78.8% versus 65.5% for the CONUT score, 89.1% versus 61.2% for the GNRI, 18.5% versus 9.1% for the PNI; P<0.001 for all comparisons) and was also higher in patients with a CFS≥4 than in those with a CFS≤3 (78.5% versus 66.0% for the CONUT score, 84.1% versus 66.5% for the GNRI, 19.7% versus 8.0% for the PNI; P<0.001 for all comparisons) (Table 2).
Table 2.
Prevalence of Malnutrition by Sex, Body Mass Index, and Clinical Frailty Scale
| Male sex (N=342) | Female sex (N=698) | P value | BMI≤22 kg/m2 (N=524) | BMI>22 kg/m2 (N=516) | P value | CFS≤3 (N=523) | CFS≥4 (N=517) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| CONUT score | |||||||||
| Normal | 72 (21.1) | 217 (31.1) | 0.001 | 111 (21.2) | 178 (34.5) | <0.001 | 178 (34.0) | 111 (21.5) | <0.001 |
| Mild | 197 (57.6) | 382 (54.7) | 303 (57.8) | 276 (53.5) | 284 (54.3) | 295 (57.1) | |||
| Moderate | 68 (19.9) | 91 (13.0) | 101 (19.3) | 58 (11.2) | 58 (11.1) | 101 (19.5) | |||
| Severe | 5 (1.5) | 8 (1.2) | 9 (1.7) | 4 (0.8) | 3 (0.6) | 10 (1.9) | |||
| GNRI | |||||||||
| Normal | 95 (27.8) | 162 (23.2) | 0.214 | 57 (10.9) | 200 (38.8) | <0.001 | 175 (33.5) | 82 (15.9) | <0.001 |
| Mild | 42 (12.3) | 112 (16.1) | 52 (9.9) | 102 (19.8) | 84 (16.1) | 70 (13.5) | |||
| Moderate | 175 (52.2) | 355 (50.9) | 327 (62.4) | 203 (39.3) | 236 (45.1) | 294 (56.9) | |||
| Severe | 30 (8.8) | 69 (9.9) | 88 (16.8) | 11 (2.1) | 28 (5.4) | 71 (13.7) | |||
| PNI | |||||||||
| Normal | 292 (85.4) | 604 (86.5) | 0.877 | 427 (81.5) | 469 (90.9) | <0.001 | 481 (92.0) | 415 (80.3) | <0.001 |
| Mild | … | … | … | … | … | … | |||
| Moderate | 27 (7.9) | 50 (7.2) | 49 (9.4) | 28 (5.4) | 25 (4.8) | 52 (10.1) | |||
| Severe | 23 (6.7) | 44 (6.3) | 48 (9.2) | 19 (3.7) | 17 (3.3) | 50 (9.7) | |||
Values are number (percentage). BMI indicates body mass index; CFS, Clinical Frailty Scale; CONUT, Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; and PNI, Prognostic Nutritional Index.
Compared with patients with normal nutrition, those with malnutrition according to any of the 3 nutritional indexes were older, leaner, and frailer and had worse New York Heart Association functional class and higher Society of Thoracic Surgeons scores. They also had a higher prevalence of atrial fibrillation, anemia, and kidney dysfunction. Moreover, echocardiographic data showed that lower LVEF and mean aortic gradient and higher degrees of mitral and tricuspid regurgitation were observed in patients with worse nutritional status (Tables S1A through S1C).
Procedural Characteristics and In‐Hospital Outcomes
The procedural characteristics and in‐hospital outcomes are listed in Table 3. Most patients (86.6%) underwent TAVI via the transfemoral approach. TAVI was performed using balloon‐expandable and self‐expandable valves in 801 (77.0%) and 239 (23.0%) patients, respectively. Emergent and urgent procedures were performed in 45 (3.3%) patients and were more frequently performed in patients with worse nutritional status according to any of the 3 nutritional indexes. In‐hospital death was identified in 15 (1.4%) patients, including cardiovascular death in 7 patients and noncardiovascular death in 8 patients. Compared with patients with normal nutrition, those with malnutrition had a higher in‐hospital mortality rate and a longer hospital stay after TAVI (Tables S2A through S2C).
Table 3.
Procedure Characteristics and In‐Hospital Outcomes
| Total (N=1040) | CONUT score | GNRI | PNI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal/mild, 0–4 (N=868) | Moderate/severe, 5–12 (N=172) | P value | Normal/mild, ≥97.5 (N=411) | Moderate/severe, <97.5 (N=629) | P value | Normal, >38 (N=896) | Moderate/severe, ≤38 (N=144) | P value | ||
| Local anesthesia | 635 (61.1) | 537 (61.9) | 98 (57.0) | 0.232 | 253 (61.6) | 382 (60.7) | 0.789 | 541 (60.4) | 94 (65.3) | 0.260 |
| Emergent and urgent procedure | 45 (3.3) | 21 (2.4) | 24 (14.0) | <0.001 | 4 (1.0) | 41 (6.5) | <0.001 | 20 (2.2) | 25 (17.4) | <0.001 |
| Access site | ||||||||||
| Transfemoral | 901 (86.6) | 753 (86.8) | 148 (86.1) | 0.805 | 362 (88.1) | 539 (85.7) | 0.266 | 768 (85.7) | 133 (92.4) | 0.021 |
| Alternative | 139 (13.4) | 115 (13.3) | 24 (14.0) | 49 (11.9) | 90 (14.3) | 128 (14.3) | 11 (7.6) | |||
| Prosthesis type | ||||||||||
| SAPIEN XT | 214 (20.6) | 184 (21.2) | 30 (17.4) | 0.563 | 95 (23.1) | 119 (18.9) | 0.179 | 197 (22.0) | 17 (11.8) | 0.016 |
| SAPIEN 3 | 587 (56.4) | 482 (55.5) | 105 (61.1) | 234 (56.9) | 353 (56.1) | 491 (54.8) | 96 (66.7) | |||
| CoreValve | 17 (1.6) | 14 (1.6) | 3 (1.7) | 6 (1.5) | 11 (1.8) | 15 (1.7) | 2 (1.4) | |||
| Evolut R/PRO | 222 (21.3) | 188 (21.7) | 34 (19.8) | 76 (18.5) | 146 (23.2) | 193 (21.5) | 29 (20.1) | |||
| Prosthesis size | ||||||||||
| 20 mm | 15 (1.4) | 11 (1.3) | 4 (2.3) | 0.004 | 5 (1.2) | 10 (1.6) | 0.799 | 11 (1.2) | 4 (2.8) | 0.061 |
| 23 mm | 437 (42.1) | 379 (43.7) | 58 (33.9) | 180 (43.8) | 257 (40.9) | 387 (43.2) | 50 (35.0) | |||
| 26 mm | 437 (42.1) | 367 (42.3) | 70 (40.9) | 168 (40.9) | 269 (42.8) | 377 (42.1) | 60 (42.0) | |||
| 29 mm | 150 (14.4) | 111 (12.8) | 39 (22.8) | 58 (14.1) | 92 (14.7) | 121 (13.5) | 29 (20.3) | |||
| Length of hospital stay after TAVI, days | 9 (6–14) | 9 (6–13) | 13 (7–21) | <0.001 | 8 (6–12) | 10 (7–15) | <0.001 | 9 (6–13) | 14 (8–25) | <0.001 |
| All‐cause mortality | 15 (1.4) | 9 (1.0) | 6 (3.5) | 0.030 | 4 (1.0) | 11 (1.8) | 0.291 | 9 (1.0) | 6 (4.2) | 0.012 |
| Acute kidney injury | 57 (5.5) | 36 (4.2) | 21 (12.2) | <0.001 | 20 (4.9) | 37 (5.9) | 0.479 | 40 (4.5) | 17 (11.8) | 0.001 |
| Disabling stroke | 14 (1.3) | 11 (1.3) | 3 (1.7) | 0.632 | 3 (0.7) | 11 (1.8) | 0.146 | 10 (1.1) | 4 (2.8) | 0.149 |
| PVL≥moderate | 2 (0.2) | 1 (0.1) | 1 (0.6) | 0.275 | 2 (0.5) | 0 | 0.054 | 2 (0.2) | 0 | 0.440 |
| Life‐threatening/disabling bleeding | 41 (3.9) | 32 (3.7) | 9 (5.2) | 0.361 | 10 (2.4) | 31 (4.9) | 0.037 | 30 (3.4) | 11 (7.6) | 0.027 |
| Coronary obstruction | 7 (0.7) | 5 (0.6) | 2 (1.2) | 0.426 | 3 (0.7) | 4 (0.6) | 0.857 | 6 (0.7) | 1 (0.7) | 0.973 |
| Major vascular complications | 44 (4.2) | 37 (4.3) | 7 (4.1) | 0.908 | 17 (4.1) | 27 (4.3) | 0.903 | 36 (4.0) | 8 (5.6) | 0.413 |
| Conversion to open surgery | 11 (1.1) | 8 (0.9) | 3 (1.7) | 0.369 | 2 (0.5) | 9 (1.4) | 0.125 | 7 (0.8) | 4 (2.8) | 0.060 |
| New pacemaker implantation | 80 (7.7) | 65 (7.5) | 15 (8.7) | 0.588 | 26 (6.3) | 54 (8.6) | 0.174 | 72 (8.0) | 8 (5.6) | 0.279 |
Values are number (percentage) or median (interquartile range). CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; PNI, Prognostic Nutritional Index; PVL, paravalvular leakage; and TAVI, transcatheter aortic valve implantation.
Postprocedural Echocardiographic Data
All patients underwent the postprocedural echocardiographic follow‐up (Table 4). On the whole, acceptable THV function was obtained (indexed effective orifice area of 1.17 cm2 [IQR, 1.00–1.36 cm2]; mean pressure gradient of 10.5 mm Hg [IQR 8.0–13.5 mm Hg]), whereas moderate and severe prosthesis–patient mismatches were identified in 76 (7.3%) and 8 (0.8%) patients, respectively. Moderate to severe paravalvular leakage was observed in only 2 (0.2%) patients. Compared with patients with normal nutrition, those with malnutrition had a lower mean pressure gradient for any of the 3 nutritional indexes, whereas larger indexed effective orifice area was observed in those with worse nutritional status according to the GNRI only. The incidence of prosthesis–patient mismatch was comparable between patients with each nutritional status based on any of the 3 indexes (Tables S3A through S3C).
Table 4.
Postprocedural Echocardiographic Data
| Total (N=1040) | CONUT score | GNRI | PNI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal/mild, 0–4 (N=868) | Moderate/severe, 5–12 (N=172) | P value | Normal/mild, ≥97.5 (N=411) | Moderate/severe, <97.5 (N=629) | P value | Normal, >38 (N=896) | Moderate/severe, ≤38 (N=144) | P value | ||
| Effective orifice area, cm2 | 1.65 (1.43–1.95) | 1.65 (1.42–1.95) | 1.66 (1.43–1.97) | 0.604 | 1.65 (1.44–1.96) | 1.63 (1.41–1.93) | 0.165 | 1.65 (1.42–1.95) | 1.67 (1.48–1.96) | 0.612 |
| Indexed effective orifice area, cm2/m2 | 1.17 (1.00–1.36) | 1.17 (1.00–1.35) | 1.19 (1.01–1.38) | 0.176 | 1.12 (0.99–1.31) | 1.20 (1.01–1.39) | 0.001 | 1.16 (1.00–1.35) | 1.22 (1.04–1.42) | 0.021 |
| Mean pressure gradient, mm Hg | 10.5 (8.0–13.5) | 10.8 (8.2–13.6) | 9.5 (7.7–12.4) | 0.001 | 11.1 (8.6–14.3) | 9.9 (7.8–12.9) | <0.001 | 10.7 (8.2–13.7) | 9.5 (7.5–12.3) | 0.001 |
| Left ventricular ejection fraction, % | 61.9 (55.6–65.7) | 62.1 (56.9–65.8) | 60.1 (47.6–64.4) | <0.001 | 63.0 (58.4–66.3) | 61.4 (54.0–65.0) | <0.001 | 62.1 (56.9–65.8) | 59.8 (46.1–64.4) | <0.001 |
| PPM | 84 (8.1) | 75 (8.6) | 9 (5.2) | 0.115 | 36 (8.8) | 48 (7.6) | 0.516 | 77 (8.6) | 7 (4.9) | 0.105 |
| Moderate PPM | 76 (7.3) | 69 (8.0) | 7 (4.1) | 0.056 | 35 (8.5) | 41 (6.5) | 0.230 | 70 (7.8) | 6 (4.2) | 0.095 |
| Severe PPM | 8 (0.8) | 6 (0.7) | 2 (1.2) | 0.541 | 1 (0.2) | 7 (1.1) | 0.089 | 7 (0.8) | 1 (0.7) | 0.911 |
| Moderate to severe PVL | 2 (0.2) | 1 (0.1) | 1 (0.6) | 0.275 | 2 (0.5) | 0 | 0.054 | 2 (0.2) | 0 | 0.440 |
Values are number (percentage) or median (interquartile range). CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; PNI, Prognostic Nutritional Index; PPM, prosthesis–patient mismatch; and PVL, paravalvular leakage.
Nutritional Scores and Long‐Term Clinical Outcomes
At a median follow‐up of 985.9 days (IQR, 556.0–1402.3 days), a total of 274 patients with all‐cause death were identified; 59 (21.5%) patients died for cardiac reasons, and the remaining 215 (78.5%) patients died for noncardiac reasons (Figure 3). Rehospitalization attributed to heart failure was required in 91 patients. The results of the univariate analysis for the association between all‐cause mortality and clinical findings are presented in Table S4. Worsening malnutrition status as a continuous variable was associated with a higher incidence of all‐cause mortality for any malnutrition indexes (Figure 4). The Kaplan–Meier analysis also showed that moderate to severe malnutrition was related to a higher incidence of all‐cause mortality than the other nutritional groups regardless of the malnutrition index used. Patients with mild malnutrition had a higher mortality rate than those with normal nutritional status by the CONUT score, whereas comparable mortality rates between the 2 groups were observed by the GNRI. Similarly for cardiovascular mortality and heart failure hospitalization, worsening malnutrition status was associated with worse prognoses by the CONUT score and the PNI, whereas there was a trend for a higher event rate in the worse malnutrition groups, albeit with no significant group difference by the GNRI (Figure 5).
Figure 3. Causes of death after transcatheter aortic valve implantation.
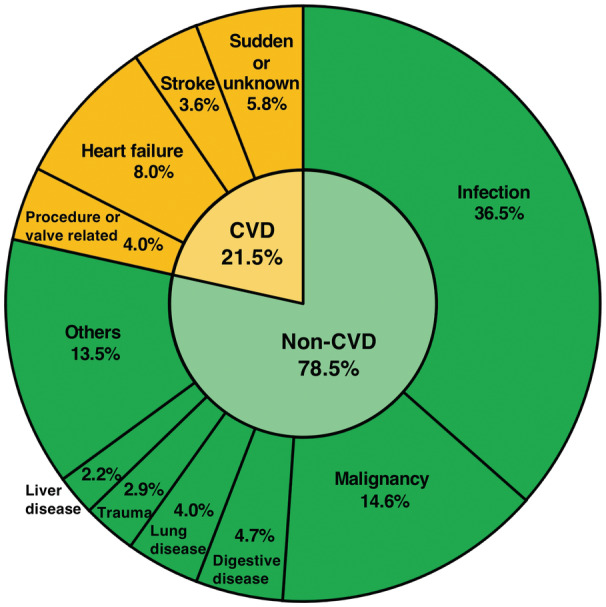
The pie chart showing the distribution of diseases causing mortality during the follow‐up period. CVD indicates cardiovascular death.
Figure 4. Association between nutritional indexes and incidence rate of all‐cause mortality.
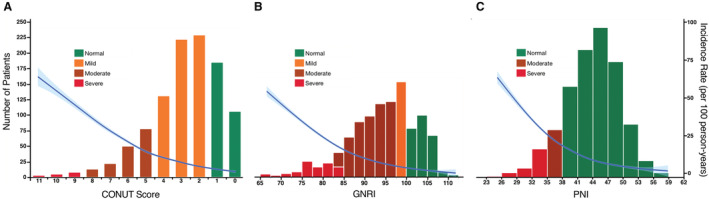
The incidence rate of all‐cause mortality is shown after adjustment for age, sex, body mass index, and Society of Thoracic Surgeons Predicted Risk of Mortality score for each nutritional index: (A) CONUT score, (B) GNRI, and (C) PNI. The right y‐axis shows the incidence rate (events per 100 person‐years). The x‐axis shows the score of malnutrition indexes. The blue curves show the incidence with 95% CIs of the estimates. Poisson models were used to estimate the incidence rates. Histograms show the population distribution of malnutrition indexes. CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; and PNI, Prognostic Nutritional Index.
Figure 5. Malnutrition degrees and clinical outcomes.
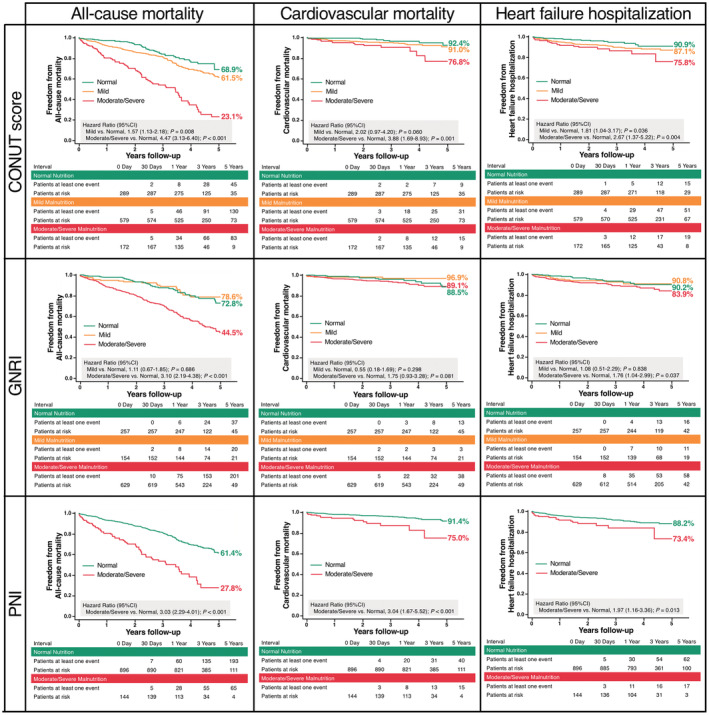
Kaplan–Meier curves for (left) all‐cause mortality, (middle) cardiovascular mortality, and (right) heart failure hospitalization by the CONUT score, GNRI, and PNI. CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; and PNI, Prognostic Nutritional Index.
In the multivariable analyses, compared with normal nutritional status, moderate or severe malnutrition was independently associated with the risk of all‐cause death during the follow‐up period, irrespective of the malnutrition indexes used (Table 5). In addition, we dichotomized patients according to the median age of 85 years, the median BMI of 22 kg/m2, the median CFS of 3, the LVEF of 50%, chronic renal failure status, and sex. Regardless of the stratification based on these factors, worsening malnutrition status was independently associated with a higher incidence of mortality without significant interactions (Figure S1A through S1C).
Table 5.
Multivariable Cox Regression Analyses of Malnutrition for All‐Cause Mortality
| Multivariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CONUT score | GNRI | PNI | |||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Model 1 | |||||||||
| Normal nutrition | … | … | Reference | … | … | Reference | … | … | Reference |
| Mild malnutrition | 1.22 | 0.87–1.72 | 0.26 | 1.06 | 0.63–1.79 | 0.82 | … | … | … |
| Moderate malnutrition | 2.22 | 1.46–3.35 | <0.001 | 2.02 | 1.36–3.01 | 0.001 | 1.64 | 1.09–2.46 | 0.018 |
| Severe malnutrition | 6.20 | 2.76–13.91 | <0.001 | 3.26 | 1.87–5.69 | <0.001 | 2.32 | 1.50–3.60 | <0.001 |
| Model 2 | |||||||||
| Normal nutrition | … | … | Reference | … | … | Reference | … | … | Reference |
| Mild malnutrition | 1.18 | 0.83–1.66 | 0.36 | 1.07 | 0.63–1.81 | 0.80 | … | … | … |
| Moderate malnutrition | 2.04 | 1.34–3.11 | 0.001 | 1.99 | 1.33–2.97 | 0.001 | 1.53 | 1.02–2.31 | 0.041 |
| Severe malnutrition | 5.97 | 2.67–13.33 | <0.001 | 3.23 | 1.86–5.62 | <0.001 | 2.27 | 1.47–3.51 | <0.001 |
Model 1 was adjusted for the following preprocedural variables: age, sex, body mass index, Clinical Frailty Scale, New York Heart Association class III/IV, Society of Thoracic Surgeons Predicted Risk of Mortality score, dyslipidemia, atrial fibrillation, peripheral artery disease, active cancer, hemoglobin, estimated glomerular filtration rate, brain natriuretic peptide, left ventricular ejection fraction, moderate to severe mitral regurgitation, transfemoral approach, and moderate to severe left ventricular outflow tract calcification. Model 2 was additionally adjusted for the following postprocedural variables: conversion to open surgery, new pacemaker implantation, prosthesis–patient mismatch, and moderate to severe paravalvular leakage. CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; HR, hazard ratio; and PNI, Prognostic Nutritional Index.
The c‐statistics did not differ among the 3 nutritional indexes for all‐cause mortality, cardiovascular mortality, and heart failure hospitalization. However, the CONUT score and the PNI have higher sensitivity than the GNRI for all of these outcomes (Figure 6).
Figure 6. Discrimination ability of each nutritional index for all‐cause mortality, cardiovascular mortality, and heart failure hospitalization.

Receiver operating characteristic curves with comparative analyses of the discrimination of each nutritional index for (A) all‐cause mortality, (B) cardiovascular mortality, and (C) heart failure hospitalization. CONUT indicates Controlling Nutritional Status; GNRI, Geriatric Nutritional Risk Index; NPV, negative predictive value; PNI, Prognostic Nutritional Index; and PPV, positive predictive value.
Nutritional Status at 1‐Year Follow‐Up
We also retrospectively collected data on the nutritional status at 1 year after TAVI of 934 patients who survived at 1‐year follow‐up and assessed temporal changes of nutritional status according to each nutritional index (Figures S2A to S2C). Of a total of 934 patients, 98 (10.5%) patients had no follow‐up data on nutritional status, who also tended to be in worse preprocedural nutritional status. Improved, stable, and worsened nutritional categories were, respectively, observed in 212 (22.7%), 483 (51.7%), and 141 (15.1%) patients for the CONUT score; in 212 (22.7%), 452 (48.4%), and 172 (18.4%) patients for the GNRI; and in 64 (6.9%), 707 (75.7%), and 65 (7.0%) patients for the PNI.
Discussion
In this study, we evaluated the prevalence and prognostic impact of malnutrition, which was assessed by 3 existing indexes, in patients undergoing TAVI. Malnutrition was common but was associated with a poor prognosis after TAVI, regardless of the nutrition index used and irrespective of age, sex, BMI, frailty, kidney function, and LVEF.
Few previous studies have assessed the prevalence of malnutrition in patients undergoing TAVI. Honda et al 11 reported that two‐thirds of 150 patients undergoing TAVI were malnourished using the CONUT score, and Doi et al 23 also reported that 65% of 288 patients undergoing TAVI were malnourished or at risk of malnutrition using the Mini Nutritional Assessment–Short Form. Aortic stenosis is a chronic, progressive disease with a prolonged inflammatory process that may cause reduced mobility, loss of muscle mass, decreased appetite, and poor nutritional status. 24 Moreover, most of the patients undergoing TAVI are elderly, although indications for TAVI have recently been expanded to include younger patients. Aging also decreases one's metabolic reserve of albumin, and therefore, the nutritional status of the elderly can be easily affected by relatively small stresses. 25 Nevertheless, malnutrition in candidates for TAVI is often unrecognized and, thus, untreated in clinical practice. In our study, comparing the clinical significance of 3 nutritional indexes in a large cohort of 1040 patients, the prevalence of malnutrition depended on the nutritional index used, ranging from 13.8% with the PNI, to 72.2% with the CONUT score, and to 75.3% with the GNRI. In particular, the PNI identified far fewer patients as malnourished compared with the other indexes. Because the PNI only identifies patients as moderately or severely malnourished, it may therefore underestimate the overall prevalence of malnutrition. Indeed, a higher concordance for the prevalence of moderate to severe malnutrition was observed between the PNI (13.8%) and the CONUT score (16.6%), perhaps reflecting the similarity of the variables on which these 2 indexes are based; on the other hand, a higher prevalence of moderate to severe malnutrition was found with the GNRI (60.5%), which considers anthropometric factors and serum markers. Therefore, clinical cardiologists should understand that malnutrition is prevalent in candidates for TAVI and that the nutritional indexes are often not interchangeable.
Lower BMI, which is believed to reflect undernourishment, is associated with worse prognosis in patients with heart failure, a phenomenon that is often termed the obesity paradox. In our study, patients with a lower BMI had higher degrees of malnutrition; however, it should be noted that malnutrition was highly prevalent also in those with a BMI >22 kg/m2 using the CONUT and GNRI criteria (65.5% and 61.2%, respectively). A high prevalence of malnutrition in patients who were overweight or obese has also been reported in previous investigations on acute coronary syndrome and heart failure, highlighting that malnutrition does not simply manifest as being underweight. In addition, it is important to note that malnutrition is not only common in patients who are frail with CFS≥4 but also is relatively prevalent in patients with CFS≤3. Indeed, as shown in Figures S1A to S1C, multivariable analyses revealed no significant interaction between the malnutrition groups and BMI groups and between the malnutrition groups and CFS groups regarding overall survival. Therefore, we should be quantitatively screening nutritional status using objective indexes without being bound by anthropometric and visual factors.
This study demonstrated that worsening malnutrition status was associated with increased long‐term mortality in patients undergoing TAVI, irrespective of age, sex, BMI, frailty, kidney function, and LVEF. The predictive role of malnutrition indexes in mortality was also confirmed after adjusting by clinical variables with known poor prognosis, including CFS, Society of Thoracic Surgeons Predicted Risk of Mortality score, and kidney function. These results are mostly consistent with those of previous reports; however, previous studies have tended to be small and/or focused on a single nutritional index. Honda et al 11 showed an association between malnutrition assessed by the CONUT score and 1‐year mortality after TAVI in a cohort of 150 patients. Doi et al 23 also demonstrated that malnutrition assessed by the Mini Nutritional Assessment–Short Form independently is an independent predictor of 2‐year mortality after TAVI in a cohort of 288 patients. However, the results of these 2 studies were not completely in agreement with the research of Okuno et al, 12 who revealed that the GNRI was not associated with 1‐year prognosis after TAVI, whereas the CONUT score and the PNI were useful predictors of 1‐year mortality. Although this research is the first and only study to compare the clinical significance of malnutrition in patients undergoing TAVI among the 3 nutritional indexes, it should be interpreted with caution because of the small sample size (n=95) and mortality events (n=9). One potential explanation for the relationship between malnutrition and poor prognosis in patients undergoing TAVI is that nutritional status may be a surrogate parameter for systemic inflammation and immune function. Indeed, previous studies reported that the causes of death after TAVI were predominantly noncardiac and mostly attributed to infection and cancer, 26 , 27 whereas in the present study, infection and cancer accounted for more than half of all deaths. In addition, systemic inflammation reportedly induces atherosclerosis, 28 which may contribute to the higher incidence of cardiovascular mortality in patients with worse nutritional status.
Finally, we should discuss the futility of performing TAVI in patients who are malnourished. Although the recent development of devices and procedures has provided tremendous survival rates after TAVI, a considerable number of patients do not fully benefit from TAVI despite a technically successful procedure. The option to consider palliative care instead of a futile invasive procedure for these patients is increasingly recognized. 29 Futility risk models after TAVI, consisting of many comorbidities such as atrial fibrillation, chronic renal failure, liver disease, pulmonary disease, anemia, and cancer, were previously reported; however, these models did not include a detailed evaluation of nutritional status. 30 , 31 Our study showed a prohibitive risk of mortality (80%) at 5 years in patients with preprocedural moderate or severe malnutrition, which was observed in 20% of the study population. We ought to acknowledge that worse nutritional status as a potential surrogate marker for diminished physiological reserve is associated with early mortality after TAVI. Objective nutritional indexes such as the CONUT score, GNRI, and PNI would be useful for the preprocedural risk stratification.
Limitations
This study has several limitations that warrant discussion. First, this is a single‐center observational study including a retrospective analysis with all inherent limitations. Data are limited to Asian patients, without information regarding educational background, family structure, or socioeconomic status, which may improve understanding of the causes of malnutrition. Therefore, confirmation of our findings by other investigators and in other countries with different health care and social systems is important. Second, the nutritional status assessed by the 3 simple objective scoring systems was not validated by more complex comprehensive nutritional assessments, such as subjective evaluation (eg, the Subjective Global Assessment and Mini Nutritional Assessment). In addition, we did not compare the prognostic value of these 3 scoring tools with comprehensive nutritional assessments. However, some subjective nutritional evaluations may be inaccurate for older patients with cognitive impairments, most of whom have indication for TAVI rather than surgical aortic valve replacement. From this perspective, the objective nutritional indexes assessed in this study will be useful for the nutritional screening of candidates for TAVI. Third, although nutritional status was assessed not only at the TAVI procedure but also at the 1‐year follow‐up, the clinical effect of nutritional interventions for patients who were undernourished was not determined. Fourth, we did not routinely perform the follow‐up computed tomography. Therefore, the presence of leaflet thrombosis on THV, which might have affected outcomes after TAVI, was not assessed in this study.
Conclusions
Malnutrition is common in patients undergoing TAVI and is associated with a poor prognosis after TAVI. Adequate nutritional assessment before TAVI may allow clinicians to identify patients at elevated risk for all‐cause mortality and cardiovascular events and who may benefit from nutritional support. These findings warrant further investigation into the clinical effect of nutritional interventions for patients who are undernourished.
Sources of Funding
None.
Disclosures
Dr Shinichi Shirai is the proctor of transfemoral‐TAVI for Edwards Lifesciences and Medtronic. Dr Yoshio Arai is the proctor of transapical‐TAVI for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supporting information
Table S1–S4.
Figure S1–S2.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026294
For Sources of Funding and Disclosures, see page 15.
See Editorial by Pompeu et al.
References
- 1. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, et al. 5‐year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7 [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 3. Kodali S, Thourani VH, White J, Malaisrie SC, Lim S, Greason KL, Williams M, Guerrero M, Eisenhauer AC, Kapadia S, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high‐risk and intermediate‐risk patients with aortic stenosis. Eur Heart J. 2016;37:2252–2262. doi: 10.1093/eurheartj/ehw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 6. Vandvik PO, Otto CM, Siemieniuk RA, Bagur R, Guyatt GH, Lytvyn L, Whitlock R, Vartdal T, Brieger D, Aertgeerts B, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ. 2016;354:i5085. doi: 10.1136/bmj.i5085 [DOI] [PubMed] [Google Scholar]
- 7. Kalantar‐Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti‐inflammatory interventions in chronic heart failure. Am J Cardiol. 2008;101:S89–S103. doi: 10.1016/j.amjcard.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, Clark AL. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. 2018;6:476–486. doi: 10.1016/j.jchf.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 9. Raposeiras Roubin S, Abu Assi E, Cespon Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, Perez DD, Blanco Prieto S, Rossello X, Ibanez B, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. 2020;76:828–840. doi: 10.1016/j.jacc.2020.06.058 [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama M, Watanabe T, Otaki Y, Watanabe K, Toshima T, Sugai T, Takahashi T, Kinoshita D, Tamura H, Nishiyama S, et al. Impact of objective malnutrition status on the clinical outcomes in patients with peripheral artery disease following endovascular therapy. Circ J. 2018;82:847–856. doi: 10.1253/circj.CJ-17-0731 [DOI] [PubMed] [Google Scholar]
- 11. Honda Y, Yamawaki M, Shigemitsu S, Kenji M, Tokuda T, Tsutumi M, Mori S, Sakamoto Y, Kobayashi N, Araki M, et al. Prognostic value of objective nutritional status after transcatheter aortic valve replacement. J Cardiol. 2019;73:401–407. doi: 10.1016/j.jjcc.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 12. Okuno T, Koseki K, Nakanishi T, Sato K, Ninomiya K, Tomii D, Tanaka T, Sato Y, Horiuchi Y, Koike H, et al. Evaluation of objective nutritional indexes as predictors of one‐year outcomes after transcatheter aortic valve implantation. J Cardiol. 2019;74:34–39. doi: 10.1016/j.jjcc.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 13. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. doi: 10.1016/j.jtcvs.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 14. Ishizu K, Shirai S, Kawaguchi T, Taniguchi T, Hayashi M, Isotani A, Arai Y, Soga Y, Kakumoto S, Ando K. Effect of radiolucent line‐guided balloon‐expandable transcatheter aortic valve implantation on subsequent pacemaker rate. Am J Cardiol. 2022;165:72–80. doi: 10.1016/j.amjcard.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 15. Okuno T, Asami M, Heg D, Lanz J, Praz F, Hagemeyer D, Brugger N, Gräni C, Huber A, Spirito A, et al. Impact of left ventricular outflow tract calcification on procedural outcomes after transcatheter aortic valve replacement. JACC: Cardiovasc Interv. 2020;13:1789–1799. doi: 10.1016/j.jcin.2020.04.015 [DOI] [PubMed] [Google Scholar]
- 16. Ignacio de Ulíbarri J, González‐Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 17. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent J‐P, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 18. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, Lewis EF, Shah SJ, Sweitzer NK, Fang JC, et al. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC: Heart Fail. 2019;7:664–675. doi: 10.1016/j.jchf.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 19. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–167. doi: 10.1016/0002-9610(80)90246-9 [DOI] [PubMed] [Google Scholar]
- 20. Ishizu K, Shirai S, Isotani A, Hayashi M, Kawaguchi T, Taniguchi T, Ando K, Yashima F, Tada N, Yamawaki M, et al. Long‐term prognostic value of the Society of Thoracic Surgery Risk Score in patients undergoing transcatheter aortic valve implantation (from the OCEAN‐TAVI registry). Am J Cardiol. 2021;149:86–94. doi: 10.1016/j.amjcard.2021.03.027 [DOI] [PubMed] [Google Scholar]
- 21. Ludman PF, Moat N, De Belder MA, Blackman DJ, Duncan A, Banya W, Maccarthy PA, Cunningham D, Wendler O, Marlee D, et al. Transcatheter aortic valve implantation in the United Kingdom. Circulation. 2015;131:1181–1190. doi: 10.1161/CIRCULATIONAHA.114.013947 [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 23. Doi S, Ashikaga K, Kida K, Watanabe M, Yoneyama K, Suzuki N, Kuwata S, Kaihara T, Koga M, Okuyama K, et al. Prognostic value of mini nutritional assessment—short form with aortic valve stenosis following transcatheter aortic valve implantation. ESC Heart Fail. 2020;7:4024–4031. doi: 10.1002/ehf2.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatia N, Basra SS, Skolnick AH, Wenger NK. Aortic valve disease in the older adult. J Geriatr Cardiol. 2016;13:941–944. doi: 10.11909/j.issn.1671-5411.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Sandbank J, Stav K, Efrati S. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin Nutr. 2016;35:1522–1529. doi: 10.1016/j.clnu.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 26. Van Mieghem NM, Van Der Boon RM, Nuis R‐J, Schultz C, Van Geuns R‐J, Serruys PW, Kappetein A‐P, Van Domburg RT, De Jaegere PP. Cause of death after transcatheter aortic valve implantation. Catheter and Cardiovasc Interv. 2014;83:E277–E282. doi: 10.1002/ccd.24597 [DOI] [PubMed] [Google Scholar]
- 27. Saia F, Latib A, Ciuca C, Gasparetto V, Napodano M, Sticchi A, Anderlucci L, Marrozzini C, Naganuma T, Alfieri O, et al. Causes and timing of death during long‐term follow‐up after transcatheter aortic valve replacement. Am Heart J. 2014;168:798–806. doi: 10.1016/j.ahj.2014.07.023 [DOI] [PubMed] [Google Scholar]
- 28. Lehrke M, Millington SC, Lefterova M, Cumaranatunge RG, Szapary P, Wilensky R, Rader DJ, Lazar MA, Reilly MP. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol. 2007;49:442–449. doi: 10.1016/j.jacc.2006.09.034 [DOI] [PubMed] [Google Scholar]
- 29. Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE, Vassileva CM. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the Management of Adults with Aortic Stenosis: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2017;69:1313–1346. doi: 10.1016/j.jacc.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 30. Zusman O, Barbash MI, Guetta V, Finkelstein A, Assali A, Segev A, Orvin K, Barsheshet A, Younis A, Witberg G, et al. Predicting the risk of late futile outcome after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2020;96:E695–E702. [DOI] [PubMed] [Google Scholar]
- 31. Lantelme P, Lacour T, Bisson A, Herbert J, Ivanes F, Bourguignon T, Quilliet L, Angoulvant D, Harbaoui B, Babuty D, et al. Futility risk model for predicting outcome after transcatheter aortic valve implantation. Am J Cardiol. 2020;130:100–107. doi: 10.1016/j.amjcard.2020.05.043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4.
Figure S1–S2.


