Abstract
Immunization of mice by the intranasal route with influenza virus hemagglutinin in combination with the mutant Escherichia coli heat-labile enterotoxin R72 (LT-R72) induced significantly enhanced serum and mucosal antibodies, surpassing, in most cases, responses achieved by traditional intramuscular immunization using inactivated split influenza vaccine. Furthermore, intranasal immunization with LT-R72 induced a potent serum immunoglobulin G2a response, indicating that this adjuvant has Th1 character.
Yearly outbreaks of influenza cause significant morbidity in the general population and high mortality among individuals in high-risk groups (10). Several commercially available inactivated whole- and split-virus vaccines exist to control the spread and severity of influenza (8, 22). However, these vaccines suffer from limited efficacy in generating long-lasting immunity, particularly in the elderly, and are not sufficiently cross-reactive to protect against antigenic variants (5, 14, 17). Although these vaccines are known to induce serum immunoglobulin G (IgG) antibodies, they are poor stimulators of secretory IgA at respiratory mucosal sites and show sporadic CD8+ cytotoxic T-lymphocyte (CTL) activation (3, 10, 21). Efforts are currently under way to develop influenza vaccines that generate significant secretory IgA, as well as maintain high serum IgG titers, by exploiting mucosal immunization (5, 6, 16, 28). Our group has focused on investigating the activity of influenza virus hemagglutinin (HA) administered intranasally (i.n.) with mutant heat-labile enterotoxins (LTs). One such mutant toxin, LT-R72, shows significantly reduced toxicity in vitro and in vivo yet maintains potent mucosal adjuvanticity (9). In the current studies, i.n. administration of influenza virus HA to mice with LT-R72 was compared to intramuscular (i.m.) immunization for induction of serum antibody responses, generation of IgG1 and IgG2a antibody subclasses, HA inhibition titers, and IgA antibody levels in mucosal secretions.
Vaccines.
Purified monovalent A/Beijing8-9/93 (H3N2) influenza virus antigen was provided by Chiron Vaccines, Siena, Italy. Dosing was based on HA content as assayed by single radial immunodiffusion as described previously (15). LT-R72 was prepared as described previously (18). All immunogens were prepared in phosphate-buffered saline.
Immunization and sample collection.
Groups of 10 female BALB/c mice (Charles River Laboratories, Wilmington, Mass.), 6 to 10 weeks old, were immunized as described in Table 1. Immunizations were made by i.m. (50 μl) injection into the posterior thigh muscle and by i.n. (25 μl) dropwise additions into the alternate nares of unanesthetized animals. Serum, saliva wash (SW), vaginal wash (VW), and terminal nasal wash (NW) samples were collected from individual animals in accordance with the sample collection schedule in Table 1 by using methods described previously (27).
TABLE 1.
Study design
| Group | Amt (μg) of:
|
Immunization schedule (days) | Route | Day(s) of sample collection
|
|||
|---|---|---|---|---|---|---|---|
| HA | LT-R72 | Serum | SW, VW | NW | |||
| IM | 5 | None | 0, 21, 35 | i.m. | 21, 35, 49 | 49 | 63 |
| IN low | 1 | 10 | 0, 21, 35 | i.n. | 21, 35, 49 | 49 | 63 |
| IN high | 10 | 25 | 0, 21, 35 | i.n. | 21, 35, 49 | 49 | 63 |
Antibody ELISA.
Serum samples from individual animals were assayed for total anti-HA Ig (IgG plus IgA plus IgM) titers by a 3,3′,5,5′-tetramethylbenzidine-based colorimetric enzyme-linked immunosorbent assay (ELISA) as previously described with A/Beijing93 HA as the coating antigen (11). A490 was measured by using a standard ELISA reader. The titers represent reciprocal serum dilutions giving an A490 of 0.5 and were normalized to a serum standard assayed in parallel. SW, VW, and NW samples from individual animals were assayed for HA-specific IgA titers, and pooled serum samples were assayed for relative levels of HA-specific IgG1 and IgG2a antibodies by using a bioluminescence immunosorbent assay as previously described with A/Beijing93 HA as the coating antigen (27). A goat anti-mouse IgA-biotin conjugate (EY Laboratories, San Mateo, Calif.) was presaturated with purified mouse IgG (Sigma Chemical Co., St. Louis, Mo.) to reduce cross-reactivity. Quantitation was based on the number of relative light units representing the total luminescence integrated over 3 s (arbitrary units). Titers represent log dilution values linearly extrapolated from the log of the number of relative light units to a cutoff value at least 2 standard deviations above the mean background.
HI assay.
Serum samples pooled by group were assayed for hemagglutination inhibition (HI) titer by the Viral and Rickettsial Disease Laboratory (Department of Health Services, Berkeley, Calif.) using a standard ELISA. The HI assay is based on the ability of sample sera to inhibit the agglutination of goat erythrocytes in the presence of HA antigen, and results are expressed as the reciprocal dilution required for complete inhibition (12, 13).
Statistics.
Log anti-A/Beijing93 HA titers from individual animals (see Fig. 1) were analyzed by using a Fisher least-significant-difference procedure (1). Comparison intervals were presented such that nonoverlapping bars imply a statistically significant difference between means of greater than 5% (P ≤ 0.05). Log anti-A/Beijing93 HA IgA titers from individual animals (see Fig. 3) were analyzed for significant differences between groups (P ≤ 0.05) by using a median sign test. Results for antibody subclass analysis (see Fig. 4) represent means and standard deviations of replicate assay determinations (n ≥ 6) of pooled samples.
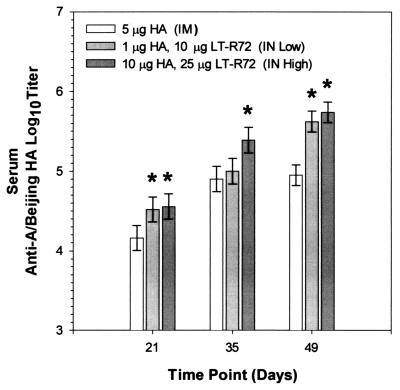
FIG. 1.
Comparison of the effects of i.m. and i.n. administrations of A/Beijing93 HA on antigen-specific serum antibody responses. Shown are mean anti-A/Beijing93 HA serum Ig antibody titers of groups of 10 mice immunized as shown in Table 1. Asterisks indicate groups whose values are significantly greater than those of the corresponding i.m. immunized group (P ≤ 0.05).
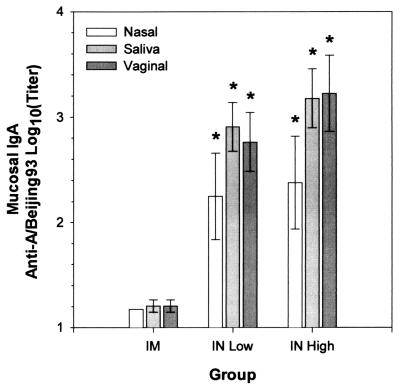
FIG. 3.
Comparison of the effects of i.m. and i.n. administrations of A/Beijing93 HA on antigen-specific IgA responses. Mean anti-A/Beijing93 HA IgA antibody titers of MW samples from groups of 10 mice immunized as shown in Table 1 (± 95% confidence intervals) are shown. Asterisks indicate groups whose titers are significantly greater than those of the corresponding i.m. immunized groups (P ≤ 0.05).
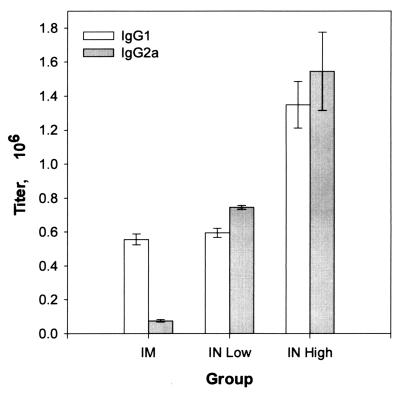
FIG. 4.
Comparison of the effects of i.m. and i.n. administrations of A/Beijing93 HA on the ratio of antigen-specific IgG1 to IgG2a antibodies in the sera of groups of 10 mice immunized as shown in Table 1.
Comparison of i.n. and i.m. immunizations.
Groups of 10 mice were immunized i.n. with LT-R72–HA formulated at two dose levels and compared to mice immunized i.m. with HA alone (Table 1). Serum antibody responses after i.n. immunization with A/Beijing93 HA and LT-R72 (Fig. 1) were significantly higher than responses obtained with i.m. immunization in most cases. Of the groups tested, immunization with 10 μg of HA and 25 μg of LT-R72 i.n. (IN high) resulted in the strongest serum antibody responses, which, after three doses, were approximately a log higher than those achieved by i.m. vaccination (P = 0.0157, 0.0044, and ≤0.0001 at days 21, 35, and 49, respectively). Notably, the low-dose (1 μg of HA with 10 μg of LT-R72) i.n. group had significantly higher responses than did the i.m. immunization group, in most cases (P = 0.0272 and ≤0.0001 at days 21 and 49, respectively).
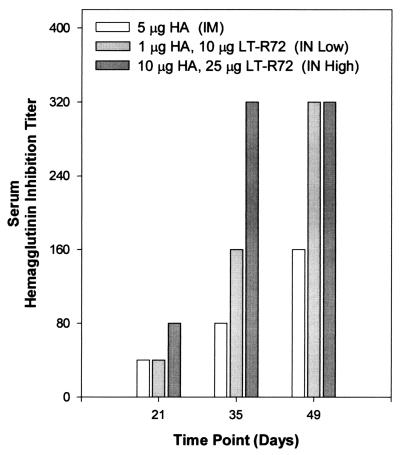
Serum HI titers (Fig. 2) closely paralleled the serum antibody responses; i.n. immunization with HA and LT-R72 resulted in HI titers similar to, and often greater than, those achieved by i.m. immunization. As before, the HI titers of the high-dose i.n. group were the highest tested, exceeding the i.m. group HI titers at all points. Immunization i.n. with 1 μg of HA and 10 μg of LT-R72 resulted in HI titers that were equivalent to those of the i.m. group after a single immunization and exceeded those of the i.m. group after two or three immunizations. However, it was apparent that at least two immunizations were required to induce potent HI titers.
FIG. 2.
Comparison of the effects of i.m. and i.n. administrations of a bivalent vaccine containing A/Beijing93 HA on serum HI titers. The data shown is for pooled serum from groups of 10 mice immunized as shown in Table 1.
Mice immunized i.n. had significantly higher IgA antibody responses than did those immunized i.m. (Fig. 3). VW extracts, from a mucosal site distant from the immunization site, showed significant IgA antibody responses in groups immunized i.n. Immunization i.m. did not result in significant IgA responses in any of the mucosal samples tested.
Immunization i.n. with HA in combination with LT-R72 resulted in significant IgG2a subclass antibody responses (Fig. 4). The ratio of IgG1 to IgG2a was approximately 10:1 after i.m. immunization with three doses of 5 μg of HA. After the third i.n. immunization, the ratio of IgG1 to IgG2a measured approximately 1:1. The absolute levels of IgG2a in serum samples from groups immunized i.n. increased 10- to 20-fold over those of groups immunized i.m., while the IgG1 subclass responses increased less than 3-fold.
We have demonstrated that i.n. immunization with influenza virus HA in combination with LT-R72 induces not only serum antibodies and viral neutralization (as measured by HI titers) comparable to or stronger than those induced by i.m. immunization but also mucosal IgA and serum IgG2a responses. This broad response to i.n. immunization may result in better protection than current inactivated whole- or split-virus vaccines can offer. Mucosal IgA has been shown to increase protection from respiratory viruses because of its location as a front-line defense, at the point of viral entry, as well as better cross-protection against antigenic variants (21, 23). The shift in dominance of antibody subclasses from IgG1 after i.m. immunization to IgG2a after i.n. immunization is notable. IgG2a has been reported to be a marker for an increased Th1 response and illustrates the potential for the induction of cell-mediated immunity with mutant LTs. Induction of cell-mediated immunity may improve vaccine efficacy, particularly if i.n. immunization boosts CTL responses in aged subjects, a population in which CTL responses are known to be weakened (4, 20).
Live, cold-adapted influenza virus vaccines are currently in development for i.n. administration. However, this approach has a number of shortcomings, including difficulty in the manufacture and storage of the viruses, high patient-to-patient variability in responses, and a significant reduction of efficacy in seropositive individuals, particularly the elderly (19, 26). Our group has previously demonstrated that antibody responses to i.n. immunization with HA-mutant LT formulations are stronger in primed or seropositive animals than in naive animals, indicating that this approach may have broad applicability in the general population (2). Preliminary observations indicate that the efficacy of LT mutants is not adversely affected by the presence of pre-existing immunity to the adjuvant (unpublished observations).
Considerable work has been undertaken to develop cholera toxin (CT) subunit B as a mucosal adjuvant, but studies have demonstrated that CT subunit B must contain small amounts of whole CT to potentiate adjuvanticity at low doses (7, 7a, 23–25). However, the toxicity of CT has severely limited this approach for use in humans. LTR-72 has been shown to have less than 1% of the ADP-ribosylation activity of wild-type LT, approximately 100,000-fold less toxicity as measured against Y1 cells in vitro, and approximately 20-fold less toxicity in vivo as measured in a rabbit ileal-loop assay (9). The safety of LTR-72 in humans needs to be resolved in the clinic. However, preclinical studies suggest an adequate margin of safety for use as an effective mucosal adjuvant.
Acknowledgments
We acknowledge Rino Rappuoli and Mariagrazia Pizza for advice and technical support for LT-R72, Mildred Ugozzoli for developing the luminometer-based ELISA used extensively in these studies, and Diana Achley for her skill in animal husbandry.
REFERENCES
- 1.Andrews H P, Snee R D, Sarner M H. Graphical display of means. Am Statistician. 1980;34:195–199. [Google Scholar]
- 2.Barchfeld G L, Hessler A L, Chen M, Pizza M, Rappuoli R, Van Nest G A. The adjuvants MF59 and LT-K63 enhance the mucosal and systemic immunogenicity of subunit influenza vaccine administered intranasally in mice. Vaccine. 1998;17:695–704. doi: 10.1016/s0264-410x(98)00252-7. [DOI] [PubMed] [Google Scholar]
- 3.Bender B S, Johnson M P, Small P A. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514–519. [PMC free article] [PubMed] [Google Scholar]
- 4.Bender B S, Taylor S F, Zander D S, Cottey R. Pulmonary immune response of young and aged mice after influenza challenge. J Lab Clin Med. 1995;126:169–177. [PubMed] [Google Scholar]
- 5.Clements M L, Stephens I. New and improved vaccines against influenza. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd edition. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 545–570. [Google Scholar]
- 6.De Haan A, Geerligs H J, Huchshorn J P, Van Scharrenburg G J M, Palache A M, Wilschut J. Mucosal immunoadjuvant activity of liposomes: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995;13:155–162. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- 7.Dickingson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press, Inc.; 1996. pp. 73–87. [Google Scholar]
- 7a.Elson C O. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press, Inc.; 1996. pp. 59–72. [Google Scholar]
- 8.Ghendon Y. The immune response of humans to live and inactivated influenza vaccines. Adv Exp Med Biol. 1989;257:37–45. doi: 10.1007/978-1-4684-5712-4_6. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of Escherichia coli heat-labile enterotoxin (LT) mutants with partial or total knock-out of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glezen P W. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 11.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. Immunoassay; pp. 553–612. [Google Scholar]
- 12.Hierholzer J C, Suggs M T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969;18:816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hierholzer J C, Suggs M T, Hall E C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969;18:824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskins T W. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet. 1979;i:33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 15.Johannsen R, Moser H, Hinz J, Freisen H J, Gruschkau H. Quantitation of haemagglutinin of influenza Tween-ether split vaccines by immunodiffusion. Vaccine. 1985;3:235–240. doi: 10.1016/0264-410x(85)90114-8. [DOI] [PubMed] [Google Scholar]
- 16.Oh Y, Ohta K, Kuno-Sakai H, Kim R, Kimura M. Local and systemic influenza haemagglutinin-specific antibody responses following aerosol and subcutaneous administration of inactivated split influenza vaccine. Vaccine. 1998;10:506–511. doi: 10.1016/0264-410x(92)90348-n. [DOI] [PubMed] [Google Scholar]
- 17.Patriarca P A, Weber J A, Parker R A, Hall W N, Kendal A P, Bregman M S, Schonberger L B. Efficacy of influenza vaccine in nursing homes: reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985;253:1136–1139. [PubMed] [Google Scholar]
- 18.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 19.Powers D C, Sears S D, Murphy B R, Thumar B, Clements M L. Systemic and local antibody responses in elderly subjects given live or inactivated influenza A virus vaccines. J Clin Microbiol. 1989;27:2666–2671. doi: 10.1128/jcm.27.12.2666-2671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powers D C. Influenza A virus-specific cytotoxic T-lymphocyte activity declines with advancing age. J Am Geriatr Soc. 1993;41:1–5. doi: 10.1111/j.1532-5415.1993.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 21.Renegar K B, Small P A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddiough M A, Sisk J E, Bell J C. Influenza vaccination: cost-effectiveness and public policy. JAMA. 1983;249:3189–3195. [PubMed] [Google Scholar]
- 23.Tamura S, Funato H, Hiroabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 25.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T, Aizawa C, Oya A, Kurata T. Escherichia coli heat-labile enterotoxin B subunit supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:1083–1089. doi: 10.1016/0264-410x(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 26.Treanor J, Dumyati G, O’Brien D, Riley M A, Riley G, Erb S, Betts R. Evaluation of cold-adapted, reassortant influenza B virus vaccines in elderly and chronically ill adults. J Infect Dis. 1994;169:402–407. doi: 10.1093/infdis/169.2.402. [DOI] [PubMed] [Google Scholar]
- 27.Ugozzoli M, O’Hagan D T, Ott G S. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. 1998;93:563–571. doi: 10.1046/j.1365-2567.1998.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldman R H, Wood S H, Torres E J, Small P A., Jr Influenza antibody response following aerosal administration of inactivated virus. Am J Epidemiol. 1970;91:575–584. [PubMed] [Google Scholar]