ABSTRACT
BACKGROUND:
A positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) for SARS CoV-2, from nasopharyngeal swabs, is the current gold standard diagnostic test for this virus and has sensitivity of 60-70%. Some studies have demonstrated a significant number of false-negative RT-PCR tests while displaying significant tomographic findings, in the early days of symptoms of COVID-19.
OBJECTIVE:
To compare accuracy between RT-PCR and computed tomography (CT) for detecting COVID-19 in the first week of its symptoms during the pandemic.
DESIGN AND SETTING:
Systematic review of comparative studies of diagnostic accuracy within the Evidence-based Health Program of a federal university in São Paulo (SP), Brazil.
METHODS:
A systematic search of the relevant literature was conducted in the PubMed, EMBASE, Cochrane Library, CINAHL and LILACS databases, for articles published up to June 6, 2020, relating to studies evaluating the diagnostic accuracy of RT-PCR and chest CT for COVID-19 diagnoses. The QUADAS 2 tool was used for methodological quality evaluation.
RESULTS:
In total, 1204 patients with COVID-19 were evaluated; 1045 had tomographic findings while 755 showed positive RT-PCR for COVID-19. RT-PCR demonstrated 81.4% sensitivity, 100% specificity and 92.3% accuracy. Chest CT demonstrated 95.3% sensitivity, 43.8% specificity and 63.3% accuracy.
CONCLUSION:
The high sensitivity and detection rates shown by CT demonstrate that this technique has a high degree of importance in the early stages of the disease. During an outbreak, the higher prevalence of the condition increases the positive predictive value of CT.
REGISTRATION NUMBER:
DOI: 10.17605/OSF.IO/UNGHA in the Open Science Framework.
KEY WORDS (MeSH terms): COVID-19 [supplementary concept], Coronavirus infections, Real-time polymerase chain reaction, Polymerase chain reaction, Tomography, Radiology
AUTHORS’ KEY WORDS: Ground-glass opacities, CT scan, Accuracy
INTRODUCTION
Since COVID-19 pneumonia emerged in Wuhan, China, there has been a search for knowledge that might prevent or minimize its spread.1,2,3 In just over three months after its initial breakout, it gained worldwide reach such that it affected more than 2.5 million people, with more than 180,000 deaths in more than 200 countries. COVID-19 is caused by the SARS-CoV-2 virus, a member of the Coronaviridae family.3 Its transmission occurs mainly through respiratory droplets.1
The clinical spectrum of the disease is variable, and the majority of cases are asymptomatic or oligosymptomatic. The most severe cases, with acute respiratory distress syndrome, commonly affect elderly patients with comorbidities.3
A positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2, from nasopharyngeal swabs, is the current gold standard diagnostic test. The sensitivity of RT-PCR for SARS-CoV-2 is 50-70%;4,5,6,7,8,9,10 around 30-40% of patients with early-stage COVID-19 are false-negative.4 An inadequate technique for collecting sampling material or low viral load, limited development of nucleic acid detection technology and variation in the detection rate between different manufacturers may all be determinants for false negative results.4,11
Use of computed tomography (CT) is based on the clinical context and time taken to make the diagnosis, especially in relation to use of RT-PCR and other clinical and laboratory investigations.4,12,13 CT findings do not alter the diagnosis of COVID-19 in cases in which RT-PCR is positive, but they are useful for grading pulmonary involvement and its evolution.4,6,8 CT has 56-98% sensitivity,7 and according to Ai et al., 25% specificity and 68% accuracy.14
Ai et al. found that out of 64 patients with an initially negative RT-PCR test, 15 (23.4%) subsequently had a positive RT-PCR (mean time interval of 5.1 ± 1.5 days); ten of these patients (15.6% of those with initial negative RT-PCR) had typical CT findings at the time of the initial negative RT-PCR.14 Fang et al. described a 29.4% rate of abnormal CT in patients with initially negative and subsequently positive RT-PCR.4,11
In the minority of patients with high clinical suspicion in the context of the current pandemic, but with negative initial RT-PCR, the presence of typical CT findings could indicate the possibility of COVID-19 earlier, i.e. before sufficient RT-PCR runs have been done to rule out or confirm the diagnosis.4,10
OBJECTIVES
To determine the accuracy of RT-PCR and CT over the first seven days of symptoms of COVID-19 and which method is more sensitive for early case detection.
METHODS
Study model
The study model followed the guidelines for systematic reviews on diagnostic accuracy studies, i.e. Cochrane Diagnostic Reviewer’s Handbook version 5.1.
Inclusion criteria
The search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We included comparative studies on diagnostic accuracy among patients who underwent both CT and RT-PCR for making the diagnosis of COVID-19 in the initial days of its evolution, regardless of the severity of the disease. We did not put any restrictions on patient age, origin, language or publication status of the study. There was no exclusion regarding population size or patient age. In the case of missing information, the authors of the study in question were contacted by e-mail.
Participants
The participants were men and women of all ages with suspected COVID-19 who underwent chest CT and RT-PCR during their first week of symptoms.
Selection of studies and data extraction
The studies selected were those potentially eligible for inclusion in terms of relevance of the articles or abstracts in indexed journals. Two authors performed independent selections for eligibility. In cases of disagreement, a third author was consulted. Data extraction was performed using a standardized form. The selection process was carried out using the Rayyan platform (https://rayyan.qcri.org).15
Evaluation of methodological quality
The QUADAS 2 tool, which is used to evaluate bias and precision, was used in relation to all the eligible studies.16 All analyses and diagrams were completed using RevMan 5.3 and MetaDisc 1.4. The study was approved by our institutional review board, under approval number: 8483190420; date: May 4, 2020. The review was registered in the Open Science Framework database.
Research methods for selecting studies
A thorough systematic search of the relevant literature was conducted in the PubMed, EMBASE, Cochrane Library, CINAHL and LILACS online scientific publication databases, for original articles published up to June 6, 2020, with no language restrictions. The search used the following Medical Subject Headings (MeSH terms): COVID-19; SARS virus; coronavirus infection; Real-Time Polymerase Chain Reaction; Polymerase Chain Reaction; and Tomography, X-Ray Computed. The reference lists of the studies included and the main reviews on the subject were also evaluated. Manual searches were also carried out in these reference lists. The full search strategy is displayed in Table 1.
Table 1. Search strategy according to the corresponding database.
| Database | Search strategy |
|---|---|
| Cochrane Library |
|
| MEDLINE |
|
| EMBASE (OvidSP) |
|
| LILACS |
|
| CINAHL |
|
RESULTS
Studies selected
The systematic review yielded 168 studies. At the end of the analysis, five studies. 9,11,14,17,18 were deemed to meet the inclusion criteria and presented acceptable quality according to the QUADAS 2 tool. These studies were thus included in the systematic review (Figure 1). Among these, two studies were included in the meta-analysis.9,17
Figure 1. PRISMA 2009 flow diagram.
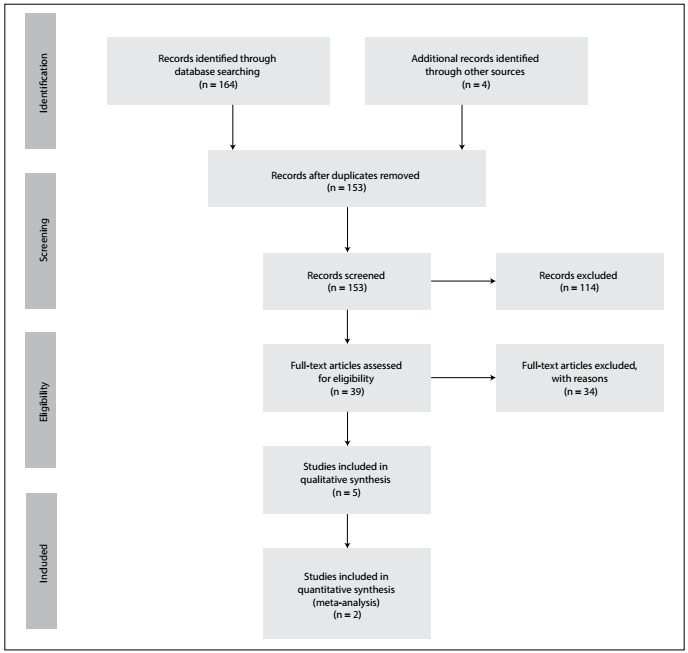
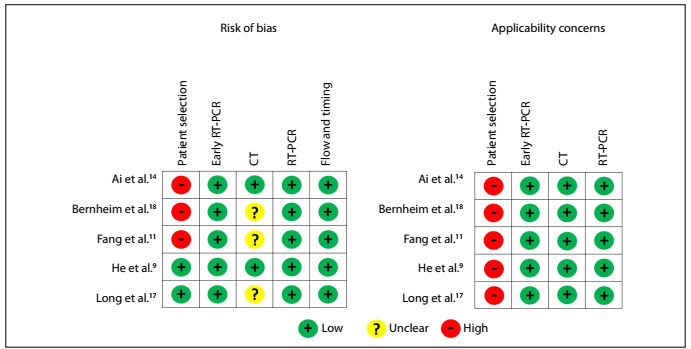
In all the studies, there was high concern about applicability. Moreover, in three of the five studies, a high risk of bias was also perceived. It was not clear in most studies whether the radiologist who reported the CT scan had access to the RT-PCR results (Figure 2).
Figure 2. QUADAS 2 risk of bias and applicability concerns.
Analysis on the studies
Table 2 provides a summary of the findings from the main studies included. In total, 1204 patients with COVID-19 that were evaluated. Among these, 1045 had tomographic findings (detection rate of 86.7%) and 755 showed positive RT-PCR for COVID-19 (detection rate of 62.7%), with a significant difference in detection rate of 24.0%.
Table 2. Summary of study findings.
| Study | Total number of COVID-19 patients included | Positive early RT-PCR | Positive early CT | Comments |
|---|---|---|---|---|
| Ai 202014 | 1014 | 601 | 888 |
|
| Bernheim 202018 | 121 | 61 out of 69 tested in the early and intermediate group | 46 out of 69 tested in the early and intermediate group |
|
| Fang 202011 | 51 | 36 | 50 | Average time between symptoms and CT or RT-PCR: 3 days |
| He 20209 | 34 | 27 | 26 |
|
| Long 202017 | 36 | 30 | 35 |
|
Regarding tomographic pattern changes, Ai et al.14 found that out of their 888 patients with positive CT results, 409 showed ground-glass opacities and 447 had consolidations; 801 had bilateral findings. Also, 42% of the patients showed improvement on CT before the RT-PCR became negative; and 3.5% showed worsened CT with negative RT-PCR. The CT sensitivity was 96.5% and its specificity was 25.4%; the positive predictive value was 65.3% and the negative predictive value was 83.3%. The first RT-PCR performed on the patients presented sensitivity of 59.2%.
Bernheim et al. evaluated patients divided into three groups concerning the onset of symptoms: early (0-2 days), intermediate (3-5 days) and late (6-12 days). They found that 56% (20 patients out of 36) had an absence of ground-glass opacity and consolidation in the first two days, while 9.0% (three patients out of 33) showed this in the intermediate group and 4% (one patient out of 25) in the late group. RT-PCR was positive in 91.6% of the patients in the early group (33 patients out of 36); 84.4% in the intermediate group (28 patients out of 33); and 92.0% in the late group (23 patients out of 25). One patient with absence of ground-glass opacity and consolidation in the early group showed negative RT-PCR findings. RT-PCR presented sensitivity of 88.4% and CT of 66.6% over the first five days of symptoms.
In the study by Fang et al.,11 study, 36 CT-positive cases showed typical changes: sparse, subpleural and peripheral ground-glass opacities, commonly in the lower lobes. The CT sensitivity was 98.0%. The first RT-PCR performed on the patients presented sensitivity of 70.5%.
He et al.9 compared use of CT and RT-PCR among 82 patients with suspected pneumonia, including COVID-19 pneumonia. The two experienced radiologists who evaluated all chest CT scans demonstrated good interobserver agreement. All the patients underwent chest CT and initial RT-PCR on the same day. The 34 COVID-19 patients had confirmation through RT-PCR, but not necessarily from the initial RT-PCR. The initial RT-PCR had 79% sensitivity, 100% specificity and 92% accuracy. The chest CT had 77% sensitivity, 96% specificity and 88% accuracy. He et al. also analyzed the two tests used in conjunction, and concluded that jointly they presented 88% sensitivity, 100% specificity and 98% accuracy.9 In the study by He et al.,9 eight patients with tomographic changes had pneumonia other than COVID-19. It was possible to calculate the positive predictive value of CT, which was 85.1%.
Long et al.17 also compared the tomographic findings of patients with COVID-19 pneumonia and non-COVID-19 pneumonia. The upper lobes of the lungs were more affected on CT in COVID-19 cases (right: 52.7% versus 37.3%; left: 55.6% versus 33.3%); the other lobes did not show any significant difference. There was also a difference in peripheral involvement, which was more common in cases of pneumonia caused by COVID-19. The sensitivity of CT was 97.2%. The first RT-PCR performed on the patients presented sensitivity of 84.6% and the negative predictive value was 89.4%. In the study by Long et al.,17 51 patients with tomographic findings had pneumonia other than COVID-19. This makes it possible to infer that CT presents high specificity. The positive predictive value for CT was calculated as 58.6%.
Accuracy assessment
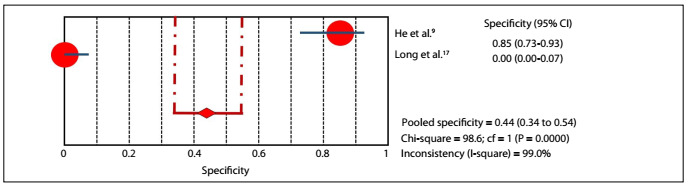
In the accuracy evaluations of the studies by He and Long,9,17 RT-PCR demonstrated 81.4% sensitivity (95% confidential interval: 70.3-89.7%) and 100% specificity (95% confidential interval: 96.3-100%), with P-value lower than 0.05, and 92.3% accuracy (Figures 3and4). In the same studies, CT demonstrated 95.3% sensitivity (95% confidential interval: 86.9-99.0%) and 43.8% specificity (95% confidential interval: 34.1-53.8%), with P-value lower than 0.05, and 63.3% accuracy (Figures 5and6).
Figure 3. Sensitivity graph: RT-PCR.
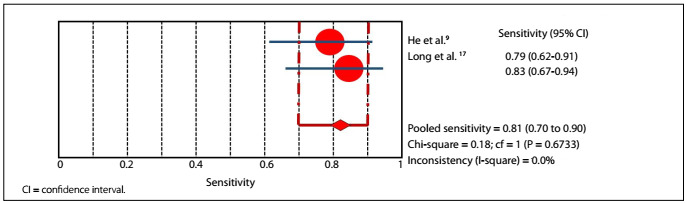
Figure 4. Specificity graph: RT-PCR.
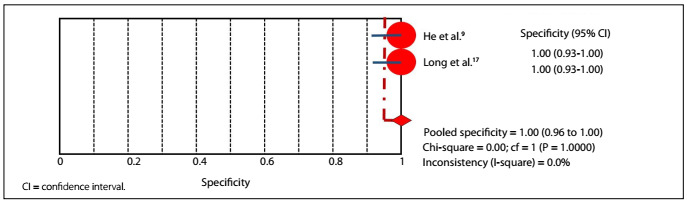
Figure 5. Sensitivity graph: chest CT.
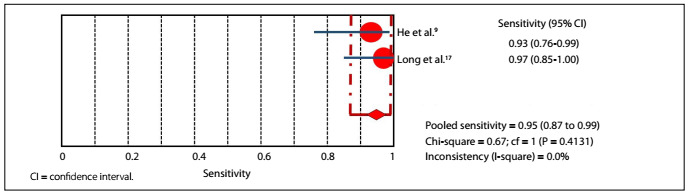
Figure 6. Specificity graph: chest CT.
All the data in these five studies were retrospective and were obtained during the epidemic period in the regions where these studies were conducted.
DISCUSSION
The symptoms of COVID-19 consist mainly of fever, fatigue and dry cough, with gradual dyspnea in some cases, and acute respiratory distress syndrome and multiple organ dysfunction in severe cases requiring intensive treatment.1,2,19,20 While the majority of patients, about 80%, have mild symptoms; older patients, especially those above 70 years old and those with underlying conditions, such as cardiovascular disease, diabetes, chronic respiratory diseases and oncological diseases, have a higher mortality rate of up to 15%.3
In addition to the most common pattern of peripheral and bilateral ground-glass injuries, other patterns of lung injury may be observed.6,7,20,21,22,23,24,25 Pulmonary consolidations are present in 2-64% of the cases and form an indicator of disease progression, thus serving as a warning sign for the severity of the patient’s condition. Reticular pattern lesions have lower incidence than consolidations and opacities.6,26
The crazy-paving pattern is present in about 5-36% of the cases, while bronchial wall thickening is present in 10-20%.6,27 Pleural changes are present in 32%, with pleural thickening; however, pleural effusion occurs in only 5% of the cases.6,24 Pulmonary fibrosis occurs in 17% of the cases and pulmonary nodules smaller than three centimeters in size, in 3-13%.6 The incidence of lymph node enlargement is about 4-8% and pericardial effusion occurs in approximately 5%. The latter is an indicator of severity.6,19 Vascular thickening is characterized in 59% of the cases.7 The radiological findings tend to become worse seven days after the onset of symptoms and show improvement 14 days after the onset of symptoms.3
In the current pandemic situation, despite the low specificity of CT (25%), this technique can be used to isolate patients and institute treatment at an early stage, since it presents sensitivity of about 88.9%, starting from the early day of symptoms.4,10,20,28 In comparison with this, chest X-ray shows abnormalities in 59.1% of the cases and in 76.7% among serious cases.4,23
Xie et al.29 reported on a case series in which they performed RT-PCR and CT on the same day, regardless of the duration of the patients’ symptoms. They found that out of their 167 patients, 162 were positive according to RT-PCR and 160 were positive according to CT. Seven patients were positive on RT-PCR and negative on CT; and five patients were positive on CT and negative on RT-PCR. CT presented 95.0% of sensitivity, while RT-PCR presented 97.0%. Concerning false-negative data, CT showed 4.0%.
Barbosa et al. evaluated 91 patients with suspected pneumonia until 30 days after their initial symptoms and performed RT-PCR and chest CT on the same day. Sixty-three of their patients had symptoms for seven days or less and, among these patients, two were positive on RT-PCR and negative on CT, with a CT false-negative rate of 3.1%. For 28 patients, both tests were negative; and for 15, both tests were positive.10
It should, however, be noted that a normal CT scan cannot be used to rule out a diagnosis of COVID-19,21,26,30 although there is some evidence to suggest that the negative predictive value of CT is greater for symptoms lasting longer than one week.4 It also needs to be taken into account that CT may be normal in cases with positive RT-PCR in 2-20% cases, according to studies by Yang et al., Guan et al. and Chung et al.4,19,23,31
Moreover, 54-70.8% of asymptomatic people who have had contact with symptomatic patients and who are COVID-19-positive according to RT-PCR may present a change on CT.4,5,32 Long et al. analyzed 37 asymptomatic individuals, who had come into contact with RT-PCR-confirmed patients, and reported that 21 (56.7%) of these individuals had positive CT findings.33 Inui et al.5 detected pulmonary opacity on CT in 24 out of 30 symptomatic patients (80%); however, in 82 asymptomatic patients, 44 (54%) had opacities on CT.5 Shi et al. also reported occurrences of CT abnormalities in asymptomatic patients.24 Furthermore, in symptomatic cases, they found greater extent of the lesion, along with areas of consolidation predominating over ground-glass opacities.5,24
Bai et al. assessed the performance of radiologists in differentiating CT results between those from patients with COVID-19 pneumonia and those from patients with non-COVID-19 pneumonia.7 These radiologists achieved accuracy ranging from 72 to 97%, with sensitivity of 70-94% and greatly varying specificity (24-94%).7
Therefore, the role of CT in confirmed cases of COVID-19 after the results from RT-PCR have been obtained is the same as in relation to any other viral infection, in that it can be used to do the following:4
Add diagnostic value for patients with pre-existing lung diseases.
Help diagnose complications or investigate a clinically discordant condition: positive to negative turnover RT-PCR, but increased hypoxia.
Find coexisting or underlying diagnoses.
Although CT is very sensitive at the onset of symptoms, in comparison with RT-PCR, it still may not reveal the characteristic pattern of COVID-19 in all cases. Hence, it remains difficult to differentiate COVID-19 from other viral causes of pneumonia.7 According to Bai et al., although making the diagnosis of pneumonia due to COVID-19 is possible via CT, subtle or atypical presentations can lead to a wrong diagnosis.7
Our findings showed that CT outperformed RT-PCR in making an early diagnosis of COVID-19 in suspected cases. Both from previous findings and ours, we suggest that an early evaluation protocol should include applying CT when RT-PCR is negative. This could guide clinicians’ treatment and patient isolation criteria, in order to avoid virus dissemination. Our meta-analysis showed that CT had specificity of 43.8% and sensitivity of 95.3%, and both of these values are higher than those in the recent literature.
All the studies evaluated were conducted among in patients with COVID-19 that confirmed within the first seven days of symptoms by means of RT-PCR. However, this test was not necessarily the first to be performed on suspected patients, within the epidemic period in the country in which these tests were performed. Therefore, the positive predictive value and detection rate of CT findings in patients with COVID-19 will be higher than it would be outside the epidemic period.
Although RT-PCR is the gold standard for diagnosing COVID-19, it presents a significant percentage of false-negative tests in the early days of symptoms of the disease (0-7 days). On the other hand, even though CT is a test with presumably low specificity,26 thereby allowing several differential diagnoses,8 it detects patterns compatible with COVID-19. It has presented very high sensitivity and significant positive predictive value and detection rate in the epidemic period.8,26
CONCLUSION
The high sensitivity and detection rate of CT demonstrate that it has a high degree of importance in the early stages of the disease, even greater than RT-PCR. During an outbreak, the higher prevalence of the condition raises the positive predictive value of CT. However, the low specificity of CT (43.8%) also needs to be considered. Outside of pandemic times, its positive predictive value for this condition should decrease proportionally with the decline in the prevalence of the disease in the population.
Acknowledgments:
Our group would like to thank the medical teams of the doctors Barbosa, He and Long for sending extra data by e-mail to improve our systematic review, especially with regard to data for performing the meta-analysis
Evidence-Based Health Department, Universidade Federal de São Paulo (UNIFESP), São Paulo (SP), Brazil
Sources of funding: No funding was received for this study
REFERENCES
- 1.Wei J, Xu H, Xiong J, et al. 2019 Novel Coronavirus (COVID-19) Pneumonia: Serial Computed Tomography Findings. Korean J Radiol. 2020;21(4):501–504. doi: 10.3348/kjr.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon SH, Lee KH, Kim JY, et al. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J Radiol. 2020;21(4):494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burhan E, Prasenohadi P, Rogayah R, et al. Clinical Progression of COVID-19 Patient with Extended Incubation Period, Delayed RT-PCR Time-to-positivity, and Potential Role of Chest CT-scan. Acta Med Indones. 2020;52(1):80–83. [PubMed] [Google Scholar]
- 4.Nair A, Rodrigues JCL, Hare S, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75(5):329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inui S, Fujikawa A, Jitsu M, et al. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19) Radiology: Cardiothoracic Imaging. 2020;2(2):e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020:1–9. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823.200823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020:1–9. doi: 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He JL, Luo L, Luo ZD, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168 doi: 10.1016/j.rmed.2020.105980.105980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa PNVP, Bitencourt AGV, Miranda GD, Almeida MFA, Chojniak R. Chest CT accuracy in the diagnosis of SARS-CoV-2 infection: initial experience in a cancer center. Radiol Bras. 2020:1–5. doi: 10.1590/0100-3984.2020.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Zhang H, Xie J, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432.200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Tawfiq JA, Memish ZA. Diagnosis of SARS-CoV-2 infection based on CT scan vs RT-PCR: Reflecting on Experience from MERS-CoV. J Hosp Infect. 2020;105(2):154–155. doi: 10.1016/j.jhin.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Hou H, Wang W, Wang W. Combination of CT and RT-PCR in the screening or diagnosis of COVID-19. J Glob Health. 2020;10(1):010347–010347. doi: 10.7189/jogh.10.010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 doi: 10.1148/radiol.2020200642.200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210–210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–526. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108961.108961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200463.200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ES, Chin BS, Kang CK, et al. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35(13):e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT During Recovery From Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hani C, Trieu NH, Saab I, et al. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araujo-Filho JAB, Sawamura MVY, Costa AN, Cerri GG, Nomura CH. COVID-19 pneumonia: what is the role of imaging in diagnosis? J Bras Pneumol. 2020;46(2) doi: 10.36416/1806-3756/e20200114.e20200114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenda E, Yulianti M, Asaf MM, et al. The Importance of Chest CT Scan in COVID-19. Acta Med Indones. 2020;52(1):68–73. [PubMed] [Google Scholar]
- 29.Xie X, Zhong Z, Zhao W, et al. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020 doi: 10.1148/radiol.2020200343.200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Yan F. Patients with RT-PCR Confirmed COVID-19 and Normal Chest CT. Radiology. 2020;295(2):E3. doi: 10.1148/radiol.2020200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]


