Abstract
Hemodialysis patients often develop constipation. We analyzed the advantage of synbiotics over prebiotics based on the stool form due to the intestinal environment in hemodialysis patients. Patients received either synbiotics or prebiotics for four weeks. The synbiotics group was treated with partially hydrolyzed guar gum containing Bifidobacterium longum BB536, while the prebiotics group was treated with the same fiber alone. The defecation status was assessed using the Bristol Stool Form Scale and abdominal bloating was evaluated using a visual analog scale. The fecal microbiota, measured by a terminal restriction fragment length polymorphism analysis, and the fecal short-chain fatty acid concentrations, measured by gas chromatography, were compared between the two groups. Synbiotics ingestion improved the individual stool form and abdominal bloating, and increased Bifidobacterium, which produces short-chain fatty acid, 13.9-fold after ingestion. In particular, acetic acid increased 2.5-fold in the synbiotics group. On the other hand, butyric acid increased 3.0-fold in the prebiotics group. Synbiotics improved the stool form in hemodialysis patients due to the composition of the intestinal microbiota and short-chain fatty acid concentrations.
Keywords: Synbiotics, Prebiotics, Hemodialysis, Bifidobacterium longum BB536, Short-chain fatty acid
INTRODUCTION
Hemodialysis (HD) patients are often constipated due to dietary restrictions and the side effects of their medications. Severe constipation induces anorexia, and the subsequent decreased dietary intake then causes malnutrition. Healthy defecation habits are thus an important factor that influences the quality of life of HD patients.
A dysbiotic microbiota is a factor associated with susceptibility to kidney disease (and vice versa), with the progressive loss of the renal function worsening the intestinal dysbiosis in patients with chronic kidney disease (CKD)/end-stage renal disease (ESRD) (15). In HD patients, the proportions of the microbiota drastically differ from those in healthy individuals. While the percentage of culturable aerobes in healthy individuals ranges from 0.1% to 0.2%, that in HD patients ranges from 11.2% to 15.6% (3, 10). This increase in the proportion of aerobes causes opportunistic infections due to the presence of uremic toxins, such as phenol, indican, and ammonia (2, 3).
Probiotics are bacteria that are thought to provide benefits for the host by improving the intestinal microbial balance, and non-digestible food constituents that are beneficial to the host are referred to as prebiotics. Probiotics and prebiotics are collectively referred to as synbiotics. Useful information on probiotics treatment for chronic constipation has been provided in recent reviews (14, 23). However, only a few studies—which have reported promising results—have evaluated the effects of probiotics or synbiotics on constipation in HD patients. For example, the ingestion of probiotics, such as Bifidobacterium longum (B. longum), or prebiotics such as oligofructose-enriched inulin and partially hydrolyzed guar gum (PHGG), which is a soluble dietary fiber, have been reported to increase the amount of Bifidobacterium in feces, to decrease uremic toxins in blood and feces, along with the serum BUN level, and to relieve constipation in CKD/HD patients (2, 7, 9, 10, 11, 18, 20). Indeed, the ingestion of PHGG for six weeks improved the presence of constipation and the stool form in HD patients (8). We also reported that PHGG for four weeks improved constipation and increased Bifidobacterium in feces in HD patients (11). Synbiotics, including Lactobacillus and Bifidobacterium species, such as B. bifidum and B. breve strain Yakult, also increase the fecal concentrations of Bifidobacterium, decrease serum p-cresol levels, and tend to improve the stool form in HD patients (1, 13).
B. longum is a strain originally discovered in humans that is commonly used as a probiotic. It is likely the most commonly used probiotic, exerting beneficial effects on the severity of constipation in elderly populations (8). B. longum BB536 increases the proportion of butyrate-producing Eubacterium rectale and has been reported to have various physiological effects, including anti-allergy effects, reductions in harmful bacteria, and improvements in the intestinal environment, defecation frequency, and stool characteristics (16).
Short-chain fatty acids (SCFAs), the end product of anaerobic bacterial fermentation of carbohydrates in the colon, play important roles in the biology of colonocytes and exert effects on various cell types. SCFAs regulate tissue-specific homeostasis, including gastrointestinal motility, colitis, metabolic syndrome, airway disease, and even carcinogenesis (16). In addition, many studies have reported that SCFAs improved the renal function in experimental animal models, including models of acute kidney injury and CKD (6).
We therefore hypothesized that a change in the intestinal environment induced by synbiotics with B. longum BB536 would improve the stool form and relieve constipation in HD patients. However, the definition of constipation still lacks objectivity. We therefore analyzed the effects of synbiotics with B. longum BB536 on the stool form and the severity of constipation in a broad sense and assessed the composition of the fecal microbiota in HD patients based on high-throughput terminal restriction fragment length polymorphism (T-RFLP) analyses and the fecal SCFA concentrations.
MATERIALS AND METHODS
Participants
This study included 11 patients receiving maintenance dialysis (males, mean age: 72.9 ± 2.6 years old) on an outpatient basis. The patients did not have fever or other signs of infection. The mean duration of HD was 32.4 ± 9.2 months. Their primary diseases were diabetic nephropathy (4 patients), chronic glomerulonephritis (3 patient), nephrosclerosis (1 patient), and unknown (3 patients). This study was approved by the Ethics Committee of Kobe University School of Health Sciences (permission No: 596). All participants were included in this study after providing their informed consent.
Study design
The study was a two-arm, non-randomized controlled trial. Since the intestinal microbiota is affected by age, we assigned the participants to two groups so that they would be of approximately the same age. As synbiotics, we used G fine® (Aido Co, Mie, Japan), containing 5×109 colony-forming units of B. longum BB536 and 5 g PHGG. Of note, 5.6 g of G fine® contains 12 kcal, 0.6 g carbohydrate, 0 g protein, 0 g lipid, 5 g fiber, and 40 mg sodium. The prebiotic used in the control group was Sunfiber® (Taiyo Kagaku Co, Tokyo, Japan). A 6 g/packet contained 5.1 g PHGG. Of note, 6 g of Sunfiber® contains 12.2 kcal, 5.3–5.9 g carbohydrate, 0–0.06 g protein, 0 g lipid, 5.1 g fiber, and 0–0.04 mg sodium. The participants ingested two packets of synbiotics or prebiotics daily for four weeks. During the examination period, the participants’ concomitant medications and diet contents were not changed; however, Lactobacillus preparations and foods containing Lactobacillus were discontinued. Blood samples for the biochemical analysis were collected every two weeks from two weeks before ingestion until the end of ingestion and then again at three weeks after the end of ingestion. Fecal samples and a questionnaire on the defecation status were collected before and after ingestion.
Biochemical analyses
The laboratory data collection was outsourced to a clinical laboratory testing facility (Fukuyama Medical Laboratory, Hiroshima, Japan).
Defecation status
The stool form was evaluated using the Bristol Stool Form Scale, which classifies samples into seven types based on the shape and hardness of the stool: Type 1, separate hard lumps, like nuts (hard to pass); Type 2, sausage-shaped but lumpy; Type 3, like a sausage but with cracks on its surface; Type 4, like a sausage or snake, smooth and soft; Type 5, soft blobs with clear-cut edges (passed easily); Type 6, fluffy pieces with ragged edges, a mushy stool; Type 7, watery, no solid pieces, entirely liquid.
Abdominal bloating with ingestion of synbiotics or prebiotics was evaluated using a visual analog scale (VAS).
Fecal bacteriological examinations and determination of SCFA concentrations
Fecal samples were collected in a test tube and maintained until use at −30°C. Analyses of the intestinal microbiota and SCFA concentrations were outsourced to TechnoSuruga Laboratory Co., LTD. (Shizuoka, Japan). The intestinal microbiota were analyzed by a T-RFLP analysis (12) and compared by a hierarchical cluster analysis of the ratio of the peak area to the total area of each operational taxonomic unit (OTU), after which a dendrogram was constructed.
The concentrations of SCFAs, namely acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and caproic acid, were analyzed by gas chromatography with a flame ionization detector. These results were expressed as micromoles per gram of feces.
Statistical analyses
Statistical analyses were conducted using the Statcel2 software program (OMS Publishing Inc., Saitama, Japan). Data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using a repeated measure analysis of variance, Pearson’s product-moment correlation, Student’s t-test, or Mann- Whitney’s U test. P values of < 0.05 were considered to indicate statistical significance.
RESULTS
Participant characteristics
The average age of the patients in the synbiotics and prebiotics groups was 78.6 ± 1.6 years and 68.2 ± 3.6 years, respectively (p = 0.037). The HD period was 24.6 ± 5.6 months in the synbiotics group and 38.8 ± 16.5 months in the prebiotics group (p = 0.447). There were no significant differences in blood data, defection status, fecal microbiota, or SCFA concentrations.
Blood biochemical tests
The blood biochemistry before and after ingestion did not differ to a statistically significant extent in either group (Table I). The renal function values, including the creatinine, BUN, and electrolyte levels, also remained unchanged.
Table I.
Effects of the ingestion of synbiotics or prebiotics on laboratory test parameters in hemodialysis patients.
| Prebiotics (n = 6) | Synbiotics (n = 5) | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| 2 weeks before ingestion | 2 weeks after ingestion | 4 weeks after ingestion | 3 weeks after the end of ingestion | 2 weeks before ingestion | 2 weeks after ingestion | 4 weeks after ingestion | 3 weeks after the end of ingestion | 4 weeks after ingestion | 3 weeks after the end of ingestion | |
| Glucose (mg/dL) | 159.3 ± 40.8 | 144.5 ± 26.4 | 142.5 ± 12.8 | 91.6 ± 22.2 | 93.4 ± 22.4 | 100.2 ± 14.9 | 0.06 | |||
| Total Protein (g/dL) | 6.8 ± 0.3 | 6.8 ± 0.4 | 6.4 ± 0.2 | 6.5 ± 0.2 | 0.67 | |||||
| Albumin (g/dL) | 3.8 ± 0.2 | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.2 | 3.5 ± 0.1 | 3.5 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.2 | 0.90 | 0.35 |
| Total Bilirubin (mg/dL) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.98 | |||||
| ALP(U/L) | 358.2 ± 101.8 | 349.5 ± 84.9 | 356.2 ± 85.9 | 223.6 ± 42.3 | 224.4 ± 44.7 | 226.8 ± 51.5 | 0.25 | |||
| AST (U/L) | 12.3 ± 1.3 | 12.0 ± 1.8 | 9.7 ± 1.5 | 10.8 ± 2.9 | 13.6 ± 2.7 | 13.6 ± 3.1 | 0.25 | |||
| ALT (U/L) | 15.3 ± 3.1 | 12.3 ± 2.5 | 11.0 ± 1.0 | 9.2 ± 1.3 | 10.4 ± 1.5 | 8.8 ± 1.2 | 0.20 | |||
| γ-GT (U/L) | 42.0 ± 19.5 | 49.5 ± 26.1 | 48.0 ± 28.2 | 31.8 ± 16.0 | 33.4 ± 17.6 | 30.2 ± 14.9 | 0.61 | |||
| Creatine kinase (U/L) | 71.8 ± 7.6 | 73.5 ± 8.7 | 68.2 ± 7.7 | 81.2 ± 12.5 | 89.0 ± 16.0 | 150.6 ± 64.9 | 0.28 | |||
| BUN (mg/dL) | 51.9 ± 3.2 | 57.8 ± 4.0 | 64.3 ± 6.1 | 59.7 ± 5.7 | 54.8 ± 6.4 | 58.0 ± 7.4 | 56.8 ± 5.7 | 56.9 ± 7.4 | 0.40 | 0.77 |
| Creatinine (mg/dL) | 8.9 ± 1.0 | 9.2 ± 1.2 | 9.4 ± 1.2 | 9.3 ± 1.3 | 9.1 ± 0.5 | 9.3 ± 0.6 | 9.7 ± 0.8 | 10.4 ± 0.7 | 0.80 | 0.53 |
| Uric acid (mg/dL) | 5.9 ± 0.7 | 5.7 ± 0.7 | 6.2 ± 0.4 | 6.6 ± 0.5 | 0.36 | |||||
| Na (mEq/L) | 139.5 ± 1.5 | 139.8 ± 1.9 | 139.2 ± 1.7 | 139.0 ± 2.2 | 140.0 ± 2.9 | 139.6 ± 2.6 | 139.4 ± 2.5 | 138.6 ± 1.2 | 0.94 | 0.88 |
| K (mEq/L) | 4.7 ± 0.5 | 4.3 ± 0.3 | 4.7 ± 0.3 | 4.7 ± 0.3 | 4.4 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.4 | 0.65 | 0.49 |
| Cl (mEq/L) | 103.5 ± 1.8 | 102.2 ± 2.3 | 101.8 ± 2.5 | 102.0 ± 2.3 | 105.2 ± 3.0 | 103.8 ± 3.2 | 104.4 ± 2.9 | 104.0 ± 1.6 | 0.52 | 0.51 |
| Ca (mEq/L) | 8.9 ± 0.4 | 8.3 ± 0.3 | 8.3 ± 0.4 | 8.4 ± 0.3 | 8.5 ± 0.4 | 8.6 ± 0.2 | 8.7 ± 0.3 | 8.5 ± 0.2 | 0.45 | 0.78 |
| P (mEq/L) | 4.9 ± 0.5 | 5.9 ± 0.9 | 5.8 ± 0.6 | 5.4 ± 0.6 | 4.9 ± 0.4 | 4.8 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.2 | 0.39 | 0.93 |
Mean ± SEM, p-value; Prebiotics vs. Synbiotics by t-test
ALP, Alkaline phosphatase; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; γ-GT, γ-glutamyl transferase; BUN, blood urea nitrogen
Defecation status
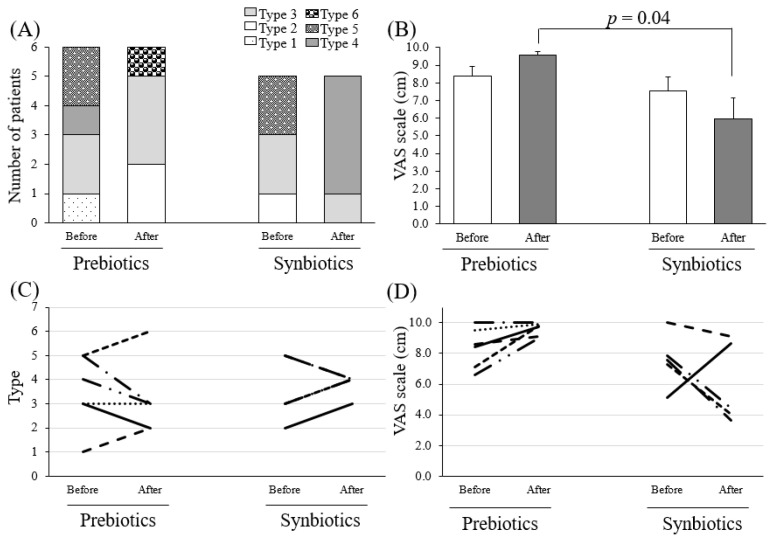
Based on the Bristol Stool Form Scale, types 3, 4, and 5 are thought to be ideal in Japan (5). Synbiotics improved the stool form to type 3, 4, and 5 in most subjects, indicating an improvement in constipation (N.S; not significant). However, some subjects in the prebiotics group showed extremely disordered properties, such as types 1, 2, 6, or 7 (N.S., Figure 1A, C).
Figure 1.
The defecation status before and after the ingestion of synbiotics or prebiotics in hemodialysis patients.
(A, C) The Bristol Stool Form Scale. Type 1, separate hard lumps, like nuts (hard to pass); Type 2, sausage-shaped but lumpy; Type 3, like a sausage but with cracks on its surface; Type 4, like a sausage or snake, smooth and soft; Type 5, soft blobs with clear-cut edges (passed easily); Type 6, fluffy pieces with ragged edges, a mushy stool; Type 7, watery, no solid pieces, entirely liquid. (B, D) Abdominal bloating by a visual analog scale (VAS) scale.
Abdominal bloating was evaluated using a VAS (Figure 1B, D). The score in the synbiotics group decreased from 7.5 ± 0.8 cm to 6.0 ± 1.2 cm after ingestion (N.S.), while that in the prebiotics group increased from 8.4 ± 0.5 cm to 9.6 ± 0.2 cm (N.S.). Since the VAS did not show large individual differences, the prebiotics and synbiotics groups were compared after ingestion. After ingestion, the degree of abdominal bloating in the synbiotics group was significantly less than that in the prebiotics group (p = 0.040).
The fecal microbiota
A previous study that described the baseline microbiota in healthy individuals, based on a T-RFLP analysis, reported that Bifidobacterium and Enterobacteriales comprised 8.0% and 10.1% of the baseline microbiota, respectively (4). On the other hand, in our HD patients, the same analysis revealed that Bifidobacterium and Enterobacteriales comprised 5.2% and 10.4% of the baseline microbiota (before ingestion), respectively. We therefore consider that the amounts of Bifidobacterium and Enterobacteriales are comparable between healthy individuals and HD patients. However, we were unable to address the amount of SCFA-producing Clostridium clusters IV and IX before ingestion in healthy individuals. The composition of the intestinal microbiota changed over the course of the study (Table II). Since the intestinal microbiota differs in each person, we calculated the “after ingestion to before ingestion” ratio. In the synbiotics group, Bifidobacterium and Bacteroides significantly increased 13.9-fold and 19.8-fold after ingestion, respectively. Furthermore, SCFA-producing Clostridium clusters IV and IX also increased after the ingestion of synbiotics. On the other hand, we did not detect any change in the microbiota of the prebiotics group.
Table II.
The after/before ratios of the fecal microbiota determined using T-RFLP in hemodialysis patients who received synbiotics or prebiotics.
| Prebiotics (n = 6) | Synbiotics (n = 5) | p-value | |
|---|---|---|---|
| Bifidobacterium | 3.0 ± 1.2 | 13.9 ± 3.8 | 0.01 |
| Bacteroides | 1.8 ± 0.7 | 19.8 ± 10.1 | 0.15 |
| Clostridium cluster IV | 0.7 ± 0.3 | 2.9 ± 2.0 | 0.36 |
| Clostridium subcluster XIVa | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.96 |
| Clostridium cluster IX | 1.5 ± 0.9 | 2.6 ± 2.6 | 0.71 |
| Clostridium cluster XVIII | 0.9 ± 0.5 | 1.0 ± 0.6 | 0.88 |
| Lactobacillales | 1.9 ± 0.8 | 0.6 ± 0.1 | 0.16 |
| Enterobacteriales | 0.8 ± 0.2 | 1.5 ± 0.3 | 0.13 |
| Others | 1.2 ± 0.1 | 0.7 ± 0.1 | 0.001 |
Mean ± SEM (after/before ratio), p-value; Prebiotics vs. Synbiotics by t-test
Bacterial family abundance was calculated as the percentage of the peak area to the total area of each operational taxonomic units (OUT).
Fecal SCFA concentrations
The total SCFA concentrations in the synbiotics and prebiotics groups increased 1.8-fold and 2.1-fold, respectively (Table III) The major SCFAs of acetic acid, propionic acid, and butyric acid, increased more than 1.5-fold. Caproic acid was not detected.
Table III.
The after/before ratios of the fecal short-chain fatty acid concentrations in hemodialysis patients who received synbiotics or prebiotics.
| Prebiotics (n = 6) | Synbiotics (n = 5) | p-value | |
|---|---|---|---|
| Total short-chain fatty acids | 2.1 ± 0.4 | 1.8 ± 0.1 | 0.60 |
| Acetic acid | 1.9 ± 0.3 | 2.5 ± 0.4 | 0.20 |
| Propionic acid | 2.3 ± 0.7 | 1.6 ± 0.1 | 0.35 |
| Butyric acid | 3.0 ± 0.7 | 1.6 ± 0.3 | 0.10 |
| Isobutyric acid | 3.0 ± 1.9 | 1.2 ± 0.3 | 0.37 |
| Valeric acid | 2.8 ± 1.1 | 0.8 ± 0.2 | 0.15 |
| Isovaleric acid | 3.9 ± 2.8 | 1.2 ± 0.5 | 0.40 |
Mean ± SEM (after/before ratio), p-value; Prebiotics vs. Synbiotics by t-test
We next evaluated the correlation coefficient between the fecal microbiota and SCFAs. After ingestion, total SCFAs, acetic acid, and propionic acid, showed positive correlations with Bifidobacterium (r = 0.83 [p = 0.002], r = 0.91 [p = 0.0001], and r = 0.80 [p = 0.003], respectively). Butyric acid was positively correlated with Lactobacillales (r = 0.70 [p = 0.02]).
DISCUSSION
The primary aim of the present study was to evaluate the effects of synbiotics with B. longum BB536 on the stool form in HD patients. The ingestion of synbiotics or prebiotics for four weeks considerably improved the stool form and altered the composition of the intestinal microbiota in HD patients. The total amount of fecal SCFAs produced by the intestinal microbiota also increased. Only a few studies have used the T-RFLP method for the analysis of HD patients, despite this approach being a high-throughput method that is superior to culture-based methods. To our knowledge, this is the first report to describe the simultaneous evaluation of the stool form, the intestinal microbiota (as determined by a T-RFLP analysis), and the fecal SCFA concentration in HD patients after the administration of synbiotics.
The oral administration of microbiota as probiotics shows extremely short persistence. This ecological framework is dependent on (1) the characteristics of the colonist, (2) microbiome-related mechanisms, and (3) host-related mechanisms (21). From this perspective, B. longum AH1206 and SBT2928, as well as Lactobacillus showed high levels of persistence as probiotics. However, it remains unclear whether or not synbiotics with B. longum BB536 can reconfigure gut communities that are perturbed in HD patients.
Bifidobacterium; Clostridium clusters IV and IX; and the Bacteroides fragilis (B. fragilis) group produce SCFAs, and dietary fiber stimulates their growth (20). In the present study, the ingestion of synbiotics also increased the concentrations of these species in the microbiota and the fecal concentrations of total SCFAs, and showed a positive correlation between Bifidobacterium and SCFAs. The ingestion of synbiotics or prebiotics also increased the concentrations of these species in the microbiota, and the effect of synbiotics was more apparent than that of prebiotics. This might be due to the synergistic effect of probiotics and prebiotics. The ingestion of synbiotics with B. longum BB536 or prebiotics for four weeks increased the fecal concentrations of total SCFAs, particularly acetic acid. Acetic acid is the main metabolite of Bifidobacterium and B. fragilis. Tsuji et al. reported that the oral administration of Bifidobacterium induces Bifidobacterium-dominant intestinal microbiota within 7 to 10 days (19). Bifidobacterium produces lactic acid and acetic acid. Intestinal acidification suppresses the growth of aerobes and improves the intestinal microbiota. Acetic acid is positively correlated with moisture (22). A reduction of aerobes reduces uremic toxins, such as phenol, indican, and ammonia (2, 3). Our results showed that synbiotics with B. longum BB536 increased the acetic acid concentrations and components of the microbiota, such as Bifidobacterium and Bacteroides. We considered that the improvement of the intestinal microbiota by synbiotics may reduce the uremic toxins and subsequent abdominal bloating. Taken together, these present and previous findings suggest that an increase in SCFAs, followed by an increase in SCFA-producing microbiota, can improve the stool form and constipation in HD patients.
Our study is associated with one major limitation. Synbiotics improved both the stool form and the intestinal environment, such as the composition of the microbiota and SCFA concentration; however, the mechanism underlying how the microbiota change the stool form is still unknown. Further studies will be necessary to clarify this point.
We concluded that synbiotics with B. longum BB536 improved the stool form by increasing the proportions of SCFA-producing Bifidobacterium, Bacteroides, and Clostridium (IV, IX), which subsequently increased the SCFA levels in HD patients. The improvement of the stool form by synbiotics will help to relieve constipation, thus improving the quality of life in HD patients.
ACKNOWLEDGEMENTS
This work was partially supported by JSPS KAKENHI (Grant-in-aid for Scientific Research), Grant Number 25450450 to MM.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest in association with the present study.
REFERENCES
- 1.Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, García-García G, Parra-Rojas I, Castro-Alarcón N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24:330–335. doi: 10.1053/j.jrn.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Di Cerbo A, Pezzuto F, Palmieri L, Rottigni V, Iannitti T, Palmieri B. Clinical and experimental use of probiotic formulations for management of end-stage renal disease: an update. Int Urol Nephrol. 2013;45:1569–1576. doi: 10.1007/s11255-012-0335-5. [DOI] [PubMed] [Google Scholar]
- 3.Fukuuchi F, Hida M, Aiba Y, Koga Y, Endoh M, Kurokawa K, Sakai H. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin Exp Nephrol. 2002;6:99–104. [Google Scholar]
- 4.Iwatsuki S, Kijima Y, Shionoya H. Effect of natural milk antibodies on intestinal flora. Nippon Shokuhin Kagaku Kogaku Kaishi. 2011;58:236–244. (in Japanese) [Google Scholar]
- 5.Kasugai K, Iwai H, Kuboyama N, Yoshikawa A, Fukudo S. Efficacy and safety of a crystalline lactulose preparation (SK-1202) in Japanese patients with chronic constipation: a randomized, double-blind, placebo-controlled, dose-finding study. J Gastroenterol. 2019;54:530–540. doi: 10.1007/s00535-018-01545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Ma L, Fu P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Devel Ther. 2017;11:3531–3542. doi: 10.2147/DDDT.S150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H, Uemura T, Nasu M, Iwata N, Yoshimura J, Sakai S. Partially hydrolyzed guar gum intake ameliorates constipation, improves nutritional status and reduces indoxylsulfuric acid in dialysis patients. Kidney Res Clin Practice. 2012;31:A53. [Google Scholar]
- 8.Martínez-Martínez MI, Calabuig-Tolsá R, Cauli O. The effect of probiotics as a treatment for constipation in elderly people: A systematic review. Arch Gerontol Geriatr. 2017;71:142–149. doi: 10.1016/j.archger.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in hemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25:219–224. doi: 10.1093/ndt/gfp414. [DOI] [PubMed] [Google Scholar]
- 10.Minami K, Tanaka S, Takahashi K, Tsujino M, Tanimura H, Ishimono K, Tsunoda T, Umemoto Y, Tanaka N, Masaki K, Mibu Y, Bandou Y, Minamino I. The effect of Bifidobacterium on intestinal bacterial flora and putrefactive products in hemodialysis. J Jpn Sci Dial Ther. 1999;32:349–356. (in Japanese) [Google Scholar]
- 11.Miyoshi M, Shiroto A, Kadoguchi H, Usami M, Hori Y. Prebiotics improved the defecation status via changes in the microbiota and short-chain fatty acids in hemodialysis patients. Kobe J Med Sci. 2020;66:E12–E21. [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashima K, Mochizuki J, Hisada T, Suzuki S, Shimamura K. Phylogenetic analysis of 16S ribosomal RNA gene sequences from human fecal microbiota and improved utility of terminal restriction fragment length polymorphism profiling. Biosci Microflora. 2006;25:99–107. [Google Scholar]
- 13.Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. 2011;26:1094–1098. doi: 10.1093/ndt/gfq624. [DOI] [PubMed] [Google Scholar]
- 14.Rao TP, Quartarone G. Role of guar fiber in improving digestive health and function. Nutrition. 2019;59:158–169. doi: 10.1016/j.nut.2018.07.109. [DOI] [PubMed] [Google Scholar]
- 15.Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr Diab Rep. 2017;17:16. doi: 10.1007/s11892-017-0841-z. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep. 2015;5:13548. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi T, Naito Y, Higashimura Y, Ushiroda C, Mizushima K, Ohashi Y, Yasukawa Z, Ozeki M, Tokunaga M, Okubo T, Katada K, Kamada K, Uchiyama K, Handa O, Itoh Y, Yoshikawa T. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br J Nutr. 2016;116:1199–1205. doi: 10.1017/S0007114516003068. [DOI] [PubMed] [Google Scholar]
- 18.Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41:S142–S145. doi: 10.1053/ajkd.2003.50104. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji Y, Yoshioka K, Kohno M, Suzuki N, Tajiri N, Hitomi Y, Kato K, Yoshida T, Mizuno-Matsumoto Y. Physio–psychological study on enteric capsules containing bifidobacteria for hemodialysis patients. J Jpn Sci Dial Ther. 2015;48:413–422. (in Japanese) [Google Scholar]
- 20.Uemura T, Maeda H, Iwata N, Yoshimura J, Sakai S. Partially hydrolyzed guar gum intake ameliorates constipation and improves nutritional conditions in dialysis patients. J. JSPEN. 2014;29:857–862. (in Japanese) [Google Scholar]
- 21.Walter J, Maldonado-Gómez MX, Martínez I. To engraft or not to engraft: an ecological framework for gut microbiome modulation with live microbes. Curr Opin Biotechnol. 2018;49:129–139. doi: 10.1016/j.copbio.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watabe J. Carbohydrate fermentation in the colon. J Intestinal Microbiology. 2005;19:169–177. (in Japanese) [Google Scholar]
- 23.Yu T, Zheng YP, Tan JC, Xiong WJ, Wang Y, Lin L. Effects of prebiotics and synbiotics on functional constipation. Am J Med Sci. 2017;353:282–292. doi: 10.1016/j.amjms.2016.09.014. [DOI] [PubMed] [Google Scholar]