Abstract
Purpose.
Genomic screening for Lynch syndrome (LS) could prevent colorectal cancer (CRC) by identifying high-risk patients and instituting intensive CRC screening. We estimated the cost-effectiveness of population-wide LS genomic screening versus family history-based screening alone in an unselected U.S. population.
Methods.
We developed a decision-analytic Markov model including health states for pre-cancer, stage-specific CRC, and death, and assumed an inexpensive test cost of $200. We conducted sensitivity and threshold analyses to evaluate model uncertainty.
Results.
Screening unselected 30-year-olds for LS variants resulted in 48 (95% credible range [CR]: 35 to 63) fewer overall CRC cases per 100,000 screened individuals, leading to 187 quality-adjusted life-years (QALYs; 95% CR: 123 to 260) gained at an incremental cost of $24.6 million (95% CR: $20.3 million to $29.1 million). The incremental cost-effectiveness ratio was $132,200, with an 8% and 71% probability of being cost-effective at $100,000 and $150,000 per QALY willingness-to-pay thresholds, respectively.
Conclusions.
Population LS screening may be cost-effective in younger patient populations under a $150,000 willingness-to-pay per QALY threshold and with a relatively inexpensive test cost. Further reductions in testing costs and/or the inclusion of LS testing within a broader multiplex screening panel are needed for screening to be highly cost-effective.
INTRODUCTION
Lynch syndrome (LS) is the most common inherited cause of colorectal cancer (CRC), accounting for approximately 3–4% of incident CRC cases.1–3 People with LS (LS heterozygotes) have a heterozygous, pathogenic germline variant in at least one DNA mismatch repair (MMR) gene, including MLH1 (OMIM 120436), MSH2 (OMIM 609309), MSH6 (OMIM 600678), and PMS2 (OMIM 600259).3 The risk of CRC by age 70 in LS heterozygotes is 38% in men and 31% in women.4 LS heterozygotes tend to develop disease at younger ages compared to the general population,5,6 with a mean age of CRC onset in the mid-40s.7 In addition, LS is associated with increased risk for other cancers such as endometrial, ovarian, and prostate.8–10
While current guidelines agree that decennial colonoscopy surveillance for CRC should begin at age 45–50 years for the general population,11 confirmed LS heterozygotes are encouraged to undergo more intensive colonoscopy surveillance initiated at an early age, such as annual or biennial colonoscopy surveillance beginning at age 20–25 years, with variations in recommendations based on individual MMR gene status.12,13 A primary challenge for the current U.S. testing paradigm is that LS heterozygotes may not have a family history meeting current criteria for testing, thus many high-risk individuals go undetected until CRC diagnosis.14 Furthermore, CRC patients may have first-degree relatives who are LS heterozygotes but are unaware of it.15 Therefore, the benefits of earlier and more intensive colonoscopy surveillance for these individuals go unrealized.
Although the Centers for Disease Control and Prevention (CDC) lists genetic testing for LS as a Tier 1 genomic application, with “significant potential for positive impact on public health based on available evidence-based guidelines and recommendations,”16 this test is not performed universally.17 Pathogenic LS variant screening is recommended for all patients with CRC at diagnosis and typically starts with tumor surveillance using immunohistochemistry staining or microsatellite instability analysis.3,18 For unselected individuals without CRC, identification of potential LS heterozygotes for risk identification and prevention of CRC relies primarily on family history-based screening, including the Amsterdam criteria and revised Bethesda criteria, among others.19 Individuals meeting family-history screening criteria (3 or more relatives with an associated cancer, 2 generations affected, and 1 relative diagnosed before age 50) then undergo germline testing on MMR genes for LS diagnosis. To date, there is no recommendation for detecting LS in individuals with an absence of family history of LS and/or LS-associated cancer.20
Advances in genomic sequencing – particularly falling costs – raise several questions. Would the health benefits of population-wide genomic screening conferred to a relatively small number of LS heterozygotes justify the cost required of such a significant public health endeavor? And is there an ideal age at which to be screened that maximizes the potential health benefits and minimizes healthcare costs? Conversely, could the potential benefits of genomic screening for LS become negligible to the target population or lead to over-screening and/or reduced uptake of decennial colonoscopy in the vast majority of individuals who are found not to possess a LS variant? Our objective was to address these questions by estimating the cost-effectiveness of germline genetic screening among unaffected, unselected individuals in the U.S. population for LS heterozygote status followed by cascade genetic testing of first-degree family members.
MATERIALS AND METHODS
Modeled Population
The model tracked hypothetical, unselected, age-based cohorts of U.S. adults from the age of model entry until death from any cause. Cohorts ranged in age at model entry from 20 to 75, and each age cohort was analyzed separately. The overall prevalence of the pathogenic LS variant (0.3%) was based on data from the Geisinger MyCode Community Health Initiative, a healthcare system-based genomic medicine research project with more than 280,000 patient-participants in Pennsylvania.21 Confirmed LS heterozygotes of any age immediately began intensive annual colonoscopy surveillance for CRC and continued until age 75. People confirmed to not have a LS variant received decennial surveillance for CRC starting at age 45.11
Population Screening Model
We developed a decision analytic model to compare (a) population screening for LS in an unselected population of individuals previously undiagnosed with CRC versus (b) family history-based testing alone. Routine family history-based testing (i.e., the status quo policy) was available in both strategies (Figure 1). Patients enter the model via a decision tree, which was used to stratify individuals by LS heterozygote status and their knowledge of that status. A Markov model was then used to simulate the cohort’s screening actions, clinical events, health-related quality of life, and healthcare costs over a lifetime. We used a U.S. health care sector perspective (i.e., focused on direct medical care costs only) in our base case analysis and discounted all cost and health outcomes by 3% per year. The model was developed in Microsoft® Excel®. The described method followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS, available in supplemental appendix).
Figure 1.
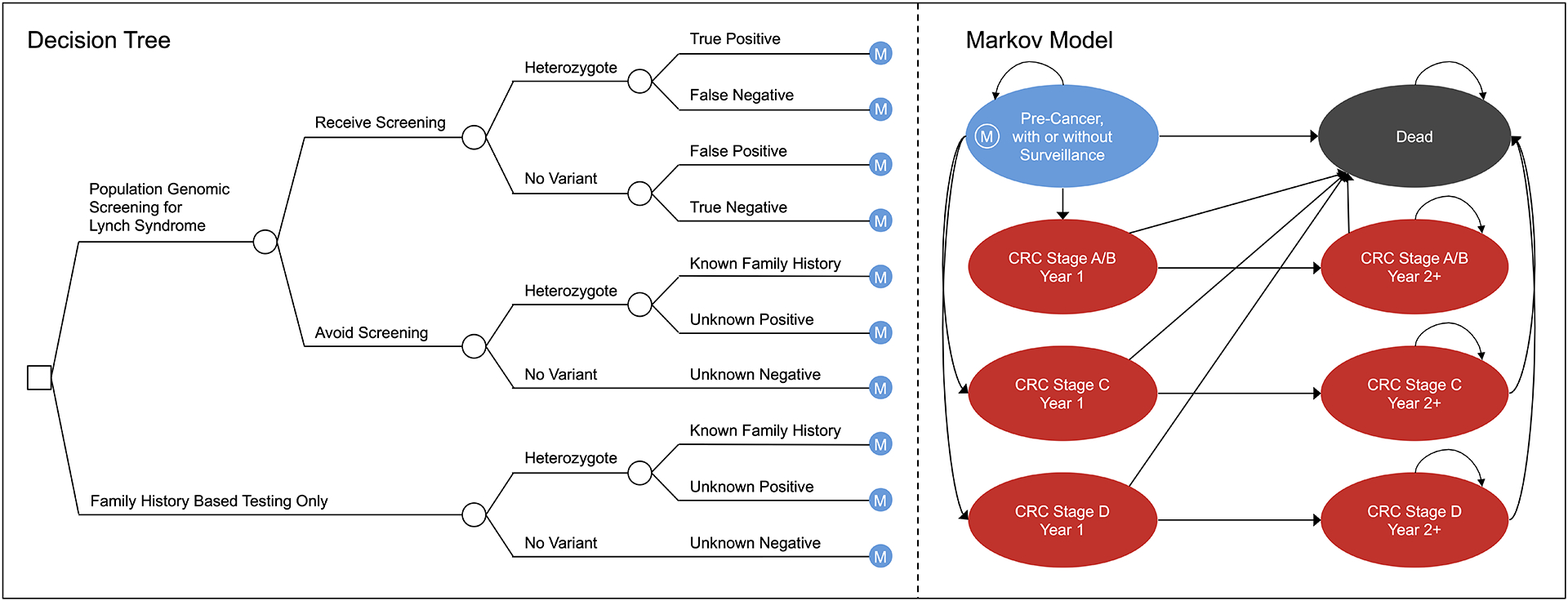
Model Schematic. CRC, colorectal cancer; M, long-term Markov model.
The decision tree accounted for (1) screening participation on the part of the individual, (2) LS heterozygote status, and (3) screening results (parameterized by test sensitivity and specificity) and/or family history-based results. The long-term Markov model included health states for pre-cancer, single-cycle health states for stage-specific incidence of CRC (Figure 2), stage-specific post-CRC health states, and all-cause death. In the pre-cancer health state, a proportion of identified LS heterozygotes adhered to high intensity annual colonoscopy surveillance between ages 20 and 75 instead of the recommended decennial interval for average risk individuals age 50 and above.20 We used annual model cycles, and patients could remain in their current state or transition to another state each year as depicted by the arrows in Figure 1. All patients could transition to death from any other health state based on CRC stage-specific and/or background mortality.16,22
Figure 2.
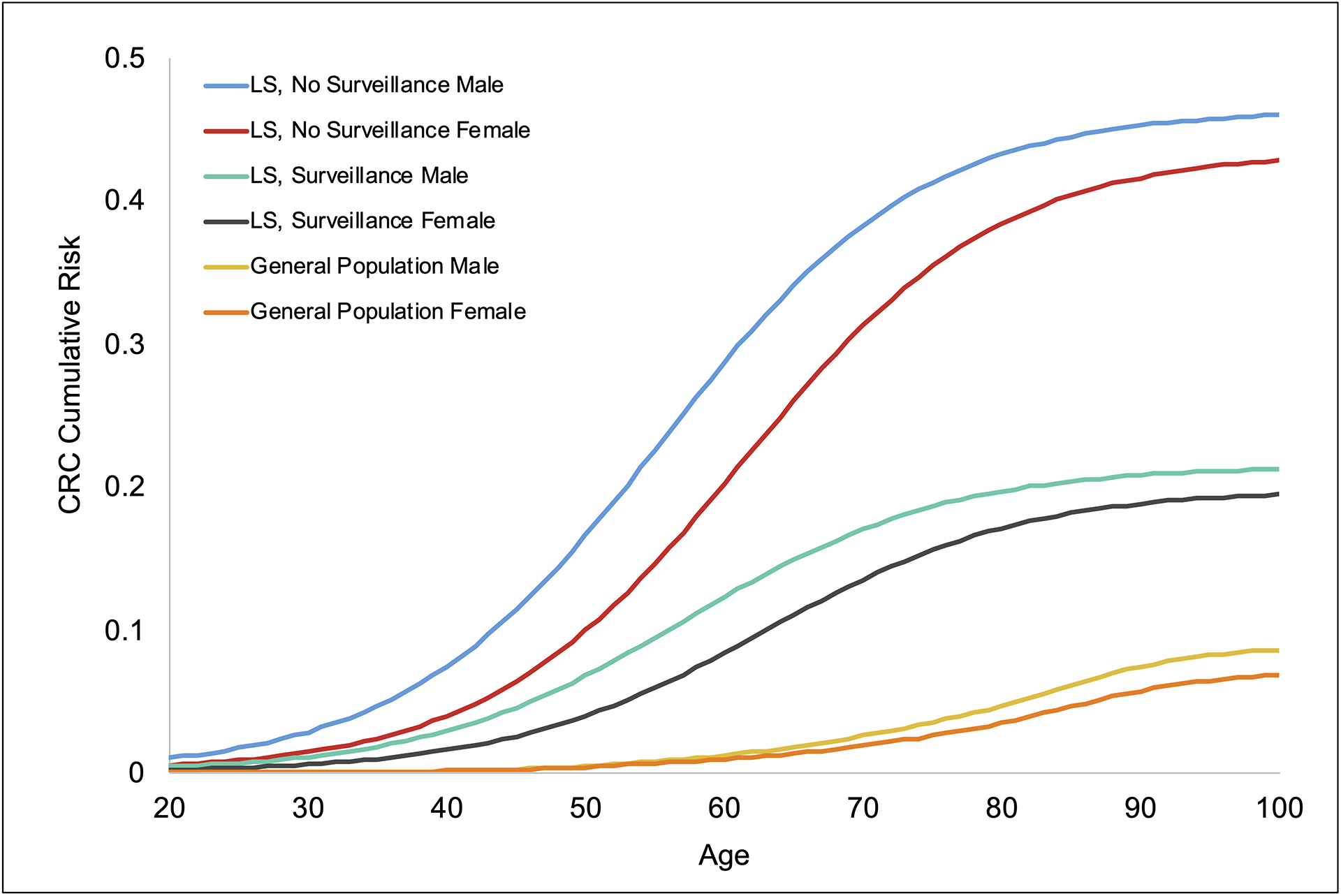
Cumulative CRC risk by age (model input). CRC, colorectal cancer; LS, Lynch syndrome.
Cascade Testing Module
We developed an accompanying module for cascade testing of population-based screening-identified heterozygotes’ family members (Figures e1 and e2 in supplementary appendix). The cascade testing calculations were dynamically tied to the primary population screening model, in that the number of surviving parents, siblings, and children available for cascade testing were dependent on the modeled age of patients entering the primary population screening model. The distribution of surviving first-degree relatives by age was based on a nationally representative sample of the U.S. population.23 The cascade testing module outcomes included testing cost among tested individuals and the incremental cost and quality-adjusted life year (QALY) outcomes generated by newly identified heterozygotes through the cascade testing process. These outcomes were then added back into the primary population screening model to calculate combined overall results.
Clinical Parameters
Colonoscopy surveillance uptake among screening-identified LS heterozygotes was derived from an Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (EWG) evidence review by Palomaki et al. who examined genetic testing, diagnosis, and health outcomes of LS heterozygotes (note: all model parameters and their references are available in Table e1 in the supplementary appendix). Surveillance uptake among family history confirmed heterozygotes was derived from a National Institute for Health Research-funded health technology assessment study by Snowsill et al., who evaluated the cost-effectiveness of testing for LS in CRC patients in the UK.
We derived non-surveilled LS heterozygotes’ annualized, age-based cancer incidence using the logistic model by Snowsill et al., which was fit to age-based cumulative CRC incidence among families with pathogenic/likely pathogenic variants in LS genes estimated by Bonadona et al; we subsequently validated this approach versus the Prospective Lynch Syndrome Database (PLSD).9 Surveilled LS heterozygotes’ CRC incidence was then derived by applying Snowsill et al.’s incidence-reducing surveillance hazard ratio to the non-surveilled LS incidence; the hazard ratio was derived from Järvinen et al., who evaluated CRC incidence and survival in two cohorts of at-risk members of 22 families with hereditary nonpolyposis CRC. CRC incidence among the general population was obtained from the Surveillance, Epidemiology, and End Results (SEER) Program.
We modeled CRC disease stage at diagnosis among high and normal intensity surveillance LS heterozygotes based on Stupart et al., who conducted a prospective cohort study of 200 LS heterozygotes followed up for a mean of four years (range 0–18) post-genetic counseling; of 71 heterozygotes who did not utilize high intensity surveillance, 36 (51%) developed CRC and 7 were diagnosed with stage D CRC, while only 11% of heterozygotes who did utilize high intensity surveillance developed CRC and none were stage D CRC. We used SEER data to model CRC disease stage in the general population. Ten-year CRC mortality from SEER was used to model all CRC-associated transitions to death; CRC stage-specific mortality in the first year post-diagnosis was used to model the transition to death from the single-cycle CRC incidence health states, and year 2+ stage-specific transition probabilities were derived from the remaining nine years of mortality estimates.
Quality of Life Parameters
We assumed a health state utility of 1.0 for healthy (pre-cancer) patients with or without colonoscopy surveillance. We derived CRC disutility estimates from Djalalov et al., who systematically reviewed 26 articles published from 1980 to 2013 including CRC health states elicited from 6,546 unique respondents, then used a linear mixed-effects model accounting for CRC type, stage, and time since initial care, among other variables. Based on their findings, a 0.05 disutility was applied to CRC stages A-C in year 1 only, while CRC stage D had a 0.24 disutility applied in year 1 and a 0.20 disutility in subsequent years. A previous systematic review of CRC economic models by Snowsill et al. concluded that the negative psychological effects of genetic testing are small (0.00–0.04) and last no longer than four months; we conservatively assumed a 4-month disutility of 0.04 applied to screened heterozygotes in the first model cycle to recognize this potentially important impact.
Cost Parameters
We modeled a population screening test cost of $200 based on currently available, low-cost testing options available to the public.24 All direct medical costs obtained from the published literature were inflated to February 2021 U.S. dollars. We derived costs for colonoscopy surveillance procedures from Dinh et al., who performed an economic analysis of 20 primary LS screening strategies. Stage-specific CRC costs were applied for the first year of treatment, for continuing treatment in subsequent years, and as palliative therapy for the last year of life, and were based on an Agency for Healthcare Research and Quality (AHRQ)-funded cost-effectiveness analysis of DNA stool testing to screen for CRC by Zauber et al. We also used the stage-specific other death palliative treatment costs from the Zauber et al. study for the transition to death via background mortality.
Analysis
We calculated lifetime cancer incidence, life years, QALYs, and direct medical costs for genomic screening in an unselected population versus family history-based testing only. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in cost between strategies divided by the difference in QALYs between strategies. We also calculated the CRC stage differences in cases prevented and total cancer cases prevented.
We performed one-way and probabilistic sensitivity analyses to assess the impacts of uncertainty in model parameters on the results. In one-way sensitivity analysis, one parameter at a time is varied to its low and high value while keeping all other parameters constant; in probabilistic sensitivity analysis, all model parameters were simultaneously, randomly varied according to an assigned probability distribution over 5,000 simulations, and 95% credible ranges (CR) were calculated for each model result.
External Validation
We validated our model by comparing the benefits of identifying an individual with Lynch syndrome to previously published studies that estimated the cost-effectiveness of genetic testing strategies to identify LS among newly diagnosed CRC patients and to offer targeted genetic testing to their relatives.25–27 Our model differed from previous models in that we used an unselected population in the U.S; we facilitated comparisons by setting pathogenic LS heterozygote prevalence to 100% and assuming 100% compliance with intensified colonoscopy surveillance among heterozygotes, allowing us to isolate the life years gained and cost per life year gained per adherent individual.
Scenario Analyses
We performed scenario analyses to evaluate model assumptions. First, we conducted an analysis excluding the effects of cascade testing. Second, we conducted threshold analyses with different genomic assay costs to estimate what costs are needed to meet conventional cost-effectiveness thresholds in the US. Third, we evaluated the potential for harm to people who do not harbor a pathogenic variant (99.7% of individuals), i.e., the potential for them to decrease adherence to routine recommended colonoscopy surveillance after receiving a negative genomic screening result. Specifically, we estimated the proportion of screened individuals without a variant who would have to avoid normal CRC surveillance to result in no overall QALY benefit for the population as a whole. This proportion received a −0.10 QALY loss due to avoiding normal colonoscopy surveillance at age 50 (discounted by 20 years for 30-year-olds), based on a wide range (0.02 to 0.20) of screening-derived QALY gains estimated within previous cost-effectiveness analyses (references available in supplementary appendix).
Fourth, given a recent PLSD study showed no benefit of annual over triennial colonoscopy in LS heterozygotes,28 we explored the potential cost impacts of reduced colonoscopy screening frequency by reducing the model’s annual colonoscopy cost by 1/2 and 2/3 to approximate biennial and triennial visits. Fifth, we evaluated the cost-effectiveness of LS population screening using a societal perspective incorporating estimates from Zheng et al., who assessed the excess economic burden attributable to CRC including per-person excess annual medical expenditures and productivity losses (employment disability, missed work days, and days stayed in bed).29 Societal costs were stratified by age and inflated to 2021 dollars (nonelderly: 18–64 years, $7,074; elderly: ≥65 years, $1,948), and were applied annually to all year 1 and post-CRC patients. Last, we evaluated the impacts of reduced access to care among traditionally underserved communities in the US by performing a two-way sensitivity analysis of CRC surveillance uptake and screening assay cost.
RESULTS
Base Case
Screening unselected 30-year-olds for LS resulted in 48 (95% credible range [CR]: 35 to 63) fewer overall CRC cases per 100,000 screened individuals (Figure 3), including 20 (95% CR: 9 to 34) fewer cases of early stage (A & B) CRC, 12 (95% CR: 2 to 23) fewer cases of stage C CRC, and 16 (95% CR: 8 to 25) fewer cases of stage D CRC (Table 1 and supplementary appendix). LS screening resulted in 187 QALYs (95% CR: 123 to 260) gained per 100,000 screened individuals at an incremental cost of $24.6 million (95% CR: $20.3 million to $29.1 million) per 100,000 screened individuals versus family history-based testing, resulting in an ICER of $132,200 (8% probability of cost-effectiveness at a $100,000/QALY willingness-to-pay ratio). The ICER decreased with age until age 40 (ICER = $123,900), after which the ICER gradually increased until age 50 (39 fewer CRC cases, 161 QALYs gained, ICER = $140,400) then more sharply increased thereafter due to decreasing benefits of screening.
Figure 3.
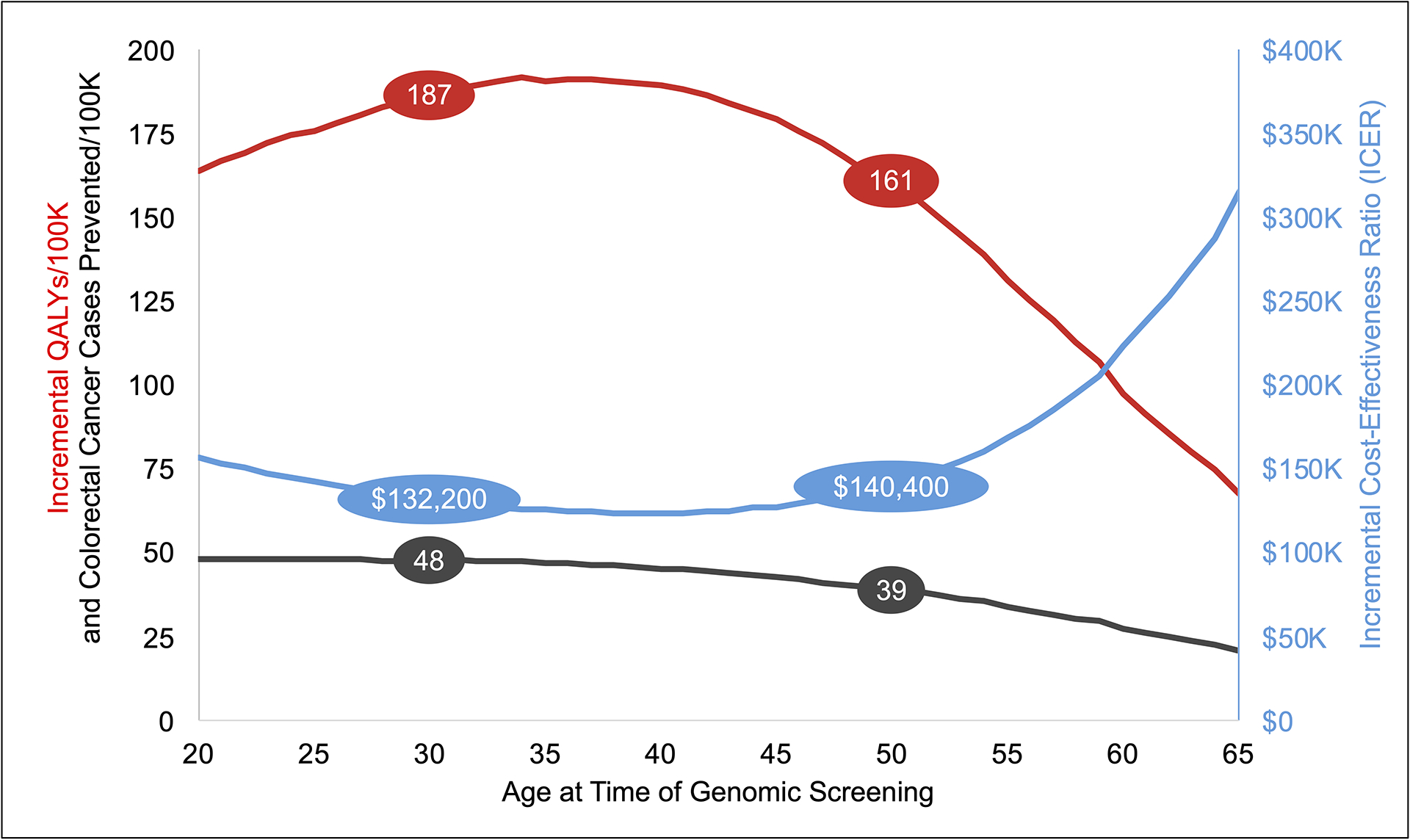
Incremental results by age at time of genomic screening. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Table 1.
Model Results
| CRC Stage A+B Cases/100K Screened | CRC Stage C Cases/100K Screened | CRC Stage D Cases/100K Screened | Cost/Screened Individual | Quality-adjusted Life Years/Screened Individual | Life Years/Screened Individual | Incremental Cost-Effectiveness Ratio (ICER) | ||
|---|---|---|---|---|---|---|---|---|
| Screened at 30 Years Old | ||||||||
| Population Screening | Base Case | 1,992 | 1,526 | 898 | $2,147 | 25.504 | 25.507 | |
| 95% Credible Range (CR) | (1,704 to 2,269) | (1,272 to 1,780) | (733 to 1,086) | ($1,988 to $2,311) | (25.498 to 25.509) | (25.502 to 25.512) | ||
| Family History-Based Testing | Base Case | 2,008 | 1,535 | 911 | $1,913 | 25.502 | 25.506 | |
| 95% CR | (1,719 to 2,285) | (1,281 to 1,790) | (743 to 1,100) | ($1,759 to $2,074) | (25.496 to 25.508) | (25.501 to 25.511) | ||
| Incremental | Base Case | −20 | −12 | −16 | $246 | 0.002 | 0.002 | $132,151 |
| 95% CR | (−34 to −8) | (−23 to −2) | (−26 to −8) | ($203 to $292) | (0.001 to 0.003) | (0.001 to 0.003) | ($86,867 to $208,010) | |
| Screened at 50 Years Old | ||||||||
| Population Screening | Base Case | 1,908 | 1,462 | 862 | $3,073 | 19.711 | 19.716 | |
| 95% CR | (1,629 to 2,174) | (1,223 to 1,723) | (696 to 1,051) | ($2,834 to $3,332) | (19.703 to 19.718) | (19.709 to 19.723) | ||
| Family History-Based Testing | Base Case | 1,921 | 1,469 | 872 | $2,860 | 19.710 | 19.715 | |
| 95% CR | (1,639 to 2,190) | (1,230 to 1,731) | (707 to 1,060) | ($2,626 to $3,127) | (19.701 to 19.717) | (19.708 to 19.722) | ||
| Incremental | Base Case | −17 | −10 | −13 | $226 | 0.002 | 0.002 | $140,371 |
| 95% CR | (−27 to −7) | (−19 to −2) | (−21 to −7) | ($182 to $267) | (0.001 to 0.002) | (0.001 to 0.002) | ($88,974 to $221,820) | |
Sensitivity Analyses
In the one-way sensitivity analysis, the ICER was most sensitive to the CRC stage at diagnosis in non-surveilled and surveilled LS heterozygotes, the cumulative risk of CRC by age 70, and screening assay cost (Figure 4). We utilized cost-effectiveness acceptability curves to represent the results of the probabilistic sensitivity analysis (Figure e4 in supplementary appendix). Population screening had a 0%, 8%, and 71% probability of being cost-effective versus family history-based testing at the $50,000, $100,000, and $150,000 per QALY thresholds, respectively.
Figure 4.
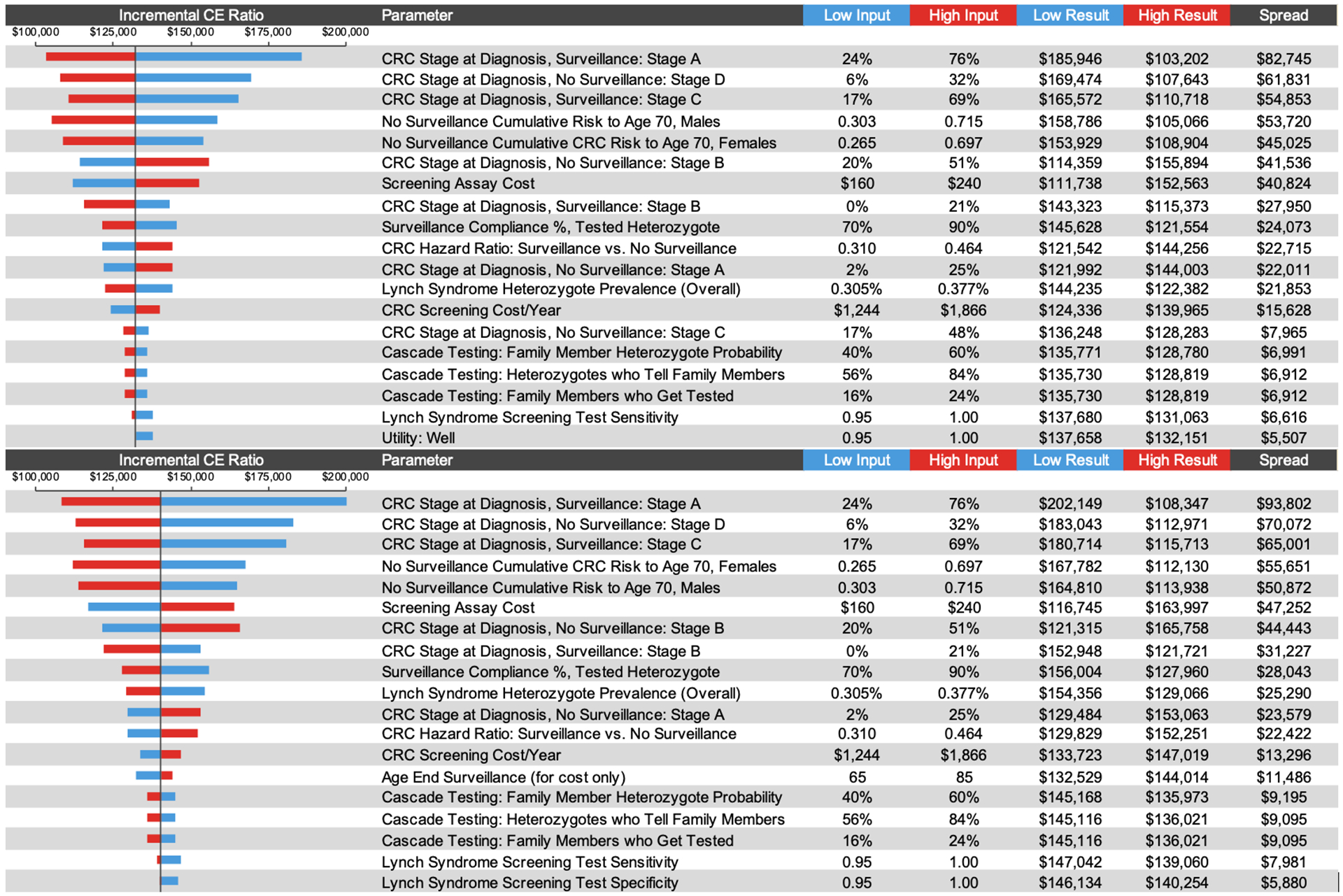
Results of 1-way sensitivity analyses. CE, cost-effectiveness; CRC, colorectal cancer.
External Validation
The life years gained per adherent LS heterozygote in the Grosse et al.26 and Severin et al.30 cost-effectiveness models of LS testing in newly diagnosed CRC patients were 0.80 and 0.52, respectively, while our model estimated 0.66 life years gained. Zhang et al.27 examined the value of population screening for young adults in Australia for multiple cancers including CRC, however the reported incremental disability-adjusted life years (DALYs) prevented for CRC due to population screening was equivalent to 5.6 DALYs prevented per identified heterozygote. When we replicated their decision model, we successfully reproduced the number of CRC cases prevented but not the benefits (73.3 DALYs/case prevented and 121.8 DALYs/death prevented).31
Scenario Analyses
Effect of Cascade Testing.
When we excluded cascade testing of first-degree relatives of screening-identified heterozygotes, the incremental cost and QALYs gained per 100,000 screened individuals decreased by approximately $1.2 million and 33, respectively, and increased the ICER to $153,200.
Potential harm.
When we used a threshold analysis to consider the potential harm of genomic screening to people who do not harbor a variant and might avoid routine normal intensity CRC surveillance after being informed of a negative result, we found that if greater than 3.6% of nonvariant 30-year-olds go on to avoid routine colonoscopy surveillance at age 50 then population screening for LS will have negative incremental health benefit.
Impact of testing cost.
Using historical genetic testing costs of $500 and $1,000, the incremental cost of screening increased to $53.2 million and $101 million, respectively, and the ICER increased to $285,200 and $540,400, respectively. To attain ICERs of $50,000 and $100,000 per QALY, the cost of genetic testing would need to fall to $39 and $137, respectively.
Reduced colonoscopy frequency in LS heterozygotes.
Reducing the annual surveillance cost by 1/2 and 2/3 to approximate biennial and triennial intervals resulted in reduced ICERs of $113K and $106K, respectively.
Societal Perspective.
The addition of societal costs for excess annual medical expenditures and productivity losses led to an overall decrease in incremental cost ($23.5 million) compared to the base case health sector perspective ($24.6 million) due to a greater number of CRC patients in the family history testing alone comparator, leading to an ICER of $126,000.
Access to Care.
The ICER tended to increase as uptake of intensive colonoscopy surveillance decreased and the cost of a genomic assay increased; conversely, increased uptake and lower genetic testing cost led to improved ICERs (Figure e5 in supplementary appendix). The ICER was generally below $100,000 per QALY gained in instances where all LS heterozygotes had equal access to increased surveillance regardless of race or socioeconomic status and the testing cost was below $125. The lower the uptake of intensive colonoscopy surveillance, the lower the genomic assay cost needed to be for LS population screening to be cost-effective at $100,000 per QALY gained.
DISCUSSION
We conducted a cost-effectiveness analysis of a hypothetical national population genomic screening program to detect LS in an unselected US population. We utilized age-specific CRC incidence and stage at diagnosis among LS non-surveilled and surveilled heterozygotes and the impacts of cascade testing of first-degree relatives. Our results showed that population genomic screening is potentially cost-effective in younger populations only if testing costs are $200 or below and policymakers are willing to pay a higher cost per QALY gained than the widely acknowledged $100,000 per QALY threshold in the U.S.32 To attain ICERs of $50,000 and $100,000 per QALY, the cost of genetic testing would need to fall to $39 and $137, respectively. We also found that cascade testing adds modest clinical and economic value, and that the potential harm conferred by screening people who do not harbor a variant should be considered.
Previous cost-effectiveness analyses have focused primarily on proband screening approaches and/or predictive models followed by germline or somatic genetic testing to confirm LS among affected CRC patients and/or their first-degree relatives (references available in supplementary appendix); our results generally align with previous analyses with regards to magnitude of incremental life years gained per identified LS heterozygote. Similar to our previous cost-effectiveness analysis of population screening for hereditary breast and ovarian cancer (HBOC),33 we accounted for the dynamics of age at time of screening and competing risk over time. As with HBOC we found that screening at younger ages leads to better health outcomes and greater cost-effectiveness, however the difference moving from younger to older ages was less notable than in the HBOC analysis, primarily owing to the lower incidence and later onset of LS-associated CRC compared to HBOC (Figure e6 in supplementary appendix).
At the population level, we found that the incremental impacts of cascade testing were potentially important. When cascade testing was removed, it decreased incremental cost and QALYs per 100,000 screened individuals by approximately $1.2 million and 33, respectively, and increased the ICER to $153,200. This difference was larger than the impact described in our HBOC analysis (ICER increase from $87,700 to $92,600) due to the inclusion of male family members, effectively doubling the pool of relatives who stand to benefit, but was not sufficient to improve the LS population screening ICER to less than $100,000. Our study suggests cascade testing should be implemented where feasible, and efforts should be made to improve rates of family communication and follow-up testing, as higher uptake of cascade testing improves the overall value of population genomic screening.
Our study has several important limitations. First, we assumed an optimistic genomic assay cost of $200 based on currently available testing options offered by private enterprise. To be fiscally feasible, population screening will likely require similar scalable approaches including the use of web-based return of results, phone counseling, and high volume to defray test processing and reporting costs. Second, we assumed high adherence to intensified CRC surveillance among screening-identified heterozygotes (80% in the base-case analysis) and family history-identified heterozygotes (70% in the base-case analysis) based on published estimates.18,34 Previous studies have reported a wide range of proportions (50–100%) of LS individuals who received a colonoscopy within 6 months to 2 years after genetic diagnosis or being identified as high risk (references available in supplementary appendix). This wide range reflects factors such as differences in education, family communication, and patients’ perception of risk. Regardless, in one-way sensitivity analysis we found that adherence to colonoscopy among LS heterozygotes was only moderately influential on the ICER. Third, we assumed that LS heterozygotes’ adherence to surveillance was constant over time; although longitudinal adherence to colonoscopy surveillance in the general population is known to vary,35 we did not identify commensurate longitudinal adherence estimates among LS heterozygotes. Our two-way sensitivity analysis exploring the impacts access to care showed that any reduction in surveillance adherence over time would lead to reduced health benefits and a corresponding increase in the ICER.
Fourth, our analysis did not directly evaluate the impacts of patient diversity, health-related, and healthcare disparities. Although MMR genes and MSI are not known to differ by race/ethnicity,36,37 on average African Americans have the highest incidence of CRC and mortality compared to other racial/ethnic groups, which can partially be explained by health-related inequalities including socioeconomic disadvantages and inequitable access to colonoscopy surveillance and genetic testing, diagnosis, and treatment.38–40 Future studies should further examine the impacts of these disparities in the distribution of health and healthcare on the value of population screening relating to cost-effectiveness and health inequality (i.e., distributional cost-effectiveness).41,42 Fifth, our estimate of CRC risk among LS heterozygotes was based on a combined incidence of 4 MMR genes. However, individual MMR genes have different penetrance and therefore confer to different risk of CRC. Lastly, our study did not include other cancers associated with LS such as endometrial, ovarian, and prostate cancer; we anticipate that inclusion of other cancers in assessing value of genetic screening would likely increase the value of population-based genetic screening.
In conclusion, population genomic screening for LS using clinical sequencing may be cost-effective in younger patient populations. In addition, we found that cascade testing is important for achieving true population-level reach but does not provide fundamentally impactful clinical and economic value, and that the potential harm conferred by screening those who turn out not to have pathogenic variants should be considered. Ultimately, the value of LS screening of the US general population should be assessed within the context of a broader multiplexed screening panel, and such analyses are ongoing.
Supplementary Material
Acknowledgment:
We thank Hadley Stevens Smith, PhD, MPSA, for contributions to the cascade testing module.
Footnotes
Ethics Declaration:
No patient-level data was used in this analysis. Patient consent for publication not required.
Data Availability:
All data relevant to the study are included in the article or uploaded as supplemental information.
REFERENCES
- 1.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. Jama. 2012;308(15):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. Jama. 2011;305(22):2304–2310. [DOI] [PubMed] [Google Scholar]
- 5.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clinic proceedings. 2014;89(2):216–224. [DOI] [PubMed] [Google Scholar]
- 6.Kastrinos F, Stoffel EM, Balmaña J, Steyerberg EW, Mercado R, Syngal S. Phenotype comparison of MLH1 and MSH2 mutation carriers in a cohort of 1,914 individuals undergoing clinical genetic testing in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2044–2051. [DOI] [PubMed] [Google Scholar]
- 7.Jasperson KW, Vu TM, Schwab AL, et al. Evaluating Lynch syndrome in very early onset colorectal cancer probands without apparent polyposis. Fam Cancer. 2010;9(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ten Broeke SW, Brohet RM, Tops CM, et al. Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol. 2015;33(4):319–325. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Valentin M, Sampson JR, Seppälä TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med. 2020;22(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson KW, Barry MJ, Mangione CM, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama. 2021;325(19):1965–1977. [DOI] [PubMed] [Google Scholar]
- 12.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147(2):502–526. [DOI] [PubMed] [Google Scholar]
- 13.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380–391. [DOI] [PubMed] [Google Scholar]
- 14.Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/Familial High-Risk Assessment: Colorectal Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(8):1010–1030. [DOI] [PubMed] [Google Scholar]
- 15.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11(9):1093–1100. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention, 2014. https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm. Accessed 12/27/2019.
- 17.Vindigni SM, Kaz AM. Universal Screening of Colorectal Cancers for Lynch Syndrome: Challenges and Opportunities. Dig Dis Sci. 2016;61(4):969–976. [DOI] [PubMed] [Google Scholar]
- 18.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastrinos F, Uno H, Ukaegbu C, et al. Development and Validation of the PREMM(5) Model for Comprehensive Risk Assessment of Lynch Syndrome. J Clin Oncol. 2017;35(19):2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan AH, Lester Kirchner H, Schwartz MLB, et al. Clinical outcomes of a genomic screening program for actionable genetic conditions. Genet Med. 2020;22(11):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Program SEaERS, 2020. https://seer.cancer.gov/explorer/application.html?site=20&data_type=4&graph_type=6&compareBy=sex&chk_sex_3=3&chk_sex_2=2&race=1&age_range=1&stage=101&advopt_precision=1. Accessed 12/13/2020 2020.
- 23.Survey Research-Center Institute for Social Research-University of Michigan, 2019. https://simba.isr.umich.edu/data/data.aspx.
- 24.Color Genomics Inc, https://home.color.com/purchase/ordering-physician?sku=combo%203. Accessed 3/1/2020.
- 25.Wang G, Kuppermann M, Kim B, Phillips KA, Ladabaum U. Influence of patient preferences on the cost-effectiveness of screening for Lynch syndrome. The American journal of managed care. 2012;18(5):e179–185. [PubMed] [Google Scholar]
- 26.Grosse SD, Palomaki GE, Mvundura M, Hampel H. The cost-effectiveness of routine testing for Lynch syndrome in newly diagnosed patients with colorectal cancer in the United States: corrected estimates. Genet Med. 2015;17(6):510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Bao Y, Riaz M, et al. Population genomic screening of all young adults in a health-care system: a cost-effectiveness analysis. Genet Med. 2019;21(9):1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seppälä T, Pylvänäinen K, Evans DG, et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: a Prospective Lynch Syndrome Database report. Hered Cancer Clin Pract. 2017;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z, Yabroff KR, Guy GP Jr., et al. Annual Medical Expenditure and Productivity Loss Among Colorectal, Female Breast, and Prostate Cancer Survivors in the United States. J Natl Cancer Inst. 2016;108(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severin F, Stollenwerk B, Holinski-Feder E, et al. Economic evaluation of genetic screening for Lynch syndrome in Germany. Genet Med. 2015;17(10):765–773. [DOI] [PubMed] [Google Scholar]
- 31.Veenstra DL, Guzauskas G, Peterson J, et al. Cost-effectiveness of population genomic screening. Genet Med. 2019;21(12):2840–2841. [DOI] [PubMed] [Google Scholar]
- 32.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. The New England journal of medicine. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 33.Guzauskas GF, Garbett S, Zhou Z, et al. Cost-effectiveness of Population-Wide Genomic Screening for Hereditary Breast and Ovarian Cancer in the United States. JAMA Netw Open. 2020;3(10):e2022874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snowsill T, Coelho H, Huxley N, et al. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2017;21(51):1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cyhaniuk A, Coombes ME. Longitudinal adherence to colorectal cancer screening guidelines. The American journal of managed care. 2016;22(2):105–111. [PubMed] [Google Scholar]
- 36.Ashktorab H, Ahuja S, Kannan L, et al. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget. 2016;7(23):34546–34557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berera S, Koru-Sengul T, Miao F, et al. Colorectal Tumors From Different Racial and Ethnic Minorities Have Similar Rates of Mismatch Repair Deficiency. Clin Gastroenterol Hepatol. 2016;14(8):1163–1171. [DOI] [PubMed] [Google Scholar]
- 38.Brawley OW. Colorectal cancer control: providing adequate care to those who need it. J Natl Cancer Inst. 2014;106(4):dju075. [DOI] [PubMed] [Google Scholar]
- 39.Jackson CS, Oman M, Patel AM, Vega KJ. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(Suppl 1):S32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller C, Lee SM, Barge W, et al. Low Referral Rate for Genetic Testing in Racially and Ethnically Diverse Patients Despite Universal Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2018;16(12):1911–1918.e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asaria M, Griffin S, Cookson R. Distributional Cost-Effectiveness Analysis: A Tutorial. Medical decision making : an international journal of the Society for Medical Decision Making. 2016;36(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asaria M, Griffin S, Cookson R, Whyte S, Tappenden P. Distributional cost-effectiveness analysis of health care programmes--a methodological case study of the UK Bowel Cancer Screening Programme. Health Econ. 2015;24(6):742–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.


