Abstract
Chronic rhinosinusitis (CRS) is characterized by persistent locoregional mucosal inflammation of the paranasal sinuses and upper airway that has substantial associated health care costs1. Personalized approaches to care that incorporate use of molecular biomarkers, phenotypes and inflammatory endotypes is a major focus of research at this time, and the concurrent rise of targeted therapeutics and biologic therapies has the potential to rapidly advance care and improve outcomes. Recent findings suggest that improved understanding of CRS phenotypic and endotypic heterogeneity, and incorporation of these therapeutic interventions2 Ultimately, these personalized approaches have the potential to target specific inflammatory pathways, increase efficacy, reduce costs, and limit side effects. This review summarizes recent advances in the identification and characterization of CRS phenotypes, endotypes, and biomarkers and reviews potential implications for targeted therapeutics.
Keywords: endotypes, rhinosinusitis, biomarkers, phenotypes, cytokines, biologics
Current Challenges in CRS Diagnosis and Management
Accumulating scientific and clinical evidence over the last decade clearly indicates that CRS is not a discrete diagnostic entity. This is largely unsurprising given that current diagnostic criteria rely largely on patient-reported symptoms that correlate poorly with objective findings of disease (nasal endoscopy, CT imaging)3. This heterogeneity can be further inferred from observed variability in both medical and surgical treatment outcomes4. Unfortunately, the ‘syndromic’ nature of CRS creates challenges for both clinicians and patients, who seek a more defined algorithmic approach to disease management. The impacts of this shortcoming were comparatively restricted in the past, largely due to a limited number of available interventions (typically some combination of antibiotics, corticosteroids, topical therapies, and surgery). However, the last decade has brought with it the emergence of targeted therapeutics with the potential to permanently change both CRS diagnosis and management. Given the high costs associated with many of these therapeutics, as well as the presumed need for long-term use, it is now imperative that clinicians identify additional methods for delineating which patients may be most appropriate for specific therapies.
Classically, CRS has been differentiated by phenotypic characteristics (most commonly polyp status) but this dichotomy and other phenotypic classifications have generally failed to account for the increasingly appreciated inflammatory heterogeneity among patients who are otherwise phenotypically similar, and have lacked the granularity to establish personalized care pathways. Much interest has likewise been placed on the use of molecular biomarkers and their perceived potential to accurately predict either outcomes or optimal therapy. But to date, no single biomarker has demonstrated the sensitivity and specificity to support widespread adoption in clinical practice. Finally, recent research suggests that a host of molecular biomarkers can be used to define inflammatory CRS endotypes with hypothetically greater potential to impact patient management and define clinically relevant disease subgroups. However, this new concept has brought with it new questions, including: 1) What are the most appropriate biomarkers for endotype characterization, 2) How many endotypes are there? 3) What are the effects of medications, comorbid disease, and other environmental changes on endotype assignment, and 4) What is the role of endotypes with respect to therapeutic selection or disease prognostication. These points highlight some of the challenges in CRS diagnosis and management in this rapidly evolving field, but point to a potential paradigm shift that may change clinical practice in years to come.
CRS Phenotypes and Implications
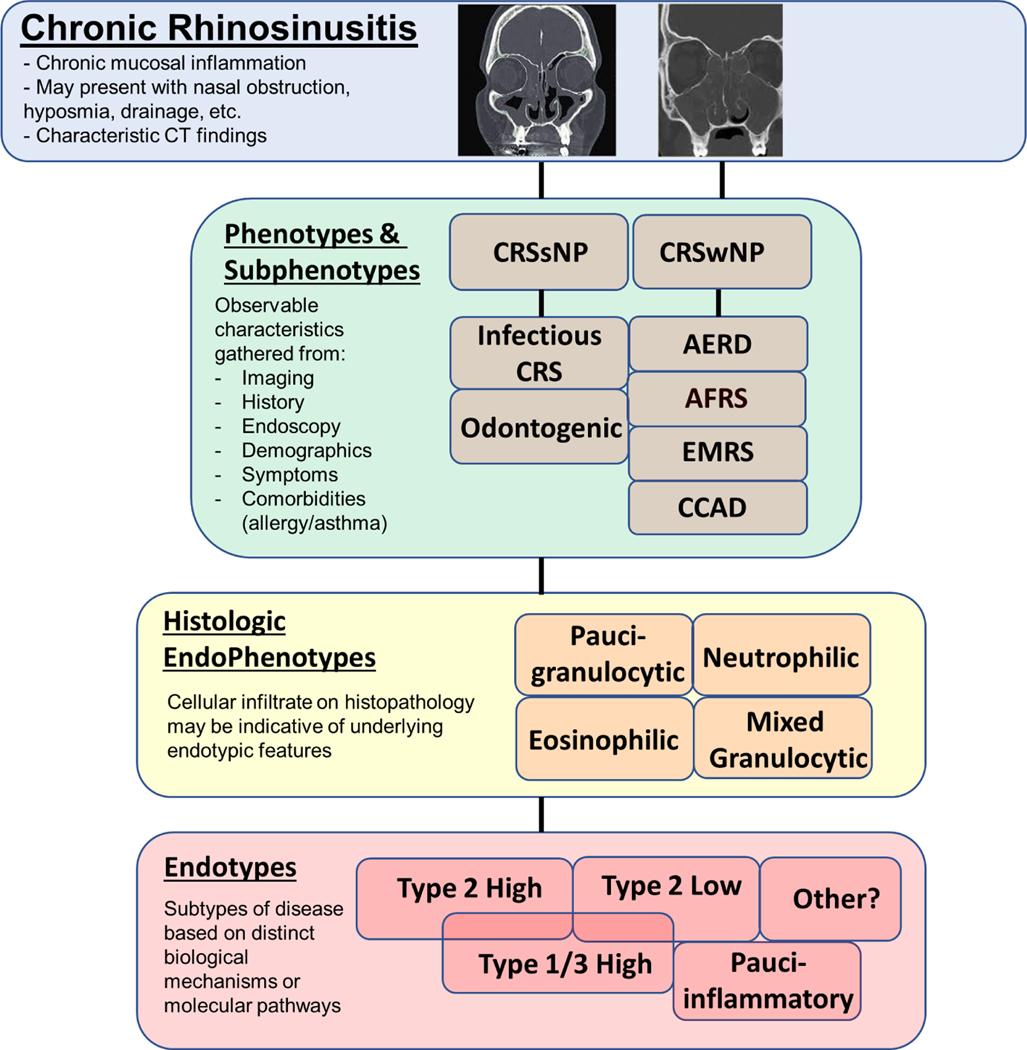
Definitions for phenotypes and endotypes are frequently confused both by clinicians and researchers. In simplest terms, phenotypic classifications are based on observable features of a disease, while endotypic classifications are based on underlying pathophysiological mechanisms of the disease (Fig. 1). Each approach has its own strengths, benefits, and limitations. CRS phenotypes have often been derived from clinical practice and experience and can incorporate a fairly diverse combination of characteristics, including patient demographics, comorbidities, endoscopic findings, clinical presentation, and patient-reported symptoms (Table 1).
Figure 1. Chronic Rhinosinusitis (CRS) Phenotypes and Endotypes.
CRS is a clinical syndrome characterized by chronic sinonasal mucosal inflammation, variable symptom presentation, and characteristic CT findings. Disease heterogeneity is evidenced by contrasting CT images in CRS patients with nasal polyps (left), without nasal polyps (middle) and AFRS (right). CRS patients can be grouped into phenotypes and subphenotypes based on observable characteristic features or endotypes based on distinct biological mechanisms or molecular pathways. Endotypic features can sometimes be inferred by tissue histology or type of cellular infiltrate. Abbreviations: AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis; CCAD, central compartment atopic disease; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; EMRS, eosinophilic mucin rhinosinusitis.
Table 1:
Overview of Chronic Rhinosinusitis Common Phenotypes and Key Studies Highlighting their Utility in Prognostication of Outcomes.
| Phenotype | Author | Year | Population and Sample Size | Study Design | Effect on Outcome |
|---|---|---|---|---|---|
| Nasal polyposis (CRSwNP) | Van Zele et al.32 | 2010 | 47 patients with CRSwNP, European population | Placebo controlled, randomized control trial | Methylprednisolone taper and doxycycline each significantly associated with decreased nasal polyp size, nasal symptoms, and markers of inflammation compared with placebo |
| Chapurin et al.26 | 2017 | Total of 17,828 patients with CRS in the United States, of which 2,363 patients had CRSwNP | Cross-sectional/epidemiologic | Increased odds (OR 4.28) of patients with CRSwNP failing medical therapy and undergoing sinus surgery compared to patients with CRSsNP | |
| Ference et al.25 | 2018 | 97,228 patients with CRS undergoing FESS in the United States between 2009–2011. 29.3% of surgeries were for patients with CRSwNP, 66.0% of patients with CRSsNP, and 4.8% for other indications | Cross-sectional/epidemiologic | Higher rates of surgery (RR 1.88) and significantly more extensive surgery for patients with CRSwNP compared to CRSsNP | |
| Bayer et al.27 | 2022 | Total of 667 patients undergoing FESS for CRS between 2012 and 2015, European cohort. 478 patients with CRSwNP | Retrospective cohort | Increased odds (OR 1.32) of patients with CRSwNP needing revision surgery compared to patients with CRSsNP | |
| Allergic Fungal Rhinosinusitis (AFRS) | Younis et al.21 | 2016 | 117 patients with AFRS (2005–2009), 2-year follow-up. Comparison of patients needing revision surgery vs not | Retrospective cohort | High proportion of AFRS patients needed revision surgery (22%) within 2 years. The presence of eosinophilic mucin was significantly associated a higher rate of revision surgery (OR 3.15) |
| Loftus et al.31 | 2020 | Subset of 467 patients with AFRS across all literature (45 papers 34,220 subjects with CRSwNP total) up to 2019 | Systematic review and meta-analysis | Significantly higher rate of surgical revision in patients with AFRS (28.7%), compared to CRSwNP overall revision rate of 18.6% | |
| Aspirin Exacerbated Respiratory Disease (AERD) | Loftus et al.31 | 2020 | Subset of 465 patients with AERD across all literature (45 papers, 34,220 subjects with CRSwNP total) up to 2019 | Systematic review and meta-analysis | Significantly higher rate of surgical revision (27.2%) compared to CRSwNP overall rate of 18.6% |
| Bayer et al.27 | 2022 | Total of 667 patients undergoing FESS for CRS between 2012 and 2015, European cohort. 16 patients had AERD | Retrospective cohort | Increased odds (OR 8.33) of patients with AERD needing revision surgery compared to patients without AERD | |
| Allergy/Atopic Disease | Robinson et al.33 | 2006 | 193 patients with CRS, New Zealand cohort | Cross sectional study | No significant difference in SNOT22, Lund-Mackay scores and rate of surgical revision between atopic and nonatopic patients |
| Wilson et al.34 | 2014 | 24 articles in English language of all available literature identified up to 2014 | Systematic review | No clear association in role of allergy in CRSwNP and CRSsNP (11 articles showing association, 7 without), poor level of evidence in the literature overall | |
| Li et al.35 | 2016 | 210 patients with CRSwNP, Chinese population | Prospective cohort study | No association between atopy status and disease severity or recurrence | |
| Central Compartment Atopic Disease (CCAD) | Steehler et al.39 | 2021 | 132 patients with CRSwNP, 38 patients with CCAD compared to non-CCAD patients (AERD, AFRS, CRSwNP NOS) | Retrospective cohort | CCAD patients demonstrated polyp recurrence (7.9%) and surgical revision (5.3%) rates lower than patients with other CRSwNP subtypes |
| Gender | Lal et al.29 | 2016 | 248 patients with CRS undergoing FESS | Retrospective cohort | Women had higher preop SNOT-22 scores prior to surgery, and within 6 months after surgery but after 1-year post-FESS, both genders showed similar symptom scores |
| Chapurin et al.26 | 2017 | Total 17,828 patients with CRS in the United States | Cross-sectional/epidemiologic | Female gender independently associated with decreased odds of undergoing sinus surgery (OR 0.82) | |
| Bayer et al.27 | 2022 | Total of 667 patients undergoing FESS for CRS between 2012 and 2015, European cohort | Retrospective cohort study | Increased odds of needing revision surgery in men (OR 1.73) compared to women with CRS | |
| Age | Crosby et al.42 | 2019 | 1,252 adult CRS patients electing to undergo FESS (2007–2018) | Retrospective cohort study | Patient younger than 50 years had a higher mean pre-ESS SNOT-22 score (44.0) compared to those of at least 50 years of age (38.9), but showed similar improvement after surgery |
| Yancey et al.30 | 2019 | 403 adults with medically refractory CRS who underwent functional endoscopic sinus surgery | Retrospective cohort study | Middle-aged patients (40–59 y/o) showed highest SNOT-22 scores, elderly patients (over 60 y/o) having the lowest. Elderly patients report smaller improvements in disease-specific and general health QOL after surgery. | |
| Holmes et al.28 | 2020 | 421 patients undergoing sinus surgery. Young adults = age 18–39 (63 patients), adults = age 40–64 (209 patients), and elderly = age 65+ (159 patients) | Retrospective cohort study | Young adults had statistically higher SNOT-22 scores (33.2) compared to adults (23.5) and elderly (25.3). Short- and long-term postoperative improvement holds across all age groups | |
| Complex/Cluster-Based Phenotypes | Soler et al. 40 | 2015 | 382 patients with CRS who had failed medical therapy. Clustering was largely determined by age, severity of patient reported outcome measures, depression and fibromyalgia | Multi-institutional, prospective study | 5 distinct clusters identified, which were predictive of medication usage and therapeutic treatment choice (medical versus surgical) |
| Adnane et al. 41 | 2017 | 131 patients with medical refractory CRS who elected FESS. Clustering methodology used nasal polyp status, gender, mucosal eosinophilia profile | Prospective cohort study | 3 district clusters identified. Cluster 1 had lowest SNOT-22 improvement after surgery in comparison with clusters 2 and 3. All patients in cluster 1 presented CRSwNP with the highest mucosal eosinophilia endotype. | |
| Lal et al. 42 | 2018 | 146 adult CRSsNP patients who underwent FESS. Unsupervised clustering methodology used patient demographics, SNOT-22 scores, CT imaging and disease comorbidities | Retrospective cohort study | 4 district clusters identified. Asthma prevalence and tissue eosinophilia were highest in the cluster with highest SNOT-22 scores. Patients with high sleep-psychosocial symptoms tended to show worsening at 6 months post-FESS |
Polyp Status
Historically, the most common approach to CRS phenotyping has been the differentiation of patients based on the presence (CRSwNP) or absence (CRSsNP) of nasal polyps. This feature is readily identifiable using nasal endoscopy or CT imaging and can be suggestive of underlying inflammatory mechanisms. In clinical practice, CRSwNP is frequently associated with obstructive symptoms, olfactory dysfunction, steroid responsiveness, and need for multiple surgeries. Conversely, CRSsNP is more commonly linked to osteomeatal complex obstruction, dental disease, and/or infection by bacterial pathogens. There was early recognition, at least in Western countries, that nasal polyps were associated with tissue and peripheral eosinophilia, and that nasal polyp tissue often contained elevated levels of type 2 inflammatory biomarkers like IL-4, IL-5, and IgE 5–7. CRSsNP was instead associated with neutrophilia and elevations in IFN-γ and IL-88. However, while polyp status can be suggestive of inflammatory endotypic features, it is not fundamentally grounded on any underlying pathophysiology, and thus has limited value in prognostication and therapeutic development. Additionally, the underlying inflammatory characteristics linked to this rigid dichotomy is now recognized as overly simplistic, with a combination of Type 1 (IFN-γ, IL-12), Type 2 (IL-4, IL-5, IL-13), and Type 3 (IL-17, IL-22) associated signatures often being present regardless of polyp status 9–11. Nonetheless, current management of CRS patients has long been driven, at least in part, by polyp status, and this differentiation does have some limited prognostic value. As an example, both topical and systemic corticosteroids can have beneficial effects in CRS, but appear to be preferentially impactful among patients with CRSwNP 12,13. Among patients with CRSwNP, other characteristics like pretreatment disease-specific quality of life (22-item Sino Nasal Outcome Test, SNOT-22) scores or comorbid asthma may be predictive of steroid responsiveness 14. Selection of other therapeutics can likewise be driven by polyp status, with a handful of studies suggesting that long-term low-dose macrolides may be more effective in CRSsNP patients. Even the use of newly developed type 2 biologic medications is largely governed by polyp status, and this characteristic is among the primary inclusion criteria for most clinical trials 15,16, and is now typically a requirement for the initiation of this class of therapeutics in clinical practice.
AERD, AFRS, EMRS, and other Subphenotypes
Several commonly accepted subphenotypes of CRS (particularly among CRSwNP) have been identified and can impact patient management. Aspirin-exacerbated respiratory disease (AERD) is a clinical syndrome that includes nasal polyposis, asthma, and sensitivity to non-steroidal anti-inflammatory drugs (NSAIDs). AERD is prevalent in up to 30% of asthmatic patients and is linked with more severe CRSwNP17 and a greater need for revision surgery18. AERD is generally associated with elevation of type 2 inflammatory mediators compared to non-AERD CRSwNP19 but also appears to have some heterogeneity with respect to inflammatory signatures19,20. Allergic fungal rhinosinusitis (AFRS) presents with often unilateral or bilateral extensive nasal polyposis and bony remodeling of the paranasal sinuses, non-invasive fungal colonization, specific IgE sensitization to fungal antigens, and presence of allergic mucin. AFRS is generally linked to profound type 2 inflammatory responses and high rates of revisions surgery18,21. Eosinophilic mucin rhinosinusitis (EMRS) was proposed as being histologically similar to AFRS except for the absence of fungal elements on histopathology22. Patients most commonly present with bilateral rather than unilateral disease. There are persistent questions with respect to this phenotypic distinction and it has been proposed that it may be reflective of low sensitivity for fungal detection with conventional Gomori methenamine silver (GMS) staining of histopathological specimens23,24. Though not well characterized, CRS presenting with comorbid allergy is often recognized as a distinct CRS phenotype, though most evidence suggests that atopic status is not directly associated with disease severity or recurrence21,25–35. Lastly, central compartment atopic disease (CCAD) is a newly described variant of CRS characterized by central sinonasal compartment polypoid changes and a high prevalence of allergic rhinitis36–38. The association of this phenotype with disease severity or prognosis is unclear with one study suggesting lower rates of revision surgery and polyp recurrence compared to other patients with nasal polyps 39. It has been hypothesized that allergy management may be especially beneficial in these particularly patients36.
Phenotypes Based on Demographics
Several studies suggest that demographic characteristics can also provide important prognostic information. Lal et al. found that women electing to proceed with endoscopic sinus surgery for CRS had worse baseline symptom scores but lower objective measures of disease than men 29,40,41, and the disparity in symptom scores was maintained after surgical intervention at most timepoints 29. Age represents an increasingly important phenotypic characteristic, given the aging world population and increased incidence of adverse events with medical and/or surgical intervention in the elderly. CRS patients of advanced age typically have lower baseline symptom burden, but also experience smaller improvements in symptom control after surgery 30,42. Most importantly, many elderly patients share endotypic features like tissue neutrophilia, bacterial colonization, and elevations of inflammatory mediators associated with the innate immune response, features that could impact their responsiveness to corticosteroids and newer targeted therapeutics 43. Despite these early studies, potential effects of demographic factors like age and gender on responses to medical intervention have not yet been well characterized.
Complex or Multi-variable Phenotypes
The above examples demonstrate the historical and contemporary importance of phenotypes in CRS management but highlight the limitations of individual phenotypic characteristics in disease prognostication. To navigate these weaknesses, Soler et al., in a multi-institutional prospective study, instead integrated multiple clinical and demographic factors to identify phenotypic subgroups in CRS patients44. Phenotypic clusters were largely determined by age, severity of patient reported outcome measures, and the presence of comorbid diagnoses such as depression and fibromyalgia. Clusters were also highly predictive of medication usage. Most importantly, this approach was later used to determine patient-reported outcomes after continued medical or surgical intervention 4. Using a similar approach, Adnane et al. performed unsupervised cluster analysis of several demographic, subjective, and objective clinical findings to identify phenotypic characteristics in a population of CRS patients undergoing endoscopic sinus surgery45. They identified three patient clusters that were predictive of disease-specific quality-of-life improvement at one year after surgery. The most discriminating factors between clusters were nasal polyp status, female sex, mucosal eosinophilia, and a history of prior sinus surgery. Finally, Lal et al used a symptom-based approach to identify phenotypically distinct clusters of CRSsNP patients, which also had distinct prognostic relevance46. These examples further highlight the continued relevance of CRS phenotypes in clinical practice and the potential ability of such approaches to inform patient decision making.
Emergence of CRS Inflammatory Endotypes
Phenotypic classifications group CRS patients with similar disease behavior or presentation, but do not incorporate differences in disease mechanism. Until recently, CRS basic and translational research has rigidly adhered to these phenotypic assignments, often in a futile attempt to link them directly with aspects of CRS pathophysiology. For example, more than 30 years ago several histopathologic studies of CRS tissue found that CRSwNP was commonly associated with elevated levels of eosinophils, while CRSsNP was commonly associated with elevated levels of neutrophils 47–50, with CRSwNP and CRSsNP sometimes referenced as ‘eosinophilic’ and ‘neutrophilic’ diseases, respectively. However, this simple dichotomy has since been disproven and it is now recognized that both CRSwNP and CRSsNP can present with eosinophilia, neutrophilia, or both, in addition to a complex infiltrate of other inflammatory cell types51–54. In a cohort of CRS patients undergoing endoscopic sinus surgery, Succar et al. found that approximately 40% of CRSwNP patients had either a neutrophilic or a mixed neutrophilic/eosinophilic infiltrate 55. Similarly, less than 20% of CRSsNP patients had neutrophilia alone. Curiously, 20% of CRS patients did not have elevated eosinophils or neutrophils. This paucigranulocytic phenotype is ill-defined, but its counterpart in asthma is associated with airway hyperresponsiveness and steroid insensitivity 56. The paucigranulocytic inflammatory phenotype may be indicative of the ‘untypeable’ endotype described by Klinger et al 57. Further complicating the roles of inflammatory phenotypes in CRS is a clear geographic divergence among patients in Western countries compared to those in Asia 58–60. This is best exemplified among Asian CRSwNP patients, who frequently lack the eosinophilia seen in many Western patients, while instead exemplifying substantial tissue neutrophilia in many cases 58.
The concept of the inflammatory endotype has only recently emerged, though many of the fundamental aspects of CRS endotyping have been incrementally introduced over the past two or more decades. The first step undoubtedly was a migration in the understanding of CRS pathophysiology from one centered on osteal obstruction and abnormal drainage, to one instead focused on chronic mucosal and locoregional tissue inflammation due to a range of etiologies 61–65. This dogmatic change increased focus on identifying inflammatory mediators and biomarkers associated with the disease. For example, important early studies found that CRSwNP was associated with elevated tissue levels of IL-5 61–63,66 and IgE 62, while CRSsNP was linked with elevated IL-8 64 and IFN-γ 5. These cytokine signatures were subsequently associated with increased numbers and activation of T cells 5, a characteristic that paved the way for subsequent understanding of CRS inflammatory heterogeneity and endotypes.
These early studies had clear limitations, chiefly that no single inflammatory mediator or biomarker was able to fully characterize individual CRS phenotypes, and that there was apparent heterogeneity in T cell polarization and patterns of cytokine expression. In a landmark study, Tomassen et al. navigated this complexity by incorporating 14 different inflammatory markers and using unsupervised statistical approaches to identify CRS disease clusters in a European population 11. These clusters subsequently became synonymous with inflammatory endotypes in what has now become a paradigm shift with respect to the prevailing view of CRS subtyping. Key findings were up to 10 unique endotypes with distinct inflammatory mechanisms and apparent correlation with phenotypic characteristics. Patients displayed a combination of type 1, type 2, and type 3 immune signatures, with the relative contributions of each subsequently being found to depend substantially on geographic differences59. Confirmatory studies in North American 9,10,43,57,67 and Asian 58,68–70 populations clearly highlighted the relevance of these putative endotypes to CRS pathophysiology and clinical care (Table 2)
Table 2:
Overview of Key Studies Establishing Chronic Rhinosinusitis Endotypes and Their Associated Disease Characteristics and Prognostic Value.
| Author | Year Published | Population and Sample Size | Endotypes Studied | Associated Prognostic Value |
|---|---|---|---|---|
| Tomassen et al.11 | 2016 | 173 CRS patients undergoing endoscopic surgery and 89 controls | 10 clusters grouped as IL-5 negative, IL-5 positive, and IL-5 high | 1. Most IL-5 negative endotypes had a CRSsNP prominence with lower incidences of asthma 2. IL-5 positive clusters tend to have a mixed phenotype 3. IL-5 high clusters were predominantly made up of CRSwNP and had a higher incidence of comorbid asthma |
| Turner et al.10 | 2018 | 90 CRS undergoing endoscopic sinus surgery | 6 clusters, 2 with low inflammation and 4 with high inflammation | 1. Low inflammation clusters were heterogenous and associated with low CT scores, good olfactory function, and longstanding improvements in SNOT-22 scores one year postoperatively 2. 2 clusters with dominant Type 2 inflammatory markers were associated with CRSwNP, comorbid asthma, and temporary improvement in SNOT-22 scores after surgery 3. 2 clusters with nondominant Type 2 inflammatory markers were associated with CRSsNP and mixed polyp statuses respectively, mild to moderate disease, less comorbid asthma, and temporary improvement in SNOT-22 scores after surgery |
| Liao et al.58 | 2018 | 246 CRS patients and 16 controls | 6 clusters | 1. High eosinophilia was associated with severe disease and high proportions of difficult-to-treat CRSwNP 2. High neutrophilic inflammation was associated with older patients with high proportions of difficult-to-treat CRSwNP while moderate neutrophilic inflammation with IL-8 was associated with severe CRSwNP and poor treatment outcomes 3. IL-10 dominant disease was associated with mild CRSwNP with good treatment outcomes |
| Stevens et al.71 | 2019 | 121 CRSsNP and 134 CRSwNP patients | 3 endotypes defined as T1, T2, or T3 dominant inflammation | 1. T1 dominant disease is heterogenous and is more common in females 2. T2 dominant disease is associated with nasal polyps, asthma, and smell loss 3. T3 dominant disease is heterogenous and is associated with increased presence of pus and purulent nasal discharge |
| Nakayama et al.70 | 2021 | 8 white CRSwNP patients from the US, 9 Japanese patients with CRSwNP born and raised in Japan, and 8 control patients | 2 CRSwNP clusters defined as non-Type 2 inflammation and eosinophilic, Type 2 dominant inflammation | 1. CRSwNP with non-Th2 inflammation is associated with lower asthma comorbidity 2. CRSwNP with eosinophilic, Type 2 dominant inflammation is associated with high asthma comorbidity |
Inflammatory Endotypes and their Relationship to Phenotypic Classifications
The disease clusters or endotypes identified using unsupervised statistical approaches should be interpreted with caution, and in many ways represent simplified modeling of the inflammatory heterogeneity of CRS. Such approaches are highly dependent upon 1) sample size and geographic location of the study population, 2) inherent bias in selection of input variables, 3) appropriate differentiation between similar groupings or clusters of patients, and 4) often subjective decisions about clustering parameters during the modelling process. Prior studies have identified a wide range of CRS endotypes, from as few as three to as many as 10, but in reality these are just artificial representations of what are likely overlapping endotypic features present in a relative continuum (Table 2). However, these representative endotypes may have important links to CRS phenotypes or disease course that could ultimately have powerful ramifications in clinical practice. Tomassen et al. found that CRS clusters were differentially enriched in patients with and without nasal polyps, and likewise varied with respect to comorbid asthma11. Other demographic and clinical variables (age, sex, allergy, aspirin sensitivity) did not vary between clusters. Subsequent work in the United States showed similar variability between endotypes with respect to CRS phenotype and asthma, but also showed significant differences in many clinical measures of disease severity (SNOT-22 score, CT score, olfactory function, prior surgery) 10. Stevens et al., in a U.S. population, characterized three putative CRS endotypes characterized by Type 1, Type 2, and Type 3 inflammatory markers, and found significant differences between endotypes in smell loss, asthma prevalence, sex, and presence of purulence 71. Collectively, these studies suggest that putative CRS endotypes share similar but overlapping immunologic characteristics regardless of 1) the geographic region of the patient population, 2) the biomarkers utilized for cluster identification, or 3) clustering methodology. Likewise, consistency between studies with respect to phenotypic and demographic characteristics within each putative endotype further suggests substantial clinical and scientific relevance.
Recent investigation into CRS endotypes has been largely exploratory and the impact of endotypic assignment on disease course remains largely hypothetical. One study to date has reported the impact of CRS endotypes on disease course using prospectively collected data 10, with endotypes being predictive of both prior surgery and postoperative improvement in SNOT-22 scores following endoscopic sinus surgery. An additional study by Liao et al. found significant differences in ‘difficult to treat’ CRS between endotypes 58, defined by failure to reach an acceptable level of disease control at one year despite adequate surgery, intranasal corticosteroid treatment, and up to two courses of antibiotics or systemic corticosteroids. Conversely, no differences in ‘difficult to treat’ cases were apparent based on phenotypic (CRSwNP vs. CRSsNP) differentiation alone, suggesting that endotypic classifications may have greater power to predict disease course when compared to more commonly used phenotypic differences. Both of these studies take an important step toward linking CRS endotypes with clinical outcomes, potentially identifying a clinical pathway whereby the inflammatory characteristics and expected outcomes of a patient can be identified prior to surgical or medical intervention. However, the limited sample sizes, lack of long-term patient follow-up, and variability in outcome measures between studies, clearly suggests that additional investigation is needed in this area.
Possible Implications for Current and Future Therapies
Recent advances in CRS phenotyping and molecular endotyping along with the development of therapeutics that target specific immune pathways have collectively created an evolving opportunity to personalize approaches to care and improve outcomes. There are many advantages inherent to CRS when compared to other inflammatory airway diseases, with perhaps the most important being ready access to the nasal cavity and paranasal sinuses that is facilitated by office-based procedures like nasal endoscopy. In many cases, biospecimens can be collected in awake patients, and both cell and mucus sampling can be performed with relative ease. Endotyping using mucus samples can be particularly advantageous as it is 1) less subject to regional tissue differences in inflammatory mediators and biomarkers, 2) minimally invasive, and 3) easily repeatable during and after therapeutic interventions. A U.S. study that utilized mucus biomarkers to identify putative CRS endotypes identified similar inflammatory disease clusters as studies that instead used tissue biopsies 10, and these clusters were found to be highly predictive of disease course. Several individual mucus biomarkers or groups of biomarkers have been found to have some prognostic value in CRS, including IL-17A 72, IL-5 and IL-13 73, cystatins 74,75, and periostin 74,75.
Despite the potential prognostic value of CRS endotypes and molecular markers, clinical care pathways are still governed largely by phenotypic status. Additionally, clinical trials designed to determine efficacy of targeted therapeutics for CRS have universally relied on disease phenotype as inclusion criteria. It can be hypothesized that enrolling patients with phenotypic (or even endotypic) classifications that are overly broad could compromise study findings, particularly for therapeutics that target specific inflammatory pathways or endotypes. Nonetheless, current practice patterns are moving rapidly toward an endotype-based approach for both selecting and evaluating therapeutic interventions.
Corticosteroids and Antibiotics
There is debatable clinical benefit of antibiotics for the treatment of CRS, stemming in part from a lack of high-quality studies. Current consensus guidelines recommend against routine use of topical and IV antibiotics for CRS and note minimal evidence to support efficacy of short (i.e. less than 3 weeks) or long (> 3 weeks) courses of non-macrolide antibiotics, regardless of polyp status. Macrolide antibiotics have anti-inflammatory characteristics including effects on cytokine secretion and neutrophil chemotaxis 76–79. A recent prospective cohort study found that low total IgE in nasal secretions was predictive of response to low-dose macrolide therapy over a 12-week period 80, while another study identified patient subgroups with low tissue eosinophilia as receiving the greatest benefit 81. These studies suggest that patients with non-Type 2 disease may be most likely to derive benefit from this class of medications. Current evidence for macrolides are mixed, with consensus guidelines suggesting them as an option for CRSsNP, as well as both neutrophil-dominant and/or steroid-insensitive CRSwNP 82. The apparent lack of substantial benefit with antibiotics may be reflective of growing scientific evidence that CRS is a chronic inflammatory disease, rather than a persistent infection. However, endotypic analysis clearly shows a subset of CRS patients with elevated markers of Type 1 and/or Type 3 inflammatory burden, which may indicate more pronounced innate immune activation by microbial pathogens. Two distinct studies in the U.S. identified inflammatory CRS endotypes characterized, in part, by endoscopic evidence of purulence or infection39,71. These findings suggest that select endotypes may benefit from antibiotic therapy, with further study clearly needed.
Surgery
Commonly accepted indications for surgery include persistent sinus disease that is refractory to medical therapy, but effective mechanisms for patient selection are poorly defined, and a subset of patients often require one or more revision procedures. Approximations of revision rates are highly dependent on adequate follow-up, but studies incorporating analysis of large population databases suggest an overall rate of approximately 15–20% after 5–10 years 83–85. Improvements in sinonasal quality-of-life and revision surgery rates are fairly similar between CRSsNP and CRSwNP, suggesting that polyp status alone may be poorly predictive of surgical success 85. However, other phenotypic classifications may be independent predictors of revision surgery, with one study reporting revision rates of 31–42% for patients with AERD or AFRS 18. Finally, Soler et al. used discriminant analysis of clinical and demographic characteristics to effectively provide prognostic information regarding surgical treatment versus continued medical management in a multi-institutional prospective study of CRS patients 4. Description of putative CRS endotypes has been fairly recent, and thus limited prospective data is available to determine whether CRS endotypes can be predictive of revision surgery or relative improvements in quality-of-life. In a U.S. study, Turner et al. found that endotype status was predictive of postoperative improvement in SNOT-22 scores at one year after surgery, while several studies have reported prognostic value for individual inflammatory biomarkers 58,74. Interpreting these studies is highly difficult, in part due to geographic differences in study populations that may be indicative of underlying variability in endotypic characteristics. Additionally, surgery itself may have impacts on endotypic classification, with small prospective studies showing a general reduction in Type 2 inflammatory mediators postoperatively 86,87, as well as ‘endotype switching’ in more than half of patients86.
Monoclonal Antibodies and Biologic Medications
Several humanized monoclonal antibody therapies are increasingly being used for CRS and represent perhaps the most paradigm-shifting approach to treatment algorithms in decades. Currently utilized biologics universally target type 2 inflammation in CRSwNP patients with or without asthma and are typically reserved for patients with severe or refractory disease that has failed standard medical or surgical interventions. However, the cost of these medications is high, and the cost-effectiveness and costutility of these promising therapeutics is yet to be fully defined 88,89.
Dupilumab is a humanized monoclonal antibody that binds the alpha subunit of the interleukin-4 receptor (IL-4Rα) and works by blocking both IL-4 and IL-13 signaling 90. In recent phase 3 studies (SINUS-24 and SINUS-52), dupilumab improved nasal polyp scores, sinonasal symptoms, and extent of disease on CT imaging in the overall CRSwNP cohort 15. The effect of dupilumab on nasal polyp score was approximately two points, while the decrease in SNOT-22 scores was comparable to that observed after endoscopic sinus surgery 91. In pooled analysis of SINUS-24 and SINUS-52, 38–54% of patients were classified as non-responders based on changes in polyp score 15, though a greater response rate was observed for secondary symptom-based endpoints like nasal congestion and sense of smell. Similar patterns have been observed with real world use, with published and anecdotal results suggesting potentially greater impacts on patient symptoms than polyp burden92,93 94. Nonetheless, trial data clearly suggests a subgroup effect, perhaps based on endotypic assignment given that all patients enrolled were similar with respect to polyp status. The magnitude of improvement for both primary and secondary endpoints were likewise similar for other subgroups, including patients with comorbid asthma and AERD.
Mepolizumab, as well as reslizumab and benralizumab, are humanized monoclonal antibodies that inhibit IL-5 signaling, a cytokine known to regulate eosinophil recruitment and survival. In the recently published phase 3 SYNAPSE study, mepolizumab met its primary endpoints (change in polyp score and nasal obstruction symptoms) at 52 weeks 95. However, the treatment effect for polyp score was a marginal 0.73 points and 50% of patients had an improvement in polyp score of less than one point. Nine percent of patients in the mepolizumab group still needed surgery within the 52-week study period and 25% required systemic steroids. These results again suggest a large number of non-responders, even among a cohort of patients with severe, refractory bilateral CRSwNP. Similar to clinical trial results for dupilumab, mepolizumab had a similar effect on primary endpoints across comorbid subgroups (asthma, AERD) 96. Treatment response was not impacted by baseline serum eosinophil counts.
Omalizumab is a monoclonal antibody that binds IgE in the blood and blocks its interaction with the high-affinity IgE receptor, resulting in inhibition of the IgE-mediated inflammatory cascade in multiple cell types. In phase 3 clinical trials (POLYP 1 and POLYP 2) omalizumab met its primary endpoints (change in nasal polyp score and nasal congestion from baseline) at 24 weeks 16. Mean change in polyp score compared to placebo was approximately one point, with 44% of patients receiving omalizumab failing to achieve an improvement greater than 1-point and 69% of patients failing to experience an improvement greater than 2-points. Patients with comborbid asthma or AERD had similar improvements in primary endpoints.
Newly developed biologic therapies clearly show great promise for CRS and other chronic inflammatory diseases, however, comparative efficacy and the ideal target population are yet to be clearly defined and the impact of different biologics is also variable between currently available therapeutics. It should be noted that patients enrolled in completed clinical trials had severe, refractory CRS with nasal polyps, thus limiting the generalizability of the findings to the wider population with CRS, or even to those with CRSwNP. Studies identifying inflammatory CRS endotypes suggests that only 15–30% of patients present with strong and uniform type 2 disease 59. This caveat is likely reflected in collective clinical trial results for biologics targeting type 2 inflammation, wherein phenotypically similar patients often have variable responses. A recent Cochrane review analysis of pooled data highlighted some persisting questions with respect to this class of therapeutics, including lack of clarity as to which patients will respond, how to choose the optimum biologic, when they should be considered, and how they may benefit patients with less severe disease 97. Additionally, there are currently no widely available targeted therapeutics for CRSsNP or for non-type 2 disease. This deficit may be highly clinically relevant given recent evidence suggesting that disease severity may be closely linked to neutrophilic and/or non-type 2 inflammation 59,98,99 or other yet to be known factors.
Knowledge Gaps, Future Research, and Conclusions
CRS is a complex inflammatory disease with a rapidly increasing selection of available therapeutic interventions. The geographic and genetic heterogeneity of the patient population and the varied and often convoluted inflammatory milieu of the disease support the need for improved and personalized approaches to management. Incorporation of disease phenotypes, endotypes, and molecular biomarkers into care decisions is beginning to transform treatment algorithms and offers promise for potential improvements in long-term outcomes. Despite encouraging advancements, many questions remain and there are substantial knowledge gaps with respect to how these tools could or should be incorporated into clinical care. For example, only a handful of studies have evaluated the potential role of phenotypes and/or endotypes as predictors of medical and/or surgical outcomes. The comparative prognostic effectiveness of phenotypes vs. endotypes is also not clear and the vast majority of outcomes research in CRS does not incorporate collection of biomarkers or other biological data that would allow for post hoc evaluation of endotypes and their potential role in disease trajectory. With respect to endotypes, the most effective biomarkers for identifying inflammatory clusters is not well established and has varied considerably between studies, and the true number of clinically relevant cluster-based endotypes is also unknown. Finally, the stability of putative endotypes remains unclear and questionable, and there is a clear lack of prospective studies that evaluate disease and biomarker trajectory among individual patients. Clinical and scientific logic would suggest that many environmental and patient-specific factors could substantially modify endotypic assignment over time, including, but not limited to age, environmental exposures, comorbid systemic or locoregional inflammatory diseases, and infection. Clarifying many of these knowledge gaps will require precise longitudinal studies that incorporate phenotypic and endotypic characteristics of each patient in a controlled setting. Further understanding of how phenotypes and endotypes affect disease trajectory and therapeutic choice will ultimately be what facilitates true personalized and precision care in CRS.
Acknowledgments
This research was supported in part by grant support from the National Institute of Allergy and Infectious Disease (R21 AI142321 to JHT) and National Institute on Aging (R01 AG065550 to JHT) Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health.
R.K. Chandra is a consultant for Optinose and Regeneron. J.H. Turner has received grant support from the NIH/National Institute of Deafness and Communication Disorders (NIDCD) and additional support from the NIH/National Institute of Allergy and Infectious Diseases (NIAID).
ABBREVIATIONS:
- AERD
aspirin-exacerbated respiratory disease
- AFRS
allergic fungal rhinosinusitis
- CCAD
central compartment atopic disease
- CRS
chronic rhinosinusitis
- CRSsNP
chronic rhinosinusitis without nasal polyps
- CRSwNP
chronic rhinosinusitis with nasal polyps
- CT
computed tomography
- IFN
interferon
- IL
interleukin
- Ig
Immunoglobulin
- IV
intravenous
- SNOT-22
22-item Sino Nasal Outcome Test
Footnotes
Disclosure of potential conflicts of interest: The remaining authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope. Sep 2014;124(9):2007–12. doi: 10.1002/lary.24630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol. Jan 2021;147(1):29–36. doi: 10.1016/j.jaci.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 3.Ta NH, Gao J, Philpott C. A systematic review to examine the relationship between objective and patient-reported outcome measures in sinonasal disorders: recommendations for use in research and clinical practice. Int Forum Allergy Rhinol. May 2021;11(5):910–923. doi: 10.1002/alr.22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler ZM, Hyer JM, Rudmik L, Ramakrishnan V, Smith TL, Schlosser RJ. Cluster analysis and prediction of treatment outcomes for chronic rhinosinusitis. J Allergy Clin Immunol. Apr 2016;137(4):1054–1062. doi: 10.1016/j.jaci.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. Nov 2006;61(11):1280–9. doi: 10.1111/j.1398-9995.2006.01225.x [DOI] [PubMed] [Google Scholar]
- 6.Hamilos DL, Leung DY, Wood R, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. Oct 1995;96(4):537–44. doi: 10.1016/s0091-6749(95)70298-9 [DOI] [PubMed] [Google Scholar]
- 7.Hamilos DL, Leung DY, Wood R, et al. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulating factor and interleukin-3. J Allergy Clin Immunol. Jul 1993;92(1 Pt 1):39–48. doi: 10.1016/0091-6749(93)90035-e [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Takahashi Y, Wataya H, et al. Mechanism of neutrophil recruitment induced by IL-8 in chronic sinusitis. J Allergy Clin Immunol. Sep 1996;98(3):659–70. doi: 10.1016/s0091-6749(96)70100-8 [DOI] [PubMed] [Google Scholar]
- 9.Tan BK, Klingler AI, Poposki JA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. Feb 2017;139(2):699–703 e7. doi: 10.1016/j.jaci.2016.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JH, Chandra RK, Li P, Bonnet K, Schlundt DG. Identification of clinically relevant chronic rhinosinusitis endotypes using cluster analysis of mucus cytokines. J Allergy Clin Immunol. May 2018;141(5):1895–1897 e7. doi: 10.1016/j.jaci.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. May 2016;137(5):1449–1456 e4. doi: 10.1016/j.jaci.2015.12.1324 [DOI] [PubMed] [Google Scholar]
- 12.Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AG. Short-course oral steroids alone for chronic rhinosinusitis. Cochrane Database Syst Rev. Apr 26 2016;4:CD011991. doi: 10.1002/14651858.CD011991.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang MT, Noel J, Ayoub NF, et al. Oral Corticosteroids Following Endoscopic Sinus Surgery for Chronic Rhinosinusitis Without Nasal Polyposis: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. May 1 2021;147(5):434–441. doi: 10.1001/jamaoto.2021.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epperson MV, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Predictors of efficacy for combination oral and topical corticosteroids to treat patients with chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. Dec 2019;9(12):1436–1442. doi: 10.1002/alr.22431 [DOI] [PubMed] [Google Scholar]
- 15.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. Nov 2 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 16.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. Sep 2020;146(3):595–605. doi: 10.1016/j.jaci.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 17.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. Mar 2015;135(3):676–81 e1. doi: 10.1016/j.jaci.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 18.Loftus CA, Soler ZM, Desiato VM, et al. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. Mar 2020;10(3):289–302. doi: 10.1002/alr.22505 [DOI] [PubMed] [Google Scholar]
- 19.Scott WC, Cahill KN, Milne GL, et al. Inflammatory heterogeneity in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. Apr 2021;147(4):1318–1328.e5. doi: 10.1016/j.jaci.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakiela B, Soja J, Sladek K, et al. Heterogeneity of lower airway inflammation in patients with NSAID-exacerbated respiratory disease. J Allergy Clin Immunol. Apr 2021;147(4):1269–1280. doi: 10.1016/j.jaci.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 21.Younis RT, Ahmed J. Predicting revision sinus surgery in allergic fungal and eosinophilic mucin chronic rhinosinusitis. Laryngoscope. Jan 2017;127(1):59–63. doi: 10.1002/lary.2624822. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. May 2000;110(5 Pt 1):799–813. doi: 10.1097/00005537-200005000-00010 [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Ghadersohi S, Kephart GM, et al. Improving the detection of fungi in eosinophilic mucin: seeing what we could not see before. Otolaryngol Head Neck Surg. Nov 2012;147(5):943–9. doi: 10.1177/0194599812451010 [DOI] [PubMed] [Google Scholar]
- 24.Ur, Rone O, Marsha T, Parpara O, Nashashib M, Grube M. Allergic fungal sinusitis and eosinophilic mucin rhinosinusitis: diagnostic criteria. J Laryngol Otol. Sep 2013;127(9):867–71. doi: 10.1017/S0022215113001680 [DOI] [PubMed] [Google Scholar]
- 25.Ference EH, Suh JD, Tan BK, Smith SS. How often is sinus surgery performed for chronic rhinosinusitis with versus without nasal polyps? Am J Rhinol Allergy. Jan 1 2018;32(1):34–39. doi: 10.2500/ajra.2018.32.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapurin N, Pynnonen MA, Roberts R, et al. CHEER National Study of Chronic Rhinosinusitis Practice Patterns: Disease Comorbidities and Factors Associated with Surgery. Otolaryngol Head Neck Surg. Apr 2017;156(4):751–756. doi: 10.1177/0194599817691476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer K, Hamidovic S, Besser G, Mueller CA, Liu DT. Factors Associated with Revision Sinus Surgery in Patients with Chronic Rhinosinusitis. J Pers Med. Jan 27 2022;12(2)doi: 10.3390/jpm12020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes T, Makary C, Unsal AA, Biddinger P, Reyes-Gelves C, Kountakis SE. How Does Age Impact Presentation and Outcomes in Chronic Rhinosinusitis? Ann Otol Rhinol Laryngol. Sep 2020;129(9):872–877. doi: 10.1177/0003489420919124 [DOI] [PubMed] [Google Scholar]
- 29.Lal D, Golisch KB, Elwell ZA, Divekar RD, Rank MA, Chang YH. Gender-specific analysis of outcomes from endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. Sep 2016;6(9):896–905. doi: 10.1002/alr.21773 [DOI] [PubMed] [Google Scholar]
- 30.Yancey KL, Lowery AS, Chandra RK, Chowdhury NI, Turner JH. Advanced age adversely affects chronic rhinosinusitis surgical outcomes. Int Forum Allergy Rhinol. Oct 2019;9(10):1125–1134. doi: 10.1002/alr.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loftus CA, Soler ZM, Koochakzadeh S, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol. Feb 2020;10(2):199–207. doi: 10.1002/alr.22487 [DOI] [PubMed] [Google Scholar]
- 32.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. May 2010;125(5):1069–1076 e4. doi: 10.1016/j.jaci.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 33.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. Nov-Dec 2006;20(6):625–8. doi: 10.2500/ajr.2006.20.2907 [DOI] [PubMed] [Google Scholar]
- 34.Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. Feb 2014;4(2):93–103. doi: 10.1002/alr.21258 [DOI] [PubMed] [Google Scholar]
- 35.Li QC, Cheng KJ, Wang F, Zhou SH. Role of atopy in chronic rhinosinusitis with nasal polyps: does an atopic condition affect the severity and recurrence of disease? J Laryngol Otol. Jul 2016;130(7):640–4. doi: 10.1017/S0022215116008112 [DOI] [PubMed] [Google Scholar]
- 36.DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. Jul 1 2017;31(4):228–234. doi: 10.2500/ajra.2017.31.4443 [DOI] [PubMed] [Google Scholar]
- 37.Marcus S, Schertzer J, Roland LT, Wise SK, Levy JM, DelGaudio JM. Central compartment atopic disease: prevalence of allergy and asthma compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. Feb 2020;10(2):183–189. doi: 10.1002/alr.22454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards TS, DelGaudio JM, Levy JM, Wise SK. A Prospective Analysis of Systemic and Local Aeroallergen Sensitivity in Central Compartment Atopic Disease. Otolaryngol Head Neck Surg. Mar 1 2022:1945998221082554. doi: 10.1177/01945998221082554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steehler AJ, Vuncannon JR, Wise SK, DelGaudio JM. Central compartment atopic disease: outcomes compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. Nov 2021;11(11):1549–1556. doi: 10.1002/alr.22819 [DOI] [PubMed] [Google Scholar]
- 40.Phillips KM, Bergmark RW, Hoehle LP, et al. Differential perception and tolerance of chronic rhinosinusitis symptoms as a confounder of gender-disparate disease burden. Int Forum Allergy Rhinol. Oct 2019;9(10):1119–1124. doi: 10.1002/alr.22390 [DOI] [PubMed] [Google Scholar]
- 41.Jurlin L, Greguric T, Baudoin T, et al. Cluster Analysis of Chronic Rhinosinusitis Suggests Gender-Based Differences. ORL J Otorhinolaryngol Relat Spec. 2019;81(1):1–9. doi: 10.1159/000492966 [DOI] [PubMed] [Google Scholar]
- 42.Crosby DL, Jones J, Palmer JN, Cohen NA, Kohanski MA, Adappa ND. Impact of age on outcomes following endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. Dec 2019;9(12):1456–1461. doi: 10.1002/alr.22444 [DOI] [PubMed] [Google Scholar]
- 43.Morse JC, Li P, Ely KA, et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J Allergy Clin Immunol. Mar 2019;143(3):990–1002 e6. doi: 10.1016/j.jaci.2018.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SoleM, HyeM, RamakrishnaV, et al. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Int Forum Allergy Rhinol. May 2015;5(5):399–407. doi: 10.1002/alr.21496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adnane C, Adouly T, Khallouk A, et al. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. Eur Arch Otorhinolaryngol. Feb 2017;274(2):879–885. doi: 10.1007/s00405-016-4315-8 [DOI] [PubMed] [Google Scholar]
- 46.Lal D, Hopkins C, Divekar RD. SNOT-22-based clusters in chronic rhinosinusitis without nasal polyposis exhibit distinct endotypic and prognostic differences. Int Forum Allergy Rhinol. Jul 2018;8(7):797–805. doi: 10.1002/alr.22101 [DOI] [PubMed] [Google Scholar]
- 47.Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. Feb 1993;91(2):616–22. doi: 10.1016/0091-6749(93)90267-j [DOI] [PubMed] [Google Scholar]
- 48.Harlin SL, Ansel DG, Lane SR, Myers J, Kephart GM, Gleich GJ. A clinical and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol. May 1988;81(5 Pt 1):867–75. doi: 10.1016/0091-6749(88)90944-x [DOI] [PubMed] [Google Scholar]
- 49.Jankowski R, Bene MC, Moneret-Vautrin AD, et al. Immunohistological characteristics of nasal polyps. A comparison with healthy mucosa and chronic sinusitis. Rhinol Suppl. 1989;8:51–8. [PubMed] [Google Scholar]
- 50.Fokkens WJ, Holm AF, Rijntjes E, Mulder PG, Vroom TM. Characterization and quantification of cellular infiltrates in nasal mucosa of patients with grass pollen allergy, non-allergic patients with nasal polyps and controls. Int Arch Allergy Appl Immunol. 1990;93(1):66–72. doi: 10.1159/000235281 [DOI] [PubMed] [Google Scholar]
- 51.Demoly P, Crampette L, Mondain M, et al. Assessment of inflammation in noninfectious chronic maxillary sinusitis. J Allergy Clin Immunol. Jul 1994;94(1):95–108. doi: 10.1016/0091-6749(94)90076-0 [DOI] [PubMed] [Google Scholar]
- 52.Poposki JA, Klingler AI, Stevens WW, et al. Elevation of activated neutrophils in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. Dec 22 2021;doi: 10.1016/j.jaci.2021.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delemarre T, Bochner BS, Simon HU, Bachert C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J Allergy Clin Immunol. Aug 2021;148(2):327–335. doi: 10.1016/j.jaci.2021.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delemarre T, Holtappels G, De Ruyck N, et al. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. Jan 2021;147(1):179–188 e2. doi: 10.1016/j.jaci.2020.08.036 [DOI] [PubMed] [Google Scholar]
- 55.Succar EF, Li P, Ely KA, Chowdhury NI, Chandra RK, Turner JH. Neutrophils are underrecognized contributors to inflammatory burden and quality of life in chronic rhinosinusitis. Allergy. Mar 2020;75(3):713–716. doi: 10.1111/all.14071 [DOI] [PubMed] [Google Scholar]
- 56.TlibO, Panettier RA Jr., Paucigranulocytic asthma: Uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. Apr 2019;143(4):1287–1294. doi: 10.1016/j.jaci.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klingler AI, Stevens WW, Tan BK, et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol. Apr 2021;147(4):1306–1317. doi: 10.1016/j.jaci.2020.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao B, Liu JX, Li ZY, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. Jul 2018;73(7):1459–1469. doi: 10.1111/all.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. Nov 2016;138(5):1344–1353. doi: 10.1016/j.jaci.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Gevaert E, Lou H, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. Nov 2017;140(5):1230–1239. doi: 10.1016/j.jaci.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 61.Bachert C, Wagenmann M, Rudack C, et al. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy. Jan 1998;53(1):2–13. doi: 10.1111/j.1398-9995.1998.tb03767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. Sep 2005;35(9):1186–91. doi: 10.1111/j.13652222.2005.02316.x [DOI] [PubMed] [Google Scholar]
- 63.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. Jun 1997;99(6 Pt 1):837–42. doi: 10.1016/s0091-6749(97)80019-x [DOI] [PubMed] [Google Scholar]
- 64.Rhyoo C, Sanders SP, Leopold DA, Proud D. Sinus mucosal IL-8 gene expression in chronic rhinosinusitis. J Allergy Clin Immunol. Mar 1999;103(3 Pt 1):395–400. doi: 10.1016/s0091-6749(99)70462-8 [DOI] [PubMed] [Google Scholar]
- 65.Schleimer RP, Kato A, Peters A, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. May 1 2009;6(3):288–94. doi: 10.1513/pats.200808-088RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP). Clin Exp Allergy. Sep 1998;28(9):1145–52. doi: 10.1046/j.1365-2222.1998.00380.x [DOI] [PubMed] [Google Scholar]
- 67.Soler ZM, Schlosser RJ, Bodner TE, et al. Endotyping chronic rhinosinusitis based on olfactory cleft mucus biomarkers. J Allergy Clin Immunol. May 2021;147(5):1732–1741 e1. doi: 10.1016/j.jaci.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei B, Liu F, Zhang J, et al. Multivariate analysis of inflammatory endotypes in recurrent nasal polyposis in a Chinese population. Rhinology. Sep 1 2018;56(3):216–226. doi: 10.4193/Rhin17.240 [DOI] [PubMed] [Google Scholar]
- 69.Ruan JW, Zhao JF, Li XL, et al. Characterizing the Neutrophilic Inflammation in Chronic Rhinosinusitis With Nasal Polyps. Front Cell Dev Biol. 2021;9:793073. doi: 10.3389/fcell.2021.793073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakayama T, Lee IT, Le W, et al. Inflammatory molecular endotypes of nasal polyps derived from Caucasian and Japanese populations. J Allergy Clin Immunol. Dec 1 2021;doi: 10.1016/j.jaci.2021.11.017 [DOI] [PubMed] [Google Scholar]
- 71.Stevens WW, Peters AT, Tan BK, et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J Allergy Clin Immunol Pract. Nov - Dec 2019;7(8):2812–2820 e3. doi: 10.1016/j.jaip.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chapurin N, Li P, Chandra RK, Turner JH, Chowdhury NI. Elevated mucus interleukin-17A levels are associated with increased prior sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. Feb 2021;11(2):120–127. doi: 10.1002/alr.22652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner JH, Li P, Chandra RK. Mucus T helper 2 biomarkers predict chronic rhinosinusitis disease severity and prior surgical intervention. Int Forum Allergy Rhinol. Oct 2018;8(10):1175–1183. doi: 10.1002/alr.22160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu D, Yan B, Wang Y, Wang C, Zhang L. Prognostic and pharmacologic value of cystatin SN for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. Aug 2021;148(2):450–460. doi: 10.1016/j.jaci.2021.01.036 [DOI] [PubMed] [Google Scholar]
- 75.MuelleK, WendleO, NocerA, et al. Escalation in mucus cystatin 2, pappalysin-A, and periostin levels over time predict need for recurrent surgery in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. Oct 2019;9(10):1212–1219. doi: 10.1002/alr.22407 [DOI] [PubMed] [Google Scholar]
- 76.Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, place-bocontrolled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. Feb 2006;116(2):189–93. doi: 10.1097/01.mlg.0000191560.53555.08 [DOI] [PubMed] [Google Scholar]
- 77.Fujita K, Shimizu T, Majima Y, Sakakura Y. Effects of macrolides on interleukin-8 secretion from human nasal epithelial cells. Eur Arch Otorhinolaryngol. 2000;257(4):199–204. doi: 10.1007/s004050050222 [DOI] [PubMed] [Google Scholar]
- 78.Suzuki H, Shimomura A, Ikeda K, Furukawa M, Oshima T, Takasaka T. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope. Dec 1997;107(12 Pt 1):1661–6. doi: 10.1097/00005537-199712000-00016 [DOI] [PubMed] [Google Scholar]
- 79.Suzuki H, Asada Y, Ikeda K, Oshima T, Takasaka T. Inhibitory effect of erythromycin on interleukin-8 secretion from exudative cells in the nasal discharge of patients with chronic sinusitis. Laryngoscope. Mar 1999;109(3):407–10. doi: 10.1097/00005537-199903000-00012 [DOI] [PubMed] [Google Scholar]
- 80.Seresirikachorn K, Kerr SJ, Aeumjaturapat S, et al. Predictive factors for identifying macrolide responder in treating chronic rhinosinusitis. Rhinology. Jun 1 2021;59(3):284–291. doi: 10.4193/Rhin20.649 [DOI] [PubMed] [Google Scholar]
- 81.Lin CF, Wang MC, Merton AT, et al. Add-on effect of clarithromycin to oral steroids as post-operative therapy for chronic rhinosinusitis with nasal polyps: a randomised controlled trial. Rhinology. Dec 1 2020;58(6):550–558. doi: 10.4193/Rhin19.325 [DOI] [PubMed] [Google Scholar]
- 82.Gurrola J 2nd, Borish L. Chronic rhinosinusitis: Endotypes, biomarkers, and treatment response. J Allergy Clin Immunol. Dec 2017;140(6):1499–1508. doi: 10.1016/j.jaci.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 83.Luu K, Tellez PA, Chadha NK. The effectiveness of mitomycin C in Otolaryngology procedures: A systematic review. Clin Otolaryngol. Jan 2022;47(1):1–13. doi: 10.1111/coa.13839 [DOI] [PubMed] [Google Scholar]
- 84.Smith KA, Orlandi RR, Oakley G, Meeks H, Curtin K, Alt JA. Long-term revision rates for endoscopic sinus surgery. Int Forum Allergy Rhinol. Apr 2019;9(4):402–408. doi: 10.1002/alr.22264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hopkins C, Slack R, Lund V, Brown P, Copley L, Browne J. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. Dec 2009;119(12):2459–65. doi: 10.1002/lary.20653 [DOI] [PubMed] [Google Scholar]
- 86.Yancey KL, Li P, Huang LC, et al. Longitudinal stability of chronic rhinosinusitis endotypes. Clin Exp Allergy. Dec 2019;49(12):1637–1640. doi: 10.1111/cea.13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jonstam K, Alsharif S, Bogaert S, et al. Extent of inflammation in severe nasal polyposis and effect of sinus surgery on inflammation. Allergy. Mar 2021;76(3):933–936. doi: 10.1111/all.14550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yong M, Wu YQ, Howlett J, Ballreich J, Walgama E, Thamboo A. Cost-effectiveness analysis comparing dupilumab and aspirin desensitization therapy for chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. Dec 2021;11(12):1626–1636. doi: 10.1002/alr.22865 [DOI] [PubMed] [Google Scholar]
- 89.Scangas GA, Wu AW, Ting JY, et al. Cost Utility Analysis of Dupilumab Versus Endoscopic Sinus Surgery for Chronic Rhinosinusitis With Nasal Polyps. Laryngoscope. Jan 2021;131(1):E26–E33. doi: 10.1002/lary.28648 [DOI] [PubMed] [Google Scholar]
- 90.Bachert C, Mannent L, Naclerio RM, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA. Feb 2 2016;315(5):469–79. doi: 10.1001/jama.2015.19330 [DOI] [PubMed] [Google Scholar]
- 91.Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. Apr 2019;74(4):743–752. doi: 10.1111/all.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wangberg H, Spierling Bagsic SR, Osuna L, White AA. Appraisal of the Real-World Effectiveness of Biologic Therapies in Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. Feb 2022;10(2):478–484 e3. doi: 10.1016/j.jaip.2021.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.PateB, KudlatA, GuA, et al. Impact of type 2 targeting biologics on acute exacerbations of chronic rhinosinusitis. Allergy Asthma Proc. Sep 1 2021;42(5):417–424. doi: 10.2500/aap.2021.42.210058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bavaro N, Gakpo D, Mittal A, Bensko JC, Laidlaw TM, Buchheit KM. Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Ralpha in a real-world setting. J Allergy Clin Immunol Pract. Jul 2021;9(7):2910–2912 e1. doi: 10.1016/j.jaip.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. Oct 2021;9(10):1141–1153. doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 96.Bachert C, Sousa AR, Han JK, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps: treatment efficacy by comorbidity and blood eosinophil count. J Allergy Clin Immunol. Jan 7 2022;doi: 10.1016/j.jaci.2021.10.040 [DOI] [PubMed] [Google Scholar]
- 97.Chong LY, Piromchai P, Sharp S, et al. Biologics for chronic rhinosinusitis. Cochrane Database Syst Rev. Mar 12 2021;3:CD013513. doi: 10.1002/14651858.CD013513.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Bruaene N, Perez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. Jun 2008;121(6):1435–41, 1441 e1–3. doi: 10.1016/j.jaci.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 99.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. Sep 2009;124(3):478–84, 484 e1–2. doi: 10.1016/j.jaci.2009.05.017 [DOI] [PubMed] [Google Scholar]