Abstract
Therapeutic hypothermia improves neurological outcome after out-of-hospital cardiac arrest or neonatal hypoxic-ischemic injury. Although supported by preclinical evidence, therapeutic hypothermia for acute stroke remains under study. In the Intravascular Cooling in the Treatment of Stroke (ICTuS) trial, awake stroke patients were successfully cooled using an endovascular cooling catheter and a novel antishivering regimen. In the ICTuS-L study, the combination of endovascular hypothermia and thrombolysis proved feasible; while hypothermia was associated with no increased risk of bleeding complications, there was an increased association with pneumonia. Despite efforts to expedite, cooling began on average six-hours after stroke onset. We designed a novel Phase 2/3 trial to further test the safety of combined thrombolysis and endovascular hypothermia and to determine if the combination shows superiority compared with thrombolysis alone. ICTuS 2 (n = 400) will assess four hypotheses, and if milestones are met, ICTuS 3 (n = 1200) will begin as a seamless continuation for a total sample of 1600 patients. The ICTuS 2 milestones include (1) target temperature reached within six-hours of symptom onset; (2) no increased risk of pneumonia; (3) no increase in signs/symptoms of fluid overload due to chilled saline infusions; and (4) sufficient recruitment to complete the trial on time. The ICTuS 2/3 protocol contains novel features – based on the previous ICTuS and ICTuS-L trials – designed to achieve these milestones. Innovations include scrupulous pneumonia surveillance, intravenous chilled saline immediately after randomization to induce rapid cooling, and a requirement for catheter placement within two-hours of thrombolysis. An Investigational Device Exemption has been obtained and an initial group of sites initiated.
Keywords: acute stroke therapy, cerebral infarction, clinical trial, hypothermia, methodology, neuroprotection
Introduction
Body temperature powerfully influences outcome after experimental focal ischemia and acute stroke in humans. Hyperthermia adversely affects outcome after stroke (1–5), and a body temperature of less than 36·5°C is associated with improved outcome and lower mortality (6). Therapeutic hypothermia has emerged as a potentially promising modality in the treatment of acute ischemic stroke, but to date has not been proven effective.
In experimental models, therapeutic hypothermia reduces infarct volume in acute stroke (recently reviewed (7,8)). The mechanisms of protection during hypothermia are multiple and include reduced cerebral metabolism, diminished release of excitatory amino acids, stabilization of the blood–brain barrier and neuronal membranes, reduced release of matrix metalloproteinases, diminished inflammatory infiltration into ischemic zones, and decreased cerebral edema (9–13). Hypothermia applied very soon after stroke is presumably targeting the early ischemic cascade, while later hypothermia would be expected to mostly reduce cerebral edema (14).
In patients with severe middle cerebral artery stroke, using surface cooling to 33°C for 48 to 72 hours followed by gradual rewarming over 24 hours can reduce intracranial pressures from 21 to 13 mmHg (15). In a trial of endovascular cooling for up to 48 hours after stroke, hypothermia appeared to reduce cerebral edema (16). Use of hypothermia for cerebral edema management remains unproven, but anecdotally has been used in patients admitted to intensive care units with intracerebral hemorrhage, subarachnoid hemorrhage, and traumatic brain injury (17,18).
In contrast, urgent cooling for neuroprotection from acute ischemic stroke remains under development. In the COOL AID study, a cooling blanket was used to treat patients who had received thrombolytic therapy after acute ischemic stroke, resulting in a trend toward improved outcome (19). To gain greater cooling power and better temperature control, we devised a stroke cooling protocol using an endovascular catheter (20). In a pilot study of patients who did not receive thrombolysis (Intravascular Cooling in the Treatment of Stroke; ICTuS), we showed that acute stroke patients could be cooled effectively and that, although they were awake, their shivering could be controlled with a combination of intravenous meperidine, oral buspirone, and surface counterwarming (21). To assure safety of the central venous line placement and endovascular cooling (ICTuS-L), we studied 58 patients eligible for thrombolysis, half of whom were randomized to hypothermia (22). We observed no increased risk of peripheral bleeding, intracerebral hemorrhage, catheter-related complications, sepsis, pancreatitis, coagulopathy, or liver dysfunction in ICTuS-L. We observed transient oliguria in the hypothermia treatment group that reversed completely (23).
We did, however, detect an increase in the risk of pneumonia in the patients who received cooling in ICTuS-L. The study may have been flawed by case ascertainment bias, as the control group did not receive the same close clinical monitoring as the hypothermia patients, and no strict criteria or definitions were set for the diagnosis of pneumonia in the trial.
To determine if hypothermia provides neuroprotective benefit, we designed a randomized, controlled clinical trial of hypothermia combined with thrombolysis versus thrombolysis alone. Before proceeding to a definitive Phase 3 trial, however, it was deemed necessary to test several remaining questions in a smaller Phase 2 trial. To improve efficiency and speed development of this potential therapy, we designed a Phase 2/3 protocol that – if the Phase 2 milestones are met – will allow a combined analysis of patients from the Phase 2 and Phase 3 trials. Here we describe the rationale and design of this novel clinical trial.
Development of endovascular cooling
Early trials of therapeutic hypothermia utilized a variety of surface cooling techniques, including cooling blankets, ice packs, ice water lavage, and alcohol rubs, typically in intubated and paralyzed patients (24–26). The time to reach target temperature was long (15,19), and surface methods did not allow precise temperature control or prevention of rebound hyperthermia (19,27). Surface cooling requires sedation and frequently the use of paralytic agents to prevent and combat shivering. Paralysis and sedation are easily applied in patients who have suffered cardiac arrest who are comatose and intubated. Patients who have suffered acute ischemic stroke, in contrast, are rarely comatose and do not receive endotracheal intubation except when pneumonia or other secondary pulmonary complications arise. Intubation and paralysis to implement therapeutic hypothermia would likely introduce additional complexity and risk, and render serial neurological examination difficult. Therefore, we chose to develop an endovascular approach to therapeutic hypothermia in acute ischemic stroke, intending to treat awake patients without the use of neuromuscular blockade. This approach was made possible by the development of powerful cooling catheters that dwell in a large central vein and allowed us to include skin warming as part of an aggressive antishivering protocol.
In the COOL AID 2 trial, a flexible catheter was inserted into the inferior vena cava, and iced saline was circulated through three balloons to induce therapeutic hypothermia in patients with stroke or intracranial hemorrhage (28,29). Target temperature was reached in most patients, although shivering was encountered. In a similar trial, we used a metallic cooling catheter coated with a covalently bonded anticoagulant; grooves in the shaft of the catheter caused flowing blood to swirl past the catheter, promoting mixing and greater heat transfer (21). In the pilot trial ICTuS, such catheters were placed and left in situ for up to 48 hours after stroke onset. After removal, venous ultrasound confirmed no increased incidence of deep venous thrombosis. During ICTuS, an antishivering protocol was developed that eventually included intravenous meperidine infusion, oral buspirone, and surface counter-warming. Buspirone has been noted by others to provide synergistic shivering suppression when combined with meperidine (30). Although other agents besides meperidine are useful in suppression shivering, we chose to continue development of our protocol with meperidine because it is widely available, relatively inexpensive, and exhibits antishivering potential at relatively low plasma levels (31,32). Surface counterwarming activates cutaneous temperature receptors and provides additional centrally mediated shivering suppression (33,34).
We next sought to determine if endovascular catheterization could be performed safely in patients treated with thrombolytic therapy (20). The ICTuS-L trial was also designed to lengthen the therapeutic time window for thrombolysis, but this goal was mooted by the publication of SITS-ISTR, a pooled analysis of trials using longer time windows, and ECASS 3, all of which supported the use of intravenous recombinant tissue plasminogen activator (rt-PA) as late as 4·5 hours after stroke onset (35,36). Comparing 28 patients in whom cooling was attempted against 30 patients in whom no cooling was attempted, we showed several key findings in ICTuS-L: most importantly, there were no bleeding complications noted in patients who underwent central venous catheterization after thrombolytic therapy. The median time from the end of the rt-PA infusion to catheterization was three-hours, and no catheter placement was attempted prior to 30 minutes after the end of rt-PA infusion. The rate of asymptomatic or symptomatic intracerebral hemorrhage was not different between the cooled and not-cooled patients. Extensive laboratory monitoring showed transient and not clinically significant rises in serum creatinine, amylase, alanine transaminase, and aspartate transaminase; these findings resolved in all patients after hypothermia was completed. Coagulation parameters did not change during hypothermia. While there was no increased incidence of urinary tract infection or sepsis, there was an increase in the detection of pneumonia in the treated group, although there was no impact on three-month clinical outcome. As only the hypothermia patients were monitored continuously by study personnel at the bedside, there was a possible ascertainment bias toward finding adverse events in the cooled patients. Nevertheless, there remains the possibility that either cooling or the antishivering regimen, or both, increased the risk of pneumonia.
The determinants of pneumonia risk in the ICTuS-L study population remain unclear. Although sputum and blood cultures did not provide definitive guidance, the majority of cases appeared related to aspiration. The study team was not using a precise definition or guidelines for the diagnosis of pneumonia, however, and there may not have been systematic search for pneumonia in all cases. Given that other infections were not increased, it appears unlikely that a systemic immunosuppressive effect caused the rise in pneumonia rate (37–39). The most likely cause for an increase in lung infections, therefore, may be reduced airway protection and dysphagia after stroke or vomiting related to the meperidine. The ICTuS 2 protocol contains important measures, focused on lung function and aspiration prevention, to prevent pneumonia: the head of the bed will be elevated and gastric contents will be drained using a nasogastric tube to reduce aspiration risk; all study patients will undergo surveillance for respiratory compromise to reduce ascertainment bias; and Centers for Disease Control definitions of pneumonia will be used to improve diagnostic reliability. While prophylactic antibiotics are not mandated – the literature supporting prophylaxis remains ambiguous – patients with suspected pneumonia will be promptly started on broad-spectrum antibiotics pending culture results. We expect these measures to reduce pneumonia risk, compared with ICTuS-L, and this risk reduction is a key milestone between Phase 2 and Phase 3 of the ICTuS 2/3 trial.
In addition to assessing safety, the ICTuS-L trial also confirmed the cooling efficacy of the endovascular cooling catheter and the antishivering regimen. Not all patients reached target temperature, however, and we examined a number of variables known to impact cooling rate or effectiveness. Greater weight (and especially body surface area) significantly delayed cooling and made it more difficult to maintain temperature at target, despite the use of a more powerful catheter in larger patients. The antishivering regimen appeared to reduce shivering effectively in those patients in whom the protocol was followed; omitting part of the regimen impaired cooling rate and control. Older patients cooled more quickly, after adjusting for body weight, but age was less critical than body weight in determining cooling rate. We noted that in some patients, target temperature could not be reached even with greater cooling power and full adherence to the antishivering regimen (34). In fact, in an effort to gain greater cooling, occasionally the meperidine infusion was increased to levels that caused respiratory suppression and other complications. As pushing the antishivering therapy to toxicity failed to yield better cooling, we modified the protocol to allow ‘permissive hypothermia’: patients will be cooled to a target temperature of 33°C, but if after full application of the antishivering regimen, the patient does not reach target, the patient will be permitted to remain at the previously achieved temperature without increasing the meperidine to levels causing respiratory suppression. Using permissive hypothermia will result in a population of patients at various final target temperatures, likely centered around 34·5°C. Such a heterogeneous endpoint is desirable because we expect permissive hypothermia to contribute to a reduction in meperidine-related pneumonia risk; at this time there is no scientific justification for preferring deeper levels of hypothermia.
In the ICTuS-L pilot study, we did not attempt central venous catheterization until 30 minutes after the end of intravenous (IV) rt-PA, and we allowed up to three-hours from the end of IV rt-PA to begin catheterization. This approach yielded a median time from stroke onset to cooling initiation of over six-hours. As preclinical data suggest that early cooling is more effective, we sought ways to initiate hypothermia very soon after stroke onset and reduced the allowed time from IV rt-PA to cooling catheter insertion to a mean of one-hour (maximum two-hours) per study center in ICTuS 2/3. The simplest and most widely available cooling method is intravenous infusion of chilled saline. After cardiac arrest, large volumes of 4°C saline are infused without complications to achieve target temperatures before placement of cooling devices. Chilled saline can be infused safely during thrombolysis and may allow more time to prepare the patient for venous catheterization. On the other hand, stroke patients tend to be older than patients with cardiac arrest, and large fluid volumes may not be tolerated as well. In ICTuS 2, therefore, we will test the hypothesis that volume loading with chilled saline will help reduce the time to reach target temperature without causing complications.
Evolution of the ICTuS 2/3 trial
Following ICTuS-L, several options for further development of endovascular hypothermia were considered. As there is no evidence that one target temperature should be preferred over another or one treatment duration over another, we considered a Phase 2 trial to compare 33°C against 35°C and 12 hours against 24 hours of cooling. A Phase 2 comparison of endovascular and surface cooling methods was considered as well. After consultation with the funding groups and an expert panel of advisors, the decision was made to attempt a definitive efficacy demonstration using a traditional Phase 3 design. As there remained a question regarding safety, a preliminary Phase 2 experience was embedded into the clinical trial design, yielding a Phase 2/3 design. While the definitive trial sample, following our power calculations, will include 1600 patients, the initial 400 patients will constitute a Phase 2 trial. If the Phase 2 milestones are achieved without creating a need to modify the protocol significantly, the data obtained from these 400 patients will be combined with those obtained from the final 1200 patients to yield a full sample. The milestones for ICTuS 2 are:
Target temperature will be reached within six-hours. We expect to see a majority of patients at target temperature within six-hours of symptom onset. Target temperature is defined as 33°C. Operationally, the patient is considered at target when the core temperature is 33.x, that is, less than 34·0°C.
Pneumonia rate not significantly higher than in the control group. We expect to see no significant increase in pneumonia risk in cooled patients compared with normothermic patients.
Saline bolus given during thrombolytic therapy does not cause signs or symptoms of volume overload. Patients randomized to hypothermia will receive a loading dose of 4°C saline and patients randomized to normothermia will receive a loading dose of room temperature saline. We will examine all patients for signs/symptoms of fluid overload. We expect to see no increased incidence of cardiovascular serious adverse events in the hypothermia group compared with the normothermia group.
Study-wide recruitment sufficient to complete the trial on time. The rate of patient accrual must be at least 0·4 patients per site per month to allow completion of the combined Phase 2/3 trial in a reasonable time frame.
For the ICTuS 2/3 trial, an endovascular cooling method was selected for three reasons. First, based on ICTuS-L, there were considerable prior data regarding safety and feasibility. Second, while newer surface methods have not been tested yet, direct comparisons of older surface cooling methods suggested less cooling power and less control of the target temperature, compared with endovascular cooling. Third, the ICTuS and ICTuS-L study results suggested that the antishivering regimen effectively controlled shivering without neuromuscular blockade; it remains uncertain if shivering can be controlled during surface cooling in awake stroke patients even when newer surface cooling methods such as hypothermia pads are used.
We chose a target temperature of 33°C and a planned 24-h treatment duration for ICTuS 2/3 based on the previous safety and feasibility data. While it remains an open question, preclinical data tend to suggest that 33°C may be more effective than 35°C. Further, our previous experience suggests that it may not be possible to reproducibly target 33°C versus 35°C; final achieved temperatures in ICTuS-L ranged from 33°C to 35°C, and the ‘permissive hypothermia’ approach we developed is based on the observation that finer precision may not be possible in awake patients. If neuroprotective efficacy can be demonstrated using the chosen parameters in ICTuS 2/3, follow-up studies may examine such questions as optimum depth and duration of cooling.
Statistical analysis plan
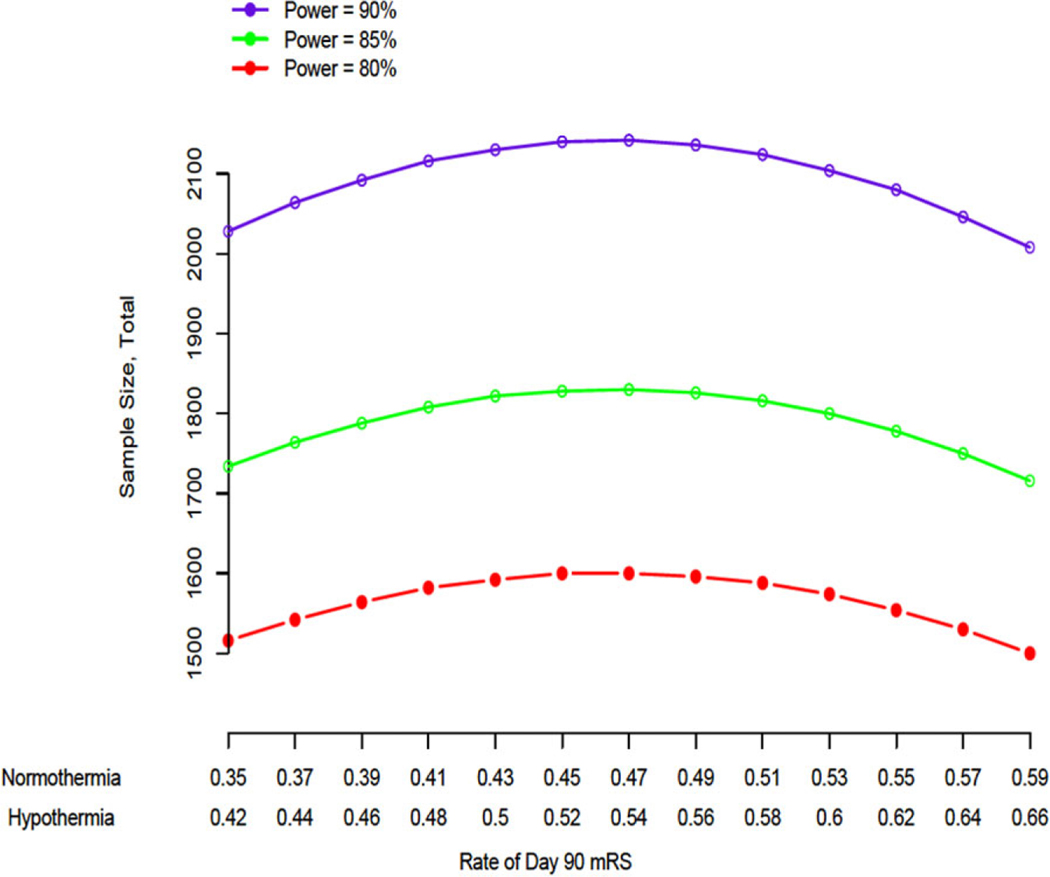
The ICTuS 2/3 trial statistical plan is based on several assumptions. We chose a standard primary outcome measure: the proportion of patients achieving a modified Rankin score of 0 or 1 by three-months after stroke. While more powerful outcome measures are available, these were found to be unacceptable to regulatory authorities; these more powerful statistical approaches will be used as secondary outcomes (Table 1). Using the ECASS 3 trial data, we estimated there would be a positive response rate in the normothermia group of 47%. This assumption is likely an overestimate, as the ECASS 3 study included patients with milder deficits [National Institutes of Health Stroke Scale (NIHSS) score less than 7] than we intend to enroll. We chose to err on the side of over-powering the ICTuS 2/3 trial because several previous clinical trials failed, in part owing to insufficient sample size. We chose a 7% absolute treatment response rate because this was the effect size noted in ECASS 3 and because recent neuroprotective trials (SAINT 2, NEST 3) were powered to detect a 7% absolute response rate after expert consultation, focus groups with treating neurologists, and discussion with regulatory authorities. As shown in Fig. 1, with these assumptions, an 80% power and an alpha of 0·05, we require 800 patients per treatment group. We then had to account for the widely recognized immediate treatment response to intravenous rt-PA. We modeled the potential loss to ‘early response’ by selecting patients from the treated group of the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA for Acute Stroke Trial who matched our intended study population (NIHSS score 7 to 20, age less than 80). In the NINDS study sample, approximately 20% of patients showed an NIHSS score of 0 at two-hours following rt-PA, suggesting the ‘early response rate’ would be as high as 20%. Therefore, we inflated our intended sample size by 20% of 800 to 1000 per group. Patients who respond to intravenous rt-PA prior to catheter insertion (or within two-hours of rt-PA infusion in the normothermia group) are considered ‘early responders’ and will be segregated in the database. Early responders will have received the fluid loading bolus and therefore will be considered with the intention-to-treat analysis. A separate per-protocol analysis, however, will examine only those patients who do not show early response. The projected early response rate of 20% is an overestimate, as the NINDS trial included patients in the 0- to 90-min time-to-treatment cohort who showed a higher likelihood of early response; we included this assumed rate of 20% to err on the side of over-powering the trial.
Table 1.
Secondary analyses: the secondary efficacy outcomes for both phase 2 alone and the combined phase 2/3 analysis
| Shift analysis of 90-day mRS (40). |
| NIHSS and Barthel, as well as global odds ratio test. |
| Modified efficacy analysis, excluding early responders, of the three-month mRS, dichotomized 0,1 and using the Cochran–Mantel–Haenszel test, stratified by study site. |
| Per-protocol analysis of the three-month mRS, dichotomized 0,1 and using the Cochran–Mantel–Haenszel test, stratified by study site, comparing patients who were randomized into Group 1 with patients in whom cooling below 35°C was achieved within six-hours from stroke onset. |
| Early responders analysis (‘normothermia’ early responders versus the ‘hypothermia’ early responders) of 36-h NIHSS. The average and percentage of subjects with a four-point improvement or score of 0 or 1 will be compared between the two groups. For patients who die prior to 36 hours, the baseline NIHSS score will be imputed as the 36-h NIHSS score. |
| 24-h AUC34. The area-under-the-curve measurement allows confirmation that the patient received a sufficient dose of cooling during the entire 24-h cooling period (34). Secondary efficacy analyses will be adjusted for 24-h AUC34. |
| 24-h AUC35. Will be done as above. This measurement may allow us to detect a positive effect of mild hypothermia. |
| 36-h edema volume on computed-tomographic scan. This serves as an early activity marker of the effectiveness of therapy and was shown to be significantly altered by hypothermia in ICTuS (16). If no edema effect is seen at the end of Phase 2, the steering committee may choose to eliminate this measurement from the Phase 3 protocol. |
mRS, Modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; AUC34, Area Under the Curve 34°C; AUC35, Area Under the Curve 35°C.
Fig. 1.
The power curves represent the total sample size required to detect an absolute difference of 7% in the proportion of parents achieving a modified Rankin (mRS) score of 0 or 1 by three-months after stroke between the hypothermia and normothermia treatment groups. Sample sizes were calculated over a range of expected outcome rates in the normothermia group and power. Power analysis was conducted using a two-sided binomial test of proportions with a normal approximation and an overall Type I error rate of 5%. mRS, Modified Rankin Scale score.
Given the possibility that the ICTuS 2/3 trial is over-powered, and given the time and financial cost of the trial, two interim analyses are planned. After the 400 patients are enrolled in ICTuS 2, there will be a futility analysis and an interim efficacy analysis. Another futility analysis and interim efficacy analysis are planned after 800 patients. Efficacy boundaries are estimated using a Lan–DeMets (O’Brien–Fleming boundary) alpha spending function (41). Stopping boundaries for futility are estimated using a conditional probability for success of 20% using the Lan–Simon–Halperin method (40).
Several decisions were made based on contemporaneous standards of care that may change during the course of the ICTuS 2/3 trial. The time window for thrombolysis recognized by the U.S. Food and Drug Administration is three-hours, despite European Medicines Agency approval and voluntary professional society endorsement of a 4·5-h window. A longer time window is allowed at European sites during the ICTuS 2/3 trial. Similarly, intra-arterial mechanical embolectomy is not approved for stroke therapy but is widely used for ‘clot retrieval’. Voluntary professional societies have widely endorsed intra-arterial mechanical embolectomy for acute ischemic stroke, and many experts believe patients with large clot burdens should be so treated (42–44). From the standpoint of neuroprotection, early recanalization is better, regardless of technique, and hypothermia appears to protect best when combined with early recanalization (45). Whether to allow patients with intra-arterial stroke treatment into neuroprotection trials remains a difficult decision for investigators and regulatory authorities.
Clinical trial protocol
The ICTuS 2/3 trial is a controlled, prospective, randomized trial designed to investigate the superiority of a combination of therapeutic hypothermia with thrombolysis compared with thrombolysis alone in patients presenting with acute ischemic stroke.
Inclusion and exclusion criteria
Male and female patients presenting with acute stroke symptoms will be enrolled in the study based on the specific criteria shown in Table 2. These criteria are based on our experience in ICTuS and ICTuS-L and have been modified only slightly. After ICTuS, we lowered the upper age limit to 80 from 85 after we encountered difficulties with study procedures in the older patients. After ICTuS-L, we decided to modify the allowed range of NIHSS scores to 7 to 20, given recent data showing that above NIHSS score of 20, even thrombolysis is less likely to benefit than in milder strokes (47). Further, we hope to reduce the likelihood of enrolling a patient who will go on to develop malignant edema and require aggressive treatment such as hemicraniectomy. We added an exclusion criterion based on posterior fossa presentations, as we have little prior experience treating strokes in such locations with hypothermia. Also, brainstem or cerebellar lesions are more likely to impair consciousness, which would make it difficult to titrate the meperidine infusion. We added an exclusion for lacunar syndromes because preclinical data address neuroprotective effects of hypothermia on the cortex, but we remain open to the notion that neuroprotection may benefit white matter injuries in the primate brain (48,49).
Table 2.
ICTuS 2/3 inclusion and exclusion criteria
| Inclusion criteria |
| Age 22 to 82 inclusive |
| Patient receiving intravenous rt-PA using standard guidelines |
| NIHSS score ≥ 7 and ≤ 20 (right brain) or ≤ 24 (left brain) at the time of randomization |
| Prestroke modified Rankin score of 0–1 |
| Able to begin endovascular phase of hypothermia within two-hours of rt-PA completion |
| Written informed consent, signed and dated by the patient (or patient’s authorized representative) |
| Exclusion criteria |
| Etiology other than ischemic stroke |
| Item 1a on NIHSS > 1 at the time of randomization |
| Clinical symptoms consistent with brainstem or cerebellar stroke |
| Classic lacunar syndrome with imaging confirmation of small deep ischemia, but randomization will not be delayed for neuroimaging other than initial scan to exclude hemorrhage |
| Known contraindications to hypothermia, such as known hematologic dyscrasias that affect thrombosis (cryoglobulinemia, sickle cell disease, serum cold agglutinins), or vasospastic disorders such as Raynaud’s or thromboangiitis obliterans |
| Known comorbid conditions that are likely to complicate therapy in the opinion of the investigator, e.g., Heart failure (New York Heart Association class III and IV) (46) |
| Uncompensated arrhythmia |
| Severe liver disease |
| History of pelvic or abdominal mass likely to compress inferior vena cava Inferior vena cava filters |
| HIV positive |
| Clinically active hypo- or hyperthyroidism |
| Renal insufficiency likely to impair meperidine clearance |
| Chronic ethanol abuse |
| Pregnancy (all women of child-bearing potential must have a negative pregnancy test, urine or blood, prior to therapy) |
| Medical conditions likely to interfere with patient assessment |
| Known allergy to meperidine or buspirone |
| Currently taking monoamine oxidase inhibitors or used them within previous 14 days |
| Life expectancy less than six-months |
| Not likely to be available for long-term follow-up |
| Use or planned use of intra-arterial thrombolysis, mechanical clot removal, or other experimental or approved acute therapy for this stroke event |
| Chest radiograph or clinical presentation suggestive of pneumonia at baseline or clinically significant pulmonary edema at baseline |
| Temperature upon admission greater than or equal to 38°C |
rt-PA, recombinant tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale.
Therapeutic hypothermia protocol
The study will be approved by the local institutional review board or ethics committee at each trial site. All study personnel who will perform consent or study enrollments must complete six-hours of training on the protocol, good clinical practice, and proper use of the endovascular catheter and control equipment. Site personnel will be trained in person by one of the trial principal investigators. If any site personnel cannot attend the in-person training, they will view a prepared set of instructional videos and participate in a phone call with one of the principal investigators to assure thorough understanding. Local physicians who will insert the endovascular catheter need not be study personnel, but they must participate in direct hands-on training under the guidance of approved instructors.
No study procedures will occur prior to intravenous rt-PA therapy initiation. After the rt-PA bolus and during the ensuing infusion, consent and prestudy procedures may begin. The Accutrol™ temperature control catheter, made by Philips/Innercool of San Diego, CA, USA, is an endovascular cooling device that utilizes a continuous, closed-circuit circulation of a chilled solution through a relatively flexible metallic heat-exchange element that, when positioned optimally within a patient, sits within the infra-diaphragmatic portion of the inferior vena cava. The element is part of a catheter that is connected to a programmable external device console containing a heat-exchange bath that cools and recirculates the saline returning from the catheter (20). Venous core blood is cooled as it mixes and flows past the chilled element on its return to the heart. The console utilizes feedback from an intravascular thermistor (thermometer) that is built into the catheter in order to precisely and rapidly cool core blood to a programmed target temperature.
After a screening evaluation that includes a complete blood count, liver enzymes and chemistries, urine pregnancy, an electrocardiogram, and cranial computed tomography, a patient randomized to undergo therapeutic hypothermia will be pretreated with 30 mg dose of buspirone orally or by nasogastric tube. A fluid loading bolus will be administered intravenously. An initial plan to base the bolus infusion on weight proved infeasible; all patients will receive 2000 ml as fast as possible. In patients randomized to hypothermia, the saline will be chilled to 4°C and administered as rapidly as possible using an infusion pump and an ultra-short connecting tube to minimize warming. The intravenous site should be located as proximal as possible and should use an 18g- or larger-bore needle. Patients not randomized to hypothermia will receive an equal amount of room temperature saline.
Patients randomized to hypothermia will have a central venous sheath placed using standard sterile technique, followed by insertion of the Accutrol catheter over a guide wire. In a patient weighing less than or equal to 90 kg, a 10·7-French catheter will be used; in a patient weighing more than 90 kg, or in whom the investigator suspects body habitus may preclude effective cooling, a 14-French version is used. The patient is given a loading dose of meperidine 1 mg/kg (maximum 100 mg) over 10 mins intravenously, and cooling is initiated with a target temperature of 33°C programmed into the console. The catheter is secured in place after its position is confirmed by a kidney, ureter, and bladder X-ray or ultrasound (20).
Hypothermia maintenance in the awake patient
As cooling is initiated, the patient is started simultaneously on a meperidine infusion at 30 mg/h intravenously. To reduce input from dermal thermoreceptors, surface counterwarming will be applied using a convective blanket. During ICTuS-L, attempts to influence shivering by manipulating the blanket setting failed. Higher settings defeat the endovascular cooling, while lower settings do not prevent shivering. The optimum strategy, therefore, is to set the blanket on the ‘medium’ setting (about 36·5° to 39·5°C) and leave that setting unchanged until the rewarming phase. Therapeutic hypothermia at a core temperature of 33°C, or at a reset target temperature if needed is maintained for a total of 24 h from initiation of the cooling procedure. The patient is monitored for shivering using a validated scale: 0 for no shivering; 1 for facial or masticatory fasciculations (most often the first manifestation); 2 for shivering in the periphery; and 3 for uncontrolled rigors (33). If shivering is detected, the patient is given a bolus of meperidine (10 to 25 mg) intravenously, followed by an increase in the meperidine drip rate by 5 mg/h. If meperidine-related sedation or respiratory depression is noted, the infusion rate is decreased. To mitigate narcotic-related nausea or vomiting, the patient is kept NPO (nothing per os), and a nasogastric tube is inserted and attached to continuous low wall-suction. Throughout the cooling period, the patient receives 15 mg dose of buspirone every eight-hours. If the core temperature cannot be maintained or decreased without significant shivering, the console target temperature is increased by 0·5°C to allow permissive hypothermia at whatever temperature can be achieved without respiratory compromise. Core blood temperature is directly assessed by means of a thermistor that is built into the endovascular catheter.
Rewarming
After 24 hours of therapeutic hypothermia induction and maintenance, the patient begins a controlled rewarming phase over 12 hours to a target temperature of 36·5°C. After 12 hours of rewarming, the cooling console is turned off and the catheter and introducer sheath are removed in the standard manner for removal of vascular introducer sheaths and catheters. During this phase, the meperidine drip is carefully reduced, and when tolerable, the warming blanket is removed.
Pneumonia and ICH monitoring
At present, there is no accepted biomarker that reliably predicts which acute stroke patients are at greatest risk for pneumonia. Previous data suggest that in some stroke patients, a cerebral immune suppression syndrome predisposes to infections. A widely accepted biomarker for early stress is the high sensitivity C-reactive protein (hs-CRP) (50). While hs-CRP is generally elevated on admission in all stroke patients, some patients show considerable increase in hs-CRP level by 24 hours after stroke (51). Those patients who suffered pneumonia after stroke showed the greatest increase by 24 hours, generally greater than a three-fold increase in hs-CRP. To test the hypothesis that comparing 24-h hs-CRP to baseline hs-CRP may predict pneumonia risk, we will collect blood for hs-CRP at baseline and 24 hours. An investigator will examine every patient daily (regardless of group assignment) and assess for any signs of pneumonia. While daily chest radiographs and empiric antibiotics are not protocol-mandated, the investigator is encouraged to confirm any suspicious physical findings with a radiograph and initiate empiric antibiotics while waiting for cultures. A computed-tomographic or magnetic resonance imaging brain scan will be obtained at 36 hours after stroke in all patients to assess for hemorrhagic transformation.
Consent is obtained in person by a certified investigator from the patient or responsible family member or both. After consent, the patient is randomized via a web-based interactive data management system. A block randomization is used, stratified by study site. Minimal data is required prior to randomization; data entry is facilitated by a web-based, interactive system with quality screens for data validity and consistency. Monitors from the coordinating centers perform on-site review of the entire care record after the first patient is treated with hypothermia. After that, all primary endpoints and serious adverse events are monitored ‘remotely’ by centralized medical record review. During the trial, an additional 10% of cases will be selected randomly for on-site monitoring of the entire case.
All adverse events are reviewed by an internal medical monitor. Inconsistencies in the data report are resolved with the site, and a preliminary judgment of severity and attribution is made. The event is then sent to an external safety monitor. If the external monitor agrees with the site and the internal monitor, the event is finalized and confirmed in the database. If there is a disagreement, a conference call is held with the site to reach consensus. All serious adverse events are reported to an external, independent Data and Safety Monitoring Board that also receives semiannual reports.
Discussion
Over the course of previous trials, we developed a protocol to induce therapeutic hypothermia in awake, nonparalyzed patients presenting with acute stroke (21,22). The approach uses an endovascular cooling catheter to induce therapeutic hypothermia, and a combination of pharmacological treatment and surface counterwarming to suppress shivering. Meperidine and buspirone interact synergistically to alter thermoregulation in humans and lower the shivering threshold (30). The efficacy and use of meperidine to suppress shivering has been well described (31,52–54). During ICTuS-L, this antishivering regimen proved remarkably effective when used correctly, and it will be implemented in ICTuS 2 without modification. To minimize respiratory suppression and possible aspiration pneumonia, we refined the protocol to allow ‘permissive hypothermia’ – patients are cooled only to the depth they tolerate without excessive meperidine, as judged by level of consciousness and respiratory rate.
A controlled trial of therapeutic hypothermia is warranted – despite the overwhelming evidence of effect in preclinical experimental studies and demonstrated efficacy in cardiac arrest patients – because equipoise remains concerning therapeutic hypothermia in adult stroke patients. Preclinical studies typically utilize young subjects free of comorbid conditions such as diabetes and hypertension. Cardiac arrest produces global cerebral ischemia with little proven penumbra; there is considerable and diffuse reperfusion with possible secondary injury. Stroke patients, on average, are 10 years older than patients suffering cardiac arrest. Further, the region of ischemia is focal, and there may be large areas of penumbral flow. Reperfusion affects a smaller portion of the brain. Despite isolated and anecdotal reports of the use of therapeutic hypothermia for ischemic stroke, there is no weight of evidence or persuasive clinical experience to justify its use until a properly powered, randomized, controlled clinical trial demonstrates safety and efficacy. A very similar trial of hypothermia is planned in Europe, the EuroHYP-1 trial (41,55). The authors of ICTuS 2/3 and those of EuroHYP-1 have coordinated data collection and plan a pooled analysis after the conclusion of both trials (personal communication, M. Macleod).
Conclusions
During ICTuS and ICTuS-L, we developed a reliable method for the induction of therapeutic hypothermia in awake stroke patients. We demonstrated feasibility. The technique may be safe, but a question of possible pneumonia risk remains to be answered. We devised a definitive, properly powered, randomized clinical trial with an embedded Phase 2 experience that addresses several milestones. If the milestones are passed, there will be a seamless transition to the Phase 3 trial, allowing for a combined analysis of the Phase 2 and Phase 3 data that will constitute the largest trial of therapeutic hypothermia yet attempted and one of the largest neuroprotective trials ever mounted.
Footnotes
Conflict of interest: None declared.
References
- 1.Castillo J, Martinez F, Leira R, Prieto JM, Lema M, Noya M. Mortality and morbidity of acute cerebral infarction related to temperature and basal analytic parameters. Cerebrovasc Dis 1994; 4:66–71. [Google Scholar]
- 2.Dietrich WD, Busto R, Valdes I, Loor Y. Effects of normothermic versus mild hyperthemic forebrain ischemia in rats. Stroke 1990; 21:1318–25. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Chopp M, Welch KM. Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artery occlusion in the rat. Neurology 1991; 41:1133–5. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma 1992; 9(Suppl. 2):S475–85. [PubMed] [Google Scholar]
- 5.Wang Y, Lim LL, Levi C, Heller RF, Fisher J. Influence of admission body temperature on stroke mortality. Stroke 2000; 31:404–9. [DOI] [PubMed] [Google Scholar]
- 6.Kammersgaard LP, Jorgensen HS, Rungby JA et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke 2002; 33:1759–62. [DOI] [PubMed] [Google Scholar]
- 7.Lyden PD, Krieger D, Yenari M, Dietrich WD. Therapeutic hypothermia for acute stroke. Int J Stroke 2006; 1:9–19. [DOI] [PubMed] [Google Scholar]
- 8.van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130(Pt 12):3063–74. [DOI] [PubMed] [Google Scholar]
- 9.Maher J, Hachinski V. Hypothermia as a potential treatment for cerebral ischemia. Cerebrovasc Brain Metab Rev 1993; 5:277–300. [PubMed] [Google Scholar]
- 10.Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev 1992; 4:189–225. [PubMed] [Google Scholar]
- 11.Morikawa E, Ginsberg MD, Dietrich WD et al. The significance of brain temperature in focal cerebral ischemia: histopathological consequences of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 1992; 12:380–9. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–8. [DOI] [PubMed] [Google Scholar]
- 13.Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–7. [DOI] [PubMed] [Google Scholar]
- 14.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22:391–7. [DOI] [PubMed] [Google Scholar]
- 15.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998; 29:2461–6. [DOI] [PubMed] [Google Scholar]
- 16.Guluma KZ, Oh H, Yu SW, Meyer BC, Rapp K, Lyden PD. Effect of endovascular hypothermia on acute ischemic edema: morphometric analysis of the ICTuS trial. Neurocrit Care 2008; 8:42–7. [DOI] [PubMed] [Google Scholar]
- 17.Kollmar R, Juettler E, Huttner HB et al. Cooling in intracerebral hemorrhage (CINCH) trial: protocol of a randomized German-Austrian clinical trial. Int J Stroke 2012; 7:168–72. [DOI] [PubMed] [Google Scholar]
- 18.Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005; 33:2744–51. [DOI] [PubMed] [Google Scholar]
- 19.Krieger D, De Georgia M, Abou-Chebl A et al. Cooling for Acute Ischemic Brain Damage (COOL AID). Stroke 2001; 32:1847–54. [DOI] [PubMed] [Google Scholar]
- 20.Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med 2006; 13:820–7. [DOI] [PubMed] [Google Scholar]
- 21.Lyden PD, Allgren RL, Ng K et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis 2005; 14:107–14. [DOI] [PubMed] [Google Scholar]
- 22.Hemmen TM, Raman R, Guluma KZ et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke 2010; 41:2265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guluma KZ, Liu L, Hemmen TM et al. Therapeutic hypothermia is associated with a decrease in urine output in acute stroke patients. Resuscitation 2010; 81:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemmen TM, Lyden PD. Induced hypothermia for acute stroke. Stroke 2007; 38(2 Suppl.):794–9. [DOI] [PubMed] [Google Scholar]
- 25.Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke 2010; 41(10 Suppl.):S72–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klassman L. Therapeutic hypothermia in acute stroke. J Neurosci Nurs 2011; 43:94–103. [DOI] [PubMed] [Google Scholar]
- 27.Felberg RA, Krieger D, Chuang R et al. Hypothermia after cardiac arrest. Feasibility and safety of an external cooling protocol. Circulation 2001; 104:1799–804. [DOI] [PubMed] [Google Scholar]
- 28.Georgiadis D, Schwarz S, Kollmar R, Schwab S. Endovascular cooling for moderate hypothermia in patients with acute stroke: first results of a novel approach. Stroke 2001; 32:2550–3. [DOI] [PubMed] [Google Scholar]
- 29.Abou-Chebl A, DeGeorgia MA, Andrefsky JC, Krieger DW. Technical refinements and drawbacks of a surface cooling technique for the treatment of severe acute ischemic stroke. Neurocrit Care 2004; 1:131–43. [DOI] [PubMed] [Google Scholar]
- 30.Mokhtarani M, Mahgoub AN, Morioka N et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg 2001; 93:1233–9. [DOI] [PubMed] [Google Scholar]
- 31.Doufas AG, Lin CM, Suleman MI et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke 2003; 34:1218–23. [DOI] [PubMed] [Google Scholar]
- 32.Kimberger O, Ali SZ, Markstaller M et al. Meperidine and skin surface warming additively reduce the shivering threshold: a volunteer study. Crit Care 2007; 11:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badjatia N, Strongilis E, Prescutti M et al. Metabolic benefits of surface counter warming during therapeutic temperature modulation. Crit Care Med 2009; 37:1893–7. [DOI] [PubMed] [Google Scholar]
- 34.Lyden P, Ernstrom K, Cruz-Flores S et al. Determinants of effective cooling during endovascular hypothermia. Neurocrit Care 2012; 16:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hacke W, Kaste M, Bluhmki E et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N EnglJ Med 2008; 359:1317–29. [DOI] [PubMed] [Google Scholar]
- 36.Wahlgren N, Ahmed N, Davalos A et al. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372:1303–9. [DOI] [PubMed] [Google Scholar]
- 37.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 2002; 15:355–60. [DOI] [PubMed] [Google Scholar]
- 38.Meisel C, Prass K, Braun J et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke 2004; 35:2–6. [DOI] [PubMed] [Google Scholar]
- 39.Harms H, Prass K, Meisel C et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE 2008; 3:e2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology 2009; 72:1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macleod MR, Petersson J, Norrving B et al. Hypothermia for stroke: call to action 2010. Int J Stroke 2010; 5:489–92. [DOI] [PubMed] [Google Scholar]
- 42.Thomassen L, Bakke SJ. Endovascular reperfusion therapy in acute ischaemic stroke. Acta Neurol Scand Suppl 2007; 187:22–9. [DOI] [PubMed] [Google Scholar]
- 43.Smith WS, Sung G, Saver J et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008; 39:1205–12. [DOI] [PubMed] [Google Scholar]
- 44.Lin R, Vora N, Zaidi S et al. Mechanical approaches combined with intra-arterial pharmacological therapy are associated with higher recanalization rates than either intervention alone in revascularization of acute carotid terminus occlusion. Stroke 2009; 40:2092–7. [DOI] [PubMed] [Google Scholar]
- 45.Hemmen TM, Lyden PD. Multimodal neuroprotective therapy with induced hypothermia after ischemic stroke. Stroke 2008; 40(3 Suppl.):S126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels. Nomenclature and Criteria for Diagnosis, 6th edn. Boston, Little, Brown and Co., 1964:114. [Google Scholar]
- 47.Mishra NK, Lyden P, Grotta JC, Lees KR. Thrombolysis is associated with consistent functional improvement across baseline stroke severity: a comparison of outcomes in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010; 41:2612–7. [DOI] [PubMed] [Google Scholar]
- 48.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res 1998; 792:105–13. [DOI] [PubMed] [Google Scholar]
- 49.Murphy BD, Fox AJ, Lee DH et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 2008; 247:818–25. [DOI] [PubMed] [Google Scholar]
- 50.Di Napoli M, Schwaninger M, Cappelli R et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP pooling project members. Stroke 2005; 36:1316–29. [DOI] [PubMed] [Google Scholar]
- 51.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke 2001; 32:133–8. [DOI] [PubMed] [Google Scholar]
- 52.Kurz A, Ikeda T, Sessler DI et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology 1997; 86:1046–54. [DOI] [PubMed] [Google Scholar]
- 53.Alfonsi P, Sessler DI, Du Manoir B. The effects of meperidine and sufentanil on the shivering threshold in postoperative patients. Anesthesiology 1998; 89:43–8. [DOI] [PubMed] [Google Scholar]
- 54.Froehler MT, Ovbiagele B. Therapeutic hypothermia for acute ischemic stroke. Expert Rev Cardiovasc Ther 2010; 8:593–603. [DOI] [PubMed] [Google Scholar]
- 55.Kollmar R, Gebhardt B, Schwab S. [EuroHYP-1 trial: EU-funded therapy study on the effectiveness of mild therapeutic hypothermia for acute ischemic stroke]. Nervenarzt 2012; 83:1252–9. [DOI] [PubMed] [Google Scholar]