Abstract
Plasmodium berghei sporozoites delivered by mosquito bite were more infectious to outbred CD-1 mice than were sporozoites delivered by intravenous inoculation. The route of challenge also affected vaccine efficacy. In view of these findings and the fact that mosquito bites are the natural mode of sporozoite delivery, infectious mosquito bites should be considered the challenge protocol of choice for sporozoite vaccine efficacy trials.
There is continued interest in the development of preerythrocytic malaria vaccines. The efficacy of a preerythrocytic vaccine relies on the outcome of challenge with live sporozoites. Two different routes of sporozoite challenge have been used: intravenous (i.v.) injection of sporozoites and infectious mosquito bites. Both methods have practical advantages and disadvantages. For example, although infectious mosquito bite is the natural mode of sporozoite delivery, the dose cannot be controlled. In contrast, sporozoite dose can be predetermined with i.v. inoculation, even though it is not the natural mode of sporozoite infection. We were interested in examining the qualitative difference in the infectivity of sporozoites that have been isolated from mosquito salivary glands and injected directly into the bloodstream of a host versus the infectivity of sporozoites that are delivered by infectious mosquito bite. More importantly, we wanted to know whether either of these routes of infection could determine the outcome of a sporozoite vaccine trial. We chose a relatively refractory model system, namely, Plasmodium berghei sporozoite-induced infection in mice (23, 51), in order to detect differences in sporozoite infectivity between these two methods of sporozoite challenge more readily.
Female outbred CD-1 mice weighing 20 to 25 g and the ANKA clone or the parent NK65 isolate of P. berghei were used throughout the experiments. To produce sporozoites, 4- to 7-day-old, nulliparous Anopheles stephensi (Dutch strain) were fed on gametocytemic mice and maintained thereafter at 21°C for 18 to 21 days. The parasite infection status within mosquito cages was monitored at 8 to 11 days by examining 5 to 10 mosquito midguts for oocysts.
Comparative infectivity of sporozoites to naive mice.
For infectious mosquito bites, mice were anesthetized and individually exposed to the bites of between 1 and 10 sporozoite-infected mosquitoes. After ca. 30 min, mosquitoes were examined for the presence or absence of blood in the gut. Absence of blood implied no feeding and, in those instances where mosquito probing was not monitored throughout the exposure period, such transmission attempts were not included in the data set. Mosquitoes that either blood fed or were known to have inserted their mouthparts and probed were dissected, and salivary glands were squashed under a coverslip and examined with phase-contrast microscopy for the presence or absence of sporozoites. Only transmission attempts in which mosquitoes had detectable sporozoite gland infections were included in the data set. To quantify the infectivity of mosquito bites, groups of 25 mice each were exposed to increased numbers of infectious mosquito bites, ranging from 1 to 10 bites per mouse. After 4 to 9 days, blood smears were taken, fixed in methanol, Giemsa stained, and examined for the presence or absence of blood-stage parasites. A minimum of three infectious bites from P. berghei-infected A. stephensi mosquitoes were required to produce blood-stage infections in naive CD-1 mice (Table 1). Essentially half of the mice bitten by four mosquitoes acquired blood-stage infection (50% infective dose [ID50] ≈ four bites), and all mice bitten by five or more mosquitoes acquired infections. The quantitative infectivity of mosquito bites to outbred CD-1 mice was compared to two previously published findings on the quantitative infectivity of gland-dissected sporozoites injected i.v. into CD-1 mice (21, 40). When the data from these studies were plotted on a log-probit scale, the estimated ID50s for i.v. inoculated sporozoites to CD-1 mice ranged from 1,700 (21) to 11,250 sporozoites (40).
TABLE 1.
Infectivity of P. berghei sporozoites to nonimmunized CD-1 outbred mice when sporozoites were delivered by infectious mosquito bite
| No. of mosquito bites | % Infected (n = 25 mice) |
|---|---|
| 1 | 0 |
| 2 | 0 |
| 3 | 8 |
| 4 | 48 |
| 5 | 100 |
| 6 | 100 |
| 7 | 100 |
| 8 | 100 |
| 9 | 100 |
| 10 | 100 |
Passive immunizations.
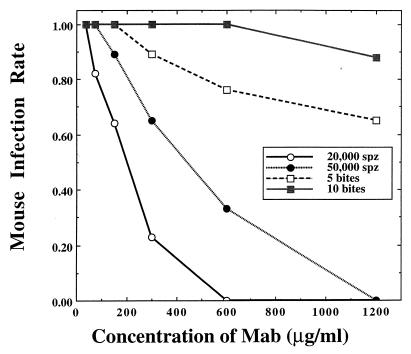
Passive transfer studies were conducted with two monoclonal antibodies (MAb Pb 3.28.1 and MAb Pb 3.213), both specific to the circumsporozoite (CS) protein of P. berghei. Twofold dilutions of MAbs were inoculated i.v. into six groups of 5 to 10 mice each. Mice were challenged with sporozoites 30 min after passive immunization. Sporozoite challenges consisted of either i.v. inoculation of sporozoites (20,000 or 50,000 sporozoites per animal) or infectious mosquito bites (5 or 10 bites per animal). The outcomes of sporozoite challenges were assessed by observing the presence or absence of blood-stage parasites in Giemsa-stained blood smears taken 4 to 9 days after challenge. There were no significant differences between the effects of the two MAbs within their respective challenge regimes (χ2 = 1.31; degrees of freedom [df] = 1; P > 0.05). For simplicity of presentation, the data for the MAbs at each concentration were pooled. Mice that were passively immunized with MAbs against the P. berghei CS protein were more easily protected against challenge from i.v. inoculated sporozoites than from challenge by infectious mosquito bite (Fig. 1). At the highest MAb concentration tested (1,200 μg/ml), all of the passively immunized mice were protected (i.e., failed to become infected) when challenged i.v. with 20,000 and 50,000 sporozoites. Less than half of similarly immunized mice were protected against sporozoites delivered by infectious mosquito bite, and blood-stage infections occurred in 65% (11 of 17) and 88% (15 of 17) of immunized mice exposed to the bites of 5 and 10 mosquitoes, respectively. Significant deviations in immunization efficacy between the two routes of challenge (bite versus i.v. inoculation) occurred at MAb concentrations of ≥300 μg/ml (χ2 = 6.09; df = 1; P < 0.05) and ≥150 μg/ml (χ2 = 6.86; df = 1; P < 0.05) for the high sporozoite challenge (50,000 sporozoites versus 10 bites) and the low sporozoite challenge (20,000 sporozoites versus 5 bites), respectively.
FIG. 1.
Comparative infectivity of P. berghei sporozoites (spz) to immunized CD-1 outbred mice when challenge sporozoites were delivered by either infectious mosquito bite or syringe inoculation into mouse tail vein. Mice were passively immunized with an i.v. injection of dilutions of MAbs Pb 3.28.1 and Pb 3.213 30 min prior to challenge. Blood smears were taken 4 to 9 days after challenge to detect blood-stage parasites.
Active immunization.
Mice were immunized with either irradiated sporozoites or a synthetic peptide based on the P. berghei CS protein. With the former, cages containing ca. 200 sporozoite-infected mosquitoes were irradiated with 12,000 rads from a cesium-137 source. Irradiated mosquitoes were then allowed to feed on anesthetized mice. Mice were boosted once every 2 weeks with a fresh batch of irradiated, sporozoite-infected mosquitoes, for a total of five boosts. With the latter, mice were immunized with a 16-amino-acid peptide, DPAPPNANDPAPPN (D-16-N), containing two tandemly repeated octapeptides from the repeat region of the P. berghei CS protein, conjugated to keyhole limpet hemocyanin (KLH) (1:5, wt/wt) (Peninsula Laboratories, Belmont, Calif.). The immunization procedure followed the protocol described previously (13). Briefly, 50 μg of peptide was emulsified in Freund’s complete adjuvant and administered in 0.15-ml doses, intraperitoneally. Up to six boosts were given two weeks apart by using 25 μg of peptide emulsified in Freund’s incomplete adjuvant. Control mice consisted of either naive mice or mice immunized with 50 μg of KLH in Freund’s complete adjuvant. Antibody titers were monitored by indirect immunofluorescence assay (IFA) on P. berghei sporozoites or by enzyme-linked immunosorbent assay on pB1tet32, a recombinant fusion protein derived from the CS protein of P. berghei (13, 54). Two weeks after their last boost, immunized mice were challenged with sporozoites. Sporozoite challenges consisted of either i.v. inoculation of sporozoites (20,000 or 50,000 sporozoites per animal) or infectious mosquito bites (5 or 10 bites per animal). The outcomes of sporozoite challenges were assessed by observing the presence or absence of blood-stage parasites in Giemsa-stained blood smears taken 4 to 9 days after challenge. Active immunizations of 40 mice with either irradiated sporozoites (IFA titer ≥1:1,000) or synthetic peptide (IFA titer ≥1:3,200) provided complete protective immunity (i.e., 0% infected) against i.v. inoculation of 20,000 and 50,000 P. berghei sporozoites (Table 2). Immunization with irradiated sporozoites was also protective against low numbers of infectious mosquito bites. There was no significant difference in vaccine efficacy between immunized mice challenged with five mosquito bites (1 infected out of 41 challenged) and mice i.v. inoculated with 20,000 sporozoites (0 infected out of 20 challenged) (χ2 = 0.5; df = 1; P > 0.05). However, the protection afforded by immunization with irradiated sporozoites was overcome when immunized mice were challenged with a higher number of infectious mosquito bites. There was a significant difference in vaccine efficacy between immunized mice challenged with 10 mosquito bites (6 infected out of 14 challenged) and mice i.v. inoculated with 20,000 sporozoites (0 infected out of 20 challenged) (χ2 = 10.4; df = 1; P < 0.05). Peptide-immunized mice were fully protected when challenged with an i.v. inoculation of 20,000 sporozoites (zero infected out of six challenged), but significantly fewer mice were protected (five infected out of six challenged) when exposed to the bites of five infectious mosquitoes (χ2 = 8.6; df = 1; P < 0.05).
TABLE 2.
Comparative infectivity of P. berghei sporozoites to immunized CD-1 outbred mice when sporozoites were delivered by either infectious mosquito bite or syringe inoculation into mouse tail veina
| Immunizing agent | Host immunoglobulin G titer | % of mice infected after:
|
|||
|---|---|---|---|---|---|
| 5 Mosquito bites | 10 Mosquito bites | Intravenous inoculation of:
|
|||
| 20,000 spz | 50,000 spz | ||||
| Irradiated spz | >1:1,000 | 2 (41) | 43 (14) | 0 (20) | 0 (14) |
| Peptide-KLH | >1:3,200 | 83 (6) | ND | 0 (6) | ND |
| KLH only | <1:20 | 100 (19) | ND | ND | ND |
| None | <1:20 | ND | ND | ND | 100 (12) |
Mice were immunized against either irradiated sporozoites (spz) or the synthetic peptide D-16-N coupled to KLH. Negative-control mice received KLH only. Sample sizes are indicated within parentheses. ND, not done.
In conducting the research described in this report, the investigators adhered to the Guide for the Care and Use of Laboratory Animals, as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council.
Conclusions.
P. berghei sporozoites were more infectious to outbred CD-1 mice when delivered by infectious mosquito bite than by i.v. inoculation. Based on two separate studies, the ID50 of i.v. delivered sporozoites ranged from 1,700 to 11,250 sporozoites, whereas the ID50 of bite-delivered sporozoites was four mosquito bites. The actual number of sporozoites deposited into the host by blood-feeding mosquitoes is low, with median estimates ranging from 6 to 11 sporozoites per mosquito (3, 4, 32). There are several reasons for this. Sporozoites must begin their exit from the salivary glands in single file, because the diameter of the gland ducts in the salivary lobes is not much larger than the diameter of a sporozoite (4). Once a mosquito begins to engorge, many of the sporozoites injected into the host may be ingested back into the mosquito with the blood meal (4, 56). Furthermore, not all gland-infected mosquitoes transmit sporozoites (3–5, 32, 39). Thus, even if we assume a fairly liberal estimate of 15 sporozoites transmitted during blood feeding, the ID50 of four infectious mosquito bites would still represent only 60 sporozoites—over 1 to 2 orders of magnitude less than the number of sporozoites required when delivered i.v.
One reason why mosquito-injected sporozoites are more infectious than i.v. inoculated sporozoites may have to do with sporozoite viability. Sporozoites may remain viable for hours to days in vitro, depending on handling conditions, media, and temperature. Sporozoite viability is best maintained in saline solutions containing at least 5 to 8% serum or blood and at temperatures above 6 and below 37°C (27, 48) and can be assessed visually by using a vital dye containing fluorescein diacetate and ethidium bromide (42) or microscopic examination of sporozoite gliding motility (50). In addition to viability, it has been shown that sporozoites from mature oocysts and the hemolymph may be less infectious to the vertebrate host than salivary gland sporozoites (49). This is a consideration when sporozoites used in i.v. inoculations are mass isolated from whole mosquitoes or mosquito thoraces (6, 29–30).
Another factor contributing to the differential infectivity of sporozoites may be related to the very different routes of entry between mosquito-delivered versus inoculated sporozoites. Most sporozoites injected into a host by an infected mosquito are deposited within the skin, usually in clumps (31). Sporozoites remain in the skin and/or subcutaneous tissues for at least 5 min after mosquito feeding terminates (31, 43). As a result of their intradermal placement into the host by the mosquito, sporozoites may migrate to the liver via the lymphatic system rather than by the blood capillaries (16, 31). Conversely, i.v. inoculation delivers sporozoites directly into the bloodstream, bypassing potentially important cutaneous interactions and possible lymphatic drainage.
A third potential factor contributing to the greater infectiousness of mosquito-delivered sporozoites may be the bioactive substances contained within mosquito saliva. Anopheline saliva has been shown to contain a variety of antihemostatic substances, including apyrase, anticoagulants, and vasodilators (35–37, 44). The relationship between these substances and infectivity of saliva-borne pathogens is only now being appreciated. The salivas of sand flies (47) and ticks (22, 25) have been shown to enhance pathogen infectivity to vertebrates. Indeed, mosquito feeding has recently been shown to potentiate the infectivity of Cache Valley virus to mice (12). The effect of bioactive substances in anopheline saliva on sporozoite infectivity has yet to be demonstrated, but any potential enhancing activity of anopheline saliva undoubtedly becomes highly diluted during the dissection and trituration of salivary glands prior to sporozoites being used in i.v. inoculation.
The route of sporozoite challenge had an impact on the efficacy of our sporozoite immunization trials. Mice passively immunized with anti-CS MAbs (Fig. 1) and mice actively immunized with anti-CS peptides (Table 2) were afforded significantly greater protection when challenged by i.v. inoculation than when challenged by bite. To date, the most efficacious sporozoite vaccine is the irradiated sporozoite. Mice immunized with irradiated sporozoites were fully protected against i.v. challenge of 20,000 and 50,000 sporozoites (approximately five times the ID50) and against a challenge of five mosquito bites each (approximately 75 sporozoites [see above]), but these mice were only partially protected against a challenge of 10 mosquito bites each (approximately 150 sporozoites).
The differential infectivities of P. berghei sporozoites to mice when delivered by mosquito bite versus i.v. inoculation are of significance to sporozoite vaccine trials in general. In vaccine trials which utilize rodent models (e.g., P. berghei, Plasmodium yoelii, and Plasmodium chaubaudi), most sporozoite challenges are administered as i.v. inoculations either of triturated salivary glands from sporozoite-infected mosquitoes or from whole mosquito thoraces that have been filtered (1, 2, 7, 10, 13, 19, 24, 26, 33, 34, 38, 41, 52, 53, 55). In contrast, sporozoite vaccine trials with humans (e.g., Plasmodium falciparum) invariably utilize infectious mosquito bites (usually five) as their challenge model system (8, 9, 11, 14, 15, 17, 18, 20, 28, 45, 46). It is often difficult to extrapolate between results of rodent and human vaccine studies, but standardization of methodologies can only serve to clarify discrepancies. In view of our findings and the fact that mosquito bite is the natural mode of sporozoite delivery, we feel that infectious mosquito bites should henceforth be used as the standard challenge protocol for all sporozoite vaccine efficacy trials, regardless of whether such trials utilize rodent or human plasmodial species.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health and the United Nations Development Program-World Bank-World Health Organization special program for research and training in tropical diseases.
MAbs were kind gifts from Yupin Charoenvit, United States Naval Medical Research Center.
REFERENCES
- 1.Anders R F, Crewther P E, Edwards S, Margetts M, Matthew M L, Pollock B, Pye D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 2.Becker S I, Wang R, Hedstrom R C, Aguiar J C, Jones T R, Hoffman S L, Gardner M J. Protection of mice against Plasmodium yoelii sporozoite challenge with P. yoelii merozoite surface protein 1 DNA vaccines. Infect Immun. 1998;66:3457–3461. doi: 10.1128/iai.66.7.3457-3461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beier J C, Davis J R, Vaughan J A, Noden B H, Beier M S. Quantitation of Plasmodium falciparum sporozoites transmitted by experimentally infected Anopheles gambiae and Anopheles stephensi. Am J Trop Med Hyg. 1991;44:564–570. doi: 10.4269/ajtmh.1991.44.564. [DOI] [PubMed] [Google Scholar]
- 4.Beier J C, Beier M S, Vaughan J A, Pumpuni C B, Davis J R, Noden B H. Sporozoite transmission by Anopheles freeborni and Anopheles gambiae experimentally infected with Plasmodium falciparum. J Am Mosq Control Assoc. 1992;8:404–408. [PubMed] [Google Scholar]
- 5.Beier M S, Davis J R, Pumpuni C B, Noden B H, Beier J C. Ingestion of Plasmodium falciparum sporozoites during transmission by anopheline mosquitoes. Am J Trop Med Hyg. 1992;47:195–200. doi: 10.4269/ajtmh.1992.47.195. [DOI] [PubMed] [Google Scholar]
- 6.Bosworth A B, Schneider I, Freier J E. Mass isolation of Anopheles stephensi salivary glands infected with malarial sporozoites. J Parasitol. 1975;61:769–772. [PubMed] [Google Scholar]
- 7.Chatterjee S, Francois G, Druilhe P, Timperman G, Wery M. Immunity to Plasmodium berghei exoerythrocytic forms derived from irradiated sporozoites. Parasitol Res. 1996;82:297–303. doi: 10.1007/s004360050117. [DOI] [PubMed] [Google Scholar]
- 8.Church L W, Le T P, Bryan J P, Gordon D M, Edelman R, Fries L, Davis J R, Herrington D A, Clyde D F, Shmuklarsky M J, Schneider I, McGovern T W, Chulay J D, Ballou W R, Hoffman S L. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175:915–920. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 9.Clyde D F, Most H, McCarthy V C, Vanderberg J P. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman R, Hoffman S L, Davis J R, Beier M, Sztein M B, Losonsky G, Herrington D A, Eddy H A, Hollingdale M R, Gordon D M, Clyde D F. Long-term persistence of sterile immunity in a volunteer immunized with X-irradiated Plasmodium falciparum sporozoites. J Infect Dis. 1993;168:1066–1070. doi: 10.1093/infdis/168.4.1066. [DOI] [PubMed] [Google Scholar]
- 12.Edwards J F, Higgs S, Beatty B J. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 13.Egan J E, Weber J L, Ballou W R, Hollingdale M R, Majarian W R, Gordon D M, Maloy W L, Hoffman S L, Wirtz R A, Schneider I, Woollett G R, Young J F, Hockmeyer W T. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987;236:453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- 14.Fries L F, Gordon D M, Schneider I, Beier J C, Long G W, Gross M, Que J U, Cryz S J, Sadoff J C. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect Immun. 1992;60:1834–1839. doi: 10.1128/iai.60.5.1834-1839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon D M, McGovern T W, Krzych U, Cohen J C, Schneider I, LaChance R, Heppner D G, Yuan G, Hollingdale M R, Slaoui M, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths R B, Gordon R M. An apparatus which enables the process of feeding by mosquitoes to be observed; together with an account of the ejection of saliva and its significance in malaria. Ann Trop Med Parasitol. 1952;46:311–319. doi: 10.1080/00034983.1952.11685536. [DOI] [PubMed] [Google Scholar]
- 17.Heppner D G, Gordon D M, Gross M, Wellde B, Leitner W, Krzych U, Schneider I, Wirtz R A, Richards R L, Trofa A, Hall T, Sadoff J C, Boerger P, Alving C R, Sylvester D R, Porter T G, Ballou W R. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996;174:361–366. doi: 10.1093/infdis/174.2.361. [DOI] [PubMed] [Google Scholar]
- 18.Herrington D A, Clyde D F, Murphy J R, Baqar S, Levine M M, do Rosario V, Hollingdale M R. A model for Plasmodium falciparum sporozoite challenge and very early therapy of parasitaemia for efficacy studies of sporozoite vaccines. Trop Geogr Med. 1988;40:124–127. [PubMed] [Google Scholar]
- 19.Hoffman S L, Sedegah M, Hedstrom R C. Protection against malaria by immunization with a Plasmodium yoelii circumsporozoite protein nucleic acid vaccine. Vaccine. 1994;12:1529–1533. doi: 10.1016/0264-410x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman S L, Edelman R, Bryan J P, Schneider I, Davis J, Sedegah M, Gordon D, Church P, Gross M, Silverman C, et al. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalane as adjuvant. Am J Trop Med Hyg. 1994;51:603–612. doi: 10.4269/ajtmh.1994.51.603. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe R I, Lowell G H, Gordon D M. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am J Trop Med Hyg. 1990;42:309–313. doi: 10.4269/ajtmh.1990.42.309. [DOI] [PubMed] [Google Scholar]
- 22.Jones L D, Kaufman W R, Nuttall P A. Modification of the skin feeding site by tick saliva mediates virus transmission. Experientia. 1992;48:779–782. doi: 10.1007/BF02124302. [DOI] [PubMed] [Google Scholar]
- 23.Khan Z M, Vanderberg J P. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect Immun. 1991;59:2529–2534. doi: 10.1128/iai.59.8.2529-2534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz F W, Scheller L F, Seguin M C, Kumar N, Marletta M A, Green S J, Azad A F. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J Immunol. 1995;154:3391–3395. [PubMed] [Google Scholar]
- 25.Labuda M, Jones L D, Williams T, Nuttall P A. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med Vet Entomol. 1993;7:193–196. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 26.Marussig M, Renia L, Motard A, Miltgen F, Petour P, Chauhan V, Corradin G, Mazier D. Linear and multiple antigen peptides containing defined T and B epitopes of the Plasmodium yoelii circumsporozoite protein: antibody-mediated protection and boosting by sporozoite infection. Int Immunol. 1997;9:1817–1824. doi: 10.1093/intimm/9.12.1817. [DOI] [PubMed] [Google Scholar]
- 27.Maynes B. The injection of mosquito sporozoites in malaria therapy. Public Health Rep. 1933;48:909–916. [Google Scholar]
- 28.Ockenhouse C F, Sun P F, Lanar D E, Wellde B T, Hall B T, Kester K, Stoute J A, Magill A, Krzych U, Farley L, Wirtz R A, Sadoff J C, Kaslow D C, Kumar S, Church L W, Crutcher J M, Wizel B, Hoffman S, Lalvani A, Hill A V, Tine J A, Guito K P, de Taisne C, Anders R, Ballou W R, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis. 1998;177:1664–1673. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki L S, Gwadz R W, Godson G N. Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J Parasitol. 1984;70:831–833. [PubMed] [Google Scholar]
- 30.Pacheco N D, Strome C P A, Mitchell F, Bawden M P, Beaudoin R L. Rapid, large-scale isolation of Plasmodium berghei sporozoites from infected mosquitoes. J Parasitol. 1979;65:414–417. [PubMed] [Google Scholar]
- 31.Ponnudurai T, Lensen A H, Van Glemert G J, Bolmer M G, Meuwissen J H. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans R Soc Trop Med Hyg. 1991;85:175–180. doi: 10.1016/0035-9203(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 32.Pumpuni C B, Mendis C, Beier J C. Plasmodium yoelii sporozoite infectivity varies as a function of sporozoite loads in Anopheles stephensi mosquitoes. J Parasitol. 1997;83:652–655. [PubMed] [Google Scholar]
- 33.Renia L, Rodrigues M M, Nussenzweig V. Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. Immunology. 1994;82:164–168. [PMC free article] [PubMed] [Google Scholar]
- 34.Renia L, Ling I T, Marussig M, Miltgen F, Holder A A, Mazier D. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun. 1997;65:4419–4423. doi: 10.1128/iai.65.11.4419-4423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro J M, Nussenzveig R H. The salivary catechol oxidase/peroxidase activities of the mosquito Anopheles albimanus. J Exp Biol. 1993;179:273–287. doi: 10.1242/jeb.179.1.273. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro J M, Nussenzveig R H, Tortorella G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1994;31:747–753. doi: 10.1093/jmedent/31.5.747. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro J M. NAD(P)H-dependent production of oxygen reactive species by the salivary glands of the mosquito Anopheles albimanus. Insect Biochem Mol Biol. 1996;26:715–720. doi: 10.1016/s0965-1748(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues E G, Zavala F, Eichinger D, Wilson J M, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 39.Rosenberg R, Wirtz R A, Schneider I, Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 1990;84:209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- 40.Scheller L F, Wirtz R A, Azad A F. Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect Immun. 1994;62:4844–4847. doi: 10.1128/iai.62.11.4844-4847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seguin M C, Ballou W R, Nacy C A. Interactions of Plasmodium berghei sporozoites and murine Kupffer cells in vitro. J Immunol. 1989;143:1716–1722. [PubMed] [Google Scholar]
- 43.Sidjanski S, Vanderberg J P. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am J Trop Med Hyg. 1997;57:426–429. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- 44.Stark K R, James A A. Salivary gland anticoagulants in culicine and anopheline mosquitoes (Diptera:Culicidae) J Med Entomol. 1996;33:645–650. doi: 10.1093/jmedent/33.4.645. [DOI] [PubMed] [Google Scholar]
- 45.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 46.Sturchler D, Berger R, Rudin C, Just M, Saul A, Rzepczyk C, Brown G, Anders R, Coppel R, Woodrow G, et al. Safety, immunogenicity, and pilot efficacy of Plasmodium falciparum sporozoite and asexual blood-stage combination vaccine in Swiss adults. Am J Trop Med Hyg. 1995;53:423–431. doi: 10.4269/ajtmh.1995.53.423. [DOI] [PubMed] [Google Scholar]
- 47.Titus R G, Ribeiro J M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 48.Tonkin I M. The influence of the suspending fluid on the survival of sporozoites in vitro. Trans R Soc Trop Med Hyg. 1947;41:259–262. doi: 10.1016/s0035-9203(47)80010-0. [DOI] [PubMed] [Google Scholar]
- 49.Touray M G, Warburg A, Laughinghouse A, Krettli A U, Miller L H. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J Exp Med. 1992;175:1607–1612. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanderberg J P. Studies on the motility of Plasmodium sporozoites. J Protozool. 1974;21:527–537. doi: 10.1111/j.1550-7408.1974.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 51.Vanderberg J P, Khan Z M, Stewart M J. Induction of hepatic inflammatory response by Plasmodium berghei sporozoites protects BALB/c mice against challenge with Plasmodium yoelii sporozoites. J Parasitol. 1993;79:763–767. [PubMed] [Google Scholar]
- 52.Wang R, Charoenvit Y, Corradin G, Porrozzi R, Hunter R L, Glenn G, Alving C R, Church P, Hoffman S L. Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J Immunol. 1995;154:2784–2793. [PubMed] [Google Scholar]
- 53.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke E D, Hoffman S L. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4071. [PubMed] [Google Scholar]
- 54.Weber J L, Egan J E, Lyon J L, Wirtz R A, Charoenvit Y, Maloy W L, Hockmeyer W T. Plasmodium berghei: cloning of the circumsporozoite protein gene. Exp Parasitol. 1987;63:295–300. doi: 10.1016/0014-4894(87)90176-7. [DOI] [PubMed] [Google Scholar]
- 55.White K L, Snyder H L, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156:3374–3381. [PubMed] [Google Scholar]
- 56.Yorke W, Macfie J W. The action of the salivary secretion of mosquitoes and of Glossina tachinoides on human blood. Ann Trop Med Parasitol. 1924;18:103–108. [Google Scholar]