Abstract
Bats are known natural reservoirs of several highly pathogenic zoonotic viruses, including Hendra virus, Nipah virus, rabies virus, SARS-like coronaviruses, and suspected ancestral reservoirs of SARS-CoV-2 responsible for the ongoing COVID-19 pandemic. The capacity to survive infections of highly pathogenic agents without severe disease, together with many other unique features, makes bats an ideal animal model for studying the regulation of infection, cancer, and longevity, which is likely to translate into human health outcomes. A key factor that limits bat research is lack of breeding bat colonies. To address this need, a captive bat colony was established in Singapore from 19 wild-caught local cave nectar bats. The bats were screened for specific pathogens before the start of captive breeding. Custom-made cages and an optimized diet inclusive of Wombaroo dietary formula, liquid diet, and supplement of fruits enabled the bats to breed prolifically in our facility. Cages are washed daily and disinfected once every fortnight. Bats are observed daily to detect any sick bat or abnormal behavior. In addition, bats undergo a thorough health check once every 3 to 4 mo to check on their overall wellbeing, perform sampling, and document any potential pregnancy. The current colony houses over 80 bats that are successfully breeding, providing a valuable resource for research in Singapore and overseas.
Abbreviations: AVS, Animal & Veterinary Service; E. spelaea, Eonycteris spelaea; MERS-CoV, Middle East respiratory syndrome-related coronavirus; NLARF, National Large Animal Research Facility; NParks, National Parks Board; SARS-CoV, Severe acute respiratory syndrome coronavirus
Introduction
Bats are known reservoirs of several zoonotic pathogens that pose a threat to human and animal health. These pathogens include Marburg virus, Hendra virus, Nipah virus, and rabies virus.7 In addition, they are likely the ancestral reservoirs for Severe Acute Respiratory Syndrome – Coronavirus (SARS-CoV 1 and SARS-CoV-2), Middle East Respiratory Syndrome coronavirus (MERS-CoV), and Ebola virus.5,7,29 Bats’ immunologic response to infection from viruses that are highly pathogenic in other mammals, the lack of tumorigenesis, and their longevity make bats a popular model for infectious disease research.1,6,16 Conducting in vitro studies creates a continuous need to collect samples from bats. However, significant challenges exist when sampling wild bats. In addition to disturbing the bat’s natural habitat, wild bats may introduce numerous confounding variables, such as large diversity in immune response, unknown disease status, and pathogen exposure history, which may negatively impact the study.2,15 To address some of these challenges, we established a breeding colony of Eonycteris spelaea in Singapore for research.
Eonycteris spelaea, also known as cave nectar bat, belongs to the subfamily Rousettinae in the family Pteropodida.25 E. spelaea is an excellent choice for the establishment of a breeding colony for several reasons. They are classified as ‘least concern’ on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species.25 In addition, these bats roost in large dense colonies within a small area, and we posit that these bats will be able to adapt quickly to our proposed colony setup (Figure 1). Also, the genome and transcriptome have already been well characterized.26 Furthermore, the availability of a continuous source of samples from the colony will help to address gaps through in vivo functional analysis of genes or pathways of interest. Finally, the E. spelaea roosts in Singapore are accessible for researchers to safely trap the bats.
Figure 1.
Eonycteris spelaea roosting in the artificial housing.
Here, we describe the establishment and subsequent reproductive success of a captive colony of E. spelaea. To our knowledge, this is the first nectivorous captive, reproductive bat colony in Asia.
Methods
Animals.
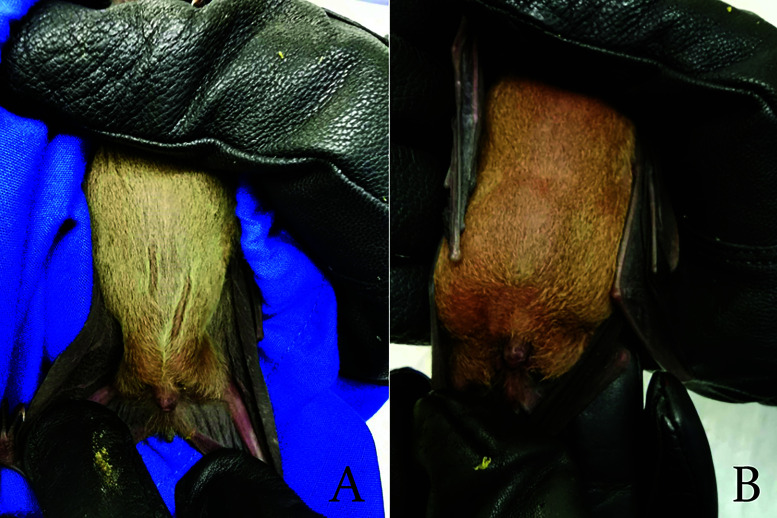
Animal ethics approval for the study was granted by SingHealth Institutional Animal Care and Use Committee (IACUC; Permit # 2015/SHS/1088). Regulatory approval for the trapping of bats was granted by the National Parks Board, Singapore (NParks; Permit# NP/RP15-087). Bats were captured between 1900 and 2100 h at 2 sites in Singapore using elevated mist nets supported by a pair of 3.00 m to 3.50 m telescopic poles. Trapped bats were removed from mist nets, placed into custom-made, breathable opaque cotton mesh holding bags, and inspected for age and sex. Bats weighing above 47.0 g with a visible distension of the stomach were suspected to be pregnant and released (Figure 2). Juvenile bats were also released. Bat mites were removed using blunt-end forceps, and any physical wounds were treated with antiseptic cream (Spectracon, New Delhi, India). Wild caught bats were quarantined for 1 mo in a separate cage before introduction to colony. All personnel wore Tyvek suits with safety goggles, N95 masks, head lamp, and bite-resistant leather gloves over nitrile gloves when trapping bats.
Figure 2.
Size of lower abdomen in a) normal and b) gravid bats. Visible distention around the lower abdomen can be observed in gravid bats.
Care and Welfare
Animal housing.
The bat facility includes an air-conditioned anteroom (3.00 m by 2.65 m) connected to an Animal Biosafety Level-1 (ABSL-1) lab (3.00 m by 2.98 m) that is connected to the bat holding area. The holding area (7.90 m by 5.66 m) is sheltered, naturally ventilated, exposed to natural photoperiod, and enclosed by wire mesh to prevent the entry of rodents, birds, and wild animals.
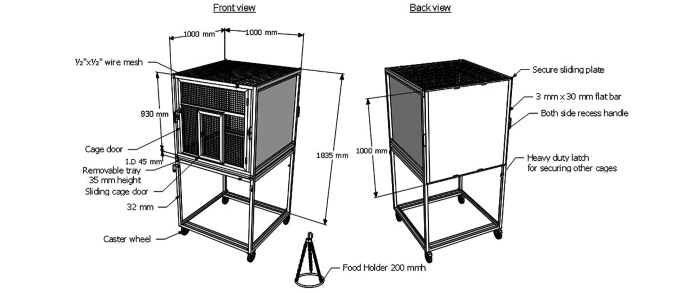
Custom cages were designed and fabricated (Steelmatic, Singapore) (Figure 3). Cages were 100 cm long, 100 cm wide, and 183 cm high. The front, top, and bottom panels of the cages were made of heavy-duty stainless steel wire mesh with no sharp edges. The top panel allowed the bats to hang freely and personnel to hang the feeding pan. The wire mesh has a 1-cm space opening, and the 2 side metal sheets are retractable. When removed, additional cages can be added on either side or on the back of the cage, allowing the bats to roam freely between adjoining cages. A removable metal collection pan was also incorporated under the bottom wire mesh panel to allow for the daily removal of waste. Hessian fabric was hung in the cage supported by a tree branch to allow bats to roost together. Outside the cage, an additional drape was used to cover one end of the cage for extra privacy and shade (Figure 4). Each enclosure consisted of 2 cages joined back-to-back and could house a maximum of 25 bats. This arrangement provided ample space for the bats to fly within the enclosure.
Figure 3.
Schematic representation of the bat cage. The bat cage is specifically designed, and custom made to accommodate living conditions of E. spelaea. The cage is designed to allow combination of 2 or 4 cages and castor wheels with brakes were also included. Frame, panels, and food holder were made of SUS 304 stainless steel.
Figure 4.
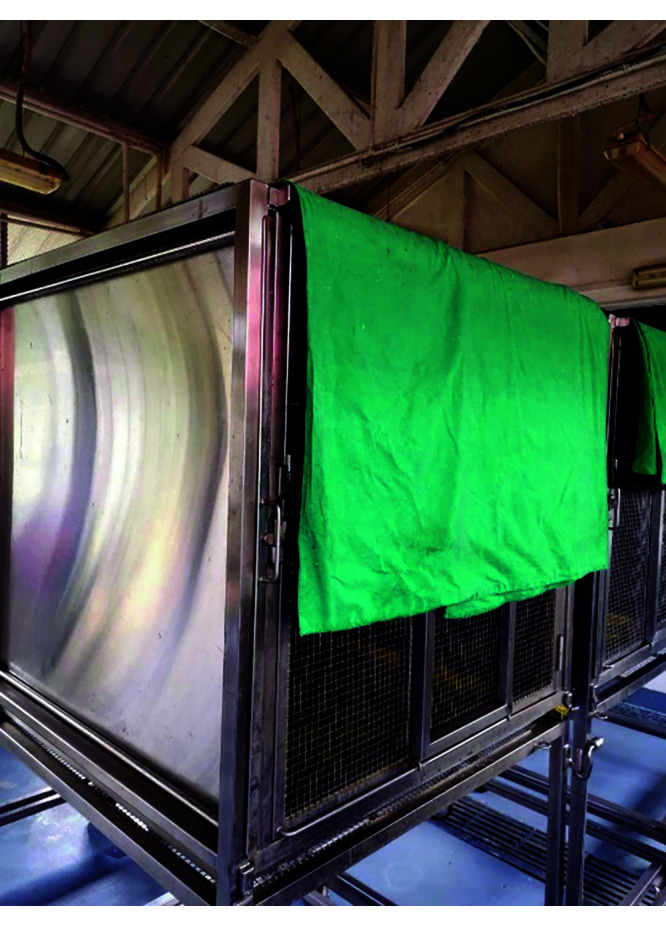
Drape hanging over top corner of bat cage to provide more shade and seclusion.
Diet.
E. spelaea’s natural diet consists primarily of nectar and pollen.14 To replicate their natural diet, water, milk powder, powder pollen, and a commercially available diet (Wombaroo Lorikeet and Honeyeater Food, Glen Osmond, SA, Australia) were provided. Fresh fruit was also provided based on seasonal availability (Table 1). The bats were fed once daily in the evening and feed was left overnight.
Table 1.
Bat colony daily dietary amounts of optimized diet and supplement. Dietary amount listed below are for 20–25 bats
| Diet components | Formulation |
|---|---|
| Liquid diet Equation (500 mL total volume) | 250 mL water 36 g low fat milk powder 0.25 g powdered pollen Top up to 500 mL with warm water |
| Commercial diet (500 mL total volume) | 150 g Wombaroo diet 1 g powdered pollen Top up to 500 mL with warm water |
| Supplement | 10 slices of watermelon / papaya / mango (To sprinkle some fruits with approximately 10 g of milk powder) |
| Water | 250 mL |
| Salt water | 250 mL of water 5 g table salt |
Sanitation.
Leftover feed from the previous evening and waste were removed. External cage surfaces were washed with water every morning. Bats were transferred to clean cages every 2 wks for cage disinfection. Cages were disinfected with 1% Virkon for 30 min of contact time followed by a rinse with water. Bat holding bags were washed after each use with detergent and disinfected using 1% Virkon for 30 min of contact time followed by a rinse with water.
Veterinary Check and surveillance.
Bats were monitored daily from outside the cage for any sign of isolation or abnormal behavior. Bats that appeared weak and emaciated were assessed by the facility veterinarian. Thorough medical health checks were performed once every 3 to 4 mo to collect samples (oral, rectal, and body swabs, and blood), detect physical injury, perform morphometric measurement, identify pregnant bats, and microchip newborn pups that had been weaned.
Bats were captured using a butterfly catch net and immediately transferred into holding bags and moved to the Biosafety Cabinet (BSC) in the ABSL-1 laboratory. Bats that appeared weak, emaciated, lost more than 20% of previously measured body weight, or had a poor prognosis were euthanized. For euthanasia, bats were anesthetized in the BSC with isoflurane gas and once anesthetized, were given an overdose of Sodium Pentobarbital 90 to 100 mg/kg body weight via the intramuscular or intraperitoneal route. This procedure was discussed and selected by the facility veterinarian and research personnel prior to starting the colony.
Sample collection.
External (head and body) and internal (oral and rectal) swabs were collected. External swabs were collected by swabbing the head and body of the bats in an up and down motion with phosphate buffered saline (PBS) (Hyclone, Logan, UT) soaked sterile polyester tipped swabs (Puritan Medical Products, Maine, United States of America). Oral swabs were collected by swabbing the inner cheeks, tongue, and palate. Rectal swabs were obtained by inserting a PBS-soaked sterile polyester tipped swab into the rectum, rotating gently, and removing. All swabs were stored in 2-mL screw cap microtubes (Sarstedt, Nümbrecht, Germany) with 500 µL of viral transport media (VTM) made up with 10% Bovine Serum Albumin (Sigma-Aldrich, St. Louis, MO) and 20% Antibiotics-Antimycotic (Thermofisher Scientific, Waltham, MA in sterile PBS. The samples were stored at −80°C (−112°F). Blood samples were collected by puncturing the cephalic vein of the wing after wiping the puncture site with an alcohol swab. Blood emerging from site of puncture was aspirated with a pipette, suspended in 10 µL (8 mm) of EDTA, and stored at −20°C (−4°F). Pressure was applied to site of puncture with sterile gauze to stop the bleeding, followed by application of antiseptic cream to prevent infection. Samples were collected from each bat prior to introduction into the colony and during a health check every 3 to 4 mo. After the samples were collected, bats were given 1 mL of fruit juice before release back into their cage. Samples were not taken from bats that were pregnant or nursing. All personnel wore personal protective equipment (PPE), bouffant cap, mask, face shield, and shoe cover when performing sampling.
Measurements.
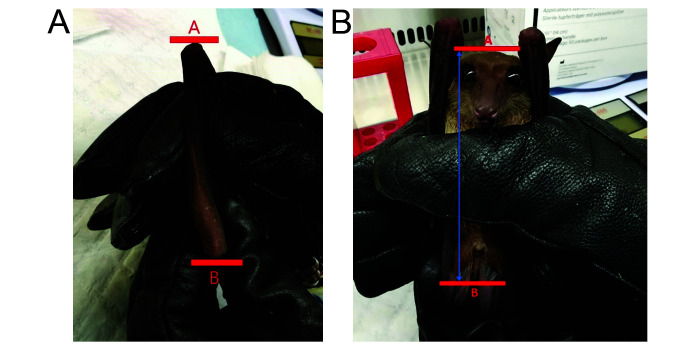
Morphometric measurements (weight, forearm length, and body length) were recorded from each bat prior to introduction into the colony and during medical checks every 3 to 4 mo using a vernier caliper (Coolant-Proof Digimatic Caliper, Mitutoyo, Kawasaki, Kanagawa, Japan). Forearm length was measured from the ulna to the wrist (Figure 5a). The total body length was taken by extending the body of the bat and measuring it from the top of the head to the end of the tail (Figure 5b).
Figure 5.
Length of a) forearm and b) body. Forearm length is determined by measuring the ulna from point A to B. Body length is determined by measuring from the point A (top of the head) to point B (tip of the tail).
Microchipping.
Bats were microchipped using ID-100B (1.4) Mini Transponder in Canula (Trovan, Isle of Man, United Kingdom). The back of the neck was cleaned with an alcohol swab and the microchip was injected subcutaneously. Pressure was applied to stop any bleeding, followed by application of antiseptic cream to prevent infections.
Extractions, cDNA Synthesis, and PCR
RNA Extraction.
RNA was extracted from whole blood, oral, rectal, body, and head swab samples using an RNA Blood Mini Kit (QIAGEN, Hilden, NRW, Germany) and Qiagen RNeasy Mini Kit (QIAGEN) according to manufacturer’s instructions. RNA was eluted in RNase-free water and stored at −80°C (−112°F). RNA concentration and quality were measured using the DeNovix DS-11+ Spectrophotometer.
cDNA Synthesis.
cDNA was generated from 1 µg of RNA using QuantiTect Reverse Transcription kit (QIAGEN) according to manufacturer’s instructions and stored at −20°C (−4°F).
PCR Screening.
PCRs were performed to detect Filovirus, Coronavirus, Nipah virus, and Rhabdovirus using GoTaq Green Master Mix (Promega, Madison, WI) in a 15 µL reaction with 40 ng of cDNA.4,8,21 Primers and annealing temperature can be found in (Table 2).
Table 2.
PCR primers for Filovirus L gene, Coronavirus RdRp region, Nipah Virus N gene, Rhabdovirus L gene and their expected amplicon size4,8,21
| Target | Primer | Sequence (5' to 3') | Annealing temperature | Size (base pair) |
|---|---|---|---|---|
| Filovirus L gene | FV-F1 | GCMTTYCCIAGYAAYATGATGG | 460 bp | |
| FV-R1 | GTDATRCAYTGRTTRTCHCCCAT | |||
| FV-F2 | TDCAYCARGCITCDTGGCAYC | 54 °C | 350 bp | |
| FV-R2 | GIGCACADGADATRCWIGTCC | |||
| Coronavirus RdRp region | IN-6 | GGTTGGGACTATCCTAAGTGTGA | 56 °C | 440 bp |
| IN-7 | CCATCATCAGATAGAATCATCATA | |||
| Nipah N gene | Forward | ATGCCATGGATCCCGAATGAGTGATAT TTTTGAAGAGGCGGCTAG | 51 °C | 1500 bp |
| Reverse | CGTAGCACTCGAGGATTCACACATCAG CTCTGACAAAGTCAAG | |||
| Rhabdovirus L gene | Forward | CCADMCBTTTTGYCKYARRCCTTC | ||
| Reverse | RAAGGYAGRTTTTTYKCDYTRATG | |||
| Forward 2 | ATGACAGACAACCTGAACAA | 58 °C | 340 bp | |
| Reverse 2 | CCATTCCAGCAGGTTGGACC |
Results
Introduction of founding population.
In October 2015, 3 female bats were captured and introduced into the facility as a pilot run. After 6 mo of successful integration, 12 female and 4 male bats were captured in April 2016. Wild caught E. spelaea weight ranged from 25 to 65 g. Forearm length ranged from 56 to 73 mm (Table 3). Wild caught bats were PCR negative for rabies virus, coronaviruses, filoviruses, and Nipah virus.
Table 3.
Wild Eonycteris values for introduction date, sex, and morphometric measurements
| Eonycteris spelaea (ES#) | Date Introduced (dd-mm-yy) | Sex | Weight (grams) | Forearm (mm) |
|---|---|---|---|---|
| 6 | 13-04-16 | Male | 65 | 71.5 |
| 7 | 13-04-16 | Male | 65 | 73.4 |
| 10 | 14-04-16 | Male | 45 | 66.9 |
| 13 | 27-04-16 | Male | 61 | 68.1 |
| 1 | 22-10-15 | Female | 29 | 59.7 |
| 2 | 22-10-15 | Female | 38 | 63.5 |
| 3 | 22-10-15 | Female | 46 | 65.6 |
| 4 | 13-04-16 | Female | 28 | 58.1 |
| 8 | 13-04-16 | Female | 29 | 58.4 |
| 9 | 14-04-16 | Female | 42 | 64.7 |
| 11 | 14-04-16 | Female | 33 | 65.8 |
| 12 | 27-04-16 | Female | 29 | 59.7 |
| 14 | 27-04-16 | Female | 30 | 59.8 |
| 15 | 27-04-16 | Female | 25 | 59.5 |
| 16 | 27-04-16 | Female | 46 | 65.5 |
| 17 | 27-04-16 | Female | 43 | 68.5 |
| 18 | 27-04-16 | Female | 51 | 68.4 |
| 19 | 27-04-16 | Female | 38 | 62.5 |
| 20 | 27-04-16 | Female | 26 | 55.9 |
Morphometric monitoring of integrated bats.
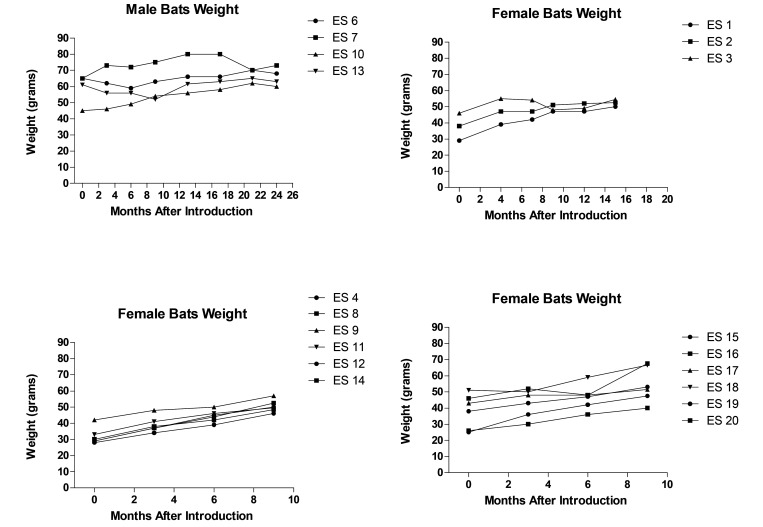
To track the bats’ growth and adaptation to the artificial housing, their baseline weight was measured before introduction and at health checks every 3 to 4 mo. At the first health check, 3 mo after introduction to the colony, 16 out of the 19 founding bats showed a weight gain of 1 to 11 g, whereas 3 bats had a weight loss of 1 to 5 g. For female bats, a net gain in weight was recorded 9 to 15 mo after introduction into the colony before pregnancy or pups latching on (Figure 6) were observed. After 24 mo, male bats showed a net gain in weight. Bats appeared to be alert, and responsive during physical handling and showed no signs of stress, injury, loss of fur, or weakness.
Figure 6.
Weight trend of bats in captivity. Increase in body weight of female bats across 9–15 mo and male bats across 24 mo.
Pregnancy.
The gestation period of E. spelaea bat is 3 to 4 mo.3 No pregnancy was detected in female bats during the first 6 mo after introduction. An observable enlarged abdomen was the primary indicator of pregnancy in the colony followed by a gain of weight. ES 16 was suspected to be pregnant due to an observable enlarged abdomen during the 9 mo after introduction health check in January 2017. From October 2016 to January 2017, ES 16 gained 19.5 g followed by a 2.5 g decrease in weight in May 2017. In May 2017, one pup without a microchip was found that potentially belonged to ES 16. Pups were also found latching on ES 1, 9, and 19 during the health check in May 2017; females were weighed with the pups attached, which accounted for at least some of the weight gain in females. Bats ES 1, 9, and 19 were not suspected to be pregnant due to the absence of an enlarged abdomen during the January 2017 health check. Multiple pregnancies and pups have been detected (Table 4) since January 2017. E. spelaea are documented to be polyestrous and can give birth to 2 pups a year.12 However, ES 1 produced 3 pups within a year (January 2018 to December 2018). Pups were found to be latching on ES 1 on April, July and December 2018. (Table 5). Changes in behavior were observed during the early and middle stages of pregnancy. Pregnant bats were generally more aggressive and vocal during handling, whereas bats closer to parturition were more docile and had a larger bulge on the abdomen. Pregnant and nursing bats were returned to the colony immediately after weighing and feeding. A total of 35 bats were born and implanted with microchips between May 2017 and December 2018.
Table 4.
Female Eonycteris spelaea pregnancy tracking, abdomen enlargement and weight measurement up to 23 mo in captivity
| Eonycteris spelaea (ES#) | Months in captivity | Health check date | Weight (grams) | Enlarged abdomen | Status |
|---|---|---|---|---|---|
| 1 | 12 | October 2016 | 47 | No | Normal |
| 15 | January 2017 | 50 | No | Normal | |
| 19 | May 2017 | 80 | – | With pup | |
| 23 | September 2017 | 66 | No | Normal | |
| 2 | 12 | October 2016 | 52 | No | Normal |
| 15 | January 2017 | 52.5 | No | Normal | |
| 19 | May 2017 | 54 | Yes | Pregnant | |
| 23 | September 2017 | 52 | No | Normal | |
| 3 | 12 | October 2016 | 49 | No | Normal |
| 15 | January 2017 | 54.5 | No | Normal | |
| 19 | May 2017 | 54 | Yes | Pregnant | |
| 23 | September 2017 | 75 | – | With pup | |
| 4 | 6 | October 2016 | 39 | No | Normal |
| 9 | January 2017 | 46 | No | Normal | |
| 13 | May 2017 | 57 | Yes | Pregnant | |
| 17 | September 2017 | 49 | No | Normal | |
| 8 | 6 | October 2016 | 44 | No | Normal |
| 9 | January 2017 | 52.5 | No | Normal | |
| 13 | May 2017 | 58 | No | Normal | |
| 17 | September 2017 | 70 | – | With pup | |
| 9 | 6 | October 2016 | 50 | No | Normal |
| 9 | January 2017 | 57 | No | Normal | |
| 13 | May 2017 | 84 | – | With pup | |
| 17 | September 2017 | 74 | – | With pup | |
| 11 | 6 | October 2016 | 46 | No | Normal |
| 9 | January 2017 | 49.5 | No | Normal | |
| 13 | May 2017 | 51 | No | Normal | |
| 17 | September 2017 | 72 | – | With pup | |
| 12 | 6 | October 2016 | 45 | No | Normal |
| 9 | January 2017 | 50 | No | Normal | |
| 13 | May 2017 | 53 | No | Normal | |
| 17 | September 2017 | 53 | No | Normal | |
| 14 | 6 | October 2016 | 42 | No | Normal |
| 9 | January 2017 | 48.5 | No | Normal | |
| 13 | May 2017 | 52 | No | Normal | |
| 17 | September 2017 | 57 | No | Normal | |
| 15 | 6 | October 2016 | 42 | No | Normal |
| 9 | January 2017 | 47.5 | No | Normal | |
| 13 | May 2017 | 51 | No | Normal | |
| 17 | September 2017 | 72 | – | With pup | |
| 16 | 6 | October 2016 | 48 | No | Normal |
| 9 | January 2017 | 67.5 | Yes | Pregnant | |
| 13 | May 2017 | 65 | No | Normal | |
| 17 | September 2017 | 58 | No | Normal | |
| 17 | 6 | October 2016 | 48 | No | Normal |
| 9 | January 2017 | 51.5 | No | Normal | |
| 13 | May 2017 | 63 | No | Normal | |
| 17 | September 2017 | 58 | No | Normal | |
| 18 | 6 | October 2016 | 59 | No | Normal |
| 9 | January 2017 | 66.5 | No | Normal | |
| 13 | May 2017 | 76 | Yes | Pregnant | |
| 17 | September 2017 | 65 | No | Normal | |
| 19 | 6 | October 2016 | 47 | No | Normal |
| 9 | January 2017 | 53 | No | Normal | |
| 13 | May 2017 | 84 | – | With pup | |
| 17 | September 2017 | 78 | – | With pup | |
| 20 | 6 | October 2016 | 36 | No | Normal |
| 9 | January 2017 | 40 | No | Normal | |
| 13 | May 2017 | 45 | No | Normal | |
| 17 | September 2017 | 45 | No | Normal |
Table 5.
Female Eonycteris spelaea #ES1. This animal had 3 pregnancies and 3 pups in a span of 1 y *Weight was not taken during June 2018 check
| Months in captivity | Date | Eonycteris spelaea (ES#1) | |
|---|---|---|---|
| Weight (g) | Status | ||
| 26 | January 2018 | 64.0 g | Pregnant |
| 29 | April 2018 | 88.0 g | With pup |
| 30 | June 2018* | — | Pregnant |
| 31 | July 2018 | 75.0 g | With pup |
| 35 | October 2018 | 66.0 g | Pregnant |
| 37 | December 2018 | 69.0 g | With pup |
Morphometric tracking of pups born in the colony.
Colony-born bats were first identified as bats without a microchip. Nine of 35 pups born on different days were chosen for the morphometric study (Table 6). At approximately 3 to 4 mo old, their weights ranged from 31 to 43 g, total lengths ranged from 78 to 95 mm, and forearm lengths ranged from 55 to 66 mm (Table 6). After 7 to 15 mo in the colony, all pups had experienced a 12 to 27 g increase in weight (Table 6). Forearm length increased by 0.9 mm to 11.2 mm (Table 6). Female E. spelaea were fertile from 6 mo old.9,18 Of the 2 female pups, ES 37 was suspected to be pregnant 15 mo after birth due to the presence of an enlarged abdomen and weight of 66 g. All pups observed after health checks were bright, alert, and responsive after release into the cage.
Table 6.
Weight and forearm length gains of Eonycteris spelaea pups at 3 to 4 mo of age and again at 7 to 15 mo with difference between the 2 tests
| ES# | Sex | Weight (g) at 3–4 mo of age (g) | Weight (g) at 15 mo of age | Weight difference (g) between 15 and 3–4 mo of age | Forearm length (mm) at 3–4 mo of age | Length (mm) at 7 to 15 mo of age | Forearm length (mm) between values from 7 to 15 and 3 to 4 mo of age | Forearm length differences (mm) between 7 to 15 and 3 to 4 mo of age |
|---|---|---|---|---|---|---|---|---|
| 5 | Male | 36 | 53 | 17 | 57.3 | 66.7 | 57.3 (15 mo) | 9.4 |
| 37 | Female | 43 | 55 | 12 | 61.3 | 65.2 | 61.3 (7 mo) | 3.9 |
| 38 | Male | 35 | 62 | 27 | 58.9 | 65.9 | 58.9 (11 mo) | 7 |
| 39 | Male | 42 | 60 | 18 | 66 | 66.9 | 66.0 (11 mo) | 0.90 |
| 40 | Male | 40 | 62 | 22 | 61.4 | 67.5 | 61.4 (11 mo) | 6.1 |
| 52 | Male | 42 | 63 | 21 | 63.4 | 69.5 | 63.4 (11 mo) | 6.1 |
| 53 | Female | 38 | 54.8 | 16.8 | 60.4 | 67.2 | 60.4 (11 mo) | 6.8 |
| 54 | Female | 31 | 51 | 20 | 55.8 | 67 | 55.8 (11 mo) | 11.2 |
| 55 | Male | 43 | 66.5 | 23.5 | 62.1 | 70.3 | 62.1 (11 mo) | 8.2 |
Diet.
The first diet used was adapted from a previous description.14 That formulation featured a blend of 2 mashed bananas, 500 mL apple juice, 20 g sucrose, 15 g glucose, 54 g infant formula (Abbott, Singapore), and 500 mL water. To complement this diet, store bought pollen (HoneyWorld, Singapore) can be incorporated into the blend. Each bat is estimated to feed on 20 mL of this diet per day.13 In the initial stage for the captive colony, a combination of a liquid diet consisting of apple juice blended with glucose powder, white sugar, and milk powder was given from Oct 2015 to June 2016. Water was given separately in another feeding bowl. The volume of this concoction was provided in surplus to be sure the bats had enough food. This formulation was palatable to bats as little leftover was recorded (Table 7). However, apple juice caused the milk to solidify when mixed, and sediments formed on the bottom of the feed pan. This can potentially cause impaired protein intake from milk powder. After a May 2016 consultation with Tolga Bat Hospital, the diet was further optimized by including Wombaroo Lorikeet and Honeyeater Food and salt water prepared inhouse and removing white sugar and apple juice (Table 1). Although the original diet provided the bats with enough sugar, bats require minerals including sodium, which are lacking in fruits.10,11 The Wombaroo diet contains 12% protein, 5% fat, 76% carbohydrate, 4% ash, 3% moisture, and 17 MJ/kg energy.27 The final formulation provided carbohydrates, protein, fat, essential amino acids, vitamins, and salt. After introduction of the new formulation, the food provided was consumed with little leftover. (Table 7).
Table 7.
Eonycteris spelaea diet given over amount eaten over a 3-mo average after optimization
| Diet intake during a 3-mo period before Diet Optimization | Diet intake during a 3-mo period after Diet Optimization | ||||
|---|---|---|---|---|---|
| Substance | Amount Provided | Amount Consumed | Substance | Amount Provided | Amount Consumed |
| Liquid diet | 250 mL | 247mL | Liquid diet | 500 mL | 250 mL |
| Fruit slices | 4 | 4 | Wombaroo diet | 500 mL | 500 mL |
| Water | 250 mL | 122 mL | Fruit slices | 10 | 10 |
| Salt water | 250 mL | 62 mL | |||
| Water | 750 mL | 379 mL | |||
Discussion
We identified a number of factors that are essential in establishing an E. spelaea breeding colony. The first challenge was to create housing conditions that allowed the bats to breed, roost, and feed. A breeding colony of Carollia perspicillata in Germany used cages 81 cm in long, 170 cm wide, and 77 cm high. Cages were kept in rooms with regulated light cycles. During the light phase, black plastic sheets were hung on top of cages to provide shade and concealment in the cage.23 In comparison, our housing featured 2 cages with combined measurements of 200 cm long, 100 cm wide, and 76 cm high, providing ample space for bats to fly. Hessian fabric retains the scent of the bats, providing an additional sense of security and familiarity. Our cage design allows cages to be linked together to accommodate colony expansion. No injuries were seen during health checks, indicating that the cage design is not detrimental to the safety of juvenile and adult bats.
The artificial environment (cages) must be coupled with a suitable diet. Therefore, the diet was optimized to provide adequate nutrients for the bats, as a commercially available feed is not available for nectivorous bats. The optimized diet included Wombaroo diet, which contains the required nutrients for all nectar-eating birds. The Wombaroo diet was designed to recreate the texture and nutritional contents of nectar and provide sufficient protein like pollen.27
After introduction, bats were monitored for abnormal behavior such as self-isolation and weight loss, which is often an indication of stress or illness. Bats ES 6, 13, and 18 lost weight, potentially due to problems acclimatizing to the diet and housing. Over 9 to 24 mo, these 3 bats gained weight, suggesting that they had successfully adapted to the colony. Generally, fruit eating bats are bimodally polyestrous, generating 2 offspring per year.22 Our E. spelaea colony showed signs of pregnancy after 9 to 15 mo in the colony. Similarly, in the C. perspicillata breeding colony, breeding started after 11 to 12 mo.23 ES 1 produced 3 offspring from 3 separate birthing events between January and December 2018, potentially implying that given proper housing conditions and ample food, breeding can be aseasonal. Also, given an abundance of resources and the lack of natural threats, the number of pups an E. spelaea female could produce in any given year is solely restricted by the bat’s ovulation cycle. Another plausible explanation was that ES 1 had undergone superfetation which means a second conception occurred in a pregnant mammal.3 Bats are known to have developed multiple reproductive strategies such as sperm storage, delayed fertilization, and delayed implantation.17,22 More in-depth studies will need to be conducted to investigate superfetation and possible delayed reproduction in the colony.
A healthy fur coat and responsiveness were used as markers of general good health of the bats. No signs of injury, joint inflammation, or drastic loss of weight were seen. In the 16 mo period from introduction of the wild bats into the colony to the birth of new pups, no sign of trauma was observed in adult bats or pups. Taken together, the artificial housing and diet created a conducive environment for the bats to thrive.
The study of bats and their responses to infection is essential in understanding the transmission and infection dynamics of viruses. Despite experiencing viral infections, bats generally do not display any clinical signs of disease. Learning more about the mechanisms used by the bat immune system to coexist with or evade viruses is relevant to both human and animal health.1 The immunologic profile of bats varies during migration seasons and among species.2,24 Previous and ongoing studies on bats have relied on wild caught bats whose health and immune status varies among the sampled bats.15 Thus, the results of these studies may be skewed because the baseline is unclear. Access to a breeding colony mitigates some of these challenges. Furthermore, the study of bat-virus interaction is presently hindered the lack of species-appropriate molecular biology reagents, indicating the urgent need to allocate resources and collaborative efforts to further investigate the synergy and relationship between bats and bat-borne viruses.2 While hurdles still exist in bat research, this breeding colony represents a step forward in understanding the bat immune system. This study concludes the successful establishment of the first E. spelaea breeding colony in Asia. Bats produced in the colony and their samples are currently being used in ongoing studies.19,20,26,28
Acknowledgments
We would like to thank the Veterinary team from NLARF, especially Dr Marvin Mataquel Taguiam, Ryan Cabo Maniquiz, and Ranjit Kumar for the assistance in veterinary needs and execution of daily Bat animal care program. We also thank Chun Kwong Chan for technical support in the bat facility. We would like to acknowledge Dr Xing Lou Yang (Wuhan Institute of Virology) for providing the filovirus L gene positive control. We thank Jennefer Mclean from Tolga Bat Hospital for her consultation in husbandry requirements. We also thank Dr Danielle Anderson, Dr Tanu Chawla, and Mr Ben Tan Kian Tong for constructive review of this manuscript. These studies were funded by NRF-CRP (NRF-CRP10-2012-05). We thank SingHealth IACUC, NParks and its AVS cluster for support in establishment of the bat colony.
Author contributions
L.-F.W., J.H.J.N. and Y.T.C. conceptualized and designed the study. R.F. and Y.Y.H. prepared the manuscript. L.-FW, J.H.J.N., I.H.M., Y.T.C and W.N.C revised the manuscript. Y.T.C., R.F., Y.Y.H., Lee P.Y-H., I.H.M., D.L.H.W., S.A.B., W.N.C., K.P.S., R.F. and Ng J.H.J. caught wild bats. J.H.J.N., Y.T.C., R.F., Y.Y.H., W.N.C., A.E.Z.K. and K.P.S. performed health screens and experiments. E.M.P and R.Y. perform health screens and husbandry. N.B.S. manages the facility operations.
References
- 1.Ahn M, Anderson DE, Zhang Q, Tan CW, Lim BL, Luko K, Wen M, Chia WN, Mani S, Wang LC, Ng JHJ, Sobota RM, Dutertre CA, Ginhoux F, Shi ZL, Irving AT, Wang LF. 2019. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat Microbiol 4:789–799. 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Baker ML, Kulcsar K, Misra V, Plowright R, Mossman K. 2020. Novel Insights Into Immune Systems of Bats. Front Immunol 11:26. 10.3389/fimmu.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat H, Sreenivasan M, Jacob PG. 1980. Breeding cycle of Eonycteris spelaea (Dobson, 1871) (Chiroptera, Pteropidae, Macroglossinae) in India. Mammalia 44:343–348. 10.1515/mamm.1980.44.3.343. [DOI] [Google Scholar]
- 4.Bourhy H, Cowley JA, Larrous F, Holmes EC, Walker PJ. 2005. Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. J Gen Virol 86:2849–2858. 10.1099/vir.0.81128-0. [DOI] [PubMed] [Google Scholar]
- 5.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 6.Gamage AM, Zhu F, Ahn M, Foo RJH, Hey YY, Low DHW, Mendenhall IH, Dutertre CA, Wang LF. 2020. Immunophenotyping monocytes, macrophages and granulocytes in the Pteropodid bat Eonycteris spelaea. Sci Rep 10:309. 10.1038/s41598-019-57212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayman DT, Bowen RA, Cryan PM, McCracken GF, O’Shea TJ, Peel AJ, Gilbert A, Webb CT, Wood JL. 2013. Ecology of zoonotic infectious diseases in bats: Current knowledge and future directions. Zoonoses Public Health 60:2–21. 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He B, Feng Y, Zhang H, Xu L, Yang W, Zhang Y, Li X, Tu C. 2015. Filovirus RNA in Fruit Bats, China. Emerg Infect Dis 21:1675–1677. 10.3201/eid2109.150260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heideman PD, Utzurrum RC. 2003. Seasonality and synchrony of reproduction in three species of nectarivorous Philippines bats. BMC Ecol 3:11–25. 10.1186/1472-6785-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iudica CA, Bonaccorso FJ. 2003. Anecdotal observations of seawater ingestion by flying foxes of the genus Pteropus (Chiroptera: Pteropodidae). Mammalia 67:455–458. [Google Scholar]
- 11.Klaus G, Schmidg B. 1998. Geophagy at natural licks and mammal ecology: a review. Mammalia 62:482–498. 10.1515/mamm.1998.62.4.482b. [DOI] [Google Scholar]
- 12.Krutzsch PH. 2005. Reproductive anatomy and cyclicity of the bat Eonycteris spelaea Dobson (Chiroptera: Pteropodidae) in West Malaysia. Acta Chiropterol 7:51–64. 10.3161/1733-5329(2005)7[51:RAACOT]2.0.CO;2. [DOI] [Google Scholar]
- 13.Law B. 1993. Sugar preferences of the Queensland blossom bat, Syconycteris australis: a pilot study. Aust Mammal 16:17–21. 10.1071/AM93003. [DOI] [Google Scholar]
- 14.Law B. 2011. Bats in captivity. Washington (DC): Logos Press. [Google Scholar]
- 15.Martínez Gómez JM, Periasamy P, Dutertre CA, Irving AT, Ng JH, Crameri G, Baker ML, Ginhoux F, Wang LF, Alonso S. 2016. Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto. Sci Rep 6:37796. 10.1038/srep37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng JH, Tachedjian M, Deakin J, Wynne JW, Cui J, Haring V, Broz I, Chen H, Belov K, Wang LF, Baker ML. 2016. Evolution and comparative analysis of the bat MHC-I region. Sci Rep 6:21256. 10.1038/srep21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak RM, Walker EP. 1994. Walker’s bats of the world. Johns Hopkins University Press. [Google Scholar]
- 18.Nowak RM, Walker EP. 1999. Walker’s Mammals of the World, 6th edition. Johns Hopkins University press. [Google Scholar]
- 19.Paskey AC, Ng JH, Rice GK, Chia WN, Philipson CW, Foo RJ, Cer RZ, Long KA, Lueder MR, Frey KG. 2020. The temporal RNA virome patterns of a lesser dawn bat (Eonycteris spelaea) colony revealed by deep sequencing. Virus Evol 6:veaa017. 10.1093/ve/veaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paskey AC, Ng JHJ, Rice GK, Chia WN, Philipson CW, Foo RJH, Cer RZ, Long KA, Lueder MR, Lim XF, Frey KG, Hamilton T, Anderson DE, Laing ED, Mendenhall IH, Smith GJ, Wang LF, Bishop-Lilly KA. 2020. Detection of Recombinant Rousettus Bat Coronavirus GCCDC1 in Lesser Dawn Bats (Eonycteris spelaea) in Singapore. Viruses 12:539. 10.3390/v12050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon LL, Chu DK, Chan KH, Wong OK, Ellis TM, Leung YH, Lau SK, Woo PC, Suen KY, Yuen KY, Guan Y, Peiris JS. 2005. Identification of a novel coronavirus in bats. J Virol 79:2001–2009. 10.1128/JVI.79.4.2001-2009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racey PA, Entwistle AC. Life-history and reproductive strategies of bats, p. 363–414. In: Crichton EG, Krutzsch PH, editors. Reproductive biology of bats. Elsevier. [Google Scholar]
- 23.Rasweiler JJ, Badwaik NK. 1996. Improved procedures for maintaining and breeding the short-tailed fruit bat (Carollia perspicillata) in a laboratory setting. Lab Anim 30:171–181. 10.1258/002367796780865718. [DOI] [PubMed] [Google Scholar]
- 24.Voigt CC, Fritze M, Lindecke O, Costantini D, Petersons G, Czirjak GA. 2020. The immune response of bats differs between pre-migration and migration seasons. Sci Rep 10:17384. 10.1038/s41598-020-74473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldien DLAS, Wilson Z. [Internet]. 2020. Eonycteris spelaea. The IUCN Red List of Threatened Species 2020. [Cited 1 August 2020]. Available at: https://www.iucnredlist.org/species/7787/22128326
- 26.Wen M, Ng JHJ, Zhu F, Chionh YT, Chia WN, Mendenhall IH, Lee BP, Irving AT, Wang LF. 2018. Exploring the genome and transcriptome of the cave nectar bat Eonycteris spelaea with PacBio long-read sequencing. Gigascience 7:giy116. 10.1093/gigascience/giy116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wombaroo Food Products. [Internet]. 2022. Food Products Wombaroo [Cited 1 August 2020]. Available at: https://www.wombaroo.com.au/product/lorikeet-honeyeater-food/.
- 28.Yong KSM, Ng JHJ, Her Z, Hey YY, Tan SY, Tan WWS, Irac SE, Liu M, Chan XY, Gunawan M, Foo RJH, Low DHW, Mendenhall IH, Chionh YT, Dutertre CA, Chen Q, Wang LF. 2018. Bat-mouse bone marrow chimera: a novel animal model for dissecting the uniqueness of the bat immune system. Sci Rep 8:4726. 10.1038/s41598-018-22899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7.Validation of Sanitization Practices in Single-use Individually Ventilated Mouse Cages at Standard and Thermoneutral Temperatures [DOI] [PMC free article] [PubMed] [Google Scholar]