Abstract
Isoflurane has been characterized as a distressing agent for rodents, causing both physiologic and behavioral effects. Using a “darkened home cage” has been recommended during CO2 administration for rodent euthanasia; this is arguably a similar animal experience to anesthetic induction with isoflurane. Based on the premise that rodents perceive red light as darkness via the primary optic tract, we compared physiologic and behavioral markers of stress in 2 inbred strains of mice (C57BL/6J and BALB/cJ) anesthetized with isoflurane in either a red-tinted (dark) induction chamber or a traditional translucent induction chamber. Physiologic stress was assessed based on plasma levels of norepinephrine, epinephrine, and corticosterone. Stress-related behaviors (rearing, face wiping, and jumping) were recorded on video and scored from initiation of induction to loss of consciousness. No significant correlations were found between chamber type and physiologic stress hormones. As compared with the translucent chamber, stress-related behaviors were more frequent in the red-tinted chamber, including: 1) significantly higher rearing frequencies in BALB/cJ mice; 2) higher behavioral stress scores in BALB/cJ and male C57BL/6J mice; and 3) more face wiping behavior when considering all mice combined. These findings suggest that mice do not experience significant alleviation of physiologic indices of stress when anesthetized in a red-tinted induction chamber. Furthermore, isoflurane induction in the red-tinted chamber appeared to increase the expression of stress-related behaviors, particularly in BALB/cJ mice. Based on our findings and a growing body of literature on the unintended effects of red light, we do not recommend using red-tinted chambers for induction of anesthesia in mice.
Abbreviations and Acronyms: RPE, retinal pigment epithelium; SAM, sympathetic-adrenomedullary
Introduction
Isoflurane has been described as a distressing agent capable of inducing an acute physiologic stress response in rats and mice. Numerous publications using these species show that exposure to isoflurane anesthesia can increase circulating corticosterone, a common marker of physiologic stress.10,37 Compared with those that did not receive isoflurane, female rats that had been anesthetized with isoflurane prior to rapid decapitation had significantly higher plasma corticosterone levels.10 In another publication, serum corticosterone levels in mice were significantly higher within 5 min after exposure to isoflurane alone and were not significantly different from the isoflurane plus vasectomy group.37 When directly compared with other methods of anesthesia, the findings on isoflurane are less conclusive. Rats anesthetized using isoflurane for blood collection had elevations of both plasma corticosterone and blood glucose and reduction in melatonin levels as compared with rats anesthetized using CO2.67 In contrast, another publication found a reduction in corticosterone in rats exposed to repeated isoflurane as compared with CO2-O2 anesthesia.1 Another study found that catecholamine (norepinephrine and epinephrine) levels were significantly lower in mice anesthetized with isoflurane or sevoflurane as compared with CO2 at 60 and 80% filling rates.46 Given these mixed findings and the common use of isoflurane anesthesia in rodents, further characterization of the physiologic stress response is warranted.
Isoflurane exposure in rodents has also been shown to affect behavior. In mice, a single exposure to isoflurane has been shown to increase anxiety, induce attention deficits, and impair learning in the postanesthetic period.69 Beyond experimental parameters, normal behaviors such as nest-building and burrowing can be negatively affected after one anesthetic event with isoflurane.23,32 Furthermore, as compared with a single exposure, repeated exposure to isoflurane can be even more stressful.23 Particularly in female mice, repeated exposure to isoflurane reduces exploratory behavior and decreases food intake.23 In light-dark preference tests using both mice and rats, animals withdrew to the light compartment more quickly after repeated exposures to isoflurane in the dark compartment, indicating a possible learned aversion over time.50,64 Thus, evidence indicates that isoflurane anesthesia induces physiologic and behavioral manifestations of stress that could affect both rodent welfare and research integrity.
To our knowledge, only one study has evaluated the use of environmental manipulation, specifically home cage bedding, to reduce stress during isoflurane anesthesia of mice.56 In that study, adding home cage bedding to the induction chamber did not significantly reduce cortisol or corticosterone during isoflurane anesthesia.56 No published studies to date have evaluated the use of another purported stress modification tool for rodents: red tint. The use of commercially available, red-tinted products is based on the rodent retinal cell composition, which lacks long wavelength cone opsins. Because red light is comprised of long wavelengths (≥ 620 nm), rodents are postulated to be unable to perceive red light (that is, for rodents, red light is equivalent to darkness).39,52,54 Rodent facilities around the world use red window film or red overhead lighting to safely observe animals during dark cycles, and structural enrichment is often provided in the form of red-tinted huts or tunnels. Although this practice persists, numerous publications indicate that chronic exposure to red light and red-tinted products have significant effects on neurobehavioral and neuroendocrine parameters of rodents.24,34,43,48 Use of dark phase red ‘safelights’ for one week in a facility caused significant reductions of plasma melatonin levels in rats.18 Circadian plasma levels of corticosterone were also shown to be affected by red lights.18 Similarly, housing nude rats in red-tinted caging for one week led to significant reductions in plasma melatonin levels and other analytes.17 Although the photobiology of red light or red tint is not fully understood in rodents, mice and rats do display a preference for darkness and exhibit anxiety-like behavior with room light exposure.6,42,47 Under the premise that rodents perceive red light as darkness, companies continue to manufacture and sell red-tinted products advertised as ‘low stress,’ including anesthetic induction chambers. In addition, the 2020 AVMA Guidelines for the Euthanasia of Animals states that when inhaled agents are being administered, rodents should be housed where they are most comfortable, such as in a “darkened home cage.”45 This guidance is based on limited data indicating that reduced light exposure during euthanasia, such as dimly lit procedure rooms, can reduce stress-related behaviors.55
The overall aim of the current study was to compare the physiologic and behavioral effects of isoflurane anesthesia in mice undergoing isoflurane anesthetic induction in either a red-tinted induction chamber or a traditional translucent induction chamber. Physiologic stress measures included the functional components of the sympathetic-adrenomedullary system (SAM; norepinephrine, epinephrine) and hypothalamic–pituitary–adrenal axis (HPA axis; corticosterone) to allow direct comparison between each pathway.13,31 Behavioral parameters of stress were selected based on previously published ethograms as indicators of anxiety (jumping, face wiping) or exploration (rearing).13,31 We hypothesized that mice anesthetized in a red-tinted chamber would exhibit lower levels of physiologic stress markers and fewer stress-related behaviors as compared with mice anesthetized in a traditional translucent induction chamber. Furthermore, we hypothesized that this effect would be greater in C57BL/6J mice as compared with BALB/cJ mice due to their differences in retinal pigmentation and visual capacity.29,54
Materials and Methods
Animal care and use was approved by the IACUC and performed at Vanderbilt University Medical Center (VUMC), an AAALAC-accredited program. Studies were conducted in accordance with standards set forth in the Guide for Care and Use of Laboratory Animals, 8th edition.35
Mice.
Two inbred mice strains (BALB/cJ and C57BL/6J) were evaluated using experimentally naïve male and female mice (10 wk of age). These strains were selected due to differences in retinal pigmentation and their common use in research.29,57 All mice were obtained directly from The Jackson Laboratory (Bar Harbor, ME) and housed in VUMC’s SPF barrier facility. Initial and quarterly sentinel testing was performed to ensure that mice remained negative for ectromelia virus, GDVII (Theiler) virus, Hantavirus, K virus, LDH elevating virus, lymphocytic choriomeningitis virus, mouse adenovirus, mouse cytomegalovirus, murine chapparvovirus, mouse hepatitis virus, mouse minute virus, mouse norovirus, mouse parvovirus, mouse thymic virus, pneumonia virus of mice, polyoma virus, reovirus 3, rotavirus, Sendai virus, Bordetella spp. Citrobacter rodentium, Clostridium piliforme, Corynebacterium bovis, Corynebacterium kutscheri, Filobacterium rodentium, Mycoplasma pulmonis, Mycoplasma spp., Salmonella spp., Streptobacillus moniliformis, Helicobacter spp., Klebsiella pneumoniae, Klebsiella oxytoca, Pasteurella multocida, Rodentibacter pneumotropicus, Pneumocystis murina, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, Beta-hemolytic Streptococcus spp., Yersinia enterocolitica, Yersinia pseudotuberculosis, Encephalitozoon cuniculi, Toxoplasma gondii, dermatophytes, fleas, lice, fur mites, follicle mites, tapeworms, pinworms and other helminths, trichomonads, and protozoa (specifically Giardia and Spironucleus).
Mice were evenly divided by sex and strain across 2 shipments, with approximately the same acclimation time for all mice prior to experimentation. Upon arrival, mice were group-housed (5 per cage) in sterile individually ventilated cages from Allentown (XJ polysulfone translucent clear, catalog number 410821-1; Allentown, NJ). Stainless steel wire bar lids (catalog number 311639; Allentown) and polysulfone translucent microfilter tops (catalog number 224423-1, Allentown) were used for all caging. The housing room was maintained at 68 to 76 °F (20 to 24 °C), 30% to 70% humidity, on a 12:12-h light:dark cycle (with lights on at 0600). Lighting in the housing room consisted of 28 ballasts with 2 white fluorescent lamps each (model no. ESEFI4332PB277PAF2HI, 277V, Kenall Manufacturing, Kenosha, WI); the room was completely devoid of light contamination during the dark phase. Mice were housed on paper-based bedding with cotton squares (ALPHA-dri PLUS, Shepherd Specialty Papers, Watertown, TN). Irradiated food (LabDiet 5LOD, Purina, St. Louis, MO) and reverse-osmosis, acidified, autoclaved water were provided ad libitum. Three days after arrival, cage change was performed in a standard laminar flow hood (Nuaire, Plymouth, MN), with mice picked up by their tail and separated into individual housing. Once individually housed, mice were maintained under the same conditions with the addition of a shreddable mouse hut (Otto Environmental, Greenfield, WI). A peroxide-derived spray (Peroxigard, Virox Technologies, Oakville, ON, Canada) was used to disinfect gloves and the laminar flow hood between cages.
Experimental design.
To minimize the impact of social isolation on the acute stress response caused by experimental treatment, mice were acclimated to individual housing for at least 10 d prior to experimentation. The entire study population (24 male and 24 female BALB/cJ, 24 male and 24 female C57BL/6J) was randomized within sex and strain per shipment using a permuted block design to randomize experimental day and treatment. On each experimental day, 6 mice were randomly assigned to the red-tinted chamber (Large Red Passive Induction Chamber, product no. IR-3005, Somni Scientific, South Park Township, PA) and 6 were assigned to the traditional translucent chamber (Large Clear Passive Induction Chamber, product no. IP-3004, Somni Scientific South Park Township, PA). Spectral transmission of each chamber was measured at room temperature using a spectrophotometer (Cary 5000 UV-VIS-NIR, Agilent Technologies, Santa Clara, CA). In addition, a light meter (Extech Instruments, Nashua, NH) placed at the bottom and center of each chamber (roughly eye level of the mouse) provided readings of 35 lux in the red chamber and 650 lux in the same location in the traditional chamber. This one-time measurement was performed in the procedure room in the same biologic safety cabinet (BSC; Nuaire, Plymouth, MN) used for isoflurane induction.
Isoflurane anesthesia.
Anesthesia was performed in a procedure space adjacent to the housing room within the barrier facility. At the beginning of each experimental day, the biologic safety cabinet (Nuaire, Plymouth, MN), anesthetic tubing, and adjacent counterspace were disinfected using a peroxide-derived spray (Peroxigard, Virox Technologies, Oakville, ON, Canada). Per manufacturer recommendations, MB10 (Quip Labs, Wilmington, DE) was used to disinfect each induction chamber between mice. One at a time, mice from the daily assigned sex and strain were randomly selected from the rack and taken into the adjacent procedure space. Once placed in the biologic safety cabinet, each mouse was immediately picked up by the tail and transferred on the operator’s hand from its home cage into the assigned anesthesia chamber. Once placed into the chamber, mice were given 2 min for acclimation prior to induction. After this acclimation period, a standard vaporizer (RC2 Rodent Circuit Controller, VetEquip, Livermore, CA) was set to 4% and isoflurane was immediately administered at a volume displacement rate of 20% (about 1 L/min). The isoflurane concentration and volume displacement rate were selected to allow sufficient time for observation of acute behavioral and physiologic responses while still mimicking anesthetic settings that would be used in a typical research environment. At the start of induction, a camcorder (Handycam HDR-CX405, Sony, Tokyo, Japan) adjacent to the chamber was set to record. Recording continued until the mouse displayed a ‘head bob,’ or the point at which the head dropped down and intentional movement ceased. This parameter has been previously correlated with loss of righting reflex and subsequent loss of consciousness.31,49 After confirming a surgical plane of anesthesia via loss of the paw withdrawal reflex, the mouse was removed from the chamber and placed on a nosecone. Isoflurane was maintained at 4% and the flow setting was adjusted to 0.5 L/min. After a second confirmation of absent paw withdrawal, blood was collected via terminal cardiac puncture using a heparinized syringe; cervical dislocation was then performed to ensure death. Blood samples were divided into 2 tubes, one treated with EGTA-glutathione for catecholamine analysis and one untreated for corticosterone analysis. Blood samples were then centrifuged, the supernatant was transferred to a clean tube, and samples were placed on wet ice. To maintain consistency and reduce circadian variation across days, data collection occurred between 0900 to 1400 on each experimental day.
Plasma catecholamines and corticosterone.
Plasma for measurement of catecholamines and corticosterone were stored at −80 °C and −20 °C respectively until further processing. Catecholamine levels were measured using HPLC, and corticosterone levels were measured using the Corticosterone Double Antibody RIA Kit (MP Biomedicals, Irvine, CA). Both assays were performed by the Hormone Assay and Analytical Services Core (Vanderbilt University, Nashville, TN; supported by NIH grants DK059637 and DK020593).
Behavioral scoring.
Two observers independently viewed the digital recordings to score behaviors for each mouse. Due to the obvious difference in chamber color and coat color, blinding the observers to treatment or strain was impossible. To address this, one of the observers was not familiar with the study and received no prior information regarding study hypothesis or objectives. Behavioral scoring was based on the frequency of 3 well-defined behaviors: rearing, jumping, and face wiping. Rearing was defined as both forelimbs in the air while the mouse remained on its hindlimbs. Jumping was characterized by all 4 limbs in the air at the same time. Face wiping was considered to be intentional touching of the muzzle or eyes using the front paws. Each behavior has been previously defined in ethograms and used as behavioral indicators of stress or anxiety.31 Once the frequency of these behaviors was determined, the frequencies were averaged between observers and given a score (Figure 1). All 3 scores were then added and totaled to determine a ‘Behavioral Stress Score,’ defined as mild, moderate, or severe. Under the assumption that rearing at low frequency is a normal exploratory behavior, rearing scores were adjusted posthoc to encompass the range of rearing frequency observed. The rearing scores were then factored into our calculations to obtain an adjusted behavioral stress score for each mouse.
Figure 1.
(A) Mouse behavioral scoring sheet used for assessment during video recordings. Rearing scores were adjusted posthoc to better capture the range of rearing frequency. (B) Original behavioral scoring for readers’ reference.
Statistical analysis.
A sample size of 12 animals per treatment group was selected based on similar behavioral studies and a power analysis based on corticosterone measurement (α 0.05; 90% power; 1 SD of 39 ng/mL).31 For statistical purposes, each mouse was treated as one unit of analysis.
We assessed normality of each data set using Shapiro-Wilk tests. To analyze our primary outcome measures, we performed a linear regression analysis. In this model, we separately regressed each hormone (norepinephrine, epinephrine, and corticosterone) against treatment and adjusted for both sex, and strain (StataCorp. Stata Statistical Software: Release 17, College Station, TX). We compared the frequency of both specific and composite behavioral scores between treatment, sex, and strain as secondary outcome measures using one-way ANOVA (for normally distributed measures) or Kruskal-Wallis (for nonnormally distributed measures). When significant effects were found, pairwise comparisons were performed using either the Tukey test (for normally distributed measures) or the Mann-Whitney U test (for nonnormally distributed measures). For infrequent individual behaviors (jumping, face wiping), we performed the Fisher exact test of proportions. To assess interobserver agreement, weighted κ statistics were determined for each behavioral measure (StataCorp. Stata Statistical Software: Release 17, College Station, TX). All behavioral statistical analyses and pairwise comparisons for both primary and secondary outcomes were performed using Prism 9.2 (GraphPad Software, La Jolla, CA). Differences were only considered significant at P values less than 0.05.
Results
Chamber lighting measurements.
The spectral transmittance distribution of each chamber is shown in Figure 2. While the traditional chamber displayed high transmittance throughout the visible spectrum, the red-tinted chamber displayed high transmittance only for wavelengths above 600 nm. Calculated radiometric and photometric values are shown in Figure 3.54 Due to differences in illuminance (lux) between the chambers, both irradiance and photon flux were markedly higher in the translucent chamber as compared with the red-tinted chamber.
Figure 2.

Transmission spectrum of both the red-tinted and traditional chambers used. Values plotted include the transmittance (%) and wavelength (nm).
Figure 3.
Radiometric and photometric values for the traditional and red-tinted chambers. Calculations made using the Rodent Toolbox spreadsheet.45
Plasma catecholamines and corticosterone.
A total of 12 hemolyzed plasma samples were excluded from catecholamine (n = 84) and corticosterone (n = 81) analysis due to potential interference.53 An additional 3 female C57BL/6J mice were excluded from corticosterone analysis due to inadequate sample volume. One male BALB/cJ mouse was excluded from behavioral analysis due to video recording failure (n = 95).
Regression of plasma norepinephrine, epinephrine, and corticosterone levels against treatment were adjusted for both sex and strain (Table 1). Mean plasma norepinephrine, epinephrine, and corticosterone levels across all groups are shown in Figures 4 and 5. No significant correlations were found based on chamber type among all mice. Significant correlations were found for norepinephrine based on strain and for epinephrine based on sex, but these differences were not significant according to pairwise Mann Whitney U tests. However, significant correlations and differences were found for epinephrine based on strain (P < 0.001; Figure 5C) and for corticosterone based on both strain (P < 0.0001, Figure 5D) and sex (P ≤ 0.05, Figure 5E). Further information on significant differences in panels C, D, and E can be found in Table 2.
Table 1.
Linear regression of plasma catecholamines and corticosterone.
| Hormones | Chamber Type | Sex | Strain |
|---|---|---|---|
| Norepinephrine | −293 | 597 | 828* |
| R2 = 0.076 | [−1090,503] | [−203,1398] | [26,1631] |
| F(3, 80)= 2.19 | |||
| Epinephrine | −39 | 1998** | 682** |
| R2 = 0.462 | [−531,454] | [1503,2493] | [186,1178] |
| F(3, 80)= 22.85 | |||
| Corticosterone | −7 | 213** | 133** |
| R2 = 0.640 | [−48,35] | [172,255] | [91,175] |
| F(3, 77)= 45.75 | |||
| Correlation coefficient
and [95% confidence interval] shown for each hormone
level against treatment, sex, and strain. *=
P < 0.05
and **= P < 0.01. | |||
Figure 4.
Comparison of mean plasma norepinephrine level across all groups. No significant correlations were found based on chamber type, sex, or strain.
Figure 5.
(A) Comparison of mean plasma epinephrine level across all groups. No significant correlation was found based on chamber type or sex. (B) Comparison of mean plasma corticosterone level across all groups. No significant correlation was found based on chamber type. (C) Comparison of epinephrine levels between strains indicated significantly higher epinephrine levels in BALB/cJ mice (P < 0.0001). Comparison of corticosterone levels by (D) strain and (E) sex found significantly higher corticosterone levels in both BALB/cJ (P < 0.0001) and female (P ≤ 0.05) groups, respectively.
Table 2.
Grouped plasma epinephrine and corticosterone values are shown as mean ± SD.
| Chamber Type | |||
|---|---|---|---|
| Hormone | Red-Tinted | Traditional | |
| Epinephrine | C57BL/6J | 204 ± 69 | 183 ± 84 |
| BALB/cJ | 2169.5 ± 1832** | 2080 ± 1442** | |
| Corticosterone | C57BL/6J | 206.7 ± 94 | 165 ± 70 |
| BALB/cJ | 380.3 ± 104** | 400 ± 165** | |
| Males | 240.6 ± 117 | 223 ± 130 | |
| Females | 357.9 ± 121* | 344 ± 191* | |
| Following ANOVA for significance, P values were generated from post hoc testing (Tukey’s test for multiple comparisons). Differences were considered highly significant and significant at P values less than 0.0001 (2 ** per result) and 0.05 (one * per result), respectively. Significant differences were only seen between strains or sexes within a given chamber type and not between chambers. | |||
Average rearing frequency.
To obtain frequency per minute, rearing frequency for each mouse was divided by time from isoflurane initiation to loss of consciousness (‘head bob’).31,49 This value was then averaged between observers and compared across chamber type, sex, and strain.
Shapiro-Wilk test indicated a normal distribution of frequency data (P = 0.4011). One-way analyses of variance revealed a highly significant difference among average rearing frequencies (P < 0.0001). The Tukey test for multiple comparisons indicated that C57BL/6J mice did not display differences in rearing frequency based on chamber type for either males (P = 0.45) or females (P = 0.90); however, both male (P < 0.0001) and female (P < 0.0001) BALB/cJ mice displayed more rearing behavior in the red-tinted chamber as compared with the translucent chamber (Figure 6). When comparing between strains, BALB/cJ males in the red-tinted chamber had higher rearing frequencies than did C57BL/6J males (P = 0.0023). In the translucent chamber, C57BL/6J females showed more rearing than did the BALB/cJ females (P < 0.0001). Within BALB/cJ and C57BL6/J strains respectively, males and females showed no significant differences in rearing when tested in either red-tinted (P = 0.44; P = 0.56) or translucent chambers (P > 0.99; P = 0.15).
Figure 6.
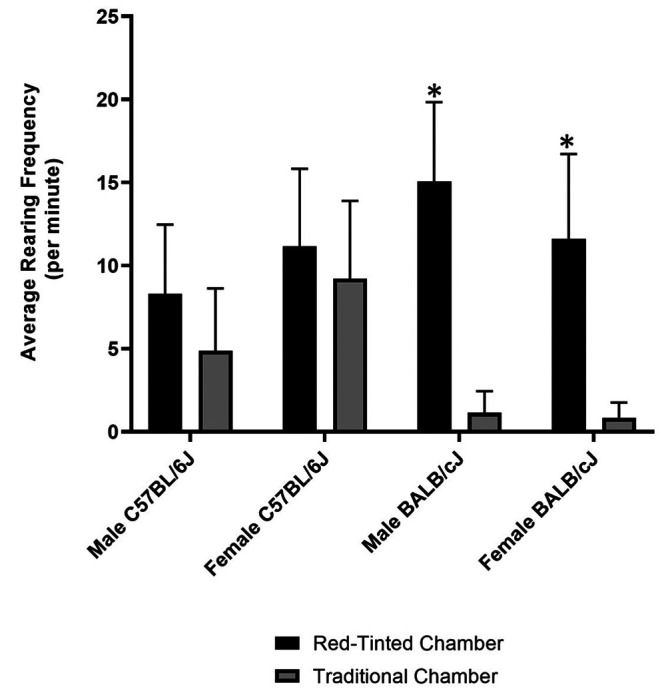
Comparison of average rearing frequency (per minute) across all groups. Asterisks indicate highly significant differences (P < 0.0001) between the red-tinted chamber compared with the traditional chamber.
Adjusted behavioral stress scores.
The Shapiro-Wilk test indicated that behavioral stress scores had a nonnormal distribution (P = 0.0024). After performing a Kruskal Wallis test for significance (P < 0.0001), multiple Mann-Whitney U tests were used. Numerous significant interactions were found between experimental groups as presented in Table 3, which also includes median behavioral stress scores for all 8 experimental groups. Higher stress scores were found for BALB/cJ males (P < 0.0001), BALB/cJ females (P < 0.0001), and C57BL/6J males (P = 0.017) when using the red-tinted chamber as compared with the translucent chamber (Figure 7). In the translucent chamber groups, C57BL/6J females had higher stress scores than both BALB/cJ females (P = 0.0001) and C57BL/6J males (P = 0.013).
Table 3.
Mann-Whitney U test for adjusted behavioral stress scores across all groups.
| Mann-Whitney U | ||||
|---|---|---|---|---|
| Group Comparison | Median | Interquartile Range | P value | |
| Red-Tinted (R) compared with Traditional (T) Chamber | Male C57BL/6J | R: 2.0 | R: 1.75 | 0.0171* |
| T: 0.0 | T: 1.0 | |||
| Female C57BL/6J | R: 2.5 | R: 1.0 | 0.2535 | |
| T: 2.0 | T: 2.0 | |||
| Male BALB/cJ | R: 3.0 | R: 1.0 | <0.0001* | |
| T: 0.0 | T: 0.0 | |||
| Female BALB/cJ | R: 2.0 | R: 2.0 | <0.0001* | |
| T: 0.0 | T: 0.0 | |||
| C57BL/6J compared with BALB/cJ: Males | Red-Tinted | 0.1339 | ||
| Traditional | 0.4786 | |||
| C57BL/6J compared with BALB/cJ: Females | Red-Tinted | 0.3265 | ||
| Traditional | 0.0001* | |||
| C57BL/6J (M compared with F) | Red-Tinted | 0.1714 | ||
| Traditional | 0.0126* | |||
| BALB/cJ (M compared with F) | Red-Tinted | 0.2295 | ||
| Traditional | 0.4783 | |||
| Normality was assessed using the Shapiro-Wilk test then followed by multiple Mann-Whitney U tests. Adjusted behavioral stress score used to assess chamber effect across all groups. Differences were only considered significant at P values less than 0.05 (one * per significant result). | ||||
Figure 7.

Comparison of adjusted behavioral stress scores across all groups. Asterisks indicate highly significant differences (P < 0.0001) between the red-tinted chamber compared with the traditional chamber.
Frequency of other behaviors and interobserver agreement.
The Fisher exact test indicated that total face wipe frequency differed significantly between chambers (P = 0.022; Figure 8). Mice in the red-tinted chamber had a relative risk of 2.86 for face-wiping as compared with those in the translucent chamber. Jumping did not differ significantly between the chambers (P = 0.44) and occurred so rarely across all groups that we do not report the data here. Interobserver agreement for each behavioral measure is presented in Table 4.
Figure 8.

Comparison between chamber types of the proportion of mice displaying face wipe behavior. Asterisks indicate significant differences (P < 0.05) between chambers.
Table 4.
Interobserver agreement between observers 1 and 2 shown for each behavioral measure.
| Observers 1 and 2 | ||
|---|---|---|
| Behavioral Measures | % Agreement | Kappa |
| Adjusted Rearing Score | 92.3 | 0.81 |
| Jumping Frequency | 97.4 | 0.27 |
| Face Wipe Frequency | 92.9 | 0.35 |
| Behavioral Stress Score | 94 | 0.73 |
Discussion
Euthanasia and anesthesia are 2 critical processes in animal research as they both aim to prevent undue pain and distress. Euthanasia is defined as a “good death” while anesthesia is defined as a “loss of sensation.”21,62 Although their outcomes are different, the initial induction process for anesthesia and euthanasia is often a similar experience for the animal. For example, to produce a “good death,” the euthanasia method should include immediate insensibility with minimal distress.13 To achieve insensibility, anesthesia (loss of sensation) must occur first. The most common method of euthanasia for rodents used in research is CO2 inhalation, either in a commercial chamber or home cage. The 2020 AVMA Guidelines for the Euthanasia of Animals describe this specific method with conditions to ensure a safe and effective euthanasia process.45 These guidelines are based on multiple studies that include the impact of CO2 flow rate and stress.12,13,30,31,49,62 In contrast, despite the routine use of inhalant anesthesia in rodents, little information is available on the isoflurane chamber induction and modifications purported to improve animal welfare.56
The objective of this study was to compare physiologic and behavioral markers of stress in mice undergoing isoflurane induction of anesthesia in a red-tinted chamber as compared with a traditional translucent chamber. Our first hypothesis was that mice undergoing isoflurane induction in the red-tinted chamber would show less physiologic stress and fewer stress-related behaviors as compared with those in a traditional translucent chamber. Contrary to our hypothesis, mice in the red-tinted chamber did not show reduced markers of physiologic stress. Furthermore, no significant differences were identified in physiologic stress based on chamber type. To assess the acute physiologic stress response, plasma catecholamines (norepinephrine and epinephrine) and corticosterone were evaluated in this experiment. To our knowledge, this is the first study evaluating all 3 of these parameters in the context of isoflurane anesthetic induction in mice.
Although corticosterone has been used historically to evaluate activity of the HPA axis, it alone does not fully capture the acute physiologic stress response in rodents. Rats exposed to stressful events can take as long as 4 min for corticosterone levels to rise.13,30,59 Furthermore, corticosterone does not achieve maximal levels until 30 min after exposure to a stressor, including handling and novel environments in rats.22,59 Due to this late onset, ACTH has been recommended as a more sensitive indicator of stress during acute stressors such as euthanasia.13,30 ACTH is also a component of the HPA axis that stimulates the release of corticosterone from the adrenal cortex.13 In response to acute CO2 exposure, ACTH has been shown to dramatically increase within 30 s in rats and mice.13,30,59 While the response can be rapid, marginal increases in ACTH have been shown to maximally increase corticosterone levels.41 This nonlinear translation to functional plasma concentrations of corticosterone limits its use in quantifying stress responses. The sympathetic-adrenal-medullary (SAM) system is a more sensitive measure of acute stress. This system is comprised of norepinephrine and epinephrine, which are collectively known as catecholamines. When animals are exposed to a variety of stressors, catecholamines are released almost immediately from sympathetic nerve endings and the adrenal medulla.13 Norepinephrine levels increase within 5 s in response to CO2 asphyxiation, a major method of laboratory rodent euthanasia.14 However, despite a growing body of evidence suggesting catecholamines may be a more appropriate measure of acute stress, publications remain largely limited to corticosterone evaluation in rodents. Given that these same studies are limited to CO2 euthanasia,12-14,30,31,62 we measured catecholamines and corticosterone to evaluate both the HPA axis and the SAM system during induction of isoflurane anesthesia.
Our second hypothesis was that using a red-tinted induction chamber would produce greater reductions in physiologic stress and stress-related behaviors in C57BL/6J as compared with BALB/cJ mice. As discussed above, neither strain exhibited less physiologic stress or stress-related behaviors during induction in the red-tinted or the translucent chamber. However, when comparing by chamber type within strain, BALB/cJ mice, in contrast to C57BL/6J, showed more stress-related behaviors in the red chamber than in the translucent chamber. In addition, average rearing frequency was not significantly affected by chamber type for either sex of C57BL/6J mice and only males displayed a significant increase in the adjusted behavioral stress score. Therefore, consistent with our hypothesis, our results suggest that BALB/cJ mice were more stressed by induction in the red-tinted chamber than were C57BL/6J mice. Importantly, for C57BL/6J mice, there was no significant reduction in stress-related behaviors in the red-tinted chamber and thus, a portion of this hypothesis was not supported.
We selected C57BL/6J and BALB/cJ mice for our study because visual disparities between pigmented and albino strains, respectively, are well documented.29,68 In albino strains, the absence of pigmentation (including in the retina) is attributed to tyrosinase mutations, resulting in melanin deficiency. Melanin plays a vital role in light absorption and reduction of scatter through the retinal pigment epithelium (RPE).35,52 Beyond light control, melanin is critical for retinal neurogenesis. In developing albino mice, disruption of the RPE leads to impairment of retinal ganglion cells, reduced photoreceptor numbers, and thinner nuclear layers.36–38,54 Functionally, these alterations reduce visual performance and cortical activity in BALB/c mice as compared with C57BL/6 mice.29,68 Independent of visual perception, the mouse retinohypothalamic tract (RHT) is essential for circadian regulation and relies on intrinsically photosensitive retinal ganglion cells (ipRGCs). Distinct from other parts of the retina, ipRGCs respond to light using the photopigment, melanopsin, and project to the suprachiasmatic nucleus (SCN) via the RHT. Entrained by light and dark cycles, the SCN acts as the biologic clock and transmits to numerous neuroendocrine centers, leading to circadian expression of corticosterone, melatonin, and other hormones.11,15,25-27 Although pigmented and albino mice may have similar total numbers of ipRGCs,63 direct comparison of this critical nonvisual pathway between strains remains largely unexplored.
Given these differences, we hypothesized that C57BL/6J mice, as compared with BALB/cJ mice, would be better able to perceive lighting differences in the darker, red-tinted chamber and thus would experience less stress. However, as discussed above, the red-tinted chamber did not reduce stress-related behaviors in any of the sexes or strains of mice that we tested. In behavioral testing, BALB/c mice are known for their anxious disposition.2,47 As compared with other strains, BALB/c spend significantly less time exploring during novel open field testing.47 As compared with C57BL/6J mice, BALB/cJ spend significantly less time in the open arms or central area of the elevated plus maze and open field test, respectively.2 Thus, in our study, BALB/cJ mice should have theoretically been more comfortable in the darkened, red-tinted chamber than in the traditional translucent chamber. However, in our study, BALB/cJ females induced in the traditional translucent chamber experienced increased absolute light exposure and yet displayed fewer stress-related behaviors than did their pigmented female counterparts.
Our finding of more stress-related behaviors in BALB/cJ mice when induced in the red-tinted chamber as compared with the translucent chamber is consistent with some literature that suggests differences in how albino rodents perceive red light. Few studies have directly compared the effects of red light or red-tinted products on albino compared with pigmented rodents. One such study found that the albino mouse strains BALB/c and NMRI spent significantly less time in red-tinted huts as compared with the pigmented strains C57BL/6 and CBA.61 Using electroretinography, another publication reported that acute red light exposure produced significantly larger evoked visual amplitudes in albino Wistar rats as compared with the pigmented Brown Norway rats.52 These findings suggest possible differences in how albino rodents perceive red light as compared with their pigmented counterparts. Numerous parameters related to optics, such as the quality and quantity of light, further complicate interpretation. Red light is considered long-wavelength light, or light above approximately 620 nm.52,54 Although multiple methods exist of measuring light quality (for example spectrophotometry) and quantity (for example, illuminance in lux), another important consideration is the shade of red incorporated into red-tinted products, as this can be highly variable among commercial vendors. In our study, the red-tinted induction chamber was made of dark red acrylic with a transmittance pattern well above 600 nm according to spectrophotometric analysis. In addition, a light meter in the BSC indicated that only 35 lx reached the bottom of the red-tinted chamber as compared with 650 lx in the translucent chamber, resulting in markedly reduced irradiance and photon flux due to the dark red tint. Altogether, these measurements indicate that significantly less light reached mice in the red-tinted chamber and allowed us to adequately evaluate the responses of mice in a truly darkened chamber environment. While our study did not find physiologic effects indicative of stress based on the acute use of a red-tinted chamber, some evidence suggests that chronic use of red light and red-tinted products has both physiologic and behavioral effects in rodents.17,18,24,34,43,48,52,55,66,67 Some of these studies have documented significant effects in albino strains despite their visual limitations. For example, albino Sprague–Dawley (SD) rats have disruptions in sleep patterns, locomotor activity, and ovulatory timing after exposure to red light during the dark cycle.24,34,48 In addition, the use of red-tinted enrichment devices and caging in SD rats significantly alters other circadian parameters, including food and water intake, plasma melatonin, and corticosterone.18,66,67 Our findings here add to a growing body of evidence suggesting that red light and red-tinted products can have wide-ranging (and strain specific) effects on rodents and research.
Our study had multiple limitations that are important to consider. First, our experimental design relied on behavioral quantification of stress and combining the frequencies of several specific behaviors (rearing, face wiping, and jumping) into a single score. Although this behavioral stress score has not been validated, it is based on behaviors that are well characterized in established mouse ethograms and a similar study.16,31,60 Furthermore, this scoring system was adjusted posthoc to encompass the behaviors that we observed more accurately. For instance, upon collating data from both observers, we found that rearing frequency greatly exceeded expected numbers across our study population. Therefore, to account for the higher rates of rearing, our adjusted behavioral stress scores assumed that low rearing frequencies were associated with normal exploratory behavior. Assessment of anxiety-related behaviors in mice often involves the use of novel, well-lit spaces such as an elevated plus maze or a light-dark box. These testing paradigms rely on the understanding of rodents as nocturnal, prey species and evaluates their willingness to explore illuminated and unprotected areas.5,16,57,58 As experimentally naïve subjects, the novel chamber environments in our study provided a unique stressor for these mice and may explain why rearing behavior was displayed far more frequently than expected. In addition, to allow for brief exploration of the chamber and adjustment to any perceived lighting differences, all mice were given a 2-min acclimation period based on another study.51 Another limitation to our study was a slight discrepancy in interior chamber dimensions, corresponding to a 4% difference in chamber volume between chamber types. In effect, this chamber volume difference corresponded to a 1% increase in the traditional chamber volume displacement rate as compared with the red-tinted chamber. While this discrepancy did extend the time to loss of consciousness for mice in the red-tinted chamber, we do not believe a 1% difference in chamber volume displacement rate accounts for the behavioral differences we observed. Likewise, the potential impact on physiologic measures was controlled by collecting blood at a set timepoint after beginning isoflurane induction.
In addition to the induction chamber environment, our mice experienced several other potential stressors that could influence our study findings. Although many studies indicate that isoflurane is a stressful agent,1,20,31,63,65 evaluating its effects during the acute stress response is challenging. Isoflurane is known for its distinct odor and for causing irritation to mucous membranes.1,20,32 In humans, isoflurane irritation and discomfort can occur within 10 s of inhalation.1,19 In comparison to humans, rodents rely primarily on olfaction to sense the environment.56 Thus, isoflurane initiation alone may have stressed the mice within seconds and immediately activated hormonal stress pathways. In addition, the rodent visual system is not highly advanced, providing an estimated human equivalent of 20/2,000 vision.4,54 Therefore, isoflurane’s olfactory stimulation or irritation may have masked the differences in chamber type, thus limiting our overall physiologic stress assessment. To capture individual stress parameters and eliminate emotional contagion, mice were anesthetized individually in a separate procedure room.8,9 Social isolation, such as single housing, is a well-documented stressor for mice and is associated with anxiety-like behaviors, changes in neuroplasticity, and elevations in stress hormones.3,28,33,40 However, single housing of mice can be beneficial, such as for territorial male strains, and adaptations in stress hormones occur over time. While the literature is inconclusive regarding the time necessary for acclimation to single housing, most studies indicate that physiologic and behavioral adaptation occur within 7 to 14 d.3,28,40 We used a 10-d single housing period to allow adaptation to individual housing prior to study. Finally, we did not evaluate the impact of circadian hormone expression on our physiologic findings. Although we found significant differences in corticosterone levels between each sex and strain, interpretation of these results may be limited due to the experimental time chosen. In unstressed mice, corticosterone levels normally remain low in the early portions of the light phase and begin to rise before the onset of the dark phase.7,44 However, in our study, despite our beginning blood collection early and ending in the middle of the light phase, corticosterone levels were significantly higher than those previously reported for unstressed mice.7,44 Although mice were randomized and this effect was evenly distributed across each group, the numerous stressors and potential time-of-day effects complicate interpretation of our results.
In conclusion, we did not find that red-tinted chambers provided any significant reduction in stress during anesthetic induction. We found no physiologic benefit to using red-tinted chambers for isoflurane anesthetic induction in mice. In addition, contrary to our hypothesis, our behavioral results indicate that red-tinted chambers may have caused greater expression of stress-related behaviors in mice during isoflurane anesthetic induction, particularly the albino mouse strain BALB/cJ. Therefore, we do not recommend the use of red-tinted induction chambers for isoflurane anesthesia in mice. Because isoflurane induction is commonly used in rodents, additional studies investigating environmental modifications and stress mitigation in mice and other rodent species are warranted. Furthermore, the use of red-tinted products as a stress mitigation strategy should continue to be critically examined as evidence of their benefit to rodents is currently lacking.
Acknowledgments
This work was funded by the Vanderbilt University Medical Center Division of Comparative Medicine. We would like to thank Dr. Debra Hickman for her consultation and expertise in preparation of this study design. In addition, we thank Dr. Dmitry Koktysh and the Vanderbilt Institute of Nanoscale Science and Engineering (VINSE) Analytical Laboratory for their assistance in the spectral transmission analysis. Lastly, we thank Dale Plummer of VUMC’s Department of Biostatistics for his role in providing statistical analysis of our data.
References
- 1.Altholtz LY, Fowler KA, Badura LL, Kovacs MS. 2006. Comparison of the stress response in rats to repeated isoflurane or CO2:O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci 45:17–22. [PubMed] [Google Scholar]
- 2.An XL, Zou JX, Wu RY, Ying Y, Tai FD, Zeng SY, Rui J, Xia Z, Liu EQ, Hugh B. 2011. Strain and sex Differences in Anxiety-Like and Social Behaviors in C57Bl/6J and BALB/cJ Mice. Exp Anim 60:111–123. 10.1538/expanim.60.111. [DOI] [PubMed] [Google Scholar]
- 3.Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van’t Klooster J, Ohl F. 2009. Individual housing of mice - Impact on behaviour and stress responses. Physiol Behav 97:385–393. 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Baker M. 2013. Neuroscience: through the eyes of a mouse. Nature 502:156–158. 10.1038/502156a. [DOI] [PubMed] [Google Scholar]
- 5.Bailey KR, Crawley JN. 2009. Chapter 5: Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis. [Google Scholar]
- 6.Bedrosian TA, Vaughn CA, Weil ZM, Nelson RJ. 2013. Behaviour of laboratory mice is altered by light pollution within the housing environment. Anim Welf 22:483–487. 10.7120/09627286.22.4.483. [DOI] [Google Scholar]
- 7.Barriga C, Martín MI, Tabla R, Ortega E, Rodríguez AB. 2001. Circadian rhythm of melatonin, corticosterone and phagocytosis: Effect of stress. J Pineal Res 30:180–187. 10.1034/j.1600-079X.2001.300307.x. [DOI] [PubMed] [Google Scholar]
- 8.Beery AK, Kaufer D. 2015. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress 1:116–127. 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beery AK, Holmes MM, Lee W, Curley JP. 2020. Stress in groups: lessons from non-traditional rodent species and housing models. Neurosci Biobehav Rev 113:354–372. 10.1016/j.neubiorev.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekhbat M, Merrill L, Kelly SD, Lee VK, Neigh GN. 2016. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behav Brain Res 305:122–125. 10.1016/j.bbr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 12.Boivin GP, Bottomley MA, Dudley ES, Schiml PA, Wyatt CN, Grobe N. 2016. Physiological, behavioral, and histological responses of male C57BL/6N mice to different CO2 chamber replacement rates. J Am Assoc Lab Anim Sci 55:451–461. [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin GP, Hickman DL, Creamer-Hente MA, Pritchett-Corning KR, Bratcher NA. 2017. Review of CO2 as a euthanasia agent for laboratory rats and mice. J Am Assoc Lab Anim Sci 56:491–499. [PMC free article] [PubMed] [Google Scholar]
- 14.Borovsky V, Herman M, Dunphy G, Caplea A, Ely D. 1998. CO2 asphyxia increases plasma norepinephrine in rats via sympathetic nerves. Am J Physiol 274:R19–R22. 10.1152/ajpregu.1998.274.1.R19. [DOI] [PubMed] [Google Scholar]
- 15.Brainard GC, Hanifin JP. 2005. Photons, clocks, and consciousness. J Biol Rhythms 20:314–325. 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- 16.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. 2013. Animal models of anxiety disorders and stress. Braz J Psychiatry 35 Suppl 2:S101–S111. 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 17.Dauchy RT, Wren MA, Dauchy EM, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Hill SM, Mao L, Dupepe LM, Ooms TG, Blask DE. 2013. Effect of Spectral Transmittance through Red-Tinted Rodent Cages on Circadian Metabolism and Physiology in Nude Rats. J Am Assoc Lab Anim Sci 52:745–755. [PMC free article] [PubMed] [Google Scholar]
- 18.Dauchy RT, Wren MA, Dauchy EM, Hoffman AE, Hanifin JP, Warfield B, Jablonski MR, Brainard GC, Hill SM, Mao L, Dobek GL, Dupepe LM, Blask DE. 2015. The influence of red-light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci 54:40–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson JM, Jones LE, Levine S. 1968. Feedback regulation of adrenocorticotropin secretion in ‘basal’ and “stress” conditions: acute and chronic effects of intrahypothalamic corticoid implantation. Endocrinology 82:655–663. 10.1210/endo-82-4-655. [DOI] [PubMed] [Google Scholar]
- 20.Doi M, Ikeda K. 1993. Airway irritation produced by volatile anaesthetics during brief inhalation: comparison of halothane, enflurane, isoflurane and sevoflurane. Can J Anaesth 40:122–126. 10.1007/BF03011308. [DOI] [PubMed] [Google Scholar]
- 21.Flecknell P. 2016. Chapter 1—Basic principles of anaesthesia, p 1. In: Flecknell P, editor. Laboratory animal anaesthesia. 4th ed. Boston: Academic Press. [Google Scholar]
- 22.Gärtner K, Büttner D, Döhler K, Friedel R, Lindena J, Trautschold I, Liebowitz MR, Gorman J. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14: 267–274. 10.1258/002367780780937454. [DOI] [PubMed] [Google Scholar]
- 23.Gjendal K, Ottesen JL, Olsson IAS, Sørensen DB. 2020. Effect of Repeated Exposure to Isoflurane on Nest Building and Burrowing in Mice. J Am Assoc Lab Anim Sci 59:30–36. 10.30802/AALAS-JAALAS-19-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González MMC. 2018. Dim light at night and constant darkness: Two frequently used lighting conditions that jeopardize the health and well-being of laboratory rodents. Front Neurol 9:609. 10.3389/fneur.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanifin JP, Brainard GC. 2007. Photoreception for circadian, neuroendocrine, and neurobehavioral regulation. J Physiol Anthropol 26:87–94. 10.2114/jpa2.26.87. [DOI] [PubMed] [Google Scholar]
- 26.Hanifin JP, Dauchy RT, Blask DE, Hill SM, Brainard GC. 2020. Relevance of electrical light on circadian, neuroendocrine, and neurobehavioral regulation in laboratory animal facilities. ILAR J 60:150–158. 10.1093/ilar/ilaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. 2002. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070. 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hebda-Bauer EK, Dokas LA, Watson SJ, Akil H. 2019. Adaptation to single housing is dynamic: Changes in hormone levels, gene expression, signaling in the brain, and anxiety-like behavior in adult male C57Bl/6J mice. Horm Behav 114:104541. 10.1016/j.yhbeh.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimel JA, Hartman RJ, Hermans JM, Levelt CN. 2007. Screening mouse vision with intrinsic signal optical imaging. Eur J Neurosci 25:795–804. 10.1111/j.1460-9568.2007.05333.x. [DOI] [PubMed] [Google Scholar]
- 30.Hickman DL. 2018. Interpreting neuroendocrine hormones, corticosterone, and blood glucose to assess the wellbeing of anesthetized rats during euthanasia. J Am Assoc Lab Anim Sci 57:725–728. 10.30802/AALAS-JAALAS-17-000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickman DL. 2021. Wellbeing of Mice Euthanized with Carbon Dioxide in Their Home Cage as Compared with an Induction Chamber. J Am Assoc Lab Anim Sci 60:72–76. 10.30802/AALAS-JAALAS-20-000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, et al. 2017. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice—Assessing the degree of distress. PLoS One 12:e0179588. 10.1371/journal.pone.0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ieraci A, Mallei A, Popoli M. 2016. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast 2016:1–13. 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda M, Sagara M, Inoué S. 2000. Continuous exposure to dim illumination uncouples temporal patterns of sleep, body temperature, locomotion and drinking behavior in the rat. Neurosci Lett 279:185–189. 10.1016/S0304-3940(99)00943-X. [DOI] [PubMed] [Google Scholar]
- 35.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 36.Iwai-Takekoshi L, Ramos A, Schaler A, Weinreb S, Blazeski R, Mason C. 2016. Retinal pigment epithelial integrity is compromised in the developing albino mouse retina. J Comp Neurol 524:3696–3716. 10.1002/cne.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KSP. 2012. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen Comp Endocrinol 179:406–413. 10.1016/j.ygcen.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Jeffery G. 1997. The albino retina: An abnormality that provides insight into normal retinal development. Trends Neurosci 20:165–169. 10.1016/S0166-2236(96)10080-1. [DOI] [PubMed] [Google Scholar]
- 39.Jennings M, Batchelor GR, Brain PF, Dick A, Elliott H, Francis RJ, Hubrecht RC, Hurst JL, Morton DB, Peters AG, Raymond P, Sales GD, Sherwin CM, West C. 1998. Refining rodent husbandry: the mouse: report of the rodent refinement working party. Lab Anim 32:233–259. 10.1258/002367798780559301. [DOI] [PubMed] [Google Scholar]
- 40.Kamakura R, Kovalainen M, Leppäluoto J, Herzig KH, Mäkelä KA. 2016. The effects of group and single housing and automated animal monitoring on urinary corticosterone levels in male C57BL/6 mice. Physiol Rep 4:e12703. 10.14814/phy2.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller-Wood ME, Shinsako J, Dallman MF. 1983. Integral as well as proportional adrenal responses to ACTH. Am J Physiol 245:R53–R59. 10.1152/ajpregu.1983.245.1.R53. [DOI] [PubMed] [Google Scholar]
- 42.Kulesskaya N, Voikar V. 2014. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: Role of equipment and procedure. Physiol Behav 133:30–38. 10.1016/j.physbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 43.LaFollette MR, Swan MP, Smith RK, Hickman DL, Gaskill BN. 2019. The effects of cage color and light intensity on rat affect during heterospecific play. Appl Anim Behav Sci 219:104834. 10.1016/j.applanim.2019.104834. [DOI] [Google Scholar]
- 44.Lassi M, Tomar A, Comas-Armangué G, Vogtmann R, Dijkstra DJ, Corujo D, Gerlini R, Darr J, Scheid F, Rozman J, Aguilar-Pimentel A, Koren O, Buschbeck M, Fuchs H, Marschall S, Gailus-Durner V, De Angelis MH, Plösch T, Gellhaus A, Teperino R. 2021. Disruption of paternal circadian rhythm affects metabolic health in male offspring via nongerm cell factors. Sci Adv 7:eabg6424. 10.1126/sciadv.abg6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. [Internet]. 2020. AVMA Guidelines for the Euthanasia of Animals. [Cited 30 September 2021]. Available at: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf.
- 46.Marquardt N, Feja M, Hünigen H, Plendl J, Menken L, Fink H, Bert B. 2018. Euthanasia of laboratory mice: Are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS One 13:e0803793. 10.1371/journal.pone.0203793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalikova S, van Rensburg R, Chazot PL, Ennaceur A. 2010. Anxiety responses in Balb/c, c57 and CD-1 mice exposed to a novel open space test. Behav Brain Res 207:402–417. 10.1016/j.bbr.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 48.McCormack CE, Sontag CR. 1980. Entrainment by red light of running activity and ovulation rhythms of rats. Am J Physiol 239:R450–R453. 10.1152/ajpregu.1980.239.5.R450. [DOI] [PubMed] [Google Scholar]
- 49.Moody CM, Chua B, Weary DM. 2014. The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. 10.1177/0023677214546509. [DOI] [PubMed] [Google Scholar]
- 50.Moody CM, Weary DM. 2014. Mouse aversion to isoflurane versus carbon dioxide gas. Appl Anim Behav Sci 158:95–101. 10.1016/j.applanim.2014.04.011. [DOI] [Google Scholar]
- 51.Nichols KE, Holliday-White KL, Bogie HM, Swearingen KM, Fine MS, Doyle J, Tiesma SR. 2020. Cardiovascular and metabolic responses to carbon dioxide euthanasia in conscious and anesthetized rats. J Am Assoc Lab Anim Sci 59:742–749. 10.30802/AALAS-JAALAS-19-000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niklaus S, Albertini S, Schnitzer TK, Denk N. 2020. Challenging a myth and misconception: Red-light vision in rats. Animals (Basel) 10:422. 10.3390/ani10030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parra MD, Bernal LJ, Cerón JJ. 2004. Cortisol and free thyroxine determination by time-resolved fluorometry in canine serum. Can J Vet Res 68:98–104. [PMC free article] [PubMed] [Google Scholar]
- 54.Peirson SN, Brown LA, Pothecary CA, Benson LA, Fisk AS. 2018. Light and the laboratory mouse. J Neurosci Methods 300:26–36. 10.1016/j.jneumeth.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powell K, Ethun K, Taylor DK. 2016. The effect of light level, CO2 flow rate, and anesthesia on the stress response of mice during CO2 euthanasia. Lab Anim (NY) 45:386–395. 10.1038/laban.1117. [DOI] [PubMed] [Google Scholar]
- 56.Reiter CP, Christy AC, Olsen CH, Bentzel DE. 2017. Effect of home cage bedding in the induction chamber on serum cortisol and corticosterone levels in response to isoflurane-induced anesthesia in C57BL/6J mice. J Am Assoc Lab Anim Sci 56:118–121. [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers RJ, Shepherd JK. 1993. Influence of prior maze experience on behaviour and response to diazepam in the elevated plus-maze and light/dark tests of anxiety in mice. Psychopharmacology (Berl) 113:237–242. 10.1007/BF02245704. [DOI] [PubMed] [Google Scholar]
- 58.Rodgers RJ, Dalvi A. 1997. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21:801–810. 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 59.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- 60.Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. 2013. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip Toxicol 6:126–135. 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soerensen DB, Moeller MR, Larsen LR. 2009. The use of the Techniplast Mouse House in four strains of mice. Scand J Lab Anim Sci 36:179–183. 10.23675/sjlas. v36i2.183 [DOI] [Google Scholar]
- 62.Turner PV, Hickman DL, van Luijk J, Ritskes-Hoitinga M, Sargeant JM, Kurosawa TM, Agui T, Baumans V, Choi WS, Choi YK, Flecknell PA, Lee BH, Otaegui PJ, Pritchett-Corning KR, Shimada K. 2020. Welfare impact of carbon dioxide euthanasia on laboratory mice and rats: A systematic review. Front Vet Sci 7:411. 10.3389/fvets.2020.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valiente-Soriano FJ, García-Ayuso D, Ortín-Martínez A, Jiménez-López M, Galindo-Romero C, Villegas-Pérez MP, Agudo-Barriuso M, Vugler AA, Vidal-Sanz M. 2014. Distribution of melanopsin positive neurons in pigmented and albino mice: evidence for melanopsin interneurons in the mouse retina. Front Neuroanat 8:131. 10.3389/fnana.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong D, Makowska IJ, Weary DM. 2013. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 9:20121000. 10.1098/rsbl.2012.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wren MA, Dauchy RT, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Blask DE, Hill SM, Ooms TG, Bohm RP. 2014. Effect of different spectral transmittances through tinted animal cages on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci 53:44–51. [PMC free article] [PubMed] [Google Scholar]
- 66.Wren-Dail MA, Dauchy RT, Ooms TG, Baker KC, Blask DE, Hill SM, Dupepe LM, Bohm RP. 2016. Effects of colored enrichment devices on circadian metabolism and physiology in male Sprague-Dawley rats. Comp Med 66:384–391. Available at https://pubmed.ncbi.nlm.nih.gov/27780005/. [PMC free article] [PubMed] [Google Scholar]
- 67.Wren-Dail MA, Dauchy RT, Blask DE, Hill SM, Ooms TG, Dupepe LM, et al. 2017. Effect of isoflurane anesthesia on circadian metabolism and physiology in rats. Comp Med 67:138–146. [PMC free article] [PubMed] [Google Scholar]
- 68.Yeritsyan N, Lehmann K, Puk O, Graw J, Löwel S. 2012. Visual capabilities and cortical maps in BALB/c mice. Eur J Neurosci 36:2801–2811. 10.1111/j.1460-9568.2012.08195.x. [DOI] [PubMed] [Google Scholar]
- 69.Yonezaki K, Uchimoto K, Miyazaki T, Asakura A, Kobayashi A, Takase K, Goto T. 2015. Postanesthetic effects of isoflurane on behavioral phenotypes of adult male C57BL/6J mice. PLoS One 10:e0122118. 10.1371/journal.pone.0122118. [DOI] [PMC free article] [PubMed] [Google Scholar]






