Abstract
Background:
Reliable biomarkers that can be serially monitored to predict treatment response to immune checkpoint inhibitors (ICIs) are still an unmet need. Here, we present a multiplex immunofluorescence (IF) assay that simultaneously detects circulating tumor cells (CTCs) and assesses CTC expression of programmed death ligand-1 (PD-L1) and interferon regulatory factor 1 (IRF-1) as a candidate biomarker related to ICI use.
Objective:
To assess the potential of CTC PD-L1 and IRF-1 expression as candidate biomarkers for patients with advanced epithelial solid tumors receiving ICIs.
Patients and Methods:
We tested the IF CTC assay in a pilot study of 28 patients with advanced solid tumors who were starting ICI. Blood for CTC evaluation was obtained prior to starting ICI, after a single cycle of therapy, and at the time of radiographic assessment or treatment discontinuation.
Results:
At baseline, patients with 0–1 CTCs had longer progression-free survival (PFS) compared to patients with ≥2 CTCs (4.3 vs 1.3 months, p = 0.01). The presence of any PD-L1+ CTCs after a single dose of ICI portended shorter PFS compared to patients with no CTCs or PD-L1- CTCs (1.2 vs 4.2 months, p = 0.02); the presence of any PD-L1+ or IRF-1+ CTCs at time of imaging assessment or treatment discontinuation also was associated with shorter PFS (1.9 vs 5.5 months, p < 0.01; 1.6 vs 4.7 months, p = 0.05). CTC PD-L1 and IRF-1 expression did not correlate with tumor tissue PD-L1 or IRF-1 expression. Strong IRF-1 expression in tumor tissue was associated with durable (≥1 year) radiographic response (p = 0.02).
Conclusions:
Based on these results, CTC PD-L1 and IRF-1 expression is of interest in identifying ICI resistance and warrants further study.
1. Introduction
Immune checkpoint inhibitors (ICIs) target and prevent immune checkpoint interactions (e.g., programmed death receptor 1 (PD-1) and programmed death ligand 1 (PD-L1)) and have the potential to restore endogenous anti-tumor immune responses. Over the last decade, ICIs have had expanding regulatory approval and use across multiple solid tumor malignancies because of their demonstrable efficacy, durability of response, tolerability, and improvements in survival. ICIs have played a tremendous role in the treatment of tumors, such as melanoma and renal cell carcinoma, where an immunologic inflammatory tumor microenvironment is prominent. Their role is expanding in additional solid tumor types such as non-small cell lung cancer (NSCLC) and urothelial carcinoma. However, in cancers of epithelial origin, ICIs produce objective responses in only select cohorts of patients [1, 2], and, therefore, biomarkers to predict and monitor ICI response are urgently needed.
Tumor microenvironment PD-L1 expression has been extensively explored as a putative predictive biomarker of ICI response. The Food and Drug Administration has approved PD-L1 tissue-based assays as companion/complementary diagnostics for certain ICIs and tumor types. These assays are not standardized across tumor types, and different ICI agents have unique PD-L1 assays that vary by antibody clone, staining methods, cell of interest (tumor cell, immune cell, or both), algorithms and cutoff points used for scoring [3–5]. While these approved assays can enrich for a group of patients with higher probability of objective response to ICI, they are not accurately predictive of response. For example, NSCLCs with PD-L1 expression ≥50% by the companion PD-L1 assay (Dako 22C3) identifies a subgroup of patients who are more likely to respond to pembrolizumab monotherapy, but less than half of the selected patients have an objective response (ORR 45%) [6]. Moreover, a small proportion of patients with low or no PD-L1 expression still can obtain clinical benefit in the form of a partial response or stable disease that can be associated with prolonged survival [7].
In cancer, PD-L1 expression can become constitutive through genomic and/or signaling alterations that allow the cancer cells to harness PD-L1 expression for immune evasion [8, 9]. In normal tissues, PD-L1 expression is inducible through the interferon gamma pathway and JAK/STAT signaling, which leads to the transcription factor interferon regulatory factor 1 (IRF-1) binding the PD-L1 promoter [10]. PD-L1 inducibility, which is suggested by high IRF-1 expression, may be a feature associated with a higher likelihood of response to ICIs. High melanoma cell IRF-1 expression has been associated with longer progression-free survival (PFS) and objective response to ICI [11]. In NSCLC, IRF-1 expression is associated with the presence of granzyme A and perforin-1, which are biomarkers of effective cytotoxic immune cell activity [12]. All of this suggest that IRF-1 has potential as a novel candidate biomarker of ICI response.
All currently approved PD-L1 assays are tissue-based, which limits the utility of these assays in monitoring treatment response due to the need for serial tissue procurement. In a number of cases, obtaining sufficient tissue to perform these assays is also technically limited due to the location or size of the tumor and other clinical factors. Liquid biopsies from peripheral blood have the potential to overcome these limitations due to the ease of acquisition. In addition, because the expression of PD-L1 in a given tissue is dynamic with treatment and also varies by tumor site (primary vs metastasis) [13–18], a liquid biopsy-based assay could allow serial assessment that may more accurately reflect the heterogeneity of the patient’s overall tumor burden in real time.
Circulating tumor cells (CTCs) collected by liquid biopsy can be interrogated for protein biomarkers using a multiplexed imaging strategy [19]. A recent report showed that PD-L1 expression on CTCs collected from peripheral blood is both dynamic with cancer treatment and may have implications for prognosis [20]. Here, we present a pilot study using the RareCyte CTC analysis platform, which can simultaneously examine PD-L1 and IRF-1 expression on CTCs in patients with advanced cancers receiving ICIs. We hypothesized that PD-L1 and/or IRF-1 expression on CTCs would be dynamic and that changes in PD-L1/IRF-1 expression would reflect the development of ICI resistance. We also evaluated the association of tissue PD-L1 and IRF-1 expression with CTC PD-L1 and IRF-1 expression. To our knowledge, this is the first study that evaluates both IRF-1 and PD-L1 expression on CTCs.
2. Methods
2.1. Blood collection and processing
A total of 10 cc of blood was collected at each timepoint in an AccuCyte® collection tube (RareCyte, Seattle, WA). Blood for spike-in samples was collected from volunteer donors. Samples were processed 24–72 hours after collection using the AccuCyte kit to generate buffy coat slides and quantify CTCs as previously described [21, 22]. Briefly, 7.5 cc of blood was transferred to an AccuCyte separation tube containing a float, followed by density-based separation of buffy coat cells from red blood cells and plasma. The buffy coat was then resuspended in 700 uL of Transfer Fluid (RareCyte) and spread evenly across 8 SuperFrost® Plus slides (VWR) and allowed to dry for 45 minutes. Slides were transferred to −20°C and banked until staining.
2.2. Circulating tumor cell staining
Slides were transferred to room temperature for 30 minutes, then fixed for 60 minutes in 10% neutral buffered formalin (Sigma-Aldrich). Slides were stained with the RarePlex 0912-VB PD-L1 CTC Panel Kit with IRF-1 added using a modified RarePlex® Developer Kit 405-A-V (RareCyte). PD-L1 and IRF-1 antibody clones were selected for optimal signal-to-background fluorescence intensity during the optimization phase of assay development. Using the Ventana DISCOVERY ULTRA autostainer, slides were stained for PD-L1 using the 0912-VB CTC Panel Kit and IRF-1 (D5E4, Cell Signaling), each with an enzyme-based amplification. The 0912-VB CTC Panel Kit includes staining for CTC identification markers pan-cytokeratin (CK) cocktailed together with Epithelial Cell Adhesion Molecule (EpCAM), a leukocyte exclusion marker CD45, and a nuclear dye.
2.3. Enumeration and characterization of CTCs
A positive control slide containing SW900 mCTCs was stained and reviewed with every staining run to ensure staining quality. Using the CyteFinder® software, candidate CTCs were identified by positive staining for CK/EpCAM and negative staining for CD45 by a ranking algorithm that utilizes machine learning. Candidate CTCs were then confirmed by a reviewer.
PD-L1 and IRF-1 staining were then assessed on the identified CTCs. CTC PD-L1 status was determined based on a decision-tree scheme (Supplemental Figure 1) to differentiate thePD-L1 staining of CTCs from that of surrounding platelets, which are collected as part of the sample preparation process. Platelet staining is specific, as it has been reported that platelets express PD-L1 protein [23]. To differentiate between CTC and platelet staining, the decision-tree scheme incorporates PD-L1 stain localization. The expectation is that PD-L1 staining of the CTC should overlay and/or surround the identified CTC. PD-L1 staining that both a) overlaid the CTC and b) was more intense than the surrounding platelet PD-L1 staining was designated as “positive.” PD-L1 staining that a) overlaid the CTC and b) was comparable to PD-L1 staining intensity of the surrounding platelets was designated as “equivocal”. Because “equivocal” staining intensity is rationally consistent with low-level PD-L1 expression, equivocal cells were included together with “positive” cells in the statistical analyses. Thus, all samples with any “equivocal” or “positive” cells were classified as having positive PD-L1 status for purposes of the statistical analysis. CTC IRF-1 status was determined based on staining intensity relative to background and co-localization with the nuclear compartment.
Images were evaluated by two initial independent reviewers and then subsequently confirmed by two additional independent reviewers that reached consensus decisions on CTC biomarker determinations. Although reviewers were not formally blinded, consensus decisions on CTC biomarker positivity were made independently of statistical outcome evaluations.
2.4. Analytic Validation of RarePlex PD-L1 Panel
The first part of validation was to establish the accurate identification and enumeration of CTCs by the base RarePlex 0912-VB PD-L1 CTC Panel Kit. This was accomplished by comparing the 0912-VB CTC Panel Kit to a comparator 3-channel 0900-VA CTC enumeration kit (CK/EpCAM, CD45, nuclear dye) using both spike-in model CTCs (mCTC) and patient-derived CTCs. A known number of PC3 cells (ATCC, Manassas, VA) were directly printed on slides and then stained, scanned, and counted using both kits. CTC enumeration accuracy of the RarePlex PD-L1 kit was determined by staining 10 limit-of-detection slides with 3 to 6 printed mCTCs each and 10 limit-of-the-blank slides with no mCTCs, analyzed by a blinded reviewer. A similar clinical sample enumeration comparison to the comparator CTC enumeration kit was made using three paired clinical samples from breast, lung and prostate cancer patients.
PD-L1 staining on CTCs was validated using both spike-in and clinical samples. Studies were blinded and performed according to RareCyte standard operating procedures under its quality management program. Cell lines with well characterized expression of PD-L1 were used in the validation studies as model CTCs (mCTC): SW900 (high – homogenously positive), H1650 (low – variably positive near the analytic validation scoring threshold) and HAP1 (negative). Cells were cultured according to instructions from supplier (ATCC) and spiked into healthy donor blood or printed onto slides in model CTC systems. The H1650 line was selected intentionally to assess replicate variation and ensure robust CV measurements.
For the analytic validation studies, the threshold for positive staining was set by evaluating the mean fluorescence intensity (MFI) of PD-L1 on mCTCs for each model cell line by using individual cell area masks. Sensitivity of PD-L1-positive staining of mCTCs (SW900, homogenously positive control) was plotted together with specificity of staining (HAP1 mCTCs, negative control) to establish the threshold that best separated the two populations.
For accuracy and precision studies, approximately 800 mCTCs were spiked into each of 3 blood collection tube samples and 5 slides were analyzed per sample over 3 autostainer runs. SW900 cells were used as the positive control and HAP1 cells were used as the negative control. A minimum of 50 analyte replicates per slide were measured for statistical robustness. Precision-repeatability was assessed by coefficient of variation (CV) using the low-positive H1650 line. Inter-donor precision was assessed by spiking H1650 cells into blood from three different donors.
2.5. Prospective Study Patient Enrollment
Patients with metastatic or locally advanced inoperable epithelial marker-positive solid tumors initiating a palliative ICI treatment regimen at the Fred Hutchinson Cancer Center (following the merger of the Seattle Cancer Care Alliance and Fred Hutchinson Research Center) or University of Washington, both in Seattle, WA, were eligible for enrollment. ICI treatment regimens for the patients on this study were allowed to include combinations with chemotherapy or investigational agents. Patients with prior history of ICI exposure were excluded. Patients with melanoma or renal cell carcinoma were also excluded from the study due to absent or low epithelial biomarker expression.
All patients had a baseline blood draw for CTC analysis prior to starting ICI treatment (Supplemental Figure 2, Timepoint A). Patients had an additional blood draw after completing a single cycle of ICI-containing therapy (Supplemental Figure 2, Timepoint B). The third and final blood draw was collected at the time of restaging imaging (3–6 months after therapy start) or at time of ICI treatment discontinuation for progression (Supplemental Figure 2, Timepoint C). Study data were collected and managed using a REDCap electronic database hosted at the University of Washington [24, 25].
2.6. Antibody conjugation for tissue staining
Prior to tissue staining, purified CD68 (D4B9C, Abcam) was conjugated with biotin (33018, Biotium) and CD3D (EP4426, Abcam) was conjugated with DY720-NHS-ester (720–01A, Dyomics) according to the manufacturer’s instructions.
2.7. Tumor tissue staining for PD-L1 and IRF-1
Archival tumor tissue availability was evaluated for all patients on the study. For patients with tumor tissue available, unstained formalin-fixed and paraffin embedded slides (4-micron thickness, unbaked, on charged slides) were obtained. All staining steps were performed at room temperature and followed by 3 washes in PBS + 0.025% Triton-X 100 unless noted otherwise. Slides were deparaffinized in xylene twice for 10 minutes each, then rehydrated sequentially in 100%, 95%, 75% ethanol and lastly in PBS for 3 minutes each. Antigen retrieval was completed in a pH 9 Tris/EDTA based buffer (RE7119-CE, Leica Biosystems) for 10 minutes at 95°C using a Dako PT Link and washed in PBS. Slides were then photobleached by submerging in 4.5% hydrogen peroxide and 24 mM sodium hydroxide in PBS for 1 hour under white light then washed 3x. The first round of primary antibody staining included IRF-1 (D5E4, Cell Signaling) and CK (AE1 and AE3, Millipore Sigma) in 6% BSA and 10% goat serum for 1 hour. Secondary antibodies against rabbit (A32733, ThermoFisher) and mouse (563846, BD) in 6% BSA and 10% goat serum were then added for 1 hour. The second round of primary antibody staining included antibodies against CD68-biotin, CD3D-DY720 and PD-L1-HRP (EPR19759, Abcam) in 6% BSA, 5% mouse serum and 5% rabbit serum for 1 hour. Slides were then stained with Streptavidin-CF405L (29056, Biotium) and SYTOX™ Orange (S11368, ThermoFisher) in 6% BSA, 5% mouse serum and 5% rabbit serum for 1 hour. Finally, slides were stained with CF488A-Tyramide (92171, Biotium) for 20 minutes. After 3 final washes, an additional 2 washes in PBS were performed, then the slides were mounted with EverBrite™ mounting medium (23001, Biotium) and allowed to dry overnight prior to imaging on a CyteFinder® II instrument.
2.8. Tissue imaging and scoring of PD-L1 and IRF-1
Tissue slides were scanned on the CyteFinder II HT system (RareCyte) with pathology software module. In brief, slides were loaded into the scanner and a preview image at 4x magnification was obtained. A full scan at 20x magnification was then completed. Cytokeratin-positive epithelial tumor cells were identified and differentiated from associated macrophages and T-cells by CD68 and CD3 staining, respectively. The number of biomarker-positive tumor cells was estimated after review of the entire tissue section, and each sample was placed into one of the following categories: Absent – less than 1% tumor staining; Positive – 1 to 50% tumor staining; Strongly Positive – greater than 50% tumor staining. Tissue images were reviewed by a primary reviewer (EL) and secondarily by a board-certified anatomic pathologist (EPK).
2.9. Statistical Analysis
Patients were dichotomized into two groups by CTC count: 2 or more CTCs vs 0–1 CTCs. The optimal CTC cut-off was chosen by using the SAS macro created by Mandrekar [26], which is based on the log rank test statistic of the progression-free survival (PFS) time. Specifically, the patients were divided into two groups by the baseline CTC at a cut-off point, and the log-rank test was performed to calculate the p-value for each cut-off point. The cut-off point with the smallest p-value was selected as the optimal one. The number of prior lines of therapy was dichotomized into 0–1 vs greater than 1. Frequency tables were created to show the association between two categorical features. Line plots were generated to show the CTC trend across patients with different best responses to ICI therapy. Kaplan-Meier curves for PFS and overall survival (OS) were plotted to present graphically the survival outcome after the therapy in each of the two different groups, respectively. Then PFS and OS were compared between two groups using Log-rank test. PFS and OS were measured from the start of ICI therapy. PFS was defined as time to clinical or radiographic progression or death of any cause. OS was defined as time to death of any cause. Fisher’s exact test was employed to test whether there is an association between being CTC+ and other categorical features. The Mann-Whitney U test was applied to test whether there is an association between being CTC+ and age. The Kruskal-Wallis test was used to test whether the CTC count or CTC trend correlates with immunotherapy treatment response. Subgroup analysis was conducted in patients with a specific type of cancer (NSCLC and urothelial cancers). Then similar analyses were conducted for the presence of PD-L1+ CTC and IRF-1+ CTC, respectively. For purposes of these analyses, patients with no identified CTCs were grouped in the PD-L1- and/or IRF-1- group. PD-L1+ or IRF-1+ subgroups denote patients with at least one identified PD-L1+ or PD-L1 equivocal CTC or at least one IRF-1+ CTC at that timepoint (see Methods, Enumeration and Characterization of CTCs, and Discussion). The significance levels were set at 0.05 for all tests. The SAS University Edition (SAS Institute, Inc., Cary, North Carolina) was used for data management and analyses.
3. Results
3.1. RarePlex PD-L1 Panel Validation
First, the sensitivity and specificity of the PD-L1 biomarker assay (RarePlex 0912-VB PD-L1 CTC Panel Kit) to enumerate CTCs was established by comparison with the validated comparator enumeration assay (RarePlex 0900-VA CTC Panel Kit). The ratio of enumerated spike-in mCTCs between the two kits (0912-VB/0900-VA) was 1.05. CTC enumeration accuracy of the PD-L1 kit was quantified by testing 10 slides printed with 3–6 mCTCs and 10 slides with no mCTCs. On this test set of 20 slides, no false positives or false negative mCTCs were detected, leading to CTC enumeration accuracy of 100%. Clinical samples from three different patients with three different tumor types (breast cancer, NSCLC, and prostate cancer) were then evaluated. The ratio of enumerated CTCs between the PD-L1 kit and the comparator kit was 1.01 in the clinical CTC samples. Representative mCTC are shown in Supplemental Figure 3.
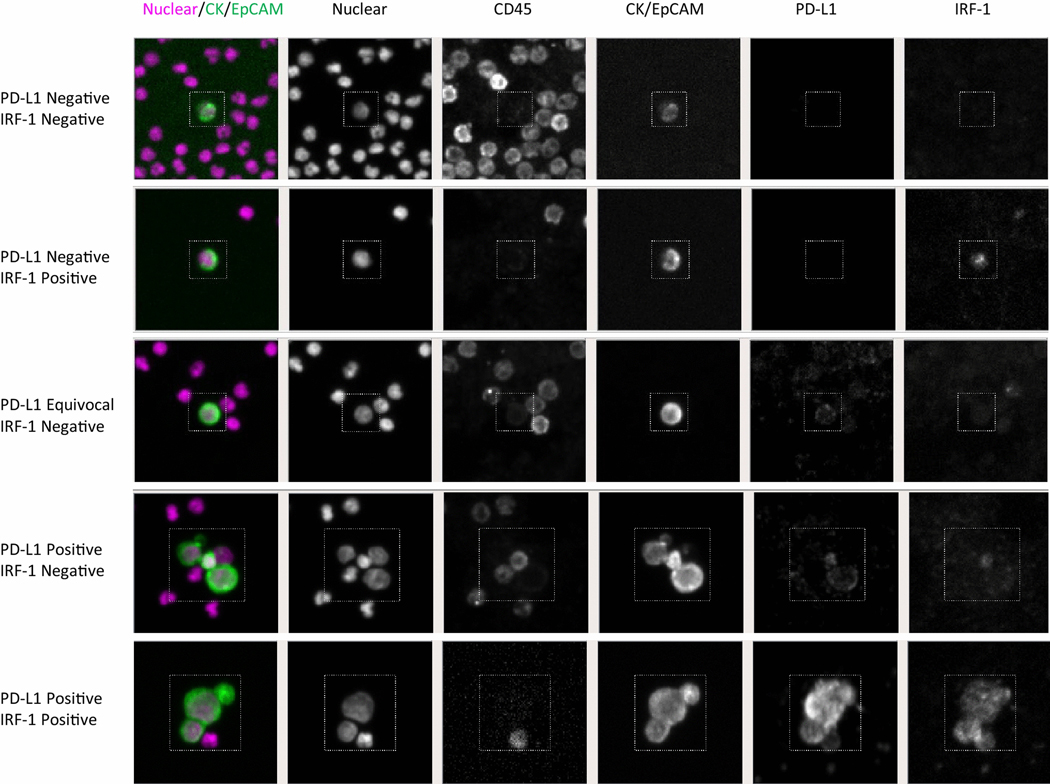
The PD-L1 kit then underwent a thorough assessment for analytic reliability. During this testing, MFI threshold of 500 was selected as it provided an optimum balance of sensitivity and specificity. This MFI positivity cut-off yielded sensitivity of 99% and specificity of 97% (Supplemental Figure 4a). The kit accuracy was 98% as determined by truth matrix and the mean coefficient of variability across 3 independent staining runs was 12.8% (Supplemental Figure 4b and 4c). When H1650 cells (low PD-L1 expression) were spiked into blood from three different donors, the mean coefficient of variation between the different donor samples was 7.6% (data not shown). Figure 1 shows representative patient-derived CTC images. As described in the Methods section under “CTC enumeration and characterization”, a decision tree was developed for CTC PD-L1 expression due to high background levels of PD-L1 staining related to platelets, which was seen on some samples. This PD-L1 staining is on-target as platelets are known to express PD-L1 [23], and was commonly seen in the clinical samples.
Fig. 1.
Representative images of circulating tumor cells (CTCs) identified by the RarePlex PD-L1 assay. CTCs were identified based on the presence of nuclear staining, the presence of CK/EpCAM staining, and the lack of CD45 staining. Representative patient samples showing negative PD-L1 (a, b), equivocal PD-L1 expression with surrounding speckled PD-L1 expression related to platelet coating (c), and strongly positive PD-L1 expression (d) are shown. Representative negative (a, c) and positive (b, d) IRF-1 staining are also shown
3.2. Patient Characteristics
A total of 30 patients enrolled in the study from July 2018 to April 2019 and started ICI therapy (Supplemental Table 1). Date of final study follow-up was April 30, 2020. One patient had insufficient sample from the baseline blood draw for CTC enumeration and one patient consented for the study but did not start ICI due to rapid clinical progression. These patients were excluded from the CTC analysis, leaving 28 patients available for analysis. All 28 patients had baseline blood draws, 96% had timepoint B blood draws (27 patients), and 54% had timepoint C blood draws (15 patients). Of the patients who had timepoint C blood draws, 40% (six patients) had the blood draw early (prior to 3 months) due to ICI discontinuation for progressive disease. The single patient who had a baseline blood draw only had rapid disease progression and did not undergo further research blood draws after Timepoint A.
The median age of the patient cohort was 63 years (range 28 – 83 years) and had 13 (46%) women and 8 (29%) never smokers. The cohort had several cancer types represented, with non-small cell lung cancer (43%), urothelial cancer (21%), and breast cancer (14%) making up the largest subgroups of patients with CTCs for evaluation (Supplemental Table 3). Most patients were starting ICI as first or second line treatment for metastatic disease and received single agent ICI as monotherapy or in combination with chemotherapy or a clinical trial agent; one patient received dual immune checkpoint blockade with ipilimumab/nivolumab. Most patients (23) had visceral metastases and only a small number of patients (4) had only lymph node metastases. A notable minority (10) had hepatic metastases. Pembrolizumab (anti-PD-1), was the most common ICI used (17). The median number of days on ICI therapy was 46; six patients received only 1 dose of ICI therapy – four due to rapid cancer progression, one due to toxicity, and one due to molecular testing that led to a different course of therapy.
In terms of best radiographic response, 25% of patients achieved partial response (PR), 29% achieved stable disease, and 46% of patients had progressive disease as best response. One patient rapidly declined after treatment and never underwent radiographic assessment. Seven patients (25%) had durable benefit, defined as sustained stable disease or partial response by imaging for at least 1 year after starting ICI. At time of final study follow-up, five of these patients continued to respond and did not require a change in therapy. A total of 23 patients had progression and 18 died during the study follow-up period. The median follow-up time was 1.3 years (range 1.0 – 1.8 years) from time of starting ICI therapy. The median PFS of the study cohort was 2.3 months (range 0.2 months to NR) and the median OS of the study cohort was 9.9 months (range 0.5 months to NR).
3.3. Tumor Tissue PD-L1 and IRF-1 Expression
Fourteen patients had archival tissue available for PD-L1 and IRF-1 staining. Representative images of tissue staining are shown in Figure 2. The tissue available was a mix of primary and metastatic biopsy samples (Supplemental Table 4). Most tumors had positive PD-L1 staining (57%), defined as at least 1% of tumor cells staining, and two patients had strongly positive PD-L1 staining, defined as 50% or more of tumor cells staining. Positive IRF-1 staining was present in 93% of tumors. Strongly positive IRF-1 staining was present in 43% of tumors.
Fig. 2.
Representative tissue images with PD-L1 and IRF-1 staining. Images for tissue with a) <1%, b) 1–49% and c) >50% PD-L1 positive staining, shown in green and d) <1%, e) 1–49% and f) >50% IRF1 staining, shown in turquoise. Cytokeratin (magenta) and Sytox (blue) staining shown as reference. Scale bars are 50 mm
Neither PD-L1 nor IRF-1 tumor staining in the archival tissue correlated to best radiographic response (progressive disease, stable disease, or partial response) in this small heterogeneous cohort (Figure 3). However, strongly positive IRF-1 tissue staining did correlate with durable benefit, defined as radiographic response for at least one year from starting ICI-based therapy (p = 0.02, Fisher’s exact test). Of the four patients with durable benefit and tissue available for IRF-1 staining, all had strong IRF-1 tissue staining; 3 also had tumor tissue that was PD-L1 positive.
Fig. 3. Association between tumor PD-L1 and IRF-1 expression with immunotherapy treatment response.
Each bar represents one patient in the study. The bar starts at time of treatment initiation with ICI and ends at the observation of progressive disease. Patients who died prior to radiographic assessment were considered to have progressive disease. A total of five patients in the study had sustained responses to ICI therapy and had not initiated another line of therapy at time of last follow-up. The time of last dose of ICI therapy is denoted by a green triangle. Tumor PD-L1 expression was denoted as negative (−) if tumor proportion score was ≤ 1%, positive (+) if tumor proportion score was 2–49%, and strongly positive (++) if tumor proportion score was ≥ 50%. Tumor IRF-1 expression was denoted as negative (−) if tumor proportion score was ≤ 1%, positive (+) if tumor proportion score was 2–49%, and strongly positive (++) if tumor proportion score was ≥ 50%. PD (red) = progressive disease, SD (purple) = stable disease, PR (green) = partial response by RECIST 1.1 criteria. A durable benefit was defined as a patient who maintained a radiographic response to treatment for greater than 1 year. If there is no symbol for PD-L1 or IRF-1, there was insufficient tissue available for PD-L1 and/or IRF-1 staining
3.4. CTC Enumeration
3.4.1. Pre-Treatment Timepoint
At baseline, 16 of 28 (57%) patients had at least 1 CTC identified. In patients with any identified CTCs, the median CTC count at baseline was 4.5 (range 1–326) (Supplemental Table 2). Cancer type and the number of lines of prior therapy were not significantly correlated with having 2 or more CTCs (Supplemental Table 3), but these clinical features were significantly associated with five or more identified CTCs at baseline (p = 0.05, p = 0.05 respectively). There was insufficient data to evaluate the interaction between cancer type and number of prior lines of therapy. There was no correlation between locations of metastatic disease (Supplemental Table 5), gender, or age with CTC count in this dataset. There was also no correlation by Kruskal-Wallis test between baseline CTC count and the best radiographic response as defined by RECIST v1.1 criteria (Supplemental Figure 5).
CTC count at the baseline timepoint appeared to have prognostic value in patients. As described in the methods section, the CTC count dichotomization point was statistically determined for this exploratory pilot study. Patients with 2 or more CTCs prior to starting ICI had lower PFS compared to patients with 0–1 CTC at baseline (Supplemental Figure 6a, PFS 1.3 months vs 4.3 months, log-rank test, p = 0.01). There was no significant difference in OS based on CTC count at baseline (Supplemental Figure 6b).
3.4.2. Post-Treatment Initiation Timepoints
At the early and late post-treatment initiation timepoints, 16 of 26 (62%) and 12 of 15 (80%) patients had identifiable CTCs. The median CTC count in patients with identified CTCs at the early post-treatment timepoint (Timepoint B) and the late post-treatment timepoint (Timepoint C) was 2.5 and 2.5, respectively, with a range of 1–1043 CTCs and 1–137 CTCs, respectively (Supplemental Table 6). Patients with 2 or more CTCs at Timepoint B had a trend towards shorter median PFS compared to patients with ≤1 CTCs (Supplemental Figure 6c, 1.5 vs 4.4 months, p = 0.07). There was no significant difference in OS between patients based on CTC count at the early post-treatment timepoint (Timepoint B) despite numerically longer OS in patients with ≤1 CTCs vs ≥2 CTCs (Supplemental Figure 6d, 4.2 vs 13.8 months, p = 0.14). There was no correlation by Kruskal-Wallis test between the CTC counts at Timepoint B or C and the patient’s best radiographic response as defined by RECIST criteria.
3.5. PD-L1 and IRF-1 status of CTCs
At baseline, 25% of the patients had at least one PD-L1+ CTC and 21% of patients had at least one IRF-1+ CTC. In patients with identified CTCs, 44% had at least one PD-L1+ or PD-L1 equivocal CTC and 38% had at least one IRF-1+ CTC (Supplemental Table 7). In patients with identified CTCs, 2 out of the 16 patients had CTCs that were concurrently PD-L1+ and IRF-1+, and 2 other patients had PD-L1 equivocal staining with concurrent IRF-1+ staining. The remaining patients had either isolated PD-L1 staining or isolated IRF-1 staining on their CTCs. In the patients with tumor tissue PD-L1 status available (from either clinical or research PD-L1 assay), PD-L1 tumor tissue status did not correlate with positive CTC PD-L1 status (Fisher exact test, p = 0.65). There were no clinical features (tumor type, location of metastases, gender, age) that correlated with the presence of CTC PD-L1 or IRF-1 positivity.
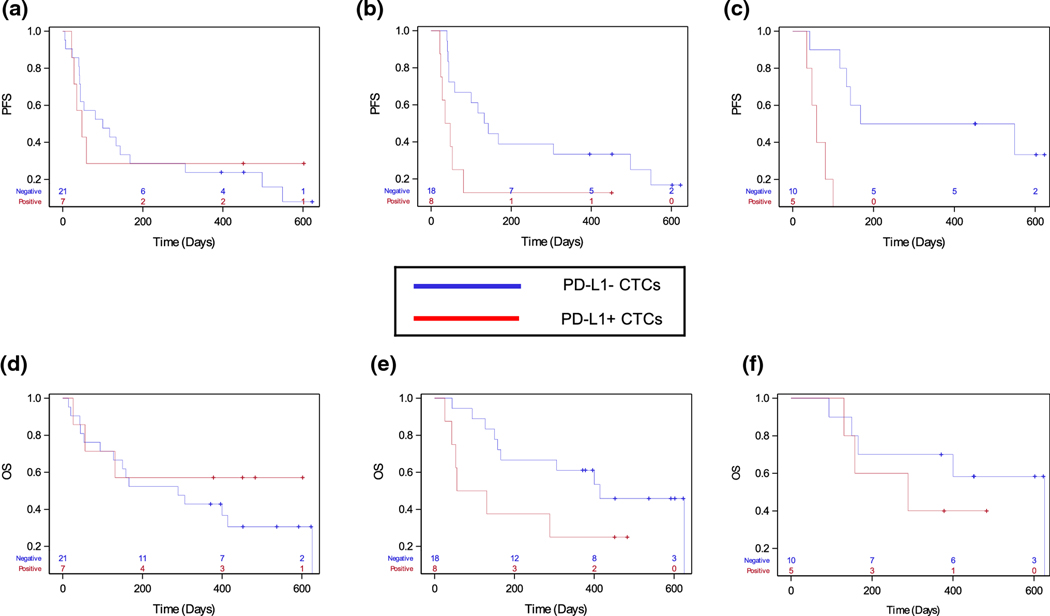
To evaluate the prognostic implications of having PD-L1+ CTCs present, we defined the patients with one or more identified PD-L1+ or PD-L1 equivocal CTCs as part of the “PD-L1+ CTC” group. Patients with either no identified CTCs or only PD-L1- CTCs were defined as part of the “PD-L1- CTC” group. At the pre-treatment timepoint, there was no difference in median PFS or OS for patients with any PD-L1 equivocal or positive CTCs vs patients with PD-L1 CTC negative status (Figure 4a and 4d). The presence of any PD-L1 equivocal or PD-L1+ CTC at the early or late post-treatment timepoints was associated with shorter PFS (Figure 4b and 4c, 1.2 vs 4.4 months and 1.9 vs 5.5 months, log-rank test, p < 0.05 and p < 0.01, respectively). There was also a trend towards shorter median OS for patients with positive CTC PD-L1 status at the early post-treatment timepoint (Figure 4e, 1.9 vs 13.8 months, p = 0.07). These data suggest that the presence of any detectable PD-L1 equivocal or PD-L1 positive CTC may have prognostic implications in the setting of ICI therapy.
Fig. 4.
The presence of PD-L1+ CTCs by timepoint in patients receiving immunotherapy. For this analysis, PD-L1 equivocal CTCs were analyzed as PD-L1+ CTCs and the PD-L1- CTC group includes patients with no identified CTCs and patients with only PD-L1- CTCs. a) At the baseline timepoint, the median PFS for patients with PD-L1+ CTCs identified versus patients who did not have PD-L1+ CTCs identified was 1.6 vs 3.3 months. b) At the early post-treatment timepoint, patients with PD-L1+ CTCs vs PD-L1- CTCs had a median 1.2 vs 4.4 months (p = 0.02) progression-free survival. c) At the late post-treatment timepoint, the median progression-free survival was 1.9 vs 5.5 months (PD-L1+ vs PD-L1-, p<0.01). d) Baseline (median overall survival (OS) in PD-L1- vs PD-L1+ 9.6 months vs NR, p = 0.42), e) early post-treatment (median OS in PD-L1- vs PD-L1+ 13.8 vs 1.7 months, p = 0.07), and f) late post-treatment (median OS in PD-L1- vs PD-L1+ 13.8 vs 20.6 months, p = 0.42) Kaplan-Meier curves
We similarly split the patients by IRF-1 CTC status. The presence of positive CTC IRF-1 status at the late post-treatment timepoint was associated with shorter PFS (Figure 5, 1.6 months vs 4.7 months, p = 0.05), but there was no significant difference in PFS or OS at the other timepoints. Neither PD-L1 nor IRF-1 status was associated with best radiographic response.
Fig. 5. Effect of IRF-1+ CTCs on survival by timepoint.
Progression-free survival (PFS) a) at baseline (IRF- vs IRF+ median PFS 2.7 vs 2.0 months, p = 0.827), b) early post-treatment (4.4 vs 1.6 months, p = 0.43), c) late post-treatment (4.7 vs 1.6 months, p = 0.05). Overall survival for IRF-1- vs IRF-1+ CTCs at d) baseline (median 5.5 vs NR months, p = 0.31), e) early post-treatment (median 13.8 vs 4.4 months, p = 0.34), or f) late post-treatment (median 20.6 vs 9.5 months, p = 0.82)
4. Discussion
PD-L1 is a dynamic surface biomarker present on both tumor and immune cells that tumor cells can utilize for protection from the immune system. PD-L1 expression in tumor sites can change over the course of therapy [27]. This increases the appeal of a liquid biopsy-based assay, which can be collected longitudinally with minimal risk to the patient [28]. In this study, we present a pilot study of a novel dual biomarker liquid biopsy assay that can characterize PD-L1 and IRF-1 protein expression on epithelial circulating tumor cells (CTCs) using a multiplexed immunofluorescence imaging strategy. This assay was initially validated using spiked-in model CTCs and clinical samples, then was used as a serial exploratory biomarker in a small pilot study of patients with advanced epithelial-origin solid tumors who were receiving therapy with ICI. To our knowledge, this is the first study to report on CTC IRF-1 expression.
In this pilot study with a heterogeneous mix of solid tumor types, a high fraction of patients had detectable CTCs at baseline although most had an overall low CTC count. Patients with two or more CTCs identified at baseline had shorter PFS compared to patients with one or no detected CTCs at baseline. Patients with one or no CTCs detected at baseline generally had fewer prior treatment lines and a lower number of metastatic sites. One concern around CTC-based assays as a clinical biomarker has been a concern that these assays may have limited utility because only a proportion of patients have detectable CTCs. Our results suggest that lack of CTCs may be a favorable prognostic feature. This has been confirmed in a larger study with the Rarecyte CTC assay that focused on patients with metastatic breast cancer. In patients with metastatic breast cancer, the identification of 0–4 CTCs was associated with a better overall survival compared to the identification of 5 or more CTCs.[29]
In addition to considering CTC counts, expression of PD-L1 or IRF-1 on CTCs seems to provide additional prognostic information. In this study, we found that the presence of any PD-L1+ or PD-L1 equivocal CTCs post-ICI treatment was associated with patients having shorter PFS compared to patients without identified CTCs or with PD-L1- CTCs. This was concordant with overall survival at these post-treatment timepoints. However, there was discordance between the PFS and OS findings for PD-L1- and PD-L1+ CTC groups at baseline. At this timepoint, patients with PD-L1+ CTCs at baseline had a shorter median PFS but a longer median OS compared to patients with PD-L1- CTCs. This was due to the presence of two patients with metastatic breast cancer who had PD-L1+ CTCs at baseline. These patients had rapid progression through ICI-containing therapy followed by multiple additional treatment regimens after ICI progression, resulting in a short PFS but long OS.
Patients with any IRF-1+ CTCs at either time of restaging imaging or treatment discontinuation also had shorter PFS. This suggests that the identification of PD-L1+ or IRF-1+ CTCs in a patient undergoing ICI therapy may suggest resistance to ICI-based therapy, particularly since the presence of PD-L1 equivocal or positive CTCs or IRF-1+ CTCs at the pre-treatment timepoint did not have the same association with PFS. These hypothesis-generating data are intriguing, but additional validation in larger and more homogenous patient cohorts will be required to further characterize the impact of PD-L1+ or PD-L1 positive or equivocal CTCs on ICI response and outcomes.
Our results generally align with data in the literature regarding CTC PD-L1 expression and prognostic implications. Previous studies evaluating CTC PD-L1 expression in non-small cell lung cancer found that PD-L1 was expressed on most CTCs, and that CTC PD-L1 expression at baseline was associated with shorter PFS in patients treated with nivolumab [30, 31]. PD-L1 expression was also identified in a high number of CTCs in urothelial cancer [32]. Our study found a lower prevalence of PD-L1+ CTCs; we ultimately identified the presence of PD-L1+ CTC in nearly half of the patients over all timepoints, but at baseline, PD-L1+ CTCs made up only 11% of the total CTCs. This is a much lower prevalence of PD-L1+ CTCs than has been reported in NSCLC: in a previous study, 95% of patients with identified CTCs had PD-L1+ CTCs [30]. This difference may be due to the diversity of tumor types and patient populations enrolled, but also various technical differences in the assays. One challenge in identifying PD-L1-positive CTCs is differentiating them from immature myeloid cells, which stain similarly to CTCs: low to absent CD45 expression, non-specific staining for cytokeratin/EpCAM, and the potential to stain positively for PD-L1 [33]. These immature myeloid cells may mimic CTCs, potentially creating false positive PD-L1+ CTCs. In our study, these immature myeloid cells were excluded based on low level CD45 expression as well as weaker cytokeratin/EpCAM staining.
Another interesting finding that was observed during the validation process was the association of platelets with the CTCs. Platelets are known to express PD-L1, and it can be influenced by ICI therapy [23]. There are also subpopulations of CTCs that are coated with platelets and may regulate CTC and immune cell interactions and therefore ICI response [34]. While assays that are dependent on surface biomarkers to isolate CTCs may miss these platelet-coated cells, the RarePlex assay is able to isolate and visualize platelet-coated CTCs because platelets are collected as part of the buffy coat fraction in the AccuCyte sample preparation process.
In the validation studies, we confirmed that platelets have low signal intensity PD-L1 staining. For the initial validation of PD-L1, we set quantitative fluorescence signal intensity thresholds that optimally separate CTC PD-L1 signal from the surrounding platelet PD-L1 background signal. However, this stringent threshold may miss CTCs that stain at the level of the platelet background. Because of this, we implemented a decision tree that allowed us to identify platelet-associated CTCs with potential low-level PD-L1 staining. We termed these cells “equivocal” due to the difficulty in delineating the platelet from CTC PD-L1 staining. Nevertheless, we classified these equivocal CTCs as PD-L1+ for the pilot study data analysis based on the previous pre-clinical and clinical data that platelet-coated CTCs may be important for ICI response [23, 34, 35]. The presence of these PD-L1 equivocal CTCs at the post-treatment initiation timepoints B and C appeared to be associated with shorter PFS as CTCs with strong PD-L1 staining at these timepoints.
As in previous studies, we did not identify a significant correlation between tumor tissue PD-L1 status and PD-L1 status of identified CTCs [30], and we also did not identify a significant correlation between tumor IRF-1 and CTC IRF-1 status. Unlike tumor PD-L1 status, there are limited data on how tumor tissue IRF-1 status may influence ICI response. In our small cohort of patients, tumor tissue IRF-1 staining showed promise for being associated with durable benefit from ICI. We found that the patients with strong tumor tissue IRF-1 staining were more likely to have sustained radiographic response (defined as ≥1 year) to ICI. Strong IRF-1 staining was more common in PD-L1-positive tumors, but it was also observed in two patients with PD-L1-negative tumors, one of whom had durable response. This suggests that activity of the interferon pathways, as indicated by IRF-1 expression, may identify a group of tumors that are particularly responsive to ICI-based therapy possibly independent of PD-L1 status.
There are several limitations inherent to the study nature, including small sample size. Lack of randomization, possible selection and confounding biases, as well as variability in cancer types, metastatic disease burden and location, performance status, organ function, medical comorbidities, prior and current therapies used, surveillance scan frequency and follow up times, can all impact the results. There was no central radiology review of scans and we relied on investigator review of records. We were not able to assess other biomarkers in this specific pilot study. Due to the small sample size with a diverse group of tumor types, we also did not perform any multivariate analysis to look at potential interactions between variables such as CTC count and the presence of PD-L1 or IRF-1 staining. In future larger studies, this type of analysis will be important to evaluate if the PD-L1 status of CTCs is an independent biomarker. Despite these limitations, our hypothesis-generating data are intriguing, suggest feasibility of the CTC-based assay, and warrant further study in larger and more homogeneous cohorts.
Based on the results of this pilot study, CTC expression of PD-L1 and/or IRF-1 may potentially serve as a biomarker of ICI response and resistance, upon further evaluation. In addition, our investigation suggests that tumor IRF-1 status may possibly enrich for patients that are particularly responsive to ICI. Additional clinical studies focusing on particular tumor types with larger number of patients, specific therapies and longer follow up should be conducted to further validate our hypothesis-generating findings.
Supplementary Material
Key Points.
Two or more circulating tumor cells (CTCs) detected in a liquid biopsy sample prior to treatment with immune checkpoint inhibitors (ICIs) portends a worse prognosis than the detection of 0–1 CTCs
The presence of any CTCs with PD-L1 staining after initiation of ICI treatment may portend a shorter duration of response to ICI therapy
Strong interferon regulator factor 1 (IRF-1) staining in tumor tissue was associated with durable responses to ICI therapy
Acknowledgements
We would like to thank Alisa Clein for her assistance in designing the REDCap database. We would like to thank Grace Durenberger for assistance with sample processing. We would like to thank Dr. Victor Chow for patient recruitment and consenting. Training support for Dr. Kennedy was provided through T32-CA009515 and the ASCO/CCF Hayden Family Foundation Young Investigator Award.
Funding:
RareCyte, Inc.
LQMC has no direct conflicts of interests but has received minor de minimus personal advisory board consulting fees and prior institutional research grant funding from Merck, Pfizer, Dynavax and Novartis, Astra-Zeneca in the last three years, de minimus advisory board consulting fees and current institutional research grant funding from Alkermes. She reports current institutional grant funding only from Oncorus. She reports minor de minimus consulting/advisory board fees from Cullinan and Elicio, Gilead, Regeneron, Sanofi-Genzyme and Daiichi Sankyo, Ipsen, Nanobiotix, Beigene, Jazz Pharmaceuticals. Research grant funding only provided to her prior institution from Bristol Myers Squibb, Genentech, Seattle Genetics, Lily/Imclone. Over the last 3 years (all unrelated to this study). PG has received consulting fees from AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Dyania Health, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Guardant Health, Gilead Sciences, Infinity Pharmaceuticals, Janssen, Lucence Health, Merck, Mirati Therapeutics, Pfizer, QED Therapeutics, Regeneron Pharmaceuticals, Seattle Genetics, Silverback Therapeutics, UroGen, 4D Pharma PLC. His institution has received grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, EMD Serono, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck, Mirati Therapeutics, Pfizer, QED Therapeutics. VKG has no direct conflicts of interests with this work but has equity in SEngine Precision Medicine, Novilla, and 3rdEyeBio. VKG is on the scientific boards for Puma Biotechnology, New Equilibrium Biosciences, Phoenix Molecular Designs; he is also on speakers’ bureaus for Puma Biotechnology, Genentech/Roche, Hologics; and receives research support to his institution from Agendia.
Footnotes
Code availability: N/A
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the IRB Committee the Fred Hutchinson Cancer Research Center Cancer Consortium (#10031).
Consent to participate: Patients agreed to participate in this study under informed consent as approved by the Fred Hutchinson Cancer Center Cancer Consortium IRB.
Consent to publish: As part of informed consent for this study, patients agreed to allow the publication of deidentified results.
Declarations:
Conflicts of interest/competing disclosures: LCK, SK, JL, and ZC have no conflicts of interest to report. ABR, EL, YS, LU, EPK are employees of RareCyte, Inc.
Data availability:
Deidentified patient data is shared in aggregate within the paper supplement.
5. References
- 1.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein M, Cimadamore A, Hartmann A, Lopez-Beltran A, Cheng L, Scarpelli M, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med. 2019;7(22):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo H, Ding Q, Gong Y, Gilcrease MZ, Zhao M, Zhao J, et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020;22(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 7.Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, Carpeno JdC, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non–Small-Cell Lung Cancer. J Clin Oncol. 2021;39(7):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gato-Cañas M, Zuazo M, Arasanz H, Ibañez-Vea M, Lorenzo L, Fernandez-Hinojal G, et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017;20(8):1818–29. [DOI] [PubMed] [Google Scholar]
- 9.Ju X, Zhang H, Zhou Z, Wang Q. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. 2020;10(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y, Zheng L, Du Q, Yan B, Geller DA. Interferon regulatory factor 1 (IRF-1) and IRF-2 regulate PD-L1 expression in hepatocellular carcinoma (HCC) cells. Cancer Immunol Immunother. 2020;69(9):1891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smithy JW, Moore LM, Pelekanou V, Rehman J, Gaule P, Wong PF, et al. Nuclear IRF-1 expression as a mechanism to assess “Capability” to express PD-L1 and response to PD-1 therapy in metastatic melanoma. J Immunother Cancer. 2017;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen T, Chen Z, Zhao ZJ, Wu J. Genetic defects of the IRF1-mediated major histocompatibility complex class I antigen presentation pathway occur prevalently in the. Oncotarget. 2017;8(37):60975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haratake N, Toyokawa G, Tagawa T, Kozuma Y, Matsubara T, Takamori S, et al. Positive Conversion of PD-L1 Expression After Treatments with Chemotherapy and Nivolumab. Anticancer Res. 2017;37(10):5713–7. [DOI] [PubMed] [Google Scholar]
- 14.Yoneda K, Kuwata T, Kanayama M, Mori M, Kawanami T, Yatera K, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121(6):490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Tateishi A, Bychkov A, Fukuoka J. Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports. Int J Mol Sci. 2019;20(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozenblit M, Huang R, Danziger N, Hegde P, Alexander B, Ramkissoon S, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei XL, Luo X, Sheng H, Wang Y, Chen DL, Li JN, et al. PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer. J Transl Med. 2020;18(1):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein M, Sikic D, Strissel PL, Erlmeier F, Germany BC. Evolution of PD-1 and PD-L1 Gene and Protein Expression in Primary Tumors and Corresponding Liver Metastases of Metastatic Bladder Cancer. Eur Urol. 2018;74(4):527–9. [DOI] [PubMed] [Google Scholar]
- 19.Arafat W, Stahlfeld C, Sperger JM, Heninger E, Gopalakrishnan D, Barata PC, et al. Intra-patient heterogeneity in urothelial cancer (UC) circulating tumor cells (CTC) and PDL1 expression to identify biomarkers of response and new therapeutic targets: A pilot study. J Clin Oncol. 2017;35(15_suppl):4537. [Google Scholar]
- 20.Wang Y, Kim TH, Fouladdel S, Zhang Z, Soni P, Qin A, et al. PD-L1 Expression in Circulating Tumor Cells Increases during Radio(chemo)therapy and Indicates Poor Prognosis in Non-small Cell Lung Cancer. Sci Rep. 2019;9(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campton DE, Ramirez AB, Nordberg JJ, Drovetto N, Clein AC, Varshavskaya P, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer. 2015;15:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaldjian EP, Ramirez AB, Sun Y, Campton DE, Werbin JL, Varshavskaya P, et al. The RareCyte® platform for next-generation analysis of circulating tumor cells. Cytometry A. 2018;93(12):1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolfes V, Idel C, Pries R, Plötze-Martin K, Habermann J, Gemoll T, et al. PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget. 2018;9(44):27460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers JaM JN, editor Cutpoint Determination Methods in Survival Analysis using SAS: UPdated %FINDCUT macro. 28th SAS Users Group International Conference (SUGI; ); 2003. [Google Scholar]
- 27.Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep. 2017;7(1):11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloten V, Lampignano R, Krahn T, Schlange T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirix L, Buys A, Oeyen S, Peeters D, Liègeois V, Prové A, et al. Circulating tumor cell detection: A prospective comparison between CellSearch® and RareCyte® platforms in patients with progressive metastatic breast cancer. Breast Cancer Res Treat. 2022. doi: 10.1007/s10549-022-06585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–12. [DOI] [PubMed] [Google Scholar]
- 31.Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergmann S, Coym A, Ott L, Soave A, Rink M, Janning M, et al. Evaluation of PD-L1 expression on circulating tumor cells (CTCs) in patients with advanced urothelial carcinoma (UC). Oncoimmunology. 2020;9(1):1738798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schehr JL, Schultz ZD, Warrick JW, Guckenberger DJ, Pezzi HM, Sperger JM, et al. High Specificity in Circulating Tumor Cell Identification Is Required for Accurate Evaluation of Programmed Death-Ligand 1. PLoS One. 2016;11(7):e0159397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Wong KHK, Khankhel AH, Zeinali M, Reategui E, Phillips MJ, et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip. 2017;17(20):3498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaslavsky AB, Adams MP, Cao X, Maj T, Choi JE, Stangl-Kremser J, et al. Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci Rep. 2020;10(1):19296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified patient data is shared in aggregate within the paper supplement.