Abstract
Background:
Knowledge of detailed lymphatic anatomy in humans is limited as the small size of lymphatic channels makes it difficult to image. Most current knowledge of the superficial lymphatic system has been obtained from cadaveric dissections.
Methods:
Indocyanine green (ICG) lymphography was performed pre-operatively to map the functional arm lymphatics in breast cancer patients without clinical or objective evidence of lymphedema. A retrospective review was performed to extract demographic, ICG imaging, and surgical data.
Results:
Three main functional forearm channels with variable connections to two upper arm pathways were identified. The median forearm channel predominantly courses in the volar forearm (99%). The ulnar forearm channel courses in the volar forearm in the majority of patients (66%). The radial forearm channel courses in the dorsal forearm in the majority of patients (92%). Median (100%), radial (91%), and ulnar (96%) channels almost universally connect to the medial upper arm channel. In contrast, connections to the lateral upper arm channel occur less frequently from the radial (40%) and ulnar (31%) channels.
Conclusions:
This study details the anatomy of three forearm lymphatic channels and their connections to the upper arm in living adults without lymphatic disease. Knowledge of these pathways and variations is relevant to any individual performing procedures on the upper extremities as injury to the superficial lymphatic system can predispose patients to the development of lymphedema.
Keywords: lymphatics, anatomy, lymphatic anatomy, ICG lymphography
INTRODUCTION
In contrast to other organ systems, knowledge of normal lymphatic anatomy is limited. The small size of lymphatics limits the imaging and mapping of this organ system. For example, while the largest artery (aorta) and vein (vena cava) in the human body are 2–3cm in diameter, the largest lymphatic (thoracic duct) is only 2mm in diameter. Historically, detailed imaging of the lymphatic system was possible using lymphangiography, but it was primarily utilized in the study of the lymphatic system in patients with a diagnosis of lymphedema.1 Moreover, routine use has fallen out of favor given its invasive nature.1–3 While lymphoscintigraphy has become the gold standard of lymphatic imaging in the work-up of lymphedema (LE), it cannot be used for fine anatomic mapping, as its main utility is demonstrating lymphatic function. Current knowledge of normal lymphatic anatomy in humans comes almost exclusively from cadaveric studies. However, even in cadavers, the small size of the lymphatic channels necessitates specialized injections and months of dissection for a single extremity, limiting understanding of functional anatomy including its variations in vivo.4 The anatomy of the superficial lymphatic system and its variations are pertinent to any practitioner who performs procedures on the upper extremity, as division of lymphatics, even outside a nodal basin, can predispose patients to lymphedema.5
Cadaveric studies have provided a fundamental understanding of lymphatic anatomy, dating back to the 18th and 19th centuries when Mascagni and Sappey, respectively, first published the results of their dissections.6,7 In a landmark paper from 1993 of 300 cadaveric dissections (90% fetal), Leduc laboriously described the lymphatic pathways of the upper extremity and provided their nomenclature.8 Suami et al., using a similar method of cadaveric dissection, described these channels, but rather than name them individually, divided the extremity into lymphosomes, or lymphatic territories that drain to a particular node or nodal basin.4,9 While the findings from these cadaveric dissections have been central to our current understanding of lymphatic anatomy, a critical limitation is their lack of characterization of functional lymphatic anatomy. All vascular systems in the body are dynamic, and cadaveric studies do not allow us to understand how these pathways function in relation to one another. A relatively newer imaging modality, indocyanine green (ICG) lymphography, allows for real-time visualization of functional lymphatic channels. We have previously noted that the lymphatic system would preferentially drain to certain pathways even when other pathways were present.10 To date, ICG lymphography has been utilized primarily for the clinical staging and evaluation of patients with lymphedema and not for further elucidation of normal functional anatomy.
At our institution, all breast cancer patients who are deemed high risk for developing lymphedema undergo pre-operative ICG lymphography to map their lymphatic system as per our standard of care.11 Of significant note, none of these patients have lymphedema or a history of lymphedema prior to imaging. These ICG lymphographies, therefore, provide a unique view of the in vivo superficial and functional lymphatic anatomy of the upper extremity. In this study we describe the anatomy of the main superficial lymphatic channels that arise in the hand and forearm and drain to the torso via the upper arm.
METHODS
ICG Lymphography
Under sterile conditions, 0.1cc of stock (2.5mg/cc) ICG solution (Akorn Inc., Lake Forest, IL) with albumin was injected intradermally at three anatomic locations: 1 centimeter (cm) proximal to the first and fourth web spaces on the dorsum of the hand and 1 cm proximal to the wrist crease in the volar forearm. The location of these injections were based on the lymphosome concept described by Suami et al.4 By injecting each lymphosome, the major lymphatic drainage pathways of the hand and forearm are represented.4 A near-infrared (NIR) imaging device, the PDE-Neo II (Hamamatsu Photonics KK, Hamamatsu, Japan) was used to visualize the superficial lymphatic channels of the extremity. All ICG lymphographies were performed by one of two members of the lymphatic surgery team (ET or DS) and final interpretation was performed by a single lymphatic surgeon (DS) for all studies.
Three major superficial lymphatic pathways arising from the hand and forearm were consistently described and recorded in real-time immediately following injection. The median channel was defined as the channel arising from the volar forearm injection. The radial channel was identified as that arising from the 1st webspace injection. Finally, the ulnar channel was defined as the pathway arising from the 4th webspace injection. Continuation of these pathways into the upper arm were labelled as medial and/or lateral channels. Lateral upper arm channels were defined as those coursing along the cephalic vein (identified utilizing ultrasonography prior to injection) in the lateral upper arm. Medial upper arm channels were those that coursed along the basilic vein in the medial upper arm.
Retrospective Review
A retrospective review of our Lymphatic Surgery REDCap database was performed. Institutional Review Board (IRB) approval was obtained (Protocol # 2020P000274). Consecutive patients with a diagnosis of node positive breast cancer undergoing ipsilateral pre-operative ICG lymphography were identified. Patient demographics and cancer characteristics were extracted for analysis. Exclusion criteria included (1) any subjective or objective evidence of lymphedema prior to ICG imaging and/or (2) any prior surgical history involving the extremity. Our objective criteria for lymphedema include a volume differential of >10% or an L-Dex >10 (ImpediMed, California).
RESULTS
Patient Demographics and Cancer Characteristics
One hundred and four consecutive ICG lymphographies from November 2018 to October 2020 were analyzed. Average age at the time of axillary surgery was 55 years (SD 12), and BMI was 28.2 kg/m2 (SD 6). Most patients were female (98%). Most patients completed neoadjuvant chemotherapy (62%) at the time of imaging. All patients had node-positive disease, established by fine needle aspiration (13%), core needle biopsy (39%), or sentinel lymph node biopsy (SLNB) (48%). The median number of nodes removed during SLNB was 3 (IQR 2–4). Following the ICG lymphography, the median number of nodes removed during ALND was 15 (IQR 10.75–22) and the median number of nodes demonstrating metastatic disease was 1 (IQR 0–3).
Anatomic Analysis
Two patients were found to have non-linear (e.g. diseased) lymphatic channels, and thus were excluded from the anatomic analysis. The course of the 3 main lymphatic pathways of the hand and forearm of the remaining 102 patients are detailed below, including their variable connections to the upper arm.
Median Channel (dermal injection of the volar forearm)
A schematic of the anatomic variants of the median channel are available in Figure 1.
Figure 1 –
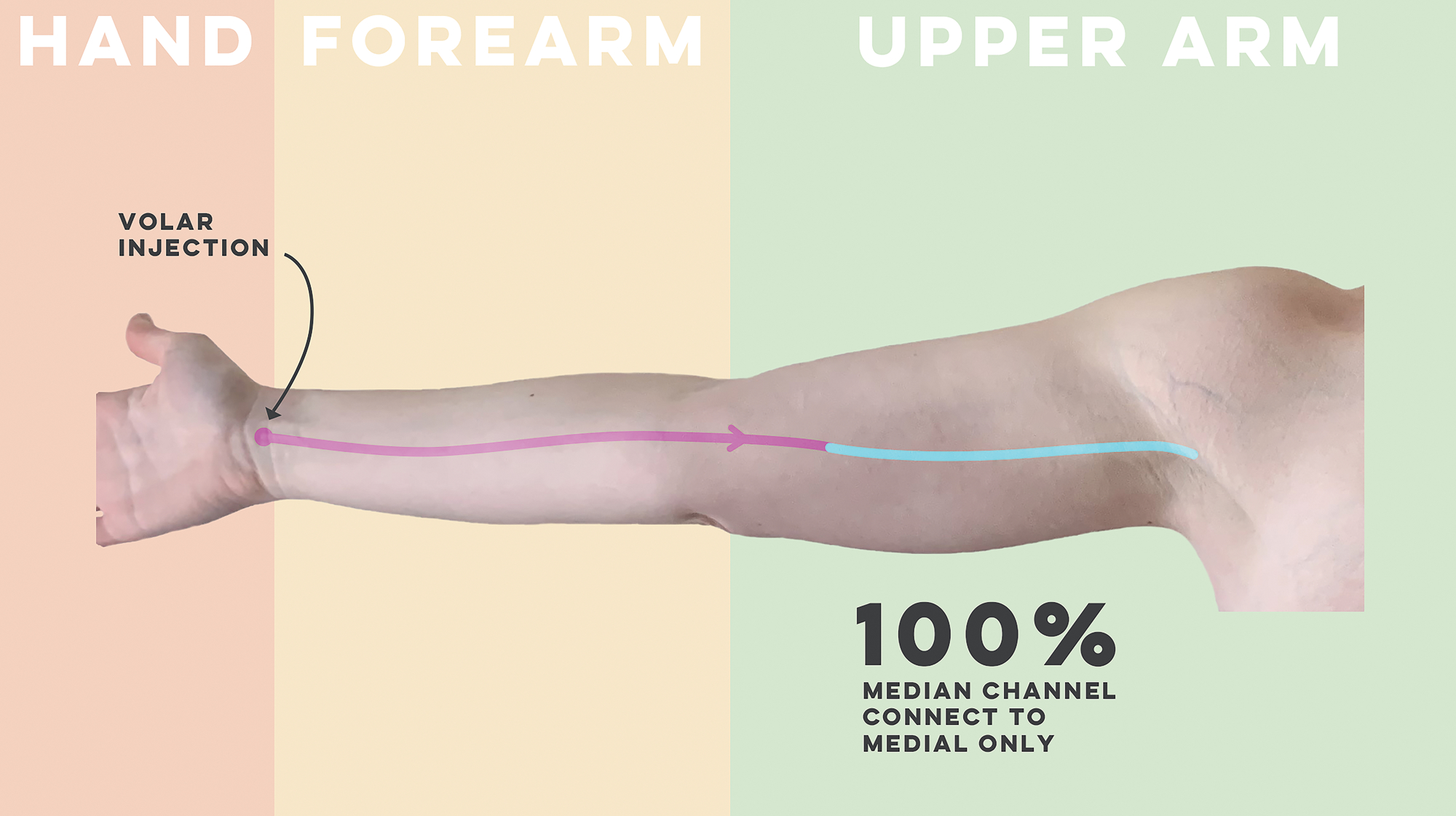
Schematic of the anatomic course of the median forearm channel
The median channel continues in the volar forearm 99% of the time (n=101). In one patient the median channel transitioned to the dorsal forearm over the radial side of the forearm. All median channels connected to only the medial upper arm channel (100%).
Radial Channel (dermal injection 1cm proximal to the 1st web space)
A schematic of the anatomic variants of the radial channel are available in Figure 2.
Figure 2 -.

Schematic of the anatomic course of the radial forearm channel
The radial channel courses in the dorsal forearm 92% (n=94) of the time. These dorsal forearm channels continued to connect to only the medial upper arm pathway in 56% (n=53), only the lateral upper arm pathway in 16% (n=15), and both the medial and lateral pathways in 28% (n=26). A branch off the dorsal radial forearm channel was identified in 30% (n=28) of cases. All branches off the dorsal radial forearm channel transitioned to the volar forearm and connected to the medial upper arm channel.
The radial channel did not continue in the dorsal forearm, transitioning to the volar forearm, 8% (n=8) of the time. All these channels connected to the medial upper arm channel only.
Including all 102 patients described, the radial forearm channel connected to only the medial upper arm pathway in 60% (n=61), only the lateral pathway in 9% (n=9), and both in 31% (n=32).
Ulnar Channel (dermal injection 1cm proximal to the 4th web space)
A schematic of the anatomic variants of the ulnar forearm channel are available in Figure 3.
Figure 3 –
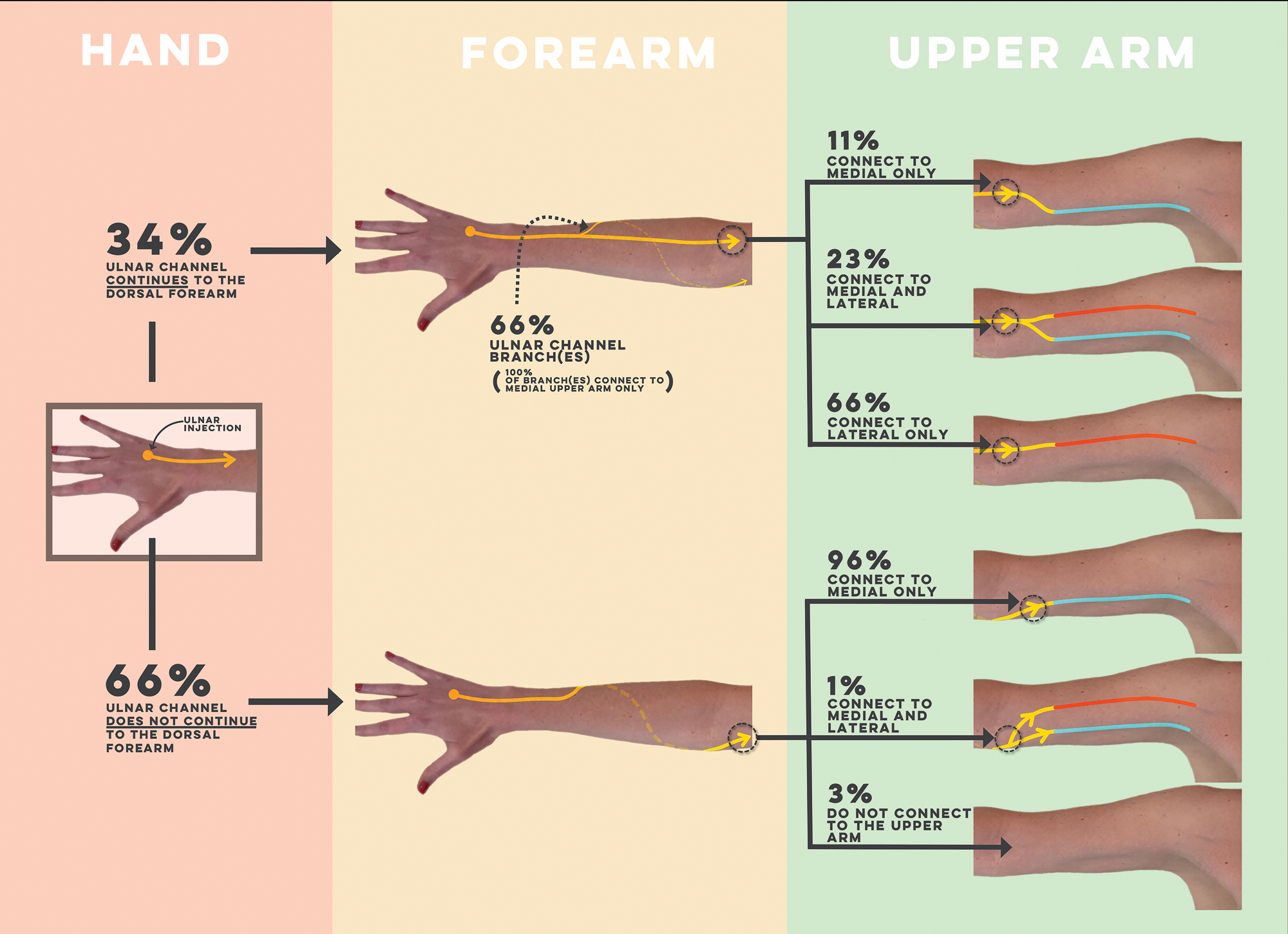
Schematic of the anatomic course of the ulnar forearm channel
The ulnar channel courses in the dorsal forearm 34% (n=35) of the time. These dorsal ulnar forearm channels continued to connect to only the medial upper arm pathway in 11% (n=4), only the lateral pathway in 66% (n=23), and both the medial and lateral pathways in 23% (n=8). A branch off the dorsal ulnar forearm channel was identified in 66% (n=23) of patients. All branches transitioned to the volar forearm and connected to the medial upper arm pathway.
The ulnar channel transitioned to the volar forearm 66% (n=67) of the time. Of these, 96% (n=64) connected to only the medial upper arm pathway, and 1% (n=1) connected to both the medial and lateral upper arm pathways. Two ulnar channels on the volar forearm did not connect to any upper arm pathways (3%).
Including all 102 patients described, the ulnar forearm channel connected to only the medial upper arm pathway in 67% (n=68), only the lateral pathway in 4% (n=4), the medial and lateral pathways in 27% (n=28), and did not connect to the upper arm in 2% (n=2).
DISCUSSION
In this paper we described the in vivo superficial and functional anatomy of the superficial lymphatic system of the upper extremity in patients without evidence of lymphedema. Of note, although the channels appear as single pathways on ICG, they represent the convergence of small tributaries in a particular lymphosome and therefore are comprised of many individual lymphatic channels. Moreover, based on the lymphosome concept, these visualized channels are representative of the course of the other non-visualized lymphatics within the given lymphosome. We tracked the course of three main pathways from the hand and forearm (median, radial, and ulnar) and their connections to two pathways in the upper arm (medial and lateral).Specifically, the median forearm channel predominantly traveled in the volar forearm (99%) (Figure 4). We found that the radial channel coursed in the dorsal forearm in the majority of patients (92%). In contrast, the ulnar forearm channel coursed in the volar forearm in the majority of patients (66%) (Figure 5). Regarding connections to the upper arm, the median channel was the most consistent, with 100% connecting to the medial upper arm channel. In contrast, for radial and ulnar forearm channels, the type of upper arm connection correlated with the course of the channel in the forearm. Specifically, regardless of injection site, if the pathway transitioned from the dorsal to the volar forearm, it almost universally connected to the medial upper arm (Figure 6). However, if the pathway coursed in the dorsal forearm, a contribution to the lateral upper arm pathway was more likely (Figure 7).
Figure 4 –
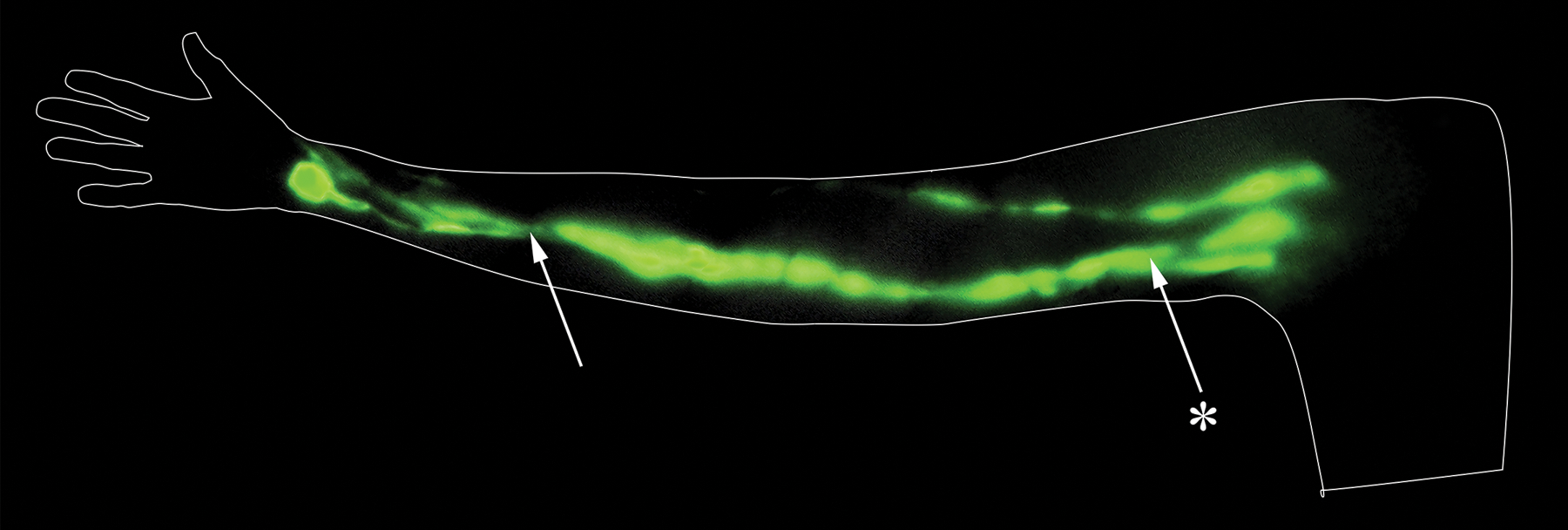
ICG lymphography of the median forearm channel of a right arm with the palm supinated. The median forearm channel is identified on the volar aspect of the forearm by the arrow. It connects to the medial upper arm pathway, identified by the asterisked arrow. This image was created from stills taken from a video captured with the PDE-Neo II. Stills were captured from the video and stitched together, and no other alterations were made.
Figure 5 -.

ICG lymphography illustrating the radial and ulnar forearm channels of a right arm with the palm pronated. The radial forearm channel in the dorsal forearm, identified by the arrow with the “R”, connects to the lateral upper arm pathway, identified by the arrow with a single asterisk. The ulnar forearm channel does not continue to the dorsal forearm, transitioning to the volar forearm, as identified by the arrow with the “U”. The ulnar channel connects to the medial upper arm pathway, as identified by the arrow with two asterisks. This image was created from stills taken from a video captured with the PDE-Neo II. Stills were captured from the video and stitched together, and only zoom was altered to create contiguous channels of similar size. All arms were reflected to standardize anatomic landmarks. No other post-production alteration was conducted, including changes in contrast.
Figure 6 –

ICG lymphography illustrating the radial forearm channel of a right arm with the palm pronated. The radial forearm channel does not continue in the dorsal forearm, but rather transitions to the volar forearm as identified by the arrow. It connects to the medial upper arm pathway as identified by the asterisked arrow. This image was created from stills taken from a video captured with the PDE-Neo II. Stills were captured from the video and stitched together, and no other alterations were made.
Figure 7 –

ICG lymphography illustrating the radial and ulnar forearm channels of a right arm with the palm pronated. The ulnar forearm channel continues in the dorsal forearm, as identified by the arrow. It connects to the lateral upper arm pathway, as identified by the asterisked arrow. The radial channel also remains in the dorsal forearm but connects to the medial upper arm channel. This image was created from stills taken from a video captured with the PDE-Neo II. Stills were captured from the video and stitched together, and no other alterations were made.
There is considerable variation in the anatomic course of the radial channel. The radial channel usually courses along the dorsal forearm (92%) as it progresses toward the upper arm. When traveling dorsally in the forearm, this channel demonstrates variability in its connections to the upper arm, with a predominance of medial upper arm connections (84%) versus lateral upper arm connections (44%). In contrast, when the radial channel transitions to the volar forearm, or a branch off a dorsal radial channel transitions to the volar forearm, these ultimately connect to the medial upper arm only.
The ulnar channel also demonstrates significant variability. The ulnar forearm channel transitions to the volar forearm in the majority of patients (66%), unlike the radial channel, and predominantly connects to only the medial upper arm. When the ulnar channel travels in the dorsal forearm (34%), connections to the lateral upper arm (89%) are most likely versus those to the medial upper arm (34%). Interestingly, in two patients the ulnar channel abruptly discontinued at the antecubital fossa. We believe that this is due to the pathway diving deeper into the extremity, perhaps connecting to the deep lymphatic system.9,12
The above findings are commensurate with reports by Suami et al.4,9 Specifically, utilizing the lymphosome concept, Suami noted that the majority of the lymphatic pathways from the hand and forearm flowed into the medial upper arm and into a sentinel lymph node in the axillary region. Moreover, Suami noted that pathways that passed along the dorsal aspect of the arm may bypass the main sentinel node in the axilla and reach smaller “sentry” nodes.4 Previously, we demonstrated that the lateral upper arm pathway, often referred to as the Mascagni-Sappey pathway, most often bypasses the main lymph nodes of the axillary basin.13 Similarly, we also demonstrated that the lymphatic pathways of the medial upper arm, overlying the basilic vein, universally drained into the axillary basin.13 Our current study provides further granularity in the mapping of the functional lymphatic pathways of the upper extremity and how their specific anatomic course affects their connections to the medial versus lateral upper arm and, subsequently, their draining nodal basins. This anatomic understanding has significant clinical implications as, if both the medial and lateral pathways are present, they can act as a “back-up” pathway for each other in the event of injury, as has been previously postulated.14
We believe that this anatomy is of utmost relevance to any provider operating on the upper extremity. Specifically, knowledge of the functional lymphatic pathways would support longitudinal incisions that would be least likely to damage the pathways which travel axially. Moreover, general knowledge of the location of the functional lymphatic pathways could inform surgeons of anatomic regions where extirpative procedures may be at high risk of causing lymphedema (e.g. in areas where the medial and lateral upper arm pathways may both be compromised). In cases where patients may have had prior compromise to one of the two upper arm pathways (e.g. prior history of axillary surgery or a lateral upper arm skin cancer excision), further caution would be warranted prior to potentially compromising the remaining pathway providing drainage for the entire arm.14 In such cases, pre-operative ICG lymphography may provide appropriate mapping of the pathways at risk and prophylactic re-routing of the functional lymphatic system could be considered. For lymphatic surgeons, the anatomic patterns described in this study may better inform the location of lymphovenous bypasses and lymph node transplantation. Specifically, in a patient with breast cancer related lymphedema (BCRL) after an axillary lymph node dissection, the radial aspect of the forearm is slightly more likely to have drainage to the lateral upper arm pathway and therefore be able to bypass the axilla than the ulnar aspect of the forearm. In fact, MRI studies from our institution have noted the edema of BCRL patients to be significantly more notable at the ulnar aspect of the forearm.15 Therefore, lymphatic interventions may be more effective when performed at the ulnar aspect of the arm for BCRL.
Of note, two patients included in our study cohort had non-linear (diseased) channels, or abnormal lymphatic anatomy. While excluded from the above anatomic analysis, further commentary on their clinical scenarios are warranted. Specifically, one patient had undergone sentinel lymph node biopsy 6 months prior in which 7 nodes were removed, of which 6 were positive. This case is interesting as it may indicate that lymphatic changes can occur very rapidly following a surgical insult. The second patient with non-linear channels received steroid injections 21 months prior for ulnar wrist pain, overlapping the location of the non-linear pattern visualized. While patients with prior surgeries of the operative extremity were excluded from analysis, we did not exclude patients with history of injections to the extremity. This case underscores the importance of delineating normal lymphatic anatomy in order to better understand and describe changes that routine intervention on the upper extremity may have on lymphatic anatomy and function.
This study has limitations. First, the study population had breast cancer with demonstrated axillary nodal metastases at the time of imaging. Therefore, an argument may be made that our findings may not represent the general population. However, we believe the anatomy described likely represents physiologic anatomy and is applicable to healthy patients, as most patients in this cohort did not have any significant axillary burden (median of 1 positive node from ALND). Moreover, it has been previously noted that the arm and breast sentinel nodes do not overlap 96% of the time.16 Therefore, the likelihood of an axilla with low disease burden significantly impacting the functional lymphatic mapping of the upper extremity is unlikely. We are currently undertaking a multi-year study in healthy volunteers to repeat the reported study in patients without cancer or any other interventions of the arm. We hope this work will corroborate that our findings in this manuscript are a good representation of normal lymphatic anatomy and its variation. Second, although ICG lymphography has many advantages as described, one disadvantage is its shallow depth of penetration (1–2–4 cm) and therefore we were only able to describe the superficial lymphatic anatomy.17 In the two patients in whom the ulnar channel was no longer visible at the antecubital fossa, we can only hypothesize that the channels coursed deep, out of range of our ICG imaging. The inability to concurrently describe the deep lymphatic system is an additional limitation of this study. Finally, while we did include patients of both biological sexes, our results are likely more relevant to women due to their overwhelming majority in our cohort (98%). Lymphatic anatomic variation between sexes is unknown.
Conclusion
We describe the in vivo superficial and functional lymphatic anatomy of the upper extremity. We consistently noted three main forearm channels (median, radial and ulnar) and two main upper arm channels (medial and lateral), and their variable inter-connections. While historic cadaveric data describes other channels in the upper extremity, our data suggests that these are the most functionally active. To minimize iatrogenic disruption of the superficial lymphatic anatomy, we believe all practitioners who perform operations on the upper extremity should be aware of its anatomy. In the future, we hope to be able to corroborate these findings with high frequency ultrasound, and correlate anatomic variants with the development of lymphatic dysfunction, to better guide all practitioners on the surgical and procedural planning of the upper extremity.
ACKNOWLEDGEMENTS
The authors wish to thank Weilung Sun for his diligence maintaining the QI database.
Financial Disclosure Statement:
Research reported in this publication was partially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (https://www.nhlbi.nih.gov/) under Award Number R01HL157991 (DS), and the Jobst Lymphatic Research Grant awarded by the Boston Lymphatic Symposium, Inc. (MG).
REFERENCES
- 1.Suami H, Koelmeyer L, Mackie H, et al. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg Oncol. 2018;27:743–750. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien BM, Das SK, Franklin JD, et al. Effect of Lymphangiography on Lymphedema. Plast Reconstr Surg. 1981;68:922–926. [DOI] [PubMed] [Google Scholar]
- 3.Bron KM, Baum S, Abrams HL. Oil Embolism in Lymphangiography. Radiology. 1963;80:194–202. [DOI] [PubMed] [Google Scholar]
- 4.Suami H, Taylor G, Pan W-R. The Lymphatic Territories of the Upper Limb: Anatomical Study and Clinical Implications. Plast Reconstr Surg. 2007;119:1813–1822. [DOI] [PubMed] [Google Scholar]
- 5.Turfe Z, Pettinga J, Leduc O, et al. Chemotherapy port related lymphedema after axillary lymph node dissection. Breast Edinb Scotl. 2016;28:145–147. [DOI] [PubMed] [Google Scholar]
- 6.Mascagni P Vasorum Lymphaticorum Corporis Humani. Historia & Iconographia. Hist Iconogr.;Carli P Edit; Senis, Switzerland. [Google Scholar]
- 7.SAPPEY P. Anatomie, physiologie, pathologie des vesseaux lymphatiques consideres chez l’homme et les vertebres. Paris A. Available from: http://ci.nii.ac.jp/naid/10012361149/. 1885. Accessed July 10, 2020. [Google Scholar]
- 8.Leduc A, Caplan I, Leduc O. Lymphatic drainage of the upper limb. Substitution lymphatic pathways. Eur J Lymphology Relat Probl. 1993;4:11–18. [Google Scholar]
- 9.Suami H, Scaglioni MF. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin Plast Surg. 2018;32:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granoff MD, Johnson AR, Lee BT, et al. A Novel Approach to Quantifying Lymphatic Contractility during Indocyanine Green Lymphangiography. Plast Reconstr Surg. 2019;144:1197–1201. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AR, Fleishman A, Tran BNN, et al. Developing a Lymphatic Surgery Program: A First-Year Review. Plast Reconstr Surg. 2019;144:975e. [DOI] [PubMed] [Google Scholar]
- 12.Pan W-R, Zeng F-Q, Wang D-G, et al. Perforating and deep lymphatic vessels in the knee region: an anatomical study and clinical implications. ANZ J Surg. 2017;87:404–410. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AR, Bravo MG, James TA, et al. The All but Forgotten Mascagni-Sappey Pathway: Learning from Immediate Lymphatic Reconstruction. J Reconstr Microsurg. 2020;36:28–31. [DOI] [PubMed] [Google Scholar]
- 14.Pissas A, Rzal K, Math ML, et al. Prevention of secondary lymphedema. Ann Ital Chir. 2002;73:489–492. [PubMed] [Google Scholar]
- 15.Kim G, Smith MP, Donohoe KJ, et al. MRI staging of upper extremity secondary lymphedema: correlation with clinical measurements. Eur Radiol. 2020;30:4686–4694. [DOI] [PubMed] [Google Scholar]
- 16.Boneti C, Korourian S, Bland K, et al. Axillary reverse mapping: mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg. 2008;206:1038–1042. [DOI] [PubMed] [Google Scholar]
- 17.Singhal D, Tran BN, Angelo JP, et al. Technological Advances in Lymphatic Surgery: Bringing to Light the Invisible. Plast Reconstr Surg. 2019;143:283–293. [DOI] [PubMed] [Google Scholar]


