Abstract
Background:
Maternal exposure to air pollution has been associated with birth outcomes; however, few studies examined biologically critical exposure windows shorter than trimesters or potential effect modifiers.
Objectives:
To examine associations of prenatal fine particulate matter (PM2.5), by trimester and in biologically critical windows, with birth outcomes and assess potential effect modifiers.
Methods:
This study used two pregnancy cohorts (CANDLE and TIDES; N = 2099) in the ECHO PATHWAYS Consortium. PM2.5 was estimated at the maternal residence using a fine-scale spatiotemporal model, averaged over pregnancy, trimesters, and critical windows (0–2 weeks, 10–12 weeks, and last month of pregnancy). Outcomes were preterm birth (PTB, <37 completed weeks of gestation), small-for-gestational-age (SGA), and continuous birthweight. We fit multivariable adjusted linear regression models for birthweight and Poisson regression models (relative risk, RR) for PTB and SGA. Effect modification by socioeconomic factors (maternal education, household income, neighborhood deprivation) and infant sex were examined using interaction terms.
Results:
Overall, 9% of births were PTB, 10.4% were SGA, and mean term birthweight was 3268 g (SD = 558.6). There was no association of PM2.5 concentration with PTB or SGA. Lower birthweight was associated with higher PM2.5 averaged over pregnancy (β −114.2, 95%CI −183.2, −45.3), during second (β −52.9, 95%CI −94.7, −11.2) and third (β −45.5, 95%CI −85.9, −5.0) trimesters, and the month prior to delivery (β −30.5, 95%CI −57.6, −3.3). Associations of PM2.5 with likelihood of SGA and lower birthweight were stronger among male infants (p-interaction ≤0.05) and in those with lower household income (p-interaction = 0.09).
Conclusions:
Findings from this multi city U.S. birth cohort study support previous reports of inverse associations of birthweight with higher PM2.5 exposure during pregnancy. Findings also suggest possible modification of this association by infant sex and household income.
Keywords: Air pollution, Fine particulate matter, Birth outcomes, Preterm birth, Birthweight, Prenatal exposure, Effect modification
1. Background
Mounting evidence suggests that exposure to ambient air pollution is related to several adverse birth outcomes including intra-uterine growth restriction (IUGR), resulting in low birthweight, small-for-gestationalage (SGA), and preterm birth (PTB) (Klepac et al., 2018; Lamichhane et al., 2015a; Stieb et al., 2012a). These outcomes are important indicators of fetal growth and development and have been associated with lifecourse health, including future cardiovascular diseases and type 2 diabetes (Barker, 2002, 2004).
Proposed mechanisms behind the association of air pollution with IUGR and PTB include systemic or local oxidative stress and inflammation, endothelial disruption, disruptions and alterations to placental structure, endocrine disruption, and epigenetic and genetic mechanisms (Lakshmanan et al., 2015; Nachman et al., 2016; Slama et al., 2008; Vadillo-Ortega et al., 2014). Prior systematic reviews and meta-analyses have consistently indicated that fine particulate matter (PM2.5) exposure is associated with decreased birthweight (Dadvand et al., 2013; Lamichhane et al., 2015b; Stieb et al., 2012b). Findings of PM2.5 exposure and PTB risk associations were inconsistent, including both null and increased risk (Klepac et al., 2018; Stieb et al., 2012b; Lamichhane et al., 2015c). These reviews identified several gaps in the literature for future studies including the need for spatially and temporally resolved exposure characterization during critical exposure windows, use of enriched data sources for outcome and covariate assessment compared to the use of administrative birth records, and investigation of potential modifiers. These considerations informed our study design.
Previous studies have largely focused on pregnancy average or trimester specific exposures, which may not necessarily align with biologically critical windows of exposure. In the current study, we considered relevant periods of exposure based on key critical biological events in pregnancy: very early pregnancy (first two weeks of gestation, around implantation), early pregnancy (10–12 weeks gestation, early placental development), and late pregnancy (last month prior to delivery, a period of accelerated fetal weight increase and a period closer to labor initiation) (Kemp, 2014; Kannan et al., 2006). Many prior studies have relied on low precision measures of PM2.5 exposure including area level measures, an approach which can reduce the variability in exposures and limit the study power (Woodruff et al., 2010). The current study uses a well validated spatially and temporally resolved PM2.5 prediction model that estimates exposures at the residential point location.
Similarity of mechanisms (e.g. oxidative stress) linking air pollution and social stressors, including socioeconomic factors, to adverse birth outcomes may lead to synergistic interactions (Eick et al., 2018; Meng et al., 2013). Additionally, socioeconomic factors can lead to differential exposure to air pollution and potentially differential vulnerability to its effects (Deguen et al., 2021). Similarly, sex-specific differences in hormone metabolism, placental response to intrauterine exposure, and fetal growth patterns may result in differences in associations of air pollution exposure with birth outcomes among males and females (Broere-Brown et al., 2016; Rosenfeld, 2015; DiPietro and Voegtline, 2017; Aibar et al., 2012). It has been shown that male fetuses have both greater exposures to prenatal toxicants and greater adverse responses (Broere-Brown et al., 2016; Rosenfeld, 2015; DiPietro and Voegtline, 2017; Aibar et al., 2012). Sex-specific differences in birth outcomes in relation to in utero toxic exposure to other chemicals have been studied in more depth, but few have evaluated differences in air pollution exposure (Barrett et al., 2016).
We leveraged information from two well-characterized cohorts to adjust for potential confounders as well as examine potential interactions of PM2.5 exposure with individual and neighborhood level socioeconomic factors and infant sex. Better understanding of the impact of air pollution on adverse birth outcomes can inform future decisions related to community wide air quality and environmental exposures during the prenatal period.
2. Methods
2.1. Study setting and population
This study included participants from two pregnancy cohorts from the ECHO PATHWAYS Consortium: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study and The Infant Development and Environment Study (TIDES) (Sontag-Padilla et al., 2015; Kobrosly et al., 2012; Barrett et al., 2014). The CANDLE cohort recruited individuals during the second trimester in Shelby County/Memphis, TN from 2006 to 2011. The TIDES cohort recruited individuals during trimester 1 from four cities in 2012 including: Seattle, WA, San Francisco, CA, Minneapolis, MN, and Rochester, NY. All recruited individuals provided informed consent upon enrollment and local IRBs approved research activities. Both cohorts enrolled pregnancies without existing complications. Additional exclusions in the current analyses included pregnancies that were multiple gestation (n = 1) and those resulting in a spontaneous abortion or stillbirth (n = 7). Participants were included in this analysis if there was a valid maternal residential address. The total study population included 2099 mother-infant dyads. The current study protocol, conducted by the ECHO PATHWAYS Consortium, was reviewed and approved by the University of Washington Human Subjects Division.
2.2. Air pollution
Exposure to particulate matter ≤2.5 μm in diameter (PM2.5) was estimated at each participant’s residential address point location (latitude and longitude) at enrollment using a national spatio-temporal prediction model (Keller et al., 2015; Kirwa et al., 2021; Wang et al., 2018). This prediction model uses data from both regulatory and research monitors across the U.S. and a large (>200) suite of geographic covariates to produce fine scale spatial predictions at a temporal resolution of two-week exposure windows. We generated concentration estimates averaged over pregnancy and each trimester. We also generated concentration estimates for pre-determined exposure windows with potential biologic relevance including very early pregnancy (0–2 weeks post estimated date of conception), late first trimester (10–12 weeks post estimated date of conception), and late pregnancy (last month prior to delivery). Exposure averages were based on estimated date of conception and were weighted according to the number of days that fell within a two-week window.
2.3. Birth outcomes
We assessed three birth outcomes: PTB, SGA, and birthweight. The calculation of gestational age in the CANDLE cohort was based on gestational age on the birth record which was confirmed by study research nurses who investigated and adjudicated discrepancies in gestational age at birth based on estimated date of conception recorded using last menstrual period (LMP) or ultrasound. In the TIDES cohort, information on gestational age was extracted from the birth record. If it was missing from the birth record, gestational age was calculated using estimated delivery date (EDD) based on ultrasound or LMP.
PTB was defined using the clinical criteria of <37 completed weeks of gestation at delivery. SGA was defined as <10th percentile of birthweight for gestational age using sex-specific percentiles based on a U.S. national reference population (Talge et al., 2014). Birthweight was measured and is reported in grams. The analyses of SGA and birthweight were restricted to term births only (≥37 completed weeks of gestation at delivery).
2.4. Effect modifiers
We assessed four potential effect modifiers: maternal education, household income, neighborhood socioeconomic factors, and infant sex. Maternal education was defined according to questionnaire response to highest level of education completed at baseline and categorized in three groups (high school degree or equivalent or less; college or technical degree; and some graduate school or more). For household income, we used a household size and regional-adjusted continuous measure. Household income category and size were based on questionnaire responses. Regional adjustment used the U.S. Bureau of Economic Analysis (BEA) regional price parity (RPP) index matched to study metro region and infant birth year (Bureau of Economic Analysis, 2020). Our adjusted income calculation used the midpoint value for the household income category from the questionnaire, divided by the square root of household size and adjusted to the relevant RPP percentile. This calculation is as follows: [Midpoint of income category/sqrt(household size)]/[(BEA RPP for study site and birth year)/100]. Neighborhood level socioeconomic status was measured using an adapted version of the neighborhood deprivation index (NDI) (Messer et al., 2006), a census tract level continuous z-score index that incorporates % <high school education, % professional employment (reverse coded), % owner occupied housing (reverse coded), % <100% poverty, and % unemployed created using principal components analysis. Effect modification with socioeconomic factors was hypothesized to have larger associations with lower education, household income, and NDI. Infant sex (male/female) determination was based on biological sex at birth.
2.5. Statistical analysis and covariates
We used descriptive statistics for study information, covariates, infant characteristics, and exposure concentrations for the whole study population and within the cohorts separately. For analyses evaluating associations of PM2.5 with PTB and SGA, we used modified Poisson regression models with robust variance to estimate a relative risk (RR). For analyses involving birthweight (measured in grams), we used linear regression models with robust standard errors. PM2.5 was modeled as a continuous exposure and presented effect estimates are scaled to the approximate concentration interquartile range (2 μg/m3).
We used a staged modeling approach for covariate adjustment by fitting minimal, partially adjusted, fully adjusted, and extended/sensitivity models. Covariates were selected a priori based on directed acyclic graphs and examination of prior literature. The minimally adjusted model included maternal age, infant sex, study city, and a time spline, including 24 knots over the six year study period, to account for temporal and seasonal trends. Though infant sex is unlikely a confounder, it is included as a precision variable. The partially adjusted model added the following: pre-pregnancy body mass index (BMI, kg/m2), Black/African American race, smoking history (yes/no based on self-report), marital status, and parity. In this analysis, in our analytic models we included a race covariate coded as self-reported Black/African American race or not; we report the distribution of race by smaller sub-categories in descriptive tables. The race variable does not have any clinical interpretation, but was used as a crude proxy for a number of potential unmeasured confounders including access to healthcare and exposure to structural racism including residential segregation (VanderWeele and Robinson, 2014; Benmarhnia et al., 2021). The fully adjusted model included variables in the partially adjusted model as well as variables related to socioeconomic factors including maternal education, household size-regional adjusted income, and NDI and is considered the primary model (Bureau of Economic Analysis, 2020; Messer et al., 2006). The partially adjusted model includes all a priori identified covariates, other than the potential effect modifiers; the fully adjusted model includes all covariates including the potential effect modifiers. The analyses of birthweight also included an adjustment for gestational age. The extended model, part of sensitivity analyses, included an adjustment for meteorological factors including temperature and relative humidity averaged over the same period as the primary exposure. Temperature and humidity are considered a sensitivity analysis given that the relationship between meteorological factors and birth outcomes is unclear, therefore the role as confounders, is uncertain.
Effect modification was assessed using models that included multiplicative interactions terms. Associations were calculated for each level of the effect modifiers from the interaction model. For continuous modifiers (household income and NDI), these were calculated at the 25th, 50th, and 75th percentile of the modifier. Due to potential differences in how socioeconomic factors function in each of our study cities, we also included an interaction term between city and education, income, and NDI in their respective modifier analyses. Statistical significance of interaction terms was assessed with p-values (p < 0.05) calculated using a Wald test based on robust standard errors.
In order to assess if any of our results were driven by a single study city or cohort, we performed sensitivity analyses using the primary model and sequentially leaving each study city out of the analyses and by leaving each study cohort of the analyses. In order to determine if effect estimates from smaller exposure windows were driven by trimester level exposures, we performed mutually adjusted analysis including trimester level exposures in analyses of biologically driven exposure windows. The results from this mutual adjustment model are designed to assess for issues related to correlated exposures and are not designed to be interpreted otherwise.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and STATA version 14 (StataCorp, College Station, TX).
3. Results
Overall and cohort specific maternal and infant characteristics are presented in Table 1 for 2099 women and infants. Overall, mean maternal age was 27.8 years (SD = 5.9) and 45.4% were nulliparous. In this study population, 46.6% of individuals self-reported as Black/African American race, 44.3% White, 2.25% Asian, 3.9% Other (including American Indian/Alaska Native, Native Hawaiian/Pacific Islander), and 3.2% reporting multiple. Mean household count and region-adjusted income was $21,822. Of all births, 9.0% were pre-term (<37 weeks). Among term births (n = 1902), 10.4% were characterized as term SGA and mean term birthweight was 3,268 g (SD = 558.6). Mean PM2.5 during pregnancy was 9.8 μg/m3 (interquartile range = 2.2 μg/m3) (Table 2). PM2.5 concentration varied by city, with Memphis having the highest concentrations and Seattle having the lowest (eTable 1). We observed a secular decreasing trend and seasonal patterns of PM2.5 levels over time reflecting known temporal trends in U.S. outdoor air pollution. Study years (2011–2013) that included more cities had more variation in concentration than earlier years (2006–2011) which included only one city, Memphis, due to earlier timing of enrollment in the CANDLE study (eFig. 1).
Table 1.
Maternal and infant characteristics.
| N | TOTAL N = 2099 Percent or Mean (SD) |
N | CANDLE N = 1417 Percent or Mean (SD) |
N | TIDES N = 682 Percent or Mean (SD) |
|
|---|---|---|---|---|---|---|
| Study City | ||||||
| Memphis, TN | 1417 | 68.1 | 1417 | 100.0 | ||
| Seattle, WA | 98 | 4.7 | 98 | 14.4 | ||
| San Francisco, CA | 167 | 8.0 | 167 | 24.5 | ||
| Minneapolis, MN | 197 | 9.4 | 197 | 28.9 | ||
| Rochester, NY | 220 | 10.5 | 220 | 32.3 | ||
| Maternal characteristics | ||||||
| Age, years, mean (SD) | 2095 | 27.8 (5.9) | 1413 | 26.3 (5.5) | 682 | 30.9 (5.6) |
| Pre-pregnancy BMI, kg/m2, mean (SD) | 2089 | 27.1 (7.1) | 1412 | 27.5 (7.5) | 677 | 26.3 (6.2) |
| Maternal Race | ||||||
| Black/African American | 978 | 46.9 | 884 | 62.5 | 94 | 14.0 |
| White | 925 | 44.3 | 437 | 30.9 | 488 | 72.6 |
| Asian | 47 | 2.2 | 13 | 0.9 | 34 | 5.1 |
| Othera | 82 | 3.9 | 75 | 5.3 | 7 | 1.0 |
| Multiple | 55 | 2.6 | 6 | 0.4 | 49 | 7.3 |
| Prior Parity | ||||||
| None | 944 | 45.4 | 579 | 40.9 | 365 | 55.0 |
| One or more | 1137 | 54.6 | 838 | 59.1 | 299 | 45.0 |
| Maternal Smoking History | ||||||
| Never | 1906 | 91.3 | 1279 | 90.3 | 627 | 93.3 |
| Former/Current | 182 | 8.7 | 137 | 9.7 | 45 | 6.7 |
| Maternal Marital Status | ||||||
| Single/living as single | 738 | 35.2 | 615 | 43.4 | 123 | 18.1 |
| Married/living as married | 1359 | 64.8 | 801 | 56.6 | 558 | 81.9 |
| Household Income | ||||||
| <$15 | 474 | 24.3 | 370 | 28.7 | 104 | 15.8 |
| $15–$24.9 | 255 | 13.1 | 196 | 15.2 | 59 | 9.0 |
| $25–$44.9 | 310 | 15.9 | 250 | 19.4 | 60 | 9.1 |
| $45–$54.9 | 144 | 7.4 | 103 | 8.0 | 41 | 6.2 |
| $55–$64.9 | 112 | 5.7 | 75 | 5.8 | 37 | 5.6 |
| $65–$74.9 | 121 | 6.2 | 80 | 6.2 | 41 | 6.2 |
| $75+ | 534 | 27.4 | 217 | 16.8 | 317 | 48.1 |
| Adjusted incomeb, mean (SD) | 1964 | 21,822 (17,381) | 1322 | 19,521 (15,337) | 642 | 33,299 (17,675) |
| Neighborhood Deprivation Indexc, mean (SD) | 2077 | −0.3 (3.6) | 1395 | 0.0 (3.7) | 682 | 0.0 (3.5) |
| Maternal Education | ||||||
| <High school, high school or equivalent | 1035 | 49.5 | 848 | 59.9 | 187 | 27.7 |
| Technical or college degree | 622 | 29.7 | 411 | 29.1 | 211 | 31.2 |
| Graduate or professional school | 435 | 20.8 | 156 | 11.0 | 279 | 41.2 |
| Infant characteristics | ||||||
| Infant Sex | ||||||
| Male | 1044 | 49.8 | 717 | 50.7 | 327 | 48.0 |
| Female | 1051 | 50.2 | 696 | 49.3 | 355 | 52.1 |
| Term birth category (weeks) | ||||||
| Very preterm (<34) | 38 | 1.8 | 29 | 2.1 | 9 | 1.3 |
| Preterm (24 to <37) | 149 | 7.1 | 93 | 6.6 | 56 | 8.2 |
| Early term (37 to <39) | 544 | 26.0 | 399 | 28.4 | 145 | 21.3 |
| Full (39–40) | 821 | 39.3 | 597 | 42.4 | 224 | 32.8 |
| Late (>40) | 537 | 25.7 | 289 | 20.5 | 248 | 36.4 |
| Pre-term birth (<37 weeks) | 187 | 9.0 | 122 | 8.7 | 65 | 9.5 |
| Term small for gestational aged | 198 | 10.4 | 143 | 11.2 | 55 | 8.9 |
| Low birth weight (<2500 g) | 136 | 6.5 | 98 | 7.0 | 38 | 5.6 |
| Term low birth weight (<2500 g, ≥37 weeks) | 48 | 2.5 | 37 | 2.9 | 11 | 1.8 |
| Birthweight (grams), mean (SD) | 2086 | 3268 (558.6) | 1405 | 3236.9 (548.0) | 681 | 3333.6 (574.7) |
| Term birthweight (≥37 weeks), mean (SD) | 1902 | 3351.8 (471.6) | 1285 | 3318.1 (462.7) | 617 | 3421.9 (482.4) |
Other includes American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, and those reported as “other”.
Regional-household count adjusted income: Household income adjusted by total household count and regional metro area parity score, calculated by the Bureau of Economic Analysis at year of birth (2008 data used for 2007).
Neighborhood Deprivation Index (NDI): Census tract level z-score index of: percent <100% poverty, percent less than high school education, percent unemployed, percent not professional employment, percent not owner occupied housing.
Small for gestational age (SGA) defined as <10th percentile based on national percentiles published in Talge et al. (2014). Restricted to births ≥37 weeks.
Table 2.
Particulate matter <2.5 (μg/m3) distributions by exposure window and cohort.
| Exposure window | TOTAL | CANDLE | TIDES | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Quartile Range | N | Mean | Quartile Range | N | Mean | Quartile Range | |
| Pregnancy | 2089 | 9.82 | 2.17 | 1407 | 10.71 | 1.26 | 682 | 7.98 | 1.48 |
| Trimester 1 | 2089 | 9.76 | 2.21 | 1407 | 10.62 | 1.52 | 682 | 7.98 | 2.17 |
| Trimester 2 | 2089 | 9.79 | 2.19 | 1407 | 10.68 | 1.43 | 682 | 7.96 | 2.05 |
| Trimester 3 | 2081 | 9.94 | 2.65 | 1402 | 10.87 | 1.87 | 679 | 8.00 | 1.83 |
| Early Trimester 1 (0–2 weeks) | 2089 | 9.89 | 2.98 | 1407 | 10.74 | 2.28 | 682 | 8.13 | 3.48 |
| Late Trimester 1 (10–12 weeks) | 2089 | 9.64 | 2.67 | 1407 | 10.50 | 1.75 | 682 | 7.86 | 2.91 |
| Last month pregnancy | 2099 | 9.95 | 3.14 | 1417 | 10.85 | 2.32 | 682 | 8.10 | 2.79 |
We did not observe a statistically significant association between maternal residential PM2.5 concentrations and PTB (Table 3), however associations indicated an increased risk particularly for PM2.5 concentrations during trimester 3, and very early pregnancy (0–2 weeks). Higher PM2.5 during very early pregnancy (0–2 weeks) was associated with a higher risk of term SGA in the partially adjusted model (RR 1.18 per 2 μg/m3 PM2.5, 95%CI 1.03, 1.36). This was slightly attenuated in the fully adjusted model (RR 1.14, 95%CI 0.98, 1.33). While associations of PM2.5 concentrations with term SGA all indicated an increased risk, no other exposure windows were statistically significant.
Table 3.
Association of ambient maternal prenatal PM2.5 exposure and birth outcomes.
| PM2.5 Exposure windowa | Model 1b | Model 2c | Model 3 (primary)d | Model 4e | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR/Beta | 95% CI | RR/Beta | 95% CI | RR/Beta | 95% CI | RR/Beta | 95% CI | |||||
| Lower limit | Upper limit | Lower limit | Upper limit | Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Preterm birth (n = 2099) | ||||||||||||
| Pregnancy | 0.98 | 0.56 | 1.72 | 0.89 | 0.48 | 1.63 | 0.97 | 0.49 | 1.94 | 0.89 | 0.45 | 1.75 |
| Trimester 1 | 1.05 | 0.77 | 1.42 | 1.00 | 0.73 | 1.36 | 1.01 | 0.72 | 1.41 | 0.97 | 0.70 | 1.33 |
| Trimester 2 | 1.04 | 0.77 | 1.42 | 0.95 | 0.68 | 1.34 | 1.02 | 0.70 | 1.47 | 0.92 | 0.63 | 1.34 |
| Trimester 3 | 1.07 | 0.74 | 1.54 | 1.11 | 0.76 | 1.62 | 1.15 | 0.78 | 1.69 | 1.12 | 0.76 | 1.65 |
| Early Trimester 1 (0–2 weeks) | 1.05 | 0.91 | 1.23 | 1.04 | 0.89 | 1.22 | 1.08 | 0.92 | 1.27 | 1.06 | 0.90 | 1.25 |
| Late Trimester 1 (10–12 weeks) | 1.06 | 0.89 | 1.28 | 1.07 | 0.89 | 1.28 | 1.05 | 0.87 | 1.27 | 1.03 | 0.85 | 1.23 |
| Month prior to delivery | 1.04 | 0.84 | 1.30 | 1.07 | 0.85 | 1.33 | 1.05 | 0.84 | 1.31 | 1.02 | 0.81 | 1.28 |
| Term Small for gestational age (n = 1902) | ||||||||||||
| Pregnancy | 1.37 | 0.90 | 2.07 | 1.37 | 0.89 | 2.12 | 1.26 | 0.79 | 2.04 | 1.25 | 0.77 | 2.02 |
| Trimester 1 | 1.21 | 0.93 | 1.57 | 1.18 | 0.91 | 1.53 | 1.12 | 0.84 | 1.48 | 1.09 | 0.82 | 1.43 |
| Trimester 2 | 1.09 | 0.84 | 1.43 | 1.10 | 0.84 | 1.43 | 1.03 | 0.76 | 1.40 | 1.03 | 0.75 | 1.41 |
| Trimester 3 | 1.06 | 0.84 | 1.34 | 1.07 | 0.84 | 1.37 | 1.07 | 0.84 | 1.37 | 1.16 | 0.88 | 1.54 |
| Early Trimester 1 (0–2 weeks) | 1.21 | 1.05 | 1.39 | 1.18 | 1.03 | 1.36 | 1.14 | 0.98 | 1.33 | 1.12 | 0.96 | 1.31 |
| Late Trimester 1 (10–12 weeks) | 1.07 | 0.91 | 1.26 | 1.09 | 0.94 | 1.28 | 1.09 | 0.92 | 1.29 | 1.04 | 0.87 | 1.25 |
| Month prior to delivery | 1.02 | 0.87 | 1.19 | 1.03 | 0.88 | 1.20 | 1.02 | 0.86 | 1.23 | 1.02 | 0.85 | 1.23 |
| Term Birthweight, g (n = 1902) | ||||||||||||
| Pregnancy | −174.2 | −239.1 | −109.3 | −148.7 | −213.7 | −83.6 | −114.2 | −183.2 | −45.3 | −114.3 | −186.5 | −42.2 |
| Trimester 1 | −59.4 | −99.4 | −19.4 | −44.4 | −83.8 | −4.9 | −23.6 | −64.5 | 17.3 | −22.7 | −65.3 | 19.8 |
| Trimester 2 | −79.6 | −119.8 | −39.4 | −67.8 | −107.1 | −28.5 | −52.9 | −94.7 | −11.2 | −54.4 | −99.3 | −9.5 |
| Trimester 3 | −57.4 | −96.7 | −18.1 | −52.3 | −91.1 | −13.5 | −45.5 | −85.9 | −5.0 | −42.9 | −84.7 | −1.0 |
| Early Trimester 1 (0–2 weeks) | −32.1 | −54.2 | −10.0 | −25.5 | −47.3 | −3.7 | −18.5 | −40.7 | 3.7 | −18.6 | −41.3 | 4.2 |
| Late Trimester 1 (10–12 weeks) | −8.4 | −33.9 | 17.0 | −7.4 | −32.3 | 17.4 | 1.7 | −23.6 | 26.9 | 5.0 | −21.3 | 31.4 |
| Month prior to delivery | −33.9 | −60.9 | −7.0 | −34.3 | −60.3 | −8.4 | −30.5 | −57.6 | −3.3 | −28.9 | −56.2 | −1.5 |
PM2.5 per 2 μg/m3 increase.
Model 1: maternal age, infant sex, city, 24df spline (4df per year). Birthweight analysis also adjusted for gestational age in all models.
Model 2: model 1 + maternal BMI, maternal Black/African-American race, smoking (ever/never), parity, marital status.
Model 3: model 2 + neighborhood deprivation index, maternal education, RPP-house hold size adjusted income.
Model 4: model 3 + average temperature, average relative humidity.
Higher concentrations of PM2.5 at the maternal residence was associated with lower birthweight at term across all exposure windows except during late first trimester (10–12 weeks) in the partially adjusted model, however this was attenuated in the fully adjusted model (Table 3). In the fully adjusted model, we found a 114.2 g decrease in birthweight with a 2 μg/m3 higher level of average PM2.5 concentration (95%CI −183.2, −45.3) during pregnancy. Similar significant associations in the fully adjusted model were found in trimester 2 (β −52.9, 95%CI −94.7, −11.2), trimester 3 (β −45.5, 95%CI −85.9, −5.0), and the month prior to delivery (β −30.5, 95%CI −57.6, −3.3). PM2.5 concentrations during trimester 1 (β −23.6, 95%CI −64.5, 17.3) and 10–12 weeks pregnancy (β 1.7, 95%CI −23.6, 26.9) were not associated with birthweight in the fully adjusted model. When accounting for meteorological factors in the extended model, effect estimates remained mostly unchanged.
In our effect modification analyses (Figs. 1–3, eTable 2), there was a significant interaction between PM2.5 concentration during very early pregnancy (0–2 weeks) and maternal education such that an association with PTB was observed only among participants with a college or technical degree (RR 1.45, 95%CI 1.16, 1.82, p-interaction = 0.004). For income, there was an indication of an exposure-response trend of a higher likelihood of term SGA corresponding to increasing PM2.5 concentration during pregnancy among those in the 25th percentile for household income compared to those in the 50th or 75th percentile, though this interaction did not reach statistical significance (p-interaction = 0.09). There was also a significant interaction between PM2.5 and NDI during the last month of pregnancy where the association of PM2.5 concentration with PTB was highest among those in the lowest 25th percentile NDI (RR 1.19, 95%CI 0.95, 1.49, p-interaction = 0.02), however none of the strata estimates were statistically significant.
Fig. 1.
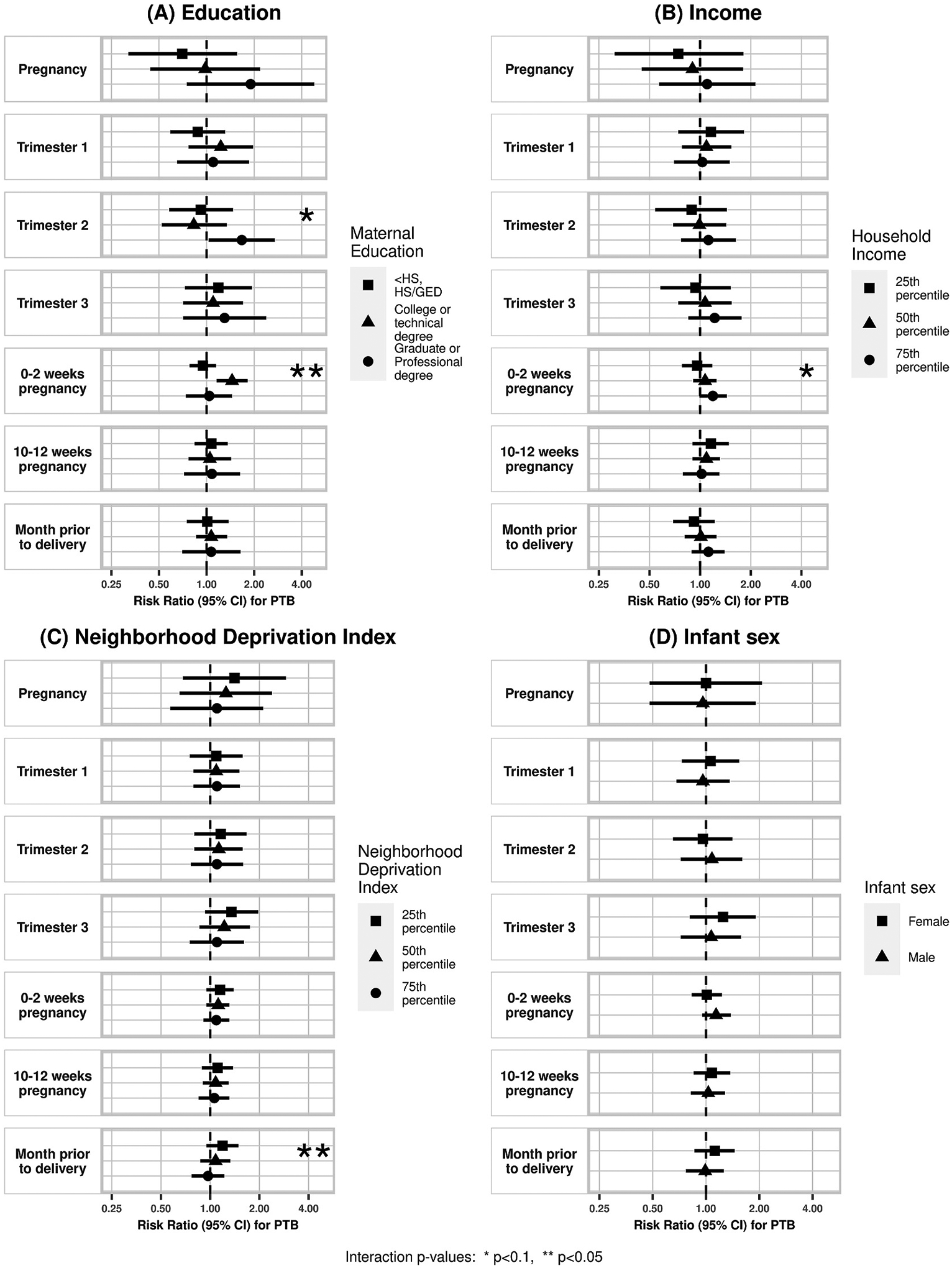
Effect modification of association between air pollution and PTB by (A) maternal education, (B) household income, (C) NDI, and (D) infant sex. Corresponding numerical results provided in eTable 2.
Fig. 3.
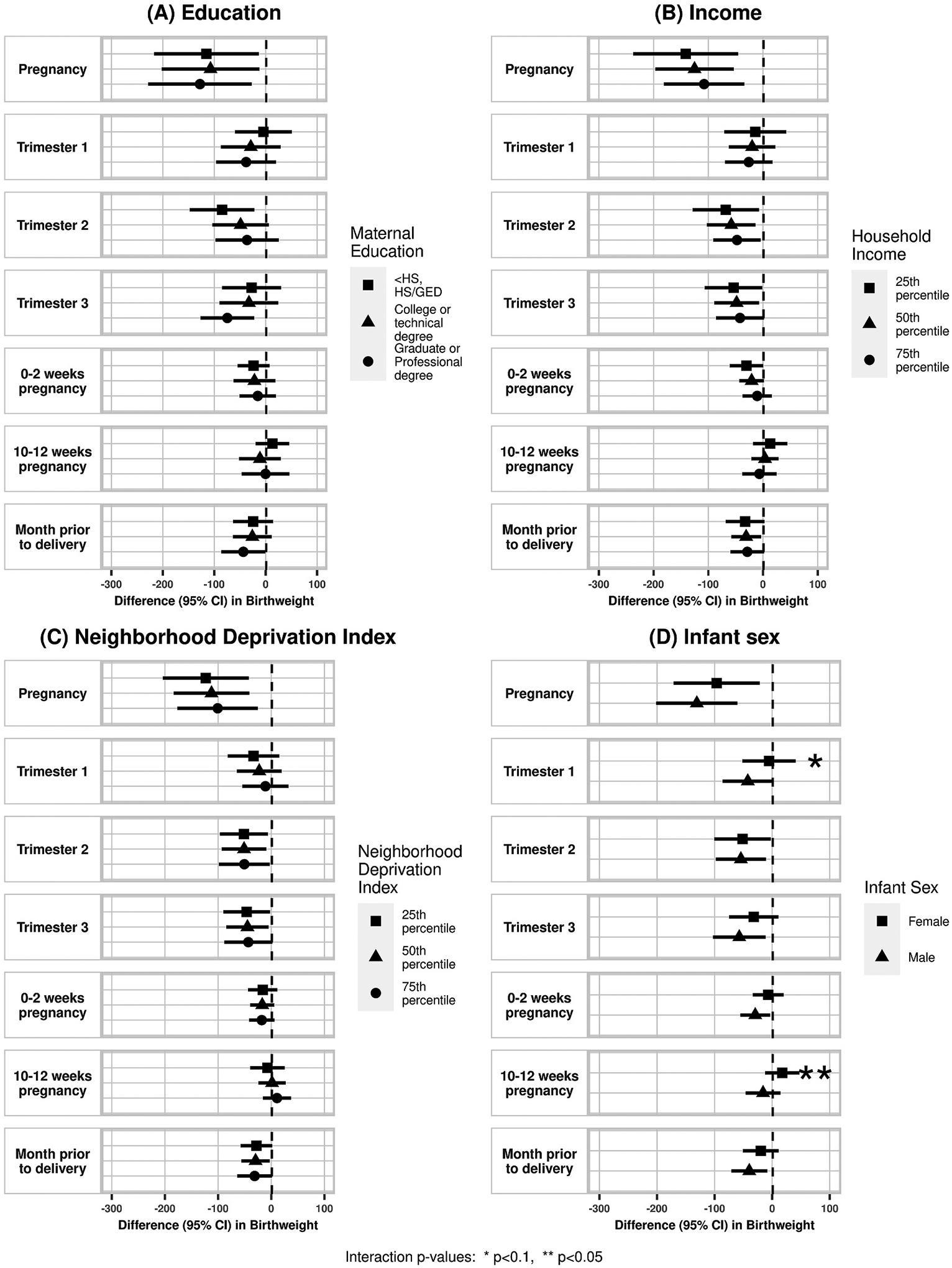
Effect modification of association between air pollution and birthweight by (A) maternal education, (B) household income, (C) NDI, and (D) infant sex. Corresponding numerical results provided in eTable 2.
There was a suggestion of effect modification by infant sex with stronger effects in males than females for term SGA and birthweight. There was a pattern of higher likelihood of SGA with higher PM2.5 concentration in males compared to females, though this was only statistically significant in the late first trimester period (10–12 weeks) (RR female 0.94, 95%CI 0.75, 1.19; RR male: 1.25, 95%CI: 1.03, 1.52; p-interaction = 0.02). There was also a pattern of larger decreases in term birthweight related to PM2.5 concentration among males compared to females during trimester 1 (β female −5.5 g, 95%CI: −51.9, 40.9; β male −42.3 g, 95%CI −86.2, 1.6; p-interaction = 0.06) and 10–12 weeks pregnancy (β female 17.6 g, 95%CI −12.3, 47.4; β male −15.8 g, 95%CI −46.2, 14.6; p-interaction = 0.05).
In the leave-one-city or cohort out sensitivity analysis, we did observe differences in findings with exclusion of certain sites or cohorts (eFig. 2a and b; eTable 3a–b). Specifically, PM2.5 concentration and birthweight associations in general were attenuated and not significant in the CANDLE cohort (vs. TIDES cohort). In our sensitivity analyses that involved including trimester level concentrations in analyses of smaller biologically driven exposure windows for trimester average exposures, we did not see meaningful changes in the PTB or SGA findings from what we reported above; for birthweight estimates were mostly attenuated and became non-significant (eTable 4).
4. Conclusions
Our findings corroborate previous reports of inverse associations of maternal PM2.5 exposure and infant birthweight in a multi-city cohort. More specifically, a 2 μg/m3 higher PM2.5 concentration during pregnancy was associated with 114.2 g lower birthweight. We also found suggestive evidence that these associations were stronger among male infants. We observed suggestive evidence for associations between PM2.5 exposure and PTB risk and SGA risk, however, potential effect modification by education and NDI was observed, where associations were stronger among college educated individuals and those with lower NDI.
In our novel assessment of biologically relevant exposure windows, we saw non-significant but decreased birthweight with exposure during very early pregnancy (0–2 weeks), but not late first trimester (10–12 weeks), indicating that observed trimester 1 effects might be driven by effects during fertilization and genetic imprinting. We also saw associations with PM2.5 concentration in the last month of pregnancy, a period of increased fetal growth. Two recent studies using non-targeted data-driven distributed model lag approaches also found late pregnancy as a potential critical window for PM2.5 effects on birthweight (Yuan et al., 2020; Yitshak-Sade et al., 2021). A recent study in Shanghai, China, explored potential susceptible windows of exposure to PM2.5 and identified late pregnancy (31–34 weeks) as a period of strongest effects on birthweight (Yuan et al., 2020). A study of PM2.5 and heat exposure effects on birthweight in Massachusetts observed that PM2.5 exposure was associated with a decrease in birthweight specifically at the later part of pregnancy (Yitshak-Sade et al., 2021).
Our findings indicate a larger decrease in birthweight and a higher likelihood of SGA with PM2.5 concentration than seen in many prior studies. A meta-analysis reported a pooled estimate of 22 g (95%CI −37.9, −6.4) lower birthweight with a 10 μg/m3 PM2.5 exposure, with estimates from individual studies included in the meta-analysis with as much as 67 g (95%CI: −77.7, −55.9) lower birthweight, while we observed an approximately 114 g lower birthweight across pregnancy with a 2 μg/m3 higher PM2.5 (Lamichhane et al., 2015a). Our study observed likelihoods for SGA with a 2 μg/m3 higher PM2.5 concentration ranging from 1.02 (95%CI 0.86, 1.23) for exposure during the last month of pregnancy up to 1.23 (95%CI 0.79, 2.04) for average exposure across pregnancy. A meta-analysis on PM2.5 and birth outcomes reported a pooled estimate of an OR for SGA 1.15 (95%CI 1.10–1.20) per a 10 μg/m3 higher PM2.5 averaged across pregnancy (Zhu et al., 2015).
PM2.5 components are known to differ across regions; the larger effects observed in our study could be explained by more toxic PM2.5 in the TIDES and CANDLE cohorts, compared to other studies (Lamichhane et al., 2015c). Differences may also reflect population differences between our study population and those of prior studies including potential differences in baseline risk factors. Another possibility is that our larger observed estimates resulted from the higher precision and accuracy in our exposure estimates compared to most prior studies. Non-differential misclassification of exposures in other studies may have resulted in attenuation of observed effect estimates. Notably, our findings, though large, are similar to effect sizes seen for other prenatal environmental exposures, including maternal smoking (Eskenazi et al., 1995; Zheng et al., 2016).
We also observed evidence of potential modification by infant sex. There are known sex-specific differences in fetal growth and development. Accumulating evidence also supports sex-specific differences in birth outcomes in relation to several maternal environmental exposures including air pollutants (DiPietro and Voegtline, 2017; Barrett et al., 2016). In a cohort study of 481 pregnancies in Krakow, Poland measuring PM2.5 via personal monitors for 48 h during the second trimester, Jedrychowski et al. found that male infants had a larger decrease in birthweight (β −189 g, 95%CI −34.2, −343) per 30 μg/m3 increase in PM2.5 compared to females (β −17 g, 95%CI: −164.8, 130.8) (Jedrychowski et al., 2009). Among a cohort of 670 pregnancies in Boston, MA, Lakshmanan et al. found a negative association of PM2.5 with birthweight specific to male infants who were born to individuals with a BMI ≥30 kg/m2 (Lakshmanan et al., 2015). The enhanced vulnerability of male infants to maternal PM2.5 exposure observed in these studies is supported in our study findings where we found stronger effects in male infants for both term SGA and birthweight. We also found significant interactions between maternal education and NDI with PM2.5 exposure on PTB risk; however given that we did not find overall PM2.5 exposure and PTB associations and the potential for spurious results in subgroup analyses, these findings need to be cautiously interpreted (Weiss, 2008).
Including an adjustment for study site likely attenuates observed effects since it limits analysis to within city variation, rather than across city variation, however given the number of unknown confounders by city, we opted to include this as an adjustment factor. In our sensitivity analyses leaving one study city or cohort out at a time, we observed differences in association in some iterations. This may indicate residual confounding by factors related to study city or cohort given that maternal characteristics differed across cohorts and cities. It could also indicate differences in PM2.5 composition by region with some regions potentially having a higher proportion of toxic components or differing vulnerabilities by cohort or city subpopulations. In particular, we saw stronger associations in the TIDES cohort compared to the CANDLE cohort, which is a single site study and has limited PM2.5 variation.
In our sensitivity analyses adjusting for exposure during each trimester while examining smaller exposure windows, associations of PM2.5 concentration with birthweight were attenuated. However, the pattern of associations of PM2.5 in very early pregnancy (0–2 weeks) and the last month of pregnancy remained, which suggests that there may be a distinct association for these time periods, even after accounting for trimester average exposures. This observation highlights the significance of examining critical windows of exposure.
Strengths of our study include the application of highly resolved exposure estimates both in time and in space, increasing our confidence in the predicted exposure estimates. This study pools together two geographically and socioeconomically diverse cohorts which recruited participants from U.S. cities with varying levels of PM2.5 exposure as well as socioeconomic distributions. This study also benefits from well characterized covariate data from these studies.
On the other hand, some limitations deserve mention. Most research to date has been conducted in pregnancy cohorts which enroll individuals during the second trimester leading to potential selection bias (Raz et al., 2018). Our study population was restricted to healthy pregnancies enrolled during the first and second trimester and resulting in a live birth. If exposure to air pollution contributes to conception or early pregnancy loss following a similar proposed causal pathway to those between air pollution exposure and birth outcomes, as supported by recent findings, selection bias may ensue (Quraishi et al., 2019; Frutos et al., 2015). Another limitation of our study relates to study power. Our study may have had limited power to detect associations in our PTB and SGA analyses, as well as in our subgroup analyses, due to small sample sizes. Our study estimated ambient exposure at residential locations and therefore may have suffered from exposure misclassification due to the lack of accounting for participant mobility or indoor sources of PM2.5, though we believe this misclassification is non-differential. Finally, though our study used data from well characterized cohort studies, there is always the possibility of residual confounding and confounding from unmeasured factors.
Our novel assessment of biologically critical windows which suggest sensitive periods to air pollution both very early in pregnancy as well as late in term pregnancies deserve further investigation in other well-characterized cohorts. The relatively large effect size of PM2.5 on birthweight and SGA among term births observed is notable and underscores the public health importance of air pollution exposure during pregnancy. Furthermore, many women may not be aware of their pregnancy in the first weeks, so ensuring healthy community wide air quality remains an important priority for fostering healthy births.
Supplementary Material
Fig. 2.
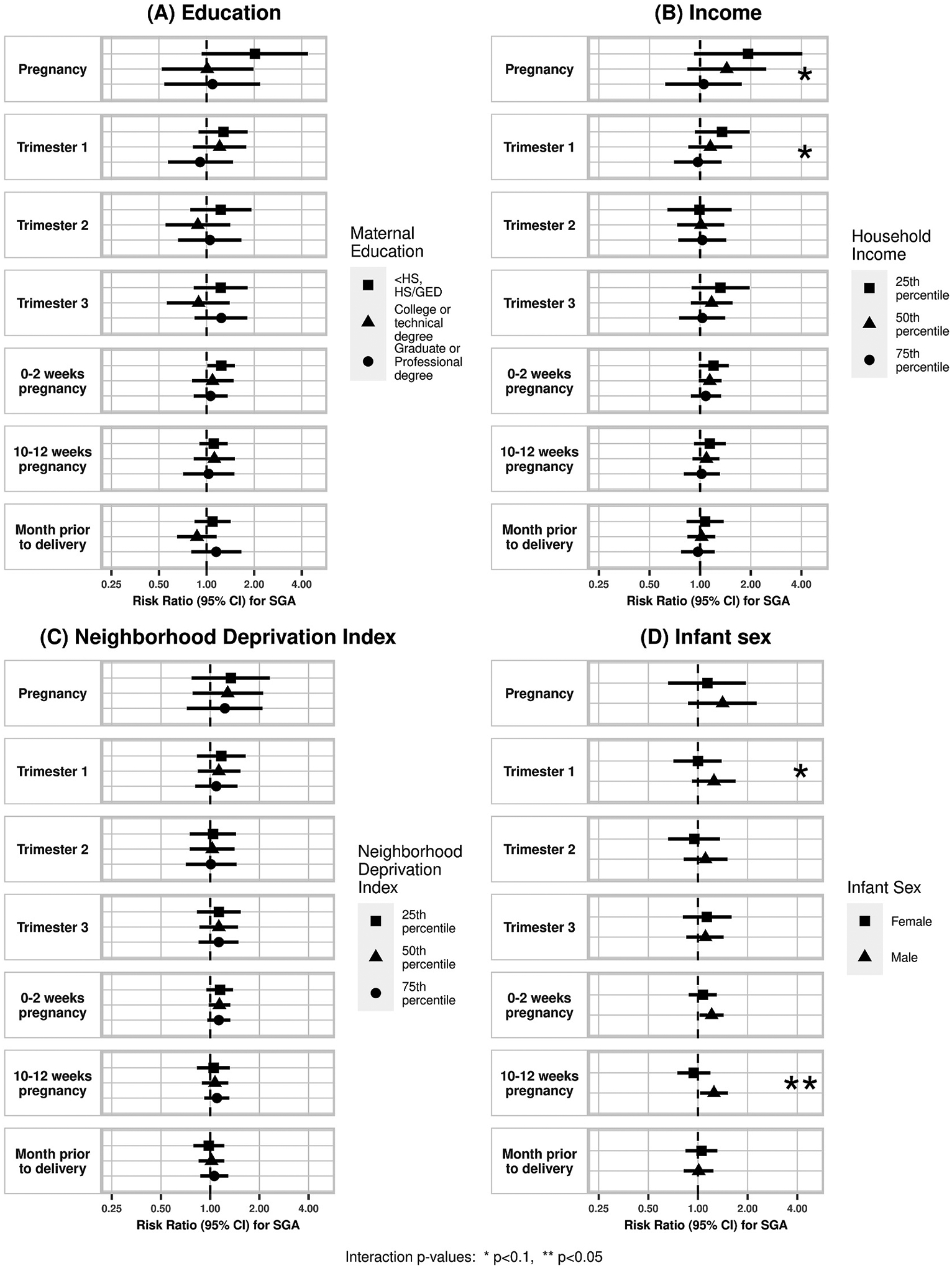
Effect modification of association between air pollution and SGA by (A) maternal education, (B) household income, (C) NDI, and (D) infant sex. Corresponding numerical results provided in eTable 2.
Acknowledgements and Funding
This work is a product of the ECHO PATHWAYS Consortium funded by NIH grants 1UG3OD023271-01 and 4UH3OD023271. CANDLE was also funded by the Urban Child Institute. TIDES is funded by NIH grants R01ES01686, 1 R01 ES25169, R01ES016863-02S4, and 3R01ES025169-04S1. This publication was developed under a STAR research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. This research was also supported by P01AG055367 from the National Institute of Aging and by R01ES025888 the National Institute of Environmental Health Sciences (NIEHS). This work was supported in part by UW NIEHS sponsored Biostatistics, Epidemiologic and Bioinformatic Training in Environmental Health (BEBTEH) Training Grant: NIEHS T32ES015459. Lastly, the research reported in this publication was supported by the University of Washington EDGE Center of the National Institutes of Health under award number P30ES007033. This article was prepared while S.M.Q. was employed at the University of Washington. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Adam Szpiro reports financial support was provided by University of Washington. Adam Szpiro reports a relationship with Health Effects Institute that includes: consulting or advisory. Adam Szpiro reports a relationship with Sonoma Technology Inc that includes: consulting or advisory. All other authors declare that they have no known competing financial interests or personal relationships.
Footnotes
CRediT authorship contribution statement
Sabah M. Quraishi: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Marnie F. Hazlehurst: Formal analysis, Data curation, Writing – review & editing. Christine T. Loftus: Data curation, Writing – review & editing, Supervision. Ruby H. N. Nguyen: Writing – review & editing. Emily S. Barrett: Writing – review & editing. Joel D. Kaufman: Writing – review & editing, Funding acquisition. Nicole R. Bush: Writing – review & editing, Funding acquisition. Catherine J. Karr: Writing – review & editing, Supervision, Funding acquisition. Kaja Z. LeWinn: Writing – review & editing, Funding acquisition. Sheela Sathyanarayana: Writing – review & editing, Funding acquisition. Frances A. Tylavsky: Writing – review & editing, Funding acquisition. Adam A. Szpiro: Methodology, Writing – review & editing. Daniel A. Enquobahrie: Conceptualization, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.113571.
References
- Aibar L, Puertas A, Valverde M, Carrillo MP, Montoya F, 2012. Fetal sex and perinatal outcomes. J. Perinat. Med 40, 271–276. [DOI] [PubMed] [Google Scholar]
- Barker D, 2002. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol 31, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Barker D, 2004. The developmental origins of adult disease. J. Am. Coll. Nutr 23, 588S–595S. [DOI] [PubMed] [Google Scholar]
- Barrett ES, et al. , 2014. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol 176, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, et al. , 2016. Prenatal stress as a modifier of associations between phthalate exposure and reproductive development: results from a multicentre pregnancy cohort study. Paediatr. Perinat. Epidemiol 30, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmarhnia T, Hajat A, Kaufman JS, 2021. Inferential challenges when assessing racial/ethnic health disparities in environmental research. Environ. Heal. A Glob. Access Sci. Source 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere-Brown ZA, et al. , 2016. Sex-specific differences in fetal and infant growth patterns: a prospective population-based cohort study. Biol. Sex Differ 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Economic Analysis, 2020. Real Personal Income and Regional Price Parities https://www.bea.gov/system/files/methodologies/RPP2020-methodology_1.pdf.
- Dadvand P, et al. , 2013. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ. Health Perspect 121, 267–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguen S, et al. , 2021. Are the effects of air pollution on birth weight modified by infant sex and neighborhood socioeconomic deprivation? A multilevel analysis in Paris (France). PLoS One 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Voegtline KM, 2017. The gestational foundation of sex differences in development and vulnerability. Neuroscience 342, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, et al. , 2018. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr. Perinat. Epidemiol 10.1111/ppe.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Prehn AW, Christianson RE, 1995. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am. J. Public Health 85, 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos V, et al. , 2015. Impact of air pollution on fertility: a systematic review. Gynecol. Endocrinol 31, 7–13. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, et al. , 2009. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ. Res 109, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A, 2006. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect 114, 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JP, et al. , 2015. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ. Health Perspect 123, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp MW, 2014. Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol 5, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwa K, Szpiro AA, Sheppard L, et al. , 2021. Fine-scale air pollution models for epidemiologic research: insights from approaches developed in the Multi-ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Curr. Environ. Health Rpt 8, 113–126. 10.1007/s40572-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A, 2018. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ. Res 167, 144–159. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH, 2012. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ. Res 115, 11–17. [DOI] [PubMed] [Google Scholar]
- Lakshmanan A, et al. , 2015. Associations between prenatal traffic-related air pollution exposure and birth weight: modification by sex and maternal pre-pregnancy body mass index. Environ. Res 137, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem J-H, Lee J-Y, Kim H-C, 2015a. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol 30, e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem J-H, Lee J-Y, Kim H-C, 2015b. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol 30, e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem J-H, Lee J-Y, Kim H-C, 2015c. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol 30, e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Thompson ME, Hall G, 2013. Pathways of neighbourhood-level socioeconomic determinants of adverse birth outcomes. Int. J. Health Geogr 12, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer LC, et al. , 2006. The development of a standardized neighborhood deprivation index. J. Urban Health 83, 1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman RM, et al. , 2016. Intrauterine inflammation and maternal exposure to ambient PM2.5 during preconception and specific periods of pregnancy: the Boston birth cohort. Environ. Health Perspect 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi SM, et al. , 2019. Ambient air pollution exposure and fecundability in women undergoing in vitro fertilization. Environ. Epidemiol 3, e036. Philadelphia, Pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Kioumourtzoglou MA, Weisskopf MG, 2018. Live-birth bias and observed associations between air pollution and autism. Am. J. Epidemiol 187, 2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Sex-specific placental responses in fetal development. Endocrinology 156, 3422–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, et al. , 2008. Meeting report: atmospheric pollution and human reproduction. In: Environmental Health Perspectives, vol. 116. Public Health Services, US Dept of Health and Human Services, pp. 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla L, et al. , 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description on JSTOR https://www.jstor.org/stable/10.7249/j.ctt19rmd99.
- Stieb DM, Chen L, Eshoul M, Judek S, 2012a. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ. Res 117, 100–111. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S, 2012b. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ. Res 117, 100–111. [DOI] [PubMed] [Google Scholar]
- Talge NM, Mudd LM, Sikorskii A, Basso O, 2014. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 133, 844–853. [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, et al. , 2014. Air pollution, inflammation and preterm birth: a potential mechanistic link. Med. Hypotheses 82, 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Robinson WR, 2014. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology 25, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. , 2018. National PM2. 5 and NO2 spatiotemporal models integrating intensive monitoring data and satellite-derived land use regression in a universal kriging framework in the United States: 1999–2016. In: ISEE Conference Abstracts. Canada, Ottawa, 2018. [Google Scholar]
- Weiss NS, 2008. Subgroup-specific associations in the face of overall null results: should we rush in or fear to tread? Cancer Epidemiol. Biomarkers Prev 17, 1297–1299. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, et al. , 2010. International collaboration on air pollution and pregnancy outcomes (ICAPPO). Int. J. Environ. Res. Publ. Health 7, 2638–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, et al. , 2021. The effect of prenatal temperature and PM2.5 exposure on birthweight: weekly windows of exposure throughout the pregnancy. Environ. Int 155, 106588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, et al. , 2020. Critical windows for maternal fine particulate matter exposure and adverse birth outcomes: the Shanghai birth cohort study. Chemosphere 240. [DOI] [PubMed] [Google Scholar]
- Zheng T, et al. , 2016. Effects of environmental exposures on fetal and childhood growth trajectories. Ann. Global Health 82, 41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, et al. , 2015. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ. Sci. Pollut. Res 22, 3383–3396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


