Abstract
Levels of interleukin 8 (IL-8), gamma interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1β (MIP-1β) were elevated in patients with tuberculosis. IP-10 and MCP-1 levels were higher in human immunodeficiency virus (HIV)-seropositive patients than in HIV-seronegative patients with tuberculosis. Lipoarabinomannan induced IL-8, MCP-1, and MIP-1β in vitro, which was partly inhibited by anti-tumor necrosis factor antibody.
The immune response in tuberculosis (TB) requires the formation of granulomas, characterized by lymphocytes, macrophages, and neutrophils (8). Chemokines induce leukocyte migration: interleukin 8 (IL-8) acts on neutrophils, and gamma interferon (IFN-γ)-inducible protein 10 (IP-10) acts on monocytes and lymphocytes. Monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) act on monocytes and T cells. IL-8 is produced after the phagocytosis of Mycobacterium tuberculosis (7). IP-10 is secreted in response to IFN-γ and is expressed in the delayed type hypersensitivity response to purified protein derivative (10). MCP-1 is produced in the lungs of mice infected with M. tuberculosis (13).
We measured IL-8, IP-10, MCP-1, and MIP-1β levels in the sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with TB (described in reference 9): 87 patients had active TB, 15 patients were HIV seropositive, 63 patients were HIV seronegative, and in 9 patients no HIV test was performed. Fever (rectal temperature above 38°C) and anorexia were scored. Sera were obtained from 15 patients with TB receiving therapy (one patient was HIV seropositive), from 27 patients who had completed therapy, from 16 persons who had been in close contact with patients with contagious pulmonary TB, and from 10 controls (all were HIV seronegative). Measurements were done by enzyme-linked immunosorbent assay, i.e., for IL-8, tumor necrosis factor (TNF) (CLB, Amsterdam, The Netherlands), IP-10, MIP-1β (R & D Systems, Abingdon, United Kingdom), and MCP-1 (Pharmingen, San Diego, Calif.). The detection limits were 2 (IL-8), 4 (TNF), 8 (MCP-1), 128 (IP-10), and 15.6 (MIP-1β) pg/ml.
Data are presented as medians (with ranges in parentheses) and were compared by using the Wilcoxon test for unmatched samples. Correlations were made by using Spearman’s test.
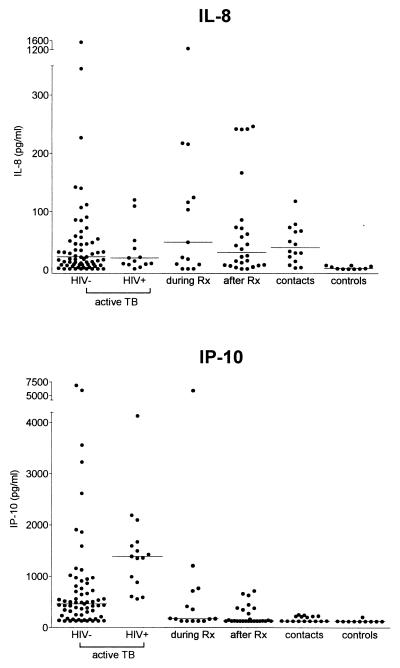
IL-8, IP-10, MCP-1, and MIP-1β levels did not differ between patients with pulmonary and extrapulmonary TB. Therefore, these groups were combined. IL-8 levels did not differ between HIV-seropositive patients and HIV-seronegative patients (Fig. 1). IL-8 levels were higher in patients and in contacts than in controls: HIV-seropositive patients with active TB, 20.7 (<2.0 to 1,657.0) pg/ml (P < 0.001); HIV-seronegative patients with active TB, 22.3 (<2 to 3,222.0) pg/ml (P < 0.001); patients during therapy, 47.7 (<2.0 to 2,168.0) pg/ml (P < 0.01); patients after therapy, 30.2 (<2.0 to 246.4) pg/ml (P < 0.001); close contacts, 38.3 (3.7 to 413.4) pg/ml (P < 0.001); controls, 2.8 (<2.0 to 8.2) pg/ml. Serum IL-8 levels remained elevated in patients in all stages of TB. Accordingly, IL-8 in bronchoalveolar lavage fluid did not decrease during the convalescent phase of TB (12). Spontaneous secretion of IL-8 from macrophages may account for high levels of IL-8 during all stages of TB, as well as in contacts (18). M. tuberculosis directly stimulates IL-8, but also IL-1 and TNF can induce IL-8 (17), which may result in high IL-8 levels in active TB.
FIG. 1.
Concentrations of IL-8 and IP-10 in sera from patients with active TB (n = 87) from patients during (n = 15) and after (n = 26) treatment (Rx), from persons who had been in close contact with contagious TB (n = 16), and from healthy controls (n = 10). Horizontal lines indicate medians.
HIV-seropositive patients with active TB had higher IP-10 levels than HIV-seronegative patients with active TB (1,387.0 [559.0 to 3,188.0] versus 462.3 [<128.0 to 6,881.0] pg/ml [P < 0.001]). Concentrations of IP-10 in serum were higher in all patient groups and in contacts than in controls: HIV-seropositive patients with active TB (P < 0.001); HIV-seronegative patients with active TB (P < 0.001); patients during therapy, 172.1 (<128.0 to 5,933.3) pg/ml (P < 0.05); patients after therapy, 130.9 (<128.0 to 712.4) pg/ml (P = 0.058); contacts, 132.0 (<128.0 to 254.8) pg/ml (P < 0.05); controls, <128.0 (<128.0 to 208.3) pg/ml. IP-10 concentrations were elevated during active TB, with higher levels in patients with fever and anorexia (1,126.0 [128.0 to 6,881.0] pg/ml) than in nonsymptomatic patients (408.8 [128.0 to 1,908.0] pg/ml [P = 0.001]) and did not decline during treatment. T helper 1 (Th1) but not Th2 cell lines respond to IP-10 (15). Consistently, IP-10 is found at sites of Th1 type immune responses (10). Therefore, elevated levels of IP-10 in serum suggest a systemic Th1 type reaction during TB. IP-10 production is under control of IFN-γ, which is an essential factor in host defense against TB (4, 6). IP-10 is chemotactic for stimulated T cells (16) and may account for the higher levels of IP-10 in HIV-seropositive patients than in HIV-seronegative patients. Whether HIV stimulates IP-10 directly remains to be determined. Furthermore, we report for the first time an association of IP-10 with fever and anorexia in TB patients. No association between IL-8, MIP-1β, or MCP-1 and fever and anorexia was found (data not shown).
HIV-seropositive patients with active TB had higher MCP-1 levels than HIV-seronegative patients with active TB (Fig. 2) (601.8 [223.7 to 1,873.0] versus 319.0 [98.1 to 2,034.0] pg/ml [P < 0.05]). MCP-1 levels were higher in all patient groups than in controls: HIV-seropositive patients with active TB (P < 0.01); HIV-seronegative patients with active TB (P < 0.05); patients during therapy, 319.1 (159 to 743.3) pg/ml (P < 0.05); patients after therapy, 355.9 (187.4 to 799.3) pg/ml (P < 0.001); contacts, 289.4 (168.7 to 500.4), pg/ml (P = 0.097); controls, 211.3 (31.2 to 161.3) pg/ml. HIV-seropositive patients with active TB had higher levels of MCP-1 than patients during therapy (P < 0.05) and after therapy (P = 0.086) and contacts (P < 0.05). Levels in HIV-seronegative patients with active TB did not differ from those in other patient groups and contacts. MCP-1 levels are elevated at the site of infection during TB (1, 12, 13) and in serum (this study). Since HIV-seropositive patients had higher levels of MCP-1 than HIV-negative patients, HIV and M. tuberculosis may have an additive effect on MCP-1 production.
FIG. 2.
Concentrations of MCP-1 and MIP-1β in sera from patients with active TB (n = 87), from patients during (n = 15) and after (n = 26) treatment (Rx), from persons who had been in close contact with contagious TB (n = 16), and from healthy controls (n = 10). Horizontal lines indicate medians.
Serum MIP-1β levels did not differ between HIV-seropositive patients and HIV-seronegative patients. During active TB, MIP-1β levels were elevated only in HIV-seronegative patients compared to controls (154.6 [31.2 to 2,197.5] versus 126.0 [31.2 to 161.2] pg/ml [P < 0.05]). HIV-seropositive patients with active TB had elevated MIP-1β levels (123.9 [38.2 to 497.7] pg/ml), but the difference with controls was not significant. MIP-1β levels did not differ between patients with active TB and patients during therapy (150.5 [61.3 to 770.1] pg/ml) and after therapy (177.3 [59.3 to 550.4] pg/ml) and close contacts (116.7 [67.9 to 316.2] pg/ml). During experimental pulmonary infection with Mycobacterium avium, MIP-1β was associated with a protective function (5). In our patient population, MIP-1β was modestly elevated in the sera of patients with TB, thereby providing the first evidence that the production of MIP-1β is enhanced during TB. Moreover, MIP-1β and IL-8 levels correlated weakly (r = 0.47; P < 0.001). No other correlations were found between chemokine concentrations. Since asymptomatic HIV-positive controls were not included in this investigation, the relative contribution of infection with HIV and TB to chemokine concentrations cannot be obtained with certainty from our measurements in HIV-seropositive TB patients.
Lipoarabinomannan (LAM) is a cell wall lipoglycan of M. tuberculosis that can induce the release of cytokines and IL-8 (17, 18). Whole blood from six healthy donors was stimulated for 24 h with mannose-capped LAM (containing 21.6 ng of lipopolysaccharide [LPS] per mg, prepared from M. tuberculosis H37Rv (3); 1 μg of LAM corresponds to 104 CFU), with or without anti-TNF-α antibody (monoclonal antibody MAK 195F; provided by Knoll, Ludwigshafen, Germany) or an isotype-matched mouse immunoglobulin G (IgG). Data are presented as means ± standard deviations and were compared by using the Student t test.
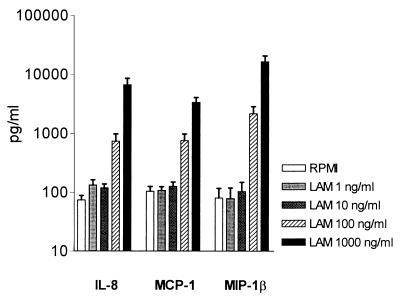
LAM induced the release of IL-8, MCP-1, and MIP-1β dose-dependently (Fig. 3). IP-10 was not produced after stimulation with LAM. Incubation with 21.6 pg of LPS/ml (i.e., the LPS content of the LAM preparation) did not induce detectable chemokine production (data not shown). This confirms earlier reports in which LAM stimulated the production of IL-8 (14, 17) and of TNF and IL-1β (18).
FIG. 3.
Effects of LAM on IL-8, MCP-1, and MIP-1β levels after stimulation of whole blood with different concentrations for 16 h. Data are means ± standard deviations (error bars) for six subjects.
TNF plays a pivotal role in mycobacterial host defense (2, 11). Anti-TNF attenuated the release of IL-8, MCP-1, and MIP-1β in whole blood stimulated with LAM, confirmative with earlier findings that the elimination of TNF inhibits LAM-induced IL-8 production (17) (Table 1). During TB, TNF may act as an intermediate factor in the release of IL-8, MCP-1, and MIP-1β.
TABLE 1.
Effect of an anti-TNF monoclonal antibody on LAM-induced chemokine productiona
| Chemokine | Mean level ± SE (ng/ml) of:
|
% Inhibition | |
|---|---|---|---|
| LAM + IgG | LAM + anti-TNF | ||
| IL-8 | 12.93 ± 4.21 | 0.12 ± 0.91* | 92 ± 3 |
| MCP-1 | 11.26 ± 12.54 | 2.28 ± 0.64* | 81 ± 4 |
| MIP-1β | 18.47 ± 29.73 | 3.91 ± 0.80* | 76 ± 6 |
Data from six different HIV-seronegative donors. Whole blood diluted 1:1 in RPMI medium was incubated for 16 h at 37°C with LAM (1 μg/ml) and anti-human TNF or control IgG (both 10 μg/ml). *, P < 0.05 (versus LAM + IgG).
Acknowledgments
This work was supported by grants from the “Mr. Willem Bakhuys Roozeboom” Foundation to N. P. Juffermans and from the Royal Dutch Academy of Arts and Sciences to T. van der Poll. The mannose-capped LAM was provided through National Institutes of Health contract NO1-A1-75320.
REFERENCES
- 1.Antony V B, Godbey S W, Kunkel S L, Hott J W, Hartman D L, Burdick M D, Strieter R M. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151:7216–7223. [PubMed] [Google Scholar]
- 2.Bermudez L E, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- 3.Chatterjee D, Lowell K, Rivoire B, McNeil M R, Brennan P J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- 4.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florido M, Appelberg R, Orme I M, Cooper A M. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology. 1997;90:600–606. doi: 10.1046/j.1365-2567.1997.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedland J S. Chemotactic cytokines and tuberculosis. Biochem Soc Trans. 1994;22:310–312. doi: 10.1042/bst0220310. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Pando R, Orozcoe H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer J M, Madrid M V. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Juffermans N P, Verbon A, van Deutekom H, van Deventer S J H, Speelman P, van der Poll T. Tumor necrosis factor and interleukin-1 inhibitors as markers of disease activity of tuberculosis. Am J Respir Crit Care Med. 1998;157:1328–1331. doi: 10.1164/ajrccm.157.4.9709126. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan G, Luster A D, Hancock G, Cohn Z A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 12.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T, Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–1477. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 13.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect & Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedel D D, Kaufmann S H. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–4623. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin D J, Oppenheim J J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom W N. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Investig. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Doerfler M, Lee T C, Guillemin B, Rom W N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Investig. 1993;91:2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]