Significance
Reducing health disparities is a high-level national priority. Dementia is a widespread, burdensome, and costly condition with substantial variation in prevalence by education, by sex, and across racial and ethnic groups. While a decline in population prevalence has been firmly established, much less is known about trends in disparities, even whether they have increased or decreased. Yet this knowledge is vital if public policy is to address these disparities. In addition to the benefit to public policy, the study of these subpopulations over time has the scientific benefit of establishing hypotheses about causal mechanisms for dementia because different subpopulations and cohorts were exposed differentially to risk factors such as education, paid work, health care delivery, and economic circumstances.
Keywords: Alzheimer’s disease, racial and ethnic inequalities, longitudinal analysis, Markov Chain Monte Carlo
Abstract
This paper presents estimates of the prevalence of dementia in the United States from 2000 to 2016 by age, sex, race and ethnicity, education, and a measure of lifetime earnings, using data on 21,442 individuals aged 65 y and older and 97,629 person-year observations from a nationally representative survey, the Health and Retirement Study (HRS). The survey includes a range of cognitive tests, and a subsample underwent clinical assessment for dementia. We developed a longitudinal, latent-variable model of cognitive status, which we estimated using the Markov Chain Monte Carlo method. This model provides more accurate estimates of dementia prevalence in population subgroups than do previously used methods on the HRS. The age-adjusted prevalence of dementia decreased from 12.2% in 2000 (95% CI, 11.7 to 12.7%) to 8.5% in 2016 (7.9 to 9.1%) in the 65+ population, a statistically significant decline of 3.7 percentage points or 30.1%. Females are more likely to live with dementia, but the sex difference has narrowed. In the male subsample, we found a reduction in inequalities across education, earnings, and racial and ethnic groups; among females, those inequalities also declined, but less strongly. We observed a substantial increase in the level of education between 2000 and 2016 in the sample. This compositional change can explain, in a statistical sense, about 40% of the reduction in dementia prevalence among men and 20% among women, whereas compositional changes in the older population by age, race and ethnicity, and cardiovascular risk factors mattered less.
Many individuals who live with dementia require costly help with activities of daily living. The total economic cost of dementia was estimated at $200 billion per year in the United States (1) and $600 billion worldwide (2)—about 1% of the global gross domestic product (GDP)—making dementia the most expensive of all medical conditions. Dementia affects many older adults. In 2021, about 6.2 million US adults age 65 or older lived with dementia (3). Because age is the strongest risk factor for dementia, it has been predicted that increasing life expectancies will substantially increase the prevalence of Alzheimer’s disease and related dementias from about 50 to 150 million worldwide by 2050 (4).
However, there is growing evidence based on data from the United States and Europe that age-adjusted dementia prevalence has been declining in developed countries, possibly because of rising levels of education, a reduction in smoking, and better treatment of key cardiovascular risk factors such as high blood pressure (5–16). Any change in these age-specific rates has important implications for projected prevalence and associated costs, such as payments for nursing care by households, insurance companies, and the government. An accurate measurement of prevalence rates and how they evolve by population subgroups is therefore paramount. The data requirements to achieve this are substantial: 1) a large enough population-representative sample with the statistical power to estimate prevalence by subgroups; 2) reliable classification as to dementia status, in total and also within subgroups; and 3) a sufficient number of waves with a longitudinally consistent measurement to identify trends.
Prior literature reported large differences in the age-adjusted prevalence of dementia by sex, education, and race and ethnicity. For example, women are more likely to live with dementia than men, especially at advanced ages (8, 17); the prevalence is higher among racial and ethnic minority groups (16, 18, 19) and among the less educated (5, 8). Less is known about how differences across subgroups in dementia prevalence and dementia risk factors have evolved, and the available evidence is mixed. Reducing health inequalities is an important public health priority, but to address inequalities, public policy should be informed by how inequalities in dementia prevalence have changed in recent decades. Whether the overall decline in dementia prevalence has occurred evenly across the entire population or whether it is found disproportionately among certain subgroups would also provide valuable information for efforts to identify causal mechanisms.
Several studies on trends in dementia are based on small community studies that are not representative of the general population, making it difficult to generalize the findings or reliably estimate dementia prevalence in smaller population subgroups, such as among racial and ethnic minority groups. The Health and Retirement Study (HRS) is a long-running longitudinal survey that satisfies many of the requirements to study dementia in subpopulations in the United States. The few studies based on the HRS have mostly used a uniform cutoff for dementia classification (5, 8, 16, 19), described by Crimmins et al. (20). In self-interviews, this methodology sums responses to items from the HRS cognitive battery and classifies individuals who score below a cutoff value as having dementia. A similar method is used for proxy-interviews. The cutoff values were selected so that the population prevalence of dementia in the HRS in a base year matched prevalence from a reliable source. This methodology guaranteed that the estimated population prevalence in the HRS was unbiased relative to that source in the base year. However, the methodology did not guarantee the unbiasedness of prevalence estimates over time in the population if population composition changes or in subpopulations. Gianattasio et al. (21) and Gianattasio, Ciarleglio, and Power (22) noted this issue in the context of racial and ethnic differences in dementia in the HRS, and we provide further evidence of such differences by age and education in Materials and Methods.
This study employs a model to assess cognitive status based on a broad set of cognitive measures elicited in a large population-representative US survey, namely, the HRS. The model increases the precision of dementia classification by using the longitudinal dimension of the data. Importantly, for the study of inequality, the model is constructed to ensure the dementia classification is calibrated accurately within population subgroups, and therefore, it is equipped to produce accurate estimates of dementia prevalence by age, sex, education, race and ethnicity, and a measure of lifetime earnings.
The HRS dataset has several unique features that strengthen the analysis, as follows: 1) it has a wide range of information about health status, cognitive abilities, socioeconomic status (SES), demographics, and other variables; 2) it has good coverage of the population with dementia because it follows individuals into nursing homes; 3) proxy-interviews are available for those who cannot complete a self-interview; and 4) a subset of HRS members were administered a clinical assessment for dementia within the Aging, Demographics and Memory Study (ADAMS) using a 3- to 4-h-long in-home cognitive assessment reviewed by an expert panel. The assessment produced a final diagnosis that we use to calibrate the cognition scores derived from the rich information available in the entire HRS sample.
We use a longitudinal latent variable model and jointly model the clinical dementia diagnosis in the ADAMS subsample and the cognitive measures in the HRS. Changes in dementia over time are primarily identified from wave-to-wave differences in the averages of individuals’ performance on the HRS cognitive tests, while the clinical diagnosis of dementia in ADAMS plays a critical role in calibrating the HRS cognitive tests to measure dementia. Importantly, our model allows the relationship between the ADAMS and HRS measures to vary across population subgroups, essentially calibrating the measures for each subgroup.
Results
Trends in Dementia Prevalence between 2000 and 2016.
We present our findings on the evolution of dementia prevalence between 2000 and 2016 in the US population age 65 or older. Because age is a powerful predictor of cognition and the age distribution in the older population may have changed over the study period, we mainly present age-adjusted prevalence, which is the result of adjusting the means of age in each survey wave to the overall sample mean obtained after pooling all survey waves.
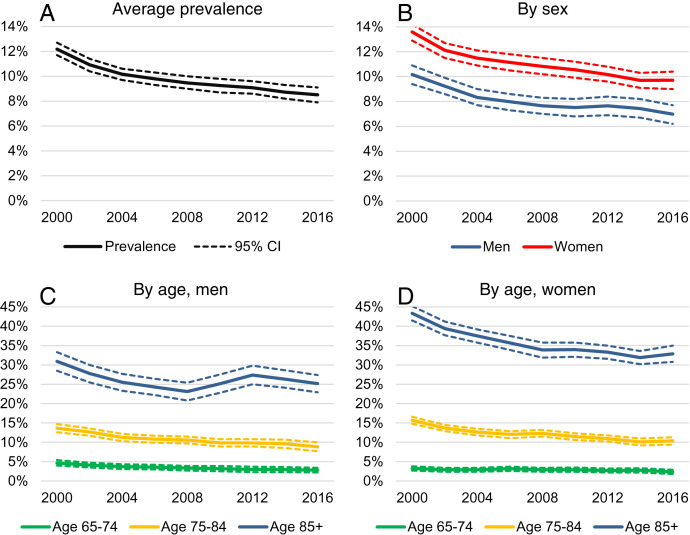
Fig. 1A shows the age-adjusted prevalence of dementia in the US population every 2 y between 2000 and 2016 among those age 65 or older (additional results and table versions of the figures may be found in SI Appendix). The age-adjusted prevalence of dementia decreased by 3.7 percentage points (ppts) from 12.2% in 2000 to 8.5% in 2016—this is a sizeable reduction, equivalent to 30.1% of the 2000 level. The prevalence of dementia decreased over the entire period, but the rate of decline was more rapid between 2000 and 2004.
Fig. 1.
Trends in the age-adjusted prevalence of dementia from 2000 to 2016 by sex and age. Sample: HRS, 2000 to 2016, Age 65+. The sample includes 21,442 individuals and 97,629 person-year observations. The dashed lines show 95% confidence intervals. (A) Average prevalence. (B) Prevalence by sex. (C) Prevalence by age for men. (D) Prevalence by age for women.
Fig. 1B shows that the prevalence of dementia was higher among women than men over the entire period, but the difference shrank between 2000 and 2016. Among men, the prevalence of dementia decreased by 3.2 ppts from 10.2 to 7.0%, while the decrease was larger among women, namely, 3.9 ppts from 13.6 to 9.7%.
Fig. 1 C and D show trends in age-adjusted dementia prevalence by sex and three age groups (65 to 74, 75 to 84, 85+ y). Age is the strongest risk factor for dementia, as evidenced by the substantially greater prevalence in older age groups. We found that dementia prevalence decreased monotonically after 2000 in all sex and age groups below 85 y. The changes were more ambiguous in the 85+ groups; prevalence fell between 2000 and 2008 for both sexes. After 2008, it stagnated or slightly increased among men, while it continued to decrease, although at a lower rate, among women. To investigate the upturn among men, we fitted linear time-trends by sex in more detailed 5-y age bands. Among men, we found a statistically significant decrease in all age groups below 90 and a small—although statistically not significant—increase in dementia prevalence above 90 (SI Appendix, Tables S8 and S9).
Decomposing the Trends in Dementia Prevalence.
Table 1 shows how much of the change in the prevalence of dementia can be explained by compositional changes in the sample over time. The first row of Table 1, labeled “Men, unadjusted,” shows dementia prevalence in 2000 and 2016 among men based on a fitted linear trend on all observations, without adjusting for compositional changes in the sample. The second row, labeled “Men, age-adjusted,” shows similar statistics after adjusting the distributions of age in the 2000 and 2016 samples to equal the overall sample mean. The difference between the first and second rows shows how much of the change in the prevalence of dementia among men can be explained by changes in the average age of men between 2000 and 2016. The third row, labeled “Men, age- & education-adjusted,” further adjusts the distribution of education in the 2000 and 2016 samples to the pooled wave sample mean. The fourth row, labeled “Men, age, education & demographics-adjusted,” adds adjustments for shifts in Social Security income quartiles, race and ethnicity, foreign-born status, and marital status. The fifth row, “Men, age, education, demographics & heath-adjusted,” also adjusts the distribution of four cardiovascular risk measures (self-reported prior diagnosis of hypertension, diabetes, stroke, and heart problems) to the sample mean. SI Appendix discusses the details of the method. The bottom panel of Table 1 shows the same statistics for women.
Table 1.
Sixteen-year change in the prevalence of dementia by sex
| Dementia prevalence | |||
|---|---|---|---|
| 2000 | 2016 | Δ2000–2016 | |
| Men, unadjusted | 0.093 | 0.069 | −0.024** |
| [0.003] | [0.003] | [0.003] | |
| Men, age-adjusted | 0.093 | 0.068 | −0.025** |
| [0.003] | [0.003] | [0.003] | |
| Men, age- & education-adjusted | 0.087 | 0.073 | −0.014** |
| [0.003] | [0.004] | [0.003] | |
| Men, age-, education-, & demographics-adjusted | 0.086 | 0.073 | −0.013** |
| [0.003] | [0.004] | [0.003] | |
| Men, age-, education-, demographics, & health-adjusted | 0.088 | 0.073 | −0.015** |
| [0.003] | [0.004] | [0.003] | |
| Women, unadjusted | 0.127 | 0.094 | −0.032** |
| [0.003] | [0.003] | [0.003] | |
| Women, age-adjusted | 0.127 | 0.093 | −0.034** |
| [0.003] | [0.003] | [0.003] | |
| Women, age- & education-adjusted | 0.123 | 0.097 | −0.026** |
| [0.003] | [0.003] | [0.003] | |
| Women, age-, education-, & demographics-adjusted | 0.123 | 0.097 | −0.026** |
| [0.003] | [0.003] | [0.002] | |
| Women, age-, education-, demographics, & health-adjusted | 0.122 | 0.097 | −0.025** |
| [0.003] | [0.003] | [0.002] | |
Dementia prevalence estimated by a fitted linear time trend on the 2000-2016 data on 65+-y-old U.S. individuals. The unadjusted models do not adjust the samples to compositional changes. The adjusted models adjust the distributions of age, education, income, race and ethnicity, foreign-born status, marital status, hypertension, diabetes, stroke, and heart problems in the 2000 and 2016 samples to the overall sample mean between 2000 and 2016. Standard errors in brackets.
**Indicates statistical significance at 1%.
According to the estimated unadjusted linear trend, the prevalence of dementia among men decreased by 2.4 ppts from 9.3% in 2000 to 6.9% in 2016. After adjusting the age composition, the estimated decrease was similar (2.5 ppts). Thus, changes in the age composition of the sample explain little of the difference in dementia prevalence between 2000 and 2016. This is because the average age did not change much over this period due to two opposing trends, as follows: although life expectancies have increased over time, the relatively large baby boom generation aged into the sample, pulling down the average age of the population age 65 or older. These two effects approximately canceled out.
Education is a very strong predictor of dementia, and there was a sizeable increase in the average level of education over the study period. For example, the average years of education among men 65+ y in 2000 was 11.8 y, and it increased to 13.4 y in 2016. Adjusting the sample composition for changes in education (third row of Table 1) accounts for almost half of the change in dementia prevalence; the decrease in dementia prevalence was only 1.4 ppts (from 8.7 to 7.3%) instead of 2.5 ppts in the second row. Thus, changes in the education composition of the US population explain, in a statistical sense, a substantial share of the difference in dementia prevalence between 2000 and 2016. However, the decrease in dementia is still meaningfully large and statistically significant after accounting for age and education trends. The estimated statistics remained largely the same when we further adjusted the sample composition for shifts in Social Security income quartile, race and ethnicity, foreign-born status, marital status (fourth row), and the four self-reported cardiovascular risk factors (fifth row).
The bottom panel of Table 1 shows a qualitatively similar picture among women. Age adjusting the sample explains little of the change in dementia prevalence (unadjusted: −3.2 ppts vs. age-adjusted: −3.4 ppts). Education explains, in a statistical sense, a substantial part of the decline in dementia prevalence. After adjusting for shifts in education, the estimated decline between 2000 and 2016 is 2.6 ppts, which is 0.8 ppts (or 24%) smaller without adjusting for education. Further adjusting the sample compositions to changes in Social Security income quartile, race and ethnicity, birthplace, marital status, and health mattered little.
In principle, entering the factors in a different order could change the estimated contribution of the different factors. SI Appendix, Table S7 shows models with the different factors added one at a time rather than in blocks: we did not find important differences from the results in Table 1.
Inequalities in Dementia Prevalence and Their Evolution between 2000 and 2016.
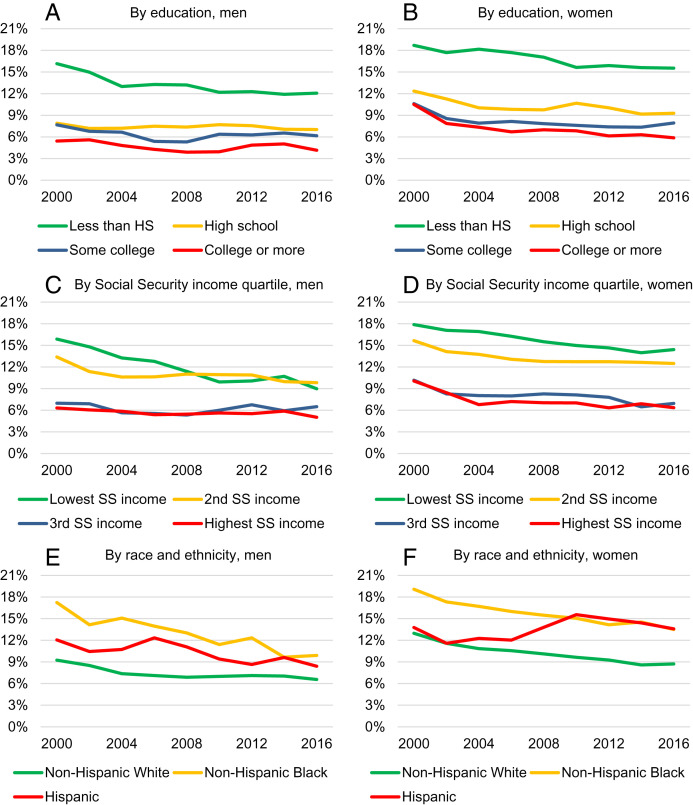
Fig. 2 shows the age-adjusted prevalence of dementia by sex, education, Social Security income quartile, and race and ethnicity. The left panels correspond to men and the right panels to women. The top, middle, and bottom panels show the relationship by education, Social Security income, and race and ethnicity, respectively. The prevalence of dementia was higher than average among less-educated men over the entire period. Still, the rate of decrease was the most pronounced among this group, leading to a narrowing of the differences in dementia prevalence between high school dropouts and the other education groups. Among women, those in the lowest education group also had the highest dementia prevalence rate, but the patterns of change were different: dementia prevalence decreased similarly among all education groups, and inequalities between the most and least educated women changed little.
Fig. 2.
Trends in the age-adjusted prevalence of dementia from 2000 to 2016 by sex, education, Social Security income, race and ethnicity. Sample: HRS, 2000 to 2016, Age 65+. The sample includes 21,442 individuals and 97,629 person-year observations. Non-Hispanic other race not shown due to small sample size, statistics reported in SI Appendix, Table S6. (A) Prevalence by education, men. (B) Prevalence by education, women. (C) Prevalence by Social Security income quartile, men. (D) Prevalence by Social Security income quartile, women. (E) Prevalence by race and ethnicity, men. (F) Prevalence by race and ethnicity, women.
Social Security income is based on the highest 35 y of earnings over the working lifetime, and so stratifying by Social Security income quartiles is a good method of stratifying by SES. We found a substantial reduction in the differences in dementia prevalence across quartiles among men, similar to the trends observed in inequalities by education; the reduction in dementia prevalence was substantially more pronounced among those in the lowest quartiles. Inequalities also decreased among women but less so than among men.
Table 2 shows by education categories estimated changes from fitting linear trends so as to smooth out the noise in Fig. 2 A and B. They adjust only for age in the first set of columns and in addition for education, demographic, and health in the second set of columns. Among men with less than a high school education, prevalence declined by 3.8 ppts, age adjusted, with little difference from further adjustments. Declines in the other education categories were small resulting in a narrowing of differences between the group with the least education and all others. Among women, prevalence declined in all education categories, and there was some narrowing, particularly between those lacking high school and the others.
Table 2.
Sixteen-year change in the prevalence of dementia by sex and education
| Age-adjusted prevalence | Age, education, demographics- & health-adjusted prevalence | |||||
|---|---|---|---|---|---|---|
| 2000 | 2016 | Δ2000–2016 | 2000 | 2016 | Δ2000–2016 | |
| Men | ||||||
| Less than high school | 0.151 | 0.113 | −0.038** | 0.15 | 0.115 | −0.035** |
| [0.005] | [0.006] | [0.006] | [0.005] | [0.006] | [0.006] | |
| High school | 0.076 | 0.072 | −0.004 | 0.077 | 0.071 | −0.006 |
| [0.004] | [0.006] | [0.007] | [0.004] | [0.006] | [0.007] | |
| Some college | 0.065 | 0.061 | −0.004 | 0.068 | 0.06 | −0.008 |
| [0.005] | [0.006] | [0.006] | [0.005] | [0.006] | [0.006] | |
| College or more | 0.049 | 0.043 | −0.006 | 0.052 | 0.042 | −0.010 |
| [0.004] | [0.004] | [0.004] | [0.004] | [0.004] | [0.004]* | |
| Women | ||||||
| Less than high school | 0.186 | 0.152 | −0.033** | 0.184 | 0.15 | −0.034** |
| [0.005] | [0.005] | [0.006] | [0.005] | [0.005] | [0.006] | |
| High school | 0.113 | 0.091 | −0.022** | 0.112 | 0.092 | −0.020** |
| [0.004] | [0.005] | [0.004] | [0.004] | [0.005] | [0.004] | |
| Some college | 0.091 | 0.072 | −0.019** | 0.091 | 0.072 | −0.019** |
| [0.004] | [0.005] | [0.005] | [0.004] | [0.005] | [0.005] | |
| College or more | 0.087 | 0.057 | −0.030** | 0.086 | 0.058 | −0.028** |
| [0.004] | [0.004] | [0.005] | [0.004] | [0.004] | [0.005] | |
Dementia prevalence estimated by a fitted linear time trend on the 2000-2016 data on 65+-y-old U.S. individuals. The age-, education-, demographics-, & health-adjusted models adjust the distributions of age, education, income, race and ethnicity, foreign-born status, marital status, hypertension, diabetes, stroke, and heart problems in the 2000 and 2016 samples to the overall sample mean between 2000 and 2016. The age-adjusted versions only adjust the distribution of age. Standard errors in brackets.
* and ** indicate statistical significance at 5% and 1%, respectively.
We found notable differences in the evolution of dementia by race and ethnicity, as shown in Fig. 2 E and F. The age-adjusted prevalence of dementia tended to be higher among racial and ethnic minority individuals, both among men and women. However, among men, the difference in the prevalence between non-Hispanic Black and White individuals narrowed while it remained stable among women. Among non-Hispanic White men, the prevalence of dementia decreased from 9.3 to 6.6%, a decline of 2.7 ppts or 29.0%. However, among non-Hispanic Black men, the rate fell from 17.2 to 9.9%, a drop of 7.3 ppts or 42.6%. Because of small sample size, we do not show results for non-Hispanic men of other races, but they can be found in SI Appendix, Table S5. Among females, the rate of decrease was similar among non-Hispanic White and Black individuals. The difference between non-Hispanic White and Hispanic individuals remained about the same over the study period among men while it grew among women. There was no change in the age-adjusted prevalence of dementia among Hispanic women between 2000 and 2016.
In SI Appendix, Fig. S1 and Table S6, we documented trends by birthplace. A larger fraction of those born abroad lived with dementia than of those born in the United States. Among men, there was little change between 2000 and 2016 in the difference, but among women the gap increased.
In SI Appendix, Fig. S2 we show education-based inequalities in dementia prevalence by sex and race and ethnicity. We found larger education-based inequalities among racial and ethnic minority individuals, often approaching 15 ppts. The differential between the lowest and highest education groups among non-Hispanic white persons ranged between 7 and 11 ppts. Those with the highest prevalence are racial and ethnic minority individuals with low levels of education.
Discussion
This paper presented estimates of the prevalence of dementia between 2000 and 2016 in the United States among individuals age 65 or older. We used a large population-representative survey, the HRS, and a longitudinal latent variable model of cognitive function, dementia, and survival. The large sample and the longitudinal design allowed us to estimate the prevalence of dementia with greater accuracy than earlier studies, permitting us to estimate trends by detailed population subgroups.
We developed a joint model of cognition, dementia, and survival in the HRS that accounted for various selection issues, such as selection into proxy- and self-interviews and selection into ADAMS—a subsample of the HRS with a clinical dementia diagnosis. We estimated the model by a Bayesian method, Markov Chain Monte Carlo. The model’s dementia prevalence estimates in 2002 and 2012 closely tracked other published estimates based on the HRS, such as those by Plassman et al. (23) and Langa et al. (8). An important advantage of our model is that the dementia prevalence rates estimated over the entire HRS sample are calibrated to detailed population subgroups. The calibration is critical to obtain accurate prevalence estimates in subgroups and to study trends in inequalities.
We found that the age-adjusted prevalence of dementia substantially decreased in the United States by 3.7 ppts from 12.2% in 2000 to 8.5% in 2016, which is equivalent to 2 ppts per decade. Similar declines have been reported in earlier studies based on US samples (7–9, 12, 13) as well as samples in some European countries (10, 14, 15).
We studied the evolution of inequalities in dementia prevalence by sex, age, education, race and ethnicity, birthplace, and by Social Security income quartiles, which is a proxy for lifetime economic resources. We observed a decrease in the prevalence of dementia in most population subgroups. We found robust evidence that over the course of the study period, differentials in the prevalence of dementia by race and ethnicity, education, and lifetime earnings narrowed among men, leading to smaller inequalities along these dimensions in 2016 compared with 2000. Among women, the prevalence of dementia is higher overall compared with men, but the decrease over the study period was greater among women, reducing the sex difference in dementia prevalence. However, education-based inequalities among women were mixed; the rate of decline was similar among the most and least educated women. Inequalities by race and ethnicity and by quartiles of lifetime earnings also narrowed among women, but not as much as among men.
Because of sizeable shifts in population characteristics throughout the study period, we estimated changes in dementia prevalence after adjusting the sample to changes in the distributions of age, education, Social Security income quartile, race and ethnicity, birthplace, and cardiovascular health. We found that the compositional changes explained about 40% of the reduction in dementia among men and about 20% among women. Education was an important factor that contributed, in a statistical sense, to the reduction in dementia; the fraction of college-educated men in our sample increased from 21.5% in 2000 to 33.7% in 2016, and the fraction of college-educated women increased from 12.3% in 2000 to 23% in 2016. After accounting for trends in education, trends in the demographic and self-reported cardiovascular risk factors explained little of the trends in dementia prevalence. It would be important in future research to complement this analysis with objectively measured cardiovascular and other risk factors because some of the self-reported measures may have biases, and these biases may vary across population groups, for example, because of differential access to the health care system.
Although it is well-known in the literature that dementia prevalence is lower among those with more education, it has not been established if the association reflects a causal mechanism. While the descriptive results presented in this paper cannot identify the causal effect of education on dementia, our findings are consistent with a causal role. They would suggest that as schooling levels continue to rise in the US population in younger generations, the prevalence of dementia would continue to decrease. Trends in the level of education differ across demographic groups, which may affect inequalities in dementia in the future. For example, while women traditionally had lower levels of education than men, in younger birth cohorts, women are more educated. While racial and ethnic minority groups still have lower education levels than non-Hispanic White individuals, the gaps across racial and ethnic groups have shrunk. Closing the education gap across racial and ethnic groups may be a powerful tool to reduce health inequalities in general and dementia inequalities in particular, an important public health policy goal.
However, other large changes occurred throughout the study period besides education, most notably, the large increase in labor force participation of women. Among 74- to 84-y-old women in 2000, 29.5% had worked for more than 30 y over their lifetimes; in 2016, 59.0% had worked more than 30 y. The effect of employment on cognitive reserve has not been well studied, but it is plausible that 30 y of exposure to the general cognitive demands of seeking and remaining employed and to the specific cognitive demands of a particular occupation could build cognitive reserve more powerfully than just a few additional years of education.
Some literature has suggested that general improvements in living conditions and social welfare have contributed to the decline in dementia prevalence (24), and we have shown that a measure of lifetime income is predictive of prevalence. During the lifetimes of the HRS subjects, there were large cohort gains in income. Consider those who were born in 1920 and 1936. They were age 80 in 2000 and in 2016, respectively, at the beginning and end of our study period. Besides having very different exposures to the Great Depression and to World War II, their economic resources and standards of living were quite different. For example, at age 30 (1966), an age at which people establish families and housing, real GDP per person of those born in 1936 was about 55% higher than of those born just 16 y earlier.
Our results differ from many of those in the literature that are based on the calibrated cutoff method. That method, discussed by Crimmins et al. (20), has been widely used (5, 8, 16, 19). It sums responses to items from the HRS cognitive battery and classifies individuals who score below a cutoff value as having dementia. The cutoff value was selected so that the population prevalence of dementia in HRS 2000 and 2002 matched estimates based on ADAMS data; that is, population prevalence in HRS was unbiased with respect to ADAMS. But using the same cutoff value for subpopulations does not guarantee unbiasedness in them. Indeed, Gianattasio, Ciarleglio, and Power (22) investigated in the HRS the variation by race and ethnicity in the cutoff value, and they found that it does vary; for example, the cutoff point for non-Hispanic Black individuals should be about 30% above that of non-Hispanic White individuals (with some variation depending on the standard of comparison). Not using a differential cutoff results in an overestimate of the difference in prevalence between non-Hispanic Black and non-Hispanic White individuals.
We compared the estimated prevalence in HRS 2002 with prevalence as directly measured in ADAMS both in the total population and in subpopulations. Our model and the cutoff method both produced prevalence estimates among non-Hispanic White individuals that were close to the estimates from ADAMS. This is to be expected because non-Hispanic White participants are 82% of the weighted sample. But among non-Hispanic Black individuals, the estimates are quite different; the cutoff method produces an estimate of dementia prevalence that is 6.3 ppts (or 28%) higher than the ADAMS measure, whereas our model’s estimate is just 0.6 ppts higher. We also found differences in trends. For example, among non-Hispanic Black men, the prevalence in the year 2000 was 8 ppts higher (or 47%) under the cutoff method than our model estimates; by 2016, it was 6 ppts (60%) higher.
Power et al. (25) documented trends in the Black–White dementia prevalence ratio among those 70 or older between 2000 and 2016, also based on HRS data. They found that the prevalence ratio decreased (i.e., inequalities narrowed) between 2000 and 2016, although the change was small. We found similar dementia prevalence ratios, and when averaging across men and women, our estimates closely tracked those of Power et al. (25). However, we found that among men and women, inequalities evolved differently; among men, the Black–White prevalence ratio substantially decreased; among women, the ratio slightly increased even though the difference measure in ppts slightly decreased.
In the Materials and Methods section, we conduct additional comparisons between the predictions of our model and of the calibrated cutoff methodology of Crimmins et al. (20). Our model produces a similar prevalence estimate in the total population but significant and substantial differences in estimates for population subgroups. For example, our model predicts a steeper age profile of dementia prevalence than the alternative method; and we find smaller differentials by education and racial and ethnic groups. We argue that it was because our model allows the relationship between dementia status and the HRS cognitive measures to vary by population subgroups, while the calibrated cutoff methodology uses the same cutoff value for the entire population, which appears to lead to inaccurate prevalence estimates for several subgroups.
Overall, we found that the prevalence of dementia substantially decreased in the older US population since 2000. The gap in dementia prevalence narrowed between men and women, and inequalities decreased between education, income, and race and ethnicity groups, especially among men. Despite these favorable trends, we still find substantial dementia inequalities across subpopulations; women, racial and ethnic minority groups, and those with lower education face substantially higher chances of living with dementia. While more research is needed to establish the causal mechanisms, our improved estimates of dementia prevalence and trends for population subgroups provide important information to public policy for quantifying the distributional burden of dementia and the associated long-term care needs.
Materials and Methods
Data and Study Sample.
The HRS is a nationally representative biennial longitudinal survey of the US population age 51 or older (26, 27). It is conducted by the University of Michigan and funded by the National Institute on Aging and the Social Security Administration. The survey collects comprehensive information about demographics, health, labor market status, wealth, income, and many other variables. The HRS stands out for their detailed information on health outcomes, including participants’ cognitive function, compared with other large population-representative US surveys.
The HRS started in 1992. It has added 51- to 56-y-old refresher cohorts to the sample every 6 y (1998, 2004, 2010, and 2016 with 2022 in process). Individuals who move to nursing homes stay in the sample, which is essential to obtain accurate estimates of dementia prevalence in the population because many cognitively impaired individuals live in nursing homes (28, 29). HRS interviewers are instructed to conduct self-interviews whenever possible. When a study member is unable to be interviewed, the HRS conducts the interview with a proxy informant, typically with a spouse or a child. Cognitively impaired persons are much more likely to be interviewed by proxy. The inclusion of proxy-interviews is critical when estimating dementia prevalence because about half of the sample who live with dementia answer through proxies (see also ref. 1). The HRS makes considerable effort to follow panel members until death. If a person drops from the sample, the HRS continues to track vital status and records the date of death or the last date the respondent was known to be alive.
In 2001 and 2003, a subsample of 856 HRS respondents age 71 or older underwent a detailed in-home clinical assessment for dementia in ADAMS (30). The duration of the evaluation was 3 to 4 h, and it concluded in a final diagnosis of the following:
-
•
dementia,
-
•
cognitively impaired but not dementia (CIND),
-
•
normal cognitive function.
ADAMS participants who were not diagnosed with dementia in the first ADAMS wave were revisited up to three times between 2003 and 2009. The follow-up visits used the same diagnostic procedure and were scheduled about 2 y apart. The process is discussed in ref. 31. We use the ADAMS classifications to calibrate our model of cognitive status both for the entire 65+-population and for subpopulations.
The HRS core survey includes an extensive set of cognitive measures that we use in the analyses to assess cognitive function and dementia status. Some of these variables are only available either in self- or proxy-interviews, while others are available in the entire sample. Self-interviews, for example, include a battery from the Telephone Interview for Cognitive Status. We use the following:
-
•Measures available in both self- and proxy-interviews
-
•Subjectively assessed memory (scale: poor, fair, good, very good, excellent),
-
•Limitations in instrumental activities of daily living (using phone, money, medications, shopping, preparing meals).
-
•
-
•Measures only available in self-interviews
-
•Immediate word recall (10 words),
-
•Delayed word recall (10 words),
-
•Serial 7 subtractions (from 100 to 65),
-
•Backward counting (from 20 to 10),
-
•Date of interview (year, month, day, day of week),
-
•Naming the president,
-
•Naming cactus after paraphrasing,
-
•Naming scissor after paraphrasing.
-
•
-
•Measures only available in proxy-interviews
-
•Interviewers’ assessment of cognitive limitations (no cognitive limitation; some limitation; cognitive limitations prevented the interview),
-
•The person ever gets lost,
-
•The person ever wanders off,
-
•The person cannot be left alone,
-
•The person sees or hears things.
-
•
We used several demographic variables in the analyses, as follows: sex, age, race and ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic other, Hispanic), whether the person was born in the United States, and marital status (married/partnered vs. single).
We used two health variables, namely, self-rated health (5-point scale from poor to excellent) and whether the person ever had a stroke prior to the survey wave (self-reported), as these may be related to individuals’ cognitive function.
For the study of inequalities, we used two measures of SES, namely, the highest level of education (less than high school, high school, some college, college, or more) and quartiles of Social Security income. Social Security income in the United States is based on an individual’s 35 highest years of earnings, and therefore, it is a good measure of the individual’s lifetime earnings. For married persons, we used the maximum of the husband’s and wife’s Social Security income, recognizing that in these cohorts some individuals had low or no earnings, and yet, they were in well-to-do households (e.g., couples with one, well-paid earner). To simplify language, we sometimes refer to this measure as household Social Security income or lifetime earnings. SI Appendix discusses the procedure we used to define this time-invariant variable in a way that reduces measurement error.
The HRS oversamples Black and Hispanic individuals to permit the estimation of race- and ethnicity-specific statistics with greater precision; survey weights are available to adjust for survey design and the demographic distribution of the HRS to the American Community Survey.
The RAND HRS Longitudinal Data file is a publicly available, cleaned, longitudinal data set based on the most commonly used HRS variables (32–34). It was developed at RAND with funding from the National Institute on Aging and the Social Security Administration. We used the RAND HRS variables whenever available.
Our analytic sample comprises 21,442 participants observed in the 2000 through 2016 core HRS survey waves at age 65 or older. SI Appendix, Table S10 shows weighted and unweighted descriptive statistics of the analytic sample. Because women live longer than men, a little over half of the person-year observations are on women. A total of 30.1% of the observations belong to the youngest group (age 65 to 69), and the numbers per age band decline with age. Non-Hispanic White participants comprise 82.4% of the weighted sample, 8.6% are non-Hispanic Black individuals, 6.8% are Hispanic individuals, and 2.2% are of other race and ethnicity. The unweighted fractions of racial and ethnic minority groups are higher because of the oversampling of these groups by HRS. Almost 1/10th of the observations (9.3%) are on people born outside the United States. About a third of the sample have a high school degree, 20.6% have some college education but not a degree, and 21.3% are college graduates. We divided individuals into four approximately equal-sized quartiles based on Social Security income. Person-year observations from the higher income quartiles are slightly overrepresented in the sample because of mortality differences.
Statistical Analysis.
The HRS has many data items that reflect cognitive status, but those items need to be aggregated in some way to produce a measure of cognition that can then be used to determine dementia status. We developed a longitudinal latent variable model of cognitive function, dementia, and survival. This section summarizes the model’s key features, and SI Appendix describes the full technical details and various diagnostic tests we performed. We used the same model in a companion paper to study the lifetime risk of dementia in the United states (35).
We assume that individual i’s cognitive function at time t, denoted by , is an unobserved latent variable, and it develops over time according to the equation
| [1] |
where xit denotes observed covariates (such as age, calendar time, sex, and education), ait is age, is a random person-specific intercept, is a random slope (with respect to age), and is a residual term.
Individuals have dementia if their cognitive function falls below 0, and they are CIND if their cognitive function is between 0 and 1. These cutoff values are normalizations. We only observe the classification as dementia, CIND, and normal in the four waves of ADAMS.
The HRS includes a set of cognitive measures, such as immediate and delayed word recall. We assume that performance on these tests depends on individuals’ latent cognitive function and other predictor variables:
| [2] |
where s indexes the different available cognitive measures. We add demographic and other predictors to xit in ref. 2. To allow for learning effects, in the equation for the cognitive measures in self-interviews, we included categorical variables indicating if it was the first time the person saw these questions (SI Appendix contains details).
Some cognitive measures are only available in self- or proxy-interviews. A selection equation models interview type using a probit framework. We model survival using a Gompertz model. Time-invariant covariates, such as sex, race and ethnicity, education, and the two random effect terms in cognition, namely, and , enter the shape parameter of survival hazards. Individuals were selected into ADAMS via stratified sampling to better cover the sample with cognitive limitations. We model selection into the ADAMS subsample based on the published selection strata (31). We also modeled nonresponse in all four ADAMS waves. We modeled missing HRS cognitive tests and missing covariates by multiple imputation.
We estimated the model by Markov Chain Monte Carlo using 2,000,000 simulation draws, and we discarded the first 10% burn-in draws. We stored the remaining 1.8 million values of each model parameter for inference. The output of the model is shown in SI Appendix, Table S13. This model is computationally intensive, but it substantially increases the precision of the estimates compared with cross-sectional models used in the literature. We also stored 901 simulated values of latent cognition for each person-year observation to permit regression-based analyses of cognition after estimating the model. A detailed description of this method is included in SI Appendix.
The model shares similarities to the biostatistics literature on joint modeling of longitudinal and survival data (36, 37), but it is adapted to the HRS setting. To the best of our knowledge, there is no state-of-the-art biostatistics approach for modeling selection into self- and proxy-interviews in a longitudinal setting.
The primary identifying assumption is that the equations describing the relationship between dementia status and the HRS cognitive measures do not change over time. That is, conditional on latent cognition and the other predictor variables, the distribution of the cognitive measures is stable in the population. We are unaware of any reasons this assumption would be violated. The measurement of trends in dementia is primarily based on wave-to-wave differences in the averages of individuals’ performance on the HRS cognitive tests. However, the clinical diagnosis of dementia in the ADAMS subsample plays a critical role in calibrating the HRS cognitive tests to measure dementia. The model establishes the statistical relationship between dementia status and the HRS cognitive measures from that relationship observed among ADAMS participants.
Validation Tests and Comparisons with Alternative Models.
We performed multiple validation tests of our methodology. First, to test our complex Bayesian estimation model, we simulated a synthetic dataset that replicated the structure of the HRS and ADAMS. We tested whether the model recovered the coefficients used to simulate the data. The model successfully recovered the coefficient values and the prevalence of dementia by sex, age, and year with great precision (SI Appendix, Tables S14–S16).
Second, we compared the model’s prevalence estimates by age and sex with established estimates by Plassman et al. (23). Plassman et al. used the first wave of ADAMS and a complex weighting procedure to estimate the prevalence of dementia in 2001 to 2003 in the United States. SI Appendix, Table S11 shows that the prevalence estimates by Plassman et al. (23) are very similar to those obtained with our model, and the corresponding confidence intervals overlapped in almost all sex and age groups (the only exception was among 90+-y-old females). However, the CIs produced by our model are substantially narrower, which highlights the value of using a larger sample and a longitudinal model. Our estimates are also in line with published dementia prevalence estimates based on the HRS for the year 2012 (7, 8).
We further compared the predictions of our model with those obtained with the calibrated cutoff method discussed by Crimmins et al. (20), which uses cutoff values chosen to match the population prevalence of dementia in HRS 2000 and 2002 to ADAMS data (23). Because the HRS cognitive measures cannot determine dementia status with 100% accuracy, the methodology leads to some false-positive and some false-negative predictions, but the calibration of the cutoff point assures that these errors cancel out in the HRS population sample. However, the errors may not cancel in every population subgroup. In the Discussion section, we showed that the calibrated cutoff methodology overpredicted dementia prevalence among racial and ethnic minority groups, while our methodology accurately recovered these rates in all groups (SI Appendix, Table S12). Age is a very strong predictor of dementia, so it is also plausible that a different cutoff value would be appropriate for older versus younger individuals; similar arguments may apply with respect to high versus low education and other classifiers. Our model addresses this issue by allowing the relationship between dementia status and the cognitive measures to vary across population subgroups. Another issue with the cutoff methodology is that its SEs do not reflect the uncertainty due to calibration; the dementia prediction is used in analyses as if it was a directly observed data point. Our model, instead, produces SEs that take calibration uncertainty into account.
We re-estimated levels and trends in dementia prevalence by sex, age, education, and race and ethnicity using the cutoff methodology. In the total 65+-y-old population, the cutoff method yielded very similar level and trend estimates compared with our method. However, we found notable differences by age, education, and race and ethnicity. Our method produced a considerably steeper age profile of dementia than the cutoff method. Thus, our model predicted that individuals at advanced ages were more likely to have dementia than what the cutoff method predicted. After investigation, we found that this result was because age is a very strong predictor of latent cognition in our model (SI Appendix, Table S13), which matches observed patterns in ADAMS. The opposite pattern applied with respect to education and race and ethnicity. Our model predicted smaller dementia differentials between educational and racial and ethnic groups than the cutoff method as shown in SI Appendix, Figs. S3 and S4. For example, according to the cutoff method, the prevalence rate among non-Hispanic Black men was 24.8% in 2000, and for non-Hispanic white men, it was 8.6% for a difference of 16.2 ppts. Our model estimated these rates to have been 17.2% and 9.3% for a difference of 7.9 ppts. By 2016, the difference between non-Hispanic Black men and non-Hispanic white men had declined to 10.9 ppts under the cutoff method and to 3.3 ppts under our model. Thus both the levels and the rates of decline are quite different.
There appears to be more noise in the cutoff series. Among men who lack a high school education, the cutoff method produced prevalence estimates about 3 ppts higher, but at the same time, the estimates are considerably noisier. For example, between 2012 and 2014, the prevalence jumped by 3 ppts according to the cutoff method but barely changed according to our model estimates.
Overall, although our model predicted that less-educated and racial and ethnic minority individuals were more likely to have dementia, the racial-, ethnic-, and education-based differentials were not as large as what the cutoff method predicted. This finding may reconcile some conflicting results in the literature. For example, Zhu et al. (16) compared the cutoff method with Medicare diagnosis codes in different racial and ethnic groups. The two methods yielded similar predictions among White participants, but the cutoff method predicted substantially higher prevalence among Black and Hispanic individuals than what the Medicare diagnosis implied. This finding would be expected if the cutoff method overpredicted dementia prevalence in racial and ethnic minority individuals.
Supplementary Material
Acknowledgments
Research support from the National Institute on Aging under Grant R01AG053972 (to PI: M.D.H.) is gratefully acknowledged.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120393119/-/DCSupplemental.
Data, Materials, and Software Availability
Program code data have been deposited in the HRS website (https://hrsdata.isr.umich.edu/data-products/trends-inequalities-prevalence-dementia-us-replication-package) (38). All datasets used in the project are available upon registration at the HRS website.
References
- 1.Hurd M. D., Martorell P., Delavande A., Mullen K. J., Langa K. M., Monetary costs of dementia in the United States. N. Engl. J. Med. 368, 1326–1334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M., et al. , The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association, 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Patterson C., World Alzheimer Report 2018 (Alzheimer’s Disease International, 2018). [Google Scholar]
- 5.Farina M. P., Zhang Y. S., Kim J. K., Hayward M. D., Crimmins E. M., Trends in dementia prevalence, incidence, and mortality in the United States (2000-2016). J. Aging Health 34, 100–108 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasset L., et al. , Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimers Dement. 12, 272–280 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Hudomiet P., Hurd M. D., Rohwedder S., Dementia prevalence in the United States in 2000 and 2012: Estimates based on a nationally representative study. J. Gerontol. B Psychol. Sci. Soc. Sci. 73 (suppl. 1), S10–S19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langa K. M., et al. , A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern. Med. 177, 51–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson E. B., Yaffe K., Langa K. M., New insights into the dementia epidemic. N. Engl. J. Med. 369, 2275–2277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews F. E., et al. ; Medical Research Council Cognitive Function and Ageing Collaboration, A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet 382, 1405–1412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince M., et al. , Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 8, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca W. A., et al. , Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 7, 80–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satizabal C. L., et al. , Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med. 374, 523–532 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrijvers E. M. C., et al. , Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78, 1456–1463 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Wu Y. T., et al. , The changing prevalence and incidence of dementia over time - current evidence. Nat. Rev. Neurol. 13, 327–339 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y., Chen Y., Crimmins E. M., Zissimopoulos J. M., Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J. Gerontol. B Psychol. Sci. Soc. Sci. 76, 596–606 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferretti M. T., et al. ; Women’s Brain Project and the Alzheimer Precision Medicine Initiative, Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Kornblith E., et al. , Association of race and ethnicity with incidence of dementia among older adults. JAMA 327, 1488–1495 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward M. D., Farina M. P., Zhang Y. S., Kim J. K., Crimmins E. M., The importance of improving educational attainment for dementia prevalence trends from 2000 to 2014, among older non-Hispanic Black and White Americans. J. Gerontol. B Psychol. Sci. Soc. Sci. 76, 1870–1879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crimmins E. M., Kim J. K., Langa K. M., Weir D. R., Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 66 (suppl. 1), i162–i171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianattasio K. Z., Wu Q., Glymour M. M., Power M. C., Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology 30, 291–302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianattasio K. Z., Ciarleglio A., Power M. C., Development of algorithmic dementia ascertainment for racial/ethnic disparities research in the US Health and Retirement Study. Epidemiology 31, 126–133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plassman B. L., et al. , Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 29, 125–132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu C., Fratiglioni L., Aging without dementia is achievable: Current evidence from epidemiological research. J. Alzheimers Dis. 62, 933–942 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Power M. C., et al. , Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 78, 275–284 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Michigan, Health and Retirement Study, Cross-wave Tracker File, 2016, public use dataset. https://hrsdata.isr.umich.edu/data-products/public-survey-data. Accessed 6 February 2019. [Google Scholar]
- 27.University of Michigan, Health and Retirement Study, Cross-Wave Imputation of Cognitive Functioning Measures 1992-2016, Version 1, public use dataset. https://hrsdata.isr.umich.edu/data-products/public-survey-data. Accessed 15 October 2019. [Google Scholar]
- 28.Magaziner J., et al. ; Epidemiology of Dementia in Nursing Homes Research Group, The prevalence of dementia in a statewide sample of new nursing home admissions aged 65 and older: Diagnosis by expert panel. Gerontologist 40, 663–672 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K., et al. , Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 287, 2090–2097 (2002). [DOI] [PubMed] [Google Scholar]
- 30.University of Michigan, Health and Retirement Study, The Aging, Demographics, and Memory Study (ADAMS), sensitive dataset. https://hrsdata.isr.umich.edu/data-products/sensitive-health. Accessed 23 May 2019. [Google Scholar]
- 31.Heeringa S. G., et al. , Aging, Demographics and Memory Study (ADAMS): Sample Design, Weighting and Analysis for ADAMS (Institute for Social Research, University of Michigan, Ann Arbor, MI, 2009). [Google Scholar]
- 32.RAND Center for the Study of Aging. RAND HRS Longitudinal File 2016, Version 1. https://hrsdata.isr.umich.edu/data-products/rand. Accessed 7 June 2019.
- 33.RAND Center for the Study of Aging. RAND HRS Fat Files 2000-2016. https://hrsdata.isr.umich.edu/data-products/rand. Accessed 7 June 2019.
- 34.RAND Center for the Study of Aging. RAND HRS Detailed Imputation File 2016, Version 1. https://hrsdata.isr.umich.edu/data-products/rand. Accessed 25 June 2019.
- 35.Hudomiet P., Hurd M. D., Rohwedder S., The Lifetime Risk of Living with Dementia for Short and Medium Durations Based on a Longitudinal Model of Cognition Estimated on Nationally Representative U.S. Data (RAND Corporation, 2022). [Google Scholar]
- 36.Lawrence Gould A., et al. , Joint modeling of survival and longitudinal non-survival data: Current methods and issues. Report of the DIA Bayesian joint modeling working group. Stat. Med. 34, 2181–2195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey G. L., Philipson P., Jorgensen A., Kolamunnage‐Dona R., A comparison of joint models for longitudinal and competing risks data, with application to an epilepsy drug randomized controlled trial. J. R. Stat. Soc. [Ser A] 181, 1105–1123 (2018). [Google Scholar]
- 38.University of Michigan, Trends in Inequalities in the Prevalence of Dementia in the U.S. (Replication Package). https://hrsdata.isr.umich.edu/data-products/trends-inequalities-prevalence-dementia-us-replication-package. Accessed 24 October 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Program code data have been deposited in the HRS website (https://hrsdata.isr.umich.edu/data-products/trends-inequalities-prevalence-dementia-us-replication-package) (38). All datasets used in the project are available upon registration at the HRS website.