Summary
Chilo suppressalis is one of the most prevalent and damaging rice pests, causing significant economic losses each year. Chemical control is currently the primary method of controlling C. suppressalis. However, the indiscriminate use of chemical insecticides increases pest resistance, pollutes the environment and poses a significant health threat to humans and livestock, highlighting the need to find safer, more pest‐specific and more effective alternatives to pest control. Plant‐mediated RNA interference (RNAi) is a promising agricultural pest control method that is highly pest‐specific and has less of an impact on the environment. Using multi‐sgRNAs/Cas9 technology to delete Fatty acyl‐CoA reductase (FAR) of C. suppressalis in the G0 generation, we show that downregulating FAR transcription may significantly increase the mortality rate and darken the epidermis of C. suppressalis compared with the control. Subsequently, we developed dsFAR transgenic rice lines using Agrobacterium‐mediated genetic transformation and then screened three strains expressing dsFAR at high levels using transcriptional level analysis. Using transgenic rice stems, a laboratory feeding bioassay indicated that at least one line (L#10) displayed a particularly high level of insect resistance, with an insect mortality rate of more than 80%. In the field trials, dsFAR transgenic rice displayed high levels of resistance to C. suppressalis damage. Collectively, these results suggest the potential of a new environment‐friendly, species‐specific strategy for rice pest management.
Keywords: Chilo suppressalis, Fatty acyl‐CoA reductase, mortality, plant‐mediated RNAi, multi‐sgRNAs/Cas9 technology
Introduction
Worldwide, insect damage causes 13–18% loss in crops annually costing approximately US$470 billion per year in lost productivity (Culliney, 2014). Chemical insecticides are the most common and widely used method for pest control (Huang et al., 2017). However, the excessive use of insecticides has resulted in pesticide resistance, food safety issues and environment pollution (Chagnon et al., 2015). The emergence of insect‐resistant genetically modified (GM) crops has been the most revolutionary method of pest control in agriculture worldwide. Following the development of the first GM cotton expressing Bacillus thuringiensis crystal (cry) gene to control Helicoverpa armigera in the mid‐1980s (Vaeck et al., 1987), there have been considerable advancements in the production of insect‐resistant plants by incorporating cry genes into transgenic plants (Bravo et al., 2011; James, 2015). Transgenic crops with enhanced pest resistance have also been created using botanically derived Galanthus nivalis agglutinin (GNA) and protein inhibitor genes (e.g. pin II, CpTi, SKTI and BTI‐CMe) (Alfonso‐Rubí et al., 2003; Duan et al., 1996; Lee et al., 1999; Rao et al., 1998; Xu et al., 1996). However, continuous large‐scale planting of GM crops has led to the development of resistance among target pests and the rapid growth of non‐target pest populations (Gahan et al., 2001; Lu et al., 2010; Xia et al., 2021).
Plant‐mediated RNA interference (RNAi) is a post‐transcriptional double‐stranded RNA (dsRNA)‐mediated gene silencing mechanism that has demonstrated excellent potential as an effective and eco‐friendly alternative method of insect pest control (Aravin et al., 2001; Baum et al., 2007; Chung et al., 2021; Mao et al., 2007; Zhang et al., 2015). Typically, the genes targeted are those required for insect growth and development. Transgenic crops expressing target‐specific dsRNA were among the first to show insect resistance (Baum et al., 2007) and Zha et al. (2011) demonstrated the potential for field‐level control of insect pests via dsRNA‐mediated knockdown of midgut genes in planthoppers. Furthermore, transgenic plants expressing dsRNA for the moult‐regulating HR3 gene and HaHMG‐CoA reductase gene demonstrated increased resistance to cotton bollworm and inhibited bollworm oviposition, respectively (Wang et al., 2013a; Xiong et al., 2013). Studies have also found that virus‐mediated dsRNA in plants reduced Manduca sexta midgut gene transcription and reduced the reproduction of Bactericera cockerelli (Kumar et al., 2012; Wuriyanghan and Falk, 2013). An increasing number of reports demonstrate a wide use of plant‐mediated RNAi pest control in the insect orders Lepidoptera and Hemiptera (Kanakala et al., 2019; Kumar et al., 2014; Luo et al., 2017), and the first commercial transgenic dsRNA product was approved by the United States Environmental Protection Agency in 2017 (US‐EPA, 2017).
Rice (Oryza sativa) is one of the most widely consumed staple foods in the world (Subudhi et al., 2006). Increasingly, insect pests are causing severe damage to rice fields. Among these is the striped stem borer Chilo suppressalis (Walker), a well‐known pest of rice crops. Outbreaks of C. suppressalis have become widespread across China as hybrid rice planting has increased and planting systems have changed (Arbab, 2014; Chen et al., 2011). The use of chemical pesticides on rice is currently the primary method for pest control, but it has resulted in pest resistance and environmental pollution (Mao et al., 2019; Zibaee et al., 2009). This has led to the development of transgenic Bt rice lines that are significantly more resistant to the main lepidopteran rice pests (Chen et al., 2005; Tang et al., 2006; Tu et al., 2000; Zhao et al., 2014). Additionally, plant‐mediated RNAi strategy has been developed and acts as a more effective and environmentally friendly approach for C. suppressalis control. For example, transgenic rice overexpressing insect endogenous Csu‐novel‐miR15 inhibits growth and delays pupation of C. suppressalis (Jiang et al., 2017), and transgenic microRNA‐14 and Csu‐novel‐260 rice are both resistant to C. suppressalis (He et al., 2019; Zheng et al., 2021). Previous studies confirm that plant‐mediated RNAi is an effective alternative pest control strategy. However, field applications present several challenges requiring further research on the practical application of plant‐mediated RNAi for pest control.
Fatty acyl‐CoA reductases (FARs) are key enzymes involved in fatty alcohol synthesis in plants, animals and microorganisms, playing a crucial role in the biology of organisms. Studies have found that FARs are required for epicuticular wax synthesis in Arabidopsis (Rowland et al., 2006), and the production of storage wax esters in Euglena and jojoba (Metz et al., 2000; Teerawanichpan and Qiu, 2010). In insects, FAR was first reported in Bombyx mori in which it is involved in the production of the sex pheromone bombykol (Moto et al., 2003). Subsequent studies have demonstrated that FARs are also responsible for synthesizing pheromone components in other insect orders (Antony et al., 2009; Carot‐Sans et al., 2015; Lin et al., 2018). Findings to dates Antony suggest FARs may play an essential role in insect reproduction. For instance, RNAi knockdown of AsFAR and NlFARs has been found to inhibit ovary development in Adelphocoris suturalis and Nilaparvata lugens, respectively (Li et al., 2019; Luo et al., 2017). All these findings suggest that FAR plays an important role in insect growth and metabolism; however, the CsFAR and its biological function remain unclear.
In this study, we found that the level of FAR is highly expressed in the second‐instar larvae and third‐day pupae stage (the crucial stage of vitellogenesis in C. suppressalis). Silencing of CsFAR performed by RNAi had no effect in vitellogenesis, strikingly, knockout of the FAR gene significantly repressed development and increased mortality of C. suppressalis larvae. These findings suggest that using plant‐mediated RNAi shows promise as a viable method to manage pest in rice. The transgenic rice expression of CsFAR dsRNA showed a high level of resistance to C. suppressalis, providing a new efficient resistance management tool for the control of C. suppressalis.
Results
Cloning and sequence analysis of C. suppressalis FAR gene
To identify one target gene for pest control based on plant‐mediated RNAi technology, currently, we attempt to find a regulatory factor that can inhibit the growth of pest population. Previous research has linked the expression profile of the Vitellogenin (Vg) gene in C. suppressalis to variations in ovarian yolk deposition during post‐pupation (PP) phase, leading to the conclusion that the fourth day following pupation is the most important node for yolk deposition (Figure S1a,c). Hence, we performed high‐throughput RNA‐seq analysis on first‐ and fourth‐day female pupa samples to determine the potential regulators in the reproductive process of C. suppressalis. Both groups included three biological replicates and relative mRNA abundance in the first‐ and fourth‐day female pupa samples was compared according to FPKM values (Figure S1b). Compared with the first‐day female pupae, the fatty acyl‐CoA reductase (FAR) gene expression in the fourth‐day female pupae was up‐regulated, which was also consistent with the results of the qRT‐PCR (Figure S1d). The open reading frame (ORF) of FAR (GenBank: MZ781303) was amplified by a pair of specific primers, which contains a 1536 open reading frames and composes of 512 amino acid residues with a calculated molecular weight of 58.78 kDa and has a theoretical isoelectric point of 6.20. According to the prediction of conserved domain database (CDD) in NCBI, CsFAR contains two conserved domains, NAD(P)H binding region and C‐terminal region (Figure S2a). Sequence alignment and identity analysis revealed that CsFAR have the highest amino acid identity with Ostrinia nubilalis (81.0%) and Bombyx mori (75.9%) (Figure S2b). Using CsFAR protein sequence and different FAR proteins from different organisms to construct a phylogenetic tree, the result showed that CsFAR was clustered together with other moths from Noctuidae and Pyralidae (Figure S3).
Temporal and spatial expression profile of FAR gene in C. suppressalis
The transcription of CsFAR in different development stages and tissues was determined by qRT‐PCR to better understand the gene function of CsFAR. CsFAR mRNA expression in 11 different life stages, including the egg to sixth‐instar larvae and the female pupae on the first, second, third, fourth day, was investigated. The expression level of CsFAR gene was higher in the second‐instar larvae and female pupae on the third day, but lower in the egg stage (Figure 1a). CsFAR was positively correlated with the transcript abundance of reproductive gene (Vg) in PP stage, leading us to speculate that it plays a reproductive regulatory function during the PP phase. It is not unexpected given the well‐known fact that the Vg gene is mainly expressed in insect fat body and then transported to the ovary via hemolymph and absorbed by the oocytes; thus, we detected expression levels of FAR in fat body, ovary and other tissues. Our results show that CsFAR was highly expressed in the head, followed by fat body, with negligible expression in the ovary (Figure 1b).
Figure 1.
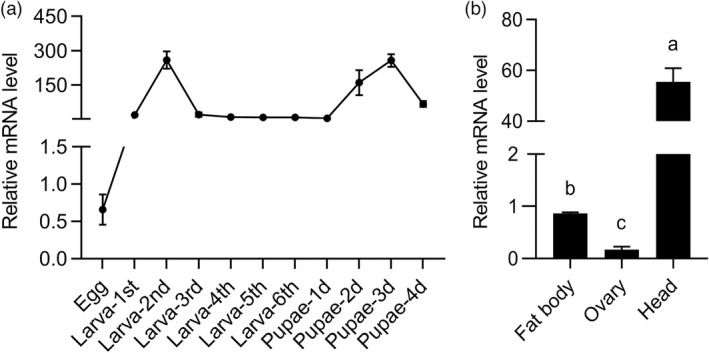
Tissue and temporal transcription analysis of CsFAR. (a) The relative expression level of CsFAR at different developmental stages of C. suppressalis. Values are represented as means ±SEM based. (b) Specific distribution of CsFAR gene in different tissues. Bars indicate the mean (±SEM) of three biological replicates. Different letters above the bars indicate significant differences, P < 0.05.
Functional identification of FAR gene in C. suppressalis vitellogenesis
In A. suturalis and N. lugens, it was reported that loss of FARs caused ovarian development and female fertility abnormalities (Li et al., 2020; Luo et al., 2017). Therefore, we examined whether CsFAR plays a role in regulating female fertility of C. suppressalis. For these studies, female pupae were injected with dsFAR or dsEGFP as a control twice at 24 h PP and 72 h PP respectively. Ovarian phenotype for CsFAR depletion was examined at 96 h PP, and interference efficiency was measured. Compared with the dsEGFP control, the transcription level of CsFAR in fat body and ovary was significantly down‐regulated, with a reduction of 89% (Figure 2b). There was no significant difference in the number of mature eggs or ovarian phenotypes (ovarian size, yolk deposition and ovarian morphology) between iFAR and iEGFP (Figure 2a,c), indicating that CsFAR knockdown had no obvious effect on ovarian development in C. suppressalis during the PP phase.
Figure 2.
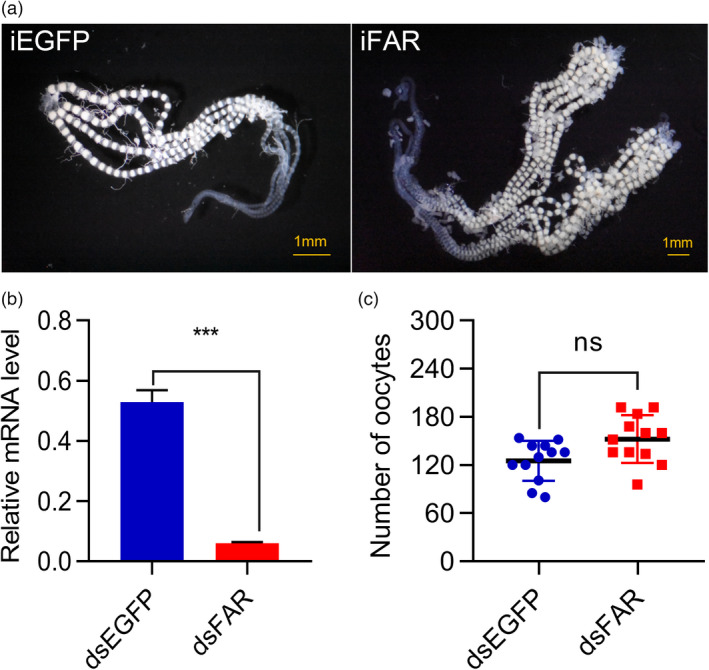
Effects of dsFAR injection on FAR gene transcription and ovarian development. (a) Ovarian phenotype after injection with dsFAR and dsEGFP were observed and photographed under a D3400 digital camera (Nikon, Tokyo, Japan). iEGFP = RNA interference for enhance green fluorescent protein; iFAR = RNA interference for FAR. (b) The CsFAR transcription level after silencing relative to the control. Error bars indicate ±SEM of three biological repeats; ***P < 0.001 (Student's t‐test). DsFAR = double‐stranded RNA for CsFAR; dsEGFP = double‐stranded RNA for EGFP. (c) Number of deposited eggs from iFAR and iEGFP female pupae. The statistical data represent 12 biological replicates and are shown as mean ± SEM.
Knockout FAR resulted in C. suppressalis lethal
FAR is a key gene involved in insect wax synthesis, sex pheromone production and epidermal construction (Teerawanichpan et al., 2010). Our next step was to determine whether the expression of CsFAR was important for other physiological functions in larval or adult stages. Gene editing technology was used to knockout CsFAR from embryonic stage to identify its function. Studies in both mammals and insects have shown that CRISPR/Cas9 system (C‐CRISPR hereafter) method can efficiently achieve complete gene knockout through simultaneous frame‐shift mutation and exon deletion to quickly identify gene function in the G0 generation using multiple sgRNAs instead of only one (Zuo et al., 2017). Therefore, we co‐injected Cas9 protein and four sgRNAs targeted exon 8 into zygotes. EGFP targeted by the same number of sgRNAs was used as a control. A total of 840 eggs for FAR and 730 eggs for EGFP were injected within 4 h after hatching, and the hatching rates of FAR‐sgRNAs injected eggs and EGFP‐sgRNAs injected eggs were 65.5% and 67.1%, respectively (Table S1).
The phenotype and behaviour of C. suppressalis were observed within 12 days after hatching. Unexpectedly, the mortality rate of the FAR‐KO group was significantly higher than that of the control group during the larval period. (Figure 3b). Furthermore, individuals with FAR‐KO showed increased blackening and transparency of the epidermis (Figure 3e). Subsequently, PCR amplification, TA cloning and DNA sequencing were performed to determine whether each dead larva in FAR‐KO group had genomic deletions (Figure 3c). Exon deletion mutations occurred in approximately 80% of dead insects (>30 bp), and each mutant carried different deletion types, including −73 bp, −87 bp and − 63 bp (Figure 3d). The transcription level of CsFAR gene in newly dead larvae with blackened epidermis or dysplasia was dramatically down‐regulated compared with the control (Figure 3f). These findings suggest that FAR mutant most likely affects the formation of the epidermis and survival of C. suppressalis in the larval stage.
Figure 3.
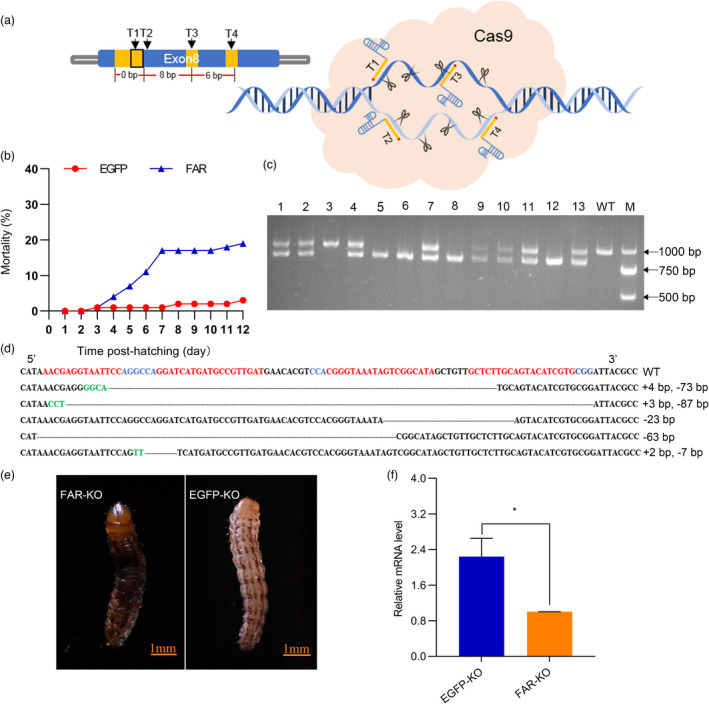
Knockout of the CSFAR gene by CRISPR/Cas9‐mediated genome editing using multiple sgRNAs. (a) Schematic diagram of C‐CRISPR technology principle and sgRNAs design of FAR gene. (b) Knockout of CsFAR led to high mortality of C. suppressalis. (c) Agarose gel electrophoresis results of PCR products from 13 mutants and one wild type. M = 2000 bp DNA marker. (d) The sequence analysis results of FAR gene from mutants and wild were obtained by TA cloning and sequencing with the sgRNA target sites (in red letters), and the PAM (protospacer adjacent motif) sequence (in blue letters). In the mutant sequences, green letters represent the inserted segment and the deletion sequence is indicated by a dashed line. The net change in sequence length is shown on the right side of each sequence (+, insertion; −, deletion). (e) Representative results of phenotypic in C. suppressalis resulting from FAR deletion at the larval stage. (f) The mRNA expression levels of FAR in the control group and the dead larvae mutants were measured using qRT‐PCR. The data shown are the mean ± SEM (n = 3). Asterisks indicate significant differences between the two groups (t‐test: *P < 0.05).
Finally, we tested whether the application of the C‐CRISPR method would result in more off‐target effects. The CasOT was used to predict potential off‐target sequences from published C. suppressalis genomic data (Ma et al., 2020). The prediction results show that sgRNA‐T1, sgRNA‐T2, sgRNA‐T3 and sgRNA‐T4 had 599, 685, 440 and 613 potential off‐target sites, respectively. We selected three off‐target sites for each sgRNA and used three random mutant gDNA as the template to detect the off‐target effects (Figure S4). Sequence phenotyping showed no mutations occurring at any of these loci.
Molecular characterization of transgenic plants
The conserved domain sequence of CsFAR was amplified as the target sequence for RNAi in rice. The pBWA(V)HS‐FAR hairpin RNA interference vector was constructed using the gateway method and was introduced into rice Zhonghua 11 (ZH11) via Agrobacterium tumefaciens‐mediated transformation (Figure 4a–f). A total of 25 regenerated T0 plants were obtained by kanamycin screening, and the positive transgenic rice plants were selected by PCR (Figure S5). To produce T1 generation transgenic rice, 12 independent T0 plant lines were transplanted into pots of soil, grown in a greenhouse and the seeds are collected. Positive PCR products were amplified from 12 T1 transgenic rice lines (Figure 5a). Besides, we found no significant difference in plant height, number of seeds per plant, panicle size or thousand‐grain weight between the control and transgenic rice (Figure S6a–e).
Figure 4.

Development of RNAi vector, genetic transformation, selection and regeneration of transgenic rice. (a) Schematic diagram of the T‐DNA region of the pBWA(v)HS‐FAR plasmid used for Agrobacterium‐mediated transformation. (b) Seed shelling and embryonic callus induction. (c) Screening of positive rice transformants using a medium containing kanamycin. (d) Somatic embryogenesis. (e) Positive transformant rooting. (f) Completely regenerated positive transgenic plants and wild‐type rice.
Figure 5.
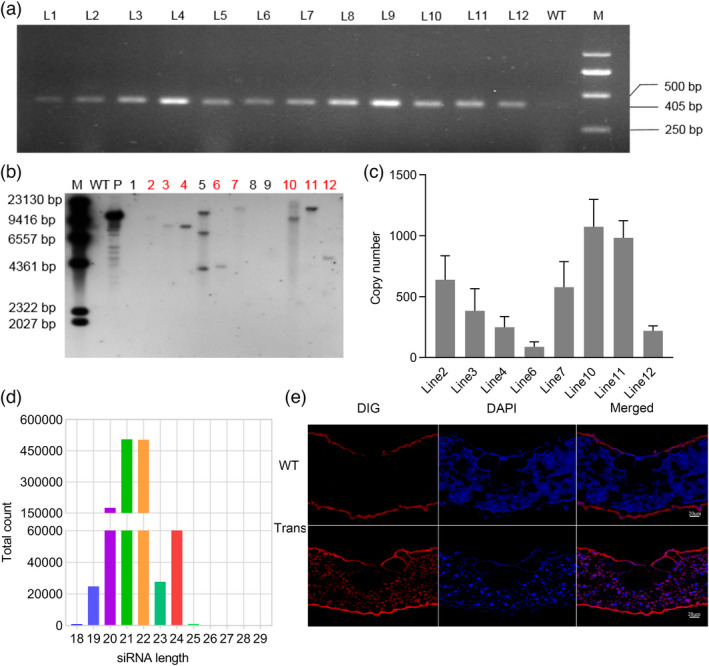
Molecular characterization of transgenic events. (a) T1 positive rice lines were identified by PCR. M = marker, 2000 bp; WT = wild‐type. (b) Southern blot analysis determined the integration of FAR transgene in the selected transgene rice lines. M = DNA molecular weight marker, P = positive control. (c) The copy number of dsFAR in leaves was determined using the absolute quantitative method. Values are represented as the mean ± SEM. (d) Length distribution and abundance of siRNAs targeting CsFAR gene in transgenic rice line. (e) Detection of siRNA in transgenic rice by FISH assay with specific probe. DAPI staining shows the nuclei. Scale bar = 20 μm.
Using southern blot analysis, absolute and relative quantitative real‐time PCR, we further determined whether the T1 rice were positive lines and whether the expression of dsFAR was stable. A digoxigenin (DIG)‐labelled probe specific to FAR fragments was used to determine the integration and number of copies of the transgene. Our results show that the FAR expression cassette had been integrated into the rice genome. Among the 12 individual plants, eight contained a single copy of T‐DNA insert, one line had multiple T‐DNA copies, and three lines had no copies of T‐DNA (Figure 5b). Absolute and relative quantitative real‐time PCR analysis was subsequently performed to measure the expression level of dsFAR in eight single‐copy transgenic lines. Three transformants (L#2, L#10 and L#11) with higher abundance of dsFAR were detected, which also corresponded with high relative expression level of dsRNA (Figures 5c and S8a). These results indicate that dsFAR was successfully integrated into rice and stably expressed. Thus, L#2, L#10 and L#11 were formulated as lines for further bioassay.
Bioinformatics analysis and visualization of siRNA
Generally, dsRNA produced by plants is cut into siRNA by the endogenous Dicer enzyme (Preall and Sontheimer, 2005). Thus, we performed high‐throughput sequencing of small RNAs from L#10 transgenic rice. The results showed that 0.001% of the total small RNAs detected were siRNAs mapped to the FAR gene, which were distributed between the 18 to 26 bp and the 21 bp siRNA was the most abundant, indicating efficient processing and production of siRNAs from the FAR hpRNA expressed in transgenic rice (Figure 5d; Table S3). The fluorescence in situ hybridization assay to determine whether siRNA was stably distributed in transgenic rice, and the fluorescent signals appeared in transgenic rice (Figures 5e and S8c). Finally, we detected that the abundance of siRNA in transgenic plant was significantly higher than that in WT by stem‐loop RT‐PCR (Figure S8b). These findings indicated that FAR dsRNA was stably expressed and successfully processed into siRNA molecules in transgenic rice plants.
Evaluation of transgenic rice for resistance to C. suppressalis
Having determined that FAR gene deletion leads to increased mortality and blackening of the epidermis of C. suppressalis, we further evaluated the resistance of dsFAR transgenic rice to C. suppressalis. The three rice lines (L#2, L#10 and L#11) showing high expression level of dsRNA were selected to feed the first‐instar larvae of C. suppressalis. Wild‐type rice was used as the control. The results showed that the mortality rate of C. suppressalis was higher on transgenic rice, especially L#10, compared with the control (Figure 6). On the eighth day of the feeding experiment, as expected, we found that approximately 80% of C. suppressali feeding on L#10 rice line were dead. The average mortality rate of C. suppressalis feeding on the L#2 and L#11 rice lines on the 8th day was 35% and 61.7%, respectively (Figure 6a). These findings indicate that of the cultivars tested, the L#10 rice line had the highest resistance to C. suppressalis, and the L#2 and L#11 rice lines had moderate resistance. We also used stem‐loop RT‐PCR to detect the abundance of siRNA in larvae fed on transgenic rice. Compared with larvae fed on wild‐type rice, larvae fed on transgenic rice significantly accumulated siRNA (Figure S9b). Moreover, RT‐PCR was performed to evaluate the effect of FAR expression level in larvae feeding on transgenic and WT plants. The results showed that the expression level of the FAR gene was significantly inhibited in larvae collected from transgenic lines (Figure 6c).
Figure 6.
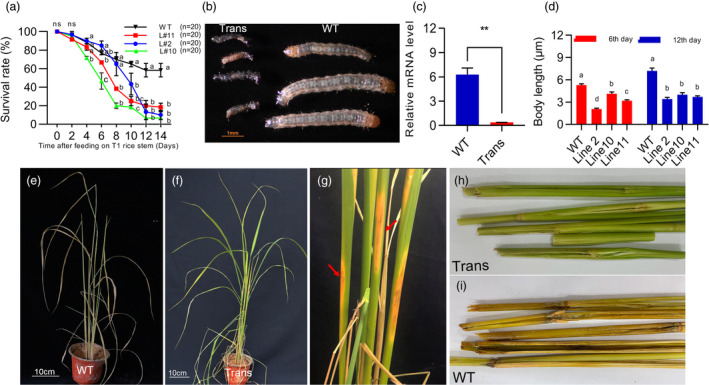
Transgenic rice lines showed high resistance to C. suppressalis larvae. (a) The survival rate of C. suppressalis in the dsFAR transgenic L#2, L#10, L#11 and control plants after 14 days of inoculation. Values are represented as the mean ± SEM and bars labelled with different lowercase letters indicate significant differences. (b) Phenotype of dead larvae after feeding on dsFAR transgenic and WT rice. (c) The silencing efficiency of CsFAR gene was evaluated by qRT‐PCR after feeding transgenic rice. (t‐test: **P < 0.01). (d) Comparison of larval body length on 6th and 12th after feeding transgenic and WT rice. Lowercase letters indicate significant differences (P < 0.05). (e–f) Comparison of damage degree between transgenic and non‐transgenic lines. (g) Local damage symptoms of C. suppressalis to transgenic rice, Red arrowhead marked local damage symptoms. (h–i) Compared with WT stems, no obvious tissue damage caused by C. suppressalis was observed in transgenic stems.
Feeding on transgenic plants resulted in larval death and blackening of the epidermis at the L2 stage (Figure 6b), which was similar to the phenotype observed for FAR‐specific knockout of C. suppressalis at the larval stage. Subsequently, we further examined the development of larvae feeding on transgenic and WT lines and found that dsFAR transgenic rice has a significant effect on the growth and development of C. suppressalis larvae (Figures 6d and S9a). Because the L#10 line showed obvious resistance to C. suppressalis in the laboratory bioassays, three wild types and three L#10 plants at the same stage of growth were selected to determine the resistance rice plants to C. suppressalis in a greenhouse environment. The level of damage caused by C suppressalis to transgenic rice within 14 days after being transferred to 30 first‐instar larvae was significantly lower than that of wild‐type rice. Furthermore, we found transgenic rice fed on by C. suppressalis only exhibited local tissue death with none of the widespread damage, such as withered heart and leaf sheaths, which is the symbol of the harm of C. suppressalis (Figure 6e–i).
Minimizing potential negative effects on non‐target insect
Plant‐mediated RNA interference typically has a high degree of specificity with minimal negative impact on non‐target insects. To assess the impact of transgenic rice on non‐target insects, we fed the same transgenic rice lines, which demonstrated strong resistance to C. suppressalis, to Sogatella furcifera (planthopper), a representative rice pest. As expected, there was no significant difference in insect damage between the transgenic rice and wild‐type rice, and the dsFAR transgenic rice had no significant effect on planthopper population or growth compared with wild‐type rice (Figure S7a–e). Our results show that the dsFAR transgenic rice has no significant effect on non‐target insects. Further research is required to determine whether transgenic rice also has similar resistance to other non‐target organisms.
Discussion
Plant‐mediated RNAi has emerged as a new pest control strategy that has the potential to reduce insect resistance caused by excessive spraying of chemical insecticides, as well as reducing the detrimental impact on the environment and human health (Christiaens et al., 2020). Transgenic plant lines such as Bt‐genes, proteinase inhibitors (Nutt et al., 1999), lectin (Zhangsun et al., 2007) and aprotinin (Christy et al., 2009) have all been shown to be highly resistant to pests. RNAi transgenic plants are also likely to face pest tolerance at some point and a single management method is unsustainable. Thus, the screening of insect target genes is crucial to achieving efficient plant‐mediated RNAi (Mamta and Rajam, 2017). At present, only a few insecticidal insect genes have been identified for the control of C. suppressalis (Tabashnik and Carriere, 2017). We found that down‐regulation of the endogenous FAR gene led to mortality and blackening of the epidermis in C. suppressalis larvae, suggesting that it is a suitable target for RNAi in pest control.
Given the importance of FAR proteins in a variety of physiological processes, we obtained a ORF of FAR from C. suppressalis and found two conservative structures, a NAD‐binding domain and a Sterile domain, which is consistent with findings in other insects, plants and even human (Li et al., 2019; Wang et al., 2021). Transcriptomic analysis showed that the transcription of FAR gene was abundant on the fourth day of the pupal stage, corresponding with Vg gene encoding during the reproductive period of C. suppressalis. Hence, our study focused on the effect of ovarian development during the PP phase. However, in contrast to the function of FAR in A. suturalis and N. lugens (Li et al., 2020; Luo et al., 2017), we found that reduction in the expression level of FAR in C. suppressalis had no effect on ovarian development. This indicates that the higher level of FAR gene expression was not related to reproduction in C. supressalis, instead it appears to be involved in other physiological and metabolic processes during the pupal stage. Based on the function of FARs reported in other insect tissues, we hypothesize that FAR plays a regulatory role in other physiological functions during the larval or adult stage, including sex pheromone production (Moto et al., 2003), cuticular hydrocarbons (CHC) formation (Li et al., 2019), lipid metabolism (Honsho et al., 2010) and wax ester biosynthesis (Rowland et al., 2006). An important finding of our study is that knockout of FAR by C‐CRISPR technology increased larval mortality in the G0 generation turning the epidermis of dead larvae black. This effect may be due to FAR‐KO directly blocking the key biological metabolic pathway during larval development, or inhibition of FAR transcription may prevent cuticular hydrocarbon (CHC) formation. Collectively, our findings highlight the importance of the FAR gene in the growth and development of C. suppressalis, and indicate the FAR gene is a promising target gene for insect resistance.
The CRISPR/Cas9 system has been used to generate genome modification in various species by directly injecting Cas9 mRNA and single‐guide RNAs (sgRNAs) into pronuclear stage zygotes, leading to a DNA double‐strand break (DSB) at a specified locus (Niu et al., 2014; Wang et al., 2013b). Nevertheless, using the traditional CRISPR/Cas9 system, a host of gene‐edited organisms show mosaicism making effective phenotypic analysis or gene deletion difficult (Jennings et al., 2016). However, by injecting multiple sgRNAs, the modified C‐CRISPR method could efficiently delete one or more genes and allow identification of gene function in the G0 generation, which may be caused by simultaneous frame‐shift mutation and exon deletion (Zuo et al., 2017). Our data demonstrate that a single gene can be knocked out of CRISPR/Cas9‐mediated C. suppressalis. Furthermore, using multiple sgRNAs demonstrates the method's effectiveness for rapidly identifying the gene function of G0 lepidoptera insects and knocking out specific genes in lepidopteran insects completely. We conducted a comprehensive off‐target analysis on insects produced by multiple sgRNAs targeting and found no obvious non‐target effect. This is consistent with previous studies where non‐target mutations are rarely found in Cas9‐modified animals (Iyer et al., 2015), and could also be due to the sgRNA, which has a low off‐target effect depending on the software selection.
Although there have been few large‐scale field applications of RNAi technology, the use of plant‐mediated RNAi to silence essential genes in pests is still being investigated as it may provide a new environmentally friendly, potentially species‐specific method of controlling pests. Some studies have shown that target gene fragments from insects are usually constructed in expression cassette, transformed into host plants and then transcribed into dsRNA in the host. Subsequently, the long transgenic dsRNA from the host genome is processed into a series of siRNAs by plant inherent Dicer‐like nuclease (DCL) (Xie et al., 2004). Previous research also has demonstrated that plant‐derived siRNA is more effective at interfering with the key genes of target insects (Bao et al., 2021). Here, we selected FAR as the target gene and successfully expressed dsFAR in transgenic rice. Furthermore, small RNA sequencing, fluorescence in situ hybridization and stem‐loop RT‐PCR results showed that dsFAR was successfully cleaved into 18–26 bp siRNAs, which was stably distributed in host plants and insect, resulting in an efficient RNAi response in C. suppressalis.
In the laboratory bioassay experiment, three rice lines with high expression level of dsFAR provided excellent control of C. suppressalis. Furthermore, field evaluation indicated that dsFAR transgenic rice could effectively prevent C. suppressalis damage. Previous studies have shown that transgenic cotton plants expressing dsAsFAR caused a significant gene silencing effect in A. suturalis, suppressing the development of populations (Luo et al., 2017). Collectively, these results suggest that dsFAR transgenic rice lines have the greatest potential for providing an alternative strategy for C. suppressalis control in terms of application. In this regard, we present preliminary evidence of the resistance of the transgenic line to C. suppressalis and its induced RNAi mechanism. To improve the RNAi effect in C. suppressalis, future research should focus on understanding the siRNA interference mechanism in greater detail and enhancing siRNA production through plant genetic engineering.
Plant‐mediated RNAi technology for pest management applications is the reliant on the availability of key insect genes that do not affect the physiological state of the host plant and are safe for humans and other non‐target organisms. In general, RNAi is less likely to damage non‐target organisms than pesticides, primarily due to the high sequence specificity of RNAi‐mediated gene expression silencing (Chung et al., 2021). Rice is one of the most important food crops globally; hence, it is essential to evaluate its agronomic characteristics as well as its impact on non‐target organisms. Here, we found no significant difference in agronomic characteristics between transgenic and wild‐type rice. We also investigated the effects of transgenic rice on the growth and development of S. furcifera. The feeding experiment showed that transgenic rice had no negative effects on the growth or reproduction of S. furcifera. Furthermore, sequence analysis showed that there were no more than 19‐21 nt nucleic acid sequences between human and CsFAR, indicating that the target for insect RNAi was highly conserved (Whyard et al., 2009). In summary, dsFAR transgenic rice may provide a safe and effective alternative strategy for the control of C. suppressalis, but a detailed safety study is needed to comply with transgenic regulations.
In conclusion, using plant‐mediated RNAi technology, we were able to produce a dsFAR transgenic rice with an excellent insect resistance. We found that inhibiting the transcription level of the FAR gene increased mortality and darkened the epidermis of C. suppressalis in the larval stage. Our findings indicate that a novel control strategy based on plant‐mediated RNAi could be used to combat this insect pest.
Methods
Insect rearing
Rice stem borer (C. suppressalis) larvae were collected from the rice fields at Hunan Agricultural University, Hunan province, China in 2017. The larvae were maintained in climate chambers at 28 ± 1 °C and 80 ± 10% relative humidity under a 16 h light: 8 h dark photoperiod. Larvae were reared on water bamboo according to the protocol described by Tang et al. (2020).
Cloning and structural analysis of CsFAR
Total RNA was isolated from pupae using TRIZOL (TaKaRa) according to the manufacturer's instructions. First‐strand cDNA was synthesized from 1 μg of total RNA using the PrimeScript RT Reagent Kit (TaKaRa) with a volume of 20 μl. Primers for amplifying the FAR protein‐coding sequence (Ma et al., 2020) were designed on NCBI Primer‐BLAST (https://www.ncbi.nlm.nih.gov/tools/primer‐blast/ ). The PCR products were purified by gel, ligated to the pEASY blunt vector (TransGen) and sequenced. NCBI CDD (https://www.ncbi.nlm.nih.gov/cdd) was used to analyse the conserved domain of FAR amino acid sequence.
Transcription analysis of CsFAR in C. suppressalis
To determine the transcription pattern of FAR for each developmental stage, a qRT‐PCR was performed on each developmental stage of C. suppressalis: egg, first‐, second‐, third‐, fourth‐, fifth‐ and sixth‐instar larvae and female pupae (at 24 h, 48 h, 72 h and 96 h after pupation). The reaction was performed with a CFX96 Touch™ Real‐Time PCR Detection System using TB Green™ Premix Ex Taq™ II (TaKaRa) in a volume of 20 μL. Primers for qRT‐PCR are given in Table S2. A minimum of three biological replicates and three technical replicates were carried out.
RNAi of CsFAR in C. suppressalis
The 432 bp fragments in the 3′ non‐coding regions of the FAR gene were used as the RNAi target sites. Double‐stranded RNA (dsRNA) was prepared using T7 RiboMAX™ Express RNAi System (Promega, Madison, WI) following the manufacturer's instructions. The dsRNA quality was evaluated by 1.2% gel electrophoresis and a spectrophotometer. Enhanced green fluorescent protein (EGFP) was used as a negative control and synthesized using the same method. Female pupae were injected twice with 2.5 μg dsRNA intra‐abdominally. After 48 h, the female pupae were dissected to observe the phenotype of the ovary. The fat bodies and ovaries were then collected, and total RNA was extracted to determine the RNAi efficiency by qRT‐PCR, and the Elongation factor gene was used as the reference gene (Qiu et al., 2017).
CRISPR/Cas9 knockout of CsFAR
To improve the chances of identification of CRISPR/Cas9‐induced gene knockouts, multiple single‐guide RNAs (sgRNAs) of target genes were injected into the embryos enabling the detection of any loss of phenotype function in the G0 generation. Four DNA fragments located in exon 8 of the FAR gene were selected as sgRNAs target sites. sgRNAs were designed using the methods described previously (Bassett and Liu, 2014), and the distance between two adjacent targets was no more than 200 bp (Figure 3a). In vitro transcription of sgRNA was performed with the MEGAscript T7 Transcription Kit (Ambion, CA) according to the manufacturer's instructions. The sgRNAs quality was tested by gel electrophoresis and stored at −80 °C.
Fresh egg masses (within 4 h of laying) were sterilized in 75% ethanol and rinsed with distilled water. Approximately 1 nL of a mixture comprising of 150 ng/μl of the four sgRNAs and 300 ng/μl Cas9 protein (Thermo Fisher Scientific,Waltham, MA, USA) was injected into each individual egg. Genomic DNA (Ezup Column Animal Genomic DNA Purification Kit, SanPrep, Shanghai, China) from dead larvae was isolated to detect mutations. PCR products were separated by gel electrophoresis, and the appearance of multiple bands indicated that a mutation had occurred. TA cloning and sequencing were used to identify the exact type of indel. We searched for potential off‐target sequences in published C. suppressalis genomic data with CasOT (Ma et al., 2020; Xiao et al., 2014) and then checked the top 3 potential off‐target (OT) sequences corresponding to each target site with PCR amplification, followed by sequencing in 3 randomly selected mutants.
Development of transgenic rice plants
A 200 bp fragment located in the FAR conserved domain was chosen for RNAi. The CsFAR fragments were amplified by two pairs of primers and cloned into pBWA(V)HS vector as a hairpin by recombination reactions to generate the RNAi vector. After sequencing, the recombinant vector was transferred into Zhonghua 11 (ZH11) rice by Agrobacterium tumefaciens‐mediated method as described previously (Jin et al., 2012). All the primers used for vector development are shown in Table S2.
Molecular analysis for the transgenic rice plants
Positive transformants were screened by PCR, Southern blot, absolute and relative quantitative real‐time PCR methods. Genomic DNA was extracted from the young leaves of transgenic and wild‐type rice using the CTAB method for PCR amplification and Southern blot analysis. To detect the transcription level of dsRNA in rice, total RNA was isolated from the young leaves of transgenic and wild‐type rice by TRIZOL according to manufacturers' directions. The cDNA was synthesized from 1 μg total RNA using the PrimeScript RT Reagent Kit (TaKaRa) and qRT‐PCR was performed as previously described. For absolute quantification, the 200 bp fragment of the FAR gene was cloned and inserted into the pMD18‐T vector. After sequencing, the plasmid was extracted as a standard plasmid and diluted at a ratio of 1:10 into 7 gradients. The diluted plasmid was used as a template for quantitative PCR to construct the standard curve, and then, the initial number of copies in the rice lines was calculated using quantitative PCR. The formula used to calculate plasmid copy number is plasmid copy number (copies/μL) = 6.02 × 1023 (copies/mol) × plasmid concentration (g/μL) / plasmid molecular weight (g/mol). Three technical replicates and three independent biological replicates were involved in each experiment. Rice U6 snRNA gene were used as the internal controls for data analysis. The primers used to detect rice are listed in Table S2.
The number of transfer DNA (T‐DNA) insertion copies in transgenic crops was investigated using southern blot analysis. Approximately 10 μg of genomic DNA was extracted from transgenic and wild‐type rice leaves at tillering stage and digested for 18 h with restriction enzyme HindIII (TaKaRa) at 37 °C. After digestion, the pBWA(V)HS‐FAR plasmid DNA positive control products were electrophoresed through 0.8% (w/v) agarose gel for 18 h at 30 V and transferred to Hybond N+ nylon membrane. The blot was hybridized at 48 °C with a DIG‐labelled probe specific to the FAR fragment sequence according to the manufacturer's instructions. The membrane was photographed with X‐ray film by exposure in a dark room at 80 °C for 4 h.
Construction of siRNA library and detection of siRNA
Small RNA libraries were generated using 1 μg of total RNA from transgenic rice leaves. The siRNA library was constructed according to the methods described previously by Bao et al. (2021). Target‐specific siRNA was screened using local BLAST, and the abundance of siRNA of different lengths in transgenic rice was analysed. The siRNA with the highest abundance in transgenic rice was selected as the probe and labelled with digoxigenin for subsequent fluorescence in situ hybridization assay, with WT rice and exogenous siRNA as a negative control (siRNA sequence is shown in Table S2). The leaves of WT and transgenic rice lines were immediately put in the fix solution (DEPC) for at least 12 h. The tissue was then dehydrated using gradient alcohol and embedded in paraffin which was subsequently sliced, dewaxed, dehydrated, digested and hybridized with the previously labelled digoxigenin probes. Finally, the slices were incubated with DAPI in the dark for 8 min and photographed with a positive fluorescence microscope. Stem‐loop RT‐PCR was performed to further confirm the effectiveness of siRNA expression in transgenic rice and whether it has successfully entered the larval body. The abundance of siRNA was detected according to the protocol of Jiang et al. (2017). The C. suppressalis 18 s rRNA and rice U6 snRNA genes were used as the reference gene.
Survival rate assay
Insect feeding and bioassays
Target (RSB) feeding test
To observe the resistance of dsFAR transgenic rice to C. suppressalis, three rice lines with a high dsFAR expression level were selected to feed C. suppressalis larvae under laboratory conditions. The stem of the rice plant was cut into 15 cm segments and inoculated with 20 newly hatched larvae and kept in a glass tube with wet cotton. There were six replicates for each rice line. Plant damage and larval survival rate were observed every 2 days. In the field trials, three transgenic and three wild‐type rice lines were inoculated with 30 first‐instar larvae and fed continuously for 14 days, and the damage degree was observed and recorded every 2 days. The body length of larvae was measured on the sixth and 12th day following inoculation, after which the larvae were collected for qRT‐PCR analysis.
Non‐target (BPH) feeding test
To evaluate the effects of transgenic rice on other non‐target insects, the planthopper was fed with transgenic and wild‐type rice. When the transgenic rice reached the tillering stage, the single plant rice was transferred to the cup and placed in an insect cage (10 cm long, 10 cm wide and 80 cm high). Sixteen planthoppers were randomly selected and released on each transgenic and control plant. The growth and survival rates of the planthopper were observed and recorded for 20 days.
Data analysis
All qRT‐PCR data were analysed using the 2−▵▵Ct method. The Student's t‐test was used to evaluate the statistical significance of the difference in gene expression between the treatment and control groups (*P < 0.05, **P < 0.01, ***P < 0.001), and all data were expressed as the means ± standard error (SE). Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA).
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
Y.Z.L. and L.Q. contributed to the experimental design and supervised the study. Q.Z.H. and Q.G. contributed to rice planting and insect feeding. Y.J.S. involved in gene editing experiment. H.L.H. and Y.W.G. involved in construction of transgenic rice. J.X. and S.J.K. involved in bioassay experiment. Y.J.S., Q.G. and W.B.D. analysed the data. L.Q and Y.J.S. involved in writing—review and editing.
Supporting information
Figure S1 Ovarian phenotype and expression profiles of CsFAR and CsVg on 1st and 4th day after pupation.
Figure S2 Structural analysis and protein sequence alignment.
Figure S3 A bootstrapped neighbour‐joining tree illustrating the phylogenetic relationships of the FAR gene in different taxa.
Figure S4 Potential off‐target sites of sgRNAs targeting FAR gene.
Figure S5 T0 positive rice lines were identified by PCR.
Figure S6 Statistics of agronomic characters of transgenic rice and wild‐type rice.
Figure S7 Safety evaluation of transgenic rice to non‐target insects (Sogatella furcifera, planthopper).
Figure S8 Molecular identification of transgenic rice.
Figure S9 Transgenic rice affects the development of C. suppressalis.
Table S1 Statistics on FAR mutation mediated by the C‐CRISPR system.
Table S2 Primers used in this study.
Table S3 The siRNA sequence information.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31801736), the Hunan Provincial Department of Education Project (Grant No. 18B096), the Changsha Municipal Natural Science Foundation (kp2007019) and the Double first‐class construction project of Hunan Agricultural University (SYL2019029).
Contributor Information
Youzhi Li, Email: liyouzhi@hunau.edu.cn.
Lin Qiu, Email: qiulin@hunau.edu.cn.
References
- Alfonso‐Rubí, J. , Ortego, F. , Castañera, P. , Carbonero, P. and Díaz, I. (2003) Transgenic expression of trypsin inhibitor CMe from barley in indica and japonica rice, confers resistance to the rice weevil Sitophilus oryzae. Transgenic Res. 12, 23–31. [DOI] [PubMed] [Google Scholar]
- Antony, B. , Fujii, T. , Moto, K. , Matsumoto, S. , Fukuzawa, M. , Nakano, R. , Tatsuki, S. et al. (2009) Pheromone‐gland‐specific fatty‐acyl reductase in the adzuki bean borer, Ostrinia scapulalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 39, 90–95. [DOI] [PubMed] [Google Scholar]
- Aravin, A.A. , Naumova, N.M. , Tulin, A.V. , Vagin, V.V. , Rozovsky, Y.M. and Gvozdev, V.A. (2001) Double‐stranded RNA‐mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Arbab, A. (2014) Spatial distribution and minimum sample size for overwintering larvae of the rice stem borer Chilo suppressalis (Walker) in Paddy Fields. Neotrop. Entomol. 43, 415–420. [DOI] [PubMed] [Google Scholar]
- Bao, W. , Li, A. , Zhang, Y. , Diao, P. , Zhao, Q. , Yan, T. , Zhou, Z. et al. (2021) Improvement of host‐induced gene silencing efficiency via polycistronic‐tRNA‐amiR expression for multiple target genes and characterization of RNAi mechanism in Mythimna separata. Plant Biotechnol. J. 19, 1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett, A. and Liu, J.L. (2014) CRISPR/Cas9 mediated genome engineering in Drosophila . Methods 69, 128–136. [DOI] [PubMed] [Google Scholar]
- Baum, J.A. , Bogaert, T. , Clinton, W. , Heck, G.R. , Feldmann, P. , Ilagan, O. , Johnson, S. et al. (2007) Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 25, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Bravo, A. , Likitvivatanavong, S. , Gill, S.S. and Soberón, M. (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carot‐Sans, G. , Muñoz, L. , Piulachs, M.D. , Guerrero, A. and Rosell, G. (2015) Identification and characterization of a fatty acyl reductase from a Spodoptera littoralis female gland involved in pheromone biosynthesis. Insect Mol. Biol. 24, 82–92. [DOI] [PubMed] [Google Scholar]
- Chagnon, M. , Kreutzweiser, D. , Mitchell, E.A. , Mitchell, E.A. , Morrissey, C.A. , Noome, D.A. , Noome, D.A. et al. (2015) Risks of large‐scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. Int. 22, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Tang, W. , Xu, C.G. , Li, X.H. , Lin, Y.J. and Zhang, Q.F. (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor. Appl. Genet. 111, 1330–1337. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Shelton, A. and Ye, G.Y. (2011) Insect‐resistant genetically modified rice in China: from research to commercialization. Annu. Rev. Entomol. 56, 81–101. [DOI] [PubMed] [Google Scholar]
- Christiaens, O. , Whyard, S. , Vélez, A.M. and Smagghe, G. (2020) Double‐stranded RNA technology to control insect pests: current status and challenges. Front. Plant Sci. 11, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy, L.A. , Arvinth, S. , Saravanakumar, M. , Kanchana, M. , Mukunthan, N. , Srikanth, J. , Thomas, G. et al. (2009) Engineering sugarcane cultivars with bovine pancreatic trypsin inhibitor (aprotinin) gene for protection against top borer (Scirpophaga excerptalis Walker). Plant Cell Rep. 28, 175–184. [DOI] [PubMed] [Google Scholar]
- Chung, S.H. , Feng, H. and Jander, G. (2021) Engineering pest tolerance through plant‐mediated RNA interference. Curr. Opin. Plant Biol. 60, 102029. [DOI] [PubMed] [Google Scholar]
- Culliney, T.W. (2014) Crop losses to arthropods. In Integrated Pest Management: Pesticide Problems ( Pimentel, D. and Peshin, R. , eds), pp. 201–225. Dordrecht, The Netherlands: Springer. [Google Scholar]
- Duan, X.L. , Li, X.G. , Xue, Q.Z. , AboElSaad, M. , Xu, D.P. and Wu, R. (1996) Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat. Biotechnol. 14, 494–498. [DOI] [PubMed] [Google Scholar]
- Gahan, L.J. , Gould, F. and Heckel, D.G. (2001) Identification of a gene associated with Bt resistance in Heliothis virescens . Science 293, 857–860. [DOI] [PubMed] [Google Scholar]
- He, K. , Xiao, H. , Sun, Y. , Ding, S. , Situ, G. and Li, F. (2019) Transgenic microRNA‐14 rice shows high resistance to rice stem borer. Plant Biotechnol. J. 17, 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsho, M. , Asaoku, S. and Fujiki, Y. (2010) Posttranslational regulation of fatty Acyl‐CoA reductase 1, FAR1, controls ether glycerophospholipid synthesis. J. Biol. Chem. 285, 8537–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Chen, Q. , Qin, W. , Sun, Y. and Qin, H. (2017) Resistance monitoring of four insecticides and a description of an artificial diet incorporation method for Chilo suppressalis (Lepidoptera: Crambidae). J. Econ. Entomol. 110, 2554–2561. [DOI] [PubMed] [Google Scholar]
- Iyer, V. , Shen, B. , Zhang, W. , Hodgkins, A. , Keane, T. , Huang, X. and Skarnes, W.C. (2015) Off‐target mutations are rare in Cas9‐modified mice. Nat. Methods 12, 479. [DOI] [PubMed] [Google Scholar]
- James, C. (2015) Global Status of Commercialized Biotech/GM Crops: 2015 ISAAA Brief no. 51. Ithaca, NY: ISAAA. [Google Scholar]
- Jennings, C.G. , Landman, R. , Zhou, Y. , Sharma, J. , Hyman, J. , Movshon, J.A. et al. (2016) Opportunities and challenges in modeling human brain disorders in transgenic primates. Nat. Neurosci. 19, 1123–1130. [DOI] [PubMed] [Google Scholar]
- Jiang, S. , Wu, H. , Liu, H. , Zheng, J. , Lin, Y. and Chen, H. (2017) The overexpression of insect endogenous small RNAs in transgenic rice inhibits growth and delays pupation of striped stem borer (Chilo suppressalis). Pest Manag. Sci. 73, 1453–1461. [DOI] [PubMed] [Google Scholar]
- Jin, S.X. , Liu, G.Z. , Zhu, H.G. , Yang, X.Y. and Zhang, X.L. (2012) Transformation of upland cotton (Gossypium hirsutum L.) with gfp gene as a visual marker. J. Integr. Agric. 11, 910–919. [Google Scholar]
- Kanakala, S. , Kontsedalov, S. , Lebedev, G. and Ghanim, M. (2019) Plant‐Mediated silencing of the Whitefly Bemisia tabaci Cyclophilin B and heat shock protein 70 impairs insect development and virus transmission. Front. Physiol. 10, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. , Pandit, S.S. and Baldwin, I.T. (2012) Tobacco rattle virus vector: a rapid and transient means of silencing Manduca sexta genes by plant mediated RNA interference. PLoS One 7, e31347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. , Pandit, S.S. , Steppuhn, A. and Baldwin, I.T. (2014) Natural history‐driven, plant‐mediated RNAi‐based study reveals CYP6B46's role in a nicotine‐mediated antipredator herbivore defense. Proc. Natl. Acad. Sci. U. S. A. 111, 1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.I. , Lee, S.H. , Koo, J.C. , Chun, H.J. , Lim, C.O. , Mun, J.H. , Song, Y.H. et al. (1999) Soybean Kunitz trypsin inhibitor (SKTI) confers resistance to the brown planthopper (Nilaparvata lugens Stal) in transgenic rice. Mol Breed. 5, 1–9. [Google Scholar]
- Li, D.T. , Chen, X. , Wang, X.Q. and Zhang, C.X. (2019) FAR gene enables the brown planthopper to walk and jump on water in paddy field. Sci. China Life Sci. 62, 1521–1531. [DOI] [PubMed] [Google Scholar]
- Li, D.T. , Dai, Y.T. , Chen, X. , Wang, X.Q. , Li, Z.D. , Moussian, B. and Zhang, C.X. (2020) Ten fatty acyl‐CoA reductase family genes were essential for the survival of the destructive rice pest, Nilaparvata lugens . Pest Manag Sci. 76, 2304–2315. [DOI] [PubMed] [Google Scholar]
- Lin, X. , Wang, B. and Du, Y. (2018) Key genes of the sex pheromone biosynthesis pathway in female moths are required for pheromone quality and possibly mediate olfactory plasticity in conspecific male moths in Spodoptera litura . Insect Mol. Biol. 27, 8–21. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Wu, K. , Jiang, Y. , Xia, B. , Li, P. , Feng, H. , Wyckhuys, K.A. et al. (2010) Mirid bug outbreaks in multiple crops correlated with wide‐scale adoption of Bt cotton in China. Science 328, 1151–1154. [DOI] [PubMed] [Google Scholar]
- Luo, J. , Liang, S. , Li, J. , Xu, Z. , Li, L. , Zhu, B. , Li, Z. et al. (2017) A transgenic strategy for controlling plant bugs (Adelphocoris suturalis) through expression of double‐stranded RNA homologous to fatty acyl‐coenzyme A reductase in cotton. New Phytol. 21, 1173–1185. [DOI] [PubMed] [Google Scholar]
- Ma, W. , Zhao, X. , Yin, C. , Jiang, F. , Du, X. , Chen, T. , Zhang, Q. et al. (2020) A chromosome‐level genome assembly reveals the genetic basis of cold tolerance in a notorious rice insect pest, Chilo suppressalis . Mol Ecol Resour. 20, 268–282. [DOI] [PubMed] [Google Scholar]
- Mamta, B. and Rajam, M.V. (2017) RNAi technology: a new platform for crop pest control. Physiol. Mol. Biol. Plants 23, 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y.B. , Cai, W.J. , Wang, J.W. , Hong, G.J. , Tao, X.Y. , Wang, L.J. , Huang, Y.P. et al. (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant‐mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 25, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Mao, K.K. , Li, W.H. , Liao, X. , Liu, C.Y. , Qin, Y. and Ren, Z.J. (2019) Dynamics of Insecticide Resistance in Different Geographical Populations of Chilo suppressalis (Lepidoptera: Crambidae) in China 2016‐2018. J. Econ. Entomol. 112, 1866–1874. [DOI] [PubMed] [Google Scholar]
- Metz, J.G. , Pollard, M.R. , Anderson, L. , Hayes, T.R. and Lassner, M.W. (2000) Purification of a jojoba embryo fatty acyl‐coenzyme A reductase and expression of its cDNA in high erucic acid rapeseed. Plant Physiol. 122, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moto, K. , Yoshiga, T. , Yamamoto, M. , Takahashi, S. , Okano, K. , Ando, T. , Nakata, T. et al. (2003) Pheromone gland‐specific fatty‐acyl reductase of the silkmoth, Bombyx mori . Proc. Natl. Acad. Sci. U. S. A. 100, 9156–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, Y. , Shen, B. , Cui, Y. , Chen, Y. , Wang, J. , Wang, L. , Kang, Y. et al. (2014) Generation of gene‐modified cynomolgus monkey via Cas9/RNA‐mediated gene targeting in one‐cell embryos. Cell 156, 836–843. [DOI] [PubMed] [Google Scholar]
- Nutt, K.A. , Allsopp, P.G. , Mcghie, T.K. , Shepherd, K.M. and Hogarth, D.M. (1999) Transgenic sugarcane with increased resistance to canegrubs. Proc. Aust. Soc. Sugar Cane Technol. 21, 171–176. [Google Scholar]
- Preall, J.B. and Sontheimer, E.J. (2005) RNAi: RISC gets loaded. Cell 123, 543–545. [DOI] [PubMed] [Google Scholar]
- Qiu, L. , Fan, J. , Zhang, B. , Liu, L. , Wang, X. and Lei, C. (2017) RNA interference knockdown of aminopeptidase N genes decrease the susceptibility of Chilo suppressalis larvae to Cry1Ab/Cry1Ac and Cry1Ca‐expressing transgenic rice. J. Invertebr. Pathol. 145, 9–12. [DOI] [PubMed] [Google Scholar]
- Rao, K.V. , Rathore, K.S. , Hodges, T.K. , Fu, X. , Stoger, E. , Sudhakar, D. , Williams, S. et al. (1998) Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J. 15, 469–477. [DOI] [PubMed] [Google Scholar]
- Rowland, O. , Zheng, H. , Hepworth, S.R. , Lam, P. , Jetter, R. and Kunst, L. (2006) CER4 encodes an alcohol‐forming fatty acyl‐coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol. 142, 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi, P.K. , Sasaki, T. , Khush, G.S. and Chittaranjan, K. (2006) Rice. In Genome Mapping and Molecular Breeding in Plants, Vol. 1 ( Cereals, K. and Millets, C. , eds), pp. 1–78. Berlin Heidelberg: Springer‐Verlag. [Google Scholar]
- Tabashnik, B.E. and Carriere, Y. (2017) Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 35, 926–935. [DOI] [PubMed] [Google Scholar]
- Tang, W. , Chen, H. , Xu, C. , Li, X. , Lin, Y. and Zhang, Q. (2006) Development of insect‐resistant transgenic indica rice with a synthetic cry1C* gene. Mol Breed. 18, 1–10. [Google Scholar]
- Tang, Y. , He, H. , Qu, X. , Cai, Y. , Ding, W. , Qiu, L. and Li, Y. (2020) RNA interference‐mediated knockdown of the transcription factor Krüppel homologue 1 suppresses vitellogenesis in Chilo suppressalis . Insect Mol. Biol. 29, 183–192. [DOI] [PubMed] [Google Scholar]
- Teerawanichpan, P. and Qiu, X. (2010) Fatty acyl‐CoA reductase and wax synthase from Euglena gracilis in the biosynthesis of medium‐chain wax esters. Lipids 45, 263–273. [DOI] [PubMed] [Google Scholar]
- Teerawanichpan, P. , Robertson, A.J. and Qiu, X. (2010) A fatty acyl‐CoA reductase highly expressed in the head of honey bee (Apis mellifera) involves biosynthesis of a wide range of aliphatic fatty alcohols. Insect Biochem. Mol. Biol. 40, 641–649. [DOI] [PubMed] [Google Scholar]
- Tu, J. , Zhang, G. , Datta, K. , Xu, C. , He, Y. , Zhang, Q. , Khush, G.S. et al. (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis delta‐endotoxin. Nat. Biotechnol. 18, 1101–1104. [DOI] [PubMed] [Google Scholar]
- US‐EPA (2017) Notice of Pesticide Registration. EPA Reg. No. 524–618, MON 87411. OPP Decision No. 533309 (Agency, U.S.E.P. eds). Washington, D.C.: Environmental Protection Agency EPA. [Google Scholar]
- Vaeck, M. , Reynaerts, A. , Hofte, H. , Jansens, S. , Debeuckeleer, M. , Dean, C. , Zabeau, M. et al. (1987) Transgenic plants protected from insect attack. Nature 328, 33–37. [Google Scholar]
- Wang, H. , Yang, H. , Shivalila, C.S. , Dawlaty, M.M. , Cheng, A.W. , Zhang, F. and Jaenisch, R. (2013a) One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.J. , Dong, Y.C. , Desneux, N. and Niu, C.Y. (2013b) RNAi silencing of the HaHMG‐CoA reductase gene inhibits oviposition in the Helicoverpa armigera cotton bollworm. PLos One 8, e67732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Xu, J. , He, Z. , Hu, N. , Luo, W. , Liu, X. , Shi, X. et al. (2021) BdFAR4, a root‐specific fatty acyl‐coenzyme A reductase, is involved in fatty alcohol synthesis of root suberin polyester in Brachypodium distachyon. Plant J. 106, 1468–1483. [DOI] [PubMed] [Google Scholar]
- Whyard, S. , Singh, A.D. and Wong, S. (2009) Ingested double‐stranded RNAs can act as species‐specific insecticides. Insect Biochem. Mol. Biol. 39, 824–832. [DOI] [PubMed] [Google Scholar]
- Wuriyanghan, H. and Falk, B.W. (2013) RNA interference towards the potato psyllid, Bactericera cockerelli, is induced in plants infected with recombinant tobacco mosaic virus (TMV). PLoS One 8, e66050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, J. , Guo, Z. , Yang, Z. , Han, H. , Wang, S. , Xu, H. , Yang, X. et al. (2021) Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 184, 3588. [DOI] [PubMed] [Google Scholar]
- Xiao, A. , Cheng, Z. , Kong, L. , Zhu, Z. , Lin, S. , Gao, G. and Zhang, B. (2014) CasOT: a genome‐wide Cas9/gRNA off‐target searching tool. Bioinformatics 30, 1180–1182. [DOI] [PubMed] [Google Scholar]
- Xie, Z. , Johansen, L.K. , Gustafson, A.M. , Kasschau, K.D. , Lellis, A.D. , Zilberman, D. , Jacobsen, S.E. et al. (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2, e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Zeng, H. , Zhang, Y. , Xu, D. and Qiu, D. (2013) Silencing the HaHR3 gene by transgenic plant‐mediated RNAi to disrupt Helicoverpa armigera development. Int. J. Biol. Sci. 9, 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D.P. , Xue, Q.Z. , McElroy, D. , Mawal, Y. , Hilder, V.A. and Wu, R. (1996) Constitutive expression of a cowpea trypsin inhibitor gene, CpTi, in transgenic rice plants confers resistance to two major rice insect pests. Mol Breed. 2, 167–173. [Google Scholar]
- Zha, W. , Peng, X. , Chen, R. , Du, B. , Zhu, L. and He, G. (2011) Knockdown of midgut genes by dsRNA‐transgenic plant‐mediated RNA interference in the hemipteran insect Nilaparvata lugens . PLoS One 6, e20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Khan, S.A. , Hasse, C. , Ruf, S. , Heckel, D.G. and Bock, R. (2015) Pest control. Full crop protection from an insect pest by expression of long double‐stranded RNAs in plastids. Science 347, 991–994. [DOI] [PubMed] [Google Scholar]
- Zhangsun, D. , Luo, S. , Chen, R. and Tang, K. (2007) Improved agrobacterium‐mediated genetic transformation of GNA transgenic sugarcane. Biologia 62, 386–393. [Google Scholar]
- Zhao, Q. , Liu, M. , Tan, M. , Gao, J. and Shen, Z. (2014) Expression of Cry1Ab and Cry2Ab by a polycistronic transgene with a self‐cleavage peptide in rice. PLoS One. 9, e110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Weng, Z. , Li, H. , Kong, Z. , Zhou, Z. , Li, F. , Ma, W. et al. (2021) Transgenic rice overexpressing insect endogenous microRNA Csu‐novel‐260 is resistant to striped stem borer under field conditions. Plant Biotechnol. J. 19, 421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibaee, A. , Sendi, J.J. , Ghadamyari, M. , Alinia, F. and Etebari, K. (2009) Diazinon resistance in different selected strains of Chilo suppressalis (Lepidoptera: Crambidae) in Northern Iran. J. Econ. Entomol. 102, 1189–1196. [DOI] [PubMed] [Google Scholar]
- Zuo, E. , Cai, Y.J. , Li, K. , Wei, Y. , Wang, B.A. , Sun, Y. , Liu, Z. et al. (2017) One‐step generation of complete gene knockout mice and monkeys by CRISPR/Cas9‐mediated gene editing with multiple sgRNAs. Cell Res. 27, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Ovarian phenotype and expression profiles of CsFAR and CsVg on 1st and 4th day after pupation.
Figure S2 Structural analysis and protein sequence alignment.
Figure S3 A bootstrapped neighbour‐joining tree illustrating the phylogenetic relationships of the FAR gene in different taxa.
Figure S4 Potential off‐target sites of sgRNAs targeting FAR gene.
Figure S5 T0 positive rice lines were identified by PCR.
Figure S6 Statistics of agronomic characters of transgenic rice and wild‐type rice.
Figure S7 Safety evaluation of transgenic rice to non‐target insects (Sogatella furcifera, planthopper).
Figure S8 Molecular identification of transgenic rice.
Figure S9 Transgenic rice affects the development of C. suppressalis.
Table S1 Statistics on FAR mutation mediated by the C‐CRISPR system.
Table S2 Primers used in this study.
Table S3 The siRNA sequence information.


