Abstract
OBJECTIVE
To quantify the effect of early rescue surfactant administration techniques for preterm infants with respiratory distress syndrome (RDS) from a health care delivery system perspective.
METHODS
A cost-consequence model was developed based on previously published literature to compare the health economic impact of implementing early surfactant administration strategies vs standard surfactant administration via endotracheal intubation and mechanical ventilation (MV).
RESULTS
Early rescue surfactant treatment strategies are associated with a decrease in the number of patients requiring MV, cumulative MV days, and rate of neonatal complications. Total annual surfactant costs are higher than standard surfactant administration, but this is offset by savings in total hospital and complication costs.
CONCLUSIONS
This cost-consequence analysis suggests selective early rescue surfactant administration strategies are associated with a lower health care burden in premature infants with RDS.
Keywords: bronchopulmonary dysplasia, cost-consequence analysis, health economics, intubate surfactant extubate, less invasive surfactant administration, premature infants, respiratory distress syndrome
Introduction
Approximately 10.2% of infants in the United States are born prematurely.1 Respiratory distress syndrome (RDS) is the most common respiratory morbidity in preterm infants.2,3 RDS occurs in about 1% of newborn infants and is the leading cause of death in premature infants, contributing 2% of all infant deaths in the United States in 2013.3,4 Additionally, more than 50% of preterm infants under 28 weeks of gestational age develop RDS.3 Noninvasive ventilation (NIV), such as continuous positive airway pressure (CPAP), is typically the first-line treatment for RDS.5 Standard care for patients with RDS who are unable to be supported with NIV is a transition to mechanical ventilation (MV) via an endotracheal tube and giving of surfactant.6,7 However, MV can be associated with long-term complications (such as bronchopulmo-nary dysplasia [BPD], severe intraventricular hemorrhage [IVH], and retinopathy of prematurity [ROP]) and short-term complications (such as pneumothorax),2,8 which potentially lead to increased costs.
In RDS treatment, early rescue surfactant administration strategies are aimed at reducing exposure to MV and involve treating for RDS early in the course of the disease, when the oxygen requirement is still relatively low to maintain acceptable oxygen saturation. Commonly, early rescue surfactant administration will be executed when the fraction of inspired oxygen (FiO2) requirement levels are less than 50%.
Early selective surfactant administration techniques include intubation-surfactant-extubation (INSURE) procedure and less invasive surfactant administration (LISA).2 With INSURE, patients are intubated to administer surfactant and extubated rapidly to avoid prolonged MV. LISA is a technique that involves the placement of a thin catheter into the trachea for surfactant administration while infants are breathing spontaneously. This approach permits infants to remain on NIV during the procedure and does not require the placement of an endotracheal tube.
Historically, infants who did not require an endotracheal tube and MV would also not receive surfactant. It is predictable that early rescue techniques may see higher rates of surfactant use, but studies have shown avoidance of MV and reduction of duration of MV and complications.8,9 Therefore, we hypothesize that early rescue surfactant administration may generate higher surfactant costs, while it is associated with improved clinical outcomes and cost savings.
The objective of this analysis is to use a health economic model built with recent applicable literature and data to estimate, from a health care delivery system perspective, the clinical and economic effects of early rescue surfactant administration versus standard (conventional) surfactant administration in infants with RDS.
Methods
To assess the potential differences in costs and clinical outcomes of treating infants with RDS using selective early rescue surfactant administration and standard methods at varying FiO2 thresholds, we developed a decision analytic model based on current clinical practices (Figure 1). The model simulates 100 infants in each strategy to reflect a single hypothetical hospital's annual volume of preterm infants with RDS.
Figure 1.
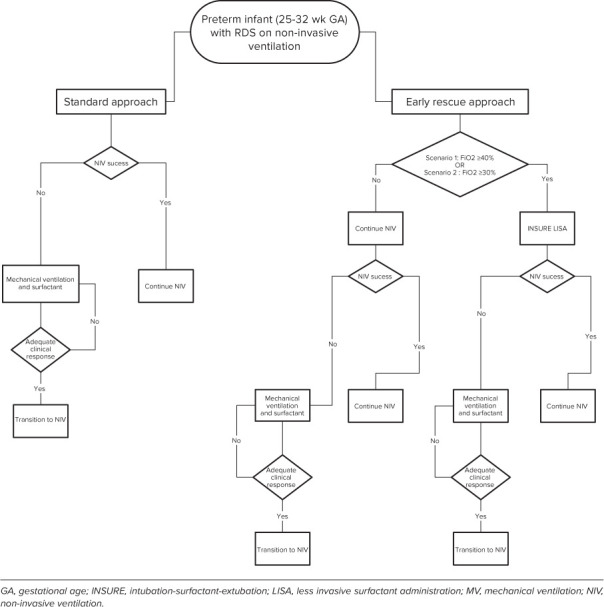
Conceptual model framework of standard vs early selective surfactant administration
Model Structure. As illustrated in Figure 1, RDS patients could undergo 3 possible scenarios: a base case and 2 alternative scenarios. Patients proceed through the model until they are discharged from the neonatal intensive care unit or deceased. In the base case (standard method), all patients received NIV support after birth. During the course of a patient's treatment, and based on literature used for standard care, clinicians followed the protocol of FiO2 requirement exceeding threshold (40%–60%), patients were placed on MV (considered NIV failure in this analysis), and then administered surfactant via endotracheal tube.10 Infants who remained intubated were administered up to 2 repeat surfactant doses when clinically required. Infants who remained clinically stable were supported non-invasively without surfactant therapy.
In the alternative scenarios treatment was standardized to a protocol where selective early rescue strategy would be initiated when FiO2 levels after birth met the predetermined target thresholds: scenario 1, FiO2 = 40%; scenario 2, FiO2 = 30%. In all scenarios, INSURE and LISA techniques were included in the model as early rescue options. The model can analyze different ratios of INSURE and LISA use. Based on contemporary survey data on clinical practices,11–13 we assumed INSURE was used for 70% of patients, and LISA was used for the other 30% of early rescue patients. Initial surfactant dose was administered via INSURE or LISA technique. After surfactant treatment, infants transitioned to or remained on NIV. If their FiO2 requirements remained stable, under the target threshold, they remained on NIV. If patients had persisting or worsening respiratory status or required MV, up to 2 repeat doses of surfactant could be administered at 12-hour intervals.
As a simplifying model assumption, CUROSURF (poractant alfa) was the assumed surfactant in all treatment strategies.
Population. Our population of interest was preterm infants, born at 25 to 32 weeks of gestation, weighing between 500 and 2000 g. The population was stratified by gestational age (25–28 weeks or 29–32 weeks) and 100-g subgroups for birth weight. The distribution of each weight band for each gestational age category was calculated based on the CDC WONDER database.1 Average weight was used for each weight subgroup in order to calculate surfactant dose. Furthermore, only infants who were stabilized on NIV in the delivery room and were spontaneously breathing were included in this model.
Clinical Inputs. Patient demographics based on birth weight and gestational age, patient management modalities, poractant alfa dosing specifications, complication rates, and hospitalization costs were derived from the literature14 and prescribing information.
Various FiO2 thresholds were assessed using different scenarios; therefore, NIV failure and success rates differed across scenarios accordingly. Base case NIV success and failure rates were derived from Dargaville et al10 and weighted according to the age distribution reported by CDC.1 NIV success and failure rates were compared for two different FiO2 levels. The NIV failure rates were informed using the sensitivity and specificity receiver operating characteristic curve for the 2 gestational ranges from Dargaville et al.10
According to the dosing instructions for poractant alfa,15 the initial dose is given at 2.5 mL/kg birth weight, and up to 2 repeat doses of 1.25 mL/kg birth weight could be administered (Table 1). Total doses were calculated based on the assumed average weight for each birth weight subgroup.15
Table 1.
Clinical Inputs for the Model to Evaluate Rate Complications With Different Surfactant Scenarios
| Key Clinical Inputs | Gestational Age, wk | LISA, % | INSURE, % | NIV Success, % | NIV Failure, % |
|---|---|---|---|---|---|
| Bronchopulmonary dysplasia | 25–28 | 13.40 | 16.80 | 14.20 | 40.30 |
| 29–32 | 1.70 | 2.20 | 2.60 | 3.00 | |
| Severe intraventricular hemorrhage | 25–28 | 2.70 | 4.30 | 6.00 | 6.00 |
| 29–32 | |||||
| Pneumothorax | 25–28 | 2.40 | 2.80 | 5.70 | 20.20 |
| 29–32 | 2.90 | 3.50 | 0.60 | 46.20 | |
| Retinopathy of prematurity | 25–28 | 4.23 | 5.16 | 1.36 | 1.36 |
| 29–32 | |||||
| Mortality | 25–28 | 1.90 | 2.80 | 0.00 | 13.40 |
| 29–32 | 0.30 | 0.40 | 0.00 | 0.00 | |
| MV requirement | 25–28 | 22.80 | 31.90 | 0.00 | 100 |
| 29–32 | |||||
| Average number of doses per patient | 25–28 | 1.48 | 1.48 | 0.00 | 1.64 |
| 29–32 |
INSURE, intubation-surfactant-extubation; LISA, less invasive surfactant administration; MV, mechanical ventilation; NIV, non-invasive ventilation
Note: Some cells are splited into two rows, but only one value was given since it applies to both rows.
Complication and mortality rates were calculated based on published literature reviews of treatment comparisons by Isayama et al8 and Rigo et al.9 Currently, there is no single head-to-head trial comparing all surfactant administration strategies. For severe IVH, LISA, INSURE, and the “continue NIV” strategy were individually compared with MV and analyzed in a network meta-analysis,8 which is an evidence synthesis technique for comparing multiple treatments that have not been tested in head-to-head trials in a single analysis by combining direct and indirect evidence within a network of clinical trials.16 We calculated the complication rates for LISA by applying the network odds ratio8 for LISA vs MV, using MV complication rates as the benchmark. Rates of mortality, BPD, and pneumothorax were stratified by gestational age by average weighting the patient population in each gestational age group and applying network odds ratio to get rates for LISA, INSURE, and continue NIV. For ROP, INSURE and continue NIV were compared with LISA in a meta-analysis. We used the complication rates of LISA as the benchmark to calculate the complication rates for continue NIV by applying the risk ratio for LISA vs continue NIV.8,9
Rates of MV were calculated based on data published in Rigo et al,9 where INSURE and continue NIV were compared with LISA. We used the MV rate of LISA as the benchmark and obtained INSURE and continue NIV rates by applying the risk ratio of LISA vs INSURE and continue NIV.
Economic Inputs. The economic model included surfactant costs, hospitalization costs stratified by MV status and treatment strategy, and adverse event costs (Table 2). Hospital costs per day were stratified by MV status and reported by the following categories: room and board, laboratory, respiratory care, radiology, supplies, and therapy, based on Guardia et al.14 Hospitalization costs for each treatment strategy were calculated based on total hospitalization days, duration of MV, and hospital costs per day for days with and without MV. The duration of MV for LISA and INSURE for was informed by published literature.2 The duration of MV for NIV failure was calculated using an average for patients continuing NIV7 and weighting the MV time by the mix of patients requiring MV and those that did not require MV.17 Complication costs were derived from the Healthcare Cost and Utilization Project database, including BPD, IVH, pneumothorax, and ROP costs. The total hospital costs for BPD and IVH were assumed to include the average RDS hospital costs. Therefore, the excess hospital costs for BPD and IVH were calculated as the net of the average RDS hospitalization. Pneumothorax and ROP costs were lower than the average RDS hospital costs and therefore considered short-term complications and additive to the average RDS hospital costs. All costs were inflated to 2020 US dollars using the medical component of the Consumer Price Index as reported by the Federal Reserve Bank.18 Drug costs were based on wholesale acquisition cost of CUROSURF (poractant alfa) from PropectoRx.com.18
Table 2.
Economic and Health Resource Inputs for the Model
| Complication and Other Costs or Time Required | ||
|---|---|---|
| Total Hospital Costs | Excess Hospital Cost Due to Adverse Events | |
| Complication costs, $ | ||
| BPD | 176,643 | 83,731 |
| IVH | 182,035 | 89,124 |
| Pneumothorax | 16,000 | 16,000 |
| ROP | 52,901 | 52,901 |
|
| ||
| Other costs, $ | ||
| LOS with MV per day | 2,999 | |
| LOS without MV per day | 2,051 | |
| Curosurf (1.5-mL vial) | 493.88 | |
| Curosurf (3-mL vial) | 973.88 | |
|
| ||
| Health resources, days | ||
| Average LOS (days) | 44.5 | |
| Duration of MV (days) | ||
| LISA | 1 | |
| INSURE | 2 | |
| NIV failure | 4 | |
BPD, bronchopulmonary dysplasia; INSURE, intubation-surfactant-extubation; IVH, intraventricular hemorrhage; LISA, less invasive surfactant administration; LOS, length of stay; MV, mechanical ventilation; NIV, non-invasive ventilation; ROP, retinopathy of prematurity
Sensitivity Analysis. To assess the effect of uncertainty of model inputs on incremental costs between scenarios, a deterministic sensitivity analysis was conducted. All population (age and weight distribution), clinical (NIV failure and success rates, surfactant dosing information, complication rates, MV requirement), and economic (hospitalization costs, MV duration and costs, and complication or adverse event costs) parameters were varied by ± 20% individually while keeping all else constant.
Results
The model was used to estimate the annual clinical consequences and budgetary effect of using early surfactant administration (LISA or INSURE) compared with standard surfactant treatment for a hypothetical US hospital treating 100 preterm infants with RDS in the base case and each scenario. Of the 100 RDS babies, we calculated 55 babies in the 25- to 28-week gestational age group and 45 babies in the 29- to 32-week gestational age group based on available national data.1 It may seem counterintuitive that there are more babies in the 25- to 28-week gestational age group. However, this is a result of overlaying the birth weight range (500–2000 g) on top of the epidemiology data from the CDC WONDER database for infants in the 25- to 32-week gestational age group.
Base Case. Predicted outcomes from the base case were: 32 patients received surfactant through standard administration after failing NIV, and all required MV within the first 72 hours of life. No surfactant was required for the 68 patients who continue NIV. The cumulative duration of stay for patients on MV was 128 days. The total surfactant volume administered was 120 mL. Fourteen patients were predicted to develop BPD (Table 3).
Table 3.
Projected Clinical and Economic Outcomes for the Different Scenarios
| Outcomes | Base Case | Scenario 1 (Threshold, 40%; LISA, 30%; INSURE, 70%) | Scenario 2 (Threshold, 30%; LISA, 30%; INSURE, 70%) |
|---|---|---|---|
| Clinical, n | |||
| Patients | 100 | 100 | 100 |
| Patients getting LISA or INSURE | 0 | 19 | 51 |
| Patients getting surfactant | 32 | 37 | 57 |
| Number of single-dose successes in early rescue | N/A | 10 | 26 |
| BPD patients, n | 14 | 12 | 10 |
| Deaths, n | 2 | 2 | 1 |
| Total doses, mL | 120 | 132 | 194 |
| Duration of stay on MV, hours | 128 | 83 | 54 |
| Patients requiring MV in first 72 hr, n | 32 | 24 | 21 |
|
| |||
| Economics, US $ | |||
| CUROSURF costs | 51,822 | 57,030 | 83,465 |
| Hospitalization (LOS and MV) costs | 9,246,156 | 9,224,109 | 9,229,695 |
| Complication costs | 1,950,392 | 1,714,824 | 1,525,973 |
| Total costs | 11,248,370 | 10,995,963 | 10,839,132 |
BPD, bronchopulmonary dysplasia; INSURE, intubation-surfactant-extubation; LISA, less invasive surfactant administration; MV, mechanical ventilation; N/A, not applicable
Base case costs included the cost of surfactant, total hospitalization cost, and excess hospital costs due to complications. The overall cost for the base case was $11,248,370 for the 100 patients in the analysis. The primary cost driver is hospitalization cost, totaling $9,246,156 and contributing 82% of overall costs. The total excess hospital costs for complications, including BPD, IVH, pneumothorax, and ROP, was $1,950,392 and contributed to 17% of overall costs. Costs of surfactant were $51,822 for the base case and contributed to less than 1% of total costs.
Scenarios. We analyzed the costs and outcomes of 2 FiO2 intervention threshold scenarios (40% and 30%). There was an inverse relationship between FiO2 thresholds and the number of patients who received surfactant. For FiO2 threshold 40% and 30%, the number of patients getting any surfactant were 37 and 57, respectively. The number of patients getting early surfactant treatment was 19 and 51, respectively. Single-dose success rate dropped as the FiO2 threshold went up. The number of patients with single-dose success were 10 and 26, respectively. Estimated cumulative surfactant volumes administered were 132 and 194 mL. The number of patients projected to require MV within 72 hours was 32 and 21; the numbers of BPD patients were estimated to be 12 and 10; and there were 2 deaths and 1 death among the 100 total patients, respectively (Table 3).
Total costs increased as the FiO2 intervention threshold increased. Similar to the base case, the major component of total costs was total hospitalization stays, which were $9,224,109 and $9,229,695, contributing 84% and 85% of total costs, respectively, for FiO2 thresholds at 40% and 30%. The second largest contributor to total costs was the excess hospital costs for complications, $1,714,824 and $1,525,973, contributing 15% and 14% of total costs, respectively. Surfactant costs for both scenarios contributed to less than 1% of total costs.
Deterministic Sensitivity Analysis. A deterministic sensitivity analysis was performed to measure the incremental total cost difference between the base case and the 30% FiO2 threshold scenario. We found that the model was most sensitive to 12 parameters, which had more than 10% effect on the baseline results when varied by ±20% individually. The parameter with the most significant effect in the model was the length of hospital stay associated with using an early rescue technique, which was primarily the INSURE method (70%) in the scenario. Other parameters with a significant effect in the model were hospital days for NIV failure, NIV success, and LISA. More extended hospital stays due to NIV failure yielded higher incremental costs. BPD also affected results, with higher BPD costs leading to more considerable incremental cost difference. Pneumothorax rates for INSURE, NIV success, and NIV failure all had a high effect on the results, with higher rates increasing the magnitude of budget effect. An increase in the number of treated infants, FiO2 threshold, and the hospital day cost all increased the incremental costs. Figure 2 includes the results from the deterministic sensitivity analysis of the top 20 influential parameters.
Figure 2.
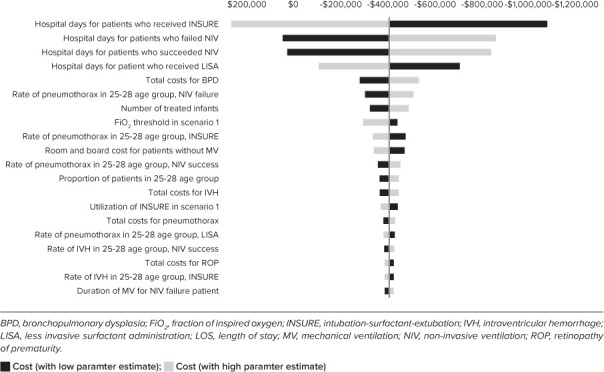
Deterministic sensitivity analysis
Discussion
We developed a cost-consequence model to project the health economic effects of using standard surfactant administration via endotracheal intubation and mechanical ventilation compared with different early rescue surfactant strategies administration based on various FiO2 requirement levels. Our results projected that scenarios with selective early surfactant rescue administration are associated with better clinical outcomes and lower total costs than a base case using standard administration. Selective early rescue surfactant administration using evidence-based CPAP failure prediction literature, using an FiO2 of 30% target threshold, resulted in improved clinical outcomes and lower total costs despite greater use of surfactant.
Early surfactant administration with either LISA or INSURE predicted a shorter duration on MV and fewer patients requiring MV in the first 72 hours compared with standard surfactant administration. Similarly, early selective surfactant administration via LISA and INSURE techniques compared with standard surfactant treatment prevented some adverse long-term complications, reduced the number of deaths, and decreased length of stay and duration of MV. The proportion of patients who met the criteria to receive surfactant treatment in the alternative scenarios was higher than the base case. The estimated duration of MV and the number of patients requiring MV in the first 72 hours were reduced, and fewer patients developed BPD. Lower FiO2 threshold led to increased early surfactant administration, which was associated with more patients receiving surfactant, fewer patients requiring MV, shorter duration of MV, and fewer BPD patients and deaths.
Our findings of clinical and economic outcomes of early surfactant treatment are in agreement with published studies. Verder et al19 showed greater clinical efficacy with early surfactant treatment in that it reduced the overall mortality and adverse events and reduced the frequency of MV compared with standard administration. Dani et al20 demonstrated that the total cost for early surfactant administration was lower than with standard administration. They found that cost of surfactant was higher in the early surfactant administration group, but this was compensated for by lower MV expenses. Our study confirmed the same cost comparison while adding to the evidence base that the decline of excess adverse events for the early treatment group also brings down costs.
To our knowledge, our study is the first cost-consequence analysis to compare the clinical and health economic outcomes of selective early surfactant treatment vs standard surfactant administration in the United States. This model adds to the evidence base on the economic effect of surfactant treatment for preterm infants with RDS. A vital strength of the model is the consideration of clinical and economic factors across various FiO2 threshold scenarios, reflecting the variability in clinical practice between hospitals in using the FiO2 threshold for determining the timing of surfactant administration.
This study has several limitations. Several simplifying assumptions were made in the development of the model that may have affected the outcomes. In our study, the patient population was limited to spontaneously breathing infants with gestational age of 25 to 32 weeks. The available evidence base could not reliably be generalized outside of this gestational age range. This model was only intended to evaluate the clinical and economic outcomes with the use of poractant alfa as the surfactant using early rescue strategies. We recognize other surfactants may have varied clinical and economic outcomes. Additionally, we used wholesale acquisition cost as a conservative proxy for a hospital's acquisition cost of surfactant. In reality, a hospital may have slightly lower acquisition cost. We assumed that patients who receive surfactant might receive more than 1 dose during the hospital stay, and we calculated the number of vials used based on minimizing wastage and costs. For clinical inputs of treatment strategies, some data are based on observational studies and retrospective data analyses rather than randomized controlled trials because of the lack of evidence.10 In terms of complications, in accordance with health economic best practice, an indirect treatment comparison was used to combine outcomes from trials that compare different treatment strategies and methods. This methodology informs comparisons among different treatment strategies not studied in a clinical trial within a single analysis.8,9 The total hospital costs for BPD and IVH are assumed to include the average RDS hospital costs. Therefore, the excess hospital costs for these complications are calculated as the net of the average RDS hospitalization. Pneumothorax and ROP are considered short-term complications and therefore additive to the average RDS hospital costs. Furthermore, the economic effect is limited to RDS infants in the initial hospitalization settings, and post-discharge costs are not included. The length of hospital stay was assumed to be the same (44.5 days) regardless of administration strategy, based on the ICD 10 code (P07.3) for preterm newborns. Besides, the health economic model simulates a hypothetical cohort using estimates from literature and as such is not a statistical simulation, and therefore the significance testing was omitted.
Reducing BPD has a long-term effect on health economic outcomes because it was shown to be the most costly complication of prematurity.21,22 Lapcharoensap et al21 reported preterm newborns with BPD generated a median hospitalization cost of $377,871 in the first year, compared with $175,836 for newborns without BPD, and that BPD is associated with substantial health care resource use, long length of stay, and high likelihood of rehospitalization. Our analysis showed a 14.3% and 28.6% reduction of BPD patients for scenarios 1 and 2 compared with the base case, which can lead to great cost savings due to BPD in the long term. In addition, our model factors in cost savings from the avoidance of other complications, such as IVH, pneumothorax, and ROP; however, we do not provide estimate for reductions in these events.
Conclusion
This cost-consequence analysis indicated that the use of selective early rescue surfactant in preterm infants with RDS in comparison with standard surfactant administration via endotracheal intubation and mechanical ventilation resulting in early surfactant administration generates the higher surfactant cost, which is offset by cost savings in total hospitalization and adverse events, which leads to lower overall cost of care.
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- CDC
US Centers for Disease Control and Prevention
- CPAP
continuous positive airway pressure
- ETT
endotracheal tube
- FiO2
fraction of inspired oxygen
- GA
Gestational Age
- INSURE
intubation-surfactant-extubation
- IVH
intraventricular hemorrhage
- LISA
less invasive surfactant administration
- LOS
Length of Stay
- MV
mechanical ventilation
- NIV
non-invasive ventilation
- RDS
respiratory distress syndrome
- ROP
retinopathy of prematurity
Funding Statement
Disclosures. Financial support for this study was provided by Chiesi USA Inc. The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report. The content and results in the manuscript were approved by all authors and have not been subject to sponsor censorship. WY and IJ are employees of PRECISIONheor, which provides consulting services to the biopharmaceutical industry, including Chiesi USA Inc. PRECISIONheor has received professional fees for the development of the model from Chiesi USA Inc. MC, KD, BB, and DF are employees of Chiesi USA Inc, and they may have equity in Chiesi USA Inc. MM is a neonatologist at the Neonatal-Perinatal Medicine Division, Children's Hospital of Orange County, who provides consultancy service and received professional fees from Chiesi USA Inc.
Footnotes
Ethical Approval and Informed Consent. Given the nature of this paper, institutional board/ethics committee review was not required.
References
- 1.Centers for Disease Control and Prevention Births data summary. Accessed March 5, 2021. https://wonder.cdc.gov/datasets.html
- 2.Buyuktiryaki M, Alarcon-Martinez T, Simsek GK et al. Five-year single center experience on surfactant treatment in preterm infants with respiratory distress syndrome: LISA vs INSURE. Early Hum Dev . 2019;135:32–36. doi: 10.1016/j.earlhumdev.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Dyer J. Neonatal respiratory distress syndrome: tackling a worldwide problem. P T . 2019;44(1):12–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Infant deaths due to respiratory distress syndrome: United States, 2007–2017. 2017. Accessed April 20, 2021 https://www.marchofdimes.org/peristats/data?reg=99&top=6&stop=119&lev=1&slev=1&obj=1.
- 5.Finer NN, Carlo WA, Walsh MC, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Early CPAP versus surfactant in extremely preterm infants. N Engl J Med . 2010;362(21):1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sammour I, Karnati S. Non-invasive respiratory support of the premature neonate: from physics to bench to practice. Front Pediatr . 2020;8:214. doi: 10.3389/fped.2020.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göpel W, Kribs A, Ziegler A et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, andomized, controlled trial. Lancet . 2011;378(9803):1627–1634. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 8.Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA . 2016;316(6):611–624. doi: 10.1001/jama.2016.10708. [DOI] [PubMed] [Google Scholar]
- 9.Rigo V, Lefebvre C, Broux I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr . 2016;175(12):1933–1942. doi: 10.1007/s00431-016-2789-4. [DOI] [PubMed] [Google Scholar]
- 10.Dargaville PA, Aiyappan A, De Paoli AG et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology . 2013;104(1):8–14. doi: 10.1159/000346460. [DOI] [PubMed] [Google Scholar]
- 11.Kurepa D, Perveen S, Lipener Y, Kakkilaya V. The use of less invasive surfactant administration (LISA) in the United States with review of the literature. J Perinatol . 2019;39(3):426–432. doi: 10.1038/s41372-018-0302-9. [DOI] [PubMed] [Google Scholar]
- 12.Klotz D, Porcaro U, Fleck T, Fuchs H. European perspective on less invasive surfactant administration-a survey. Eur J Pediatr . 2017;176(2):147–154. doi: 10.1007/s00431-016-2812-9. [DOI] [PubMed] [Google Scholar]
- 13.Heiring C, Jonsson B, Andersson S, Björklund LJ. Survey shows large differences between the Nordic countries in the use of less invasive surfactant administration. Acta Paediatr . 2017;106(3):382–386. doi: 10.1111/apa.13694. [DOI] [PubMed] [Google Scholar]
- 14.Guardia CG, Moya FR, Sinha S, Simmons PD, Segal R, Greenspan JS. A pharmacoeconomic analysis of inhospital costs resulting from reintubation in preterm infants treated with lucinactant, beractant, or poractant alfa. J Pediatr Pharmacol Ther . 2012;17(3):220–227. doi: 10.5863/1551-6776-17.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CUROSURF dosing instructions. Births Data Summary. 2019. Accessed March 5, 2021. https://curosurf.com/dosing-administration/
- 16.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med . 2017;12(1):103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kribs A, Härtel C, Kattner E et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klin Padiatr . 2010;222(1):13–17. doi: 10.1055/s-0029-1241867. [DOI] [PubMed] [Google Scholar]
- 18. ProspectoRx. Elsevier. 2021. Accessed March 5, 2021. https://prospectorx.com/login.
- 19.Verder H, Albertsen P, Ebbesen F et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics . 1999;103(2):E24. doi: 10.1542/peds.103.2.e24. [DOI] [PubMed] [Google Scholar]
- 20.Dani C, Ravasio R, Fioravanti L, Circelli M. Analysis of the cost-effectiveness of surfactant treatment (Curosurf®) in respiratory distress syndrome therapy in preterm infants: early treatment compared to late treatment. Ital J Pediatr . 2014;40:40. doi: 10.1186/1824-7288-40-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapcharoensap W, Bennett MV, Xu X, Lee HC, Dukhovny D. Hospitalization costs associated with bronchopulmonary dysplasia in the first year of life. J Perinatol . 2020;40(1):130–137. doi: 10.1038/s41372-019-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurihara C, Zhang L, Mikhael M. Newer bronchopulmonary dysplasia definitions and prediction of health economics impacts in very preterm infants. Pediatr Pulmonol . 2021;56(2):409–417. doi: 10.1002/ppul.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]


