Abstract
Background
Hospitalized patients with SARS-CoV-2 community-acquired pneumonia (CAP) and associated comorbidities are at increased risk of cardiovascular complications. The magnitude of effect of cardiovascular complications and the role of prior comorbidities on clinical outcomes are not well defined.
Research Question
What is the impact of cardiovascular complications on mortality in hospitalized patients with SARS-CoV-2 CAP? What is the impact of comorbidities and other risk factors on the risk of developing cardiovascular complications and mortality in these patients?
Study Design and Methods
This cohort study included 1,645 hospitalized patients with SARS-CoV-2 CAP. Cardiovascular complications were evaluated. The clinical course during hospitalization was described by using a multistate model with four states: (1) hospitalized with no cardiovascular complications; (2) hospitalized with cardiovascular complications; (3) discharged alive; (4) and dead. Cox proportional hazards regression was used to analyze the impact of prior comorbid conditions on transitions between these states. Hazard ratios (HRs) and 95% CIs are reported.
Results
Cardiovascular complications occurred in 18% of patients hospitalized with SARS-CoV-2 CAP. The mortality rate in this group was 45% vs 13% in patients without cardiovascular complications. Male subjects (HR, 1.32; 95% CI, 1.03-1.68), older adults (HR, 1.34; 95% CI, 1.03-1.75), and patients with congestive heart failure (HR, 1.59; 95% CI, 1.18-2.15), coronary artery disease (HR, 1.34; 95% CI, 1.00-1.79), atrial fibrillation (HR, 1.43; 95% CI, 1.06-1.95), direct admissions to the ICU (HR, 1.77; 95% CI, 1.36-2.32), and Pao2/Fio2 < 200 (HR, 1.46; 95% CI, 1.11-1.92) were more likely to develop cardiovascular complications following hospitalization for SARS-CoV-2 CAP; however, these factors are not associated with increased risk of death following a cardiovascular complication.
Interpretation
Prior comorbidities, older age, male sex, severity of illness, and hypoxemia are associated with increased risk of cardiovascular complications. Once patients develop cardiovascular complications, the risk of death is extremely high. Cardiovascular complications are the primary drivers of mortality in hospitalized patients with SARS-CoV-2 CAP.
Key Words: cardiovascular complications, COVID-19, mortality, SARS-CoV-2
Take-home Points.
Study Question: What is the impact of cardiovascular complications on mortality in hospitalized patients with SARS-CoV-2 CAP? What is the impact of comorbidities and other risk factors on the risk of developing cardiovascular complications and mortality in these patients?
Results: Cardiovascular complications occurred in 18% of patients hospitalized with SARS-CoV-2 CAP. The mortality rate in this group was 45% vs 13% in patients without cardiovascular complications. Male subjects, older adults, and patients with congestive heart failure, coronary artery disease or atrial fibrillation were more likely to develop cardiovascular complications following hospitalization; however, these prior comorbidities were not associated with an increased risk of death following a cardiovascular complication.
Interpretation: Prior comorbidities, older age, and male sex are associated with increased risk of cardiovascular complications. Once patients develop cardiovascular complications, the risk of death is extremely high. Cardiovascular complications are the primary drivers for mortality in hospitalized patients with SARS-CoV-2 CAP.
A major component of the pathogenesis of COVID-19 is the effect of the virus on the cardiovascular system. SARS-CoV-2 enters the host cell via angiotensin-converting enzyme 2 receptors, which are expressed in endothelial cells of several organ systems.1 In patients who die of COVID-19, the cardiac tissue often harbors SARS-CoV-2,2 and the lungs exhibit capillary microthrombi, endothelial cell destruction, and angiogenesis.3 The tropism of the virus for the cardiovascular system has led experts to consider COVID-19 as much a vascular ailment as a respiratory disease.4 Clinically, the effects of COVID-19 on the cardiovascular system manifest as a higher incidence of VTE,5 stroke, and myocardial infarction.6 Patients who survive the acute phase of COVID-19 are at higher risk of cardiovascular sequelae, including cardiac dysrhythmias, coronary atherosclerosis, and heart failure.7 At the University of Louisville, our group previously assessed a cohort of hospitalized patients with COVID-19.8 Cardiovascular complications occurred in 18% of the patients and were a risk factor for increased mortality.
It is now well established that cardiovascular complications are common and portend worse outcomes in patients with COVID-19. However, an in-depth analysis of the clinical hospital course of patients with COVID-19 following a cardiovascular complication is missing. Studies have assessed cardiovascular complications and mortality as independent end points but examining them in a temporal sequence is warranted. In the current study, we expanded on our prior work and aimed to describe the clinical course of patients hospitalized with SARS-CoV-2 CAP by using a time-dependent multistate model to evaluate the impact of cardiovascular complications on mortality in hospitalized patients with SARS-CoV-2 CAP. A secondary objective was to evaluate the impact of comorbidities and other risk factors on the risk of developing cardiovascular complications and mortality in these patients
Study Design and Methods
Study Design and Study Population
The burden of COVID-19 was assessed in a retrospective study that included patients ≥ 18 years of age and hospitalized with SARS-CoV-2 CAP at eight acute-care hospitals in the Louisville, Kentucky, metropolitan area. Patients were diagnosed with SARS-CoV-2 upon admission to the hospital. Patients who developed symptoms following 48 h of admission to the hospital were excluded from this study. Patients were included if they were hospitalized between March 1, 2020, and July 1, 2020 (wave 1) and between October 1, 2020, and March 31, 2021 (wave 2).
Data Collection
Data abstracted from electronic medical records included patient demographic characteristics, medical history, risk factors for cardiovascular complications, and outcomes during hospitalization.
Study Definitions
SARS-CoV-2 CAP
A patient hospitalized with: (1) a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction from a nasopharyngeal swab or other respiratory sample; (2) fever (> 37.8°C), cough, or shortness of breath; and (3) evidence of new or worsening of pulmonary infiltrates on chest radiograph or CT scan.
Predictive Variables
The rationale for choosing comorbidities and risk factors as predictive variables is outlined in the Statistical Analysis section.
Cardiovascular Complications
A patient who experienced any of the following events during hospitalization: heart failure, sudden cardiac arrest, cardiogenic shock, acute myocardial infarction, pulmonary edema, new or serious arrhythmia or acute worsening of long-term arrhythmia, cerebrovascular accident, myocarditis, or DVT.
In-Hospital Mortality
A patient who experienced all-cause mortality during hospitalization. Patients transferred to end-of-life palliative care were considered to have died during hospitalization. Date of transfer was used as date of death.
Multistate Model
A multistate model was proposed to describe the clinical course of hospitalized patients with SARS-CoV-2 CAP as depicted in Figure 1 . The four states in the model were defined as: (1) hospitalized with no cardiovascular complications; (2) hospitalized with the development of any cardiovascular complication; (3) in-hospital mortality; and (4) discharged alive. States 3 and 4 are referred to as absorbing states, as patients remain in this state when they enter it. Five potential transitions to states 2, 3, or 4 were defined as: (1) hospitalization (state 1) to development of cardiovascular complication (state 2); (2) hospitalization (state 1) to in-hospital mortality (state 3); (3) hospitalization (state 1) to discharged alive (state 4); (4) cardiovascular complication (state 2) to in-hospital mortality (state 3); and (5) cardiovascular complication (state 2) to discharged alive (state 4).
Figure 1.
Multistate model framework depicting states and transitions. CAP = community-acquired pneumonia.
Statistical Analysis
Descriptive statistics are reported for the patients with and without cardiovascular complications. Categorical variables are summarized as frequencies and percentages, with comparisons made by using χ2 tests of independence. Continuous variables are summarized as medians and interquartile ranges and were compared by using the Kruskal-Wallis rank sum test. A nonparametric Cox model was used to estimate a separate baseline hazard for each of the transitions. To estimate covariate effects on each transition, a semi-parametric Cox proportional hazards regression was used. Separate baseline hazards were specified for each transition. Analysis was performed by using R version 3.6.1 and the mstate package for multistate modeling (e-Appendix 1).9 , 10
Predictor variables were considered for inclusion in the final model based on the following criteria: (1) a biologically plausible association with cardiovascular complications or in-hospital mortality in patients with SARS-CoV-2 CAP; (2) increased rates in patients who experienced cardiovascular complications or in-hospital mortality compared with the overall cohort; and (3) general availability of predictors in a clinical setting. Likelihood ratio tests were used to determine if the effect of a predictor was the same or different across all transitions. Goodness-of-fit tests were used to assess violations to the proportional hazards assumption with violations included as a stratification variable. e-Table 1 outlines the selection process for potential predictors and the criteria for inclusion in the final model.
Protection of Human Subjects
The study was approved by the Institutional Review Board at the University of Louisville Human Subjects Research Protection Program Office (IRB #20.0257) and by the research offices at each participating hospital. The study was exempt from informed consent.
Results
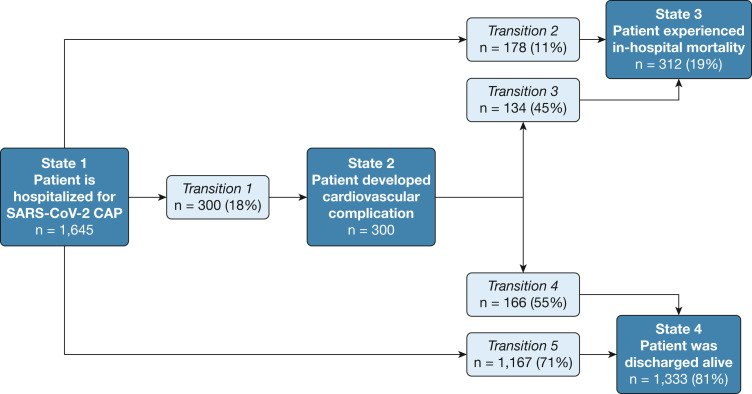
A total of 1,645 patients hospitalized for SARS-CoV-2 CAP were included in the analysis. The clinical course of these patients according to the multistate model is depicted in Figure 2 . In the overall cohort, a total of 300 patients (18%) experienced a cardiovascular complication. A Kaplan-Meier curve depicting time to cardiovascular complication for these 300 patients is shown in e-Figure 1. For those who experienced cardiovascular complications, the median time to cardiovascular complication was 1.38 days (95% confidence limit, 1.15-2.21 days). Of these 300 patients, 134 (45%) died during hospitalization. Of the 1,345 patients who did not experience cardiovascular complications, 178 (13%) died during hospitalization.
Figure 2.
Clinical course of hospitalized patients with SARS-CoV-2 CAP according to a multistate model framework. CAP = community-acquired pneumonia.
Baseline characteristics for the patients who experienced and did not experience cardiovascular complications are depicted in Table 1 . The baseline characteristics in overall cohort and transition states are illustrated in e-Table 2. The frequency of cardiovascular complications is depicted in Table 2 . The most frequent cardiovascular complication was a new arrhythmia in 121 patients, accounting for 7% of the overall cohort and 41% of those who developed cardiovascular complications. The physical examination and laboratory findings are noted in e-Table 3. Patients who experienced cardiovascular complications had higher severity of disease at presentation, with higher rates of pneumonia severity index, CURB-65 score (confusion, BUN ≥ 20 mg/dL, respiratory rate ≥ 30 breaths per minute, low systolic [< 90 mm Hg] or diastolic [≤ 60 mm Hg] BP, age ≥ 65 years), direct ICU admission, low Pao 2/Fio 2 ratio, and elevated ferritin levels.
Table 1.
Patient Characteristics: Demographic Characteristics, Medical History, and Risk Factors for Developing Cardiovascular Complications Between Patients With and Without Cardiovascular Complications
| Variable | Cardiovascular Complication (n = 300) | No Cardiovascular Complication (n = 1,345) | P Value |
|---|---|---|---|
| Demographic characteristics, social and medical history | |||
| Age, median (IQR), y | 69 (59, 76) | 63 (52, 74) | < .001 |
| Male | 177 (59) | 649 (48) | .001 |
| Nursing home resident | 43 (14) | 158 (12) | .255 |
| Race: black | 89 (30) | 353 (26) | .256 |
| Smoking history | 148 (49) | 560 (42) | .018 |
| Obesity | 160 (53) | 724 (54) | .927 |
| Diabetes | 143 (48) | 509 (38) | .002 |
| Renal disease | 83 (28) | 248 (18) | < .001 |
| Congestive heart failure | 74 (25) | 152 (11) | < .001 |
| Liver disease (noncirrhotic) | 9 (3) | 43 (3) | > .999 |
| Cirrhosis | 9 (3) | 14 (1) | .019 |
| Risk factors for cardiovascular complications | |||
| Aspirin use | 117 (39) | 364 (27) | < .001 |
| Beta-blockers | 136 (45) | 423 (31) | < .001 |
| ACE inhibitors | 60 (20) | 252 (19) | .672 |
| Antiplatelet | 39 (13) | 104 (8) | .005 |
| Statins | 140 (47) | 546 (41) | .062 |
| Family history of CAD | 64 (21) | 248 (18) | .282 |
| CAD | 97 (32) | 248 (18) | < .001 |
| Hypertension | 224 (75) | 869 (65) | .001 |
| Hyperlipidemia | 153 (51) | 562 (42) | .004 |
| Atrial fibrillation | 63 (21) | 131 (10) | < .001 |
| Prior cerebrovascular accident | 46 (15) | 148 (11) | .045 |
| Severity of disease on admission | |||
| PSI risk class IV or V | 217 (72) | 610 (45) | < .001 |
| CURB-65 score ≥ 3 | 129 (43) | 352 (26) | < .001 |
| Direct ICU admission | 113 (38) | 221 (16) | < .001 |
| Low Pao2/Fio2 (< 200) | 99 (33) | 188 (14) | < .001 |
| Elevated ferritin (> 500 ng/mL) | 111 (37) | 367 (27) | .001 |
Data are presented as No. (%) unless otherwise indicated. Bolded text indicates statistical significance. ACE = angiotensin-converting enzyme; CAD = coronary artery disease; CURB-65 = confusion, BUN ≥ 20 mg/dL, respiratory rate ≥ 30 breaths per minute, low systolic (< 90 mm Hg) or diastolic (≤ 60 mm Hg) BP, age ≥ 65 years; IQR = interquartile range; Pao2/Fio2 = ratio arterial oxygen tension over inspired oxygen; PSI = pneumonia severity index.
Table 2.
Cardiovascular Complications: Type of Cardiovascular Complication Experienced Among Patients Who Developed Cardiovascular Complications
| Cardiovascular Complication | Overall (N = 300) |
|---|---|
| New arrhythmia | 121 (40) |
| Heart failure | 52 (17) |
| Acute myocardial infarction | 44 (15) |
| Pulmonary embolism | 34 (11) |
| Acute worsening of chronic arrhythmia | 29 (10) |
| Pulmonary edema | 28 (9) |
| Sudden cardiac arrest | 25 (8) |
| DVT | 21 (7) |
| Cerebrovascular accident | 19 (6) |
| Cardiogenic shock | 20 (7) |
| Myocarditis | 3 (1) |
Data are presented as No. (%).
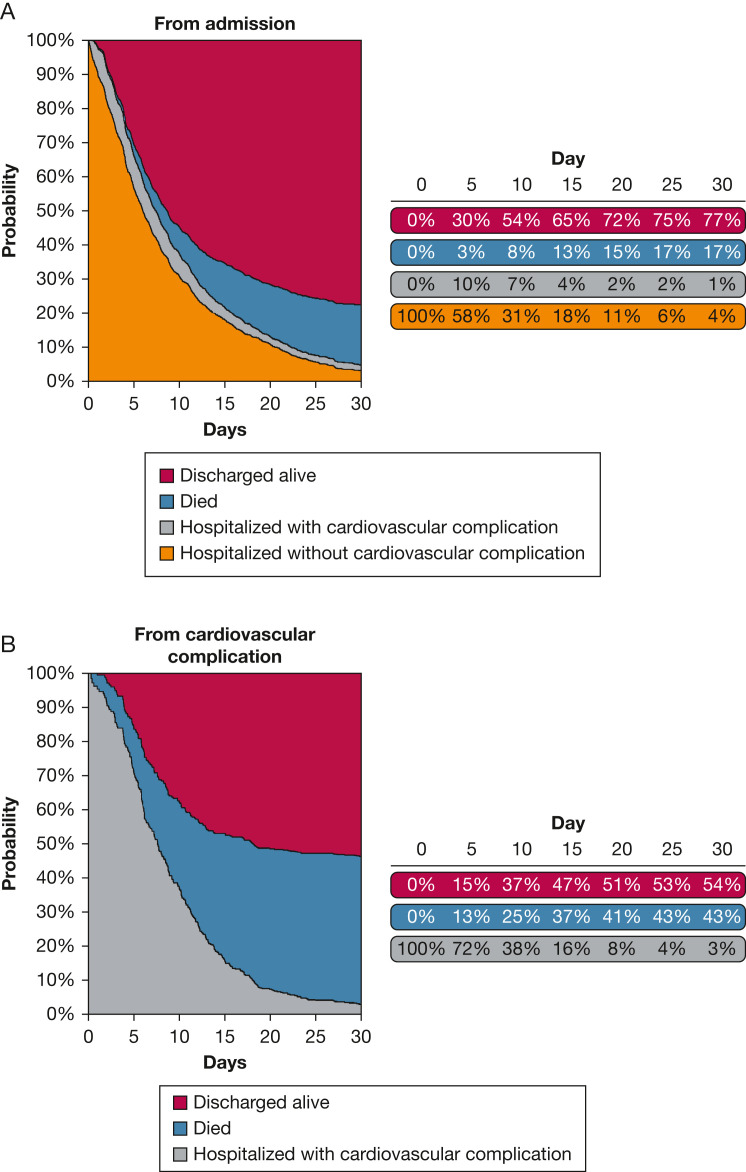
We illustrate the stacked estimates of transition probabilities during the first 30 days of hospitalization for a patient starting from hospitalization (Fig 3 A) and for a patient after developing a cardiovascular complication (Fig 3B) from the nonparametric model. The area between two curves represents the probability of occupying the corresponding state at a specified time point. Patients who occupy state 2 have experienced a cardiovascular complication, are alive, and remain hospitalized. For patients who occupy state 1 on day 0, the proportion of patients who experience a cardiovascular complication and now occupy state 2 is highest at day 5. As time passes, the proportion of patients occupying states 1 or 2 naturally decreases as patients have died or are discharged, and now occupy states 3 or 4. A higher proportion of patients who occupied state 2 died and transitioned to state 3 during hospitalization when compared to patients who occupy state 1 at admission.
Figure 3.
A, Stacked transition probabilities during the first 30 days of hospitalization starting from hospitalization. B, Stacked transition probabilities during the first 30 days following the development of a cardiovascular complication.
Transition-specific covariate estimates from the semi-parametric model are depicted in Table 3 . The final model included the following: age (categorized as ≥ 65 years of age); male sex; smoking history (current or former); obesity (BMI ≥ 30 kg/m2); antecedent aspirin use; history of diabetes, renal disease, congestive heart failure, coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, and cerebrovascular disease; direct admission to the ICU; high inflammatory status (ferritin > 500 ng/mL); and hypoxemia (Pao 2/Fio 2 < 200). Violations to the proportional hazard’s assumption were observed for age for transitions 2, 4, and 5; congestive heart failure for transition 5; smoking history for transition 2; obesity for transition 4; renal disease for transition 4; direct ICU admission for transitions 4 and 5; elevated ferritin for transition 5; and hypoxemia for transitions 2 and 5. Direct ICU admission was most strongly associated with the development of cardiovascular complications during hospitalization (HR, 1.77; 95% CI, 1.36-2.32) and also most strongly associated with dying without cardiovascular complication (HR, 1.80; 95% CI, 1.26-2.55). Male patients (HR, 1.32; 95% CI, 1.03-1.68), patients aged ≥ 65 years (HR, 1.34; 95% CI, 1.03-1.75), and patients with a history of congestive heart failure (HR, 1.59; 95% CI, 1.18-2.15), coronary artery disease (HR, 1.34; 95% CI, 1.00-1.79), or atrial fibrillation (HR, 1.43; 95% CI, 1.06-1.95) were also more likely to develop cardiovascular complications. Patients with hypoxemia were also at increased risk of developing cardiovascular complications (HR, 1.46; 95% CI, 1.11-1.92). Although cerebrovascular disease was not associated with the development of cardiovascular complications, patients with cerebrovascular disease were at increased risk of dying (HR, 1.67; 95% CI, 1.12-2.48) and were less likely to be discharged alive (HR, 0.79; 95% CI, 0.64-0.98) without cardiovascular complication. In addition, current or former smokers were less likely to be discharged alive (HR, 0.85; 95% CI, 0.75-0.96). After development of cardiovascular complication, no risk factors increased risk of death, although patients with hypoxemia had a reduced likelihood of being discharged alive (HR, 0.49; 95% CI, 0.30-0.83).
Table 3.
Cox Proportional Hazards Regression: Transition-Specific Estimates for Covariates From the Semi-parametric Cox Proportional Hazards Regression
| Transition 1: Admission to Cardiovascular Complication |
Transition 2: Admission to Died |
Transition 3: Cardiovascular Complication to Died |
Transition 4: Cardiovascular Complication to Home Discharge |
Transition 5: Admission to Home Discharge |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age ≥ 65 y | 1.34 | 1.03-1.75 | 0.80 | 0.54-1.19 | ||||||
| Male | 1.32 | 1.03-1.68 | 1.00 | 0.72-1.39 | 0.98 | 0.66-1.45 | 0.85 | 0.58-1.25 | 1.06 | 0.93-1.20 |
| Smoking history | 1.02 | 0.81-1.3 | 1.41 | 0.96-2.06 | 0.72 | 0.49-1.06 | 0.85 | 0.75-0.96 | ||
| Obese | 1.06 | 0.83-1.35 | 0.85 | 0.61-1.18 | 1.28 | 0.88-1.88 | 0.94 | 0.83-1.07 | ||
| Diabetes | 1.09 | 0.85-1.4 | 1.13 | 0.82-1.58 | 0.92 | 0.62-1.38 | 0.91 | 0.61-1.37 | 1.02 | 0.89-1.17 |
| Renal disease | 1.14 | 0.86-1.51 | 1.46 | 1.00a-2.13 | 0.64 | 0.40-1.02 | 1.01 | 0.85-1.21 | ||
| Congestive heart failure | 1.59 | 1.18-2.15 | 1.06 | 0.68-1.64 | 1.46 | 0.94-2.29 | 1.18 | 0.71-1.98 | ||
| Aspirin use | 1.08 | 0.83-1.40 | 0.72 | 0.51-1.03 | 0.87 | 0.59-1.29 | 0.97 | 0.65-1.44 | 0.95 | 0.82-1.11 |
| Coronary artery disease | 1.34 | 1.00-1.79 | 1.17 | 0.78-1.76 | 1.17 | 0.76-1.80 | 1.21 | 0.77-1.91 | 1.01 | 0.84-1.20 |
| Hypertension | 1.07 | 0.79-1.44 | 1.04 | 0.71-1.52 | 1.4 | 0.87-2.26 | 0.89 | 0.57-1.39 | 0.97 | 0.84-1.11 |
| Hyperlipidemia | 1.09 | 0.84-1.41 | 0.83 | 0.59-1.17 | 1.03 | 0.68-1.56 | 0.97 | 0.64-1.47 | 0.97 | 0.84-1.11 |
| Atrial fibrillation | 1.43 | 1.06-1.95 | 0.92 | 0.59-1.43 | 1.15 | 0.72-1.83 | 1.06 | 0.66-1.71 | 0.89 | 0.71-1.13 |
| Cerebrovascular disease | 1.08 | 0.78-1.49 | 1.67 | 1.12-2.48 | 1.11 | 0.69-1.78 | 1.13 | 0.67-1.89 | 0.79 | 0.64-0.98 |
| Direct admission to ICU | 1.77 | 1.36-2.32 | 1.80 | 1.26-2.55 | 1.56 | 1.00a-2.43 | ||||
| Elevated ferritin (> 500 ng/mL) | 1.07 | 0.84-1.37 | 0.98 | 0.71-1.37 | 0.80 | 0.54-1.18 | 0.75 | 0.50-1.13 | ||
| Low Pao2/Fio2 (< 200) | 1.46 | 1.11-1.92 | 1.00 | 0.63-1.56 | 0.49 | 0.30-0.83 | ||||
Empty cells indicate violations to the proportional hazard’s assumption. Cells with bolded text indicate statistical significance. HR = hazard ratio.
CI lower bound was < 1 but appears as 1.00 due to rounding.
Discussion
The primary finding of the current study is that once patients develop a cardiovascular complication, their risk for mortality is substantially increased. We found that cardiovascular complications occurred in approximately 18% of patients hospitalized with SARS-CoV-2 CAP, and of these patients, 45% died. In contrast, in-hospital mortality was observed in 13% of patients who did not develop a cardiovascular complication. In our multivariate analysis, we showed that male patients, older adults, and patients with congestive heart failure, atrial fibrillation, and coronary artery disease are more likely to develop cardiovascular complications following hospitalization. However, these risk factors do not translate into increased risk of death following cardiovascular complication; that is, once a cardiovascular complication is present, the probability of death is high regardless of the presence of risk factors for cardiovascular complications. It is important to recognize that cardiovascular complications appeared not only as a consequence of comorbidities but also from severity of disease and hypoxemia. As shown in this analysis, direct ICU admission and hypoxemia were both risk factors for developing cardiovascular complications. Furthermore, we found that hypoxemia following cardiovascular complication decreased the likelihood of hospital discharge at any point in time. Similar to comorbidities, we did not find an association between hypoxemia or ICU admission and mortality following cardiovascular complication.
Other studies have shown that mortality rates are higher in patients with cardiovascular complications and SARS-CoV-2 CAP.11 , 12 Shi et al11 conducted a cohort study in 416 patients hospitalized with COVID-19 infection. They assessed the effect of cardiac injury, defined as increased levels of cardiac biomarkers, on mortality. They found a prevalence of cardiac injury of 19.7%. The mortality was 51.2% in patients with cardiac injury in contrast to 4.5% in those without it. Even though they used a different approach to assess cardiovascular insult, their numbers are similar to ours. Another study that included part of the current cohort showed similar results regarding the proportion of patients with cardiovascular complications and the risk of death following a cardiovascular complication.8 A different set of risk factors for cardiovascular complications was assessed in that study, which found the following factors to be significantly associated with higher risk of cardiovascular complications: African-American race, male sex, lower SaO2/Fio 2 ratio, higher serum potassium, lower serum albumin, and cardiovascular comorbidity count. Distinctive features of the current study are the larger sample size (more than twice as large) and an assessment of the temporal evaluation of mortality following cardiovascular complication.
Other aspects of the epidemiology of cardiovascular disease in patients with COVID-19 have been addressed by studies with different designs. For instance, one study assessed the impact of a concomitant COVID-19 infection in the outcomes of patients presenting with acute cardiovascular disease.13 In patients with ST-segment elevation myocardial infarction, the presence of concomitant COVID-19 infection was associated with increased mortality compared with patients without COVID-19. Another study found that, following COVID-19 infection, the 1-year risk of several cardiovascular diseases increases compared with patients who did not have COVID-19 infection.14
Respiratory infections are known to increase the risk of developing cardiovascular complications,15 , 16 and SARS-CoV-2 is no exception. Several mechanisms have been proposed that are responsible for the cardiovascular complications in SARS-CoV-2 CAP. The novel virus can cause direct myocardial injury by using the angiotensin-converting enzyme 2 in the cardiac tissues17 or by the release of cytokines.18 In most cases, increased cardiometabolic demand coupled with systemic inflammation and ongoing hypoxia can cause myocardial damage.19 In addition, the known effect of structurally similar SARS-CoV-2 on lipid metabolism leads us to believe that SARS-CoV-2 might lead to chronic cardiovascular effects.20
As shown in Figure 3A, the proportion of patients who have experienced a cardiovascular complication and are still hospitalized is highest at day 5. Our data emphasize the importance of preventing cardiovascular complications in patients hospitalized with COVID-19. As shown in Figure 3B, experiencing a cardiovascular complication on day 0 is associated with high mortality during hospitalization, as 43% had died at day 30. Previous literature reported that the large number of cardiovascular complications occur during the first few days of CAP hospitalization.21 However, this area is still unexplored with COVID-19, and this is a unique finding of our study. In the current study, the development of arrythmias was the most common cardiovascular complication observed. Other studies have shown that development of arrhythmias in patients with SARS CoV-2 CAP can range from 6% to 17%22 and is commonly linked to poor disease outcomes.23
One strength of the current study was the use of multistate modeling to establish the relationship between cardiovascular complications and mortality. We also included patients from all adult acute-care hospitals in Louisville; consequently, the current study cohort well represents the Louisville community. We have previously shown that the demographic characteristics of the Louisville population correspond to those of the United States.24 As a result, the current study may be generalizable to the broader US population.
One limitation of our study was that cardiovascular complications were a composite outcome. As a result, we are unable to provide the magnitude of effect for each cardiovascular complication independently. In addition, we did not assess markers of cardiac injury, ECG, or echocardiogram findings into our multistate model. We did not collect the posthospitalization clinical course of the patients. Another limitation of our study is that we covered two waves of the COVID-19 pandemic, which could include patients with different variants, different characteristics, and who received different treatments. However, prior studies from our group showed that the outcomes in the two waves were not substantially different.25 , 26 Mortality was 17% during wave 1 (March 1, 2020-July 1, 2020)25 and 19% during wave 2 (October 1, 2020-March 31, 2021).26 Cardiovascular complications were experienced by 19% of patients in wave 124 and 18% of patients in wave 2.26
Future studies should focus on preventing cardiovascular complications and provide a more in-depth analysis on markers of cardiac injury. Future studies should also assess long-term sequelae in patients who experienced cardiovascular complications.
Interpretation
The current study shows that cardiovascular complications are common in patients with SARS-CoV-2 CAP. We identified risk factors for the development of cardiovascular complications. Identifying these risk factors may allow clinicians to target high-risk patients for close monitoring and manage any cardiovascular complication in a timely manner. Finally, we described the trajectory of inpatients with SARS-CoV-2 pneumonia. The highest risk for developing cardiovascular complications is within 5 days of admission. Following the development of cardiovascular complications, nearly one-half of the patients died during hospitalization.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: J. H. has a research grant from Gilead. None declared (A. S. A., D. S., T. R. C., S. F., J. A. R., F. A., R. C.).
Acknowledgments
Author contributions: R. C. takes responsibility for the content of the manuscript, including data and analysis. A. S. A. and D. S. contributed to writing and data interpretation. T. R. C. and S. F. contributed to writing and data analysis. J. H. contributed to writing and data interpretation. J. A. R. and R. C. contributed to study design, writing, and data interpretation. F. A. contributed to data interpretation.
Other contributions: The authors appreciate the researchers at the Center of Excellence for Research in Infectious Diseases for data collection and quality control.
Additional information: The e-Appendix, e-Figure, and e-Tables are available online under "Supplementary Data."
Supplementary Data
References
- 1.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindner D., Fitzek A., Bräuninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonow R.O., O’Gara P.T., Yancy C.W. Cardiology and COVID-19. JAMA. 2020;324(12):1131–1132. doi: 10.1001/jama.2020.15088. [DOI] [PubMed] [Google Scholar]
- 5.Piroth L., Cottenet J., Mariet A.S., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsoularis I., Fonseca-Rodríguez O., Farrington P., Lindmark K., Fors Connolly A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q., Samanapally H., Nathala P., et al. Outcomes and risk factors for cardiovascular events in hospitalized COVID-19 patients. J Cardiothorac Vasc Anesth. 2021;35(12):3581–3593. doi: 10.1053/j.jvca.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wreede L.C., Fiocco M., Putter H. mstate: an R package for the analysis of competing risks and multi-state models. J Statistical Software. 2011;38(7):1–30. [Google Scholar]
- 10.de Wreede L.C., Fiocco M., Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo L., Fu M., Li Y., et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: a pooled analysis. Clin Cardiol. 2020;43(12):1478–1493. doi: 10.1002/clc.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad M., Kennedy K.F., Imran H., et al. Association between COVID-19 diagnosis and in-hospital mortality in patients hospitalized with ST-segment elevation myocardial infarction. JAMA. 2021;326(19):1940–1952. doi: 10.1001/jama.2021.18890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton T.C., Thompson M., Meade T.W. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29(1):96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 17.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugert C.L., Kwiat V., Valera I.C., Bugert J.J., Parvatiyar M.S. Cardiovascular injury due to SARS-CoV-2. Curr Clin Microbiol Rep. 2021;8(3):167–177. doi: 10.1007/s40588-021-00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pieralli F., Vannucchi V., Nozzoli C., et al. Acute cardiovascular events in patients with community acquired pneumonia: results from the observational prospective FADOI-ICECAP study. BMC Infect Dis. 2021;21(1):116. doi: 10.1186/s12879-021-05781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furmanek S.P., Glick C., Chandler T., et al. The city of Louisville encapsulates the United States demographics. University Louisville J Respir Infect. 2020;4(2):4. [Google Scholar]
- 25.Ramirez J.A., Bordon J., Cavallazzi R., et al. Characteristics and outcomes of adults hospitalized with SARS-CoV-2 community-acquired pneumonia in Louisville, Kentucky. University of Louisville J Respir Infect. 2020;4(1):72. [Google Scholar]
- 26.Ramirez J.A., Chandler T., Furmanek S.P., et al. Epidemiology and outcomes of hospitalized adults with SARS-CoV-2 community-acquired pneumonia in Louisville, Kentucky. University of Louisville J Respir Infect. 2021;6(1):2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.