Abstract
Background
COVID-19 contagious health care personnel (HCP) who are self-isolating for a 10-day period increases burden to workforce shortages. Implementation of a 5-day early return-to-work (RTW) program may reduce self-isolation periods, without increasing transmission risk, during the COVID-19 pandemic.
Design and methods
This observational cohort quality improvement study included newly diagnosed COVID-19 HCP at a multifacility health care system. The program allowed HCP to return to work 6 days after date of a positive test result if they were not immunocompromised, had mild and improving symptoms, and self-reported a SARS-CoV-2 antigen negative test on day 5.
Results
Between January 4 and April 3, 2022, 1,023 HCP self-enrolled and 344 (33.6%) self-reported negative test results. Among these, 161 (46.8%) self-reported negative test results on day 5 and were eligible for early RTW on day 6. A total of 714 days were saved from missed work in self-isolation. The number of tests purchased, dispensed, and reported per day of HCP time saved was 4.4. No transmission events were observed originating from HCP who participated in early RTW.
Conclusion
Implementing a 5-day early RTW program that includes HCP self-reporting SARS-CoV-2 antigen test results can increase staffing availability, while maintaining a low risk of SARS-CoV-2 transmission.
Key Words: Workforce shortages, Contact tracing, Employee health, SARS-CoV-2 antigen test
Background
During the COVID-19 pandemic, health care personnel (HCP) self-isolation due to widely prevalent COVID-19 illness has contributed to workforce shortages. Adequate staffing in health care facilities is critical to providing a safe work environment and safe patient care. 1 Our conventional practice for HCP with a SARS-CoV-2 polymerase chain reaction (PCR) positive test result was aligned with the Centers for Disease Control and Prevention (CDC) recommendations related to the duration of COVID-19 contagiousness and minimum isolation period of 10-days (or longer in infrequent cases of moderate or severe illness or immunocompromising medical condition).2 Compared to the duration of contagiousness due to other respiratory viruses,3 this practice increases burden on the HCP workforce during high community prevalence of SARS-CoV-2 infections.
As part of evolving public health and HCP guidance during the COVID-19 pandemic, on December 23, 2021, the CDC provided updated recommendations to shorten isolation periods for HCP with a positive test result for a SARS-CoV-2 infection if they were not immunocompromised, had defervesced and feeling well enough to work, and had a SARS-CoV-2 negative test result within 48 hours prior to returning to work on day 7.4 With the context of this new public health guidance, we developed the 5-Day Early Return-To-Work (RTW) program that includes HCP self-collection and self-report of a SARS-CoV-2 antigen test result on day 5, and if negative, returning to work on day 6.5 Our intervention relies on antigen testing to be a marker of contagiousness. While the test characteristics of antigen testing to diagnose infection with SARS-CoV-2 are well described,6 other reports confirm an association between antigen test positivity and contagiousness as measured by viral culture through the course of the illness.7, 8, 9
Prior to the start of this project, we had previously developed and implemented a 7-Day Early RTW program that included a robust contact tracing application and barrier-free test distribution framework to distribute SARS-CoV-2 antigen test kits to HCP.10 Based on data from our 7-day early RTW program, we anticipated that HCP self-collecting and self-reporting antigen testing on day 5 of isolation would reduce the 10-day self-isolation period and avoid transmission events. Thus, the primary aim was to evaluate the benefit of the 5-Day Early RTW program to reduce the 10-day self-isolation period for HCP following a SARS-CoV-2 positive test result. The secondary aim was to evaluate the cost of the 5-Day Early RTW program, as measured by transmission events originating from HCP returning to work early, and number of tests performed.
Methods
Design
The observational cohort study design underwent formal review and was granted ethical approval (Project 3201) as a quality improvement project by our Quality Improvement Review Committee. Methods and results are reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement and Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines (Supplementary Table S1).11 , 12
Setting
This quality improvement project was completed during January 4 through April 3, 2022 at a 40-hospital integrated academic health care system providing care principally within central and western Pennsylvania (USA). The study period encompassed both elevated community activity and a low-prevalence period with a system-wide patient test-positivity rate ranging from a peak of 43.9% on January 9, 2022 to a nadir of 2.9% on April 3, 2022 (percentages are unadjusted values rather than a 7-day average and an aberrant low value was removed).
Data collection and SARS-CoV-2 testing
Contact tracing for COVID-19 diagnoses and SARS-CoV-2 positive test results were conducted by Infection Prevention and Control and Employee Health teams in conjunction with frontline managers. Our contact tracing application was used to collect detailed information about the exposure, symptoms, test results, and clinical guidance recommendations (provided in Supplemental Materials). SARS-CoV-2 testing (nucleic acid amplification test) for COVID-19 was offered at no cost to HCP. Human Resources policies allowed up to 10 days paid time off for confirmed COVID-19 illness and self-isolation, including cases of reinfection. Data was collected from existing institution repositories: Power Apps-based employee COVID-19 self-reporting tool, Employee Health databases with COVID-19 reported illness information, and HCP COVID-19 contact tracing application. Access to data was password protected and limited to the project team. Data documented within a secure intranet system was de-identified for analysis.
Participants
All HCP who had a SARS-CoV-2 positive test result were invited to participate in the 5-Day Early RTW program if they were determined to be ≤4 days since date of positive test result. HCP were excluded if their primary location of employment was within a long-term care community or outside the state of Pennsylvania (due to location-specific regulations). Systemwide, at the start of the study period there were 87,765 HCP with a 70% vaccination level and at the end of the study period, there were 88,183 HCP with an 89% vaccination level. During the 3-month study period, 5,937 (7%) HCP had a documented diagnosis of COVID-19.
Intervention
A multidisciplinary collaboration including the departments of Infection Prevention and Control; Quality, Safety, and Innovation; Human Resources; Employee Health; and Laboratory Services synthesized current evidence, CDC guidelines, and institution policies to design the 5-Day Early RTW program. This new program offered voluntary self-enrollment and allowed HCP, who had an initial COVID-19 positive test result, were not immunocompromised, and felt well enough to return to work, the opportunity to self-report results of a SARS-CoV-2 antigen test on day 5 of their isolation and, if negative, return to work on day 6. If the HCP delayed their self-collection of a SARS-CoV-2 antigen test later than day 5, they were eligible to RTW the subsequent day of a negative test result. Repeat testing after self-reporting a SARS-CoV-2 antigen positive test result was not considered (eg, if HCP had a positive test on day 5, they were not allowed to RTW before day 11, even with a subsequent SARS-CoV-2 antigen negative test result).
Clinical guidance was provided by Employee Health (or the HCP's personal physician) on the likelihood of a false negative symptomatic test result; retesting was recommended on a case-by-case basis. For negative test HCP with symptoms of a viral respiratory infection, clinical guidance was provided by Employee Health (or the HCP's personal physician) according to the Evaluation of Illness criteria in the Employee Viral Respiratory Infection Guidelines Including Influenza and SARS-CoV-2 (COVID-19) Viruses (provided in Supplemental Materials).
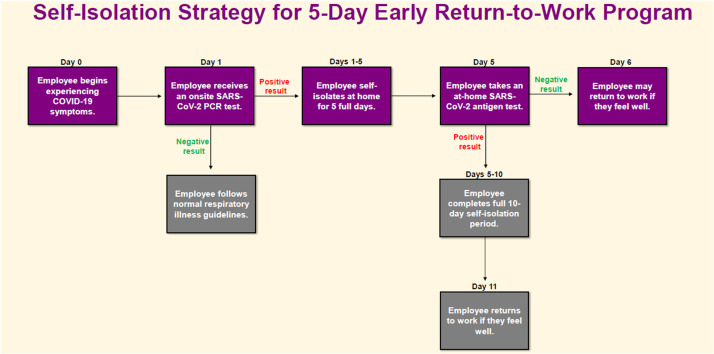
Acute care facility teams established on-site collection locations for initial onset COVID-19 testing and drive through pick up locations for SARS-CoV-2 antigen test kits; test kit distributions were recorded to reduce risk of misuse/allocation of test kits. HCP were required to use an internal web-based reporting tool to self-report their eligibility attestation and SARS-CoV-2 antigen test results (Supplementary Fig S1 and Fig S2). To aid in educating our health system leaders and project teams we designed a flowchart of the self-isolation strategy for the 5-Day Early RTW program (Fig 1 ).
Fig 1.
Health care personnel 5-day early return-to-work program flowchart. PCR, polymerase chain reaction.
Outcome measurements
Primary outcomes include the number of HCP eligible for early RTW and total number of days saved from missed work due to self-isolation. To quantify the potential benefit of the program, the total number of HCP eligible for early RTW was calculated as the sum of HCP who had a negative test on day 5 and the number of days saved from missed work in self-isolation was calculated as the sum of maximum days in self-isolation (10 days) subtracted by actual days in self-isolation (minimum = 5 days, maximum = 10 days per person).
Secondary outcomes include 2 sources of cost: transmission after early return to work and number of tests performed. To estimate this risk on the HCP, we cross-reference HCP who self-reported early return to work with HCP in the existing Infection Prevention and Control for Employee Health contact tracing application to see if any HCP participating in the 5-Day Early RTW program were subsequently part of a potential COVID-19 transmission cluster. The cost of the program was also focused on the purchase of SARS-CoV-2 antigen test kits for distribution. We calculated the number of tests purchased and dispensed that were required to save one day of HCP time.
Statistical analysis
Data collected during the project were analyzed using descriptive statistics (means, percentages, and standard deviations). No formal hypothesis testing was done.
Results
During the study analysis period between January 4 and April 3, 2022, there were 87,765 HCP and 88,183 HCP systemwide, respectively. During this time, there were 5,937 (6.7%) HCP diagnosed by Employee Health or reported an external diagnosis of COVID-19 to Employee Health, accounting for approximately 44,100 days lost to COVID-19. Of the 5,937 HCP, 1,023 (17.3%) participated in self-reported antigen testing on or after day 5. Among 1,023 self-reporting antigen test results, 679 (66.4%) were positive and 344 (33.6%) were negative. Of the 344 HCP self-reporting a negative test, 161 (46.8%) were eligible for early RTW, which is 15.7% (161 of 1,023) of all HCP self-reporting as part of the program, and 2.7% (161 of 5,937) of all HCP with a diagnosis of COVID-19 during the 3-month study period. The remaining 183 (53.2%) HCP were excluded from the analysis for the following reasons: 2 (0.6%) were not cleared by employee health for early RTW until after the study end date due to a delay in data report generation; 42 (12.2%) were not cleared by employee health for early RTW due to risk of COVID-19 contagiousness (eg, continued symptoms and not feeling well enough to return to work); and 139 (40.4%) attested to meeting all study eligibility criteria for self-enrollment, but did not follow through in providing employee health with actual documentation of their initial external positive test result. Further exploration revealed that allowing HCP to self-enroll in the study without documentation to support eligibility presented an opportunity to obtain a free SARS-CoV-2 antigen test kit for personal use (eg, upcoming air travel, large crowd events, social gatherings, or curiosity).
Among the 161 HCP who met eligibility criteria to RTW early, 79% (128 of 161) actually RTW prior to day 11, on average 4.4 days early, and actual number of days saved from missed work secondary to the 5-Day Early RTW program for the 128 HCP was 714 days. Table 1 characterizes the impact of the program during each of the 3 months with different prevalence. The trend for prevalence of monthly COVID-19 positive tests for our systemwide community (HCP and patients combined) mirrors the monthly HCP only COVID-19 positive tests during the study period.
Table 1.
Health care personnel resources recovered during the 5-day early return-to-work program by month
| Period | Number of HCP cleared to RTW early | Frequency of COVID-19 positivity in reference populations |
Program participation measured as proportion of HCP who RTW early, by COVID-19 reporting status‡ |
RTW program days saved |
||||
|---|---|---|---|---|---|---|---|---|
| Community-wide testing* | Employee PCR self-collection program† | Eligible for RTW early (self-reporting COVID-19 negative test) | Participating in self-reporting (COVID-19 positive or negative test result) | All HCP reporting COVID-19 positive to employer | Potential | Actual | ||
| January 4, 2022-February 3, 2022 | 131 | 35% | 39% | 82% (281) | 82% (843) | 5,212 | 670 | 616 |
| February 4, 2022-March 3, 2022 | 26 | 13% | 21% | 14% (49) | 13% (133) | 588 | 95 | 78 |
| March 4, 2022-April 3, 2022 | 4 | 5% | 10% | 4% (14) | 5% (47) | 137 | 20 | 20 |
| Overall study period | 161 | 25% | 34% | 34% (344) | 17% (1,023) | 5,937 | 785 | 714 |
HCP, health care personnel; PCR, polymerase chain reaction; RTW, return-to-work.
Community-wide is defined as HCP and patients combined with COVID-19 tests performed at study facilities.
Does not account for unreported at-home results or results performed outside of study facilities.
Proportions are expressed as percentages.
There were no HCP who returned earlier than 10 days and subsequently part of a potential COVID-19 transmission cluster. During the evaluation period approximately 3,118 tests were purchased and distributed. Therefore, the number of SARS-CoV-2 antigen tests purchased, dispensed, and reported per 1 day of health care worker time was 4.4 (3,118/714).
Discussion
Persistence of the COVID-19 pandemic has led to growing staffing shortages. The 10-day self-isolation guideline for HCP with a SARS-CoV-2 positive test result was established to be conservative with respect to virus transmission.1 Efficiencies in methods for testing HCP to determine eligibility for early RTW programs are especially needed during surges of COVID-19 cases. Our methods for HCP self-collection and facility lab testing typically provided test results within 1 day, and HCP self-collection SARS-CoV-2 antigen test results were available and self-reported the same day. The adaptability to flex early RTW self-isolation periods without increased incidence of COVID-19 is critical for pandemic surge response. In this quality improvement project characterizing the impact of a 5-day early RTW program for HCP who self-reported a SARS-CoV-2 antigen negative test result on day 5 of isolation, we found that of those participating in the program and eligible for early RTW, 79.3% successfully RTW, on average 4.4 days early, and over a 3-month period accruing 714 days were recovered. Although the proportion of all COVID-19 positive HCP that returned early to work was very low, the costs of implementing the program per day saved were small, particularly given the voluntary nature of the program. These data support the approach that HCP may be eligible to RTW earlier than the conservative recommended quarantine period of 10 days, and our organization has continued the program.1
Risk reduction and degree of infectiousness with SARS-CoV-2 positivity and release from isolation is an ongoing challenge. A recent study of fully vaccinated National Football League (NFL) team members (N = 173) appears to refute the current CDC public health guidance focusing on 5 days4 with their conclusion that persons with COVID-19 should continue taking precautions, including correct and consistent mask use, for a full 10 days after symptom onset or after initial positive test result if they are asymptomatic.13 Another study reporting 5-day early RTW for health care workers (N = 729) found 80% were still SARS-CoV-2 antigen positive.14 Their conclusion for ending isolation after 5 days is also cautious, suggesting that during the 10 days after infection, persons might be infectious to others and are recommended to wear a well-fitting mask when around others, and to avoid contact with those at elevated risk for severe disease.14 While the NFL description reported that no one who returned to work early became sick,13 neither of the studies report on transmission (or absence of transmission) from individuals returning to activities before day 10. Moreover, there is added risk in 5-day early RTW since a substantial number of HCP may still be contagious, that is mitigated by universal masking and SARS-CoV-2 antigen negative test result; but unfortunately, these studies don't quantify how successful antigen testing is in risk reduction.
There are several limitations to the study. Several design elements of this quality improvement intervention result in limitations in the analysis or interpretation of the findings. Specimen self-collection and the test platform we used were not validated or medically observed, which could lead to false negatives. While the test characteristics of antigen testing to diagnose infection with SARS-CoV-2 are well described,6 work environment and patient care safety based upon the qualitative data of self-collection SARS-CoV-2 antigen testing remain unknown. However, this is not a study of analytic sensitivity, it is a pragmatic trial to test for feasibility and test positivity is a continuum of contagiousness risk, likely mitigated by universal masking (which may change). We did not repeat SARS-CoV-2 testing at day 10, so we may have missed virus acquisition after returning to work. Furthermore, case finding for transmission depended on limitations of contact tracing (not active surveillance). A limiting factor in the success of the program relates to the HCP voluntary self-selection and nonparticipation process design. As a result, there was a greater divide between the number of all HCP with documented COVID-19 and the number of HCP who may have negative test results on day 5 and RTW early. Therefore, we likely under-estimated the beneficial impact (and potential risk) of the program. Yet, even with this limitation, we demonstrated successful feasibility of HCP returning to work on day 6 following a negative test result on day 5 rather than the CDC's recommendation of returning to work on day 7.4
Conclusion
To optimize HCP workforce staffing availability during a COVID-19 pandemic surge, self-report antigen testing methods are needed to implement early RTW programs, while maintaining low risk of SARS-CoV-2 transmission.
Footnotes
Funding/support: This work did not receive external funding.
Conflict of interest: None of the authors received any payments or influence from a third-party source for the work presented.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.11.006.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Centers for Disease Control and Prevention. Strategies to mitigate healthcare personnel staffing shortages. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/mitigating-staff-shortages.html. Accessed October 22, 2021.
- 2.Byrne AW, McEvoy D, Collins AB, et al. Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention strategies for seasonal influenza in healthcare settings. https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed May 4, 2022.
- 4.Centers for Disease Control and Prevention. COVID-19: interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed May 4, 2022.
- 5.UPMC COVID-19 Update: Updates to UPMC health care worker quarantine and self-isolation guidelines. Accessed May 4, 2022.https://shared-services-upmc.newsweaver.com/icfiles/2/74241/222910/909505/2796dcd7acebaa37cef7824f/covid-19%20update %2012.30.21.pdf
- 6.Xie J-W, He Y, Zheng Y-W, et al. Diagnostic accuracy of rapid antigen test for SARS-CoV-2: a systematic review and meta-analysis of 166,943 suspected COVID-19 patients. Micro Res. 2022;265:127185. doi: 10.1016/j.micres.2022.127185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu VT, Schwartz NG, Donnelly MAP, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med. 2022;182:701–709. doi: 10.1001/jamainternmed.2022.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby JE, Riedel S, Dutta S, et al. Sars-Cov-2 antigen tests predict infectivity based on viral culture: comparison of antigen, PCR viral load, and viral culture testing on a large sample cohort. Clin Microbiol Infect. 2023;29:94–100. doi: 10.1016/j.cmi.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakki S, Zhou J, Jonnerby J, et al. Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study. Lancet Respir Med. 2022;10:1061–1073. doi: 10.1016/S2213-2600(22)00226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruscetti A, Chrisman M, Wagester S, et al. Healthcare personnel early return-to-work program after higher-risk SARS-CoV-2 exposure: a learning health system quality improvement project. AJIC. 2022;50:542–547. doi: 10.1016/j.ajic.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 12.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack CD, Wasserman EB, Killerby ME, et al. Results from a test-to-release from isolation strategy among fully vaccinated National Football League players and staff members with COVID-19 – United States, December 14-19, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:299–305. doi: 10.15585/mmwr.mm7108a4. [DOI] [PubMed] [Google Scholar]
- 14.Lefferts B, Blake I, Bruden D, et al. Antigen test positivity after COVID-19 isolation – Yukon-Kuskokwim Delta Region, Alaska, January-February 2022. MMWR Morb Wkly Rep. 2022;71:293–298. doi: 10.15585/mmwr.mm7108a3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.