Abstract
Introduction
SARS-CoV, MERS-CoV, and SARS-CoV-2, later named coronavirus disease 2019 (COVID-19), are three highly pathogenic and lethal human coronaviruses that have arisen in the last two decades. Pregnant women have a higher risk due to a special state of immunosuppression. However, there is no proof that pregnant women and their babies are more vulnerable to infection, as data is limited.The primary goal of this retrospective study is to examine the effects of early COVID-19 diagnosis and to address the best method of delivery based on medical records of neonatal and maternal outcomes observed at Nepal's Paropakar Maternity Hospital. This retrospective research will determine whether vaginal delivery is healthy compared to cesarean delivery and whether the outcome of a COVID-19 pregnancy in both mother and the baby is independent of the disease's status of the mother.
Methods
Study design: The proposed study is a retrospective cross-sectional study.
Patients population
104 cases of COVID positive pregnancy with vaginal delivery or caesarean section.
Results
The neonatal outcomes of COVID pregnancy revealed at least 51% baby born with Low APGAR score, 18% born prematurely, 19% with low birth weight, 7% requiring NICU admission, 3% Neonatal asphyxia, and 2% Neonatal death. Furthermore, when normal vaginal delivery and Cesarean section were compared there was no significant between the differences found in the outcomes
Conclusion
The COVID positive status had no association with the perinatal outcomes. Moreover, COVID status rarely affected the course of pregnancy.
Guideline
STROCSS 2021.
Keywords: COVID-19, Pregnancy, Neonatal outcome
Highlights
-
•
Pregnant women have a higher risk of COVID-19 infection due to a special state of immunosuppression.
-
•
There is very little possibility of intrauterine and intrapartum transmission of COVID.
-
•
When normal vaginal delivery and Cesarean section were compared there was no significant between the differences found in the outcomes.
-
•
COVID status rarely affected the course of pregnancy.
1. Introduction
SARS-CoV, MERS-CoV, and SARS-CoV-2, later named coronavirus disease 2019 (COVID-19), are three highly pathogenic and lethal human coronaviruses that have arisen in the last two decades [1]. Corona viruses are transmitted primarily through droplets produced when an infected individual coughs or sneezes, as well as through saliva or discharge droplets. Humans are generally susceptible to COVID-19 but pregnant women have a higher risk due to a special state of immunosuppression. Studies conducted on extreme acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) suggest a substantially higher case fatality rate in pregnant women than in non-pregnant women. However, due to the small number of studies, the same could not be verified for COVID [2] (see Table 3)
Table 3.
Pregnancy, delivery, and neonatal outcomes.
| Pregnancy and delivery | No of vaginal delivery | No of elective cesarean due to COVID infection | No of elective cesarean due to obstetric complications |
|---|---|---|---|
| Presence of gestational diabetes | 2 | 3 | |
| Weeks of gestation at delivery | |||
| <34 | 3 | 3 | |
| 34–37 | 6 | 7 | |
| >37 | 41 | 44 | |
| Neonatal outcomes | |||
| 1. Premature deliver | 7 | 4 | |
| 2. Low birth weight (<2500) | 4 | 7 | |
| 3. Low Apgar score at 5 min | 1 | 19 | |
| 4. NICU admission | 2 | 2 | |
| 5. Neonatal asphyxia | 1 | 1 | |
| 6. Neonatal death | 1 | 0 | |
| 7. COVID-19 positivity | 0 | 0 | 0 |
| Breastfeeding | 50 | 0 | 54 |
| Delayed cord clamping | 0 | 0 | 0 |
| Total | 50 | 0 | 54 |
The possibility of transmission during vaginal or cesarean delivery is poorly understood. Vertical transmission of viral infection occurs most commonly during intrauterine life through placental transfer, during delivery through ingestion or aspiration of cervico vaginal secretions, and during the postpartum period through breastfeeding. Vaginal delivery carries a higher risk of ingestion or aspiration of cervical secretion, as well as contact with infected perineal tissue [3]. There is no proof that pregnant women and their babies are more vulnerable to infection, as data is limited.
The primary goal of this research is to examine the effects of early COVID-19 diagnosis and to address the best method of delivery based on medical records of neonatal and maternal outcomes observed at Nepal's Paropakar Maternity Hospital. This retrospective research will determine whether vaginal delivery is healthy compared to cesarean delivery and whether the outcome of a COVID-19 pregnancy in both mother and the baby is independent of the disease's status of the mother.
2. Objectives
General: To highlight general maternal and neonatal outcomes after vaginal delivery from COVID-19 infected mothers.
Specific: To suggest whether vaginal delivery in COVID-19 pregnancy is safe.
3. Methods
Study design: The proposed study is a retrospective cross-sectional study.
Place, site of the institution, and duration of study: Paropakar Maternity Hospital, Division of Statistics, Kathmandu, Nepal; March 2019 to April 2021.
Sample size and sampling technique: Since very limited data is available regarding this kind of research and all those studies have reported less number of candidates enrolled as a limitation, we have no set sample size estimated for this study. Non-probability convenience sampling will be used. We included 104 cases of COVID positive pregnancy with various outcomes.
Inclusion criteria: Pregnant women with a confirmed diagnosis of COVID-19 with PCR, before or within 36 h after delivery.
Exclusion criteria: Pregnant women not diagnosed with COVID-19.
Methodology: This retrospective study included all women with a confirmed diagnosis of COVID-19 infection admitted to Paropakar Maternity Hospital who underwent vaginal delivery between March 2019 to April 2021 using a standardized questionnaire. Results were analyzed using appropriate statistical methods. Continuous data were expressed as mean ± standard deviation (SD) and as frequencies and percentages for all categorical variables. For the recorded variables, averages, range or proportion, and corresponding 95% CI was computed, as appropriate. Statistically significant differences among groups were tested using the common Chi-square test for heterogeneity.
Research Hypothesis: No core outcome was set in this research. Clinical data and radiological findings of pregnant women diagnosed with COVID-19 were reviewed according to WHO guidelines5, without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or to interpret results.
Data management and analysis: Preliminary data were collected from hospital records and an expert statistician will perform data management and analysis. Data was stored in an electronic database (MS Excel Sheet) and analyzed using the IBM Statistical Package for the Social Sciences Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA).
Ethical consideration: The study protocol was approved by the Institutional Review Board of Paropakar Maternity Hospital.
4. Results
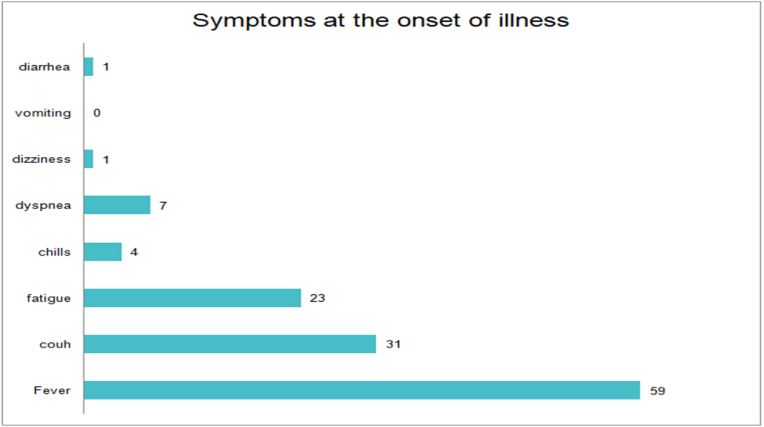
The mean age of the participants was 27 years with 91% of the patient having a history of exposure before the onset of illness. 83% of the women developed symptoms 5 days before admission. Most women complained of fever, cough, and fatigue and a significant number of participants reported shortness of breath (Table 1 Fig. 1).
Table 1.
Baseline characteristics of index pregnant patients with COVID-19.
| Median Age | 27 years |
| Exposure history within 14 days | 91% |
| Time from onset of illness to hospitalization (days) | Number of pregnant women |
| 1.0-1 | 18(17%) |
| 2.2-5 | 14(13%) |
| 3. >5 | 86 (82.69%) |
Fig. 1.
Symptoms of COVID
The hospital has a separate isolated ward for the COVID pregnancy but only 9 pregnant women reported home isolation when they started experiencing symptoms suggestive of COVID. (see Table 2)
Table 2.
Investigations.
| Confirmatory test done (SARS-CoV-2 quantitative RT-PCR) | 97 |
| Quarantine at home immediately after the onset of symptoms | 9 |
| Family member with PCR positive COVID | 1 |
| Lab findings | |
| 1. Lymphopenia(≤1100 cells/μL) | 0 |
| 2. Leukocytosis(>11,000 cells/μl) | 7 |
| 3. Elevated CRP(>10 mg/L) | 10 |
| 4. Elevated ALT(33IU/L) | 0 |
| CT evidence of pneumonia | 2 |
Most of the investigations done showed Positive RT-PCR and only a few reported deranged liver functions and reactive CRP. There is currently no clear evidence of definitive treatment for COVID-19, and the mainstay of treatment is supportive care. No specific treatments were given to the pregnant proceeding to delivery apart from symptomatic management and oxygen at a rate of 1–2L/min using a face mask to maintain saturation targeting SpO2 of 88–92%. No antibiotics, antivirals, or steroids were commenced for the treatment of SARS-COVII.
5. Outcome
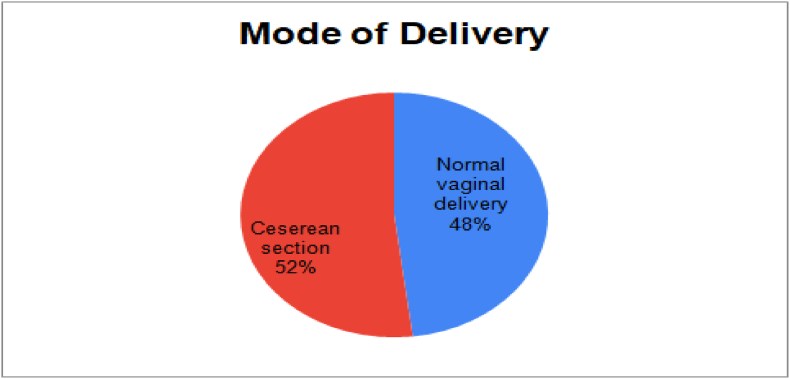
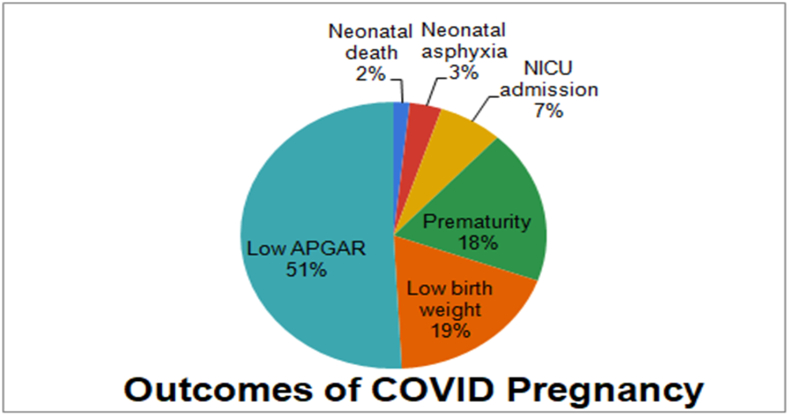
Among 104 COVID-positive women, 50 had a normal vaginal delivery and 54 underwent cesarean section due to obstetric complications and not owing to the COVID positive status (Fig. 2). Subsequently, the neonatal outcomes of COVID pregnancy are depicted in the figure below which reveals at least 51% baby born with Low APGAR score, 18% born prematurely, 19% with low birth weight, 7% requiring NICU admission, 3% Neonatal asphyxia, and 2% Neonatal death (see Fig. 3).
Fig. 2.
Delivery methods for COVID pregnancy.
Fig. 3.
Outcomes of COVID pregnancy.
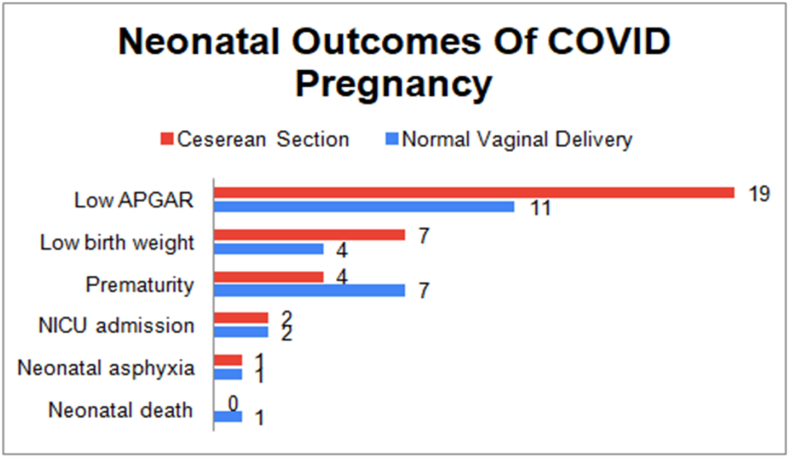
Furthermore, when normal vaginal delivery and Cesarean section were compared there was no significant between the differences found in the outcomes highlighting COVID status rarely affected the course of pregnancy (Fig. 4).
Fig. 4.
A comparison between neonatal outcomes of COVID pregnancy between Normal vaginal delivery and Cesarean section.
This study is in line with STROCSS criteria [18].
6. Discussion
We could confirm that, COVID status did not affect the mode of delivery and none underwent operative delivery for COVID. We further noticed none of the newborns delivered were COVID positive despite nearly all newborns were tested for COVID. Thus, we concluded very little possibility of intrauterine and intrapartum transmission of COVID-19 infection.
There is little evidence on the impact of the current COVID-19 outbreak on pregnant women and their infants. We found a variety of symptoms ranging from fever, malaise, headache, dizziness to shortness of breath but usual tell-tale signs like ageusia were grossly absent. Nearly 91% reported close contact history and no history of travel or family members with confirmed COVID diagnosis. A recent small series study conducted by Chen et al. showed diverse clinical presentations which varied from fever and cough. The neonatal outcomes, although four out of nine deliveries were premature, they concluded the preterm labors were independent of the disease status of the mother [4]. Our study further confirmed these findings. In addition, we found only a few pregnant women who suspected COVID undertake precautionary self–isolation measures at home pointing to difficulty in practicing social distancing at times when family care and support are most vital.
Our study included COVID-positive pregnancy of all three trimesters but unlike the study conducted by Chen et all which included third-trimester pregnancy only done by examining amniotic fluid, cord blood, and neonatal throat swab samples at birth during cesarean section, we only included hospital records. A thorough review of hospital records was made and clinical and laboratory investigations were recorded. The COVID positive status had no association with the perinatal outcomes. Only a few cases of deranged liver function were noted and few showed elevated inflammatory markers like CRP contrary to the study reported by Zhu et al. which predicted the perinatal 2019-nCoV infection may have serious consequences for mothers and newborns, including fetal distress, premature labor, respiratory distress, thrombocytopenia, abnormal liver function, and even death [5].
Our findings are further backed by the study done by Chen et al. who dismissed the possibility of intrauterine fetal infection although the third trimester, although the third trimester is associated with changes in lung physiology, such as decreases in ERV and FRC volumes and increases in respiratory resistance [4,6]. Furthermore, a study done by Ferrazzi et al. concluded, in moderate cases, vaginal delivery is acceptable, and cesarean section should be reserved for women who have serious respiratory issues, and delivering the baby would allow for better ventilation. While postpartum infection cannot be ruled out, their findings indicated that vaginal delivery is linked to a lower risk of SARS Cov2 transmission to the newborn. In addition, anecdotal evidence suggests a low risk of mother-to-child transmission [7]. According to a recent consensus, the evidence regarding optimal delivery timing, the safety of vaginal delivery, or whether cesarean delivery prevents vertical transmission at the time of delivery is unclear. The study also suggested the route of delivery and delivery timing should be individualized, based on obstetrical indications and maternal-fetal status [8].
A recommendation has been made on 14-day period isolation of neonates with advice against active breastfeeding from COVID positive mothers [9,10] but no such recommendations have been made in Nepal so far. Although some advocate early or delayed cord clamping, separation of the mother from the newborn, early cord clamping in extremely preterm babies, and no breastfeeding have not been shown to minimize the risk of transmission of other viral illnesses [11,12].The Paropakar Maternity Hospital did not use the controversial methods of neonatal isolation and delayed or early cord clamping.
The neonatal outcomes between a COVID and non- COVID pregnancy also had not very different outcomes and the pregnancy is not associated with an increased risk of spontaneous abortion and spontaneous preterm birth [13]. There were comparable numbers of newborns born prematurely with low APGAR scores requiring NICU admissions. Only 1 case of neonatal death was reported but the reason for the death could not be confirmed and the stillborn was reported to be COVID negative.
Based on these limited reports, there exist limitations due to the small size of the study, and the finite number of available data from other respiratory pathogens such as SARS and influenza, it is unknown whether pregnant women with COVID-19 will experience more severe disease [14].Our study was also a small sample study and had limited case reporting and filing. Only 104 cases of COVID pregnancy which included both the first and second waves of COVID in Nepal in one of the biggest governmental maternity hospitals is far from credible. We are skeptical if the hospital records truly represented the true number of cases in the real-time scenario.
7.Conclusion
Most of the studies found the minimal possibility of vertical transmission and perinatal transmission of COVID-19 [15,16]. Many studies indicate there is a potential threat to mother and child following infection which included fetal distress, premature labor, respiratory distress, thrombocytopenia accompanied by abnormal liver function, and even perinatal death but no such instances were reported in our study [16,17].
Ethical approval
The study protocol was approved by the Institutional Review Board of Paropakar Maternity Hospital.
Sources of funding
This retrospective study hasn't been funded by any person or any institutions.
Author contributions
-
•
Dr. Neela Sunuwar, Emergency Medical officer, Kulhuduffushi Regional Hospital, Maldives:study design and data collection
-
•
Dr. Anu Radha Twayana, MBBS graduate, Kathamdu University of Medical Sciences, Dhulikhel, Nepal: data collection
-
•
Sulav Deo, Department of medicine, Surakshya Hospital, Nepal: he has done analysis and interpretation
-
•
Prakash Paudel Jaishi, medical officer, Al Kamil Health center, Al Kamil, South Sharqiyah, Oman: he has done data analysis and interpretation
-
•
Prabhat Kiran Neupane, Internship at Department of Medicine, Kist Medical College, Kathmandu, Nepal: he has written the paper
-
•
Sandhya kiran Neupane, Medical officer, Shadhak Polyclinic, Kathmandu, Nepal.: she has written and edited the paper
Registration of research studies
-
1.
Name of the registry:
-
2.
Unique Identifying number or registration ID:
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Prakash Paudel Jaishi.
General Practitioner,Al Kamil Health center, Al Kamil, South Sharqiyah, Oman.
Consent
Written informed consent was obtained from the paropakar maternity hospital. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
There is no any conflicts of interest with this article.
Acknowledgments
We would like to thank Paroparkar Maternity Hospital and the Deputy Director of Paropakar Maternity Hospital Dr. Shree Prasad for his expert guidance and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104880.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chathappady House N.N., Palissery S., Sebastian H. Corona viruses: a review on SARS, MERS, and COVID-19. Microbiol. Insights. 2021 Jan 1;14 doi: 10.1177/11786361211002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 2020 May;222(5):415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S., et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis. BJOG An Int. J. Obstet. Gynaecol. 2020 Aug 1;127(9):1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet Lond Engl. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020 Feb;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamseddine R.S., Wahbeh F., Chervenak F., Salomon L.J., Ahmed B., Rafii A. Pregnancy and neonatal outcomes in SARS-CoV-2 infection: a systematic review. J Pregnancy. 2020 Oct 7 doi: 10.1155/2020/4592450. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Y C, J B . Arch Gynecol Obstet [Internet]; 2020 Sep. Maternal and Infant Outcomes of Full-Term Pregnancy Combined with COVID-2019 in Wuhan, China: Retrospective Case Series.https://pubmed.ncbi.nlm.nih.gov/32696241/ [cited 2021 May 18];302(3). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D., Yang H., Cao Y., Cheng W., Duan T., Fan C., et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID‐19) infection. Int. J. Gynecol. Obstet. 2020 May 1;149(2):130–136. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Shi Y., Xiao T., Fu J., Feng X., Mu D., et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann. Transl. Med. 2020 Feb;8(3) doi: 10.21037/atm.2020.02.20. 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favre G., Pomar L., Qi X., Nielsen-Saines K., Musso D., Baud D. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect. Dis. 2020 Jun 1;20(6):652–653. doi: 10.1016/S1473-3099(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid M.B., Fontijn J., Ochsenbein-Kölble N., Berger C., Bassler D. COVID-19 in pregnant women. Lancet Infect. Dis. 2020 Jun 1;20(6):653. doi: 10.1016/S1473-3099(20)30175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dashraath P., Wong J.L.J., Lim M.X.K., Lim L.M., Li S., Biswas A., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020 Jun 1;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020 Jul;223(1):111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2020 Jul;56(1):15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur. J. Med. Res. 2020 Sep 4;25(1):39. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 May;2(2) doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng Yin K., Lee K.S., Zhang J.J.Y. Potential challenges in managing obstetrical patients with coronavirus disease 2019. Am. J. Obstet. Gynecol. 2020 Nov;223(5):783–784. doi: 10.1016/j.ajog.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew G., Agha R., for the STROCSS Group STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.