Abstract
Background
Few studies have characterized methadone-involved overdose deaths in the US since 2014 despite changing patterns of opioid use, the onset of the COVID-19 pandemic, and changes to take-home dose guidance in opioid treatment programs (OTPs) in March 2020.
Methods
Data on monthly overdose deaths in the US from January 1, 2007 to March 31, 2021 were obtained through CDC WONDER. Interrupted time series models were used to assess for changes in series levels starting in April 2020. Analyses were stratified by involvement of synthetic opioids in overdose deaths.
Results
An increase in methadone-involved overdoses of 105.4 deaths per month (95 % CI: 73.8–137.0) occurred starting in April 2020 compared with prior trends (p < 0.001). Trends in methadone-involved overdose deaths showed a step increase starting in April 2020 both with (54.2 deaths per month; 95 % CI: 39.4–68.9) and without (51.7 deaths per month; 95 % CI: 23.4–78.0) synthetic opioid involvement (p < 0.001 for both). Among overdose deaths without synthetic opioids, the increase in methadone-involved overdose deaths accounted for 26.5 % of the increase between the 12-month periods before and after March 2020. The relative percentage increase in methadone-involved overdose deaths, both with and without synthetic opioid co-involvement, was highest among Hispanic and non-Hispanic Black individuals.
Conclusions
Methadone-involved overdose deaths, both with and without other synthetic opioid co-involvement, increased during the 12-month period after March 2020, compared with prior trends. These results provide a cautionary addition to previous findings of no or limited methadone-related harms after the US regulatory changes during the COVID-19 pandemic.
Keywords: Methadone, COVID-19, Overdose, Mortality
Abbreviations: OUD, opioid use disorder
1. Introduction
Methadone treatment reduces mortality among patients with opioid use disorder (OUD), yet methadone can also contribute to overdoses (Schuckit, 2016). In the US, methadone-involved overdoses have been most closely linked with the prescription of methadone for analgesia. During 2002–2014, the quantity of methadone prescribed as an analgesic was strongly associated with reports of diverted methadone and with methadone-involved overdose deaths (Jones, 2016). Following interventions to reduce methadone prescribing for analgesia, methadone-involved overdose deaths declined even as increasing numbers of individuals received methadone as treatment for opioid use disorder (Jones, 2016).
Few studies have described methadone-involved overdose deaths in the US since 2014, despite substantial changes in patterns of opioid use and the increased involvement of high-potency synthetic opioids in overdose deaths (Jones et al., 2018). Most significantly, patterns of methadone-involved overdose deaths since the onset of the COVID-19 pandemic have had limited characterization, despite large increases in total opioid-involved overdose deaths, particularly among racial and ethnic minority groups, and the increased eligibility for take-home doses through opioid treatment programs (OTPs) starting in March 2020 (Brothers et al., 2021, Amram et al., 2021, Jones et al., 2022).
Using death certificate data from the National Vital Statistics System, this study characterized methadone-involved overdose deaths throughout the US from January 1, 2007 to March 31, 2021, with a focus on the periods before and after the COVID-19 pandemic started in March 2020. Changes in methadone-involved overdose deaths were compared with changes in total overdose deaths without methadone. Given the substantial increase in synthetic opioid involved overdose deaths since 2019, analyses were stratified by involvement of synthetic opioids in overdose deaths (Hedegaard et al., 2021).
2. Methods
Overdose deaths among U.S. residents in the 50 states and the District of Columbia were identified through CDC WONDER on a monthly basis between January 2007 and March 2021 (National Center for Health Statistics). Overdose deaths prior to January 1, 2021 reflect final confirmed counts, while overdose deaths between January 1, 2021 and March 31, 2021 reflect provisional counts (National Center for Health Statistics). Overdose deaths were analyzed starting in 2007 given the release of methadone-related warnings from the Food and Drug Administration in 2006 and previous reports documenting decreasing methadone-related deaths beginning in 2007 (Jones, 2016).
Overdose deaths were identified based on ICD-10 codes of X40–44 (unintentional poisoning), X60–64 (intentional self-poisoning), X85 (homicide), and Y10–14 (undetermined intent) as underlying cause of death (Centers for Disease Control and Prevention, 2018). Methadone-involved overdose deaths were identified when ICD-10 code T40.3 was a contributing cause of death. Other involved substances were identified by ICD-10 codes listed as a contributing cause of death. Non-methadone opioids were identified based on codes T40.0 (opium), T40.1 (heroin), T40.2 (natural and semi-synthetic opioids [NSS]), T40.4 (synthetic opioids), and T40.6 (other and unspecified opioids). (Though methadone is also a synthetic opioid, the term ‘synthetic opioid’ in this manuscript will be reserved for the involvement of non-methadone synthetic opioids designated by ICD-10 code T40.4). Non-opioid involved substances were identified from codes T40.5 (cocaine), T42.4 (benzodiazepines), T43.6 (other psychostimulants), and T51.0 and T51.9 (ethanol and unspecified alcohols) (Jones et al., 2018, Kleinman and Weiss, 2022). Demographic information about the decedents was obtained through aggregated mortality reports from CDC WONDER that were stratified by demographic variable.
The demographics of decedents, co-involved substances, and trends in overdose deaths were descriptively analyzed. Monthly overdose trends were analyzed using seasonal autoregressive integrated moving average (ARIMA) modelling, with an interrupted time-series framework, to assess for changes in the level of the series starting after March 2020. Seasonal ARIMA models account for historical trends, lagged effects, seasonality, and autocorrelation between months of overdose trends (Schaffer et al., 2021). In a first step, seasonal ARIMA models were using fitted to trends in methadone-involved overdose deaths occurring from January 2007 to February 2020, using an automated procedure (Hyndman and Khandakar, 2008). We used augmented Dickey-Fuller tests to confirm stationarity after differencing and Box-Pierce tests to test for independence of residuals. We then modelled a seasonal ARIMA function using the same parameters as the model from January 2007 to February 2020, but with the addition of a step effect for months from April 2020 to March 2021. March 2020 was not included in the step effect given that OTP regulatory changes occurred part-way through the month (Substance Abuse and Mental Health Services Administration, 2020). The step effect represents the change in the level of monthly methadone-involved overdose deaths occurring starting in April 2020 compared with the expected values based on the prior trends.
For year-over-year analyses, we used chi-squared tests to compare the proportional changes in methadone-involved overdose deaths between the 12 months before and after March 2020 with the proportional changes in total overdose deaths without methadone. Given the substantial increase in synthetic opioid involved overdose deaths during the COVID-19 pandemic, analyses were stratified by involvement of synthetic opioids in overdose deaths.
To compare counts of methadone-involved overdose deaths within a demographic group between the 12-month periods before and after March 2020, we used z-tests under a normal approximation to a presumed Poisson distribution as recommended by Centers for Disease Control guidance for annual death counts > 100 (Xu et al., 2021). All statistical tests were conducted post-hoc and should be considered exploratory.
The Centre for Addiction and Mental Health Research Ethics Board determined that this study was exempt from review, in accordance with the Tri-Council Policy Statement on Research Ethics 2. The report follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Data was analyzed between January 7, 2022 and August 5, 2022 using R, version 4.1.1 (R Foundation for Statistical Computing).
3. Results
3.1. Time-series analyses
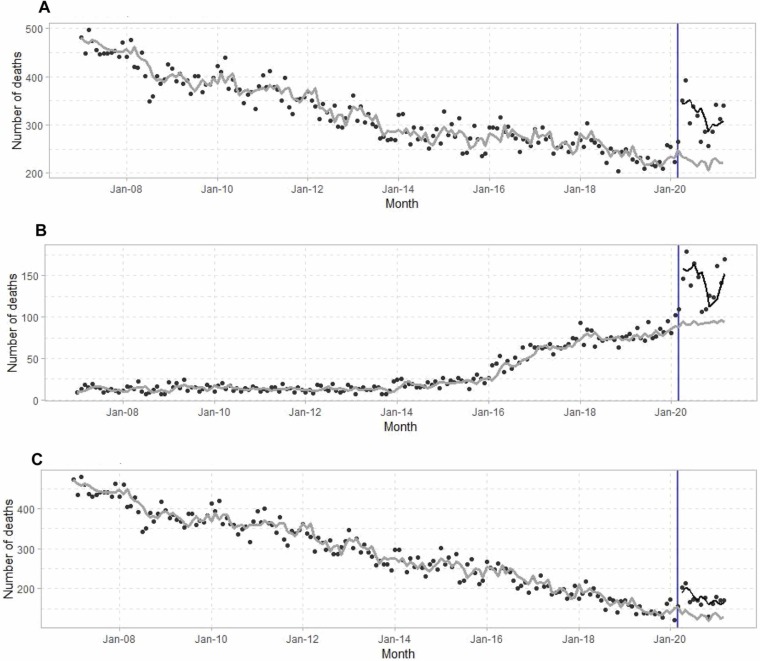
A total 55,225 methadone-involved overdose deaths occurred in the United States between January 1, 2007 and March 31, 2021 ( Fig. 1a). Monthly methadone-involved overdose deaths decreased from January 2007 to February 2020. A step effect of an additional 105.4 (95 % CI: 73.8–137.0) monthly methadone-involved overdose deaths starting after March 2020 (p < 0.001 for step effect). Monthly overdose deaths co-involving methadone and synthetic opioids increased by 54.2 deaths per month (95 % CI: 39.4–68.9) starting in April 2020 compared with the expected values based on prior trends (p < 0.001) (Fig. 1b). Monthly overdose deaths involving methadone without synthetic opioids increased 51.7 deaths per month (95 % CI: 23.4 – 78.0) starting in April 2020 compared with the expected values based on prior trends (p < 0.001) (Fig. 1c).
Fig. 1.
Time-series of monthly methadone-involved overdose deaths from January 2007 to March 2021. March 2020 is identified with the vertical line. Seasonal ARIMA models were used to generate estimates of monthly overdose deaths based on trends prior to March 2020 (grey line) and with a step effect occurring after March 2020 (black line). (A) All overdose deaths involving methadone. (B) Overdose deaths involving methadone with synthetic opioids (T40.4). (C) Overdose deaths involving methadone without synthetic opioids.
3.2. Year-over-year analyses
Methadone-involved overdose deaths increased by 1067 (39.1 %) between the 12-month periods before and after March 2020, while total overdose deaths without methadone involvement increased by 23,611 (33.7 %) (χ2 = 2.38 for proportional increases, df = 1, p = 0.12) ( Table 2). Methadone-involved overdose deaths increased by 195 (70.4 %) among Hispanic individuals, by 209 (57.1 %) among non-Hispanic Black individuals, and by 627 (31.4 %) among non-Hispanic White individuals (Table 2). Total overdose deaths without methadone increased by 23,611 (33.7 %), with increases of 3482 (46.1 %) among Hispanic individuals, 5271 (49.4 %), non-Hispanic Black individuals, and 13,610 (27.6 %,) non-Hispanic White individuals.
Table 2.
Differences in total overdose deaths, total overdose deaths without methadone, and methadone-involved overdose deaths between the 12-month periods before and after March 2020.
| Total overdose deaths | Total overdose deaths without methadone | Methadone-involved overdose deaths | p-value | |
|---|---|---|---|---|
| All | 24,678 (33.9 %) | 23,611 (33.7 %) | 1067 (39.1 %) | < 0.001 |
| Gender | ||||
| Female | 6474 (27.8 %) | 6097 (27.5 %) | 377 (32.6 %) | < 0.001 |
| Male | 18,204 (36.8 %) | 17,514 (36.5 %) | 690 (43.9 %) | < 0.001 |
| Race/ethnicity | ||||
| Hispanic | 3677 (47 %) | 3482 (46.1 %) | 195 (70.4 %) | < 0.001 |
| Non-Hispanic Black | 5480 (49.7 %) | 5271 (49.4 %) | 209 (57.1 %) | < 0.001 |
| Non-Hispanic White | 14,237 (27.7 %) | 13,610 (27.6 %) | 627 (31.4 %) | < 0.001 |
| Other | 1277 (48.9 %) | 1241 (49.1 %) | 36 (41.9 %) | 0.015 |
| With synthetic opioid involvement | 23,345 (60.7 %) | 22,631 (60.5 %) | 714 (71.4 %) | < 0.001 |
| Sex | ||||
| Female | 6193 (58.2 %) | 5971 (58.1 %) | 222 (60.7 %) | < 0.001 |
| Male | 17,152 (61.7 %) | 16,660 (61.4 %) | 492 (77.6 %) | < 0.001 |
| Race/ethnicity | ||||
| Hispanic | 3203 (77.9 %) | 3087 (77.2 %) | 116 (101.8 %) | < 0.001 |
| Non-Hispanic Black | 4833 (74.2 %) | 4673 (74.2 %) | 160 (75.8 %) | < 0.001 |
| Non-Hispanic White | 14,200 (53.1 %) | 13,796 (52.9 %) | 404 (62.1 %) | < 0.001 |
| Other | 1109 (104.9 %) | – | – | – |
| Without synthetic opioid involvement | 1333 (3.9 %) | 980 (3 %) | 353 (20.4 %) | < 0.001 |
| Sex | ||||
| Female | 281 (2.2 %) | 126 (1.1 %) | 155 (19.6 %) | < 0.001 |
| Male | 1052 (4.8 %) | 854 (4.1 %) | 198 (21.1 %) | < 0.001 |
| Race/ethnicity | ||||
| Hispanic | 474 (12.8 %) | 395 (11.1 %) | 79 (48.5 %) | < 0.001 |
| Non-Hispanic Black | 647 (14.3 %) | 598 (13.7 %) | 49 (31.6 %) | 0.01 |
| Non-Hispanic White | 37 (0.2 %) | -186 (−0.8 %) | 223 (16.5 %) | < 0.001 |
| Other | 168 (10.8 %) | – | – | – |
Relative percentage increases from the 12 months before March 2020 to the 12 months after March 2020 are shown in parentheses. P-values compare counts of methadone-involved overdose deaths between the two 12-month periods. P-values are calculated using z-tests. Omitted data could not be displayed due to data suppression rules.
3.2.1. Synthetic opioid co-involvement
Overdose deaths co-involving methadone and synthetic opioids increased by 714 (71.4 %) during the 12-month period after March 2020 compared with the 12 months prior to March 2020 (Table 2). In comparison, total overdose deaths involving synthetic opioids and without methadone increased by 22,631 (60.5 %). There was no significant difference in proportional increases (χ2 = 2.61, df = 1, p = 0.11). The number of methadone-involved overdose deaths co-involving synthetic opioids increased by 116 (101.8 %) among Hispanic individuals, 160 (75.8 %) among non-Hispanic Black individuals and 404 (62.1 %) among non-Hispanic White individuals. In comparison, total overdose deaths with synthetic opioid involvement and without involving methadone increased by 3087 (77.2 %), 4673 (74.2 %), and 13,796 (52.9 %) among Hispanic, non-Hispanic Black and non-Hispanic White individuals, respectively.
3.2.2. Without synthetic opioid co-involvement
Overdose deaths involving methadone without synthetic opioids increased by 353 (20.4 %) between the 12-month periods before and after March 2020 (Table 2). The increase in total overdose deaths without synthetic opioids and without methadone over the same period was 980 (3.0 %). The proportional increase was significantly higher among overdose deaths involving methadone without synthetic opioids (χ2 = 21.7, df = 1, p < 0.001). The increase in methadone-involved overdose deaths without synthetic opioids represented 26.5 % of the total increase in overdose deaths without synthetic opioids between the two 12-month periods.
Methadone-involved overdose deaths without synthetic opioids increased by 79 (48.5 %) among Hispanic individuals, 49 (31.6 %) among non-Hispanic Black individuals and 223 (16.5 %) among non-Hispanic White individuals. These contrast with increases of 395 (11.1 %), 598 (13.7 %) in total overdose deaths without synthetic opioid and without methadone involvement among Hispanic and non-Hispanic Black individuals, respectively, and a decrease of 186 (−0.8 %) among non-Hispanic White individuals.
3.2.3. Co-involved substances
In the 12-months prior to March 2020, (52.6 %) of methadone-involved overdoses involved another opioid (synthetic opioids: 1000 [36.7 %]; heroin: 510 [18.7 %], NSS: 470 [17.2 %]), 758 (27.8 %) involved benzodiazepines, 432 (15.8 %) involved cocaine, 372 (13.6 %) involved other psychostimulants, and 270 (9.9 %) involved ethanol or unspecified alcohols (Table 1). 638 deaths (23.4 %) did not co-involve additional opioids, benzodiazepines, cocaine, other psychostimulants or ethanol/unspecified alcohols.
Table 1.
Methadone-involved overdose deaths during March 2019 – February 2020 and April 2020 – March 2021.
| March 2019 – February 2020 |
April 2020 – March 2021a |
|||||
|---|---|---|---|---|---|---|
| All overdose deaths | All overdose deaths without methadone | Overdose deaths involving methadone | All overdose deaths | All overdose deaths without methadone | Overdose deaths involving methadone | |
| All | 72,809 (100 %) | 70,081 (100 %) | 2728 (100 %) | 97,487 (100 %) | 93,692 (100 %) | 3795 (100 %) |
| Sex | ||||||
| Female | 23,314 (32 %) | 22,158 (31.6 %) | 1156 (42.4 %) | 29,788 (30.6 %) | 28,255 (30.2 %) | 1533 (40.4 %) |
| Male | 49,495 (68 %) | 47,923 (68.4 %) | 1572 (57.6 %) | 67,699 (69.4 %) | 65,437 (69.8 %) | 2262 (59.6 %) |
| Race/ethnicity | ||||||
| Hispanic | 7822 (10.7 %) | 7545 (10.8 %) | 277 (10.2 %) | 11,499 (11.8 %) | 11,027 (11.8 %) | 472 (12.4 %) |
| Non-Hispanic Black | 11,036 (15.2 %) | 10,670 (15.2 %) | 366 (13.4 %) | 16,516 (16.9 %) | 15,941 (17 %) | 575 (15.2 %) |
| Non-Hispanic White | 51,338 (70.5 %) | 49,339 (70.4 %) | 1999 (73.3 %) | 65,575 (67.3 %) | 62,949 (67.2 %) | 2626 (69.2 %) |
| Otherb | 2613 (3.6 %) | 2527 (3.6 %) | 86 (3.2 %) | 3890 (4.0 %) | 3768 (4.0 %) | 122 (3.2 %) |
| Age | ||||||
| 0–19 | 1086 (1.5 %) | 1056 (1.5 %) | 30 (1.1 %) | 1902 (2.0 %) | 1854 (2.0 %) | 48 (1.3 %) |
| 20–29 | 11,783 (16.2 %) | 11,526 (16.4 %) | 257 (9.4 %) | 16,279 (16.7 %) | 15,928 (17 %) | 351 (9.2 %) |
| 30–39 | 18,779 (25.8 %) | 18,091 (25.8 %) | 688 (25.2 %) | 25,826 (26.5 %) | 24,913 (26.6 %) | 913 (24.1 %) |
| 40–49 | 15,523 (21.3 %) | 14,936 (21.3 %) | 587 (21.5 %) | 20,999 (21.5 %) | 20,130 (21.5 %) | 869 (22.9 %) |
| 50–59 | 15,654 (21.5 %) | 14,959 (21.3 %) | 695 (25.5 %) | 19,951 (20.5 %) | 19,043 (20.3 %) | 908 (23.9 %) |
| 60–69 | 7984 (11 %) | 7572 (10.8 %) | 412 (15.1 %) | 10,362 (10.6 %) | 9734 (10.4 %) | 628 (16.5 %) |
| 70+ | 1990 (2.7 %) | 1932 (2.8 %) | 58 (2.1 %) | 2154 (2.2 %) | 2077 (2.2 %) | 77 (2.0 %) |
| Co-involved substances | ||||||
| Synthetic opioidsc | 38,435 (52.8 %) | 37,435 (53.4 %) | 1000 (36.7 %) | 61,780 (63.4 %) | 60,066 (64.1 %) | 1714 (45.2 %) |
| Heroin | 13,916 (19.1 %) | 13,406 (19.1 %) | 510 (18.7 %) | 12,450 (12.8 %) | 11,838 (12.6 %) | 612 (16.1 %) |
| NSS | 12,044 (16.5 %) | 11,574 (16.5 %) | 470 (17.2 %) | 13,772 (14.1 %) | 13,126 (14 %) | 646 (17.0 %) |
| Benzodiazepines | 9927 (13.6 %) | 9169 (13.1 %) | 758 (27.8 %) | 12,700 (13 %) | 11,719 (12.5 %) | 981 (25.8 %) |
| Cocaine | 16,613 (22.8 %) | 16,181 (23.1 %) | 432 (15.8 %) | 20,273 (20.8 %) | 19,667 (21 %) | 606 (16.0 %) |
| Psychostimulantsd | 16,925 (23.2 %) | 16,553 (23.6 %) | 372 (13.6 %) | 26,512 (27.2 %) | 25,899 (27.6 %) | 613 (16.2 %) |
| Alcoholse | 10,318 (14.2 %) | 10,048 (14.3 %) | 270 (9.9 %) | 14,123 (14.5 %) | 13,725 (14.6 %) | 398 (10.5 %) |
Counts from January – March 2021 reflect provisional figures.
Includes non-Hispanic individuals of other races (including multiple races) and individuals for whom Hispanic ethnicity status is not stated.
ICD-10 code T40.4: synthetic opioids excluding methadone.
ICD-10 code T43.6: psychostimulants other than cocaine.
ICD-10 codes T51.0 and T51.9: ethanol and unspecified alcohols.
In the 12 months after March 2020, 2191 (57.7 %) methadone-involved overdoses involved another opioid (other synthetic opioids: 1714 [45.2 %]; heroin: 612 [16.1 %], NSS: 646 [17.0 %]), 981 (25.8 %) involved benzodiazepines, 606 (16.0 %) involved cocaine, 613 (16.2 %) involved other psychostimulants, and 613 (10.5 %) involved ethanol or unspecified alcohols (Table 1). 750 deaths (19.8 %) did not involve additional opioids, benzodiazepines, cocaine, other psychostimulants, ethanol or unspecified alcohols.
4. Discussion
Methadone-involved overdose deaths among U.S. residents increased in the 12-months after March 2020 compared with prior trends. Methadone-involved overdose deaths increased above previous trends both with and without co-involvement of synthetic opioids. In analyses with and without involvement of synthetic opioids, there was an initial spike in methadone-involved overdose deaths during March – May 2020, after which these deaths stabilized but remained elevated above their prior trends. In year-over-year analyses comparing the 12 months before and after March 2020, methadone-involved overdose deaths without synthetic opioids had a significantly larger proportional increase than total overdoses without synthetic opioids or methadone. Among overdose deaths without synthetic opioids, the increase in methadone-involved overdose deaths accounted for 26.5 % of the total increase between the two 12-month periods. The proportional increase in methadone-involved overdose deaths that co-involved synthetic was not significantly higher than overdose deaths involving synthetic opioids without methadone. The increases in methadone-involved overdose deaths occurred despite a 24 % reduction in the number of patients receiving methadone in the US between 2019 and 2020 (from 408,550 in 2019 to 311,531 in 2020) (Substance Abuse and Mental Health Services Administration, 2021).
The number of individuals with methadone-involved overdose deaths increased in each demographic group, with the most prominent percentage increases among Hispanic and non-Hispanic Black individuals (both with and without co-involvement of synthetic opioids). It is unknown whether these overdose deaths occurred among individuals receiving methadone from OTPs or from other sources. Previous analyses have highlighted numerous ways in which structural racism has been embedded within OTPs, and follow-up studies are needed to determine whether changes in OTP treatment provision during COVID-19 were implemented in a way that disproportionately harmed Hispanic and non-Hispanic Black individuals (Peterkin et al., 2021).
Previous studies have also assessed whether there were changes in methadone-associated harms associated with the onset of the COVID-19 pandemic and OTP regulatory changes in the US (Amram et al., 2021, Jones et al., 2022, Welsh et al., 2022). On March 16, 2020, the Substance Abuse and Mental Health Services Administration released guidance allowing states to request blanket exceptions to federal regulations limiting take-home methadone doses. These exceptions allowed up to 28 days of take-home doses for stable patients and 14 days of take home doses for less stable patients, who an OTP believed could handle take-home doses (Substance Abuse and Mental Health Services Administration, 2020). Previous regulations limited take-home doses to once per week over the first 90 days of treatment and 2 take-home doses over the subsequent 90 days (in addition to a single take-home dose for days in which the clinic was closed) (Substance Abuse and Mental Health Services Administration, 2015).
A nationwide study performed an interrupted time-series analysis (ITSA) to model methadone-involved overdoses before and after March 2020 (Jones et al., 2022). The study found, that as a proportion of overall ITSA-estimated overdose deaths, ITSA-estimated methadone-involved overdose deaths remained similar before and after March 2020 (Jones et al., 2022). The study also identified an increase in methadone-involved overdose deaths in March 2020 and attributed the increase to the increase in fentanyl driven deaths rather than OTP changes. However, the previous analysis did not stratify overdose deaths by synthetic opioid involvement, and in emphasizing the proportion of total overdose deaths involving methadone, obscured large relative percentage increases in methadone-involved overdose deaths.
Our analysis clarifies that methadone-involved overdose deaths without synthetic opioid co-involvement increased more rapidly than total overdose deaths without synthetic opioids, and that overdose deaths involving methadone and synthetic opioids increased at least as rapidly as total overdose deaths involving synthetic opioids. The lower base rate of synthetic opioid involvement among methadone-involved overdose deaths than among total overdose deaths accounts for the difference in results when stratifying. These results highlight the importance of stratification along synthetic opioid involvement during the period under study.
Findings from international jurisdictions provide conflicting results about an association between increased methadone take-home doses and adverse events. In England, guidance recommended that individuals prescribed opioid agonist treatments during the COVID-19 pandemic, be provided with two weeks of carries during March – May 2020. A time-series ecological study from England found that methadone-involved overdose deaths increased both among recipients (22 %) and non-recipients of prescribed opioid agonist treatments (74 %). Unsupervised dosing of methadone for OUD in the late 1990s and early 2000s was associated with high overdose rates in England and Scotland, and the rate of methadone-involved overdoses per quantity of methadone dispensed decreased after supervised dosing was instituted (Strang et al., 2010). In Ontario, Canada, where methadone can be prescribed from office-based settings and dispensed at pharmacies, a retrospective propensity-matched cohort study using administrative data found that individuals whose methadone dispensing was changed from daily to receiving 5 – 6 take home doses per week had lower rates of treatment discontinuation, opioid overdose and than matched individuals continuing daily dispensing (Kleinman et al., 2022, Gomes et al., 2022). However, residual confounding remains possible, particularly since Ontario’s guidance at the time recommended that an assessment of suitability for take-home doses be based on social stability and a patient’s ability to safely manage take-home doses, markers of clinical stability that may not be reflected in administrative data (Lam et al., 2022).
The results in this study make several important contributions to the understanding of methadone-involved overdose deaths. First, the study uses a nationwide, population-level data set and provides the most comprehensive assessment of methadone-involved overdose deaths during the year before and during the COVID-19 pandemic in the US. Second, the stratified analysis used in this study highlights the increases in methadone-involved overdose deaths both with and without synthetic opioid co-involvement. Third, this study highlights the disproportionate increased incidence of methadone-involved overdoses without synthetic opioids among Hispanic and non-Hispanic Black communities. Fourth, this study highlights the degree to which other substances are co-involved in methadone-involved overdose deaths, regardless of whether synthetic opioids were involved.
This study has several important limitations. First, when multiple substances are involved in an overdose death, death certificate data do not indicate the relative contributions of each involved substance to the overdose death. This can result in the misidentification of methadone as a contributing substance to overdose deaths that were largely caused by other substances, such as synthetic opioids. Second, this study is observational and does not allow for a causal attribution of the increase in methadone-involved overdose deaths to any specific factor. Third, the provisional data included for January – March, 2021 may be subject to revision as further information becomes available, though minimal revisions are expected (Jones et al., 2022). Fourth, this study cannot distinguish whether individuals who die from methadone-involved overdoses receive methadone through OTPs, as prescriptions for pain, or through other sources, including diverted methadone. Fifth, ICD-10 codes for alcohol likely underestimate alcohol involvement in overdose deaths. Finally, all statistical tests were conducted post-hoc and should be considered exploratory.
The current study found an increase in methadone-involved overdose deaths temporally associated with this period despite a decrease in the number of individuals receiving methadone. Numerous societal changes occurred concurrently, and the absolute increases in methadone-involved overdose deaths found in this report are small compared with the total increase in overdose deaths in the US since the beginning of the pandemic. Additionally, monthly methadone-involved overdose deaths remained lower than in the late 2000s when methadone was more frequently prescribed for analgesia. Prospective controlled studies would be necessary to fully understand whether and to what degree OTP regulatory changes contributed to these increases. The impact of any OTP changes among Hispanic and non-Hispanic Black individuals should be specifically considered, especially in light of the disproportionate increases in methadone-involved overdose deaths in these groups. In determining whether OTP regulatory changes should be extended, any potential association between the changes and an increase in methadone-involved overdose deaths would have to be balanced with the benefits of increasing access to take-home doses, including enhancing patient autonomy, destigmatizing methadone treatment, and the numerous ways that patients identify take-home doses as improving their lives (Frank et al., 2021, Kleinman et al., 2022, Treloar et al., 2007).
Funding sources
This research was supported by the Centre for Addiction and Mental Health Discovery Fund.
Disclosures of conflicts and potential conflicts of interest
RAK has received funding through the Centre for Addiction and Mental Health Discovery Fund and the Research in Addiction Medicine Scholars Program (National Institute on Drug Abuse R25-DA033211). He has received travel awards through the American Academy on Addiction Psychiatry and the American Psychiatric Association. MS reports no disclosures.
Contributor statement
The study was conceived by RAK. RAK drafted the manuscript. MS critically revised the manuscript and assisted with the statistical analysis. Both authors approved the final copy.
Acknowledgements
None.
References
- Amram O., Amiri S., Panwala V., Lutz R., Joudrey P.J., Socias E. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. Am. J. Drug Alcohol Abus. 2021;47(6):722–729. doi: 10.1080/00952990.2021.1979991. [DOI] [PubMed] [Google Scholar]
- Brothers S., Viera A., Heimer R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. J. Subst. Abus. Treat. 2021;131 doi: 10.1016/j.jsat.2021.108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Mortality Multiple Cause Files. US Department of Health and Human Services, Centers for Disease Control and Prevention. 〈https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm〉.
- Frank D., Mateu-Gelabert P., Perlman D.C., Walters S.M., Curran L., Guarino H. “It’s like ‘liquid handcuffs”: the effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm. Reduct. J. 2021;18(1):88. doi: 10.1186/s12954-021-00535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T., Campbell T.J., Kitchen S.A., et al. Association between increased dispensing of opioid agonist therapy take-home doses and opioid overdose and treatment interruption and discontinuation. JAMA. 2022;327(9):846–855. doi: 10.1001/jama.2022.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H., Miniño A.M., Spencer M.R., Warner M. Drug Overdose Deaths in the United States, 1999–2020. NCHS Data Briefs # 428. Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/nchs/data/databriefs/db428.pdf [PubMed] [Google Scholar]
- Hyndman R.J., Khandakar Y. Automatic time series forecasting: the forecast package for R. J. Stat. Softw. 2008;27:1–22. doi: 10.18637/jss.v027.i03. [DOI] [Google Scholar]
- Jones C.M. Trends in methadone distribution for pain treatment, methadone diversion, and overdose Deaths — United States, 2002–2014. MMWR Morb. Mortal. Wkly Rep. 2016:65. doi: 10.15585/mmwr.mm6526a2. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Einstein E.B., Compton W.M. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA. 2018;319(17):1819–1821. doi: 10.1001/jama.2018.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Compton W.M., Han B., Baldwin G., Volkow N.D. Methadone-involved overdose deaths in the US before and after federal policy changes expanding take-home methadone doses from opioid treatment programs. JAMA Psychiatry. 2022;79(9):932–934. doi: 10.1001/jamapsychiatry.2022.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman R.A., Weiss R.D. Benzodiazepine-Involved Overdose Deaths in the USA: 2000-2019. J Gen Intern Med. 2022;37(8):2103–2109. doi: 10.1007/s11606-021-07035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman R.A., Nielsen S., Weiss R.D. Is daily supervised buprenorphine-naloxone necessary? BMJ. 2022;378 doi: 10.1136/bmj-2022-071467. [DOI] [PubMed] [Google Scholar]
- Kleinman R.A., Brothers T.D., Danilewitz M., Bahji A. , 2022. Office-based methadone prescribing for opioid use disorder: the Canadian Model. Journal of Addiction Medicine. 16 (5), 499 - 504. 10.1097/ADM.0000000000000950. [DOI] [PMC free article] [PubMed]
- Lam V., Sankey C., Wyman J., Zhang M., 2021. COVID-19 Opioid Agonist Treatment Guidance. 〈https://www.camh.ca//-/media/files/covid-19-modifications-to-opioid-agonist-treatment-delivery-pdf.pdf?la=en&hash=261C3637119447097629A014996C3C422AD5DB05〉 (Accessed 27 January 2022).
- National Center for Health Statistics, Centers for Disease Control and Prevention. CDC WONDER. 〈https://wonder.cdc.gov/〉 (Accessed 27 January 2022).
- Peterkin A., Davis C.S., Weinstein Z. Permanent methadone treatment reform needed to combat the opioid crisis and structural racism. Journal of Addiction Medicine. 2021;16(2):127–129. doi: 10.1097/ADM.0000000000000841. [DOI] [PubMed] [Google Scholar]
- Schaffer A.L., Dobbins T.A., Pearson S.A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 2021;21(1):58. doi: 10.1186/s12874-021-01235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M.A. Treatment of opioid use disorder. N. Engl. J. Med. 2016;375(4):357–368. doi: 10.1056/NEJMra1604339. [DOI] [PubMed] [Google Scholar]
- Strang J., Hall W., Hickman M., Bird S.M. Impact of supervision of methadone consumption on deaths related to methadone overdose (1993-2008): analyses using OD4 index in England and Scotland. BMJ. 2010;341:c4851. doi: 10.1136/bmj.c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Opioid Treatment Program (OTP) Guidance. Published online March 29, 2020. 〈https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf〉 (Accessed 28 July 28 2022).
- Substance Abuse and Mental Health Services Administration . Federal Guidelines for Opioid Treatment Programs. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2015. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . National Survey of Substance Abuse Treatment Services (N-SSATS): 2020. Data on Substance Abuse Treatment Facilities. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2021. [Google Scholar]
- Treloar C., Fraser S., Valentine K. Valuing methadone takeaway doses: the contribution of service-user perspectives to policy and practice. Drug.: Educ. Prev. Policy. 2007;14(1):61–74. doi: 10.1080/09687630600997527. [DOI] [Google Scholar]
- Welsh C., Doyon S., Hart K. Methadone exposures reported to poison control centers in the United States following the COVID-19-related loosening of federal methadone regulations. Int. J. Drug Policy. 2022;102 doi: 10.1016/j.drugpo.2022.103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Murphy S.L., Kochanek K., Arias E. Deaths: Final Data for 2019. National Vital Statistics Reports. 2021;70(8) doi: 10.15620/cdc:106058. [DOI] [PubMed] [Google Scholar]