Abstract
Immune signaling pathways convert pathogenic stimuli into cytosolic events that lead to the resolution of infection. Upon ligand engagement, immune receptors together with their downstream adaptors and effectors undergo substantial conformational changes and spatial reorganization. During this process, nanometer-to-micrometer-sized signaling clusters have been commonly observed that are believed to be hotspots for signal transduction. Because of their large size and heterogeneous composition, it remains a challenge to fully understand the mechanisms by which these signaling clusters form and their functional consequences. Recently, phase separation has emerged as a new biophysical principle in organizing biomolecules into large clusters with fluidic properties. Although the field is still in its infancy, studies of phase separation in immunology are expected to provide new perspectives for understanding immune responses. Here, we present an up-to-date view of how liquid–liquid phase separation drives the formation of signaling condensates and regulates immune signaling pathways including those downstream of T cell receptor, B cell receptor and the innate immune receptors cGAS–STING and RIG-I. We conclude with a summary of the current challenges the field is facing and outstanding questions for future studies.
Introduction
Phase separation is a well-understood phenomenon in physical chemistry, but a relatively new concept for most biologists, including immunologists. It describes the segregation of biomolecules from a homogeneous environment into two distinct phases (the condensed phase and the dilute phase), of which the concentration and mobility of solutes differ significantly from each other. Because of the aqueous and fluidic environment of the intracellular space, liquid–liquid phase separation (LLPS) has been frequently observed in cells. LLPS is a concentration- and environment-dependent condensation process driven by solute–solute interactions that energetically overcome solute–solvent interactions (in cells the solute can be protein, nucleic acid, lipid, sugar, or other metabolites). In a liquid-like state, the condensed phase frequently exchanges materials with the dilute phase, and this liquid-like property has an important role in defining the composition and biochemical activity of molecules in the condensed phase. Sometimes, liquid condensates can ‘age’ and transition to a gel-like or solid state in which biomolecules are highly crosslinked and statically reside in the condensed phase. Because the cellular condensates are commonly formed through multiple types of interactions, a spectrum of intermediate states between the liquid and solid states could be observed. Interestingly, abnormal or irregular transition of condensates to a solid state is associated with certain neurodegenerative diseases. Examples include FUS (fused in sarcoma) condensates in frontotemporal lobar degeneration and TDP43 (TAR DNA-binding protein 43) condensates in amyotrophic lateral sclerosis1.
In immunology, LLPS was characterized in the context of both the cell surface receptor pathways including the T cell receptor (TCR)2, 3 and B cell receptor (BCR) pathway4, 5, and the cytosolic signaling pathways including the cGAS-STING6, 7, RIG-I8, and NF-κB pathway9. As compared to other fields of study, including stress responses and transcriptional regulation, where phase separation has been well documented, research into the relevance of phase separation to immune signaling is still limited. In this article, we discuss the possible ways in which phase separation can bring new mechanistic insights into understanding immune responses. We start by introducing the basic features of phase separation as relevant to immunologists, followed by discussion of cell-surface signaling cascades (phase separation on membranes) and intracellular signaling pathways (phase separation inside cells). We conclude with a discussion of several outstanding questions for future studies.
Phase separation in 3D vs 2D
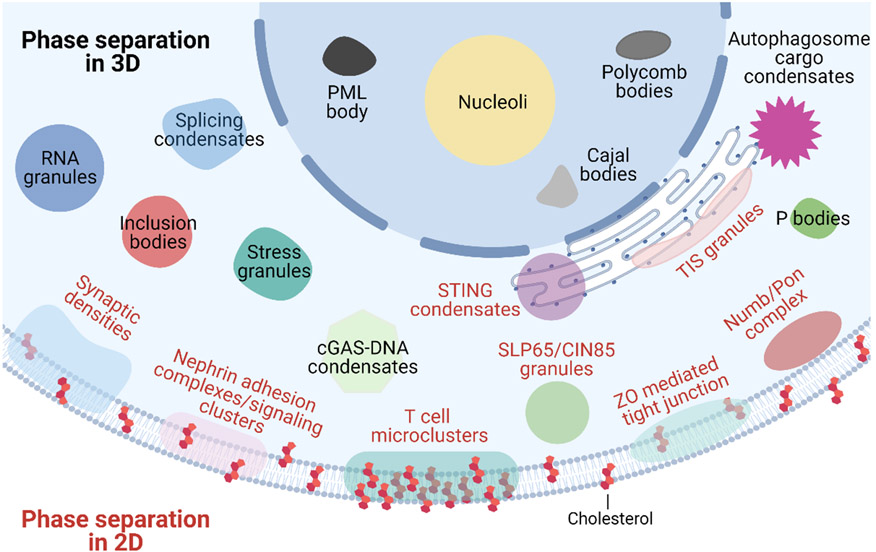
Cellular condensates formed through LLPS can be generally categorized into two types (FIG. 1): 3D structures formed in the cytoplasm and nucleus that do not have membrane wrapped around them; and 2D or near-2D structures that are formed along the cell membrane. These 3D and 2D condensates have similarities in terms of their large size (up to micrometers in magnitude), complexity in chemical composition, and dynamic exchange of materials with the environment. Indeed, a recent preprint (non-peer-reviewed data) describes that many 2D condensates can be viewed as 3D condensates that are ‘wetted’ (cover the surface with a low contact angle) on fluidic membranes, forming a prewet phase10. Although 3D condensates are the most commonly studied entities in the field of phase separation, 2D membrane-associated condensates (clusters) are well represented in immune signaling because cell-surface immune receptors frequently form micron or sub-micron sized clusters upon ligand engagement. In this section, we introduce the properties of both 3D and 2D phase-separated structures and discuss the physical mechanisms, physiological triggers, and signaling functions of these condensates.
Figure 1 ∣. Phase separation in 3D and 2D.
The intracellular space is filled with biomolecular condensates formed through phase separation. These include 3D membrane-less organelles such as nucleoli, Cajal bodies, promyelocytic leukemia (PML) bodies and polycomb bodies in the nucleus; and stress granules, cGAS–DNA condensates, inclusion bodies, P bodies, splicing condensates, RNA granules, autophagosome cargo condensates, SLP65–CIN85 granules and endoplasmic reticulum-associated TIS granules and STING condensates in the cytoplasm. 2D condensates associated with the plasma membrane include T cell microclusters, nephrin-containing adhesion complexes, pre- or post-synaptic densities, NUMB–PON complexes and zonula occludens (ZO)-mediated tight junctions.
Phase separation in 3D: membrane-less organelles.
One of the major motivations for studying phase separation is to understand how biomolecules self-assemble in space and time to form subcellular structures. The cytoplasm is filled with organelles such as mitochondria, the endoplasmic reticulum and the nucleus, which are topologically separated from the cytoplasmic environment by lipid bilayers. However, other cellular compartments exist that are not encased in membranes. How these structures are maintained and dynamically reorganized remain crucial questions for cell biologists. One of the earliest studied examples of these membrane-less organelles is that of P granules in Caenhorhabditis elegans embryos, which have liquid-like properties: they undergo spontaneous fusion, fall apart under shear force, wet on hard surfaces, and rapidly dissolve or assemble, reflecting a LLPS process11 (BOX 1). A large number of other membrane-less organelles have since been identified as structures formed through phase separation, including stress granules, P bodies, nucleoli, Cajal bodies, promyelocytic leukemia (PML) bodies, inclusion bodies, polycomb bodies and Negri bodies (reviewed in REFS12, 13). In addition, a few new membrane-less compartments have recently been identified, including splicing condensates, autophagosome cargo condensates, and endoplasmic reticulum-associated TIS granules14-16. These examples have expanded the traditional view of membrane-based compartmentalization of the cytoplasm and revealed another level of complexity of the intracellular space.
Box 1 ∣. Defining liquid–liquid phase separation.
The liquid-like properties of phase-separated proteins have classically been described as the ability of protein concentrates to form round droplets that undergo fusion and fission, exchange materials with the environment, and exhibit fluorescence recovery after photobleaching (FRAP)12, 123, 124. FRAP is a commonly used technique to assess the fluidity of biomolecular condensates involving bleaching of fluorescently tagged molecules and observing recovery of the fluorescent signal in the bleached area over time, which is typically owing to molecular diffusion of fluorescent molecules from outside the bleached area. However, there is an increasing need for these parameters to be clarified, particularly as some condensates are known to mature and harden as revealed by decreased recovery over time, becoming ‘gel-like or solid’124. This introduces a temporal component to phase-separated structures that requires further elucidation with regards to functional consequences. Because of heterogenous compositions and multiple types of interactions driving condensate formation, there could be a spectrum of states rather than defined boundaries between liquid and solid states and some condensates might be a mixture of liquid- and solid-like structures. Concerningly, liquid–liquid phase separation (LLPS) has also been used loosely to describe condensates that are actually generated through other processes, such as scaffold-driven assembly, the mechanism of which is distinguished from phase separation125. Ectopic overexpression systems are commonly used to investigate LLPS but may result in artificial phenotypes that would not occur under physiological conditions. Furthermore, although FRAP is insightful, it is also heavily exploited as the ultimate, defining experiment for LLPS. This can be fallacious as fluorescence recovery cannot always be attributed to diffusion; rather, recovery may also be a consequence of strong, high-affinity interactions between unbleached probes and macromolecules that do not undergo LLPS124, 125. The aforementioned liquid-like criteria concerning morphology and kinetics, although important, are not the only relevant features of LLPS; organization, surface tension, viscosity and turbidity are other characteristics that should be measured on nascent condensates. Therefore, multiple techniques should be used to determine liquid-like behaviors, many of which are summarized in Table 1 and in more detail in other reviews124, 126.
Phase separation in 2D: membrane-associated condensates.
In addition to 3D condensates formed in the cytoplasm or nucleus, phase separation can also occur on 2D surfaces (FIG. 1)2, 17-24. Near-micron-scale signaling domains enriched in receptors, adaptors, effectors and particular lipids are frequently observed on the plasma membrane25-28. In the late 1990s, a lipid raft model was formally proposed to explain these signaling domains, whereby lateral interactions between lipids drive the formation of a liquid-ordered phase on the plasma membrane that organizes membrane proteins into domains29. However, there is a lack of direct evidence supporting the presence of large lipid domains in live cells at physiological temperatures and so whether the lipid raft model explains signaling domain formation remains controversial30, 31. Alternatively, recent studies have shown that multivalent interactions between membrane-proximal proteins can drive the formation of liquid-like large signaling clusters (phase-separated condensates) both in vitro and in live cells2, 19, 32. Of note, although the evidence that lipids form giant domains on the native plasma membrane is lacking, they can modulate membrane-associated protein condensates; for example, cholesterol enhances TCR clustering33, potentially through the cholesterol-interacting transmembrane domain of the TCR complex. Conversely, protein condensates can influence phase separation of lipids; for example, condensates of the linker for activation of T cells (LAT), downstream of TCR triggering, promote phase separation of cholesterol on model membranes34. Together, the current evidence supports the notion that combined interactions between proteins and lipids shape phase separation on 2D membranes.
As compared to 3D phase separation forming membrane-less organelles in the cytosol, there are several unique physical and chemical features of 2D phase separation on membranes. First, membranes restrict the motion of proteins, which increases their effective macromolecular concentrations and accelerates biochemical reactions by orders of magnitude as compared to a 3D environment35. Indeed, the protein concentration threshold to induce condensates on the 2D membrane is much lower than in 3D solution19, 20, 36. Second, lipids are much smaller in size, are higher in density and are more mobile than membrane-bound proteins. These properties can lead to unique mechanisms that modulate liquid-like condensate formation. Third, cellular membranes contain numerous protrusions or invaginations with convex or concave shapes. The local membrane curvature may affect the distribution and diffusion of lipids and membrane-bound proteins and thus the formation of condensates37.
Mechanisms of phase separation.
Two major mechanisms have been proposed to drive liquid-like condensate formation, involving folded domains (secondary structures such as α helix or β strand) and/or intrinsically disordered regions (IDRs; unfolded regions that do not contain secondary structures). In the first mechanism, proteins containing tandem folded domains can be crosslinked through well-defined protein–protein interactions. A seminal example of this is the formation of nephrin clusters by multivalent interactions between phosphotyrosines and Src homology 2 (SH2) domains, and between proline-rich motifs and SH3 domains18; this was the first example of successful reconstitution of phase-separated droplets in vitro. A similar mechanism involving crosslinking applies when one of the protein components is replaced by DNA or RNA molecules. In this scenario, the length of the nucleic acid, which determines the binding valency (the number of interacting sites), has a crucial role in setting the threshold for phase separation38-40.
The second major mechanism involves IDRs41, 42, protein regions that usually do not contain folded domains and are generally unstructured. A similar term that is frequently used in the literature is low-complexity domains (LCDs). By definition, LCDs contain over-represented amino acids and are usually unstructured. IDRs and LCDs are largely overlapping, but IDRs are defined by their structure whereas LCDs are defined by their sequence. IDRs (and LCDs) can sometimes self-assemble to form homotypic or heterotypic higher-order structures in a concentration-dependent manner. For a list of databases that summarize candidate proteins for LLPS based on either experimental results or sequence predictions, see Supplementary Table 1. It should be noted that although the presence of IDRs suggests the ability of a protein to phase separate, this always requires experimental verification.
Although the protein sequences and mechanisms driving phase separation vary, one common feature present in almost all cases is that phase separation is driven by multivalent interactions. As the binding valency (number of binding sites per protein) increases, the threshold for forming phase-separated domains decreases, in a non-linear manner. For example, increasing the binding valency from 3 to 4 reduces the threshold to form condensates between proline-rich motifs and SH3 domains by about 10-fold18. Consistent with this correlation between valency and tendency to phase separation, dimerization or oligomerization domains (for example, coiled-coil domains), when coexisting with IDRs on the same polypeptide, can promote phase separation43. Of note, the interactions driving phase separation are not limited to the strong interactions that hold conventional macromolecular complexes together. Weak interactions, including cation-pi interactions and Van der Waals interactions, could also provide the driving force for LLPS because a high avidity can be achieved when combining low-affinity interactions with multivalency. The low affinity of individual interactions also enables flexibility in reorganizing structures and is crucial to the liquid-like property of condensates.
Physiologically relevant triggers.
As more examples of LLPS are discovered, we view phase separation as a basic property of biomolecules (similar to their size, charge and hydrophobicity) rather than as a special feature of certain molecules. Instead of asking which proteins can phase separate, it is more important to address under what physiological conditions phase separation occurs. Indeed, accumulating evidence suggests that liquid-like condensates are regulated by multiple physiological triggers. These regulatory processes have been extensively studied in yeasts that are directly exposed to various environmental challenges. For example, in response to high temperature or low pH, the poly(A)-binding protein Pab1 forms condensates, which increases cell survival44. It has been noted that condensates could form either above or below a threshold temperature, depending on the specific interactions mediating LLPS45. Redox metabolism is also an important factor in regulating LLPS; for example, the yeast RNA-binding protein Pbp1 phase separates when the cell is in a reduced state, which allows for the partitioning of the target of rapamycin complex 1 (TORC1) and the modulation of TOR signal transduction46. Physiological triggers are also frequently converted into post-translational modifications such as phosphorylation that either promote or inhibit phase separation. For example, antigenic stimuli to TCR trigger the downstream phosphorylation of LAT, which drives liquid-like microcluster formation to promote T cell activation2; and cell cycle-regulated, DYRK3-mediated phosphorylation of SC35 dissolves splicing speckles (nuclear domains enriched in pre-mRNA splicing factors) and prevents the trapping of mitotic regulators47. Post-translational modifications can also modulate phase separation during pathological processes. Acetylation inhibits the phase separation of the neurodegenerative disease-associated Tau protein in vitro48. Hypomethylation of FUS promotes phase separation and transition to a gel-like state, which disrupts the formation of ribonucleoprotein granules and reduces protein synthesis in neuron terminals49. Studying phase separation in the context of these physiologically or pathologically relevant triggers is important because only then can the functional, biological consequences of phase separation be revealed. This applies not only to in vivo studies but also to in vitro assays, in which individual components of phase-separated condensates are purified to reconstitute condensate formation. In vitro reconstitution can be a powerful way to dissect the mechanisms of phase separation, but it is important that the buffer conditions of in vitro assays, including salt, pH and temperature, reflect intracellular conditions. The use of crowding reagents, including polyethylene glycol (PEG) or dextran, needs to be cautiously evaluated and justified.
Functional consequences of condensation.
The extensively characterized mechanisms of condensate formation provide the foundation to address the functions of condensate assembly. These functions include effects on biochemical activities of individual molecules, intracellular signaling and metabolic pathways, and organism-level phenotypes. In the field of cell signaling research, the idea that clusters or ‘signalosomes’ of signaling molecules promote signal transduction has been prevalent and there is much evidence to support this concept. What new properties or perspectives could liquid-like condensates bring to our understanding of cell signaling? We view liquid-like condensates as one type of signaling platforms. The traditionally defined signaling clusters share some features with phase-separated condensates. For example, they both concentrate molecules. However, phase-separated condensates are usually within the upper size range (micrometer scale) of signaling clusters and thus have features, such as exclusion, that do not typically exist in the nanometer-scale clusters. Moreover, liquid-like condensates are usually formed through weak intermolecular interactions, which results in a high rate of material exchange with the environment that could potentially allow for a continues flow of substrates into the condensate to promote enzymatic reactions. Moreover, the heterogeneous composition of liquid-like condensates could enable them to interact with a wide range of effectors, having a broad spectrum of activities that might favor responses to different environmental stimuli. On a purely speculative note, as liquid-like condensates mature into gel-like structures they might transduce force more efficiently. Many immune receptors (such as TCR, BCR and the low-affinity IgG receptor FcγRIIA) are force sensitive50-52, such that a change in the material property of condensates might regulate receptor activity. We elaborate on some of these points in the following sections but many areas still require investigation.
It should be noted that data addressing the functional importance of condensation need to be carefully interpreted, with some studies showing an association between function and condensate formation rather than a causal relationship. In most cases, proteins, instead of condensates per se, are manipulated, which does not perfectly address the functional consequences of condensate formation. Fairly speaking, this caveat applies equally to any newly identified cellular structures in history, including many membrane-bound organelles. We suggest several ways in which a cause-and-effect relationship between condensate formation and function could be established. These include independent means to manipulate condensate formation (for example, generating point mutations, chemical perturbations and physical manipulation by optical trap), carefully characterizing the physical properties (Table 1) and chemical composition of condensates and quantitatively correlating them with functional outcomes, reconstituting condensates in vitro or in different cellular settings, and engineering condensates by swapping IDRs to show that the functional outcome is related to condensation rather than a specific protein sequence. As the field moves forward, more tools and techniques are expected to become available to comprehensively determine the functions of condensates.
Table 1 ∣.
Methods to characterize liquid-like condensates
| Method | Condensate properties studied |
Notes | References* |
|---|---|---|---|
| Differential interference contrast (DIC) microscopy | Morphology and dynamics | – | 106, 107 |
| Regular fluorescence microscopy | Morphology, fusion kinetics, surface tension and composition | Small-sized, monomeric fluorescent tags are preferred; use low ratio of labelled to unlabeled components to avoid optical-artifact | 108, 109 |
| Fluorescence recovery after photobleaching (FRAP) | Diffusivity | Can measure recovery and diffusion coefficients, from which viscosity can be calculated | 11, 108 |
| Fluorescence correlation spectroscopy (FCS) | Diffusivity | Can calculate diffusion coefficients and size of particles in live cells | 65, 108 |
| Distributed amphifluoric Förster resonance energy transfer (DAmFRET) | Density and degree of order/disorder | High throughput | 110, 111 |
| Photoluminescence lifetime imaging | Viscosity and internal organization | – | 112 |
| Particle tracking | Viscosity, elasticity and mesh size | Uses bead injection or expression of genetically encoded nanoparticles | 113, 114 |
| Light scattering | Morphology and structure | Includes dynamic, static, multi-angle, small-angle, small-angle x-ray and small-angle neutron scattering techniques | 18, 44 |
| Correlated electron and light microscopy (CLEM) | Organization | – | 115, 116 |
| Cryo-electron tomography (Cryo-ET) | Morphology and organization | Restricted by sample thickness | 4, 117 |
| Optical tweezers | Surface tension and viscosity | – | 118, 119 |
| Atomic force microscopy (AFM) | Stiffness and structure | – | 49, 120 |
| Nuclear magnetic resonance (NMR) imaging | High-resolution structure and chemical environment | Low complexity sequences, high droplet viscosity and droplet heterogeneity may confound resonance | 121, 122 |
Owing to reference limits, two examples are provided for each technique.
Phase separation on the immune cell surface
Cell surface receptors on immune cells, together with ligand and/or downstream binding partners, can form nanometer- to micrometer-sized clusters on the plasma membrane53-55. In this section, we discuss how phase separation regulates the formation of these membrane clusters and hence immune signaling cascades. We focus on activating signaling cascades because of the available examples though there is evidence suggesting that the inhibitory receptor PD1 forms microclusters when engaged with PD-L156. However, it remained to be determined whether PD1 microclusters are formed through phase separation.
TCR signaling.
A prominent feature of the TCR signaling pathway is that major components in the pathway have been reported to form discrete micrometer- or submicrometer-sized clusters, known as T cell microclusters, on the plasma membrane. T cell microclusters were initially described in the late 1990s57. Since then, continuous efforts from multiple groups have built up a comprehensive list of the proteins that form microclusters, which includes the transmembrane receptors TCR, CD28 and PD1; the kinases LCK and ZAP70; the adaptor proteins LAT, GRB2, GADS (also known as GRAP2), SLP76 (also known as LCP2) and NCK1; and the enzymes SOS1, PLCγ1 and CBL25, 56, 58 (FIG. 2). The formation of T cell microclusters depends on ligand binding and phosphorylation, and the composition of clusters is heterogeneous and dynamic. Components of the clusters usually have a higher density and lower mobility compared with the surrounding environment, as revealed by single-molecule imaging59. The high density can increase the likelihood of molecular interactions within clusters and the low mobility may facilitate reaching the minimal binding time that is required for interactions to occur. While investigating the key players in forming T cell microclusters, it was shown that three proteins — LAT, GRB2 and SOS1 — can form oligomers in solution through multivalent interactions60. To determine the mechanism of microcluster formation in the context of the plasma membrane, a supported lipid bilayer-based reconstitution system was established61. It was shown that LAT microclusters have liquid-like properties and are formed through LLPS of LAT and its binding partners2. Interestingly, two enzymes, SOS1 and PLCγ1, also have an enzyme-independent, scaffolding role in promoting LAT cluster formation62, 63, and the composition of LAT clusters affects whether they undergo a smooth centripetal movement, which is driven by the retrograde flow of actin, at the immunological synapse64.
Figure 2 ∣. A microcluster view of T cell receptor signaling.
Upon antigen engagement, the T cell receptor (TCR) complex is phosphorylated by LCK on the immunoreceptor tyrosine-based activation motifs (ITAMs) of its CD3 chains. The phosphorylated TCR complex recruits the kinase ZAP70, which then phosphorylates LAT, resulting in the formation of liquid-like condensates of LAT. These LAT microclusters are enriched with adaptor proteins (such as GRB2, GADS, SLP76 and NCK1) and effector proteins (such as SOS1, PLCγ1, WASp and ARP2/3) to trigger the activation of downstream pathways, including RAS signaling, calcium influx (not shown) and actin remodeling. LAT microclusters exclude the phosphatase CD45 to protect phosphotyrosines, which are an activation marker of TCR signaling. CBL, an E3 ubiquitin ligase, is recruited to LAT microclusters to attenuate clustering and hence TCR signal transduction. The TCR co-receptors CD28 and PD1 overlap with LAT microclusters when engaging their own ligands.
What are the biochemical functions of LAT condensates? LAT condensates promote tyrosine phosphorylation, which is a key activation marker for the TCR signaling pathway. This is achieved by the enrichment of kinases in clusters, but exclusion of phosphatases. Interestingly, the exclusion properties of LAT condensates are dependent on charge: LAT condensates are negatively charged and therefore partially exclude negatively charged phosphatases such as CD452. In addition to charge, the exclusion can also be mediated by other factors including protein size65. Moreover, LAT condensates promote downstream signaling pathways by increasing the membrane dwell time of SOS1 and N-WASp, which activate the RAS signaling pathway and actin remodeling, respectively3, 66.
Although the assembly of LAT condensates is well studied, the disassembly process is less well understood. Endocytosis and ubiquitylation have been proposed to decrease the concentration of LAT on the plasma membrane and promote cluster disassembly67. Also, the cytosolic phosphatases SHP1 and SHP2, in complex with THEMIS, can be recruited to LAT clusters and potentially dephosphorylate LAT to disassemble the cluster68. This forms a potential negative feedback loop to regulate LAT cluster formation, which could be important for resetting TCR signaling to a baseline level after activation. Interestingly, although LAT is the key to driving microcluster formation in the TCR signaling pathway, it is dispensable for microcluster formation of chimeric antigen receptors (CARs)69. It has been proposed that the cytosolic domain of a CAR, once phosphorylated, can establish multivalent interactions with the LAT binding partners GADS and SLP76 to form microclusters in the absence of LAT, although the specific mechanisms of CAR microcluster formation and whether these microclusters have liquid-like features need to be determined69. It also remains an open question how the co-signaling domains of CARs, including CD28 and 4–1BB domains, modulate CAR microcluster composition and downstream signaling outcomes.
In contrast to LAT microclusters, which are relatively well characterized, the mechanism of transmembrane receptor clustering (for example, of TCR, CD28 or PD1) remains much less clear. There are still many open questions about the nature of receptor microclusters in T cells and the extent to which LLPS drives their formation. On one hand, these receptors contain multiple phosphorylable tyrosines, share certain cytosolic binding partners with LAT, and partially overlap with LAT microclusters, suggesting a potential role of LLPS. On the other hand, the mobility and oligomerization states of these receptors are determined not only by their binding partners in T cells, but also by the ligands with which they interact, which are located on another cell membrane. The local membrane geometry in the immunological synapse could also affect receptor organization70. Attempts to deconstruct these individual elements and reconstitute back, although technically challenging, will be crucial to reveal the mechanism of the spatial organization of signaling receptors on the T cell surface.
The physiological function of phase separation in the TCR signaling pathway is another intriguing question to explore. One of the remarkable features of the TCR signaling machinery is the ability to differentiate between self and non-self antigens, the mechanisms of which are still not fully understood71, 72. The affinity of TCRs for self antigens compared with non-self antigens is only several-fold different, but the signaling output is all or nothing. Phase separation could provide an appealing explanation for this behavior because phase separation is a highly coordinated and collective process that results in a binary outcome. A small change in input such as a slight increase in the affinity of the antigen–TCR interaction, could trigger phase separation and activate the downstream signaling cascade. A careful titration of antigen density and affinity will be needed to test how phase separation might play a role in self versus non-self discrimination.
BCR signaling.
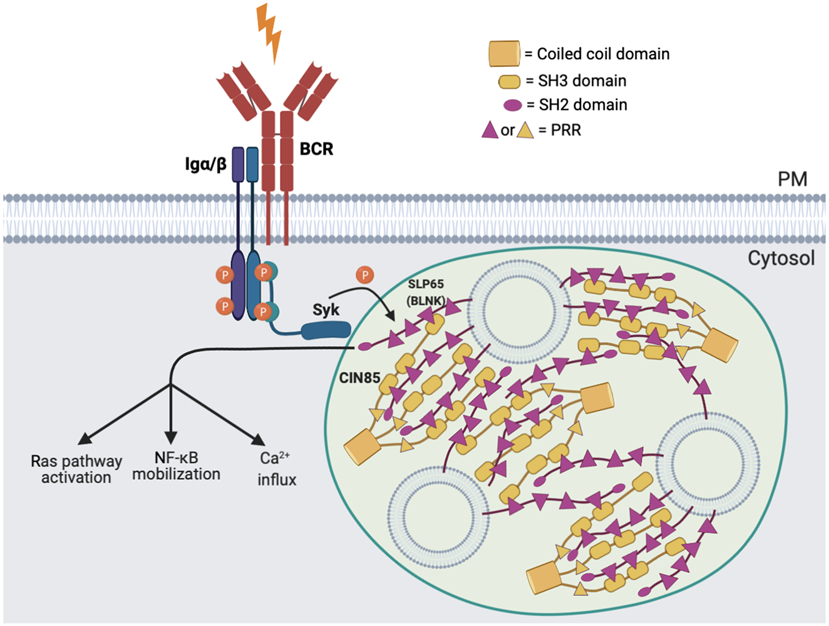
Similar to the role of LAT in the TCR signaling pathway, the scaffold protein SLP65 (also known as BLNK) drives LLPS in the BCR signaling pathway. It has been shown that SLP65 forms liquid-like condensates with its binding partner CIN85 (also known as SH3KBP1) through a classical multivalent interaction between the SH3 domains of trimeric CIN85 and the proline-rich motifs of SLP65 (FIG. 3)4, 73. In contrast to the LAT condensates that are formed on the plasma membrane following TCR activation, SLP65 condensates are pre-formed in the cytoplasm of resting B cells and associate with the plasma membrane following BCR activation74. Although SLP65 and CIN85 are sufficient to form condensates at high concentrations, liposomes (spherical vesicles) have a crucial role in promoting condensate formation at physiological cellular concentrations of SLP65 and CIN85. SLP65 contains an amino-terminal lipid-binding domain that recognizes small unilamellar vesicles with radii of 20 nm, but not large unilamellar vesicles with radii of 60 nm4. Preferential binding of SLP65 to small, highly curved vesicles in vitro parallels findings that SLP65 localizes to intracellular vesicles enriched with VAMP7, a protein that is known to contribute to the trafficking of TCR pathway components. This co-localization is selective, as SLP65 does not colocalize with RAB8-positive or RAB27-positive vesicles73. Cryo-electron tomography (Cryo-ET) imaging has shown that liposomes in the in vitro-reconstituted condensates are spaced on average 5 nm apart. This fits a model in which CIN85 molecules are sandwiched between SLP65 molecules that are, in turn, bound to adjacent vesicles4. The Cryo-ET approach revealed the internal organization of the tripartite SLP65–CIN85–vesicle condensates, and a similar approach could potentially be applied to other studies of LLPS.
Figure 3 ∣. Signaling condensates in the B cell receptor pathway.
The scaffold protein SLP65, its binding partner CIN85 and liposomes (spherical vesicles) form liquid-like signaling condensates in the cytosol of resting B cells. Condensate formation is mediated through multivalent interactions between the proline-rich motifs of SLP65 and the SH3 domains of CIN85, and between the amino-terminal lipid-binding domain of SLP65 and vesicles. CIN85 is trimerized by its coiled-coil domain, which further increases its interaction valency. Upon B cell receptor (BCR) stimulation, the kinase SYK is recruited and activated at the BCR, which phosphorylates SLP65 as the condensates approach the plasma membrane. Downstream pathways are further triggered including RAS activation, NF-κB mobilization and calcium influx.
What is the function of SLP65 condensates? SLP65 mutants lacking the N-terminal vesicle-binding domain cannot form condensates either in the cytoplasm or on the plasma membrane, which is accompanied by defects in BCR signaling (for example, calcium influx). Importantly, these defects can be rescued by fusing another vesicle-recognizing domain (N-BAR) to the N-terminal truncation mutant of SLP654. Together, these data support the function of SLP65 condensates in promoting calcium signaling and potentially other downstream signaling pathways. The functional roles of vesicles in SLP65 condensates, beyond serving as a structural component, remain to be determined, although it is proposed that SLP65 condensates could exploit the trafficking pathway of VAMP7-positive vesicles to quickly deliver a large number of BCR signaling components to the plasma membrane for efficient signal transduction. There are also a few other questions worthy of further investigation: do SLP65 condensates recruit other BCR signaling components? Do SLP65 condensates have a role in sorting vesicles and regulating vesicular trafficking? Do the condensates disassemble or degrade and, if so, how?
The lipid bilayer (vesicle)-facilitated phase separation of SLP65 concurs with other works showing that BCRs form nanoclusters and microclusters when engaged with soluble antigens and APC-presented antigens, respectively75, 76. BCRs are selectively enriched in liquid-ordered phase and BCR nanoclusters overlap with specific lipid and protein probes on the B cell membrane77. Increasing cholesterol levels promoted BCR signaling by stimulating calcium influx, whereas decreasing cholesterol levels resulted in reduced calcium influx. The liquid-ordered phase is also enriched in the Src kinase LYN and depleted of the phosphatase CD45, which thereby promoted signaling propagation5. Whether the BCR itself forms clusters through LLPS remains to be determined, and further work is also needed to analyze the condensation states of downstream BCR effectors.
Innate immune receptors.
There are a large number of innate immune receptors that cluster at the cell surface upon ligand binding, including phagocytic receptors such as Drosophila melanogaster Draper78 and dectin 179, as well as the mast cell receptor FcεRI80-82. These receptor clusters are similar to TCR and BCR signaling clusters in that they contain immunoreceptor tyrosine-based activation motifs (ITAMs) or ITAM-related sequences; are nanometer to micrometer in size; include multivalent interacting proteins that enhance signal transduction; spatially exclude inhibitors of signal propagation; and are affected by lipid composition (for example, cholesterol levels). Moving forward, it will be interesting to evaluate the mechanism by which these receptors form clusters and to confirm whether LLPS plays a role.
Liquid-like condensates in immune cells
In addition to signal transduction on the plasma membrane, phase separation also regulates intracellular immune signaling events, including the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway, which responds to double-stranded DNA (dsDNA), and the retinoic acid-inducible gene I (RIG-I) pathway, which responds to single-stranded RNA (ssRNA) or double-stranded RNA (dsRNA). Moreover, viral proteins can hijack immune pathways in host cells by trapping key signaling components in liquid-like condensates, as illustrated by the nuclear factor-κB (NF-κB) pathway.
cGAS liquid-like condensates.
cGAS senses abnormal cytosolic dsDNA derived from pathogens or from nuclear or mitochondrial damage83. dsDNA binds and activates cGAS, resulting in the synthesis of 2′3′-cyclic GMP–AMP (cGAMP). cGAMP, in turn, activates STING, leading to downstream signaling that induces the expression of type I interferons and other proinflammatory cytokines84. Recently, it has been shown that DNA binding to cGAS induces the formation of liquid-like condensates (FIG. 4a). Several essential multivalent elements are involved in the formation of cGAS–dsDNA condensates, including the positively charged N-terminal domain of cGAS, long DNA strands (>100 base pairs) and free zinc ions. A newly identified DNA-binding domain in the catalytic core of cGAS also enhances condensate formation85. Functionally, the formation of condensates can promote cGAS activity by protecting DNA from degradation by the exonuclease TREX186. In addition to DNA, cGAS also forms liquid-like condensates with dsRNA though dsRNA does not activate cGAS to produce cGAMP6. Interestingly, recent data from a preprint (not yet peer reviewed) indicate that high concentrations of dsRNA compete with dsDNA for cGAS binding and inhibit cGAS activity, whereas dsRNA at low concentrations promotes phase separation and the production of cGAMP87. The differential effect of dsDNA versus dsRNA on cGAS activation raises an intriguing question how RNA virus infection might influence the response of host cells to a DNA virus at the level of cGAS regulation.
Figure 4 ∣. Phase separation of intracellular innate immune signaling pathways.
a ∣ Cyclic GMP–AMP synthase (cGAS) forms liquid-like condensates with double-stranded DNA (dsDNA) to enhance the production of 2′3′-cyclic GMP–AMP (cGAMP) by protecting DNA from degradation by the exonuclease TREX1. Bacteria-derived streptavidin, free zinc ions, RNA and the stress granule protein G3BP1 regulate the formation of cGAS–dsDNA condensates and production of cGAMP. cGAMP, in turn, activates STING, leading to downstream signaling through TBK1 and IRF3 that induces the expression of type I interferons and other proinflammatory cytokines However, overproduction of cGAMP induces the formation of STING condensates on the endoplasmic reticulum (ER), which recruit TBK1 but exclude IRF3 and thereby prevent overactivation of the innate immune response by limiting interferon production. b ∣ The E3 ubiquitin ligase TRIM25 forms liquid-like condensates with RNA (preprint data; not yet peer reviewed), which recruit the RNA sensor retinoic acid-inducible gene I (RIG-I) and promote RIG-I activation through K63-linked ubiquitylation and downstream signaling through mitochondrial antiviral signaling protein (MAVS). In parallel, G3BP1 recruits the E3 ubiquitin ligase RNF125 into stress granules and destabilizes RNF125, which inhibits the K48-linked ubiquitylation of RIG-I that would otherwise lead to its proteasomal degradation. G3BP1 also promotes RNA binding to RIG-I to trigger RIG-I activation. c ∣ Nuclear factor-κB (NF-κB) functions downstream of RIG-I–MAVS signaling. The p65 subunit of NF-κB is trapped in the inclusion bodies that are formed by phase separation of the viral replication machinery. Trapped p65 is unable to translocate into the nucleus to induce the expression of pro-inflammatory cytokines.
Two tumor-associated mutations, G303E and K432T, at one of the DNA-binding sites of cGAS result in a reduced ability to form cGAS condensates and decreased cGAMP production85. However, these mutations may also affect DNA binding and enzymatic activity, so it is not known whether the reduced ability to form condensates explains the decreased cGAMP production. Further investigations are needed to understand how the formation of cGAS condensates affects downstream pathways in both immune cells and tumor cells and how these cGAS mutants contribute to altered immune responses during tumor progression. Manipulating cGAS condensate formation may provide a new way in which to modulate the antitumor immune response.
Moreover, pathogen proteins can also regulate the phase separation of cGAS. Streptavidin, a secreted protein from the bacterium Streptomyces avidinii, binds to cGAS to enhance cGAS–DNA interactions and promote LLPS of this complex. This results in enhanced cGAS activation and interferon-β production88. Whether or how this mechanism benefits S. avidinii remains unclear. On the other hand, herpesvirus protein ORF52 and VP22, which inhibit cGAS activity, disrupt cGAS-DNA droplet formation. This disruption depends on the formation of new droplets composed of viral proteins and DNA. Interestingly, DNA preferentially forms droplets with ORF52 other than cGAS, even though the DNA-binding affinity of ORF52 is lower than the one of cGAS89. This suggests that droplet formation can influentially alter the way that biomolecules interact with each other.
G3BP1, which is a key player in the formation of stress granules (cytosolic phase-separated structures composed of RNA and various proteins that arise under conditions of cellular stress)90, is also crucial for DNA sensing and efficient activation of cGAS. The percentage of cells containing cGAS condensates was significantly reduced in G3BP1-deficient cells. Interestingly, the regulatory function of G3BP1 on cGAS phase separation does not depend on stress granules91. It is unclear how the partitioning of G3BP1 in cGAS droplets versus stress granules is regulated.
cGAMP, once produced by cGAS, binds and activates STING, which is accompanied by polymerization of STING92. A recent study showed that STING forms condensates with stacked ER membrane in the presence of excess amount of cGAMP. The STING condensates recruit the downstream signaling kinase TBK1 but exclude the transcription factor IRF3, and thereby lose the ability to trigger robust interferon production7. Interestingly, another recent study showed that a multivalent STING agonist PC7A triggers STING condensate formation and stimulates the prolonged production of pro-inflammatory cytokines93. These examples illustrate an emerging notion that condensation could have both positive and negative roles in regulating signaling. Future studies are required to determine the structural and compositional differences between the two aforementioned STING condensates to understand their opposite signaling outcomes.
RIG-I and stress granules.
RIG-I triggers the innate immune response against ssRNA viruses or dsRNA viruses94. Tripartite motif-containing protein 25 (TRIM25), an E3 ubiquitin ligase, catalyzes the K63-linked ubiquitylation of RIG-I on its two caspase-recruitment domains (CARDs), which is required for its interaction with mitochondrial antiviral signaling protein (MAVS) and its ability to induce antiviral signal transduction and interferon production95. A recent preprint (not yet peer-reviewed) showed that RNA binding triggers LLPS of TRIM25, which recruits RIG-I to condensates and increases its ubiquitylation by TRIM258 (FIG. 4b). By contrast, RNF125, another E3 ubiquitin ligase, has been shown to negatively regulate the RIG-I pathway by catalyzing K48-linked ubiquitylation of RIG-I that leads to its proteasomal degradation96. G3BP1, the core component of phase-separated stress granules, directly interacts with RNF125 in virus-induced stress granules to promote its auto-ubiquitylation and degradation. G3BP1 also interacts with RIG-I to enhance its binding to dsRNA and downstream signaling pathways94, 97. Together, these results suggest that G3BP1 in stress granules functions as a positive regulator of RIG-I signaling. Further investigation will be needed to address how TRIM25-induced condensates and G3BP1-mediated stress granules coordinately regulate the partitioning and activity of RIG-I.
Besides RIG-I, virus-induced stress granules also colocalize with several other innate immune proteins, including melanoma differentiation-associated gene 5 (MDA5), protein kinase R (PKR), 2′-5′ oligoadenylate synthase (OAS) and ribonuclease L (RNase L)98, 99. However, the consequences of localization of these proteins to stress granules in the context of innate immune responses remain to be explored. In addition to halting protein translation to block viral replication, virus-induced stress granules98, 100 could have an important role in integrating multiple immune signaling pathways through phase separation to confer effective anti-viral responses.
NF-κB pathway.
Upon infection, some viral proteins form membrane-less liquid-like condensates known as inclusion bodies to promote viral genome replication and alter antiviral immune responses to escape host immune surveillance101-103. One example is the inhibition of the NF-κB pathway by respiratory syncytial virus (RSV). In RSV-infected cells, the NF-κB subunit p65 is rapidly sequestered into perinuclear intracytoplasmic puncta that are synonymous with inclusion bodies (FIG. 4c). The trapped p65 cannot translocate into the nucleus to activate the downstream transcription of pro-inflammatory cytokine genes and other antiviral genes9. Similarly, MAVS and MDA5, two upstream regulators of the NF-κB pathway, are recruited into RSV-induced inclusion bodies as a mechanism to inhibit the expression of interferon-β104. In another example, the sequestration of phosphorylated p38 mitogen-activated protein kinase (MAPK) and O-linked N-acetylglucosamine transferase into RSV-induced inclusion bodies can suppress MAPK-activated protein kinase 2 signaling and stress granule formation, respectively, both of which would otherwise inhibit RSV replication105. Whether other viruses that generate inclusion bodies, such as Rabies, Ebola or Nipah viruses, use similar mechanisms of immunomodulation requires further elucidation.
Conclusions and outstanding questions
Phase separation offers several new perspectives for understanding biological systems such as immune responses. At the molecular level, phase separation highlights the importance of weak interactions and unstructured protein domains, which have frequently been ignored in studying protein–protein interactions in the past but seem to have crucial functions in driving LLPS. At the subcellular level, studies of phase separation reveal new membrane-less organelles or intracellular compartments for signal transduction. At the physiological level, phase separation provides mechanistic insights for understanding cellular decision-making processes in the immune response. The role of phase separation in immune signaling has so far been shown in a few pathways, including TCR, BCR, cGAS, RIG-I, and NF-κB signaling. However, the field is still in its infancy and further investigations, both in terms of technological developments and physiological exploration, are required to comprehensively test the functions of phase separation in immunity. Looking forwards, we envision that the following areas will be exciting to explore.
Condensate structure at atomic resolution.
One challenging area in the LLPS field is to determine the internal organization and structure of the condensate components at atomic resolution. This becomes even more complicated in the presence of biomembranes. Although electron microscopy and crystallography have been used to answer such structural questions in other contexts, their application to liquid-like objects remains limited. Advances in nuclear magnetic resonance imaging and computational simulations may provide new avenues for approaching this question. Understanding the internal organization of condensates will benefit the design of agonists and antagonists for perturbing condensates and the associated immune responses. For example, STING condensates could be an interesting therapeutic target because of an important role of STING in anti-cancer immunity and because different STING condensates seem to have opposite effects on IFN production, as discussed above.
Phase separation across the immunological synapse.
So far, most studies of phase separation have focused on a single environment, either in the cytoplasm (for example, cGAS or RIG-I signaling) or on the plasma membrane (for example, TCR or BCR signaling). However, the immunological synapse is a complicated sandwich-like structure composed of five environments: the cytosol and plasma membrane of an immune cell; the intermembrane space; and the plasma membrane and cytosol of an antigen-presenting cell. These different environments are coupled through multiple ligand–receptor pairs and may influence their assembly structures as well as confer two-way signaling. A multiple-membrane reconstitution system, together with light-sheet microscopy on live-cell conjugates, will benefit a comprehensive understanding of the phase separation behavior at immunological synapses.
Engineering immune signaling by targeting phase separation.
Given the role of phase separation in promoting TCR signaling, phase separation might also be targeted to engineer T cells for cancer immunotherapy. A large number of methods have been developed to control phase separation (BOX 2). These could be exploited to engineer key signaling molecules in T cells to boost their antitumor activity.
Box 2 ∣. Methods to manipulate phase separation.
Both chemical and optogenetic tools are available to artificially induce or promote phase separation. For example, the iPOLYMER system uses heterodimerization of FKBP (FK506 binding protein) and FRB (FKBP rapamycin binding domain) fusion proteins; using this system, artificial RNA granules can be formed upon treatment with rapamycin127. A large number of optogenetic approaches have also been developed to control phase separation. In the OptoDroplets system, a light-inducible cryptochrome 2 (CRY2) domain is fused to a protein that has the potential to phase separate (for example, containing an intrinsically disordered region). Blue light induces oligomerization of CRY2, which triggers condensate formation in a reversible manner in live cells. This method has been used to induce the phase separation of ribonucleoproteins128. Other optogenetic tools include Corelet (which promotes phase separation using ferritin spheres129), PixELL (which induces condensate disassembly130) and DropletTF (which promotes gene transcription both in vitro and in mice131). One challenge of applying optogenetic approaches to in vivo studies is the low tissue penetrance and high phototoxicity of blue or other visible light. This technical hurdle could potentially be circumvented by using near-infrared nanoparticles that convert infrared light, which has deep tissue penetrance and low phototoxicity, to visible light that is compatible with current optogenetic tools132.
Supplementary Material
Acknowledgements
X.S. has received support from the American Cancer Society Institutional Research Grant, the Charles H. Hood Foundation Child Health Research Awards, the Andrew McDonough B+ Foundation Research Grant, the Gilead Sciences Research Scholars Program in Hematology/Oncology, the Rally Foundation and Bear Necessities Foundation a Collaborative Pediatric Cancer Research Awards Program, the Yale SPORE in skin cancer DRP Award, the Yale DeLuca Pilot Award, and the NIGMS MIRA (R35) program (GM138299).
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41577-021-00572-5
References
- 1.Zbinden A, Perez-Berlanga M, De Rossi P & Polymenidou M Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev Cell 55, 45–68 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Su X et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang WYC et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363, 1098–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong LE et al. Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness. Nat Commun 11, 848 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone MB, Shelby SA, Nunez MF, Wisser K & Veatch SL Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du M & Chen ZJ DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X et al. The STING phase-separator suppresses innate immune signalling. Nat Cell Biol 23, 330–340 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Haubrich K et al. RNA binding regulates TRIM25-mediated RIG-I ubiquitylation. bioRxiv (2020). [Google Scholar]
- 9.Jobe F, Simpson J, Hawes P, Guzman E & Bailey D Respiratory Syncytial Virus Sequesters NF-kappaB Subunit p65 to Cytoplasmic Inclusion Bodies To Inhibit Innate Immune Signaling. J Virol 94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouches M, Veatch S & Machta B Surface Densities Prewet a Near-Critical Membrane. bioRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brangwynne CP et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–32 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Banani SF, Lee HO, Hyman AA & Rosen MK Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappu RV Phase Separation-A Physical Mechanism for Organizing Information and Biochemical Reactions. Dev Cell 55, 1–3 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Li W et al. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat Cell Biol 22, 960–972 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun D, Wu R, Zheng J, Li P & Yu L Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res 28, 405–415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma W & Mayr C A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3'UTR-Mediated Protein-Protein Interactions. Cell 175, 1492–1506 e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao YG & Zhang H Phase Separation in Membrane Biology: The Interplay between Membrane-Bound Organelles and Membraneless Condensates. Dev Cell 55, 30–44 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Li P et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–40 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banjade S & Rosen MK Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C & Honigmann A Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell 179, 923–936 e11 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Schwayer C et al. Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow. Cell 179, 937–952 e18 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Shan Z et al. Basal condensation of Numb and Pon complex via phase transition during Drosophila neuroblast asymmetric division. Nat Commun 9, 737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng M et al. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 166, 1163–1175 e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X et al. RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol Cell 73, 971–984 e5 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Bunnell SC et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol 158, 1263–75 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Depoil D et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol 9, 63–72 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Lin WC, Petit RS & Groves JT EphA2 receptor activation by monomeric Ephrin-A1 on supported membranes. Biophys J 101, 2731–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algeciras-Schimnich A et al. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol 22, 207–20 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder T & Simons K Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol 9, 534–42 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Munro S Lipid rafts: elusive or illusive? Cell 115, 377–88 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Levental I, Levental KR & Heberle FA Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol 30, 341–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Kalappurakkal JM, Mayor S & Rosen MK Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction. Mol Biol Cell 30, 2996–3012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 531, 651–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung JK et al. Coupled Membrane Lipid Miscibility and Phosphotyrosine-Driven Protein Condensation Phase Transitions. Biophys J (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gureasko J et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol 15, 452–61 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng M et al. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 174, 1172–1187 e16 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Orbach R & Su X Surfing on Membrane Waves: Microvilli, Curved Membranes, and Immune Signaling. Front Immunol 11, 2187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain A & Vale RD RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H et al. RNA Controls PolyQ Protein Phase Transitions. Mol Cell 60, 220–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langdon EM et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato M et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han TW et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–79 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Sanders DW et al. Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324 e28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riback JA et al. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 168, 1028–1040 e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruff KM, Roberts S, Chilkoti A & Pappu RV Advances in Understanding Stimulus-Responsive Phase Behavior of Intrinsically Disordered Protein Polymers. J Mol Biol 430, 4619–4635 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Kato M et al. Redox State Controls Phase Separation of the Yeast Ataxin-2 Protein via Reversible Oxidation of Its Methionine-Rich Low-Complexity Domain. Cell 177, 711–721 e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai AK, Chen JX, Selbach M & Pelkmans L Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559, 211–216 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Ferreon JC et al. Acetylation Disfavors Tau Phase Separation. Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qamar S et al. FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-pi Interactions. Cell 173, 720–734 e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B, Chen W, Evavold BD & Zhu C Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 157, 357–368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishi H et al. Neutrophil FcgammaRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J Clin Invest 127, 3810–3826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan Z et al. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Case LB, Ditlev JA & Rosen MK Regulation of Transmembrane Signaling by Phase Separation. Annu Rev Biophys 48, 465–494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dustin ML & Groves JT Receptor signaling clusters in the immune synapse. Annu Rev Biophys 41, 543–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaqaman K & Ditlev JA Biomolecular condensates in membrane receptor signaling. Curr Opin Cell Biol 69, 48–54 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui E et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grakoui A et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–7 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Barda-Saad M et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 6, 80–9 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Douglass AD & Vale RD Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–50 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houtman JC et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol 13, 798–805 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Su X, Ditlev JA, Rosen MK & Vale RD Reconstitution of TCR Signaling Using Supported Lipid Bilayers. Methods Mol Biol 1584, 65–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kortum RL et al. The ability of Sos1 to oligomerize the adaptor protein LAT is separable from its guanine nucleotide exchange activity in vivo. Sci Signal 6, ra99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng L, Palaia I, Saric A & Su X PLCgamma1 promotes phase separation of T cell signaling components. J Cell Biol 220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ditlev JA et al. A composition-dependent molecular clutch between T cell signaling condensates and actin. Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei MT et al. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem 9, 1118–1125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Case LB, Zhang X, Ditlev JA & Rosen MK Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 363, 1093–1097 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balagopalan L et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol 27, 8622–36 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paster W et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J 34, 393–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong R et al. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR). EMBO J 39, e104730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis SJ & van der Merwe PA The kinetic-segregation model: TCR triggering and beyond. Nat Immunol 7, 803–9 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Pielak RM et al. Early T cell receptor signals globally modulate ligand:receptor affinities during antigen discrimination. Proc Natl Acad Sci U S A 114, 12190–12195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin JJY et al. Mapping the stochastic sequence of individual ligand-receptor binding events to cellular activation: T cells act on the rare events. Sci Signal 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engelke M et al. Macromolecular assembly of the adaptor SLP-65 at intracellular vesicles in resting B cells. Sci Signal 7, ra79 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Oellerich T et al. The B-cell antigen receptor signals through a preformed transducer module of SLP65 and CIN85. EMBO J 30, 3620–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gold MR & Reth MG Antigen Receptor Function in the Context of the Nanoscale Organization of the B Cell Membrane. Annu Rev Immunol 37, 97–123 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Pierce SK & Liu W The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat Rev Immunol 10, 767–77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shelby SA, Castello-Serrano I, Wisser K, Levental I & Veatch S Membrane phase separation drives organization at B cell receptor clusters. bioRxiv, 2021.05.12.443834 (2021). [Google Scholar]
- 78.Williamson AP & Vale RD Spatial control of Draper receptor signaling initiates apoptotic cell engulfment. J Cell Biol 217, 3977–3992 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodridge HS et al. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature 472, 471–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shelby SA, Holowka D, Baird B & Veatch SL Distinct stages of stimulated FcepsilonRI receptor clustering and immobilization are identified through superresolution imaging. Biophys J 105, 2343–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veatch SL, Chiang EN, Sengupta P, Holowka DA & Baird BA Quantitative nanoscale analysis of IgE-FcepsilonRI clustering and coupling to early signaling proteins. J Phys Chem B 116, 6923–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon AK, Holowka D & Baird B Small oligomers of immunoglobulin E (IgE) cause large-scale clustering of IgE receptors on the surface of rat basophilic leukemia cells. J Cell Biol 98, 577–83 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun L, Wu J, Du F, Chen X & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu J et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie W et al. Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc Natl Acad Sci U S A 116, 11946–11955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou W, Mohr L, Maciejowski J & Kranzusch PJ cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell 81, 739–755 e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen S, Rong M, Lv Y, Zhu D & Xiang Y Regulation of cGAS activity through RNA-mediated phase separation. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y et al. Streptavidin Promotes DNA Binding and Activation of cGAS to Enhance Innate Immunity. iScience 23, 101463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu G et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol Cell (2021). [DOI] [PubMed] [Google Scholar]
- 90.Yang P et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 181, 325–345 e28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu ZS et al. G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol 20, 18–28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ergun SL, Fernandez D, Weiss TM & Li L STING Polymer Structure Reveals Mechanisms for Activation, Hyperactivation, and Inhibition. Cell 178, 290–301 e10 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Li S et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomed Eng (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang W et al. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis 10, 946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gack MU et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Arimoto K et al. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A 104, 7500–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SS, Sze L, Liu C & Lam KP The stress granule protein G3BP1 binds viral dsRNA and RIG-I to enhance interferon-beta response. J Biol Chem 294, 6430–6438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Onomoto K et al. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS One 7, e43031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reineke LC & Lloyd RE The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses. J Virol 89, 2575–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rozelle DK, Filone CM, Kedersha N & Connor JH Activation of stress response pathways promotes formation of antiviral granules and restricts virus replication. Mol Cell Biol 34, 2003–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monette A et al. Pan-retroviral Nucleocapsid-Mediated Phase Separation Regulates Genomic RNA Positioning and Trafficking. Cell Rep 31, 107520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinrich BS, Maliga Z, Stein DA, Hyman AA & Whelan SPJ Phase Transitions Drive the Formation of Vesicular Stomatitis Virus Replication Compartments. mBio 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikolic J et al. Negri bodies are viral factories with properties of liquid organelles. Nat Commun 8, 58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lifland AW et al. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J Virol 86, 8245–58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fricke J, Koo LY, Brown CR & Collins PL p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J Virol 87, 1333–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brangwynne CP, Mitchison TJ & Hyman AA Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A 108, 4334–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ambadipudi S, Biernat J, Riedel D, Mandelkow E & Zweckstetter M Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat Commun 8, 275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maharana S et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitrea DM et al. Self-interaction of NPM1 modulates multiple mechanisms of liquid-liquid phase separation. Nat Commun 9, 842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khan T et al. Quantifying Nucleation In Vivo Reveals the Physical Basis of Prion-like Phase Behavior. Mol Cell 71, 155–168 e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Posey AE et al. Mechanistic inferences from analysis of measurements of protein phase transitions in live cells. J Mol Biol, 166848 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan Z et al. Dynamic Monitoring of Phase-Separated Biomolecular Condensates by Photoluminescence Lifetime Imaging. Anal Chem 93, 2988–2995 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Taylor N et al. Biophysical characterization of organelle-based RNA/protein liquid phases using microfluidics. Soft Matter 12, 9142–9150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delarue M et al. mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell 174, 338–349 e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smirnov E et al. Reproduction of the FC/DFC units in nucleoli. Nucleus 7, 203–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oberti D et al. Dicer and Hsp104 function in a negative feedback loop to confer robustness to environmental stress. Cell Rep 10, 47–61 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Franzmann TM et al. Phase separation of a yeast prion protein promotes cellular fitness. Science 359 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Patel A et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–77 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Wang J et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699 e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Louvet E, Yoshida A, Kumeta M & Takeyasu K Probing the stiffness of isolated nucleoli by atomic force microscopy. Histochem Cell Biol 141, 365–81 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Conicella AE, Zerze GH, Mittal J & Fawzi NL ALS Mutations Disrupt Phase Separation Mediated by alpha-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 24, 1537–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brady JP et al. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc Natl Acad Sci U S A 114, E8194–E8203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boeynaems S et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 28, 420–435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alberti S, Gladfelter A & Mittag T Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176, 419–434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McSwiggen DT, Mir M, Darzacq X & Tjian R Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 33, 1619–1634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitrea DM et al. Methods for Physical Characterization of Phase-Separated Bodies and Membrane-less Organelles. J Mol Biol 430, 4773–4805 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura H et al. Intracellular production of hydrogels and synthetic RNA granules by multivalent molecular interactions. Nat Mater 17, 79–89 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin Y et al. Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 168, 159–171 e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bracha D et al. Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable sds. Cell 175, 1467–1480 e13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dine E, Gil AA, Uribe G, Brangwynne CP & Toettcher JE Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst 6, 655–663 e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider N et al. Liquid-liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci Adv 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu N et al. Near-Infrared-Light Activatable Nanoparticles for Deep-Tissue-Penetrating Wireless Optogenetics. Adv Healthc Mater 8, e1801132 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.