Background:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by suppurative infection, sinus tract, and abscess formation. International management guidelines are largely consensus-based. Botulinum toxin (BTX) has been widely used in the treatment of apocrine and eccrine gland disorders, such as hyperhidrosis, although the effectiveness of BTX in the treatment of HS remains unknown. The aim of this systematic review was to understand the published evidence of BTX safety and effectiveness in the management of HS.
Methods:
We conducted a PRISMA-compliant, prospectively registered (PROSPERO, CRD42021228732), systematic review. We devised bespoke search strategy and applied it to the Cochrane Central Register of Controlled Trials, Medline, Embase, and OpenGrey up until March 2022. We included all clinical studies that reported outcomes following BTX treatment in patients diagnosed with HS (both adult and pediatric).
Results:
A total of 4658 studies were identified, of which six met full inclusion criteria reporting data on 26 patients. The six identified studies included one randomized control trial, one case series, and four case studies. The one included randomized control trial demonstrated a significant reduction in the Dermatology Life Quality Index score at 3 months following treatment with BTX.
Conclusions:
The effectiveness and safety of BTX in the treatment of HS remain unknown. This systematic review identified a paucity of high-quality clinical data. Evidence of treatment effectiveness is likely to come from registry-based cohort studies using established core outcome sets in the first instance.
Takeaways
Question: What is the evidence for the use of botulinum toxin (BTX) in the management of hidradenitis suppurativa (HS)?
Findings: Six studies were identified. All studies were low quality. One RCT demonstrated a significant improvement in patient-reported outcomes at 3 months in HS patients treated with BTX versus control. This study had a high risk of bias.
Meaning: There is biological plausibility for BTX in the treatment of HS. There is a paucity of high-quality evidence, and the effectiveness of BTX in the treatment of HS remains equivocal.
INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of follicular epithelium within apocrine glands characterized by suppurative infection, scarring, abscess, sinus tract, and fistula formation.1 HS affects 1% of the adult population and has a large, detrimental impact on quality of life.2 Previous work has demonstrated that HS is significantly more painful compared with other common dermatological conditions owing to the presence of deep inflammatory nodules and recurrent abscess formation.3 Further work has demonstrated that patients with HS have a significantly greater impairment in quality of life compared with other dermatoses.4,5 The incidence of depression among HS patients is between 21% and 42.9%,4,6 with sexual dysfunction and distress commonly reported.7
The evidence base for the management of HS is limited.8 International guidelines published by the European Academy of Dermatology and Venerology recommend that focal lesions are treated with local excision, deroofing, or radical excision with secondary reconstruction.9 Recurrence precludes local excision as a definitive treatment with reported recurrence rates of 69.9%.10 To address this, radical excision of all apocrine gland–bearing skin in the affected anatomic region has been proposed. Resultant soft tissue defects may be treated by primary closure if skin laxity permits, healing by secondary intention or through skin grafting and local flap coverage. Wound dehiscence, scars and associated contracture, secondary donor site scars, graft, and flap failure are all complications associated with a high morbidity.11
Medical therapy is recommended in the treatment of diffuse disease as definitive monotherapy or in combination with surgery.9 Medical therapies include topical and systemic antibiotics, vitamin A derivatives such as tretinoin, and biologics. Given the systemic adverse effects associated with long-term medical therapy12,13 and high morbidity associated with conventional surgical methods, there has been growing interest in minimally invasive local treatments such as laser hair removal to alter apocrine gland and follicle function. Ablative skin resurfacing using carbon dioxide lasers14 and nonablative skin resurfacing using Nd-YAG lasers15 have been used to treat HS with varying success. The use of ablative and nonablative lasers is not recommended in current guidelines, and widespread use is limited by cost.9,16 Botulinum toxin (BTX) represents an alternative minimally invasive topical treatment.
The use of botulinum toxin A (BTX-A) for the management of HS was first reported in 2005.17 BTX inhibits apocrine gland secretion, and it has been suggested that a reduction in apocrine gland secretion reduces bacterial skin flora load, follicle rupture, and associated acute inflammatory response although its direct mechanism of action in HS remains unknown.18 The aim of this systematic review was to understand the published evidence for the safety and efficacy of BTX injections compared with other active operative and nonoperative treatment strategies or placebo in the management of patients with HS.
METHODS
This systematic review was prepared and conducted using guidelines from the Preferred Reporting Items for Systematic Reviews and Meta Analyses.19 Our study was prospectively registered with PROSPERO (CRD42021228732).
Search Strategy
A bespoke search strategy was devised to identify clinical studies that reported the use of BTX in the treatment of HS. A combination of index and free-text terms was used, and full search strategies are given in Supplemental Digital Content 1. (See table, Supplemental Digital Content 1, which displays the search strategy, http://links.lww.com/PRSGO/C265.) The search strategies were applied to the Cochrane Central Register of Controlled Trials, Medline and In Process (1946–January 2021), and EMBASE (1974–January 2021). OpenGrey was searched as a source of gray literature. The search of the above databases was updated on March 24, 2022. The search was limited to human subjects, and no restrictions were placed on publication language. Reference lists of included articles were hand-searched to identify further relevant publications.
Study Selection
All primary research studies that investigated the use of BTX in the treatment of patients with HS were eligible for inclusion. This included randomized controlled trials, nonrandomized controlled trials, cohort studies, case-control studies, case series, and case reports. Expert opinion, systemic reviews, narrative reviews, and descriptions of intervention technique without accompanying outcome data were excluded.
Eligibility Criteria
All studies examining the use of BTX in either adult or pediatric patients with clinically diagnosed HS were eligible for inclusion. BTX is widely used. Two serotypes have been approved for use in humans: BTX-A and botulinum toxin B (BTX-B). BTX-A was approved by the United States Food and Drug Administration in 2002 for the treatment of glabellar wrinkles.20 It is widely used for minimally invasive facial rejuvenation, and there are many commercial brands available.21 BTX-B was initially approved by the Food and Drug Administration in 2000 for the treatment of cervical dystonia although it has also been widely used off label for cosmetic purposes.20 We are interested in all BTX preparations and brands and will refer to them collectively as BTX throughout.
In comparative studies, any clinically active comparator (including, but not limited to, surgical excision with or without surgical reconstruction and pharmacological intervention), best supportive care, or placebo was eligible for inclusion.
Outcomes
The primary outcome was treatment efficacy. This was measured in line with core domains recommended by the HISTORIC core outcome set initiative for HS, which included pain, global assessment (physician and patient reported), progression of disease course, HS-specific quality of life [as measured by disease-specific patient-reported outcome measures such as the Hidradenitis Suppurativa Quality of Life Score and generic measures such as the Dermatology Life Quality Index (DLQI)], and physical signs (such as the number of inflammatory nodules).22 Treatment safety was also assessed through quantification of treatment-related adverse events.
Data Extraction and Analysis
Standardized abstract screening was performed in duplicate by two independent reviewers (L.G. and R.R.) to identify potential studies for review using a prespecified checklist of inclusion criteria. Full-text review and data extraction were then performed, again in duplicate by two independent reviewers (L.G. and R.R.). Disagreements were resolved through consensus discussion with a third author (C.H.).
Risk of bias assessments was performed for included randomized control trials and case series. Randomized controlled trials were assessed using the revised Cochrane Risk of Bias (RoB 2) tool,23 and case series was assessed using the National Institute of Health quality assessment tool for Case Series Studies.24
RESULTS
Search Results
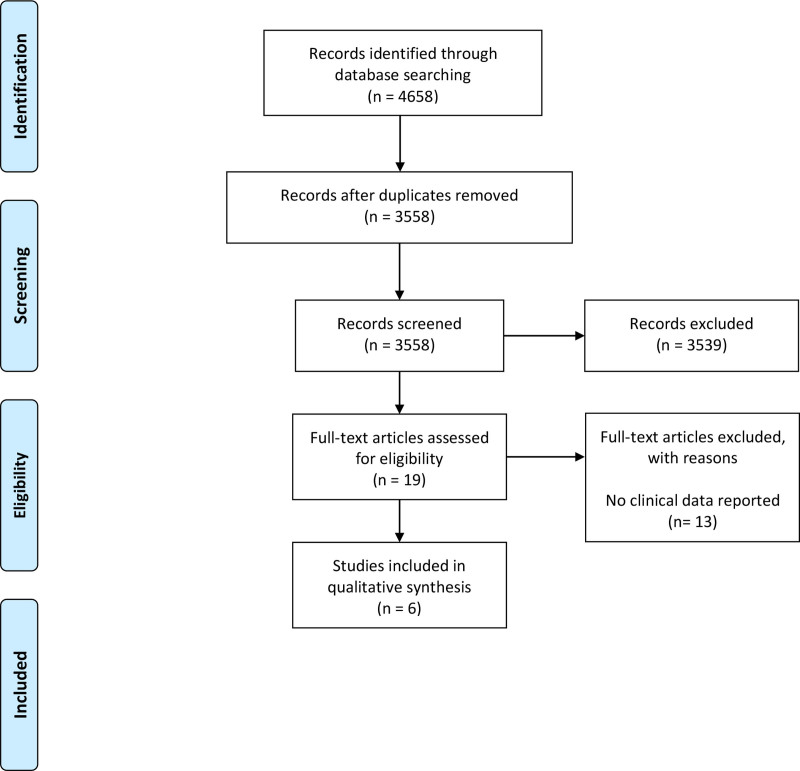
The initial search conducted up to January 2021 identified 4051 studies: 2619 from EMBASE, 1105 from Medline, 320 from Cochrane Central Register of Controlled Trials, and seven from the gray literature. A subsequent search conducted up to March 2022 identified a further 607 studies. All searches were run in parallel. Duplicate articles were identified and removed using a web-based screening and data extraction tool.25 Overall, 4658 studies were identified, 3558 were screened upon removal of duplicates, and 19 full-text articles were assessed for eligibility. The combination of both searches is outlined in Figure 1. A total of 13 studies were excluded as they did not report clinical data, and details of these studies are available on request. There were six studies that met full inclusion criteria. The six studies included one randomized control trial, one case series, and four case reports.
Fig. 1.
PRISMA flow diagram.
Study Characteristics, Demographics, and Quality
Randomized Control Trial
One randomized control trial was identified. Grimstad et al26 conducted a randomized, placebo-controlled trial that assessed the efficacy of BTX-B in the treatment of patients with HS affecting the axillae, groin, and perineum across Hurley stages I–III using the DLQI as a primary outcome measure. The study included 20 participants (three men) with a mean age of 37.5 years. Hurley staging was used to grade disease severity; 70% of patients (n = 14) had stage I disease, 25% of patients (n = 5) had stage II disease, and 5% of patients (n = 1) had stage III disease. Collectively, 40% of patients had disease affecting the groin only (n = 8), 25% of patients had disease affecting the axilla (n = 5), and 35% had multisite disease (n = 7).
After a 3-month study period, all study participants who were unblinded were treated with a single dose of BTX-B and were evaluated at 3 months after treatment. The included randomized control trial was deemed to be at high risk of bias due to unblinding of intervention at 3 months, the associated effect on outcome assessment, and bias arising from outcome reporting. (See figure, Supplemental Digital Content 2, the risk of bias assessment summary, http://links.lww.com/PRSGO/C266.)
Remaining Studies
The five remaining included studies reported data from six patients; 17% (n = 1) were men with a mean age of 34 years (standard deviation, 13). Hurley stage was reported in three studies; 75% of patients (3 of 4) had stage II disease, and 25% of patients (1 of 4) had stage III disease. Anatomic location of disease was reported by five studies, 50% of patients (3 of 6) had axillary disease, 17% of patients (1 of 6) had multisite disease (>1 anatomic area requiring treatment), and 33% (2 of 6) had disease affecting the groin.
Interventions and Comparators
Collectively, five out of six included studies reported the use of BTX-A,17,27–30 and one study reported the use of BTX-B.26 Varying doses of BTX were used. (See table, Supplemental Digital Content 3, which displays the summary table of included studies, http://links.lww.com/PRSGO/C267.) Justification of treatment dose was reported in two studies.26,29 Campanati et al29 report the use of a grid technique, dividing the affected area into equal 1.5 cm2 squares, each of which received 4 units of Botox. Grimstad et al26 report the use of a similar technique and divided affected areas into 1–1.5 cm2 squares, each of which received between 4–5 units of Botox. Grimstad et al26 limited the total dose per treatment area to 150 units in the axilla, 200 units in the groin, and 600 units in the perineum.
Outcomes
Assessments of intervention effect were reported in five out of six included studies, and further information is provided in Supplemental Digital Content 3 (http://links.lww.com/PRSGO/C267). One study used the DLQI as a primary outcome measure.26 The DLQI is a ten-item patient-reported outcome measure designed to assess the impact of dermatological conditions on quality of life. It is scored from 0 to 30 with higher scores indicating greater quality-of-life impairment.31 At 3 months, there was a statistically significant reduction in DLQI scores in the botox cohort (17 versus 8, P < 0.05). The change in DLQI scores at baseline and 3 months was also above the established minimal clinically important change of 4. This minimal clinically important change was determined in a cohort of patients with inflammatory skin conditions (including HS) using an anchor-based approach.32 Notably, there was no significant difference in DLQI scores at 3 months in the placebo arm. All patients were unblinded at 3 months and continued treatment with BTX. Following commencement on BTX therapy, the change in DLQI scores from 3 to 6 months in the control arm was not statistically significant (11 versus 6.5, P < 0.07).
Grimstad et al26 used a visual analogue scale to quantify perceived impairment of general health related to hidradenitis and “pain in the worst boil.” Further outcomes included total number of nodules and total number of lesions. A significant reduction in the total number of lesions (9 versus 4, P < 0.01), HS-related impairment of general health (8 versus 3.5, P < 0.01), and the number of nodules (7.5 versus 3.5, P < 0.01) was seen at 3 months in the Botox arm. No significant difference was noted in HS-related impairment of general health (6 versus 5.5, P > 0.05), number of nodules (6 versus 2, P > 0.05), total number of lesions (6 versus 4.5, P > 0.05), and pain (7 versus 7, P > 0.05) in the control arm at 3 months.
DISCUSSION
This systematic review identified one randomized control trial that demonstrated BTX improves patient-reported outcomes compared with a control cohort. This systematic review did not identify any other high-quality evidence of intervention effect or safety. The role of BTX in the treatment of HS remains equivocal although the limited available evidence is encouraging.
There is biological plausibility for why BTX may be an effective treatment for HS.33 There is also a body of published evidence from other apocrine/eccrine gland disorders such as hyperhidrosis that suggest BTX is well tolerated with few adverse effects.34 The current evidence gap may be due to lack of academic interest or poor perceived treatment efficacy. The former is supported by the paucity of evidence in current clinical practice guidelines9 and heterogeneity in current national care pathways.35 However, there is growing academic interest in the treatment of HS. The James Lind Alliance has recently outlined key priorities in future HS management through priority setting partnerships between key patient, clinician, and academic stakeholders.36 Furthermore, there are ongoing national cohort studies [namely, the Treatment of Hidradenitis Suppurativa Evaluation Study (THESUS)], which aim to determine current HS treatment pathways and validate instruments used to measure domains that have been identified as part of the HS core outcome set.37 Ongoing studies and recent priority setting partnerships may reduce the current evidence gap. While of limited direct use, the data presented in the current systematic review will hopefully further stimulate academic interest and lead to further studies investigating the effectiveness and efficacy of BTX in the treatment of HS.
Evaluating BTX as a novel, minimally invasive treatment for HS would be informative and feasible as demonstrated by Grimstad et al. There are a number of factors to consider. HS varies in severity, and a study investigating disease of all stages may be counterproductive. The efficacy of BTX may differ based on disease severity. Preliminary work investigating the efficacy of BTX across the range of disease severity would be informative and could be delivered through prospective cohort studies using recently established international registries.38 This preliminary work would inform power calculations to minimize the risk of type II error through incorporation of instruments identified in the THESUS study.37 The duration of intervention effect and the impact of repeated intervention over time must also be considered in future trial design. While considerations of convenience and cost are beyond the scope and remit of this review, they could be considered contributory factors in the relative paucity of literature surrounding this treatment. Moreover, there are several preparations and brands of BTX, none of which are licensed for the treatment of HS to the authors' knowledge at the time of this publication. Furthermore, there is no current standard of care across different severities of HS, making selection of a comparator challenging.8,35,39
Future clinician and patient surveys will be required to ascertain patient perceptions regarding the use of BTX, associated barriers to implementation, and whether true equipoise exists.37 The aim of THESUS is to determine current HS treatment pathways and to validate instruments used to measure domains identified as part of the HS core outcome set.22 Outcome measures identified and validated in the THESUS study could be integrated into existing international registries.38,40 This would provide preliminary evidence of intervention effect across different severities of HS through a pragmatic approach. These data could then be used to inform the design of an RCT in conjunction with the above consideration points.
In conclusion, there are limited clinical data to determine the efficacy and safety of BTX in the treatment of HS. This systematic review identified one randomized control trial that demonstrated a statistically significant and clinically meaningful difference in DLQI scores at 3 months following intervention effect. The remaining body of evidence is largely anecdotal with predominance of case reports and small case series. The efficacy of BTX in the treatment of HS is likely to come from registry-based cohort studies in the initial setting, which can then be used to inform the design of placebo-controlled randomized trials if true equipoise exists.
Supplementary Material
Footnotes
Published online 18 November 2022.
Disclosures: Dr. Harrison is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship (NIHR300684). Dr. Rodrigues is funded by an NIHR postdoctoral fellowship (PDF-2017-10-075). The other authors have no financial interest to declare. This document presents independent research funded by the NIHR. Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994–999. [DOI] [PubMed] [Google Scholar]
- 2.Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596–601. [DOI] [PubMed] [Google Scholar]
- 3.Wolkenstein P, Loundou A, Barrau K, et al. ; Quality of Life Group of the French Society of Dermatology. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621–623. [DOI] [PubMed] [Google Scholar]
- 4.Onderdijk AJ, van der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2013;27:473–478. [DOI] [PubMed] [Google Scholar]
- 5.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264–268. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurek A, Peters EM, Chanwangpong A, et al. Profound disturbances of sexual health in patients with acne inversa. J Am Acad Dermatol. 2012;67:422–428.e1. [DOI] [PubMed] [Google Scholar]
- 8.Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619–644. [DOI] [PubMed] [Google Scholar]
- 10.Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppuritiva: a study of 106 cases. Surgeon. 2005;3:23–26. [DOI] [PubMed] [Google Scholar]
- 11.Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AL, Pace ND, DeSesso JM. Teratogen update: topical use and third-generation retinoids. Birth Defects Res. 2020;112:1105–1114. [DOI] [PubMed] [Google Scholar]
- 13.David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273–288. [DOI] [PubMed] [Google Scholar]
- 14.Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208–213. [DOI] [PubMed] [Google Scholar]
- 15.Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188–1198. [DOI] [PubMed] [Google Scholar]
- 16.Alshami MA. New application of the long-pulsed Nd-YAG laser as an ablative resurfacing tool for skin rejuvenation: a 7-year study. J Cosmet Dermatol. 2013;12:170–178. [DOI] [PubMed] [Google Scholar]
- 17.O’Reilly DJ, Pleat JM, Richards AM. Treatment of hidradenitis suppurativa with botulinum toxin A. Plast Reconstr Surg. 2005;116:1575–1576. [DOI] [PubMed] [Google Scholar]
- 18.Qu H, Gao L. Botulinum toxin type A for the management of hidradenitis suppurativa. Am J Transl Res. 2021;13:14115–14120. [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadick NS, Herman AR. Comparison of botulinum toxins A and B in the aesthetic treatment of facial rhytides. Dermatol Surg. 2003;29:340–347. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury S, Baker MR, Chatterjee S, et al. Botulinum toxin: an update on pharmacology and newer products in development. Toxins (Basel). 2021;13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorlacius L, Ingram JR, Villumsen B, et al. ; HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC). A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 24.National Heart, Lung and Blood Institute. Study Quality Assessment Tools. 2013. Available at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed June 20, 2022.
- 25.Covidence. Covidence systematic review software. 2022. Available at https://www.covidence.org/. Accessed June 20, 2022.
- 26.Grimstad Ø, Kvammen BØ, Swartling C. Botulinum toxin type B for hidradenitis suppurativa: a randomised, double-blind, placebo-controlled pilot study. Am J Clin Dermatol. 2020;21:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feito-Rodríguez M, Sendagorta-Cudós E, Herranz-Pinto P, et al. Prepubertal hidradenitis suppurativa successfully treated with botulinum toxin A. Dermatol Surg. 2009;35:1300–1302. [DOI] [PubMed] [Google Scholar]
- 28.Shi W, Schultz S, Strouse A, et al. Successful treatment of stage III hidradenitis suppurativa with botulinum toxin A. BMJ Case Rep. 2019;12:e226064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campanati A, Martina E, Giuliodori K, et al. Two cases of Hidradenitis suppurativa and botulinum toxin type a therapy: a novel approach for a pathology that is still difficult to manage. Dermatol Ther. 2019;32:e12841. [DOI] [PubMed] [Google Scholar]
- 30.Khoo AB, Burova EP. Hidradenitis suppurativa treated with Clostridium botulinum toxin A. Clin Exp Dermatol. 2014;39:749–750. [DOI] [PubMed] [Google Scholar]
- 31.Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. [DOI] [PubMed] [Google Scholar]
- 32.Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230:27–33. [DOI] [PubMed] [Google Scholar]
- 33.Stuart ME, Strite SA, Gillard KK. A systematic evidence-based review of treatments for primary hyperhidrosis. J Drug Assess. 2020;10:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howes R, Ingram JR, Thomas KS, et al. ; THESEUS Survey collaborator group. The surgical management of hidradenitis suppurativa in the United Kingdom: a national survey of care pathways informing the THESEUS study. J Plast Reconstr Aesthet Surg. 2022;75:240–247. [DOI] [PubMed] [Google Scholar]
- 36.Ingram JR, Abbott R, Ghazavi M, et al. The hidradenitis suppurativa priority setting partnership. Br J Dermatol. 2014;171:1422–1427. [DOI] [PubMed] [Google Scholar]
- 37.Bates J, Stanton H, Cannings-John R, et al. Treatment of hidradenitis suppurativa evaluation study (THESEUS): protocol for a prospective cohort study. BMJ Open. 2022;12:e060815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daxhelet M, Suppa M, Benhadou F, et al. Establishment of a European Registry for hidradenitis suppurativa/acne inversa by using an open source software. J Eur Acad Dermatol Venereol. 2016;30:1424–1426. [DOI] [PubMed] [Google Scholar]
- 39.Ingram JR, McPhee M. Management of hidradenitis suppurativa: a U.K. survey of current practice. Br J Dermatol. 2015;173:1070–1072. [DOI] [PubMed] [Google Scholar]
- 40.Daxhelet M, Daoud M, Suppa M, et al. European registry for hidradenitis suppurativa: state of play. J Eur Acad Dermatol Venereol. 2021;35:e274–e276.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.