Abstract
Acute respiration distress syndrome (ARDS) is a typical complication in toxic shock-like syndrome (TSLS) caused by Streptococcus pyogenes. An isolated perfused rat lung model was used to identify the causative agent of ARDS in TSLS in this study. Some crude preparations separated from the culture supernatants of S. pyogenes isolates caused rapid increases in the weight of perfused lungs, indicating vascular permeabilization. Six samples from M type 1 and 3 isolates from TSLS and pharyngitis patients showed strong permeabilization activity, whereas preparations from isolates of other M types (although the number of isolates examined was limited) were negative. The active substance was purified to a single band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using various columns, and the N-terminal amino acid sequence was determined. The resultant sequence of eight amino acids was identical to SpeF (mitogenic factor). Moreover, the vascular permeabilization activity of the purified band was abolished with anti-SpeF antiserum prepared by immunizing with the purified SpeF. This activity was also neutralized by the antiserum prepared by immunizing with a synthetic peptide derived from the published SpeF sequence. These results suggested that streptococcal SpeF is a major cause of permeabilization of lung blood vessels and sufficient for the pathogenesis of ARDS under the conditions of TSLS caused by S. pyogenes.
Acute respiration distress syndrome (ARDS) and pulmonary hemorrhage are frequently major complications in toxic shock-like syndrome (TSLS) caused by group A streptococci (GAS). About 60% of TSLS cases exhibit ARDS (15, 26, 28). ARDS is a result of pulmonary edema, which is caused by the permeabilization of pulmonary capillaries and ultimately leads to alveolar flooding in many cases. ARDS is one of the complications in systemic bacterial infections which have been recently classified as systemic inflammatory response syndrome; however, only a few bacterial toxins and cell components have been identified as causative substances for ARDS (23, 25).
Because of the worldwide prevalence of TSLS, a number of studies have been undertaken to identify the relevant virulence factors (6, 18, 29). The proposed virulence factors include exotoxins such as streptococcal pyrogenic exotoxins A (SpeA), B (SpeB), C (SpeC), and F (SpeF; previously referred to as mitogenic factor) and streptococcal superantigen (3, 5, 17, 32, 33). These exotoxins have superantigenic activities which induce massive T-cell proliferation and release large amounts of cytokines. It has therefore been postulated that some clinical symptoms such as multiorgan failure, high fever, and shock in TSLS are caused by the action of these superantigens (11, 14, 20). SpeB is a protease and activates interleukin-1β (10). The cytokines tumor necrosis factor alpha and interleukin-1β have toxic effects on endothelial cells (4, 27), and therefore extremely high levels of these cytokines in the circulation may injure pulmonary microvascular endothelial cells, resulting in permeabilization of pulmonary capillaries. Another hypothetical mechanism for the induction of ARDS in TSLS patients is that toxins produced by GAS immediately damage pulmonary endothelial cells. Lee et al. particularly noted that this hypothesis is of major importance in the development of TSLS (12).
An isolated perfused lung model used previously for the study of ARDS induced by bacterial toxins (23) has enabled us to measure the permeabilization effect of toxins on pulmonary capillaries. We found that crude preparations from the culture supernatant of GAS isolates of M types 1 and 3 are potent inducers of ARDS in the isolated perfused rat lung model. We then further purified and characterized the active substance.
MATERIALS AND METHODS
Bacterial isolates.
Streptococcus pyogenes isolates used in this study were isolated in Japan between 1992 and 1994 (13). The isolates used in the lung vascular permeability assays and in immunoblot analysis are listed in Tables 1 and 2, respectively. The case definition of TSLS was based on the formula developed by the U.S. Working Group on Severe Streptococcal Infections (34).
TABLE 1.
S. pyogenes isolates used in lung vescular permeability assays
| Isolate | M type | Source |
|---|---|---|
| 1269 | 1 | TSLS |
| 1143 | 3 | Pharyngitis |
| 1236 | 3 | Pharyngitis |
| 1268 | 3 | TSLS |
| 1281 | 3 | Necrotizing fasciitis |
| 1286 | 3 | TSLS |
| 1259 | 4 | Pharyngitis |
| 1252 | 22 | Pharyngitis |
| 1292 | 22 | TSLS |
| 1256 | Untypeable | Pharyngitis |
TABLE 2.
S. pyogenes isolates used in immunoblot analysis
| No. of isolates | M type | Source |
|---|---|---|
| 1247 | 1 | Pharyngitis |
| 1276 | 1 | TSLS |
| 1529 | 1 | TSLS |
| O8 | 1 | Pharyngitis |
| 1239 | 3 | Pharyngitis |
| 1265 | 3 | TSLS |
| 1287 | 3 | TSLS |
| 1289 | 3 | TSLS |
| O5 | 3 | Pharyngitis |
| O9 | 3 | Pharyngitis |
| O14 | 3 | Pharyngitis |
| K5 | 2 | Pharyngitis |
| 1266 | 4 | TSLS |
| 1288 | 4 | TSLS |
| A2 | 4 | Pharyngitis |
| O6 | 4 | Pharyngitis |
| O3 | 11 | Pharyngitis |
| O7 | 12 | Pharyngitis |
| O12 | 12 | Pharyngitis |
| 1270 | 28 | TSLS |
| 1244 | 28 | Pharyngitis |
| 1285 | Untypeable | TSLS |
M typing.
M antigens of S. pyogenes were extracted by the hot HCl method, and M types were determined by the capillary precipitation reaction, as described previously (19).
Purification of the active substance.
S. pyogenes was grown in 50 ml of brain heart infusion broth (Difco Laboratories, Detroit, Mich.) at 37°C in a 5% CO2 atmosphere. The culture supernatant was collected by centrifugation and filtered through a 0.22-μm-pore-size sterile membrane filter (Millipore Corp., Bedford, Mass.). The proteins in the culture supernatant were precipitated with a 50% saturated ammonium sulfate solution at 4°C for 2 days. The precipitate was collected and resolubilized in phosphate buffer (0.1 M sodium phosphate [pH 7.0]), and the solution was dialyzed into phosphate buffer at 4°C overnight. After dialysis, the volume of the crude preparation was adjusted to a concentration of 0.5 g of protein/ml and used for the lung vascular permeability assays.
To purify the active substance, the following procedures were performed with an FPLC Standard system (Pharmacia, Uppsala, Sweden). Briefly, the precipitate with ammonium sulfate prepared from the culture supernatant of isolate 1286 was resolubilized in 0.05 M acetate buffer (pH 4.8). The solution was then applied to a DEAE-Sepharose Fast Flow column (Pharmacia) preequilibrated with 0.05 M acetate buffer (pH 4.8) in an NaCl gradient of 0 to 1.0 M, and fractionated peaks were tested for lung vascular permeability. The active fraction was then applied to a phenyl-Sepharose HP column (Pharmacia) preequilibrated with 0.05 M acetate buffer (pH 4.8) containing 2.0 M ammonium sulfate in an ammonium sulfate gradient of 2.0 to 0 M. A sharp major peak exhibited high lung vascular permeabilization activity. This fraction was further applied to a Superdex 75 column (Pharmacia) in phosphate buffer (0.05 M sodium phosphate [pH 7.0]). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 12% acrylamide gel resulted in a final peak containing a single protein band with an estimated molecular mass of 25 kDa. The purified protein was quantified with bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.). To determine the N-terminal amino acid sequence of the active protein, the purified protein was further applied to a μ Bondasphere column (Waters Japan K.K., Tokyo, Japan) for reversed-phase high-performance liquid chromatography with a linear gradient of acetonitrile (20 to 80%, 1%/min) in 0.1% trifluoroacetic acid at a flow rate of 1 ml/min. Amino acid sequencing was performed with an automated amino acid sequencer (model 476A; Applied Biosystems, Foster City, Calif.).
Lung vascular permeability assay.
Male rats Wistar weighing 250 to 300 g were anesthetized with an intraperitoneal administration of pentobarbital sodium (35 mg/kg of body weight). The lungs were isolated and perfused for the lung vascular permeability assay as described below.
Modified isolated lung perfusion models (7) were made as described by Gaar et al. (2). Briefly, after insertion of a tracheal tube, arterial and venous cannulae were inserted into the left pulmonary artery via the right ventricle and into the left atrium, respectively. Blood was removed by using a Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2 · 2H2O, 1.2 mM MgSO4 · 7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM glucose) containing 10% low-molecular-weight dextran, 3% bovine serum albumin, and 10−1 mM papaverine hydrochloride (Tokyo Kasei Kogyo Co., Tokyo, Japan). The pH was adjusted to between 7.3 and 7.5 with either hydrochloric acid or sodium bicarbonate solution. All reagents were purchased from Tokyo Kasei Kogyo.
The isolated rat lungs were placed in a moisture chamber with the temperature maintained at 35°C and were perfused under zone III conditions (venous pressure > alveolar pressure) and ventilated with constant 30-mm H2O airway pressure. Pulmonary arterial and venous pressures were measured with LPU-0.1 pressure transducers (Nihon Kohden, Tokyo, Japan) positioned at the orifices of the inflow and outflow cannulae, respectively. Pressures were zeroed at the level of the lung hilus, and most of the lung mass was below this level. Perfusion flow was measured by counting the drips from the venous outlet with an RT-5 tachometer (Nihon Kohden). Lung weight was measured with a TB-611T force-displacement transducer (Nihon Kohden) connected to an RP-5 amplifier (Nihon Kohden).
In the crude preparations, after hemodynamic equilibrium was obtained, capillary pressure and capillary filtration coefficient (Kf,c) values were determined by occluding both arterial and venous cannulae and by elevating both arterial and venous reservoirs, respectively, as described previously (2). Briefly, capillary pressure was obtained as the mean of both arterial and venous pressures while the perfusion flow was stopped. The capillary filtration coefficient value was calculated by dividing the slope ratio of the lung weight gain, i.e., outward flow of perfusion fluid from the vascular lumen, by the mean value of the changes in arterial and venous pressures (ΔPa and ΔPv, respectively) and the quotient was then divided by the lung weight at the extrapolated zero time (Wt=0), i.e., starting point of elevating both reservoirs. The outward flow at zero time (ΔW/Δt)t=0) was calculated by extrapolating the slope ratios of lung weight gain at times from 3 to 7 min after the reservoir elevation, on a semilogarithmic scale. The equation used was as follows:
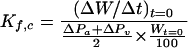 |
The samples were then added to the perfusion fluid, and the capillary pressure and capillary filtration coefficient values were measured at appropriate times. All animal experiments were performed according to the ethical guidelines of the Institute for Laboratory Animal Research, Nagoya University School of Medicine.
Preparation of antisera.
Rabbit antisera were prepared as described previously (8). Briefly, the purified antigen preparation or a synthetic peptide coupled with keyhole limpet hemocyanin at a concentration of 10 μg/ml was emulsified with an equal volume of Freund’s complete adjuvant (Difco) and subcutaneously injected into a rabbit. The second antibody response was elicited by immunization with the antigen alone, and serum was obtained. The synthetic peptide, Val-Leu-Val-Tyr-Asn-Thr-Ala-Asn-Thr-Ile-Asn-Tyr-His-Asn-Gly-Thr-Pro-Thr-Gln-Lys, was derived from the published SpeF sequence (8) and was coupled with keyhole limpet hemocyanin for immunization.
Western blot analysis.
Active fractions were separated by SDS-PAGE in 12% acrylamide and electroblotted onto a nitrocellulose filter membrane as described previously (31). After immunoreaction with rabbit antiserum, the membrane was secondarily stained with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin (Biosource, Camarillo, Calif.).
RESULTS
Lung vascular permeabilization induced by crude preparations.
We used the isolated perfused rat lung model to assay lung vascular permeability. The capillary filtration coefficient value was used as an index of permeabilization. This model utilizes an artificial solution instead of blood, enabling us to detect the direct effects of samples on pulmonary endothelial cells by minimizing the effects of blood components secreted from cells such as lymphocytes.
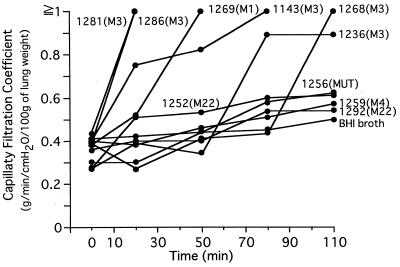
Among 10 preparations from S. pyogenes isolates examined, all of those from M type 1 and 3 isolates, irrespective of the source, had capillary filtration coefficient values over 0.89 (Fig. 1). The capillary filtration coefficient values of isolates 1281 and 1286 rapidly exceeded 1.0 within 20 min, and those of isolates 1143 and 1269 exceeded 1.0 by 80 min. The capillary filtration coefficient values of isolates 1236 and 1268 eventually peaked at the highest values. In this model, capillary filtration coefficient values exceeding 1.0, indicate that the permeability barrier function of lung vascular endothelium is destroyed by the active substance in the sample preparation and thus subsequent data are meaningless.
FIG. 1.
Lung vascular permeabilization induced by crude preparations. At time zero, 300 μl of each crude preparation was added to 50 ml of the perfusion fluid. The capillary filtration coefficient values for the preparations were measured at 20, 50, 80, and 110 min. The M type and source of each S. pyogenes isolate are described in Table 1.
In contrast, the capillary filtration coefficient values of other than M type 1 and 3 isolates increased only slightly after long-term incubation (Fig. 1, 1252, 1256, 1259, and 1292).
Purification and characterization of the active substance.
S. pyogenes isolate 1286 from a TSLS patient exhibited high activity in lung vascular permeability assays. The culture supernatant of the isolate was saturated with ammonium sulfate, and the protein fraction was precipitated.
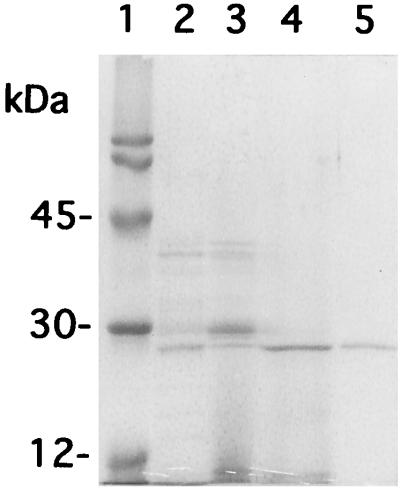
The crude precipitate was serially fractionated with a DEAE-column, a hydrophobic interaction chromatography column, and a gel filtration column. A single peak was separated, and this peak gave a single band with an estimated molecular mass of 25 kDa on SDS-PAGE (Fig. 2, lane 5). This protein exhibited strong lung vascular permeabilization activity.
FIG. 2.
The purified active substance in SDS-PAGE. The active substance was purified from the crude preparation of S. pyogenes 1286 as described in Materials and Methods and analyzed by SDS-PAGE. Lane 1, molecular weight markers; lane 2, crude preparation; lane 3, active fraction after DEAE column purification; lane 4, active fraction after phenyl-Sepharose column purification; lane 5, active fraction after Superdex 75 column purification.
Automated amino-terminal peptide sequencing was performed to determine the sequence of the N-terminal amino acids of the purified protein. The amino acid sequence identified was Gln-Thr-Gln-Val-Ser-Asn-Asp-Val. Computer-assisted homology searches revealed that this sequence is identical to the N-terminal portion of streptococcal SpeF reported by Yutsudo et al. (32). This finding was confirmed by Western blot analysis. SpeF-specific rabbit antiserum (kindly donated by T. Yutsudo) strongly bound the purified protein (data not shown). These results indicate that SpeF is a causative toxin of lung vascular permeabilization.
Lung vascular permeabilization by purified SpeF and its inhibition by anti-SpeF serum 1.
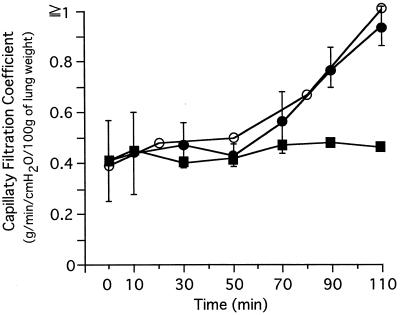
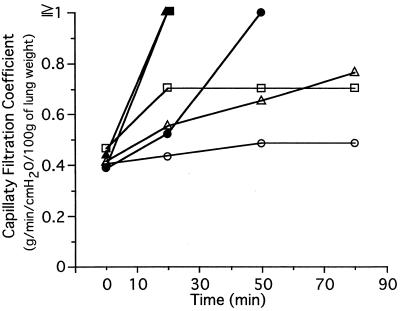
In the isolated perfused rat lung model, purified SpeF at 1.0 mg/ml in phosphate-buffered saline was added stepwise to 50 ml of the perfusion fluid to give concentrations of 10, 30, and 100 ng/ml at 0, 40, and 80 min, respectively. The capillary filtration coefficient values were then measured at 10, 30, 50, 70, 90, and 110 min (Fig. 3). As Fig. 3 shows, purified SpeF had a cumulative effect on lung vascular permeabilization, which was abolished by anti-SpeF antiserum prepared with immunization of the purified SpeF (anti-SpeF serum 1); in contrast, normal rabbit serum had no effect on the permeabilization activity of the purified SpeF.
FIG. 3.
Inhibition of lung vascular permeabilization by ant-SpeF serum 1. One milligram of the highly purified active substance (SpeF) in phosphate-buffered saline was mixed with an equivalent volume of anti-SpeF serum 1 or a normal rabbit serum, and the mixture was incubated for 30 min at room temperature. The mixtures were added to 50 ml of the perfusion fluid to reach a toxin concentration of 100 ng/ml. The capillary filtration coefficient values were then measured at each indicated time. ○, SpeF treated with a normal rabbit antiserum; ●, SpeF; ■, SpeF treated with anti-SpeF serum 1. Values are the means of four different experiments ± standard deviations (SpeF) and the means of two different experiments (SpeF + anti-SpeF serum 1).
Western blot analysis of the production of SpeF.
The relationship between lung vascular permeabilization activity and SpeF production by each S. pyogenes isolate was examined. Immunoblot analysis was performed to evaluate the production of SpeF in the culture fluid. The cultures were preadjusted to give ∼108 CFU/ml, and aliquots (0.5 ml) of the culture supernatants were prepared by centrifugation and filtration. The proteins in the culture supernatants were then precipitated with 1.0 ml of ethanol. The precipitates were collected and resolubilized in 20 μl of distilled water, and 10-μl aliquots were analyzed by Western blotting using anti-SpeF serum 1. This antiserum detected only a single band on SDS-PAGE, at the position of the purified SpeF. This band was also stained with anti-SpeF serum provided by T. Yutsudo and the antiserum prepared by immunization with the synthetic peptide (anti-SpeF serum 2) (data not shown).
In all M type 1 and 3 preparations with high lung vascular permeabilization activity, high amounts of SpeF were detected (Fig. 4). Among four isolates with no vascular permeabilization activity, 1252 had only a trace amount of SpeF production, 1256 and 1292 expressed SpeF only poorly, and 1259 showed lower but significant levels of SpeF production.
FIG. 4.
Immunoblot analysis of SpeF production from S. pyogenes isolates used in the lung vascular permeability assay. Anti-SpeF serum 1 was used as the first antibody.
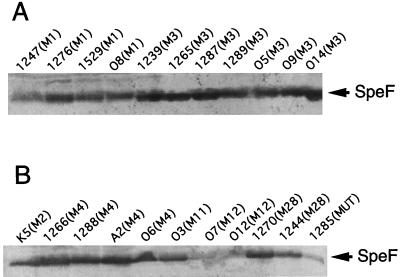
Western blot analysis was performed with a greater number of S. pyogenes isolates to determine the degree of expression of SpeF among clinical isolates. In the analysis of 22 S. pyogenes isolates belonging to seven different M types, all M type 1 and 3 isolates from TSLS and pharyngitis patients expressed high levels of SpeF, as judged by immunoblot analysis (Fig. 5A). The degree of SpeF production was variable in the isolates belonging to the other M types (Fig. 5B). For example, the isolates belonging to M types 2, 4, 11, and 28 expressed relatively large amounts of SpeF, whereas three isolates of M types 12 and MUT produced only small amounts (Fig. 5B). Interestingly, the crude preparations of isolates K5 and O3 showed no positive permeabilization activity (i.e., the capillary filtration coefficient values for the K5 and O3 isolates were 0.45 and 0.53 at 80 min, respectively, after sample application), although these preparations contained immunologically positive SpeF protein.
FIG. 5.
Immunoblot analysis of SpeF production from various S. pyogenes clinical isolates belonging to M types 1 and 3 (A) and to other M types (B). Anti-SpeF serum 1 was used as the first antibody. The M type and source of each S. pyogenes isolate are described in Table 2.
Inhibition of lung vascular permeabilization activity of crude preparation by anti-SpeF serum 2.
We suspected that the lung vascular permeabilization was also induced by other exoproteins in the crude preparations and therefore prepared anti-SpeF serum 2 by immunization with a synthetic peptide derived from the SpeF sequence. This new antiserum was highly specific to SpeF, but the antibody titer was slightly lower than that of anti-SpeF serum 1, as judged by Western blot reaction.
As Fig. 6 shows, anti-SpeF serum 2 neutralized the permeabilization activity of crude preparations from isolates 1269, 1281, and 1286, decreasing the capillary filtration coefficient values from ≧1.0 to 0.48 for 1269 at 50 min, ≧1.0 to 0.55 for 1281 at 20 min, and ≧1.0 to 0.7 for 1286 at 20 min. The suppression by anti-SpeF serum 2, however, was not complete for 1281 and 1286, and long-term perfusion with these crude preparations caused low levels of lung vascular permeabilization. These as well as the previous results clearly indicated that streptococcal SpeF was a major causative toxin for permeabilization of lung blood vessels.
FIG. 6.
Inhibition of lung vascular permeabilization activity of crude preparation by anti-SpeF serum 2. Three hundred microliter of the crude preparation of 1269 (M1), 1281 (M3), or 1286 (M3) was mixed with threefold volumes of anti-SpeF serum 2, and the mixture was incubated for 30 min at room temperature. The mixtures were added to 50 ml of the perfusion fluid, and the capillary filtration coefficient values for each sample were measured at 20, 50, and 80 min, respectively. ●, crude preparation of 1269; ○, crude preparation of 1269 treated with anti-SpeF serum 2; ▴, crude preparation of 1281; ▵, crude preparation of 1281 treated with anti-SpeF serum 2; ■, crude preparation of 1286; □, crude preparation of 1286 treated with anti-SpeF serum 2.
DISCUSSION
SpeF produced by S. pyogenes is a multifunctional protein with mitogenic, superantigenic, and nuclease activities (9, 30, 32). In the present study, we found that SpeF had vascular permeabilization activity in a rat lung model. Induction of significant reactions in the rat lung model required approximately 100 ng of purified SpeF in 1 ml of perfusion solution, a dose somewhat higher than those used for the induction of in vitro mitogenic activity by SpeF (16). Seeger and coworkers reported that several bacterial toxins including Escherichia coli hemolysin, Pseudomonas aeruginosa cytotoxin, and Staphylococcus aureus α-toxin induce lung vascular permeabilization in an isolated perfused rabbit lung model, very similar to the rat model used in this study; they showed that these toxins play important roles in the pathogenesis of ARDS caused by bacterial infections (1, 21, 24). SpeF may be a crucial factor for onset of ARDS during severe infection or TSLS caused by S. pyogenes.
It has been proposed that the mechanisms by which these toxins exert lung vascular damage in the isolated perfused rabbit lung model include transmembrane pore formation by toxins, calcium gating through the pores, calcium-mediated induction of phospholipolytic activities, and subsequent formation of cell specific arachidonic acid metabolites (22, 23).
There are a few possible mechanisms which would explain rat lung vascular permeabilization by SpeF. One is that mentioned by Seeger et al. (1, 21, 24): SpeF would form pores on lung vascular endothelial cells and then causes sequential events which trigger arachidonic acid cascades and consequently causes lung vascular permeabilization. However, this is unlikely, because so far there is no evidence that SpeF has pore-forming ability. Second, SpeF has potent superantigenic activity (30) and may activate T lymphocytes to produce excessive amounts of lymphokines and induce vascular permeabilization. However, in this isolated rat lung model, most T cells in the blood vessels were washed away with Krebs-Henseleit solution before SpeF application and permeabilization was induced within 30 min after the application, minimizing the possibility of lymphocyte involvement. Third, SpeF might immediately stimulate vascular endothelial cells to cause vascular permeabilization via an unidentified pathway. It has been demonstrated that S. pyogenes produces various extracellular toxic proteins, including pore-forming toxins and superantigenic Spe toxins (3, 5, 17, 25, 32, 33). Minor contaminants of these toxins in the purified SpeF preparation would induce permeabilization of lung blood vessels. However, anti-SpeF serum 2 prepared with a synthetic peptide based on the published SpeF sequence also inhibited the permeabilization activity. We therefore suggest that streptococcal SpeF is a major causative toxin for permeabilization of lung blood vessels. Since the inhibition by anti-SpeF serum 2 was not complete for some crude preparations, other toxins such as hemolysin in the crude preparations would also be responsible for induction of vascular permeabilization. It should be noted, however, that the anti-SpeF titer of serum 2 was lower than that of serum 1.
The amount of SpeF secreted in the culture varied among S. pyogenes isolates, but M type 1 and 3 isolates generally produced high amounts of SpeF. Isolates belonging to other M types produced varied amounts of SpeF. Isolates K5 and O3 expressed antigenically positive SpeF with an apparent molecular mass the same as that of the purified preparation, but the crude preparations of these isolates showed no positive permeabilization activity. The structure of the active domains of SpeF may be lost in K5 and O3, although sequence data for the inactive SpeF are not yet available. It is also likely that the antigenic determinant domains of SpeF and the domains relevant to the permeabilization activity are different.
ACKNOWLEDGMENTS
We thank Hideo Igarashi (Tokyo Metropolitan Research Laboratory of Public Health, Tokyo, Japan) for providing S. pyogenes isolates and Yoshikata Shimizu (Asahi General Hospital, Chiba, Japan) for case definition and a useful suggestion. We are also grateful to Teiko Murai (College of Health Professions, Toho University, Tokyo, Japan) for M typing of S. pyogenes isolates and Takashi Yutsudo (Discovery Research Laboratory I, Shionogi & Co., Ltd., Osaka, Japan) for donating anti-SpeF antiserum and for useful discussions.
This work was supported by grant 08457086 from the Science and Technology Agency of the Japanese Government.
REFERENCES
- 1.Ermert L, Rousseau S, Schutte H, Birkemeyer R G, Grimminger F, Bhakdi S, Duncker H R, Seeger W. Induction of severe vascular leakage by low doses of Escherichia coli hemolysin in perfused rabbit lungs. Lab Investig. 1992;66:362–369. [PubMed] [Google Scholar]
- 2.Gaar K A, Jr, Taylor A E, Owens L J, Guyton A C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol. 1967;213:910–914. doi: 10.1152/ajplegacy.1967.213.4.910. [DOI] [PubMed] [Google Scholar]
- 3.Goshorn S C, Bohach G A, Schlievert P M. Cloning and characterization of the gene, speC, for pyrogenic exotoxin type C from Streptococcus pyogenes. Mol Gen Genet. 1988;212:66–70. doi: 10.1007/BF00322445. [DOI] [PubMed] [Google Scholar]
- 4.Hackett S P, Stevens D L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992;165:879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- 5.Hauser A R, Schlievert P M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990;172:4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser A R, Stevens D L, Kaplan E L, Schlievert P M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991;29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirabayashi A, Nishiwaki K, Taki K, Shimada Y, Ishikawa N. Effects of neuropeptide Y on lung vascular permeability in the pulmonary circulation of rats. Eur J Pharmacol. 1994;256:227–230. doi: 10.1016/0014-2999(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki M, Igarashi H, Hinuma Y, Yutsudo T. Cloning, characterization and overexpression of a Streptococcus pyogenes gene encoding a new type of mitogenic factor. FEBS Lett. 1993;331:187–192. doi: 10.1016/0014-5793(93)80323-m. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki M, Igarashi H, Yutsudo T. Mitogenic factor secreted by Streptococcus pyogenes is a heat-stable nuclease requiring His122 for activity. Microbiology. 1997;143:2449–2455. doi: 10.1099/00221287-143-7-2449. [DOI] [PubMed] [Google Scholar]
- 10.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee P K, Deringer J R, Kreiswirth B N, Novick R P, Schlievert P M. Fluid replacement protection of rabbits challenged subcutaneously with toxic shock syndrome toxins. Infect Immun. 1991;59:879–884. doi: 10.1128/iai.59.3.879-884.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto M, Murai T, Ichiyama S, Saito M, Arakawa Y, Ohta M. Prevalence of the speA2 and speA3 alleles in Streptococcus pyogenes isolated from TSLS patients in Japan. FEMS Microbiol Lett. 1997;150:233–237. doi: 10.1016/s0378-1097(97)00120-1. [DOI] [PubMed] [Google Scholar]
- 14.Murray D L, Ohlendorf D H, Schlievert P M. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News. 1995;61:229–235. [Google Scholar]
- 15.Nakata H, Maemoto T, Kitazawa K, Honda A, Shimizu Y, Ooe K. Fulminant group A streptococcal infection accompanied by massive pulmonary hemorrhage and subsequent asphyxia: a case report. Kansenshogaku Zasshi. 1994;68:1428–1432. doi: 10.11150/kansenshogakuzasshi1970.68.1428. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 16.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reda K B, Kapur V, Mollick J A, Lamphear J G, Musser J M, Rich R R. Molecular characterization and phylogenetic distribution of the streptococcal superantigen gene (ssa) from Streptococcus pyogenes. Infect Immun. 1994;62:1867–1874. doi: 10.1128/iai.62.5.1867-1874.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichardt W, Muller-Alouf H, Alouf J E, Kohler W. Erythrogenic toxins A, B and C: occurrence of the genes and exotoxin formation from clinical Streptococcus pyogenes strains associated with streptococcal toxic shock-like syndrome. FEMS Microbiol Lett. 1992;100:313–322. doi: 10.1111/j.1574-6968.1992.tb14058.x. [DOI] [PubMed] [Google Scholar]
- 19.Rotta J, Krause R M, Lancefield R C, Everly W, Lackland H. New approaches for the laboratory recognition of M types of group A streptococci. J Exp Med. 1971;134:1298–1315. doi: 10.1084/jem.134.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlievert P M. Role of superantigens in human disease. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 21.Seeger W, Walmrath D, Neuhof H, Lutz F. Pulmonary microvascular injury induced by Pseudomonas aeruginosa cytotoxin in isolated rabbit lungs. Infect Immun. 1986;52:846–852. doi: 10.1128/iai.52.3.846-852.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeger W, Walmrath D, Menger M, Neuhof H. Increased lung vascular permeability after arachidonic acid and hydrostatic challenge. J Appl Physiol. 1986;61:1781–1789. doi: 10.1152/jappl.1986.61.5.1781. [DOI] [PubMed] [Google Scholar]
- 23.Seeger W, Suttorp N. Role of membrane lipids in the pulmonary vascular abnormalities caused by bacterial toxins. Am Rev Respir Dis. 1987;136:462–466. doi: 10.1164/ajrccm/136.2.462. [DOI] [PubMed] [Google Scholar]
- 24.Seeger W, Birkemeyer R G, Ermert L, Suttorp N, Bhakdi S, Duncker H-R. Staphylococcal α-toxin-induced vascular leakage in isolated perfused rabbit lungs. Lab Investig. 1990;63:341–349. [PubMed] [Google Scholar]
- 25.Shanley T P, Schrier D, Kapur V, Kehoe M, Musser J M, Ward P A. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect Immun. 1996;64:870–877. doi: 10.1128/iai.64.3.870-877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 27.Stevens D L, Bryant A E, Hackett S P, Chang A, Peer G, Kosanke S, Emerson T, Hinshaw L. Group A streptococcal bacteremia: the role of tumor necrosis factor in shock and organ failure. J Infect Dis. 1996;173:619–626. doi: 10.1093/infdis/173.3.619. [DOI] [PubMed] [Google Scholar]
- 28.Stevens D L. New aspects of group A streptococcal infection. Kansenshogaku Zasshi. 1997;71:52–53. . (Abstract.) [Google Scholar]
- 29.Talkington D F, Schwartz B, Black C M, Todd J K, Elliott J, Breiman R F, Facklam R R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyosaki T, Yoshioka T, Tsuruta Y, Yutsudo T, Iwasaki M, Suzuki R. Definition of the mitogenic factor (MF) as a novel streptococcal superantigen that is different from streptococcal pyrogenic exotoxins A, B, and C. Eur J Immunol. 1996;26:2693–2701. doi: 10.1002/eji.1830261122. [DOI] [PubMed] [Google Scholar]
- 31.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yutsudo T, Murai H, Gonzalez J, Takao T, Shimonishi Y, Takeda Y, Igarashi H, Hinuma Y. A new type mitogenic factor produced by Streptococcus pyogenes. FEBS Lett. 1992;308:30–34. doi: 10.1016/0014-5793(92)81043-l. [DOI] [PubMed] [Google Scholar]
- 33.Weeks C R, Ferretti J J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986;52:144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome. JAMA. 1993;269:390–391. [PubMed] [Google Scholar]