Abstract
Background and aim
Animal modelling of arthritis is often associated with pain and suffering. Severity may be reduced with the use of analgesia which is, however, often withheld due to concerns of introducing a confounding variable. It is therefore important to design and validate pain relief protocols that reduce pain without compromising the scientific objectives. The present study evaluated the effect of buprenorphine analgesia in the immediate post-induction period of an adjuvant-induced monoarthritic rat model. The aim of this study was to extend previous work on refinement of the model by alleviating unnecessary pain.
Methods
Male and female Sprague Dawley rats were injected with 20 μl of complete Freund's adjuvant (CFA) into the left ankle. Rats were treated with buprenorphine, either injected subcutaneously or ingested voluntarily, and were compared to rats given subcutaneous injections with vehicle (saline or pure nut paste) or carprofen the first three days post CFA-injection. Measurements of welfare, clinical model-specific parameters and pain-related behaviour were assessed.
Results
Buprenorphine, administered either subcutaneously (0.10 or 0.15 mg/kg, twice daily) or by voluntary ingestion in nut paste (1.0 or 3.0 mg/kg, twice daily), improved mobility, stance, rearing and lameness scores significantly 7 h post CFA-injection. Mechanical hyperalgesia peaked at 7 h and was significantly lower in buprenorphine-treated animals, compared to vehicle-treated animals. Joint circumference was highest 24–72 h after CFA injection. Animals treated with buprenorphine did not decrease in joint circumference, opposite carprofen treated animals.
Conclusion
Buprenorphine, administered either subcutaneously or by voluntary ingestion, provides adequate analgesia for both sexes within the first 24 h post CFA-injection. Buprenorphine treatment improved clinical scores and appeared not to suppress the inflammatory response. The present study supports previous findings that voluntarily ingested buprenorphine is an effective alternative to repeated injections.
Keywords: Refinement, Analgesia, Animal pain model, Adjuvant-induced monoarthritis rat model
Refinement; Analgesia; Animal pain model, Adjuvant-induced monoarthritis rat model.
1. Introduction
Maintaining high standards of research animal well-being is an ethical, legal and scientific mandate [1]. The use of analgesia in pain and inflammatory models is a neglected area since analgesics have the potential to affect model development both at the peripheral site of injury, but also mechanistically at the spinal and supra-spinal level [2, 3, 4, 5]. This concern is often used as a justification for withholding analgesia [6]. However, it is evident that unrelieved pain, and resulting distress, in animals has negative effects on many body functions and influences the outcome of experiments [7, 8, 9]. Research into pain relief protocols that are designed to eliminate unnecessary pain, with minimal impact on experimental outcomes, is therefore warranted.
The complete Freund's adjuvant (CFA)-induced monoarthritic (MA) rat model was introduced in 1992 [10] to reduce animal suffering and the systemic condition associated with the original polyarthritis model [11, 12, 13, 14]. Upon the induction of this MA model a stable arthritic condition develops, suitable to study chronic inflammatory pain in rodents [15, 16, 17]. It is evident that the induction of MA is painful to the animals, and in our recent work we demonstrated that animals induced with CFA MA exhibit most indicators of pain and reduced well-being in the acute inflammation [18] (and preliminary data).
The highly potent partial μ-opioid receptor agonist buprenorphine is a commonly used analgesic for reducing mild to moderate pain in laboratory rodents [19, 20, 21]. Traditionally, buprenorphine is administered subcutaneously, but several other effective routes of delivery have been established [22, 23, 24]. Voluntary oral (p.o. vol.) administration is an example of a useful analgesic strategy that involves reduced stress and longer lasting serum concentrations compared to traditional subcutaneous injections [23, 25, 26, 27]. Reported doses and duration of analgesic activity of buprenorphine vary extensively. Injections from 0.01 to 0.50 mg/kg, administered s.c. or i.v., and oral gavage of up to 10.0 mg/kg have been used. Dosing intervals similarly vary between 5 and 12 h [26,[28], [29], [30], [31], [32]].
Buprenorphine has been suggested to affect the inflammatory response less than other opioids, e.g. by showing minimal or no effects on corticosterone secretion, no effect on natural killer cell activity or lymphoproliferation, and no effect on proinflammatory cytokine concentrations [33, 34, 35, 36, 37]. In models of neuropathic pain in rats [38], and cerebral ischemia in rats and mice [27, 39], buprenorphine showed no significant impairment of model development, all the while improving animal well-being. In a CFA-induced polyarthritis rat model, Walker and colleagues [40] showed that buprenorphine had no significant effect on disease progression assessed by joint swelling. Recently, we demonstrated that buprenorphine was effective in reducing facial pain expression scores (rat grimace scale), and improved mobility, stance and lameness scores in the CFA MA model during the first two weeks post induction. Furthermore, it did not interfere with the inflammatory CFA-induced ankle swelling and it appeared not to interfere with the intended MA pathology (preliminary data).
In the present study, we extended on the previous study investigating the effect of buprenorphine on acute pain and inflammation in the CFA-induced MA model in rats. The current study focused on the immediate post-induction period where well-being appears to be most compromised. It was hypothesized that buprenorphine treatment would decrease pain-related behaviour and improve clinical scores in the immediate post-induction period without reducing joint swelling.
2. Materials and methods
The following study was approved by the Danish competent authority - the Animal Experiment Inspectorate under the Danish Ministry of Environment and Food of Denmark (license number 2014-15-0201-00257). Experiments were carried in an AAALAC accredited animal facility in accordance with the Guide for the Care and Use of Laboratory Animals [41] and with the Directive 2010/63/EU [42].
2.1. Animals and housing
A total of 132 RjHan:SD male and female Sprague Dawley rats from Janvier Labs (Le Genest-Saint-Isle, France) were used. They were all aged seven weeks, weighing 382–422 g (males) and 232–275 g (females) in Experiment 1, and 394–473 g (males) and 223–262 g (females) in Experiment 2, at the start of testing. Rats were pair-housed with a partner receiving the same treatment in type IV S individually ventilated cages (IVC) (size 480 × 375 × 210 mm) from Tecniplast (Varese, Italy). Each cage was provided with aspen chip bedding (Tapvei, Harjumaa, Estonia) and contained paper nesting materials (Enviro-Dri, Milford, USA), aspen wooden sticks (Tapvei), polycarbonate rat retreats (Molytex, Glostrup, Denmark) and cardboard tubes (Lillico Biotechnology, Surrey, UK). Ambient temperature was monitored and maintained at 22 °C (±2 °C), and relative humidity at 55% (±10%). The light:dark cycle was 12:12 h, with lights on between 6:00 and 18:00, with 15–30 min of twilight before and after each light cycle. Feed (Altromin 1314; Altromin GmbH & Co., Lage, Germany) and tap water was available ad libitum. Animals were acclimatized to their surroundings for 10 days after arrival from the vendor, before start of experiments.
2.2. Experimental design
The study was divided into two experiments:
-
I.
CFA-injected male and female rats received buprenorphine administered subcutaneously (s.c.) or in hazel nut paste by voluntary ingestion (p.o. vol.). These were compared to vehicle and carprofen (s.c.)-treated rats the first three days post CFA-injection.
-
II.
Since the expected analgesic effect was not observed at the time points investigated in Experiment I, but where there was suspicion that there could be an effect at an earlier stage, a second experiment was performed: CFA-injected male and female rats received buprenorphine administered as subcutaneous injections (s.c.) or in hazel nut paste by voluntary ingestion (p.o. vol.). These were compared to vehicle-treated rats for shorter intervals the first 24 h post CFA-injection.
In each experiment, animals were randomly allocated into experimental groups (Table 1) and housed in pairs receiving the same treatment. Baseline testing was performed on all animals one day prior to CFA-injection. The CFA-injection and all the following behavioural and analgesiometric assessments were performed blinded and by the same female experimenter throughout each experiment. At the end of the experiments, all animals were euthanized by blunt trauma to the head followed by cervical dislocation.
Table 1.
Overview of experimental groups in Experiment 1 and 2.
| Experiment 1 | |||
|---|---|---|---|
| Group | N | CFA | Treatment |
| Control | 6 males and 6 females | − | Isotonic saline 0.9% s.c., twice daily |
| Vehicle | 6 males and 6 females | + | Isotonic saline 0.9% s.c., twice daily |
| CAR s.c. | 6 males and 6 females | + | Carprofen 5.0 mg/kg s.c., once daily and isotonic saline 0.9% s.c., as second injection |
| BUP s.c. | 6 males and 6 females | + | Buprenorphine 0.1 mg/kg, s.c., twice daily |
| BUP p.o. vol. | 6 males and 6 females | + | Buprenorphine 0.8 mg/kg p.o. vol., twice daily |
| Experiment 2 | |||
|---|---|---|---|
| Group | N | CFA | Treatment |
| Vehicle | 6 males and 6 females | + | Isotonic saline 0.9% s.c., twice daily |
| 6 males and 6 females | + | Nut paste, twice daily | |
| BUP s.c. | 6 males and 6 females | + | Buprenorphine 0.10 mg/kg s.c., twice daily |
| 6 males and 6 females | + | Buprenorphine 0.15 mg/kg s.c., twice daily | |
| BUP p.o. vol. | 6 males and 6 females | + | Buprenorphine 1.0 mg/kg p.o. vol., twice daily |
| 6 males and 6 females | + | Buprenorphine 3.0 mg/kg p.o. vol., twice daily | |
Group sizes were determined based on previous experience from similar studies [18](and unpublished work), and by a group size estimate using the resource equation method, as described by Mead (1988) and by Festing (2002 and 2003) [43, 44, 45], resulting in a number of 6–8 experimental units per group.
2.3. Drugs and administration
Analgesic-treatment was initiated pre-emptively, 60 min before the CFA injection. Buprenorphine was administered either subcutaneously (0.10 or 0.15 mg/kg, twice daily) (Temgesic® 0.3 mg/ml; RB Pharmaceuticals Inc., Richmond, UK) or as voluntary ingestion in nut paste (0.8–3.0 mg/kg) (Temgesic® 0.4 mg sublingual tablets; Schering-Plough, Kenilworth, Canada) at 8:00 and 16:00, 60 min prior to behavioural testing. Carprofen (Rimadyl vet. 50 mg/mL; Pfizer Inc., Secaucus, USA) was administered subcutaneously (5 mg/kg) at 8:00, 60 min prior to behavioural testing. Doses used for s.c. drug treatment were based on published recommendations [21, 46, 47, 48, 49]. The preparation of buprenorphine for voluntary ingestion was carried out according to the recommendations made by Abelson and colleagues [50]. Nutella® (chocolate-hazelnut paste) of 10 g/kg body weight (BW) (Ferrero, Pino Torinese, Italy) was mixed with 0.8 mg/kg BW, 1.0 mg/kg BW or 3.0 mg/kg BW buprenorphine. Buprenorphine tablets were crushed to powder before being mixed with Nutella, assuring even concentration of the mixture. Rats were habituated to voluntary ingestion of 0.4 g of the pure nut paste, twice a day, for three days prior to start of the experiment. The nut paste was given on a piece of adhesive tape attached to the cage wall. Once habituated, each rat was allowed to ingest its own portion in a separate cage, temporarily separating the two cage-mates. The cage-mates were returned to their home cage within 10 min. Rats that did not receive any analgesia, received either 1 ml/kg of 0.9% isotonic saline administered subcutaneously or 10 g/kg nut paste at 8:00 and 16:00.
2.4. Induction of monoarthritis
Inflammation was induced by injecting 20 μL complete Freund's adjuvant (CFA) (Sigma-Aldrich, St. Louis, USA), containing 1 mg/ml heat-killed and dried Mycobacterium tuberculosis (strain H37Ra, ATCC 25177), into the left ankle (tibio-tarsal joint) during brief isoflurane anaesthesia (Attane Vet, Isoflurane 1000 mg/g, ScanVet) (3.5% isoflurane delivered in pure oxygen at a flow rate of 0.5 l/min). The injection volume used and injection procedure was performed as previously described [18].
2.5. Animal welfare assessment
Animal welfare was evaluated in their home cages at baseline and at 4, 8, 24 and 72 h post induction in Experiment 1, and at 3, 7 and 24 h in Experiment 2, using a scoring sheet presented in Table 2. The welfare assessment (WA) protocol has previously been used in monitoring pain and distress in laboratory rodents [51]. Scores were summarized and compared to a predetermined humane endpoint. If the sum of scores exceeded 0.4, the animal would be euthanized, although this humane endpoint was never reached during the studies.
Table 2.
Welfare assessment score sheet adapted from Hampshire et al. [52].
| General appearance | Reference score |
|---|---|
| Bright and alert | 0 |
| Burrowing or hiding, quiet but rousing when touched | 0.1 |
| Burrowing or hiding, quiet but rousing when touched. No exploration when lid off, burrows, hides, head presses. Might be aggressive when touched | 0.4 |
| Porphyrin staining | |
| None | 0 |
| Mild | 0.1 |
| Obvious on face or paws | 0.4 |
| Gait and posture | |
| Normal | 0 |
| Mild incoordination when stimulated, hunched posture, mild piloerection | 0.1 |
| Obvious ataxia or head tilt, hunching, severe piloerection | 0.4 |
| Body weight | |
| Up to 5% weight loss | 0 |
| 5–10% weight loss | 0.1 |
| 10–20% weight loss | 0.4 |
| Wounds | |
| Bites or scratches itself, leading to wounds | 0.4 |
As part of the welfare assessment, body weight of the animals was measured at baseline and at 24, 48 and 72 h post induction in Experiment 1, and at baseline and at 24 post induction in Experiment 2.
2.6. Model-specific parameters
Scoring of arthritis was performed according to Butler et al., 1999 (Table 3) [10], at baseline and at 4, 8, 24 and 72 h in Experiment 1, and 3, 7 and 24 h in Experiment 2, using a protocol similar to a previous study [18].
Table 3.
Model-specific parameters.
| Mobility | Reference score |
|---|---|
| The rat lies down only | 4 |
| The rat crawls using front legs only | 3 |
| The rat walks with difficulty | 2 |
| The rat walks and runs with difficulty | 1 |
| The rat walks and runs normally | 0 |
| Stance | |
| The rat stands on three paws only | 3 |
| The rat stands with the arthritic paw touching floor, toes curled under | 2 |
| The rat stands bearing some weight on the arthritic limb | 1 |
| The rat stands bearing weight equally on all four limbs | 0 |
| Rearing | |
| The rat is only bearing weight on the non-arthritic hind limb | 2 |
| The rat is bearing some weight on the arthritic limb | 1 |
| The rat is equally bearing weight on both hind limbs | 0 |
| Lameness | |
| Severe, limb carried | 3 |
| Moderate, toe touching ground | 2 |
| Mild, slight lameness | 1 |
| Normal ambulation | 0 |
2.7. Ankle circumference
The circumference of the joints was measured in order to determine the size of the inflammatory response upon CFA-injection at baseline and at 4, 8, 24 and 72 h post induction in Experiment 1, and at 3, 7 and 24 h in Experiment 2. The circumference (C) was determined by measuring two perpendicular diameters of the joint using digital callipers and estimated using the formula: C = 2 × π × , where a is the radius of dorso-plantar axis and b is the radius of the medio-lateral axis [53].
2.8. Assessment of mechanical sensitivity
An electronic von Frey (EVF) device (Model EVF3 with a hard tip from Bioseb, USA/France) was used to assess mechanical hypersensitivity at baseline and at 24 and 72 h in Experiment 1, and 3, 7 and 24 h in Experiment 2. Rats were placed in individual chambers (size 16.5 × 24.2 × 14.6 cm) on an elevated metal grid platform. Four rats were tested at a time after a 15-minute habituation period. The EVF tip was applied to the plantar surface of the paw, at an anatomical location nearest the tibio-tarsal joint, with a linearly increasing uniform force. The applied weight in gram (g) required to elicit paw withdrawal was recorded, corresponding to the mechanical threshold. The test was repeated three times for each hind paw, shifting between each paw, starting with the non-injured right paw. The EVF tip was only applied when the rat stood still in a natural position with all four paws placed on the floor and a paw withdrawal response was only considered with a complete lifting of the stimulated hind paw. Lifting of the paw due to normal locomotor activity was ignored.
2.9. Rat grimace scale scoring
A quantitative analysis of facial expressions in rats (the Rat Grimace Scale (RGS)) was applied to assess spontaneous non-evoked pain. RGS scores were assigned at baseline and at 24 and 72 h in Experiment 1. No RGS scoring was applied in Experiment 2. Animals were habituated to the observer and method for six days prior to scoring. The blinded coding of facial expressions was done when the rats were placed in the EVF chambers, prior to EVF testing. The animals were allowed to habituate for approximately 15 min prior to scoring. Three selected pictures of each rat facing the camera were considered, the remaining were discarded. Pictures were saved and later scored. One out of the three pictures of the same animal was randomly picked out and represented that individual animal in the scoring process. The scoring was done according to the original method described by Sotocinal et al. [54]. Four action units (orbital tightening, ear changes, nose/cheek flattening and whisker changes) were scored “0” if the specified feature was not present, “1” if moderately present, “2” if obvious present. The average score of all four action units was calculated giving an average score between 0-2 for each animal.
2.10. Faecal corticosterone metabolite assessment
For measuring levels of faecal corticosterone and corticosterone metabolites (FCM), faecal samples from 24 h of defecation were collected 24 and 72 h post induction in Experiment 1. The sample material (soiled bedding from a cage housing two rats of the same treatment-group) was kept at -20 °C until faecal pellets could be separated out from the bedding material. After each sorting, the pellets were placed in plastic resealable bags and stored again at -20 °C. FCM were analysed according to a previously described method [55, 56], with some modifications regarding evaporation, as reported in previous work (preliminary data). During the process, faecal pellets from each sample were separately thawed, weighed and mixed with 96% ethanol (5 ml/g faeces). Each sample was solubilised on a shaking table in 50 ml tubes and incubated at room temperature overnight. The suspension was centrifuged at 2000 x g (Hermle Labortechnik GmbH, Wehingen, Germany) for 20 min, whereafter the supernatant was collected and the pellet discarded. The supernatant was further centrifuged at 10000× g for 15 min (Eppendorf 5415D; Eppendorf AG, Hamburg, Germany). The extracted sample was then evaporated to dryness under vacuum (Genevac EZ-2 personal evaporator) and resuspended in 0.15 M phosphate buffered saline (PBS, pH 7.2). The sample was again centrifuged at 10000× g and the clear liquid was analysed by ELISA (DRG-Diagnostics corticosterone competitive enzyme-linked immunosorbent assay, Marbug, Germany), according to the manufacturer's instructions.
2.11. Data analysis
In Experiment 1, we evaluated the effects of buprenorphine treatment during the first three days after CFA-injection. Findings in Experiment 1 led to the second experiment, where two different doses and two different administration routes of buprenorphine were compared within 24 h. Data in Experiment 2 revealed clear similarities in some of the groups. Consequently, certain groups were combined for the data-analysis. Subcutaneous administration groups were combined (BUP s.c.) and voluntary oral administration groups were combined (BUP p.o. vol.). The vehicle p. o. group and the vehicle s.c. group were combined to one vehicle group (Vehicle).
Data were computed using GraphPad Prism 8.0 and SPSS (IBM SPSS statistics 27). A two-way repeated measures (RM) ANOVA was performed on combined data from male and female CFA-injected rats on body weight, EVF, circumference and FCM data, evaluating the effects of the factors sex and treatment, as well as their interaction. A subsequent two-way repeated-measures (RM) ANOVA was performed on the same data in each sex, followed by a Tukey's multiple comparisons test. Model-specific scores and RGS scores were analysed using one-way non-parametric ANOVAs (Kruskal-Wallis tests) followed by a Dunn's multiple comparisons test for each time point. P values p < 0.05 were considered significant for all statistical analyses.
3. Results
3.1. Welfare and clinical scores
All CFA-injected rats developed clinical signs of acute inflammation (erythema, edema and tenderness) localized to the injected paw, starting a few hours after the injection. However, none of the CFA-injected rats presented a severe decrease detectable by our general welfare scores during the two experiments (Table 2). Total welfare scores never exceeded 0.1, similarly to previous studies [18] (preliminary data). In addition, no significant differences in body weight (BW) were detected between treatment groups at any time in either experiment (Supplementary material, Figure S1).
Model-specific parameters were affected in all CFA-injected rats during Experiment 1 and 2. In experiment 1 (all data are shown in Figure S2), a few differences in mobility and stance scores were detected between CFA-injected groups. A Kruskal-Wallis test detected differences in mobility (males; H (4) = 16.95, p = 0.0007 and females; H (4) = 10.70, p = 0.0135), where both buprenorphine groups were significantly improved in scores compared to the vehicle group (p = 0.0053) at 72 h in males and the subcutaneously-treated buprenorphine group were significantly improved in scores compared to the group with carprofen treatment (p = 0.0283) at 48 h in females. Significant differences were detected in stance (H (4) = 16.95, p = 0.0135), where treatment with voluntary ingested buprenorphine significantly improved scores compared to the vehicle group (p = 0.0313) and the carprofen group (p = 0.0313) at 72 h. In experiment 2, affected model-specific parameters were obvious different between groups, especially at 7 h and 24 h (Figure 1A and B; selected data from Experiment 2 - all data from Experiment 2 are shown in Figure S3). At 7 h (Figure 1A), significant differences between groups were clear in both sexes. A Kruskal-Wallis test detected differences in mobility (males; H (3) = 7.964, p = 0.0186), where treatment with subcutaneous buprenorphine significantly improved scores compared to the vehicle group in males (p = 0.0226), while both buprenorphine groups were significantly improved in scores compared to the vehicle group in females (BUP p. o. p = 0.0137 and BUP s.c. p = 0.0002) (Dunn's multiple comparisons test). Significant differences were detected between groups in stance (males; H (3) = 19.20, p < 0.0001 and females; H (3) = 17.22, p = 0.0002), rearing (males; H (3) = 13.36, p = 0.0013 and females; H (3) = 20.10, p < 0.0001) and lameness scores (males; H (3) = 7.964, p = 0.0186 and females; H (3) = 17.28, p = 0.0002), where both buprenorphine groups clearly differed from the vehicle group, having significantly improved scores in both sexes. At 24 h (Figure 1B), a few significant differences were detected, however only in females. The Kruskal-Wallis test detected similar differences between groups in mobility (H (3) = 8.409, p = 0.0149) and stance scores (H (3) = 7.541, p = 0.0230), where treatment with voluntary ingestion of buprenorphine significantly improved these scores compared to the vehicle group (mobility: p = 0.0116 and stance: p = 0.0185). Significant differences in rearing (H (3) = 17.24, p = 0.0002) and lameness (H (3) = 11.94, p = 0.0026) were detected, where both buprenorphine groups significantly improved the scores compared with the control group (rearing: BUP s.c. p = 0.0007, BUP p. o. vol. p = 0.0014 and lameness: BUP s.c. p = 0.0069, BUP p. o. vol. p = 0.0100). No significant differences were detected between buprenorphine groups at any time.
Figure 1.
Model-specific parameters (Experiment 2). Mobility, stance, rearing and lameness scores at (A) 7 h and (B) 24 h post induction. Data are presented as mean ± SEM for combined vehicle groups (saline s.c. and nut paste p.o. vol.), subcutaneous buprenorphine groups (BUP 0.10 and 0.15 mg/kg s.c.) and voluntary oral buprenorphine groups (BUP 1.0 and 3.0 mg/kg p. o. vol.) in each sex to each parameter. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 vs. the CFA-injected vehicle-treated group, determined by a Kruskal-Wallis test, followed by Dunn's multiple comparisons test. All groups N = 12.
3.2. Ipsilateral circumference
Ipsilateral ankle circumferences from Experiment 1 are presented in Figure 2 A and B. In experiment 1, an overall two-way RM ANOVA revealed a significant effect of sex (F (1, 40) = 18.282, p < 0.0001) and treatment (F (3, 40) = 5.220), p = 0.004). No sex∗treatment interaction was detected. Joint circumference increased gradually within a few hours after CFA injection until 24 h post CFA-injection in both sexes. No significant differences were detected between groups the first 24 h after CFA-injection in neither Experiment 1 nor 2 (the shorter experiment 2 is therefore presented in Figure S4). However, the groups differed in both sexes from 24 h (males: F (3, 20) = 6.831, p = 0.0024; females; F (3, 20) = 3.196, p = 0.0456, using a two-way RM ANOVAs) (Experiment 1, Figure 2A and B). Treatment with subcutaneous buprenorphine showed a significantly increased circumference compared to the vehicle group at 72 h (p = 0.0475) in males (Tukey's multiple comparisons test). By contrast, the carprofen group presented a gradually decreasing circumference after 24 h. In males, the circumference was significantly lower in carprofen-treated subjects than in the buprenorphine groups at 48 h (s.c.; p = 0.0225, p. o. vol.; p = 0.0406) and 72 h (s.c.; p = 0.0008, p. o. vol.; p = 0.0160). In females, the circumference in the carprofen group was lower than in the group treated with subcutaneous buprenorphine at 48 h (p = 0.0264), in the group treated with voluntary ingested buprenorphine at 72 h (p = 0.0002), and in the vehicle group at 72 h (p = 0.0353) (Tukey's multiple comparisons test).
Figure 2.
Circumference measurements of the ipsilateral (left, CFA-injected) ankle in millimetre (mm) (Experiment 1). (A) Males and (B) females. Data were analysed by a two-way RM ANOVA followed by a Tukey's multiple comparisons test and presented as mean ± SEM. The asterisk (∗) represents a statistically significant difference from the vehicle group at ∗p < 0.05. The plus (+) represents a statistically significant difference between BUP p. o. vol. and CAR at +p < 0.05 and +++p < 0.001. The hash sign (#) represents statistically significant difference between BUP s.c. and CAR at #p < 0.05 and ###p < 0.001. All groups N = 6.
3.3. Mechanical sensitivity
In Experiment 1 (Figure 3A and B), an overall two-way RM ANOVA revealed a significant effect of sex (F (1, 40) = 4.374, p = 0.043) and treatment (F (3, 40) = 3.015, p = 0.041). However, RM ANOVAs within each sex revealed no significant differences between CFA-injected groups, meaning that the effect of treatment detected must be sex-related differences in e.g. baseline measurements instead of opposite effects of treatment.
Figure 3.
Ipsilateral (left, CFA-injected) paw withdrawal thresholds (g) as an indication of mechanical hyperalgesia. Ipsilateral paw withdrawal thresholds (A) males and (B) females in Experiment 1 and (C) males and (D) females in Experiment 2. Data were analysed by a two-way RM ANOVA followed by a Tukey's multiple comparisons test at baseline, 24 h and 72 h in Experiment 1 and at baseline, 3 h, 7 h, 24 h in Experiment 2. All groups in Experiment 1, N = 6. Groups in Experiment 2 were combined vehicle groups (saline s.c. and nut paste p. o.), BUP s.c. groups (BUP 0.10 and 0.15 mg/kg s.c.) and BUP p. o. vol. groups (BUP 1.0 and 3.0 mg/kg p. o. vol.) with N = 12 of each combined group. All data are presented as mean ± SEM. The asterisks (∗) represents statistically significant differences from the vehicle group at ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
During the first 24 h studied in Experiment 2 (Figure 3C and D), an overall two-way RM ANOVA revealed a significant effect of sex (F (1, 65) = 23.930, p < 0.0001) and treatment (F (2, 65) = 50.213, p < 0.0001), but no interactions. Decreased mechanical thresholds were observed from 3 h after CFA-injection in the vehicle group with significantly lower thresholds compared to baseline values (males: p = 0.0089 and females: p = 0.0472), (Tukey's multiple comparisons test). The lowest mechanical thresholds (i.e. the highest level of inflammatory hyperalgesia) were detected in the vehicle group at 7 h in both sexes (p < 0.0001) (Tukey's multiple comparisons test). Buprenorphine treatment resulted in significantly higher mechanical thresholds (analgesic effect) compared to the vehicle groups for both sexes (p < 0.0001), independent of route of administration. The effect of buprenorphine remained significantly different from the vehicle group to some extent at 24 h as well (males: BUP s.c.: p = 0.0137; females: BUP p. o. vol.: p = 0.0133, BUP s.c.: 0.0235). No differences were detected between buprenorphine groups at any time. A two-way RM ANOVA detected no difference between different doses of buprenorphine on EVF or any other parameter, and therefore these groups were joined. But for clarity, the full EVF dataset is presented in the supplementary (Figure S5).
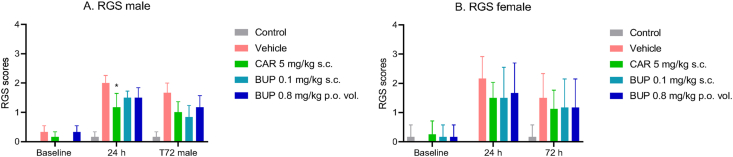
3.4. Rat grimace scale scores
Rat Grimace scale (RGS) scores (Figure 4A and B) increased following CFA-injection with overall highest scores at 24 h presented by the vehicle group in both sexes. A Kruskal-Wallis test detected significant differences between CFA-injected groups at 24 h (p = 0.0125) in males only, where the carprofen group showed a significant reduction in scores compared to the vehicle group (p = 0.0127), while no differences were detected between treatment groups at 7 h (Dunn's multiple comparisons test).
Figure 4.
Facial expressions as a measure of non-evoked pain (Experiment 1). Rat grimace scale (RGS) scoring was performed at pre-induction baseline and at 24 and 72 h post induction in (A) males and (B) females. The Kruskal-Wallis test was used for hypothesis tests, followed by Dunn's multiple comparisons test. Scores are displayed as mean ± SEM. The asterisk (∗) represents statistically significant difference from the vehicle group at ∗p < 0.05. All groups N = 6.
3.5. Faecal corticosterone metabolites
Average concentrations of faecal corticosterone metabolites (FCM) excreted in 24 h per cage were compared between treatment groups (Figure S6). An overall two-way RM ANOVA detected no significant effects of sex or treatment.
4. Discussion
The aim of this study was to evaluate the effects of buprenorphine on model-specific parameters, inflammation and pain-related behavioral readouts during the immediate post-induction period in the complete Freund's adjuvant-induced monoarthritic rat model.
Although the welfare or body weight of the animals was not greatly affected by the CFA injection in general, all CFA-injected animals were still affected clinically, with impaired mobility, stance, rearing abilities and increased lameness. Buprenorphine treatment, administrated voluntary in nut paste or subcutaneously, resulted in a reduced degree of impairment from the injury, as expected.
Ankle circumferences increased in both sexes until 24 h post CFA injection. From 24 h the groups started to differ from each other, with the carprofen group having the lowest circumferences. The results suggest that carprofen modulates the inflammatory response and decreases disease severity in this model, which was expected given the anti-inflammatory components of the drug [57]. Buprenorphine treatment (sc. and p.o. vol.), on the other hand, resulted in a significant increase in circumference compared to the vehicle group in males, and similar size of circumference as the vehicle group in females, indicating that buprenorphine, in contrast to carprofen, did not suppress the inflammatory swelling. Whether buprenorphine exacerbated the inflammatory process in males is not known and should be further investigated. This sex-dependant issue has not been evident in our previous study with buprenorphine treatment of 0.05 mg/kg in the same model (unpublished work). However, this is not unlikely given a previous report that morphine produces increased paw swelling in males exposed to an adjuvant-induced arthritis model [3].
The rat grimace scale was applied to detect and quantify spontaneous pain behaviors in this model. The RGS scores suggested moderate pain intensity, with treatment groups expressing fewer pain-related facial expressions than the vehicle group. However, only the carprofen group showed significantly decreased scores compared to the vehicle group. In our previous study (unpublished work), we demonstrated positive treatment effects of buprenorphine in this model, but this was done by calculating the AUC over a longer treatment period (Days 1–15 post-induction). The RGS method is seemingly reliable in assessing some pain-related behavior in this model. Whether it is sensitive enough to assess analgesic effectiveness in the time window tested in this study, however, is doubtful.
Significant decreases in mechanical thresholds in EVF testing were observed as early as 3 h post CFA-injection and were lowest (indicating highest pain levels) 7 h post CFA injection in both males and females. A clear analgesic effect of buprenorphine (regardless of the route of administration and dosage tested) was evident 3, 7 and 24 h post CFA injection with significantly higher thresholds compared to the vehicle group. This indicates that buprenorphine treatment provides effective pain relief during the first 24 h after CFA-injection. A decline in analgesic effect was observed at 24 h post CFA injection in Experiment 2, whereas no analgesic effects were detected in Experiment 1 (at 24 h and 72 h). The latter is consistent with previous findings (preliminary data). Significant pain-related responses in the EVF test have been demonstrated during 10 days [18] and even up to 16 days post CFA injection (preliminary data). We therefore emphasize the necessity of providing analgesia for longer than 24 h post CFA injection. Importantly, the hyperalgesia measured by EVF testing in this model is likely to be secondary hyperalgesia, since the tested location is remote to the primary injury [58]. It is generally agreed that the mechanisms mediating primary and secondary hyperalgesia are different. Whereas primary hyperalgesia is mainly attributed to peripheral nociceptor sensitization (at the injury site) and central sensitization, secondary hyperalgesia is largely due to changes in the processing of sensory input in the central sensitization (CNS) [58]. Although secondary hyperalgesia requires continuous nociceptive input from the primary injury [59], it may not be directly correlated to analgesic efficacy on pain from the ankle joint. Measures such as weight bearing [60, 61] or changes in gait [62] may provide more differentiated readouts about the impact of this injury model, rather than relying on stimulus-evoked mechanical responses alone.
Voluntary oral administration of buprenorphine has, besides the obvious advantage of being non-invasive, been found to lower plasma and fecal corticosterone levels and to improve water consumption and body weight maintenance compared to subcutaneous treatment [23, 25, 26]. However, the administration route has also been criticized as being less effective compared to subcutaneous injections in rats, because of first-pass metabolism reduced bioavailability [63, 64]. Some studies suggest using doses more than 100 times higher than those commonly used for subcutaneous injections to ensure an effect in analgesiometric tests [65]. In this study we used doses of 0.8–3.0 mg/kg buprenorphine in nut paste, where 1.0 and 3.0 mg/kg provided a similar analgesic effect as a subcutaneous treatment at doses of 0.15 and 0.10 mg/kg at least during the first 24 h. The similar analgesic effect suggests that an expected ceiling effect of buprenorphine (due to its pharmacological properties) was reached within the dosing range. Therefore the use of higher doses of buprenorphine does not seem to be necessary for alleviating pain in this model and might just result in decreased palatability of the oral formulation [65] or induce adverse effects such as reduced appetite, changes in activity, and pica behavior [20]. We did not observe any rats unwilling to eat the treatments mixed in nut paste, and none of the mentioned adverse effects were observed in any buprenorphine-treated animals in this study. However, when considering minimizing confounding factors, the lower dose of buprenorphine could preferentially be used given that there was no added effect on any parameters from the higher dose.
Administration of opioid analgesia has varying effects on immunosuppression, depending on drug and whether the model involves acute or chronic inflammation [66]. However, buprenorphine has been reported to lack immunosuppressive effects when administered in acute and chronic inflammatory reactions [67, 68], even at doses as high as 12.5 mg/kg/h delivered continuously with osmotic pumps in mice [68]. Higher levels of fecal corticosterone levels were detected post CFA injection in this study, however no significant differences were detected between groups at any time. This suggests that buprenorphine treatment, administered as 0.8 mg/kg p. o. vol. or 0.1 mg/kg s.c., does not noticeably influence corticosterone levels in this model. Whether 0.15 mg/kg s.c. or 1.0–3.0 mg/kg p. o. vol. influences corticosterone levels in the same time window remains unknown.
In conclusion, buprenorphine administered by subcutaneous injection or by voluntary ingestion in nut paste resulted in a sufficient analgesic effect in the CFA-induced monoarthritis rat model during the first 24 h post CFA injection. In addition, buprenorphine treatment improved mobility, stance, rearing and lameness scores and showed no suppressing effect on the CFA-induced inflammatory response. The data demonstrate that buprenorphine, administered for voluntary ingestion in nut paste, is an effective alternative to repeated injections of buprenorphine. Our findings highlight the importance of tailoring analgesia to periods where pain and inflammation peak to enhance animal wellbeing and to minimize potential impact on scientific objectives.
Declarations
Author contribution statement
M. S. Berke, P. Colding-Jørgensen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. Hestehave, J. Hau, D. Bratbo Sørensen, H. E. Jensen, O. Kalliokoski: Analyzed and interpreted the data.
K. S. P. Abelson: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Danish 3R Center (33010-NIFA-14-586).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Authors thank Helle Runchel Porsdal, Trine Marie Ahlman Glahder, Daniel Kylmann Hansen and Gerda Majgaard Jensen for their invaluable assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ana M.M.-s., Sandøe P., Olsson I.A.S. Painful dilemmas: the ethics of animal-based pain research. Animal Welfare. 2009;18:49–63. [Google Scholar]
- 2.Paska W., McDonald K.J., Croft M. Studies on type II collagen induced arthritis in mice. Agents Actions. 1986;18(3–4):413–420. doi: 10.1007/BF01965006. [DOI] [PubMed] [Google Scholar]
- 3.Earl J.R., et al. Proinflammatory effects of morphine in the rat adjuvant arthritis model. Int. J. Tissue React. 1994;16(4):163–170. [PubMed] [Google Scholar]
- 4.Dinda A., Gitman M., Singhal P.C. Immunomodulatory effect of morphine: therapeutic implications. Expert Opin. Drug Saf. 2005;4(4):669–675. doi: 10.1517/14740338.4.4.669. [DOI] [PubMed] [Google Scholar]
- 5.Pulichino A.M., et al. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J. Pharmacol. Exp. Ther. 2006;319(3):1043–1050. doi: 10.1124/jpet.106.110387. [DOI] [PubMed] [Google Scholar]
- 6.Richardson C.A., Flecknell P.A. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? ATLA. 2005;33:119–127. doi: 10.1177/026119290503300207. [DOI] [PubMed] [Google Scholar]
- 7.Baumans V., et al. Pain and distress in laboratory rodents and lagomorphs: report of the federation of European laboratory animal science associations (FELASA) working group on pain and distress accepted by the FELASA board of management November 1992. Lab. Animals. 1994;28(2):97–112. doi: 10.1258/002367794780745308. [DOI] [PubMed] [Google Scholar]
- 8.Flecknell P.A., Waterman-Pearson A. WB Saunders; 2000. Pain Management in Animals. [Google Scholar]
- 9.Ren K., Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler S.H., et al. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 11.Waksman B.H. Immune regulation in adjuvant disease and other arthritis models: relevance to pathogenesis of chronic arthritis. Scand. J. Immunol. 2002;56:12–34. doi: 10.1046/j.1365-3083.2002.01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Pearson C.M., Wood F.D. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheumatism. 1959;2(5):440–459. [Google Scholar]
- 13.Pearson C.M. Experimental joint disease observations ON adjuvant-induced arthritis. J. Chronic Dis. 1963;16:863–874. doi: 10.1016/0021-9681(63)90136-x. [DOI] [PubMed] [Google Scholar]
- 14.Waksman B.H., Pearson C.M., Sharp J.T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J. Immunol. 1960;85:403–417. [PubMed] [Google Scholar]
- 15.Knight B., et al. Induction of adjuvant arthritis in mice. Clin. Exp. Immunol. 1992;90(3):459–465. doi: 10.1111/j.1365-2249.1992.tb05868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo S.T., et al. The effects of pressure on arthritic knees in a rat model of CFA-induced arthritis. Pain Physician. 2013;16(2):E95–102. [PubMed] [Google Scholar]
- 17.Parvathy S.S., Masocha W. Gait analysis of C57BL/6 mice with complete Freund's adjuvant-induced arthritis using the CatWalk system. BMC Musculoskeletal Disorders. 2013;14 doi: 10.1186/1471-2474-14-14. 14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berke M.S., Abelson K.S.P. The adjuvant-induced rat model of monoarthritis: welfare implications and possible refinement strategies. Scand. J. Lab. Animal Sci. 2020;46(5):39–50. [Google Scholar]
- 19.Cowan A., Lewis J.W., Macfarlane I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br. J. Pharmacol. 1977;60(4):537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roughan J.V., Flecknell P.A. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim. 2002;36(3):322–343. doi: 10.1258/002367702320162423. [DOI] [PubMed] [Google Scholar]
- 21.Stokes E.L., Flecknell P.A., Richardson C.A. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Animals. 2009;43(2):149–154. doi: 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- 22.Kalliokoski O., et al. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet. J. 2011;187(2):251–254. doi: 10.1016/j.tvjl.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Goldkuhl R., et al. Plasma concentrations of corticosterone and buprenorphine in rats subjected to jugular vein catheterization. Lab Animals. 2010;44(4):337–343. doi: 10.1258/la.2010.009115. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen K., et al. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57bl/6 male mice. Comp. Med. 2013;63:105–113. [PMC free article] [PubMed] [Google Scholar]
- 25.Goldkuhl R., Hau J., Abelson K.S. Effects of voluntarily-ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behaviour in permanently catheterised rats. In Vivo. 2010;24(2):131–135. [PubMed] [Google Scholar]
- 26.Goldkuhl R., et al. Effect of subcutaneous injection and oral voluntary ingestion of buprenorphine on post-operative serum corticosterone levels in male rats. Eur. Surg. Res. 2008;41(3):272–278. doi: 10.1159/000142372. [DOI] [PubMed] [Google Scholar]
- 27.Kalliokoski O., et al. The effect of voluntarily ingested buprenorphine on rats subjected to surgically induced global cerebral ischaemia. In Vivo. 2010;24(5):641–646. [PubMed] [Google Scholar]
- 28.Gades N.M., et al. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp. Top. Lab. Animals Sci. 2000;39(2):8–13. [PubMed] [Google Scholar]
- 29.Abbott F.V., Bonder M. Options for management of acute pain in the rat. Vet. Record. 1997;140(21):553–557. doi: 10.1136/vr.140.21.553. [DOI] [PubMed] [Google Scholar]
- 30.Leach M.C., Forrester A.R., Flecknell P.A. Influence of preferred foodstuffs on the antinociceptive effects of orally administered buprenorphine in laboratory rats. Lab. Animals. 2010;44(1):54–58. doi: 10.1258/la.2009.009029. [DOI] [PubMed] [Google Scholar]
- 31.Martin L.B., et al. Analgesic efficacy of orally administered buprenorphine in rats. Comp. Med. 2002;51(1):43–48. [PubMed] [Google Scholar]
- 32.Hestehave S., et al. Antinociceptive effects of voluntarily ingested buprenorphine in the hot-plate test in laboratory rats. Lab. Animals. 2017;51(3):264–272. doi: 10.1177/0023677216668553. [DOI] [PubMed] [Google Scholar]
- 33.Franchi S., Panerai A.E., Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav. Immun. 2007;21(6):767–774. doi: 10.1016/j.bbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Martucci C., Panerai A.E., Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110(1-2):385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Odunayo A., et al. Immunomodulatory effects of opioids. J. Vet. Emerg. Crit. Care. 2010;20(4):376–385. doi: 10.1111/j.1476-4431.2010.00561.x. [DOI] [PubMed] [Google Scholar]
- 36.Sacerdote P., et al. Antinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity study. Br. J. Pharmacol. 1997;121(4):834–840. doi: 10.1038/sj.bjp.0701138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piersma F.E., et al. Interference of pain control employing opioids in in vivo immunological experiments. Lab. Animals. 1999;33(4):328–333. doi: 10.1258/002367799780487887. [DOI] [PubMed] [Google Scholar]
- 38.Hestehave S., et al. Is there a reasonable excuse for not providing post-operative analgesia when using animal models of peripheral neuropathic pain for research purposes? Plos One. 2017;12(11):e0188113. doi: 10.1371/journal.pone.0188113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobsen K.R., et al. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp. Med. 2013;63(2):105–113. [PMC free article] [PubMed] [Google Scholar]
- 40.Walker J., et al. Effect of μ-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm. Res. 1996;45(11):557–563. doi: 10.1007/BF02342227. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Laboratory Animal Research . eighth ed. The National Academies Press; Washington DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 42.Directive 2010/63 of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010;L246:33–77. [Google Scholar]
- 43.Festing M.F.W., Altman D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J./Nat. Res. Council, Inst. Lab. Animal Resour. 2002;43:244–258. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
- 44.Festing M.F.W., Weigler B.J. In: Handbook of Laboratory Animal Science. Hau J., van Hoosier G.L. Jr., editors. Vol. 1. CRC Press; Boca Raton: 2003. Experimental design and statistical analysis; pp. 327–350. (Essential Principles and Practices). [Google Scholar]
- 45.Mead R. Cambridge University Press; Cambridge, New York: 1988. The Design of Experiments. [Google Scholar]
- 46.Curtin L.I., et al. Evaluation of buprenorphine in a postoperative pain model in rats. Comp. Med. 2009;59(1):60–71. [PMC free article] [PubMed] [Google Scholar]
- 47.Flecknell P.A. The relief of pain in laboratory animals. Lab. Animals. 1984;18(2):147–160. doi: 10.1258/002367784780891226. [DOI] [PubMed] [Google Scholar]
- 48.Hedenqvist P., Hellebrekers L. In: Handbook of Laboratory Animal Science. Hau J S.S., editor. 2011. Laboratory animal analgesia, anesthesia, and euthanasia. [Google Scholar]
- 49.Stewart L.S.A., Martin W.J. Evaluation of postoperative analgesia in a rat model of incisional pain. J. Am. Assoc. Lab. Animal Sci. 2003;42(1):28–34. [PubMed] [Google Scholar]
- 50.Abelson K.S., et al. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab. Animals. 2012;46(4):349–351. doi: 10.1258/la.2012.012028. [DOI] [PubMed] [Google Scholar]
- 51.Bolon B., et al. Rodent preclinical models for developing Novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J. Biomed. Biotechnol. 2011 doi: 10.1155/2011/569068. Artn 56906810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hampshire V.A., et al. Retrospective comparison of rat recovery weights using inhalation and injectable anaesthetics, nutritional and fluid supplementation for right unilateral neurosurgical lesioning. Lab. Animals. 2001;35(3):223–229. doi: 10.1258/0023677011911660. [DOI] [PubMed] [Google Scholar]
- 53.Tag H.M., et al. Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. J. Basic Clin. Physiol. Pharmacol. 2016;27(1):71–78. doi: 10.1515/jbcpp-2015-0044. [DOI] [PubMed] [Google Scholar]
- 54.Sotocinal S.G., et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundbom R., et al. Post-operative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo. 2011;25(3):335–342. [PubMed] [Google Scholar]
- 56.Kalliokoski O., et al. Quantitative effects of diet on fecal corticosterone metabolites in two strains of laboratory mice. In Vivo. 2012;26(2):213–221. [PubMed] [Google Scholar]
- 57.Budsberg S. In: Handbook of Veterinary Pain Management. second ed. Gaynor J.S., Muir W.W., editors. Mosby; Saint Louis: 2009. 10 – Nonsteroidal antiinflammatory drugs; pp. 183–209. [Google Scholar]
- 58.Woolf C.J. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3) doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh M.T., Donaldson L.F., Lumb B.M. Differential contributions of A- and C-nociceptors to primary and secondary inflammatory hypersensitivity in the rat. Pain. 2015;156(6):1074–1083. doi: 10.1097/j.pain.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schött E., et al. Weight bearing as an objective measure of arthritic pain in the rat. J. Pharmacol. Toxicol. Methods. 1994;31(2):79–83. doi: 10.1016/1056-8719(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 61.Bove S., et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11(11):821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 62.Angeby-Möller K., Berge O.G., Hamers F.P. Using the CatWalk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: effects of morphine and rofecoxib. J. Neurosci. Methods. 2008;174(1):1–9. doi: 10.1016/j.jneumeth.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Thompson A.C., et al. Lack of analgesic efficacy in female rats of the commonly recommended oral dose of buprenorphine. J. Am. Assoc. Lab. Animals Sci. 2006;45(6):13–16. [PubMed] [Google Scholar]
- 64.Brewster D., Humphrey M.J., McLeavy M.A. The systemic bioavailability of buprenorphine by various routes of administration. J. Pharm. Pharmacol. 1981;33(8):500–506. doi: 10.1111/j.2042-7158.1981.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 65.Thompson A.C., et al. Analgesic efficacy of orally administered buprenorphine in rats: methodologic considerations. Comp. Med. 2004;54(3):293–300. [PubMed] [Google Scholar]
- 66.Liang X., et al. Opioid system modulates the immune function: a review. Transl. Perioper. Pain Med. 2016;1(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Flores R., Weber R.J. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology. 2000;48(2):145–156. doi: 10.1016/s0162-3109(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 68.Martucci C., Panerai A.E., Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110(1):385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.