Abstract
Background/Objective
The effects of fasted aerobic exercise on body composition and whether it causes adverse effects remain controversial. This study was to compare the effects of fasted and non-fasted aerobic exercise on body shape and blood biochemical indexes in overweight and obese young adult males, and observe whether FAE triggers adverse reactions.
Methods
Thirty overweight and obese young adult males were randomly divided into fasted aerobic exercise (FAE) group, non-fasted aerobic exercise (NFAE) group, and control group. They were subjected to indoor treadmill intervention five days a week combined with diet control for six weeks. The FAE group had breakfast 0.5 h after exercise, and the NFAE group exercised 1 h after breakfast. Both groups filled out adverse reaction questionnaires during exercise, and the control group did not have any intervention. Height, weight, body mass index (BMI), and body fat percentage of the three groups of subjects before and after the experiment were measured by the GAIA KIKO bio-resistance antibody composition analyzer in Korea; waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) were measured by the tape measure method; fasting plasma glucose (FPG), fasting insulin (FINs), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein (VLDL), and HDL-C/LDL-C were measured by Roche C8000 automatic biochemical analysis instrument.
Results
Weight, BMI, body fat percentage, WC, HC, WHR, WHtR, TG, TC, LDL-C and VLDL decreased very significantly (P < 0.01) in the FAE and NFAE groups after the 6-week experiment. The decrease in FINS was significant in the FAE group (P < 0.05) and the decrease in HDL-C was very significant in the NFAE group (P < 0.01). There was no significant difference in the frequency of adverse reactions between two groups (P > 0.05).
Conclusion
Six-week FAE and NFAE significantly improved body shape in overweight and obese young adult males, while FAE significantly reduced fasting insulin levels and increased tissue cell sensitivity to insulin. And compared to NFAE, 30 min of FAE in the morning did not increase adverse effects.
Keywords: Fasting aerobic exercise, Obesity, Body shape index, Blood biochemical index, Adverse effects
1. Introduction
Obesity is an organism state caused by excess accumulation of adipose tissue in the body, which is not only an independent risk factor for various metabolic diseases,1 but also the cause of a series of psychological and social problems.2 As a kind of chronic noncommunicable disease, the prevalence of obesity is increasing globally. In 2015 alone, overweight and obesity were responsible for nearly 4 million deaths worldwide,3 by 2020, nearly 700 million people worldwide have been affected by obesity, which seriously reduces the quality of human life and causes a heavy social and medical burden.4
Long-term obesity not only greatly increases the risk of hypertension,5 hyperlipidemia,6 knee osteoarthritis,7 type 2 diabetes,8 stroke,9 coronary heart disease,10 and other diseases in healthy individuals, but also reduces life expectancy.11 How to prevent obesity and effectively reduce the level of obesity has been the focus of medical and sports science research. As a kind of exercise therapy, fasted aerobic exercise (FAE) has attracted much attention in recent years.
Fasting generally refers to the body state of having had nothing for more than 8 h,12 while fasted aerobic exercise refers to aerobic exercise performed in this state. In 1989, Davis proposed that the thermogenic effects of pre-prandial and post-prandial exercise were different,13 leading to a trend of research on the effects of dietary restriction combined with exercise on human body composition. Recent studies have confirmed that moderate intensity exercise 1 h after meal can reduce the fat oxidation rate to about 50% of the fasting exercise, and the FAE process has higher levels of blood glycerol and fatty acid release, which can induce better lipid metabolism.14 Clinical applications in patients with type 2 diabetes and non-alcoholic fatty liver disease have also demonstrated numerous health promotion benefits.15 However, some studies have shown a different result. For instance, Schoenfeld16 found no significant difference in the effects of fasting aerobic exercise versus non-fasting aerobic exercise on body composition after a four-week diet controlled combined exercise intervention in 20 young women. After an intervention of pre-breakfast aerobic exercise and post-breakfast aerobic exercise in 10 obese male college students, Kim17 found that morning exercise after overnight fasting was more effective than post-breakfast exercise in reducing body fat, but the increase in cortisol levels after overnight fasting exercise may have a negative impact on long-term weight loss in obese men.
Many studies have evaluated the effects of aerobic exercise on the human body on an empty stomach, but relatively few have actually managed subjects in a fully enclosed environment. In addition, it is a controversial question whether FAE causes adverse reactions. Therefore, we held a fully enclosed fat loss boot camp to investigate the difference in the effects of FAE and non-fasted aerobic exercise (NFAE) on body composition and the feasibility of the application of FAE by observing the effects of FAE and NFAE on body shape and blood biochemical indexes of obese men and the incidence frequency of adverse reactions during exercise in the morning of six weeks.
2. Methods
The study was designed as a single-blind randomized controlled trial, and subjects in each group were unaware of each other's training and diet plans. All subjects were physically examined to confirm the absence of specific diseases before conducting the experiment. Subjects signed an informed consent form prior to participation in the study, and Guangxi Normal University approved the study, which met the ethical standards of the Declaration of Helsinki.
2.1. Research subjects
Thirty overweight and obese (aged 19–21, BMI ≥28 kg/m2, and body fat percentage ≥25%) young adult males who attended an enclosed fat loss training program at the Sports Medicine Laboratory of Guangxi Normal University, China, in 2020 were chosen as the study subjects. After their physical examination, medical history consultation, medications and supplement intake survey, Physical Activity Readiness Questionnaire (PAR-Q),18 resting state 12-lead Electrocardiogram (ECG) examination,19 and exercise load test, those with cardiovascular system diseases were excluded, and those included in the experiment had no history of systematic training, And all subjects had no medications or supplement intake before and during the trial. The random number table method divided them into FAE group (n = 10), NFAE group (n = 10), and blank control group (n = 10), and the basic conditions before the intervention are shown in Table 1.
Table 1.
Basic information of subjects in each group before intervention.
| Group | Quantity | Age | Height (cm) | Weight (kg) | BMI | Body fat percentage |
|---|---|---|---|---|---|---|
| FAE group | 10 | 20.15 ± 0.71 | 1.74 ± 0.07 | 90.57 ± 88.97 | 30.02 ± 1.90 | 26.38 ± 1.17 |
| NFAE group | 10 | 21.02 ± 0.58 | 1.72 ± 0.04 | 88.97 ± 10.53 | 29.96 ± 2.53 | 26.15 ± 1.19 |
| Blank control group | 10 | 20.70 ± 0.70 | 1.70 ± 0.06 | 86.84 ± 9.46 | 30.07 ± 2.52 | 26.39 ± 1.01 |
| P-value | 0.865 | 0.339 | 0.696 | 0.994 | 0.865 |
Tip 1: no statistically significant difference between three groups.
2.2. Exercise intervention methods
Subjects completed indoor treadmill brisk walking, of which the intensity was based on the results of the exercise load test and health status comprehensive development. In formal exercise we used Polar meter to monitor heart rate, so as to control the intensity of exercise, target heart rate = resting heart rate + heart rate reserve × 20%–40% (heart rate reserve = maximum heart rate - resting heart rate).20 Subjects in each group exercised five days a week, once a day, for 30 min each time, with 20 min of preparatory and finishing activities before and after exercise.21 They did not carry out any form of exercise training except for the exercise required in the experiment. The blank control group did not have any form of exercise intervention.
2.3. Provision of meals
The subjects in the FAE and NFAE groups ate all three meals at the fitness center during the experiment, and the dietitian calculated the individual basal metabolic rate according to the Harris-Benedict equation22 and then developed a diet plan. The diet plan follows the low-calorie principle of gradually limiting total calorie intake while ensuring nutritional balance. The ratio of intake of the three major nutrients is:55%–65% carbohydrate, 20%–35% protein, 10%–15% fat, three meals calorie ratio of about 3 : 4: 3,23 the blank control group was not provided with a meal and did not have any exercise intervention. In addition, dietary records of all subjects before and during the intervention were collected and analyzed to ensure the scientific validity of the diet plan. Meal times were as shown in Table 2, with the FAE group eating 0.5 h after exercise and the NFAE group eating breakfast 1 h after exercise.
Table 2.
Diet schedule.
| Breakfast |
Lunch |
Dinner |
||||
|---|---|---|---|---|---|---|
| Group | FAE group | NFAE group | FAE group | NFAE group | FAE group | NFAE group |
| Monday (Break) | 8:30 intake | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| no exercise | no exercise | |||||
| Tuesday (Break) | 8:30 intake | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| no exercise | no exercise | |||||
| Wednesday | 7:30 exercise | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| 8:30 intake | 9:40 exercise | |||||
| Thursday | 7:30 exercise | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| 8:30 intake | 9:40 exercise | |||||
| Friday | 7:30 exercise | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| 8:30 intake | 9:40 exercise | |||||
| Saturday | 7:30 exercise | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| 8:30 intake | 9:40 exercise | |||||
| Sunday | 7:30 exercise | 8:30 intake | 13:00 intake | 13:00 intake | 17:30 intake | 17:30 intake |
| 8:30 intake | 9:40 exercise | |||||
2.4. Test indexes and instruments
Body morphology and blood biochemical index values were measured before the start of training and after the end of training, respectively. Among them, height, weight, BMI, and body fat percentage were measured by the Korean GAIA KIKO bio-resistance antibody composition analyzer, and waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) were measured by the tape measurements. Fasting plasma glucose (FPG), fasting insulin (FINs), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein (VLDL), and HDL-C/LDL-C were measured using a Roche Cobas 8000 fully automated biochemical analyzer.24 All subjects were asked to maintain their usual living and eating habits prior to the blood test. Both blood tests were scheduled at the same time in the early morning and subjects were asked to fast for 12 h prior to the blood biochemistry test, with no food intake but moderate water intake during the fast. The medical history of all subjects and whether they ingested medications and supplements were investigated before the start of the experiment to ensure that all subjects included in the experiment were free of medications and supplement intake. And the fully enclosed administration excludes the influence of ingested medications or supplements on the test results. The last meal before the first blood test was requested to be consumed maintaining the pre-experimental diet and avoiding over-eating. The last meal before the post-experimental blood test was consumed maintaining the same diet plan as during the experiment.
2.5. Adverse reactions and evaluation during exercise
Subjects in the FAE and NFAE groups filled out adverse reaction questionnaires at each exercise according to the clinical trial manual criteria. The evaluation criteria for each adverse reaction were as follows.
-
(1)
Subjective hunger of the subjects was divided into three levels, and the mild expression was slightly hungry. Moderate symptoms are marked but tolerable hunger; Severe symptoms are intense hunger, dizziness, and sweating, which would be hard to bear.
-
(2)
There are three levels of thirsty, including mild performance for a little thirsty; Moderate symptoms are marked thirsty, but they are still tolerable; Severe symptoms are intense thirsty, accompanied by intolerable dry and chapped lips.
-
(3)
The degree of fatigue of the subjects was manifested as dizziness, fatigue, drowsiness.
-
(4)
For nausea and vomiting feeling judgment: there was nausea, regurgitation, and the feeling of wanting to vomit.
-
(5)
The comfort level was assessed according to the visual analogue scale (VAS), It has 10 scales. Where 0 in the table indicates high comfort and 10 indicates severe discomfort. Subjects in the FAE and NFAE groups were penciled in one-on-one by the researcher at each training session to record their feelings, allowing the exercisers to choose a score based on how they felt.
2.6. Statistical analysis
Data were analyzed by SPSS Statistics 26.0 software and expressed as mean ± standard deviation (±SD). Each index was compared before and after the intervention using paired samples t-test, and between groups using independent samples t-test and one-way ANOVA, with significance level defined as P < 0.05 and very significant level defined as P < 0.01.
3. Results
The changes in body morphological indicators and blood biochemical indicators in each group of subjects after the 6-week intervention, as well as the occurrence of adverse reactions during exercise, are presented.
3.1. Changes of body morphology indexes in each group after intervention
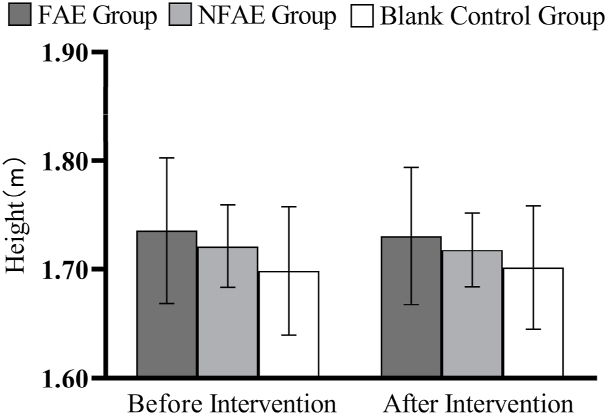
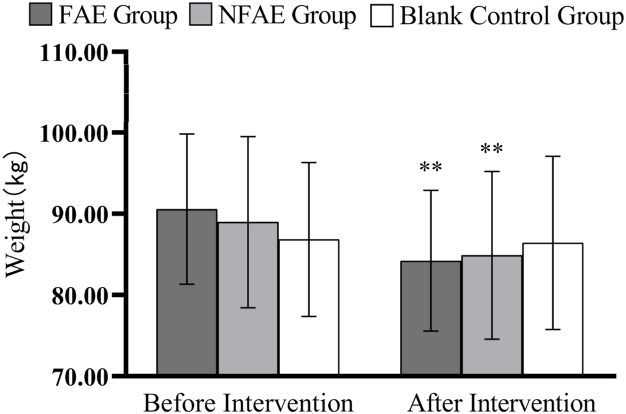
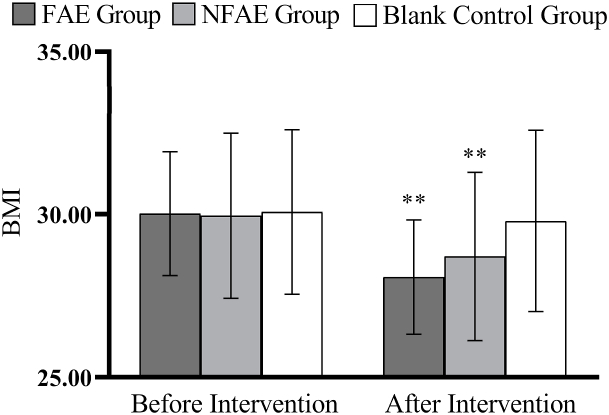
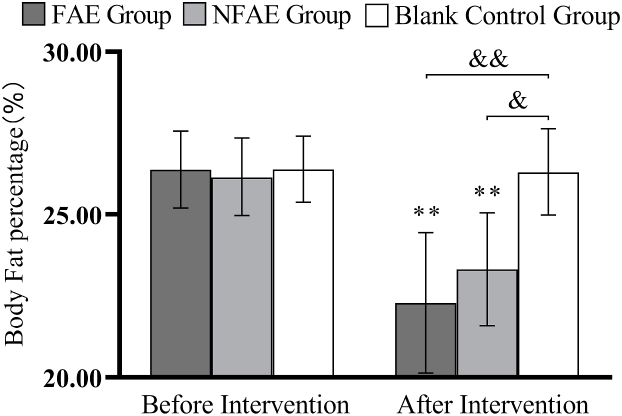
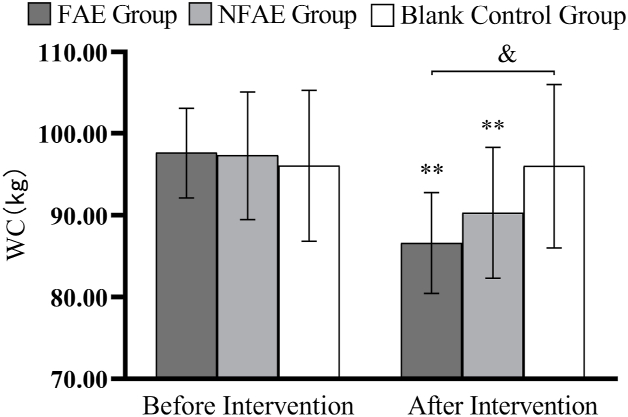
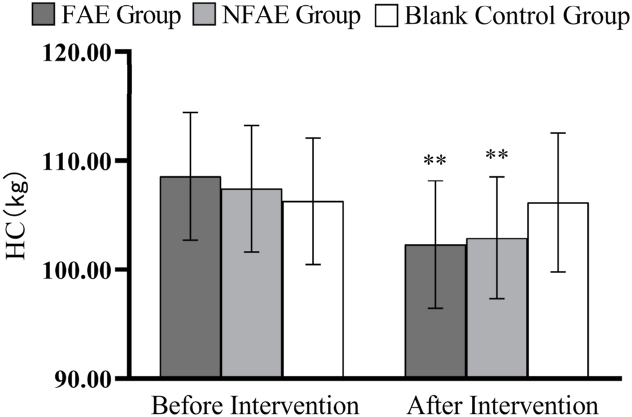
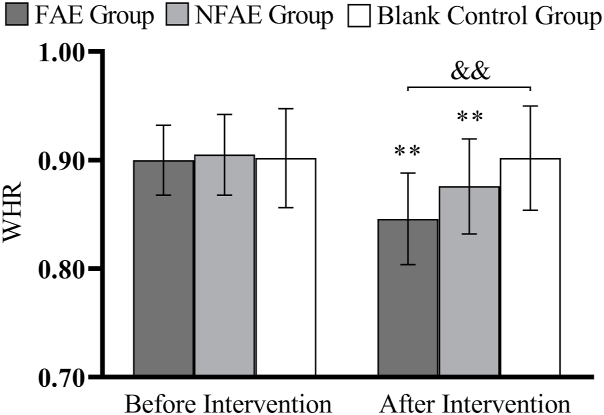
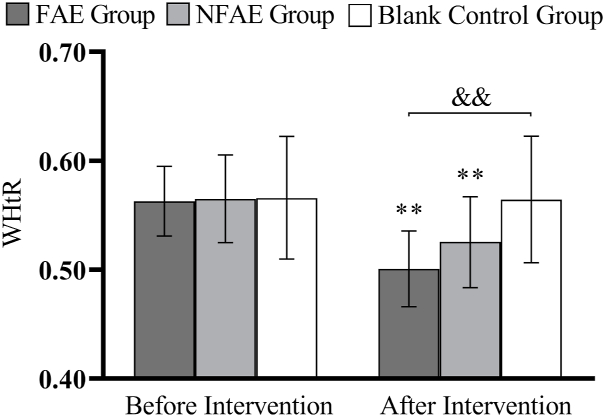
There were no significant differences in height, weight, BMI, body fat percentage, WC, HC, WHR, and WHtR among the groups before the intervention (P > 0.05). After intervention, all subjects except the blank control group had significantly lower body shape indexes than before intervention (P < 0.01). After intervention, the percentage of body fat in the FAE group, WHR, WHtR, decreased very significantly compared with the blank control group (P < 0.01), WC decreased significantly (P < 0.05) compared to the blank control group. The body fat percentage of NFAE group decreased significantly compared with blank control group (P < 0.05). There was no significant difference in height, weight and BMI between the groups after the intervention (P > 0.05). The changes of body shape indexes are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Fig. 1.
Histogram of height change before and after intervention for each group of subjects.
Fig. 2.
Histogram of weight change before and after intervention for each group of subjects
Tip 2: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01.
Fig. 3.
Histogram of the change in BMI before and after the intervention for each group of subjects
Tip 3: ∗∗ indicates a highly significant difference within the groups after the intervention compared to before the intervention, P < 0.01.
Fig. 4.
Histogram of the change in body fat percentage before and after the intervention for each group of subjects
Tip 4: ∗∗ indicates a highly significant difference within the groups after the intervention compared to before the intervention, P < 0.01; && indicates a highly significant difference between groups compared to post-intervention, p < 0.01; & indicates a significant difference between groups compared to post-intervention, p < 0.05.
Fig. 5.
Histogram of WC changes before and after intervention for each group of subjects
Tip 5: ∗∗ indicates that there is a very significant difference in the group after intervention compared with before intervention, P < 0.01. & indicates a significant difference between groups compared to post-intervention, P < 0.05.
Fig. 6.
Histogram of HC changes before and after intervention for each group of subjects
Tip 6: ∗∗ indicates that there is a very significant difference in the group after intervention compared with before intervention, P < 0.01.
Fig. 7.
Histogram of WHR changes before and after intervention for each group of subjects
Tip 7: ∗∗ indicates that there is a very significant difference in the group after intervention compared with before intervention, P < 0.01. && indicated that there was a very significant difference between groupsafter intervention, P < 0.01.
Fig. 8.
Histogram of WHtR changes before and after intervention for each group of subjects
Tip 8: ∗∗ indicates that there is a very significant difference in the group after intervention compared with before intervention, P < 0.01. && indicated that there was a very significant difference between groupsafter intervention, P < 0.01.
3.2. Changes of blood biochemical indexes in each group after intervention
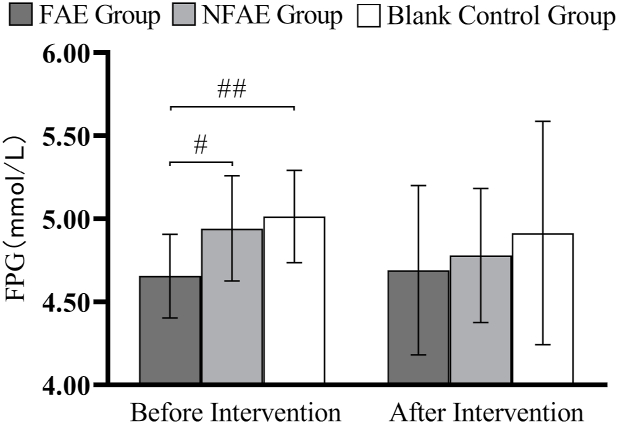
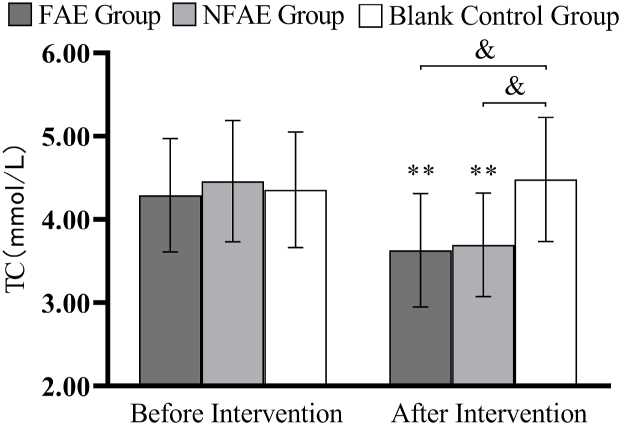
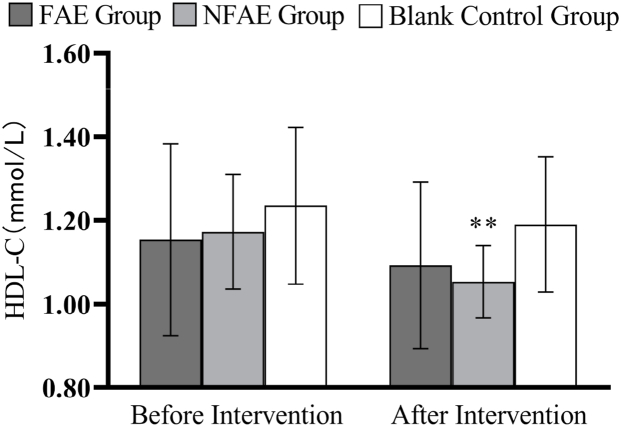
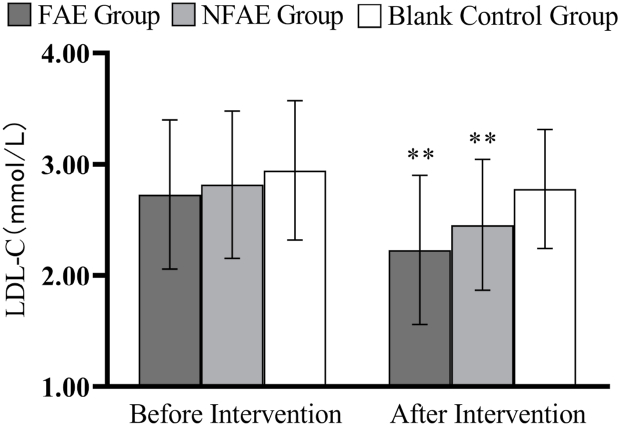
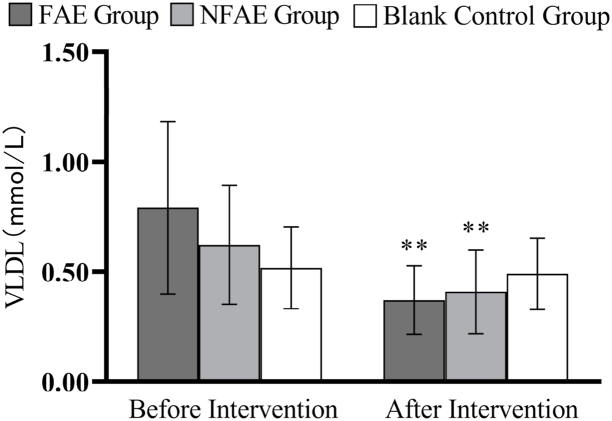
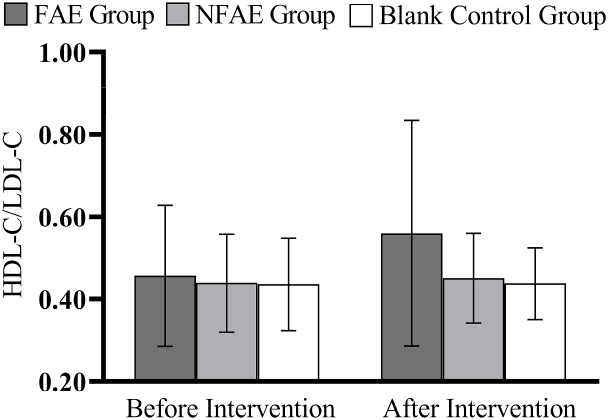
According to one-way analysis of variance, there was a significant difference in FPG values between the FAE group and the NFAE group before intervention (P < 0.05), the difference between the FAE group and the blank control group was highly significant (P < 0.01). However, there were no significant differences in FINs, TG, TC, HDL-C, LDL-C, VLDL, and HDL-C/LDL-C between the pre-intervention groups (P > 0.05). After intervention, there were no significant differences in FPG, FINS, TG, HDL-C, LDL-C, VLDL, HDL-C/LDL-C between groups (P > 0.05), but there were significant differences in TC between groups (P < 0.05).
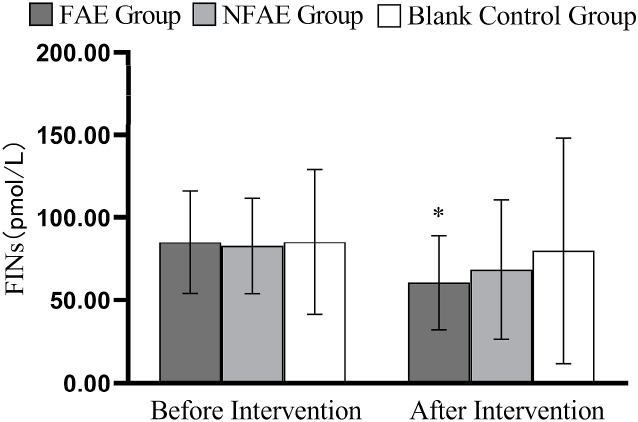
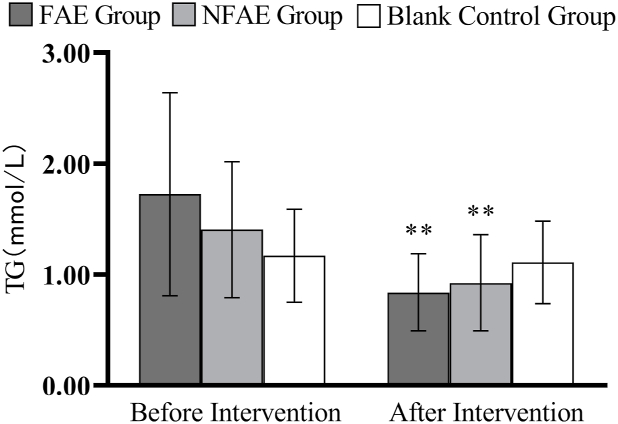
TG, TC, LDL-C, and VLDL decreased very significantly (P < 0.01) in the FAE and NFAE groups after the 6-week intervention compared with the pre-intervention period. Among them, FINS in the FAE group was significantly lower after the intervention than before the intervention (P < 0.05). HDL-C was significantly lower in the NFAE group than before the intervention (P < 0.01). Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16 are histograms of FPG, FINS, TG, TC, HDL-C, LDL-C, VLDL, HDL-C/LDL-C before and after intervention, respectively.
Fig. 9.
Histogram of FPG changes before and after intervention for each group of subjects
Tip 9: ## indicates that there is a very significant difference between groups before intervention, P < 0.01; # indicates that there is a significant difference between groups before intervention, P < 0.05.
Fig. 10.
Histogram of changes in FINs before and after the intervention for each group of subjects
Tip 10: ∗ indicates a significant difference within the group after the intervention compared to before the intervention, P < 0.05.
Fig. 11.
Histogram of TG changes before and after intervention for each group of subjects
Tip 11: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01.
Fig. 12.
Histogram of TC changes before and after intervention for each group of subjects
Tip 12: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01. & indicates a significant difference between groups compared to post-intervention, P < 0.05.
Fig. 13.
Histogram of HDL-C changes before and after intervention for each group of subjects
Tip 13: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01.
Fig. 14.
Histogram of LDL-C changes before and after intervention for each group of subjects
Tip 14: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01.
Fig. 15.
Histogram of VLDL changes before and after intervention for each group of subjects
Tip 15: ∗∗ indicates a highly significant difference within the group after the intervention compared to before the intervention, P < 0.01.
Fig. 16.
Histogram of HDL-C/LDL-C changes before and after intervention for each group of subjects.
3.3. Adverse reactions and assessment during exercise
-
(1)
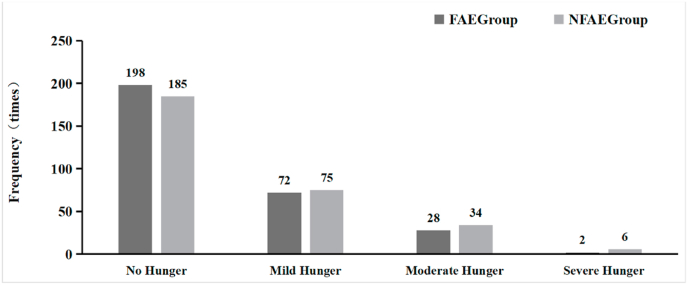
In terms of hunger, during a total of 30 exercise training sessions, there was no significant difference (P > 0.05) in the number of mild, moderate and severe hunger pains during exercise between subjects in the FAE and NFAE groups (see Fig. 17).
-
(2)
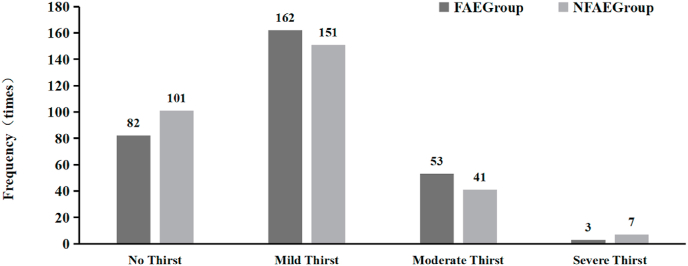
Regarding the sensation of thirst, there was no significant difference (P > 0.05) in the number of times that subjects in the FAE and NFAE groups experienced mild, moderate, or severe thirst during exercise (see Fig. 18).
-
(3)
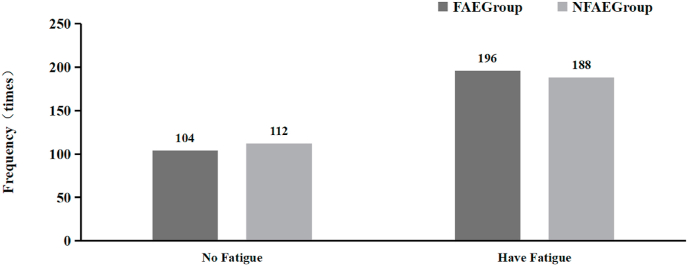
In terms of fatigue, there was no significant difference in FAE and NFAE group in the frequency of fatigue for subjects during exercise (P > 0.05) (see Fig. 19).
-
(4)
With regard to nausea symptoms, no nausea occurred in subjects in both the FAE and NFAE groups during a total of 30 exercise training sessions.
-
(5)
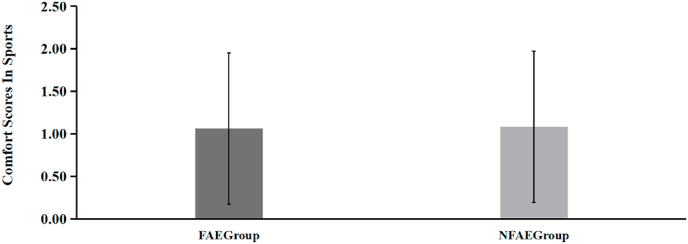
For comfort, the subjects in the FAE group had a score of (1.06 ± 0.89) and the NFAE group had a score of (1.08 ± 0.99) for comfort during exercise, and there was no significant difference between the two groups for comfort during exercise (P > 0.05) (see Fig. 20).
Fig. 17.
Comparative histogram of hunger in each group of subjects during the experiment.
Fig. 18.
Comparative histogram of thirst experienced by subjects in each group during the experiment.
Fig. 19.
Histogram comparing the fatigue experienced by the subjects in each group during the experiment.
Fig. 20.
Histogram comparing the comfort scores of subjects in each group during the experiment.
4. Discussion
This section will systematically analyze the causes of changes in body morphological indicators and blood biochemical indicators in each group of subjects and analyze the adverse effects during exercise.
4.1. Effects of FAE and NFAE on body shape indices in obese male
The results of this study showed that both 6-week FAE and NFAE combined with dietary restriction were effective in reducing body weight, BMI, body fat percentage, WC, HC, WHR, and WHtR in overweight and obese young adult males. Although FAE reduced the above indicators to a greater extent than NFAE, there was no significant difference between FAE and NFAE in improving body shape and reducing the degree of physical obesity in obese individuals. Subjects in both the FAE and NFAE groups showed height reduction after the intervention, but it was not statistically significant compared to the pre-intervention period. Therefore, we believe that the changes in the values of BMI before and after the intervention are mainly due to weight loss. In addition, the rate of change in body fat percentage was greater than the rate of change in weight in both groups, suggesting that the weight loss came primarily from a reduction in fat weight. Trabelsi25 conducted a comparative study of changes in body composition induced by FAE or NFAE during Ramadan. The researchers randomized 19 men to the Fast group (n = 10) and the Fed group (n = 9), followed by aerobic training for 40–60 min, three days a week for 30 days, and found varying degrees of weight loss in both groups, but only the FAE group showed a significant decrease in body fat percentage. Hackett26 analyzed 96 subjects in five studies and found that increased fat oxidation from aerobic training in a fasted state may lead to a greater negative energy balance in the body, enhancing the fat loss effect of exercise. Although the improvement in body morphology was greater with FAE than with NFAE in this study, the difference was not significant. This is inconsistent with the findings of the above-mentioned literature. The reason for this phenomenon may be that the duration of this experiment was 6 weeks, longer than the 4 weeks in the above study. Whether the fat loss efficiency of FAE decreases with longer time, thus showing a better fat loss effect of 4 weeks FAE than 6 weeks, is worthy of further study.
4.2. Effect of FAE and NFAE on blood biochemical parameters in obese male
The results of the study showed that FAE and NFAE combined with dietary restriction for 6 weeks were effective in reducing TG, TC, LDL-C, and VLDL values in overweight and obese young adult males. Although the reductions in these metrics were greater with FAE than with NFAE, there was no significant difference. In addition, this study found that 6-week FAE and NFAE had no significant effect on FPG, while FINS decreased significantly after FAE. The reason may be that FAE improved the biological effect of insulin, indirectly suggesting that FAE may improve the mechanism of insulin receptor or post-receptor action.27
Maughan28 believe that the body's response to fasting involves increased fat mobilization and decreased re-esterification of free fatty acids (FFA), and that fasting exercise causes an increase in blood FFA levels, which in turn causes an increase in FFA utilization by muscle tissue. INS is the most potent lipolysis inhibitory hormone secreted by islet beta cells in the pancreas, Low concentrations in the body during starvation and exercise induce an increase in the rate of lipolysis,29 and higher levels of INS will inhibit lipolysis during exercise after carbohydrate intake.
The significant reduction of TG, LDL-C, and VLDL by FAE may be related to the following factors: reduced endogenous TG synthesis level, increased lipoprotein lipase activity and thus increased serum TG hydrolysis, increased skeletal muscle use of fatty acid.30 Horowitz31 has suggested that lipolysis is inhibited after carbohydrate intake, and exercise in this state will limit the oxidation of fat during exercise. In a comparative analysis of the metabolic effects of 27 studies related to fasting exercise, Vieira et al.32 found that aerobic exercise performed in the fasted state induced higher rates of fat oxidation than aerobic exercise performed after eating. However, this study found that FAE was not superior to NFAE in improving blood lipids, which may also be related to the longer duration of the experiment.
4.3. Fasting aerobic exercise and adverse reactions
There was no significant difference in the frequency of hunger, thirst, fatigue and nausea during exercise and in the comfort scores during exercise between subjects in the FAE and NFAE groups in this experiment, indicating that FAE at 30 min in the morning does not increase the probability of the above adverse effects relative to NFAE. The biological principles that trigger the sensation of hunger in humans are controversial, and may be related to factors such as gastric contraction and emptying, changes in gastric hunger hormone concentration, changes in blood glucose, stimulation of the human digestive tract mucosa by gastrointestinal flora, and activation of the feeding center in the hypothalamus.33 In this study, the reason why fasting exercise did not trigger excessive hunger may be related to the fact that exercise increased the concentration of satiety hormones such as peptide YY and glucagon-like peptide 1, while decreasing the concentration of gastrin.34
The main causes of the feeling of thirst in the human body are the decrease in the amount of blood circulating in the body and the increase in the osmotic pressure of body fluids, in addition to psychological factors, customs and behaviors, and personal habits.35 In this study, the frequency of thirst in the FAE group was slightly higher than that in the NFAE group, but it was not statistically significant, which may be related to the presence of water within the breakfast consumed in the NFAE group. The frequency of fatigue was slightly higher in the FAE group than in the NFAE group, but the difference was not significant. The possible reason for this may be related to the fact that the FAE group performed prolonged endurance exercise after an overnight fast, which reduced the work capacity of the central nervous system and the musculoskeletal system.36-37 However, the principle of human exercise fatigue is extremely complex and is caused by the complex superposition of various fatigue-causing factors rather than being explained by a single free radical theory, exhaustion theory, protective inhibition theory, etc.38 In addition, the subjects in the FAE and NFAE groups did not experience nausea throughout the experimental cycle, and the exercise comfort scores also proved that the difference in the effect of FAE and NFAE on human subjective sensations was not significant, which may be related to the shorter exercise time, lower exercise intensity, and simpler exercise methods.
5. Conclusions
In conclusion, several key findings can be summarized based on the study presented above:
-
(1)
Six weeks of FAE and NFAE significantly reduced body weight, BMI, body fat percentage, WC, HC, WHR and WHtR in overweight and obese young adult males, while FAE did not differ significantly from NFAE in terms of improving body shape.
-
(2)
Both FAE and NFAE for six weeks were effective in improving lipid metabolism in vivo, while FAE significantly reduced fasting insulin levels and increased tissue cell sensitivity to insulin.
-
(3)
FAE at 30 min in the morning did not increase the probability of adverse effects such as hunger, thirst, fatigue and nausea relative to NFAE.
The limitations of this study are the small sample size and the fact that only young men were selected as subjects in this study, but not women, middle-aged and elderly people, or children. In the future, we need to increase the sample size to increase the precision of the experiment and further study the effect of fasting aerobic exercise on women, middle-aged and elderly people and children to verify the health promotion benefits of this exercise therapy.
Funding statement
This study was funded by the Guangxi Postgraduate Education Innovation Program, China (Project No. YCSW2019089 and Project No. YCSW2021109).
Author statement
We would like to submit the enclosed manuscript entitled “The Effects of Six Weeks of Fasted Aerobic Exercise on Body Shape and Blood Biochemical Index in Overweight and Obese Young Adult Males”, which we wish to be considered for publication in “Journal of Exercise Science & Fitness”. No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Declaration of competing interest
We submitted a manuscript titled “The Effects of Six Weeks of Fasted Aerobic Exercise on Body Shape and Blood Biochemical Index in Overweight and Obese Young Adult Males”. All authors reviewed and solemnly promised that there was no conflict of interest in the article.
Acknowledgments
The authors would like to thank the reviewers for their thoughtful comments and efforts towards improving our manuscript. We acknowledge that Guangxi Normal University and the Sports Medicine Laboratory facilitated the implementation of this research. Xiaolong Liu and Mengxiao He contributed equally to this manuscript.
Footnotes
This study was carried out at: Sports Medicine Laboratory, Guangxi Normal University, Guilin, Guangxi province, China, postal code: 541006.
Contributor Information
Xiaolong Liu, Email: 1171767934@qq.com.
Mengxiao He, Email: 929332521@qq.com.
Xiaoli Gan, Email: 2543329963@qq.com.
Yang Yang, Email: 416330321@qq.com.
Qin Hou, Email: 476989839@qq.com.
Rongbo Hu, Email: rongbo.hu@tum.de.
References
- 1.Wen J., Yang J., Shi Y., et al. Comparisons of different metabolic syndrome definitions and associations with coronary heart disease, stroke, and peripheral arterial disease in a rural Chinese population. PLoS One. 2015;10(5):1–15. doi: 10.1371/journal.pone.0126832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu D.T., Nguyet N.T., Nga V.T., et al. An update on obesity: mental consequences and psychological interventions. Diabetes Metabol Syndr. 2019;13(1):155–160. doi: 10.1016/j.dsx.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Afshin A., Forouzanfar M.H., Reitsma M.B., et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theilade S., Christensen M.B., Vilsbll T., et al. An overview of obesity mechanisms in humans: endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes Metabol. 2021;23(s1):17–35. doi: 10.1111/dom.14270. [DOI] [PubMed] [Google Scholar]
- 5.Ryu S., Frith E., Pedisic Z., et al. Secular trends in the association between obesity and hypertension among adults in the United States, 1999-2014. Eur J Intern Med. 2019;62:37–42. doi: 10.1016/j.ejim.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Shi X.D., He S.M., Tao Y.C., et al. Prevalence of obesity and associated risk factors in Northeastern China. Diabetes Res Clin Pract. 2011;91(3):389–394. doi: 10.1016/j.diabres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Ann Phys Rehabil Med. 2016;59(3):157–160. doi: 10.1016/j.rehab.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Katsiki N., Anagnostis P., Kotsa K., et al. Obesity, metabolic syndrome and the risk of microvascular complications in patients with diabetes mellitus. Curr Pharmaceut Des. 2019;25(18):2051–2059. doi: 10.2174/1381612825666190708192134. [DOI] [PubMed] [Google Scholar]
- 9.Winter Y., Pieper L., Klotsche J., et al. Obesity and abdominal fat markers in patients with a history of stroke and transient ischemic attacks. J Stroke Cerebrovasc Dis. 2016;25(5):1141–1147. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Rovella V., Anemona L., Cardellini M., et al. The role of obesity in carotid plaque instability: interaction with age, gender, and cardiovascular risk factors. Cardiovasc Diabetol. 2018;17(1):46–55. doi: 10.1186/s12933-018-0685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters A., Barendregt J.J., Willekens F., et al. Obesity in adulthood and its consequences for life expectancy: a life-table Analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 12.DeWaters A.L., Mejia D., Thomas J., et al. Patient preparation for outpatient blood work and the impact of surreptitious fasting on diagnoses of diabetes and prediabetes. Mayo Clin Proc Innov Qual Outcomes. 2020;4(4):349–356. doi: 10.1016/j.mayocpiqo.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis J.M., Sadri S., Sargent R.G., et al. Weight control and calorie expenditure: thermogenic effects of pre-prandial and post-prandial exercise. Addict Behav. 1989;14(3):347–351. doi: 10.1016/0306-4603(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 14.Enevoldsen L.H., Simonsen L., Macdonald I.A., et al. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol. 2004;561(Pt 3):871–882. doi: 10.1113/jphysiol.2004.076588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallis G.A., Gonzalez J.T. Is exercise best served on an empty stomach? Proc Nutr Soc. 2019;78(1):110–117. doi: 10.1017/S0029665118002574. [DOI] [PubMed] [Google Scholar]
- 16.Schoenfeld B.J., Aragon A.A., Wilborn C.D., et al. Body composition changes associated with fasted versus non-fasted aerobic exercise. J Int Soc Sports Nutr. 2014;11(1):54–60. doi: 10.1186/s12970-014-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T.W., Lee S.H., Choi K.H., et al. Comparison of the effects of acute exercise after overnight fasting and breakfast on energy substrate and hormone levels in obese men. J Phys Ther Sci. 2015;27(6):1929–1932. doi: 10.1589/jpts.27.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garber C.E., Blissmer B., Deschenes M.R., et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 19.Le H.M., Downey B.C., Lanois C.J., et al. Comparison of the limb-lead Electrocardiogram to the 12-lead Electrocardiogram for identifying conditions associated with sudden cardiac death in youth athletes. Am J Cardiol. 2021;152:146–149. doi: 10.1016/j.amjcard.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Gao H., Xu J., Zhang L., et al. Effects of living high-training low and high on body composition and metabolic risk markers in overweight and obese females. BioMed Res Int. 2020;2020:1–9. doi: 10.1155/2020/3279710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karami H., Dehnou V.V., Nazari A., et al. Regular training has a greater effect on aerobic capacity, fasting blood glucose and blood lipids in obese adolescent males compared to irregular training. J Exerc Sci Fit. 2021;19(2):98–103. doi: 10.1016/j.jesf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao Z.Y., Wu X.T., Liang B.M., et al. Comparison of five equations for estimating resting energy expenditure in Chinese young, normal weight healthy adults. Eur J Med Res. 2012;17(1):26–35. doi: 10.1186/2047-783X-17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajala O., English P., Pinkney J. Systematic review and meta analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 24.Paleari R., Bonetti G., Callà C., et al. Multicenter evaluation of an enzymatic method for glycated albumin. Clin Chim Acta. 2017;469:81–86. doi: 10.1016/j.cca.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Trabelsi K., el Abed K., Stannard S.R., et al. Effects of fed- versus fasted-state aerobic training during Ramadan on body composition and some metabolic parameters in physically active men. Int J Sport Nutr Exerc Metabol. 2012;22(1):11–18. doi: 10.1123/ijsnem.22.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Hackett D., Hagstrom A.D. Effect of overnight fasted exercise on weight loss and body composition: a systematic review and meta-analysis. J Funct Morphol Kinesiol. 2017;2(4):43–53. doi: 10.3390/jfmk2040043. [DOI] [Google Scholar]
- 27.Hansen D., De Strijcker D., Calders P. Impact of endurance exercise training in the fasted state on muscle biochemistry and metabolism in healthy subjects: can these effects be of particular clinical benefit to type 2 diabetes mellitus and insulin-resistant patients? Sports Med. 2017;47(3):415–428. doi: 10.1007/s40279-016-0594-x. [DOI] [PubMed] [Google Scholar]
- 28.Maughan R.J., Fallahs J., Coyle E.F. The effects of fasting on metabolism and performance. Br J Sports Med. 2010;44(7):490–494. doi: 10.1136/bjsm.2010.072181. [DOI] [PubMed] [Google Scholar]
- 29.Bennard P., Imbeault P., Doucet E. Maximizing acute fat utilization: effects of exercise,food, and individual characteristics. Can J Appl Physiol. 2005;30(4):475–499. doi: 10.1139/h05-134. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Arcos I., Hiyama Y., Drosatos K., et al. Adipose-specific lipoprotein lipase deficiency more profoundly affects brown than white fat biology. J Biol Chem. 2013;288(20):14046–14058. doi: 10.1074/jbc.M113.469270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horowitz J.F., Mora-Rodriguez R., Byerley L.O., et al. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol. 1997;273(4):E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
- 32.Vieira A.F., Costa R.R., Macedo R.C., et al. Effects of aerobic exercise performed in fasted v.fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016;116(7):1153–1164. doi: 10.1017/S0007114516003160. [DOI] [PubMed] [Google Scholar]
- 33.Tack J., Verbeure W., Mori H., et al. The gastrointestinal tract in hunger and satiety signalling. United European Gastroenterol J. 2021;9(6):727–734. doi: 10.1002/ueg2.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57(s2):36–42. doi: 10.1159/000322702. [DOI] [PubMed] [Google Scholar]
- 35.Mckinley M.J., Cairns M.J., Denton D.A., et al. Physiological and pathophysiological influences on thirst. Physiol Behav. 2004;81(5):795–803. doi: 10.1016/j.physbeh.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Li Q., Wang K., et al. Effects of long-term fasting and confinement on the cardiovascular activity. Med Biol Eng Comput. 2021;59(9):1901–1915. doi: 10.1007/s11517-021-02380-4. [DOI] [PubMed] [Google Scholar]
- 38.Hargreaves M. Fatigue mechanisms determining exercise performance: integrative physiology is systems biology. J Appl Physiol. 2008;104(5):1541–1542. doi: 10.1152/japplphysiol.00088.2008. [DOI] [PubMed] [Google Scholar]