Abstract
Type 1 diabetes (T1D) is one of the world's health problems with a prevalence of 1.1 million for children and young adults under the age of 20. T1D is a health problem characterized by autoimmunity and the destruction of pancreatic cells that produce insulin. The available treatment is to maintain blood glucose within the desired normal range. To meet bolus and basal requirements, T1D patients may receive multiple daily injections (MDI) of fast-acting and long-acting insulin once or twice daily. In addition, insulin pumps can deliver multiple doses a day without causing injection discomfort in individuals with T1D. T1D patients have also monitored their blood glucose levels along with insulin replacement treatment using a continuous glucose monitor (CGM). However, this CGM has some drawbacks, like the sensor needs to be replaced after being inserted under the skin for seven days and needs to be calibrated (for some CGMs). The treatments and monitoring devices mentioned creating a lot of workloads to maintain blood glucose levels in individuals with T1D. Therefore, to overcome these problems, closed-loop artificial pancreas (APD) devices are widely used to manage blood glucose in T1D patients. Closed-loop APD consists of a glucose sensor, an insulin infusion device, and a control algorithm. This study reviews the progress of closed-loop artificial pancreas systems from the perspective of device properties, uses, testing procedures, regulations, and current market conditions.
Keywords: Closed-loop artificial pancreas, Glucose sensor, Insulin, Type 1 diabetes
Closed-loop artificial pancreas; Glucose sensor; Insulin; Type 1 diabetes.
1. Introduction
Diabetes has become a worldwide health issue and is included as one of the top 10 causes of death in adults. About 463 million (9.3%) adults are recently living with diabetes worldwide. This number is estimated will be rosed until 700 million (10.9%) of the world population. The burden caused by diabetes increased not only from the healthcare aspect but also from the global health expenditure that reached about United States dollar (USD) 760 billion by 2019. The healthcare expenditure is forecasted to be USD 825 billion by 2030, and it will be increased to USD 845 billion by 2045 [1].
Type 1 diabetes (T1D) is one of three main types of diabetes alongside type 2 diabetes (T2D), and gestational diabetes mellitus (GDM) [2]. An amount of 1.1 million children and those aged under 20 years old have T1D by 2019 [1]. T1D is an autoimmune-associated health problem and destruction of pancreatic β cells for producing insulin [3]. Insulin is essential for the human body to reduce the concentration of blood glucose then the energy could be generated [4]. In the T1D patient, there is a significant loss of insulin and amylin secretion. Despite the tiny percentage of monogenic T1D patients, recently, there has been no therapy to overcome the root cause of diabetes. The available treatments are the only option to maintain blood glucose within the desired normal range (70–180 mg/dL) [5]. The discovery of exogenous insulin around 100 years ago has a notable positive progression to prolong the short lifespan of T1D patients. Various types of treatment have been carried out such as insulin injection and continuous insulin infusion using the insulin pump [6].
Multiple daily injection (MDI) treatment with long-acting insulin can be administered once or twice a day to T1D patients to meet the requirement of basal needs. On the other hand, MDI with rapid-acting insulin is given to meet the meal insulin needs and refine the hyperglycemia condition [7]. Another way to provide exogenous insulin for T1D patients is the insulin pump, a small external device that can continuously deliver amounts of rapid-acting insulin. Insulin pumps consist of a tube that connects the reservoir to the infusion site. Insulin pumps are more favorable than MDI for basal and bolus delivery purposes. Insulin pumps can deliver a very small amount of insulin to 0.01 units compared to syringe injections that only 0.5 units for the smallest amount. Besides, insulin pumps can provide multiple doses a day without the discomfort caused by injections to individuals with T1D [8]. From the development perspective, patch pumps for Insulin are newly developed to optimize the accuracy and flexibility of insulin delivery at lower costs. The insulin patch pump is a fully flexible device for performing the most complex regimens for insulin-treated diabetic patients. This device works on the principle of electromechanical and has a mechanical pump with an electronic controller. The mechanism of action of this patch pump is based on a needle inserter that inserts a metal needle measuring about 4 mm 30 ga into the patient's subcutaneous tissue. The device has a syringe-shaped plunger spring containing silicone oil [9].
T1D patients have also monitored their blood glucose level alongside insulin, replacing treatment by employing the continuous glucose monitor (CGM). CGM is an electrochemical sensor placed subcutaneously to measure the interstitial fluid of glucose levels. The sensor is linked to the transmitter, which transfers the current of blood glucose to a portable device every certain time and it alerts the users when the blood glucose level exceeds the hypoglycemia or hyperglycemia thresholds [10]. However, this CGM has several drawbacks, such as the sensor needs to be replaced after being inserted under the skin for seven days, and it needs to be calibrated (for some CGMs) multiply each day to maintain the accuracy and precision levels of measurement [11].
The mentioned treatments and monitoring devices produce a lot of workload for maintaining blood glucose levels in individuals with T1D. This issue has to be solved to improve the goals of therapy, improve patient comfort and compliance, and also improve cost efficiency. It also needs automated insulin delivery and monitoring to prevent short-term and long-term complications caused by T1D and minimize its daily burden.
2. Method
A structured literature search was employed using PubMed after identification of free-text terms and MeSH terms, for searches within titles and abstracts relating to the four components of the search: closed-loop artificial pancreas, glucose sensor, insulin, and type 1 diabetes. The search query was: (((closed-loop artificial pancreas) AND (glucose sensor)) AND (insulin)) AND (type 1 diabetes). The search was conducted on January 16, 2022, and updated on June 08, 2022. This search yielded 172 articles, which consisted of 57 clinical trials, 2 meta-analyses, 47 randomized controlled trials, 46 reviews, and 4 systematic reviews. Search results not related to closed-loop artificial pancreas for type 1 diabetes were excluded (12 articles). The remaining articles from the exclusion system were then read, understood, and interpreted to produce reviews relevant to closed-loop artificial pancreas. Furthermore, several studies on FDA-based regulation were also included.
3. Description of the device properties
One of the most widely used for managing blood glucose in T1D patients is the artificial pancreas. The artificial pancreas device (APD) is a closed-loop control system of blood glucose in individuals with diabetes. This closed-loop APD consists of a glucose sensor, an insulin infusion device, and a control algorithm. The early approved APD by the Food and Drug Administration (FDA) in the early 1970s is Biostator™ that by Miles Labs for managing insulin-dependent diabetes patients [12]. APD can be classified by its configuration, such as a single-hormone APD, which only delivers insulin and a dual-hormone APD, which delivers insulin and glucagon [13]. On the other hand, Based on the automation level, closed-loop APD can be categorized into three different generations. First-generation APD consists of three main stages, including (1) insulin pump turns off when there is the response from user to low glucose alarm; (2) alarm for hypoglycemia that followed by reduction of insulin delivery, and (3) similar to 2nd stage with a higher threshold. The second-generation consists of stage (4) hybrid closed-loop at all times with bolus-assisted for mealtime and stage (5) fully-automated insulin closed loop. The third generation comprises stage (6) fully-automated multi hormones (insulin added with glucagon or amylin) closed-loop APD [14].
An automated closed-loop APD is defined as an externally worn medical device system that consists of three main functions, including (1) insulin delivery carried out by an insulin infusion pump; (2) monitoring glucose levels carried out by a CGM, and (3) control center function carried out by a digital controller [15]. Several types of insulin infusion pumps are available on the market including tubeless patch pumps, automated delivery of insulin with a hybrid closed-loop system, and multiple closed-loop systems. There is also an AndroidAPS-assisted system using Bluetooth commands in a smartphone [16]. CGM improvement is proportional to sensor reliability, accuracy, and feature development. The main sensors are based on electrochemical glucose oxidase reactions to determine blood glucose levels [17]. A control algorithm system is employed for maintaining target blood glucose variables. The control strategies include (1) proportional, integral, derivative controller; (2) model predictive control; and (3) fuzzy logic [16].
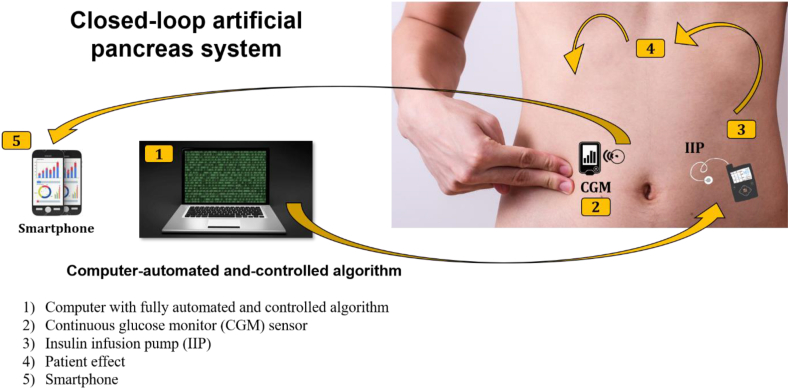
In general, the recent system consists of four main components that integrated each other as depicted in (Figure 1):
Figure 1.
Schematic implementation of closed-loop artificial pancreas systems. (1) A software installed in an external controller that obtains information from the CGM and executes a series of mathematical calculations. Then, the controller will send dosing instructions to the insulin infusion pump. (2) CGM worn subcutaneously delivers the data about interstitial glucose concentrations to a smartphone. (3) As the final step, the amounted insulin delivery is regulated in real-time by the control algorithm.
3.1. APD automated and controlled algorithm
The control algorithm is one of the vital components of APD. It regulates the correct insulin injection rate based on CGM's measured blood glucose level. The usage of a control algorithm could hinder the event of hypoglycemia and decrease time in hyperglycemia. The desired control algorithm should have the ability to overcome the problem in glucose-insulin dynamics, such as the delay associated with subcutaneous insulin infusion, time-varying dynamics, inter-individual variability, and strong non-linear phenomena [18]. Additionally, control algorithms have an essential role in closed-loop therapy by facilitating the personalization and adaptation of the AP system [19]. Several algorithms have been tested in-silico and clinically over time and improved over the years [20].
3.1.1. Model predictive control
The model predictive control (MPC) model utilizes the prediction model to predict the effects of proposed manipulated input changes on the desired output [21]. Note that MPC is not a single exact algorithm, it is instead composed of several algorithms tailored based on the desired goals. MPC is considered one of the most efficient control strategies developed in recent years. MPC could solve the problem in the presence of significant disturbances (i.e., meals and physical exercises) and delays in the effect of meals and insulin absorption [22]. In addition, MPC offers high flexibility in controlling parameters for each patient [23]. However, to utilize the MPC algorithm, one must carefully implement the mathematical models of glucose-insulin interaction. Several mathematical models have been proposed by researchers to be implemented in MPC. Generally, the models can be classified as linear and non-linear models. The non-linear model is viewed as a suitable mathematical model for glucose-insulin interaction due to this system shows a high non-linearity nature. However, the non-linear model is not applicable for portable APD as it demands high computational performance. Currently, the development of the MPC control algorithm tries to adapt customized linear models that can tailor to individual patient needs leading to better glucose control [24]. Messori et al. presented three different customized linear models which could improve glucose control performance, including the carbohydrate ratio (CR)-based model, non-parametric model, and constrained optimization model [24]. However, the proposed models were only tested in silico.
MPC has been successfully used in outpatient clinical studies and has shown promising results [25, 26, 27]. For instance, a multicenter study conducted by Brown et al. demonstrated that closed-loop control (CLC) utilizing the MPC model could improve glucose control with fewer hypoglycemia events overnight [27]. What is more, those beneficial CLC outcomes were also observed during the daytime. Furthermore, Messori et al. conducted the study using run-to-run (R2R) adaptive wearable AP under free-living conditions [26]. Here, they utilized the MPC linear model coupled with real-time corrections to the basal insulin and the meal bolus. The results show that the R2R-AP significantly improved glucose control of T1D patients during the night. Interestingly, the equivalent control performance was maintained during the day in a one-month trial under free-living conditions.
3.1.2. Proportional-integral-derivative
Proportional-integral-derivative (PID) control is based on the principle of controlling a single target quantity via a control-feedback calculated as a simple summation of the proportional, integral, and derivative term. In diabetes therapy, the PID algorithm adjusts insulin delivery by assessing glucose excursion from three perspectives, i.e., deviation from target glucose (proportional term), area under the curve between measured and target glucose (integral term), and the rate of change in measured glucose (derivative term) [28]. PID is known as the simplest way to design a robust control system for the patient with TD1. Pinsker et al. compared the performance between MPC and PID algorithm and concluded that MPC performs better than PID as it achieved nearly 75% in the target range, including the unannounced meal [29]. However, this finding was highlighted by Steil, in which he justified that the results of Pinsker et al. cannot be extended to include all available PID algorithms [30].
3.1.3. Fuzzy logic
Unlike MPC and PID, which rely on mathematical models in describing human glucoregulatory systems for interpretation of glucose data and insulin administration, fuzzy logic (FL) uses glucose management parameters to determine the insulin doses. The introduction of FL algorithm could give an alternative solution to the problem incorporating various physiological parameters such as illness and stress. As stated previously, FL relies on predetermined glucose parameters by an expert diabetes clinician. Three terms are tailored (glucose level, glucose slope or rate, and glucose acceleration) to cover all possible dosing situations that a person with diabetes would normally encounter [31]. From the study by Mauseth et al., using an FL controller could be an alternative to an MPC-based controller as a component of a closed-loop insulin delivery system [31].
Recent advanced closed-loop artificial pancreas system based on FL controller employs the novel “MD-Logic Artificial Pancreas algorithm” as a fully digitalized and advanced hybrid closed-loop system to control user glucose levels. It can change basal insulin and provide auto-correcting bolus in real-time conditions [32]. This process relies on the dynamics of the glucose sensor readings and the information from the insulin infusion pump. Furthermore, the effectiveness of the MD-Logic algorithm has been validated in many clinical studies. MD-Logic algorithm of advanced closed-loop artificial pancreas system is integrated into the Android and iOS platforms.
3.2. Continuous glucose monitor (CGM)
A wearable device that lets the users take a look at their glucose readings every single minute in real-time. CGM contains three main elements such as (1) sensor: a tiny wire inserted under the stomach's skin that measures blood glucose every minute; (2) receiver: display the obtained data from the sensor, and (3) transmitter: a wireless component of sensor that delivers blood glucose level to the receiver. Each CGMs have their calibration standard.
3.2.1. From self-blood glucose measurement to continuous glucose measurement
The discovery of insulin in the early 1920s brought a significant breakthrough in the therapy and the prognosis of diabetic patients. Insulin therapy has an integral part of diabetes treatment. Insulin plays a role in managing blood sugar and preventing diabetes complications by keeping blood sugar in the target range (normal or near-normal glycemia). It is known that the normal range of blood sugar ranges from 3.8-5.6 mmol/L. In comparison, hyperglycemia and hypoglycemia events occur when the blood sugar level up above 8 mmol/L and falls below values of 3 mmol/L, respectively [33]. Therefore, in order to keep the glucose level in the target range, researchers developed glucose sensing devices for monitoring and measuring glucose levels in physiological fluids both in-vivo and in-vitro. Besides achieving the glycemic target, glucose monitoring is also crucial for patients in (1) getting the proper treatment on symptoms, (2) assessing response to therapy, and (3) preventing or delaying the progression of complications in patients [34].
For monitoring and assessing their blood sugar at home, patients can use a self-monitoring of blood glucose (SBMG) device. By using SBMG, patients can adjust insulin doses for self-medication and detect hypoglycemia [35]. SBMG device measures patients' blood glucose levels with the assistance of conventional blood glucose meters to measure finger-prick blood samples. SBMG approach is known as a non-continuous method due to the measurement carried out only at a specific point during the day [36]. American diabetes association (ADA) recommends patients with diabetes type 1 to measure their blood glucose three times or more each day [37]. ADA also recommends diabetic patients to use SBMG as it could be a guide to successful therapy and to achieve postprandial glucose targets.
The need for obtaining a real-time blood sugar level and trend to predict a hyperglycemic and hypoglycemic event leads to the development of continuous glucose monitoring (CGM). Nowadays, CGM is becoming a major focus of research in diabetes management. Unlike SBMG, the CGM device has the capability to measure a blood sugar level every 5 min with 288 readings daily [34]. Thanks to CGM development, it can help patients and physicians see a blood sugar pattern and decide when a therapy adjustment is required. A typical CGM device consists of a sensor, electronic processing unit, and display unit. In CGM, a glucose-measuring sensor is placed under the skin in the subcutaneous tissue. Theoretically, the sensors can be placed in any part of the body, but the commonplace to put the sensors are the upper-back arm, the abdomen, or the upper-buttock area [38].
3.2.2. Overview of the developments in blood glucose sensors
The basic principle of current glucose meters is based on electrochemical measurement. Here, a small drop of blood is tested in the electrochemical strips to perform the measurement. Two standard methods are used in electrochemical measurements, i.e., amperometric and colorimetric [39]. Both approaches exploit enzyme strip tests for determining the glucose level. The former measured the electrical current produced from the reaction between glucose and enzyme. The latter measured the color change and intensity of the test strip when the blood sample was exposed to enzyme glucose oxidase [40]. Generally, a glucose monitoring system employs the interaction with the following enzymes: glucose oxidase (GOx), glucose dehydrogenase (GDH), and hexokinase [41, 42]. For self-monitoring purposes, the devices are usually constructed by using GOx and GDH. However, hexokinase is generally used in the laboratory setting as a reference glucose result [43]. This section will give an overview of electrochemical and non-electrochemical-based glucose monitoring devices.
In general, glucose biosensors can be classified into electrochemical-based and non-electrochemical-based biosensors. To date, electrochemical-based biosensors have been developed up to the fourth generation. In the first up to the third generation, GOx and GDH enzymes were mainly utilized as a redox center. In the fourth generation of biosensors, the non-enzymatic approach has been introduced. Here, the non-enzymatic approach could solve several problems faced by conventional sensors. The conventional enzyme-based electrochemical method is known to be susceptible to environmental factors, such as temperature, humidity, and pH value [44]. Several inorganic materials have been successfully employed for fourth-generation biosensors, including carbon nanotubes, reduced graphene oxide [45], graphene oxide [46], nickel microparticles [47], MXene [48], and platinum [49]. However, the critical concerns in realizing these materials as the future glucose sensors are related to the biocompatibility issue and ensuring the deposited materials grow uniformly [50].
Electrochemical-based sensors suffer instability and unsatisfactory accuracy. Therefore, researchers try to seek new solutions to overcome these issues. Non-electrochemical-based biosensors, for instance, optical measurement, were assessed as a promising method in glucose sensor development as it offers fast measurement (the data can be obtained in less than a minute) and is reagent-free [51]. Several optical technologies have been reported for glucose measurement, including near-infrared, mid-infrared, Raman, photoacoustic, fluorescence, and optical coherence tomography. Most optical methods employ light intensity change from nanomaterials or molecular recognition compounds where glucose can bind reversibly [52]. Optical sensors can be placed non-invasively or invasively, but such sensors need to be carefully constructed. Until now, only the fluorescence method has been employed in commercial CGMs. The fluorescence method is considered the most successful optical biosensor so far. Some problems related to miniaturization, cost, and improving signal-to-noise ratio need to be addressed in the future.
3.2.3. Commercial CGM in the market and the technology behind
The market of commercial CGM enjoys rapid growth of market share and it is expected to reach USD 10.36 billion by 2028 [53]. Such a high market development is owing to the capability of CGM to provide and keep track of glucose levels over a designated period. Currently, four companies have become major manufacturers of CGM, i.e., Abbott, Dexcom, Senseonic, and Medtronic. These companies offer different and unique technologies in respect to each other, aiming to give better glycemic control. Currently, in the US market, Abbott offers CGM with the name i.e., FreeStyle Libre and FreeStyle Libre 2. Dexcom introduced G5 mobile and G6, while Medtronic launched Guardian Sensor 3. Interestingly, unlike the other CGMs which use the electrochemical-based sensor, Eversense uses optical-sensors CGM with the market name i.e., Eversense XL.
Medtronic and Dexcom utilize the first-generation glucose sensor with GOx as an oxidoreductase. Dexcom uses two electrodes (working and reference), while Medtronic separates counter and reference electrodes into three electrodes. Abbott used a more sophisticated generation of biosensors i.e., second-generation glucose sensing. From the construction of the electrode, Abbots also uses a three-electrode system as we can see in Medtronic CGM. Here, the osmium complex is used as an electron mediator. One of the key advantages of this second-generation sensor is lower redox potential, making Abbott CGM less prone to interference substances compared to other CGM [54]. Senseonic constructs CGM with different sensing approaches. Instead of electrochemical-based measurement, the device uses an optical-based sensor. Here, the fluorescence method is used by utilizing the interaction between fluorophore-linked divalent boronic acid and glucose.
3.3. Insulin infusion pump (IIP)
Based on the instructions sent by the controller, the infusion pump adjusts insulin delivery to the subcutaneous tissue. IIP of advanced closed-loop artificial pancreas system uses rapid-acting insulin such as aspart, glulisine, and lispro since this IIP delivers small amounts of insulin every few minutes, thus the usage of longer-acting insulin is unnecessary.
3.4. Intervention effect
The intervention effect is defined as the effect of an advanced closed-loop artificial pancreas system on blood glucose levels. The main goal must be achieved that the blood glucose is controlled properly.
3.5. Smartphone
The smartphone acts as a data receiver from CGM that can display the entire blood glucose level and control the insulin dosing.
4. The main effect of the usage of the device
Controlling the blood glucose levels within 24 h a day as a target, preventing exercise-induced hypoglycemia, and preventing postprandial hyperglycemia are the main goals of using the ideal closed-loop APD [55]. The main effect of closed-loop APD can be classified into short-term and long-term effects:
4.1. Short-term effects
The ideal aim of closed-loop APD is to control meal-related hyperglycemia. However, there is also the biggest risk of the usage of this device which is hypoglycemia due to an over delivery of insulin, excessive physical exercise, and consumption of alcohol [56, 57]. Emergency carbohydrates can be consumed if hypoglycemia cannot be avoided by decreasing insulin delivery or increasing glucagon. Thus, it needs a reliable control algorithm to combine multiple hypoglycemia prevention procedures such as initiation of glucagon when the blood glucose reaches the threshold (50–90 mg/dL), suspension and control insulin delivery, and escalation of alarms to remind the user [58, 59]. However, a study suggested that the short-term effect of using the artificial pancreas system (combination of CGM, insulin pump, and controller) was reducing the HbA1c level by 0.6 percentage units without a possible increase in the patient's risk of developing hypoglycemia [60].
4.2. Long-term effects
The ultimate long-term benefit of the usage of closed-loop APD is to prevent diabetes-related complications and prolong the lifespan of individuals with T1D. The long-term complications are retinopathy, cardiovascular disease, and neuropathy. The result of the diabetes control and complication trial showed that a risk gradient per 1 SD (gradient risk) is higher than the HbA1c variable. For instance, a value of 1.3 for a complication of diabetes denotes that the risk elevates by 30% when the HbA1c variable increases by 1 SD [61]. A study conducted in Sweden showed that long-time use of CGM should be reimbursed in combination with an insulin pump if the level of HbA1c > 9.8% (poor glycemic control) or if ≥ 2 severe hypoglycemic events have occurred within 1 year to improve glycemic control in clinical practice (reported considerable non-severe hypoglycemic events in long-time users) [62].
5. Necessary tests to be conducted to determine the device's suitability of use
The effectiveness of closed-loop APD must be determined to achieve the main short-term and long-term goals. The effectiveness is affected by two major aspects, including the device specifications and the physiological condition of the users. Based on the device specifications, several key parameters need to be assessed, such as:
5.1. Device specifications
5.1.1. Accuracy and precision of CGM
The accuracy and precision of CGM are the essential parameters related to the reliability of measurement. The most recent available CGMs in the market can achieve the mean median absolute relative difference (MARD) of sensor <10%, which is compatible with closed-loop blood glucose control. Based on the in silico study, a sensor with MARD ≤10% can determine the decision of insulin dose accurately [63]. Proper calibration and rapid drift of sensor sensitivity also affect the accuracy of CGM. If these parameters are inappropriate, persistent deviations could have occurred, then insulin will be over-delivered, increasing the possibility of hypoglycemia [64].
To avoid hypoglycemia and hyperglycemia events, determining the blood glucose concentration of T1D patients is crucial. CGM is a novel approach to measuring glucose levels compared to conventional capillary blood glucose measurements. However, there are three challenges that we need to resolve to measure blood glucose concentration by CGM accurately, i.e., (1) uncertainty of CGM data due to noise disturbance, (2) CGM accuracy, and (3) time-lag in CGM sensing that may become longer than 10 min [65]. To deal with these CGM issues, various algorithms have been developed. For instance, Bazaev et al. developed an algorithm based on the sigma model or logistic function. The algorithm can predict the blood glucose levels 2 h in advance by utilizing the data from an invasive glucose meter as an initial blood glucose level [66]. Furthermore, Pérez-Gandía et al. proposed the usage of an artificial neural network (ANN) algorithm in conjunction with CGM data [67]. Here, patients' blood glucose level was predicted by using ANN by employing only historical CGM data as an input without the need to use an invasive method for processing the data. The results showed that the ANN algorithm gave a promising result without a significant prediction delay. However, this method has a limitation in predicting a sudden change in glucose levels, such as during meal-intake and physical exercise. Therefore, Facchinetti et al. proposed the smart sensor concept by introducing denoising, enhancement, and prediction module to CGM [68]. The denoising module could reduce the irregularity of CGM measurement and subsequently reduce the number of false hypoglycemic and hyperglycemic events. The enhancement module was shown might improve CGM accuracy. Finally, the prediction module allowed CGM to anticipate hypoglycemic and hyperglycemic events up to 15 min before they occur.
In addition, sensor fault detection and data reconciliation are needed to ensure the CGM sensor gives data with high quality and accuracy. Generally, sensor errors can be divided into two, i.e., hard errors (complete hardware failure) and soft errors (bias, drift, and outlier) [69]. In the case of hard errors, they can be solved by replacing new sensor components and recalibration. On the other hand, soft errors are handled by replacing the erroneous data with automatically estimated values. Hybrid online multi-sensor error detection and functional redundancy (HOMSED&FR) have been tested to overcome sensor errors and the results showed that the proposed method has successfully detected most of the errors and reconciled erroneous reading with the data close to the real value [70]. Here, the HOMSED&FR algorithm was constructed by combining the outlier Kalman filter and a locally-weighted partial least squares regression model. Furthermore, the more complex algorithm (smart multiple-model) for detecting CGM error was developed based on four models: Kalman filter, locally-weighted partial least squares regression model, predictor-based subspace identification, and approximate linear dependency-based kernel recursive least square [71]. In addition, an artificial neural network is used as a voting algorithm to integrate these four different models into one system. The results showed that the smart multiple-method could detect most CGM sensor faults such as missing signal, drift change, signal stuck, and pressure-induced sensor attenuation. At the same time, the smart-multiple method could reconcile the reading with model estimations that are closer to expected values.
5.1.2. Insulin absorption and delivery
Exogenous insulin from closed-loop APD needs to reach the maximum glucose-lowering capacity of approximately 1.5–2 h for subcutaneous administration. A high level of blood glucose has to be normalized during closed-loop delivery. The compatibility of insulin and glucagon also has to be determined in dual-hormone therapy to minimize the possibility of hypoglycemia [72]. Several models have been developed to assess insulin absorption and delivery. The observable glucose-insulin (OGI) dynamic model is one of them. This approach was used to analyze glucose regulation, glucose transport, and insulin absorption. This model features steps to personalize parameters in real-time and is proven to be able to analyze glucose dynamics in silico [73]. A combination of the Compartmental Model (CM) and Self Organizing Map (SOM) can also be applied for this purpose. Parameters measured included insulin infusion rate, the number of carbohydrates that had been digested, and sugar levels before the patient used the AP system. Measurements are based on glucose absorption and insulin kinetics simulated by previous glucose measurements. This method was successful in analyzing the metabolic behavior of patients with T1D [74]. Real-time state prediction of glucose and insulin levels can be done using Bergman's non-linear minimal model. This method observes conditions (plasma insulin) and parameters (glucose sensitivity) to obtain a dynamical model [75]. If you want to measure accurately, the plasma insulin concentration (PIC) estimator can be an option. This method combines the Kalman filtering algorithm and Hovorka's glucose-insulin approach. This combination model successfully predicts PIC levels in real-time based on the processing of clinical data containing significant disturbances [76].
5.1.3. Control algorithms
The most widely used control algorithms are model predictive control (MPC), proportional integral derivative (PID), and fuzzy logic (FL) controls. The controller must have the ability to handle mealtime insulin boluses in a proper term or manner. The algorithms must update the state vector when the user ingests a meal or when the insulin boluses are delivered. Thus, the bolus and meal change the predicted blood glucose levels and influence the optimum profile of insulin [77]. Bequette has critically assessed various control algorithms and concluded that MPC offers several advantages in controlling the glucose level of T1D patients owing to its flexibility to deal with many challenges in AP [78]. For instance, when a meal disturbance is detected, MPC could modify the desired glucose value (setpoint), rather than only being stuck at a constant setpoint. What is more, the MPC algorithm may also recognize that the increasing value of glucose absorption into the circulation will not continue indefinitely in the future. Thus, the insulin infusion can be decreased at any time for avoiding hypoglycemic events. Furthermore, MPC algorithm may be combined with a health monitoring system (HMS) and zone controller to prevent hypoglycemia events by sending a short and multimedia message service [79]. This system has been tested to control glucose levels upon unannounced meals and exercise. The clinical result was promising, however, there is still some limitation in this study, i.e., the meal size was very small. More importantly, in a closed-loop AP system, the degradation performance must also be carefully assessed. Usually, the degradation comes from model deficiencies, poor control design parameters, and inappropriate constraints [80]. Thus, the performance assessment of control algorithms is becoming critical. Recently, a control-performance assessment algorithm has been developed, which incorporates online learning to identify the behaviors and disturbance patterns from the historical data [81].
The adaptive control is a powerful technique for controlling the complexity and variability of the dynamics of blood glucose concentration over time. Several adaptive techniques are developed to overcome these most crucial parameters of AP including (1) Run-to-run (R2R): implementing a carbohydrate to insulin ratio (CR) during the day and a subcutaneous basal insulin delivery at night where the AP performance index is calculated based on data obtained from CGM [82]; (2) Minimum variance (MV) controller: reducing the deviation of the controlled signal around the desired value and can adapt to changes in system parameters. The initial experimental trial of an AP with a predictive MV controller was carried out by Pagurek et al. [83]. A systolic pump infused the glucose solution into a vein in one arm of the study subject and a volume of blood was drawn continuously from the other arm and monitored with an auto-analyzer to determine the glucose concentration; (3) Self-Tuning Regulator: This adaptive control is developed by Astrom and Wittenmark [84]. This controller is the extension of the MV control, which is better to improve the process of identifying unknown parameters in the initial MV model. Sarti et al. [85] developed and implemented a self-tuning control algorithm for 300 simulations of insulin concentration gain with different sampling time intervals where the results obtained were more realistic than other control methods.
Recently, Hajizadeh et al. utilized machine learning techniques and patient historical data to construct personalized multivariable multimodule artificial pancreas (PMM-AP) [86]. Here, machine learning is used to modify several vital parameters, such as controller-set points and the objective function weight. These parameters are adjusted in advance to avoid meal and exercise disturbance and importantly the parameters are defined adaptively to accommodate various situations faced by T1D patients. Here, the recursive subspace-based system identification is used in combination with PIC and CGM to give better dynamic behavior of glucose variation in the patient's body over wide time ranges. Furthermore, Li et al. constructed the control algorithm with an adaptive feedback controller to update the controller parameters [87]. Interestingly, adaptive control algorithms have been successfully applied to dual-hormone AP [88]. The simulation study on three virtual patients revealed that the MPC algorithm showed promising results within a daily variation in the model parameter. Furthermore, Resalat et al. introduced a new insulin sensitivity adaptation (ISA) algorithm and adaptive learning postprandial hypoglycemia prevention (ALPHA) algorithm [89]. By combining ISA and ALPHA into MPC, the control algorithm can be used to adapt to both non-meal and postprandial periods.
5.2. Physiological condition of the users
On the other hand, based on the user perspective (in this case, individuals with T1D) several parameters need to be evaluated such as:
5.2.1. Psychosocial assessment
The psychosocial assessment is also importantly conducted to investigate the main effect caused by the close-loop APD for the user. This assessment includes some variables such as acceptance and usage of APD, fear of hypoglycemia, and satisfaction level of APD and its treatment. These assessments can be conducted by employing the diabetes technology questionnaire and interviews with the user of closed-loop APD. The outcome of this evaluation is based on personal convenience, benefit, perceived usefulness, and perceived ease of closed-loop APD usage. A higher score can represent higher acceptance from the users [90]. Several approaches have been used to assess psychosocial factors in patients. Wristband Biosignals is a method that can be used to evaluate the presence of acute psychological stress (APS) in patients. This approach measures psychological stress signals using a classification algorithm that can distinguish physical activity (PA) from APS. The lower the APS number, the more acceptable AP systems are [91]. This approach has also been developed using machine learning which can estimate the intensity and type of APS and PA either simultaneously or individually [92].
5.2.2. Individual variability
Every individual with T1D has their inter-subject variability to factors affecting insulin sensitivity such as age, gender, body weight, body height, physical condition, and lifestyle (smoking and drinking alcohol). This can influence insulin absorption and action within users [93]. An adaptive control system in close-loop APD may be required for significant variability in insulin compensation. Various studies have been demonstrated the feasibility of adaptive control systems for normalizing glycemia levels from any initial glucose levels. For instance, Pinsker et al. conducted the study by employing personalized MPC as a control algorithm [29]. The result showed that the algorithm performed well even after challenging with a 65-g unannounced meal. The personalized MPC algorithm showed 74.4% mean time in the range 70–80 mg/dL (safe glucose range). Furthermore, Dassau et al. did the clinical evaluation of a personalized APD by using multiparametric MPC (mpMPC) [94]. Here, the fully automated APD based on mpMPC was coupled with insulin on board (IOB) as a safety algorithm. The calculation of IOB was used to prevent overdosing caused by the administration of previous insulin. The result showed that the fully automated APD had good glucose control 70% of the time in the near-normal range (80–180 mg/dL). Despite the results of glycaemic control facilitated by mpMPC-IOB showing promising results, there is a problem arising from IOB calculation for determining insulin concentration i.e., the diurnal variation in the insulin diffusion, absorption, and utilization. These parameters need to be carefully considered as there are important for the personalization of APD. As a breakthrough, Hajizadeh et al. used the estimated plasma insulin concentration (PIC) to design a predictive control algorithm that is dynamically constrained by previously estimated PIC and thus explicitly accounting for the insulin concentration in the bloodstream as part of the optimal control solution [95]. What is more, Hajizadeh et al. coupled APD with wearable devices to obtain physical activity signals. Therefore, personalized APD can prevent hyperglycemia after patients perform exercises [96].
5.2.3. Meal absorption and profile
The amount and size of food is an essential factors for AP systems. It is used to determine the appropriate insulin bolus dose [97]. Several approaches to meal detection in the AP have been developed. One of them is a fuzzy system using CGM, which estimates the amount of carbohydrate intake. This method calculates the number of food boluses based on the ratio of insulin and carbohydrate intake. This method allows the AP system to prevent the possibility of postprandial hyperglycemia in patients automatically [98]. The predictive control model (MPC) is another method that can be implemented. This model employs an estimation algorithm based on continuous observations of the first and second derivatives of glucose. This will generate an impulse to eat which will later be converted into grams of carbohydrates that can be measured [99]. Another method is the Unscented Kalman Filter. This approach measures the cross-covariance between the difference in the rate of disturbance and the measured glucose. This is done to analyze the sensitivity and the amount of carbohydrate intake in patients. This approach successfully detects this aspect of sensitivity [100].
A more comprehensive description of the necessary tests to be conducted to determine the device's effectiveness is described in the regulatory requirements section: APD system performance.
6. Regulatory requirements
The regulatory requirements for APD are based on the document of Guidance for Industry and Food and Drug Administration (FDA) Staff: The Content of Investigational Device Exemption (IDE) and Premarket Approval (PMA) Applications for Artificial Pancreas Device Systems by 2012. This guidance consists of background, scope, device description, indication for use, APDs performance, clinical study progression, labeling, manufacturing, and post-approval study. The FDA recommends the manufacturer provide these descriptions, including (1) system level; (2) functional components of glucose monitoring; (3) functional components control algorithm and signal processing; (4) functional components of the infusion pump, and (5) functional components of the communication pathway. FDA recommends all information about individual parts of the device should be provided. Requirements for current good manufacturing practice (GMP) that are included in Quality Systems (QS) regulations are also necessary. The FDA anticipates that most APD will require post-approval studies (PAS) to better assess long-term and real-world patient performance and/or outcomes. FDA recommends the PAS protocol be developed and be submitted the protocol with the original PMA [101].
7. Data about the market and competition for the device
Market and competition data of closed-loop APD is based on each structural part of the device, including (1) Insulin infusion pump (IIP) and (2) Continuous glucose monitoring (CGM) devices. There are three main types of IIP that work incorporation with CGM. In the first type, the CGM delivers the value of the glucose sensor, and then it will be displayed on the IIP. In this type, no insulin dosing is provided based on the value of CGM. In the second type, the CGM sends sensor glucose value to the IIP then it can suspend insulin when the blood value achieves the low threshold limit [102]. The last type is the hybrid closed-loop APD, where the CGM delivers sensor glucose values to the IIP, then it can decide insulin dosing on basal insulin delivery from the obtained values [103].
For many current years, the insulin infusion pump has been developed notably. Some of them, work as stand-alone insulin delivery devices without a CGM as an integrated medical device. Presently, there are three available hybrid closed-loop APDs available in the market. First, Medtronic plc commercializes the automated insulin delivery system with a hybrid closed-loop APD MiniMed™ 770G at a retail price is approximately USD 899. This price is applicable as an offer when the device is upgraded from the older Medtronic MiniMed™ 670G. For reference, the approved full price regulated by the ministry of health Canada is USD 6300 [104]. This insulin pump can be integrated with Guardian™ Sensor 3 CGM, and it has been approved by the FDA and is already available in the United States and several European countries, including Belgium, Denmark, Finland, Ireland, Italy, Netherlands, Spain, Sweden, Switzerland, and the United Kingdom [105]. The second company is Tandem® Diabetes Care with t: slim X2™ insulin pump. This insulin pump can be incorporated with Dexcom G5® or G6® CGM. This insulin pump can be obtained by upgrading the older version by USD 799 for the new condition and USD 399 for refurbished units [106]. Moreover, the full price of the new unit is the cost of USD 6300 [104]. The third hybrid APD available is developed by CamDiab with the market name CamAPS FX. CamAPS FX is an android app that can be integrated with pump and CGM. CamAPS is compatible with Dana Diabecare RS® pump and Dexcom G6® CGM. Unlike the two other APDs, CamAPS requires the user to pay for an app subscription. In summary, the market data for APD is shown in Table 1.
Table 1.
The available artificial pancreas devices in the market.
| The MiniMed™ 770G system | t:slim X2™ insulin pump with Control-IQ™ technology | CamAPS® FX with Dana Diabecare RS and Dana-i pump | |
|---|---|---|---|
| Manufacturer | Medtronic plc | Tandem® Diabetes Care | CamDiab |
| CGM system | Guardian™ 3 | Dexcom G6® | Dexcom G6® |
| Smartphone integration | Android and iOs | Android and iOs | Android |
| Price |
|
|
|
| Release year | September 2020 | January 2020 | 2020 |
| Reference | [107, 108] | [106, 108, 109] | [108, 109, 110] |
8. Future perspective
Improvements to the components of the closed-loop artificial pancreas system are likely to maximize performance and user comfort. The device workload is expected to be minimized with the development of the continuous glucose monitoring component with optimized accuracy, longer usage time, and fully integrated into insulin pump and smartphone (as a data receiver). In a recent development, for instance, the MD-Logic Artificial Pancreas algorithm (a fully digitalized and advanced hybrid closed-loop system) poses a promising performance in controlling user glucose levels and has been validated in clinical trials. A very fast-acting and accurate level of insulin is also needed to be fully automated based on the employed algorithms. A user-friendly interface of smartphone applications is required for the user and health care professionals to evaluate the benefits of using the device. Finally, cost-effectiveness studies are required for the health care system in taking reimbursement decisions regarding the use of this device.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author acknowledges the support of the Elsevier and Polish Consortium agreement for the publication.
References
- 1.International Diabetes Federation . 2019. IDF Diabetes Atlas Ninth Edition 2019. [PubMed] [Google Scholar]
- 2.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., Shaw J.E., Bright D., Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherrington A.D. Banting lecture 1997. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 5.Cinar A. Advances in artificial pancreas control systems. J. Process Control. 2019;81:221–222. doi: 10.1016/j.jprocont.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickup J.C., Keen H., Parsons J.A., Alberti K.G. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br. Med. J. 1978;1:204–207. doi: 10.1136/bmj.1.6107.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messer L.H., Berget C., Beatson C., Polsky S., Forlenza G.P. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol. Therapeut. 2018;20:S254–S264. doi: 10.1089/dia.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pala L., Dicembrini I., Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019;56:973–980. doi: 10.1007/s00592-019-01326-5. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg B.H. Patch pumps for insulin. J. Diabetes Sci. Technol. 2019;13:27–33. doi: 10.1177/1932296818786513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vettoretti M., Facchinetti A. Combining continuous glucose monitoring and insulin pumps to automatically tune the basal insulin infusion in diabetes therapy: a review. Biomed. Eng. Online. 2019;18:1–17. doi: 10.1186/s12938-019-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen N., Gupta A. Current diabetes technology: striving for the artificial pancreas. Diagnostics. 2019;9 doi: 10.3390/diagnostics9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago J.V., Clemens A.H., Clarke W.L., Kipnis D.M. Closed-loop and open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes. 1979;28:71–84. doi: 10.2337/diab.28.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Kadish A.H. Automation control OF blood sugar. I. A servomechanism for glucose monitoring and control. Am. J. Med. Electron. 1964;3:82–86. [PubMed] [Google Scholar]
- 14.Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care. 2015;38:1036–1043. doi: 10.2337/dc15-0364. [DOI] [PubMed] [Google Scholar]
- 15.Trevitt S., Simpson S., Wood A. Artificial pancreas device systems for the closed-loop control of type 1 diabetes: what systems are in development? J. Diabetes Sci. Technol. 2016;10:714–723. doi: 10.1177/1932296815617968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal R.A., Ekhlaspour L., Hood K., Buckingham B. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr. Rev. 2019;40:1521–1546. doi: 10.1210/er.2018-00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christiansen M.P., Klaff L.J., Brazg R., Chang A.R., Levy C.J., Lam D., Denham D.S., Atiee G., Bode B.W., Walters S.J., Kelley L., Bailey T.S. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: precise II. Diabetes Technol. Therapeut. 2018;20:197–206. doi: 10.1089/dia.2017.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toffanin C., Messori M., Di Palma F., De Nicolao G., Cobelli C., Magni L. 2013. Artificial Pancreas: Model Predictive Control Design from Clinical Experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thabit H., Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59:1795–1805. doi: 10.1007/s00125-016-4022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghorbani M., Bogdan P. 5th Work. Med. Cyber-Physical Syst., Schloss Dagstuhl-Leibniz-Zentrum Fuer Informatik. 2014. Challenges and opportunities in design of control algorithm for artificial pancreas. [Google Scholar]
- 21.Kushner T., Bequette B.W., Cameron F., Forlenza G., Maahs D., Sankaranarayanan S. Autom. Reason. Syst. Biol. Med. Springer; 2019. Models, devices, properties, and verification of artificial pancreas systems; pp. 93–131. [Google Scholar]
- 22.Allgöwer F., Zheng A. Birkhäuser; 2012. Nonlinear Model Predictive Control. [Google Scholar]
- 23.Mehmood S., Ahmad I., Arif H., Ammara U.E., Majeed A. Artificial pancreas control strategies used for type 1 diabetes control and treatment: a comprehensive analysis. Appl. Syst. Innov. 2020;3:31. [Google Scholar]
- 24.Messori M., Incremona G.P., Cobelli C., Magni L. Individualized model predictive control for the artificial pancreas: in silico evaluation of closed-loop glucose control, IEEE Control Syst. What Mag. 2018;38:86–104. [Google Scholar]
- 25.Incremona G.P., Messori M., Toffanin C., Cobelli C., Magni L. Model predictive control with integral action for artificial pancreas. Control Eng. Pract. 2018;77:86–94. [Google Scholar]
- 26.Messori M., Kropff J., Del Favero S., Place J., Visentin R., Calore R., Toffanin C., Di Palma F., Lanzola G., Farret A. Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol. Therapeut. 2017;19:560–571. [Google Scholar]
- 27.Brown S.A., Breton M.D., Anderson S.M., Kollar L., Keith-Hynes P., Levy C.J., Lam D.W., Levister C., Baysal N., Kudva Y.C. Overnight closed-loop control improves glycemic control in a multicenter study of adults with type 1 diabetes. J. Clin. Endocrinol. Metab. 2017;102:3674–3682. doi: 10.1210/jc.2017-00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boughton C.K., Hovorka R. New closed-loop insulin systems. Diabetologia. 2021;1–9 doi: 10.1007/s00125-021-05391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinsker J.E., Lee J.B., Dassau E., Seborg D.E., Bradley P.K., Gondhalekar R., Bevier W.C., Huyett L., Zisser H.C., Doyle F.J. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39:1135–1142. doi: 10.2337/dc15-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steil G.M. Comment on Pinsker et al. Randomized Crossover Comparison of Personalized MPC and PID Control Algorithms for the Artificial Pancreas. Diabetes Care. 2016;39:1135–1142. doi: 10.2337/dc15-2344. Diabetes Care. 40 (2017) e3–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauseth R., Hirsch I.B., Bollyky J., Kircher R., Matheson D., Sanda S., Greenbaum C. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol. Therapeut. 2013;15:628–633. doi: 10.1089/dia.2013.0036. [DOI] [PubMed] [Google Scholar]
- 32.Atlas E., Nimri R., Miller S., Grunberg E.A., Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072–1076. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch S.M., Bequette B.W. Proc. IEEE 27th Annu. Northeast Bioeng. Conf. (Cat. No. 01CH37201) IEEE; 2001. Estimation-based model predictive control of blood glucose in type I diabetics: a simulation study; pp. 79–80. [Google Scholar]
- 34.Funtanilla V.D., Caliendo T., Hilas O. Continuous glucose monitoring: a review of available systems. Pharmacol. Ther. 2019;44:550. [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk J.K., Stegner J. Self-monitoring of blood glucose: practical aspects. J. Diabetes Sci. Technol. 2010;4:435–439. doi: 10.1177/193229681000400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villena Gonzales W., Mobashsher A.T., Abbosh A. The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors. 2019;19:800. doi: 10.3390/s19040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal A., Gupta Y., Singla R., Kalra S., Tandon N. American diabetes association “standards of medical care—2020 for gestational diabetes mellitus”: a critical Appraisal. Diabetes Ther. 2020;11:1639–1644. doi: 10.1007/s13300-020-00865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maltoni G., Zucchini S. Glucose Sens. Use Child. Adolesc. Springer; 2020. Different types of continuous glucose monitoring systems on the market; pp. 13–34. [Google Scholar]
- 39.Dalvi N. Glucose meter reference design. Appl. Note. 2013 [Google Scholar]
- 40.Khandpur R.S. 3 Volume Set. John Wiley & Sons; 2020. Compendium of Biomedical Instrumentation. [Google Scholar]
- 41.Price C.P. 2003. Point-of-care Testing in Diabetes Mellitus. [DOI] [PubMed] [Google Scholar]
- 42.D’costa E.J., Higgins I.J., Turner A.P.F. Quinoprotein glucose dehydrogenase and its application in an amperometric glucose sensor. Biosensors. 1986;2:71–87. doi: 10.1016/0265-928x(86)80011-6. [DOI] [PubMed] [Google Scholar]
- 43.Carta M., Giavarina D., Paternoster A., Bonetti G. Glucose meters: what’s the laboratory reference glucose? J. Med. Biochem. 2020;39:32. doi: 10.2478/jomb-2019-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang D.-W., Lee S., Seo M., Chung T.D. Recent advances in electrochemical non-enzymatic glucose sensors–a review. Anal. Chim. Acta. 2018;1033:1–34. doi: 10.1016/j.aca.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 45.Xuan X., Kim J.Y., Hui X., Das P.S., Yoon H.S., Park J.-Y. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018;120:160–167. doi: 10.1016/j.bios.2018.07.071. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Wang Y., Jia J., Wang J. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sensor. Actuator. B Chem. 2012;171:580–587. [Google Scholar]
- 47.Toghill K.E., Compton R.G. Electrochemical non-enzymatic glucose sensors: a perspective and an evaluation. Int. J. Electrochem. Sci. 2010;5:1246–1301. [Google Scholar]
- 48.Li M., Fang L., Zhou H., Wu F., Lu Y., Luo H., Zhang Y., Hu B. Three-dimensional porous MXene/NiCo-LDH composite for high performance non-enzymatic glucose sensor. Appl. Surf. Sci. 2019;495 [Google Scholar]
- 49.Park S., Chung T.D., Kim H.C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003;75:3046–3049. doi: 10.1021/ac0263465. [DOI] [PubMed] [Google Scholar]
- 50.Yoon H., Xuan X., Jeong S., Park J.Y., Wearable robust, non-enzymatic continuous glucose monitoring system and its in vivo investigation. Biosens. Bioelectron. 2018;117:267–275. doi: 10.1016/j.bios.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Jernelv I.L., Milenko K., Fuglerud S.S., Hjelme D.R., Ellingsen R., Aksnes A. A review of optical methods for continuous glucose monitoring. Appl. Spectrosc. Rev. 2019;54:543–572. [Google Scholar]
- 52.Klonoff D.C. Overview of fluorescence glucose sensing: a technology with a bright future. J. Diabetes Sci. Technol. 2012;6:1242–1250. doi: 10.1177/193229681200600602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grand View Research, Continuous Glucose Monitoring Device Market Worth $10.36 Billion By 2028., (n.d.).
- 54.Lee I., Probst D., Klonoff D., Sode K. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 2021;181 doi: 10.1016/j.bios.2021.113054. [DOI] [PubMed] [Google Scholar]
- 55.Steil G.M., Rebrin K., Darwin C., Hariri F., Saad M.F. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz J.L., Sherr J.L., Cengiz E., Carria L., Roy A., Voskanyan G., Tamborlane W.V., Weinzimer S.A. Effect of insulin feedback on closed-loop glucose control: a crossover study. J. Diabetes Sci. Technol. 2012;6:1123–1130. doi: 10.1177/193229681200600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steil G.M., Palerm C.C., Kurtz N., Voskanyan G., Roy A., Paz S., Kandeel F.R. The effect of insulin feedback on closed loop glucose control. J. Clin. Endocrinol. Metab. 2011;96:1402–1408. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shivers J.P., Mackowiak L., Anhalt H., Zisser H. Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J. Diabetes Sci. Technol. 2013;7:789–794. doi: 10.1177/193229681300700324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doyle F.J., Huyett L.M., Lee J.B., Zisser H.C., Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191–1197. doi: 10.2337/dc13-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergenstal R.M., V Tamborlane W., Ahmann A., Buse J.B., Dailey G., Davis S.N., Joyce C., Peoples T., Perkins B.A., Welsh J.B., Willi S.M., Wood M.A. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N. Engl. J. Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 61.Lind M., Odén A., Fahlén M., Eliasson B. The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLoS One. 2009;4:1–6. doi: 10.1371/journal.pone.0004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson J., Attvall S., Sternemalm L., Pivodic A., Fahlén M., Hanås R., Ekeroth G., Lind M. Effect on glycemic control by short- and long-term use of continuous glucose monitoring in clinical practice. J. Diabetes Sci. Technol. 2011;5:1472–1479. doi: 10.1177/193229681100500622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovatchev B.P., Patek S.D., Ortiz E.A., Breton M.D. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol. Therapeut. 2015;17:177–186. doi: 10.1089/dia.2014.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thabit H., Hovorka R. Closed-loop insulin delivery in type 1 diabetes, Endocrinol. Metab. Clin. North Am. 2012;41:105–117. doi: 10.1016/j.ecl.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moser E.G., Crew L.B., Garg S.K. Role of continuous glucose monitoring in diabetes management. Av. En Diabetol. 2010;26:73–78. [Google Scholar]
- 66.Bazaev N.A. In: Blood Glucose Prediction for “Artificial Pancreas” System. Zhang K.V.P.E.-W., editor. IntechOpen; Rijeka: 2017. Ch. 5. [Google Scholar]
- 67.Pérez-Gandía C., Facchinetti A., Sparacino G., Cobelli C., Gómez E.J., Rigla M., de Leiva A., Hernando M.E. Artificial neural network algorithm for online glucose prediction from continuous glucose monitoring. Diabetes Technol. Therapeut. 2010;12:81–88. doi: 10.1089/dia.2009.0076. [DOI] [PubMed] [Google Scholar]
- 68.Facchinetti A., Sparacino G., Guerra S., Luijf Y.M., DeVries J.H., Mader J.K., Ellmerer M., Benesch C., Heinemann L., Bruttomesso D., Avogaro A., Cobelli C. On behalf of the A. Consortium, real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793–800. doi: 10.2337/dc12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McIntosh I.B.D. The University of Wisconsin; Madison: 2000. A Model-Based Fault Detection and Diagnosis Methodology for HVAC Subsystems.https://www.proquest.com/dissertations-theses/model-based-fault-detection-diagnosis-methodology/docview/304657037/se-2?accountid=27375 [Google Scholar]
- 70.Feng J., Hajizadeh I., Samadi S., Sevil M., Hobbs N., Brandt R., Lazaro C., Maloney Z., Yu X., Littlejohn E., Quinn L., Cinar A. Hybrid online multi-sensor error detection and functional redundancy for artificial pancreas control systems. IFAC-PapersOnLine. 2018;51:138–143. [Google Scholar]
- 71.Feng J., Hajizadeh I., Yu X., Rashid M., Samadi S., Sevil M., Hobbs N., Brandt R., Lazaro C., Maloney Z., Littlejohn E., Cinar A. Multi-model sensor fault detection and data reconciliation: a case study with glucose concentration sensors for diabetes. AIChE J. 2019;65:629–639. doi: 10.1002/aic.16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellingsen C., Dassau E., Zisser H., Grosman B., Percival M.W., Jovanovič L., Doyle F.J. Safety constraints in an artificial pancreatic β cell: an implementation of model predictive control with insulin on board. J. Diabetes Sci. Technol. 2009;3:536–544. doi: 10.1177/193229680900300319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W., Wang S., Geng Y., Qiao Y., Wu T. An OGI model for personalized estimation of glucose and insulin concentration in plasma. Math. Biosci. Eng. 2021;18:8499–8523. doi: 10.3934/mbe.2021420. [DOI] [PubMed] [Google Scholar]
- 74.Zarkogianni K., Litsa E., Vazeou A., Nikita K.S. 13th IEEE Int. Conf. Bioinforma. Bioeng. IEEE BIBE 2013; 2013. Personalized glucose-insulin metabolism model based on self-organizing maps for patients with type 1 diabetes mellitus. [Google Scholar]
- 75.Eberle C., Ament C. Real-time state estimation and long-term model adaptation: a two-sided approach toward personalized diagnosis of glucose and insulin levels. J. Diabetes Sci. Technol. 2012;6:1148–1158. doi: 10.1177/193229681200600520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hajizadeh I., Rashid M., Samadi S., Feng J., Sevil M., Hobbs N., Lazaro C., Maloney Z., Brandt R., Yu X., Turksoy K., Littlejohn E., Cengiz E., Cinar A. Adaptive and personalized plasma insulin concentration estimation for artificial pancreas systems. J. Diabetes Sci. Technol. 2018;12:639–649. doi: 10.1177/1932296818763959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brooker G. The artificial pancreas. Handb. Biomechatron. 2018:405–456. [Google Scholar]
- 78.Bequette B.W. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol. Therapeut. 2005;7:28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 79.Harvey R.A., Dassau E., Bevier W.C., Seborg D.E., Jovanovič L., Doyle F.J., III, Zisser H.C. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol. Therapeut. 2014;16:348–357. doi: 10.1089/dia.2013.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Z., Qin S.J., Singhal A., Megan L. Performance monitoring of model-predictive controllers via model residual assessment. J. Process Control. 2013;23:473–482. [Google Scholar]
- 81.Hajizadeh I., Samadi S., Sevil M., Rashid M., Cinar A. Performance assessment and modification of an adaptive model predictive control for automated insulin delivery by a multivariable artificial pancreas. Ind. Eng. Chem. Res. 2019;58:11506–11520. [Google Scholar]
- 82.Toffanin C., Visentin R., Messori M., Di Palma F., Magni L., Cobelli C. Toward a run-to-run adaptive artificial pancreas: in silico results. IEEE Trans. Biomed. Eng. 2018;65:479–488. doi: 10.1109/TBME.2017.2652062. [DOI] [PubMed] [Google Scholar]
- 83.Pagurek B., Riordon J.S.I., Mahmoud S. Adaptive control OF the human glucose-regulatory system. Med. Biol. Eng. 1972;10:752–761. doi: 10.1007/BF02477386. [DOI] [PubMed] [Google Scholar]
- 84.Åström K.J., Wittenmark B. On self tuning regulators. Automatica. 1973;9:185–199. [Google Scholar]
- 85.Sarti E., Cruciani P., Fabietti P.G. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS. Vol. 6. 1992. Self-tuning control algorithm for wearable artificial pancreas; pp. 2267–2269. [Google Scholar]
- 86.Hajizadeh I., Askari M.R., Sevil M., Hobbs N., Brandt R., Rashid M., Cinar A. In: Chapter 3 – Adaptive Control of Artificial Pancreas Systems for Treatment of Type 1 Diabetes. Boubaker B.E., editor. Academic Press; 2020. pp. 63–81. O.B.T.-C. T. [Google Scholar]
- 87.Li S., Tao G. 2008 7th World Congr. Intell. Control Autom. IEEE; 2008. Adaptive feedback control based artificial pancreas; pp. 2186–2191. [Google Scholar]
- 88.Boiroux D., Bátora V., Hagdrup M., Wendt S.L., Poulsen N.K., Madsen H., Jørgensen J.B. Adaptive model predictive control for a dual-hormone artificial pancreas. J. Process Control. 2018;68:105–117. [Google Scholar]
- 89.Resalat N., Hilts W., El Youssef J., Tyler N., Castle J.R., Jacobs P.G. Adaptive control of an artificial pancreas using model identification, adaptive postprandial insulin delivery, and heart rate and accelerometry as control inputs. J. Diabetes Sci. Technol. 2019;13:1044–1053. doi: 10.1177/1932296819881467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnard K.D., Hood K.K., Weissberg-Benchell J., Aldred C., Oliver N., Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their Applicability in AP research. Diabetes Technol. Therapeut. 2015;17:295–300. doi: 10.1089/dia.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sevil M., Rashid M., Hajizadeh I., Askari M., Hobbs N., Brandt R., Park M., Quinn L., Cinar A. Discrimination of simultaneous psychological and physical stressors using wristband biosignals. Comput. Methods Progr. Biomed. 2021;199 doi: 10.1016/j.cmpb.2020.105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sevil M., Rashid M., Hajizadeh I., Park M., Quinn L., Cinar A. Physical activity and psychological stress detection and assessment of their effects on glucose concentration predictions in diabetes management. IEEE Trans. Biomed. Eng. 2021;68:2251–2260. doi: 10.1109/TBME.2020.3049109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Riazi A., Pickup J., Bradley C. Daily stress and glycaemic control in type 1 diabetes: individual differences in magnitude, direction, and timing of stress-reactivity. Diabetes Res. Clin. Pract. 2004;66:237–244. doi: 10.1016/j.diabres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Dassau E., Zisser H., Harvey R.A., Percival M.W., Grosman B., Bevier W., Atlas E., Miller S., Nimri R., Jovanovič L. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36:801–809. doi: 10.2337/dc12-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hajizadeh I., Rashid M., Samadi S., Sevil M., Hobbs N., Brandt R., Cinar A. Adaptive personalized multivariable artificial pancreas using plasma insulin estimates. J. Process Control. 2019;80:26–40. [Google Scholar]
- 96.Hajizadeh I., Rashid M., Turksoy K., Samadi S., Feng J., Sevil M., Hobbs N., Lazaro C., Maloney Z., Littlejohn E., Cinar A. Incorporating unannounced meals and exercise in adaptive learning of personalized models for multivariable artificial pancreas systems. J. Diabetes Sci. Technol. 2018;12:953–966. doi: 10.1177/1932296818789951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samadi S., Turksoy K., Hajizadeh I., Feng J., Sevil M., Cinar A. Meal detection and carbohydrate estimation using continuous glucose sensor data. IEEE J. Biomed. Heal. Informatics. 2017;21:619–627. doi: 10.1109/JBHI.2017.2677953. [DOI] [PubMed] [Google Scholar]
- 98.Samadi S., Rashid M., Turksoy K., Feng J., Hajizadeh I., Hobbs N., Lazaro C., Sevil M., Littlejohn E., Cinar A. Automatic detection and estimation of unannounced meals for multivariable artificial pancreas system. Diabetes Technol. Therapeut. 2018;20:235–246. doi: 10.1089/dia.2017.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee H., Buckingham B.A., Wilson D.M., Bequette B.W. A closed-loop artificial pancreas using model predictive control and a sliding meal size estimator. J. Diabetes Sci. Technol. 2009;3:1082–1090. doi: 10.1177/193229680900300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramkissoon C.M., Herrero P., Bondia J., Vehi J. Unannounced meals in the artificial pancreas: detection using continuous glucose monitoring. Sensors. 2018;18:1–18. doi: 10.3390/s18030884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.FDA, Guidance for Industry and Food and Drug Administration Staff The content of investigational device exemption and Premarket approval applications for artificial pancreas device systems; Availability. Fed. Regist. 2012;77:70168–70169. [Google Scholar]
- 102.Forlenza G.P., Li Z., Buckingham B.A., Pinsker J.E., Cengiz E., Wadwa R.P., Ekhlaspour L., Church M.M., Weinzimer S.A., Jost E., Marcal T., Andre C., Carria L., Swanson V., Lum J.W., Kollman C., Woodall W., Beck R.W. Predictive low-glucose suspend reduces hypoglycemia in adults, Adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. PLGS Reduces Hypoglycemia PROLOG Trial. 2018:1–7. doi: 10.2337/dc18-0771. [DOI] [PubMed] [Google Scholar]
- 103.Saunders A., Messer L.H., Forlenza G.P. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: overview of its safety and efficacy. Expet Rev. Med. Dev. 2019;16:845–853. doi: 10.1080/17434440.2019.1670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ministry of health Canada . 2021. Insulin Pump/Supply Product Manual. [Google Scholar]
- 105.McDermott J., Levine Brian, Brown A. 2018. FDA Approves Medtronic MiniMed 670G Hybrid Closed Loop for 7–13 Year Olds. [Google Scholar]
- 106.Serino M., Brown A. Dexcom G5 CGM; 2017. FDA Approves Tandem’s T:slim X2 Pump with Integrated. [Google Scholar]
- 107.Medtronic, MiniMed 770G Pathway Program, (n.d.). https://www.medtronic.com/ca-en/diabetes/home/c/pathway.html (accessed December 22, 2021).
- 108.diabetesnet Comparison of Current Continuous Glucose Monitors (CGMs) https://www.diabetesnet.com/diabetes-technology/meters-monitors/compare-current-monitors/ (n.d.) (accessed December 22, 2021)
- 109.Brown G. 2018. FDA APPROVES DEXCOM G6. [Google Scholar]
- 110.Medical C. 2020. DANA Diabecare RS Insulin Pump Kit. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.