Abstract
Stimulation of Mycobacterium tuberculosis-primed lymph node cells from C57BL/6 mice with α antigen (also known as antigen 85B and MPT59) induced cell proliferation, production of interleukin 2 and gamma interferon, and expansion of Vβ11+ CD4+ T cells in conjunction with antigen-presenting cells in an I-Ab-restricted manner. Using a series of 15-amino-acid peptides that overlapped each other by 5 amino acids and spanned the mature α antigen, we identified the antigenic epitope for α antigen-specific Vβ11+ Th1 cells. That peptide (peptide-25), which corresponds to amino acid residues 240 to 254 of α antigen, contains a motif that is conserved in I-Ab and requires processing by antigen-presenting cells. Using peptide-25-reactive Vβ11+ T-cell clones and substituted peptide-25 mutants, we determined which amino acid residues within peptide-25 were critical for T-cell receptor (TCR) recognition. Our results showed that the amino acid residues at positions 245, 246, 248, 250, and 251 are important for recognition of TCRVβ11 and that residues at positions 244, 247, 249, and 252 are I-Ab contact residues. We also observed that active immunization of C57BL/6 mice with peptide-25 can lead to decreased bacterial load in the lungs of M. tuberculosis H37Rv-infected mice. These results should provide us with a useful tool for delineating the regulation of Vβ11+ Th1-cell development during M. tuberculosis infection and for developing a vaccine inducing a Th1-dominant immune response.
The interaction between specifically sensitized T cells and activated macrophage effector cells is considered to be the hallmark of protective immunity against the pathogenic mycobacteria Mycobacterium tuberculosis and Mycobacterium leprae (4, 7). Also, mice infected with live Mycobacterium bovis BCG predominantly exhibit a Th1 cytokine secretion profile, although some susceptible mouse strains activate interleukin 4 (IL-4)-producing Th2 cells to some extent (18, 20). Gamma interferon (IFN-γ), which has powerful immunomodulatory and macrophage-activating properties (14, 25, 29), also plays an essential role in the protective immunity against these intracellular pathogens (34); this was convincingly demonstrated in recent experiments using IFN-γ and IFN-γ receptor gene-disrupted mice (10, 11, 14).
The specific antigens eliciting protective T-cell responses are not yet known for tuberculosis, but considerable numbers of proteins have been identified by using both classical purification methods and, especially, recombinant DNA technology (1, 33, 51, 52). One of the antigens secreted from M. tuberculosis is α antigen, which is also known as MPT59 or antigen 85B (Ag85B). This protein has shown itself to be one of the most potent antigen species yet purified. Our major interests are to understand how Th1 cells develop after infection or immunization with M. tuberculosis, to characterize antigenic epitopes for Th1-cell activation, and to determine the efficacy of DNA vaccination against tuberculosis.
It has previously been shown that α antigen stimulation of M. tuberculosis-primed lymph node cells from C57BL/6 (B6) mice induced cell proliferation, production of IL-2 and IFN-γ, and expansion of Vβ11+ CD4+ Th cells in conjunction with antigen-presenting cells (APCs) in an I-Ab-restricted manner (54). In contrast, lymph node cells from nonprimed mice failed to proliferate in response to α antigen. We also mapped the antigenic epitope for α antigen-specific Vβ11+ T cells. That peptide (peptide-25), which corresponds to amino acid residues 240 to 254 of α antigen, contains a motif that is conserved in I-Ab and requires processing by APCs to trigger peptide-25-specific Vβ11+ CD4+ T cells.
The purpose of this study was twofold: first, we wanted to evaluate the feasibility of peptide-25 as a vaccine against M. tuberculosis H37Rv, and second, we wanted to determine which amino acid residues of peptide-25 were critical for T-cell receptor (TCR) recognition and for major histocompatibility complex (MHC) class II binding. Our results revealed that peptide-25 acts to some extent as a protective vehicle against live H37Rv infection. Peptide-25 contains distinct amino acid residues critical for TCR recognition and for MHC class II binding.
MATERIALS AND METHODS
Mice.
B6 mice were obtained from Japan SLC Inc. (Hamamatsu, Japan) and used when they were 8 to 12 weeks of age. The mice were maintained in the animal facility at the Institute of Medical Science, University of Tokyo, under strict pathogen-free conditions; laboratory chow and water were available ad libitum.
Antigens and reagents.
M. tuberculosis α antigen was purified as previously described (28) and used at selected concentrations. Peptide-25 and its substitution mutants were synthesized by Sawaday Chemicals (Tokyo, Japan) by using 9-fluorenylmethoxycarbonyl chemical strategies and an automated Applied Biosystems (Foster City, Calif.) 430A peptide synthesizer. Peptides were purified via high-pressure liquid chromatography, and composition analysis was carried out. Mouse IL-2 was purified from conditioned medium of phorbol myristate acetate-stimulated EL-4 cells as previously described (39) by using immunoaffinity chromatography and S4B6, an anti-IL-2 monoclonal antibody (MAb) (26).
MAbs.
The following MAbs were used: RL172.4 (8) and GK1.5 (12) (American Type Culture Collection [ATCC], Manassas, Va.), which recognize CD4; RA3-6B2 (9) (ATCC), which recognizes B220; 53-6.72 (23) and 3.155 (40) (ATCC), which recognize CD8; and 2.4G2 (47) (ATCC), which recognizes FcγR. In addition, M5/114.15.2 (anti-I-Ab) (5) and 28-16-8S (anti-I-Ab) (35) were kindly provided by Toshinori Nakayama (University of Tokyo). B20.6 (anti-Vβ2) (50), 44-22-1 (anti-Vβ6) (3), and F23.1 (anti-Vβ8) (43) were kindly provided by Yasunobu Yoshikai (Nagoya University, Nagoya, Japan). KJ-25 (anti-Vβ3) (36), KT4 (anti-Vβ4) (45), B21.5 (anti-Vβ10) (30), MR11-1 (anti-Vβ12) (49), MR12-3 (anti-Vβ13), and 14-2 (anti-Vβ14) (44) were purchased from PharMingen (San Diego, Calif.). MR9-4 (anti-Vβ5) (22), MR10.2 (anti-Vβ9) (48), and RR3-15 (anti-Vβ11) (6) were kindly provided by Osami Kanagawa (Washington University, St. Louis, Mo.) and Hiromitsu Nakauchi (Tsukuba University, Tsukuba, Japan). Phycoerythrin-labeled streptavidin was purchased from GIBCO BRL (Grand Island, N.Y.).
The following anticytokine MAbs were used: 11B11 (32), which neutralizes IL-4 and was kindly provided by William E. Paul (National Institutes of Health, Bethesda, Md.); BVD6-24G2 (2) (PharMingen), which recognizes IL-4; RA4-6A2 (PharMingen), which neutralizes IFN-γ; and XMG1.2 (anti-IFN-γ) (27), which was kindly provided by Maureen Howard (DNAX Research Institute, Palo Alto, Calif.).
Immunization.
Mice were immunized by subcutaneous injection at the base of the tail with 500 μg of acetone powder containing heat-killed M. tuberculosis H37Rv in paraffin oil or 10 μg of purified protein derivative (PPD) in incomplete Freund’s adjuvant (ICFA) (39, 42, 54). In some experiments, B6 mice were immunized with 1 to 10 μg of peptide-25 or its mutants with substitutions in ICFA and then boosted 7 days before experiments with 10 μg of the antigen.
Bacterial load in lungs and livers of M. tuberculosis H37Rv-challenged mice.
Mice were infected intravenously in the lateral tail vein with 106 CFU of M. tuberculosis H37Rv or 2 × 106 CFU of M. bovis BCG (Paris strain), whose α antigen contains an amino acid sequence identical to that of peptide-25 (53), and were sacrificed at various time points. To release intracellular bacteria, lungs and livers of M. tuberculosis H37Rv- or BCG-infected mice were homogenized in distilled water. Serial 10-fold dilutions were then made, and 100 μl of each was spread onto Middlebrook 7H10 Bacto-Agar (Difco, Detroit, Mich.) plates or 1% Ogawa Agar (Eiken Co., Tokyo, Japan) plates to quantitate bacterial load. The number of CFU per lung and per liver was measured as previously described but with slight modification (19). The cultures were incubated for 21 days at 37°C; thereafter, mycobacterium colony formation was assessed.
Cell culture.
Suspensions of single cells were prepared from inguinal lymph nodes of B6 mice. For proliferation assays, cells (5 × 105) were cultured in 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark) in the presence of selected concentrations of α antigen or synthetic peptides. Each well contained 200 μl of complete medium consisting of RPMI 1640 medium (Sigma) supplemented with 8% fetal calf serum, penicillin (100 IU/ml), streptomycin (50 μg/ml), and 5 × 10−5 M 2-mercaptoethanol. Cultures were set up in triplicate. The cells were pulse-labeled with [3H]thymidine (1 μCi/well) during the last 8 h of a 72-h culture period; incorporation of radioactivity was measured by tritium-sensitive avalanche gas ionization detection on a Matrix 96 direct beta counter (Packard, Meriden, Conn.) (54). The background counts per minute was obtained by culturing cells without any stimulation. We show representative results from a series of three separate experiments. For determining cytokine production, cells were cultured for either 72 h (IFN-γ or IL-4) or 24 h (IL-2).
For fluorescence-activated cell sorter analysis, cells (5 × 106) were cultured for 96 h in the presence of antigens or synthetic peptides in 24-well flat-bottom microtiter plates (Nunc). Each well contained 2 ml of complete medium. Cells were harvested after 4 days and washed, and 106 cells from each well were used for fluorescence-activated cell sorter analysis.
Cytokine quantitation assays.
IL-2 production was determined by bioassay by using an IL-2-dependent mouse cytotoxic T-cell line, CTLL-2 (54). A unit of IL-2 activity was defined as the reciprocal of the dilution yielding one-third of the maximal proliferation induced by a standard IL-2 preparation. IFN-γ and IL-4 production was measured by enzyme-linked immunosorbent assay as described previously (46, 54). Standard curves were generated, and the concentration of each cytokine was determined by using the computer program SOFTmax. We detected 1 U of IFN-γ per ml.
Establishment of cloned T cells.
α antigen- and peptide-25-reactive T-cell clones were established according to a previously described procedure (39) with slight modification. Briefly, lymph node cells (5 × 105/well) from B6 mice previously immunized and boosted with PPD or M. tuberculosis were stimulated with either PPD (5 μg/ml) or α antigen (1 μg/ml) and maintained for 7 days in a 24-well plate. Cells were then restimulated with the relevant antigen; irradiated spleen cells (2 × 106/ml) from B6 mice were also added as APCs, and culture was continued. Surviving cells were then stimulated again with antigen plus APCs. This procedure was repeated three to four times with 7-day intervals in between. Cloned T-cell lines were obtained by replating cells in 96-well microplates with one cell in each well in the presence of APCs. These cells were cultured in the presence of antigen plus APCs; 5% culture supernatant from concanavalin A-stimulated rat spleen cells was also added as an IL-2 source.
Flow cytometry.
Expression of cell surface antigens, particularly TCRVβ, before and after culture was analyzed by flow cytometry. Suspensions of single cells prepared from inguinal lymph nodes or collected from culture were stained with fluorescein isothiocyanate-labeled anti-CD4 MAb and biotinylated anti-Vβ MAbs plus streptavidin-phycoerythrin (GIBCO BRL) as described previously (54). 2.4G2 MAb (10 μg/ml) was added during the incubation to block nonspecific binding of the labeled MAbs. Fluorescence intensity was measured on a FACScan analyzer (Becton Dickinson). The gate on the lymphoid population was set by forward and side scatters. To assess particular Vβ+ T cells, we calculated the percentage of Vβ+ cells from the total CD4+ cells.
I-Ab-peptide binding assays.
I-Ab-peptide binding assays were carried out according to a previously described procedure (16) with slight modification. Aliquots (50 μl) of immunoaffinity purified I-Ab molecule (0.5 μM) were incubated for 48 h at room temperature in 1.5-ml Eppendorf tubes with 1.7 μM biotinylated 46F50E54A (mutant peptide of pigeon cytochrome c p43-58, AEGFSYTEANKAKGIT) and nonbiotinylated competitor peptides. Protein A (10 μg of phosphate-buffered saline [PBS] per ml; Wako Pure Chemicals, Osaka, Japan) was immobilized on 96-well polystyrene microtiter plates (Sumitomo Bakelite Co. Ltd., Osaka, Japan) by incubation at room temperature overnight. The ascites form of anti-I-Ab MAb (1:10 dilution) was then applied to protein A-coated plates and incubated for 24 h at 4°C. After washing the plates with PBS containing 0.05% Tween 20 (Bio-Rad, Hercules, Calif.), the preincubated I-Ab-peptide mixtures were added and incubated for 3 h at room temperature. After washing again with Tween-PBS, streptavidin-alkaline phosphatase (1:500 dilution in PBS containing 0.05% Tween 20 and 1% bovine serum albumin) was added to the plates, and the preparation was incubated for 2 h at room temperature. Thereafter, 1 mg of p-nitrophenol phosphate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) per ml of diethanolamine buffer was added to the plates, and after washing with Tween-PBS, the absorbance of each well was read at 405 nm.
The affinity with which test peptides were bound to I-Ab was expressed in terms of their ability to competitively inhibit binding of biotinylated 46F50E54A peptide. The relative binding affinity was calculated as the ratio of the concentrations (millimolar) of unlabeled 46F50E54A and unlabeled test peptides, each of which inhibits to the same degree the binding of the biotinylated standard. In practice, the following peptides and concentrations were used: I-Ab, 0.5 mM; biotinylated 46F50E54A peptide, 1.7 mM; unlabeled 46F50E54A peptide, 0.0214 to 214 mM; and unlabeled test peptides, 24 mM. All assays were carried out in duplicates.
RESULTS
Peptide-25 of α antigen induces peptide-specific Vβ11+ T-cell development in vivo.
We first evaluated whether peptide-25 is immunogenic in vivo. Two groups (n = 5) of B6 mice were immunized with either peptide-25 or α antigen emulsified in ICFA. As a control, B6 mice were immunized with S35 peptide, which is the leader peptide of α antigen (Fig. 1) (53). Seven days after immunization, peptide-25-induced proliferation and IFN-γ production by lymph node cells were examined; the results clearly showed that both peptide-25 and α antigen are immunogenic in vivo (Table 1). Lymph node cells from B6 mice immunized with either peptide-25 or α antigen responded to the respective antigen with cell proliferation and IFN-γ production, whereas S35-immunized mice did not respond to S35. The magnitudes of the proliferative responses were dose dependent, and the responses reached a plateau at a peptide-25 concentration of 100 ng/ml (data not shown). In addition, CD4+ T cells expanded by stimulation with peptide-25 and α antigen were Vβ11+ (54) (data not shown).
FIG. 1.
Amino acid sequence of α antigen and the overlapping peptides used for its analysis. The 40-amino-acid putative signal sequence is represented in italic letters.
TABLE 1.
Peptide-25-induced proliferation and IFN-γ production in peptide-25-primed lymph node cellsa
| Priming antigen | Stimulatory antigen or medium | [3H]TdR uptake (mean cpm ± SD) | IFN-γ production (U/ml) |
|---|---|---|---|
| α antigen | RPMI 1640 | 2,645 ± 110 | <1.0 |
| α antigen | 17,375 ± 1,456 | 9.0 | |
| Peptide-25 | 26,469 ± 932 | 13 | |
| Peptide-25 | RPMI 1640 | 1,179 ± 38 | <1.0 |
| Peptide-25 | 29,226 ± 2,726 | 340 | |
| S35 | RPMI 1640 | 718 ± 93 | <1.0 |
| S35 | 822 ± 254 | <1.0 |
Lymph node cells from α antigen-, peptide-25-, or S35-primed B6 mice were cultured with 1 μg of α antigen or peptide-25 per ml or with 10 μg of S35 per ml. Cultures were set up in triplicate. After 3 days, cell proliferation was assessed by [3H]thymidine ([3H]TdR) uptake and the concentrations of IFN-γ in culture supernatants were determined. Representative results of a series of three independent experiments are shown. Results are expressed as the means for triplicate cultures.
Mice immunized with peptide-25 are resistant to a subsequent challenge with M. tuberculosis H37Rv.
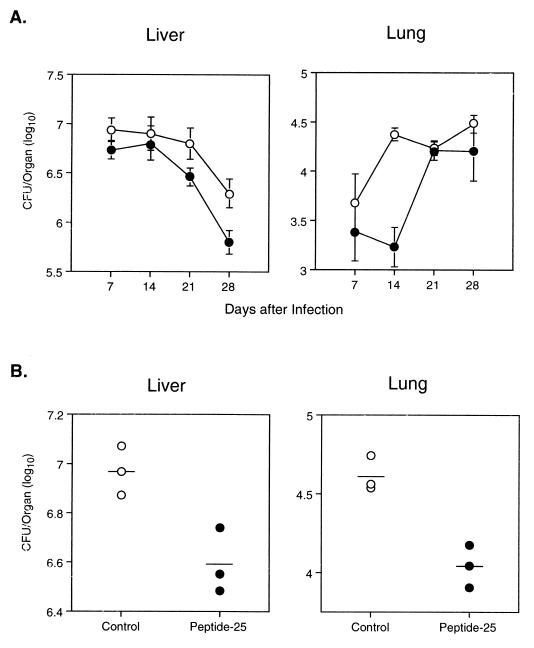
To determine whether potent peptide-25-specific immune responses would protect mice against subsequent challenges with viable M. tuberculosis H37Rv infection, we immunized a group of B6 mice with peptide-25 and boosted them 6 weeks later. The immunized mice were then challenged by intravenous injection of 106 CFU of M. tuberculosis H37Rv 7 days after the booster injection. A group of nonimmunized, age-matched B6 mice were also infected with 106 CFU of M. tuberculosis H37Rv. The CFU of M. tuberculosis H37Rv per lung or per liver were enumerated 1, 7, 14, 21, and 28 days after infection (5 mice per group per time point). On day 1 of infection, no significant difference of CFU per lung or per liver between the nonimmunized and the peptide-25-immunized groups was observed (data not shown). Interestingly, as shown in Fig. 2A, the number of CFU per lung on days 7 and 14 in mice immunized and boosted with peptide-25 was significantly lower (about 1/10) (P < 0.04) than that in nonimmunized mice. No significant difference of CFU per lung between the nonimmunized group and the group immunized with peptide-25 was observed on days 21 and 28 of infection. The number of CFU per liver was also decreased in mice immunized with peptide-25 on days 14, 21, and 28 of infection, but this was not statistically significant (Fig. 2B). We repeated the experiments three times and obtained essentially identical results. In another experiment, we examined CFU per liver and per lung 14 days after infection. As shown in Fig. 2B, the number of CFU per lung in peptide-25-immunized mice (4.04 ± 0.02 log) was again significantly lower (P < 0.02) than that in nonimmunized mice (4.61 ± 0.01 log). Furthermore, the number of CFU per liver in mice immunized with peptide-25 (6.59 ± 0.13 log) was lower than that in nonimmunized mice (7.00 ± 0.10 log) (P < 0.02). We did not see significant differences of CFU in spleens between the immunized mice and the control mice (data not shown). Thus, active immunization with peptide-25 induces marginal protection against subsequent infection with live M. tuberculosis H37Rv and a delay of the early expansion of bacterial burden.
FIG. 2.
Protective effect of peptide-25 immunization against M. tuberculosis H37Rv infection. Mice were immunized and boosted with peptide-25 in ICFA. The immunized mice and a group of nonimmunized B6 mice were intravenously infected with 106 CFU of M. tuberculosis H37Rv. The number of live H37Rv CFU in the lungs and livers of mice on the indicated days after infection (A) and 14 days after infection (B) are shown. Results are expressed as H37Rv CFU per lung and per liver in nonimmunized (○) and peptide-25-primed (●) mice (five mice per group per time point).
Establishment of peptide-25-reactive T-cell clones.
To define which peptide-25 amino acid residues are critical for Vβ11+ CD4+ T cells, lymph node cells from either M. tuberculosis-primed or PPD-primed B6 mice were cultured with PPD or α antigen, and T-cell clones were established according to procedures described in Materials and Methods. We established 15 different CD4+ T-cell clones, 12 of which expressed Vβ11. Other clones expressed Vβ14, Vβ10, and Vβ5.
Using a series of synthetic 15-amino-acid peptides, which overlapped each other by 5 amino acids and spanned the entire 285-amino-acid sequence of the mature α antigen protein, we determined the peptide specificity of the cloned T cells. Each clone was stimulated with each of the test peptides at a concentration of 1 μg/ml in the presence of irradiated B6 spleen cells as APCs, and proliferative responses were monitored. All clones expressing Vβ11 responded exclusively to peptide-25 (Table 2). The magnitude of the effect of peptide-25 was dose dependent: 5 ng of peptide-25 per ml induced significant proliferative responses and <100 ng of peptide per ml elicited the plateau level of proliferative response. In contrast, stimulation with as much as 10 μg of other synthetic peptides per ml had no effect on T-cell proliferation. Peptide-25-specific clones produced IFN-γ and IL-2 upon stimulation with peptide-25, but they did not produce IL-4 (data not shown). The clone expressing Vβ14 also responded to peptide-25, while clones expressing Vβ10 and Vβ5 did not. These results confirm our previous studies (54) showing that peptide-25 is the potent antigenic epitope of α antigen, preferentially stimulating Vβ11+ α antigen-reactive Th1 cells.
TABLE 2.
Proliferation of cloned T cells in response to α antigen and peptide-25a
| Clone | TCRVβ | [3H]TdR uptake when the following stimulatory antigen or medium was used
|
||
|---|---|---|---|---|
| RPMI 1640 | α antigen | Peptide-25 | ||
| BP1 | Vβ11 | 230 ± 177 | 47,500 ± 3,220 | 66,400 ± 6,630 |
| BP2 | Vβ11 | 69 ± 21 | 39,500 ± 4,050 | 21,600 ± 1,660 |
| BP4 | Vβ11 | 587 ± 59 | 40,700 ± 4,510 | 12,400 ± 951 |
| BM5 | Vβ11 | 52 ± 28 | 16,600 ± 3,200 | 42,200 ± 3,700 |
| BM2 | Vβ14 | 51 ± 16 | 3,200 ± 840 | 25,000 ± 3,780 |
| BP5 | Vβ5 | 89 ± 44 | 11,400 ± 885 | 50 ± 11 |
| BP7 | Vβ10 | 55 ± 11 | 30,700 ± 1,700 | 73 ± 27 |
Cloned T cells (2 × 104) were cultured in the presence of 1 μg of α antigen or peptide-25 per ml of medium. Cultures were set up in triplicate. After 3 days, proliferative responses were assessed by [3H]thymidine ([3H]TdR) uptake. Representative results of a series of three independent experiments are shown. Results are expressed as means of triplicate cultures.
Amino acid residues of peptide-25 critical for TCR recognition and MHC binding.
Peptide-25, which consists of amino acids 240 to 254 of α antigen contains a motif (Y-x-x-x-x-x-x-x-A) similar to the I-Ab motif (F-x-x-x-x-x-x-x-A) described by Itoh et al. (21). To examine the role played by individual amino acid residues in peptide-25-evoked activation of T cells, we analyzed the proliferative response of T-cell clones to a group of peptide-25 mutants that each contained a single amino acid substitution. Although the clones displayed varying patterns of reactivity to the substituted peptides, some general features of the T-cell responses were identified (Fig. 3). No T-cell clones responded to N245A mutant peptides. Other amino acid residues important to the majority of T-cell clones were 244 and 246 to 251; substitution of any one of these residues significantly reduced the capacity of peptide-25 to stimulate proliferation of cloned T cells. IFN-γ secretion induced by peptide-25 and its substitution mutants generally paralleled the proliferative responses (data not shown). Based on these results, we conclude that amino acids 244 to 252 of peptide-25 are critical for its recognition by reactive T-cell clones expressing TCRVβ11.
FIG. 3.
Features of peptide-25-reactive T-cell clones. Cloned T cells (2 × 104/well) were cultured with 1 μg of peptide-25 or various peptide-25 substitution mutants per ml in the presence of irradiated B6 spleen cells as APCs for 72 h. Proliferative responses were measured as a function of [3H]thymidine incorporation. (A) The stimulation index (SI) was calculated as the ratio of counts per minute obtained in the presence and in the absence of peptide. +, SI was >10; −, SI was <1. (B) Results are expressed as the frequency of critical residues and were calculated by dividing the number of clones responsive to a mutant peptide by the total number of clones.
Using peptide-25 substitution mutants as antigens to prime peptide-25-reactive lymph node T cells, we determined which amino acids served as T-cell or MHC contact residues by comparing proliferative responses elicited in vitro. Residues required for T-cell recognition would be expected to induce a unique population of T cells with slight cross-reactivity to peptide-25 (13, 31). We immunized mice with either peptide-25 or one of the substituted mutants and evaluated proliferative responses to either the priming peptide or peptide-25. Results obtained by using substitution mutants altered at positions 243 to 254 are shown in Fig. 4. The mutants with N245A, A246K, G248S, H250A, and N251A substitutions primed lymph node T cells with slight reactivity to peptide-25. In contrast, the peptides with Y244D, A247K, G249K, and A252R substitutions failed to generate any T-cell response either to them or to peptide-25. Taken collectively, amino acid residues at positions 245, 246, 248, 250, and 251 of peptide-25 should be important for TCR; whereas the residues at positions 244, 247, 249, and 252 may be MHC contact residues, since the relevant substituted peptides bind weakly to I-Ab (see below).
FIG. 4.
Ability of peptide-25 and substituted peptide-25 mutants to prime lymph node cells in vivo. B6 mice were immunized with 1 μg of peptide-25 or 1 μg of the substituted peptide-25 mutants. Lymph node cells (5 × 105) from the immunized mice were stimulated in vitro with 1 μg of either priming peptide ( ) or peptide-25 (
) or peptide-25 ( ) or were left unstimulated (□); their proliferative responses were assessed as a function of [3H]thymidine incorporation. Results are expressed as mean counts per minute ± SD of triplicate cultures.
) or were left unstimulated (□); their proliferative responses were assessed as a function of [3H]thymidine incorporation. Results are expressed as mean counts per minute ± SD of triplicate cultures.
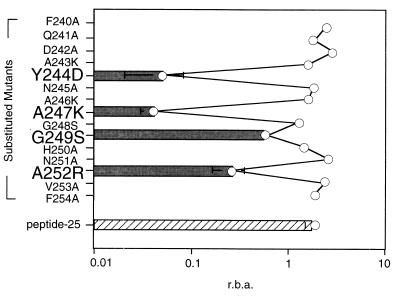
It has been shown that substitution of particular amino acids can alter a peptide’s affinity for MHC. We therefore estimated the relative binding affinity of peptide-25 and its substituted mutants for the I-Ab molecule by using a competitive binding inhibition assay (Fig. 5) (16). Results were expressed in terms of relative binding affinity and revealed that substituted mutants Y244D, A247K, and A252R possessed a significantly lower affinity for purified I-Ab than did peptide-25.
FIG. 5.
Relative binding affinity of substituted peptide-25 mutants. The binding affinity of peptide-25 and its mutants with substitutions was measured according to procedures described in Materials and Methods and is expressed as relative binding affinity (r.b.a.).
DISCUSSION
We have demonstrated that stimulation of M. tuberculosis-primed CD4+ Vβ11+ T cells from B6 mice with mycobacterium α antigen induces cell proliferation and production of IFN-γ and IL-2 (54). MHC-restricted interaction between α antigen-reactive T cells and irradiated spleen cells as APCs is required for the response. The major antigenic epitope of α antigen for Vβ11+ Th1 cells in M. tuberculosis-primed mice is mapped as peptide-25 (amino acids 240 to 254). Neither α antigen nor peptide-25 is a superantigen (54). In this study, we were able to extend our previous findings and provide a more detailed analysis of the amino acid residues of peptide-25 that are critical for TCRVβ11 and MHC class II binding with peptide-25-reactive Th1 clones. Peptide-25 was immunogenic in vivo and induced preferential generation of Vβ11+ Th1 cells. We also showed the efficacy of peptide-25 immunization in protection against a subsequent challenge with live M. tuberculosis H37Rv.
Over the past several years, a number of studies have attempted to define the T-cell determinants of various antigens; common structures or specific alignment of amino acids within peptides (motifs) is required for binding to certain MHC molecules (37). In contrast to MHC class I-bound peptides, demonstration of a distinct, specific motif among peptides binding to MHC class II molecules has proved difficult. Nonetheless, some motifs for particular MHC class II molecules have emerged. For instance, it is known that antigenic peptides comprising 10 to 12 amino acid residues are able to bind MHC class II molecules. As was previously described (54), peptide-25 consists of 15 amino acids and 11 amino acid residues at positions 244 to 252 that are sufficient to stimulate peptide-25-reactive T-cell clones expressing TCRVβ11. This was confirmed by using a deletion mutant of peptide-25 [s-peptide-25 (AA243-253)] that retains the capacity to stimulate peptide-25-reactive T-cell clones and is able to prime s-peptide-25-reactive T cells in vivo (data not shown).
Peptide-25 contained a motif (Y-x-x-x-x-x-x-x-A) similar to the I-Ab motif (F-x-x-x-x-x-x-x-A) described by Itoh et al. (21). To examine the anchoring role of Y at position 244, we made either an alanine or an aspartic acid substitution at this position (Y244A or Y244D). Y244 of peptide-25 was important for recognizing the MHC class II antigen. Evidence of this is the comparative insensitivity of peptide-25-reactive Vβ11+ T-cell clones to Y244D-substituted peptides: their effects were less than 20% that of peptide-25 and were detectable only at higher concentrations (>10 μg/ml) (Fig. 3). It has been demonstrated that there is a core sequence (P1 through P9) with nine amino acids for the interaction between the antigenic peptide and MHC class II molecule, in which P1 is the most conserved and the first anchoring amino acid interacting with the HLA-DR or mouse I-E molecule (38). Recently, it was also shown that P4 through P9, in particular P4, but not P1 are able to interact with mouse I-Ak and I-Ad molecules (15, 41). In these studies, we observed that the responsiveness of peptide-25-reactive T-cell clones to mutants of peptide-25 (Y244D and A247K) at positions P1 and P4 was significantly lower than that to peptide-25. In contrast, the cells responded similarly to A252R, a mutant of peptide-25 at position 252 (P9), as they did to peptide-25. Also, a binding assay revealed that A252R bound to the I-Ab molecule better than Y244D and A247K. P1 through P4 of peptide-25 may be important in I-Ab binding, and P9 might not be involved in the binding.
Mycobacteria has long been recognized for showing powerful immunologic adjuvant activity that augments both cell-mediated and humoral immune responses. A study of the mechanisms of pertussis and mycobacterial adjuvants showed that adjuvants induced soluble mediators—later determined to be cytokines—in T cells mediating the augmentation of antibody responses (24). It is possible that the strong immunogenicity of peptide stimuli contributes to the unique adjuvant activity of mycobacteria by providing an initial burst of cytokines favorable to the expansion of the T cells required for both antibody production and cell-mediated immunity. In this regard, it is interesting that immunization of B6 mice with 0.1 μg of peptide-25 (amino acids 240 to 254) or s-peptide-25 (amino acids 243 to 253) in ICFA induces the development of Vβ11+ T cells. Indeed, our recent studies showed that 1 μg of peptide-25 in solution was sufficient to induce the development of peptide-25-reactive Vβ11+ Th1 cells in vivo.
It is worthwhile to test the feasibility of peptide-25 as an immunogen to protect live M. tuberculosis infection. Our experiments using M. tuberculosis H37Rv revealed that fewer CFU per lung in the immunized mice are observed early following challenge (days 7 and 14) but not later (days 21 and 28) (Fig. 2). Immunization of B6 mice with peptide-25 also induced a decreased number of CFU per lung 14 days after BCG infection (data not shown). Although results were variable from experiment to experiment, the differences in the bacterial burden in the liver between the immunized mice and the control mice never exceeded more than 0.5 log at any time point. Only on day 28 after M. tuberculosis H37Rv infection was the number of CFU per liver in immunized mice significantly decreased compared with that in control mice. In the spleen, there was no significant difference between the two groups. This may be due to the fact that immunization might decrease initial infection in the lungs but does not inhibit growth of the organisms implanted in the lungs. This may not be the case, though, because the number of CFU per lung at day 1 of infection in peptide-25-immunized mice was similar to that in nonimmunized mice (3.85 ± 0.23 log versus 3.73 ± 0.15 log). Because it is apparent that CD4+ T-cell responses are required for immunity to mycobacterium infection, peptide-25 immunization may not be sufficient to induce full immunity, as reported recently for the Listeria infection system. IL-12, IL-18, and tumor necrosis factor alpha may enhance the priming effect of peptide-25 on Th1 development during the process of inducing antituberculosis immunity. We may have to apply a more efficient regimen by employing a potent vehicle or by applying a different immunizing route for peptide-25 to induce potent protective immunity against M. tuberculosis H37Rv.
We infer from the data presented above that immunization with peptide-25 does activate CD4 T cells that can participate in the antituberculosis response but that these T cells, at least on their own, are not fully protective. How may our results be extended to the human situation, since the T-cell epitope of α antigen is useful in I-Ab mice? As discussed above, P1 and P4 of a core sequence with nine antigenic amino acids for T-cell stimulation appear to be important for binding to HLA-DR. In our analysis, P1 and P4 of peptide-25 are important for mouse MHC class II binding. So, we believe that our results might be extended to the human situation by using a combination of peptide-25 antigens, each of which has amino acid substitutions at P1 and P4 that bind to the HLA-DR molecule. Further studies are needed to prove this hypothesis.
Recently, Huygen and his colleagues (19) found that a vaccination with plasmid DNA containing M. tuberculosis genes encoding hsp65, the 38-kDa PstS-1 homologue, and the Ag85 complex, which includes Ag85B, is an effective means of inducing protective immunity in animal models. Our data enhance the likelihood of using peptide-25 as a vaccine conferring antituberculosis immunity in humans.
In conclusion, the T-cell epitope mapping performed here shows that peptide-25 of the mycobacterium α antigen is able to prime and stimulate IFN-γ-producing Th1 cells in vivo. Synthetic peptide-25 is not a mycobacterial superantigen, and it may contribute to pathogenesis by inducing the local release of a spectrum of cytokines from α antigen-reactive T cells bearing Vβ11-encoded mouse TCRs. These observations underscore the immunodominant and potentially protective character of α antigen protein. Vaccination experiments with defined peptides are now needed to further elucidate the potential role of α antigen and peptide-25 in protective immunity against mycobacteria.
ACKNOWLEDGMENTS
We thank M. Howard, H. Nariuchi, H. Nakauchi, W. E. Paul, Y. Yoshikai, and O. Kanagawa for providing MAbs and S. Takaki and Y. Kikuchi for their valuable suggestions throughout this study. We are indebted to Williams F. Goldman and T. Kinashi for their critical review of this paper.
This study was supported in part by a grant-in-aid, a special grant for advanced research on molecular pathogenesis and immunointervention of immune disorders, from the Ministry of Education, Science, Sports and Culture of Japan and by research funds from the Meiji Milk Product Co. Ltd.
REFERENCES
- 1.Abou-Zeid C, Ratliff T L, Wiker H G, Harboe M, Bennedsen J, Rook G A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams J S, Roncarolo M G, Yssel H, Andersson U, Gleich G J, Silver J E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 3.Acha-Orbea H, Zinkernagel R M, Hengartner H. Cytotoxic T cell clone-specific monoclonal antibodies used to select clonotypic antigen-specific cytotoxic T cells. Eur J Immunol. 1985;15:31–36. doi: 10.1002/eji.1830150107. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P F, Abrams J S, Lu S, Sieling P A, Rea T H, Modlin R L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya A, Dorf M E, Springer T A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 6.Bill J, Kanagawa O, Woodland D L, Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of Vβ11-bearing T cells. J Exp Med. 1989;169:1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom B R, Murray C J. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 8.Ceredig R, Lowenthal J W, Nabholz M, MacDonald H R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 9.Coffman R L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 12.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 13.Evavold B D, Williams S G, Hsu B L, Buus S, Allen P M. Complete dissection of the Hb(64-76) determinant using T helper 1, T helper 2 clones, and T cell hybridomas. J Immunol. 1992;148:347–353. [PubMed] [Google Scholar]
- 14.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fremont D H, Monnaie D, Nelson C A, Hendrickson W A, Unanue E R. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 16.Fujisao S, Nishimura Y, Matsushita S. Evaluation of peptide-HLA binding by an enzyme-linked assay and its application to the detailed peptide motifs for HLA-DR9 (DRB1*0901) J Immunol Methods. 1997;201:157–163. doi: 10.1016/s0022-1759(96)00234-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 20.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, et al. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh Y, Ogasawara K, Takami K, Gotohda T, Naruse H, Good R A, Onoe K. Determination of amino acids on agretopes of pigeon cytochrome c-related peptides specifically bound to I-A allelic products. Eur J Immunol. 1994;24:76–83. doi: 10.1002/eji.1830240113. [DOI] [PubMed] [Google Scholar]
- 22.Kanagawa O, Utsunomiya Y, Bill J, Palmer E, Moore M W, Carbone F R. Conformational difference of T cell antigen receptors revealed by monoclonal antibodies to mouse Vβ5 T cell receptor for antigen determinants. J Immunol. 1991;147:1307–1314. [PubMed] [Google Scholar]
- 23.Ledbetter J A, Herzenberg L A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 24.Maillard J, Bloom B R. Immunological adjuvants and the mechanism of cell cooperation. J Exp Med. 1972;136:185–190. doi: 10.1084/jem.136.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonough K A, Kress Y, Bloom B R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. . (Erratum, 61:4021–4024.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 27.Mosmann T R, Schumacher J H, Fiorentino D F, Leverah J, Moore K W, Bond M W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- 28.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan C F, Murray H W, Wiebe M E, Rubin B Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Necker A, Rebai N, Matthes M, Jouvin-Marche E, Cazenave P A, Swarnworawong P, Palmer E, MacDonald H R, Malissen B. Monoclonal antibodies raised against engineered soluble mouse T cell receptors and specific for VαI8-, Vβ2- or Vβ10-bearing T cells. Eur J Immunol. 1991;21:3035–3040. doi: 10.1002/eji.1830211220. [DOI] [PubMed] [Google Scholar]
- 31.Ogasawara K, Maloy W L, Beverly B, Schwartz R H. Functional analysis of the antigenic structure of a minor T cell determinant from pigeon cytochrome C. Evidence against an alpha-helical conformation. J Immunol. 1989;142:1448–1456. [PubMed] [Google Scholar]
- 32.Ohara J, Paul W E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 33.Ohmen J D, Barnes P F, Grisso C L, Bloom B R, Modlin R L. Evidence for a superantigen in human tuberculosis. Immunity. 1994;1:35–43. doi: 10.1016/1074-7613(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 34.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 35.Ozato K, Sachs D H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981;126:317–321. [PubMed] [Google Scholar]
- 36.Pullen A M, Marrack P, Kappler J W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988;335:796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- 37.Rammensee H G, Falk K, Rotzschke O. MHC molecules as peptide receptors. Curr Opin Immunol. 1993;5:35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- 38.Reizis B, Eisenstein M, Mor F, Cohen I R. The peptide-binding strategy of the MHC class II I-A molecules. Immunol Today. 1998;19:212–216. doi: 10.1016/s0167-5699(97)01238-3. [DOI] [PubMed] [Google Scholar]
- 39.Sano Y, Yamada G, Dobashi K, Mizuochi T, Hamaoka T, Takatsu K. Establishment of three PPD-reactive helper T cell clones with distinct functions in B cell activation. J Immunol. 1984;133:629–635. [PubMed] [Google Scholar]
- 40.Sarmiento M, Glasebrook A L, Fitch F W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 41.Scott C A, Peterson P A, Teyton L, Wilson I A. Crystal structures of two I-Ad-peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity. 1998;8:319–329. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]
- 42.Shinomiya Y, Harada M, Kurosawa S, Okamoto T, Terao H, Matsuzaki G, Shiraakusa T, Nomoto K. Anti-metastatic activity induced by the in vivo activation of purified protein derivative (PPD)-recognizing Th1 type CD4+ T cells. Immunobiology. 1995;193:439–455. doi: 10.1016/s0171-2985(11)80429-8. [DOI] [PubMed] [Google Scholar]
- 43.Staerz U D, Rammensee H G, Benedetto J D, Bevan M J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985;134:3994–4000. [PubMed] [Google Scholar]
- 44.Sugihara S, Fujiwara H, Shearer G M. Autoimmune thyroiditis induced in mice depleted of particular T cell subsets. Characterization of thyroiditis-inducing T cell lines and clones derived from thyroid lesions. J Immunol. 1993;150:683–694. [PubMed] [Google Scholar]
- 45.Tomonari K, Lovering E, Spencer S. Correlation between the Vβ4+ CD8+ T-cell population and the H-2d haplotype. Immunogenetics. 1990;31:333–339. doi: 10.1007/BF02115007. [DOI] [PubMed] [Google Scholar]
- 46.Uehara S, Hitoshi Y, Numata F, Makino M, Howard M, Mizuochi T, Takatsu K. An IFN-γ-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int Immunol. 1994;6:1937–1947. doi: 10.1093/intimm/6.12.1937. [DOI] [PubMed] [Google Scholar]
- 47.Unkeless J C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utsunomiya Y, Kosaka H, Kanagawa O. Differential reactivity of Vβ9 T cells to minor lymphocyte stimulating antigen in vitro and in vivo. Eur J Immunol. 1991;21:1007–1011. doi: 10.1002/eji.1830210422. [DOI] [PubMed] [Google Scholar]
- 49.Vacchio M S, Ryan J J, Hodes R J. Characterization of the ligand(s) responsible for negative selection of Vβ11- and Vβ12-expressing T cells: effects of a new Mls determinant. J Exp Med. 1990;172:807–813. doi: 10.1084/jem.172.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Meerwijk J P, Iglesias A, Hansen-Hagge T, Bluethmann H, Steinmetz M. Allelic exclusion of a T cell receptor-beta minilocus. J Immunol. 1991;147:3224–3228. [PubMed] [Google Scholar]
- 51.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiker H G, Harboe M, Bennedsen J, Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. 1988;27:223–239. doi: 10.1111/j.1365-3083.1988.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 53.Wit L D, Palou M, Content J. Nucleotide sequence of the 85-B protein gene of Mycobacterium bovis BCG and Mycobacterium tuberculosis. DNA Sequence. 1994;4:267–270. [PubMed] [Google Scholar]
- 54.Yanagisawa S, Koike M, Kariyone A, Nagai S, Takatsu K. Mapping of Vβ11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int Immunol. 1997;9:227–237. doi: 10.1093/intimm/9.2.227. [DOI] [PubMed] [Google Scholar]