Abstract
In order to study the mucosal and serum antibody response to polysaccharide-encapsulated bacteria in mice, a preparation of heat-inactivated Streptococcus pneumoniae type 4 was administered, with and without cholera toxin, at various mucosal sites. It appeared that intranasal immunization of nonanesthesized animals was superior to either oral, gastric, or colonic-rectal antigen delivery with regard to the induction of serum immunoglobulin G (IgG) and IgA, as well as saliva IgA antibodies specific for pneumococci. The marked IgA antibody response in feces after intranasal, but not after oral or gastric, immunization is suggestive of a cellular link between the nasal induction site and the distant mucosal effector sites. Intranasal immunization also induced antibodies in serum and in mucosal secretions against type-specific capsular polysaccharide. IgA and IgG antibody levels in pulmonary lavage fluids correlated well with saliva IgA and serum IgG antibodies, respectively. Antibody determinations in pulmonary secretions may therefore be redundant in some cases, and the number of experimental animals may be reduced accordingly. After intraperitoneal challenge with type 4 pneumococci, mice immunized intranasally were protected against both systemic infection and death, even without the use of cholera toxin as a mucosal adjuvant. Thus, an efficient intranasal vaccine against invasive pneumococcal disease may be based on a very simple formulation with whole killed pneumococci.
Streptococcus pneumoniae is one of the major bacterial causes of respiratory tract infections and a frequent cause of bacteremia (22, 25). With increasing resistance of pneumococcal strains to antimicrobial agents (7), there is a demand for preventive measures. The presently available polyvalent polysaccharide vaccine offers protection against a large number of pneumococcal strains, and it protects against systemic pneumococcal infection (12, 24). However, the protective efficacy against pneumonia is controversial (20, 28), and the polysaccharide vaccine is not considered to be sufficiently immunogenic for use with infants and children under 2 years of age (21). There is thus a need for alternative vaccination strategies, e.g., development of polysaccharide-protein conjugate vaccines, pneumococcal protein vaccines, or mucosal vaccines.
Most pathogens enter the host through the mucosal membranes and seem to induce a local mucosal immune response, mainly represented by secretory IgA (10). Studies of carriage of pneumococci in the upper respiratory tract have shown that such carriage may induce anti-pneumococcal antibodies (15). In preliminary studies with mice, we have been able to show that a preparation of whole heat-inactivated pneumococci was immunogenic when applied to mucosal surfaces and that the nasal mucosa may be the preferred site for antigen delivery (2). It has also been shown recently that nasal immunizations with pneumococcal surface protein A could induce immunity with the power to protect against challenge with pathogenic organisms (27).
Most previous studies were done with cholera toxin (CT) or its nontoxic subunit B as a mucosal adjuvant (8, 23, 26, 27). However, it became clear from other experiments that several killed airway pathogens, i.e., Bordetella pertussis, group B streptococci, and outer membrane vesicles from group B meningococci, were strongly immunogenic when given as a nasal vaccine, even without the use of CT (9, 14, 16, 18). If this is also valid for whole inactivated pneumococci, it may be possible to create nonproliferating mucosal vaccines without additional mucosal adjuvants, which might themselves be immunogenic or tolerogenic (13).
The aim of this study was to determine in more detail the mucosal site that would be the most efficient for induction of systemic and mucosal antibody responses after application of heat-inactivated whole pneumococci. We also attempted to find out whether cholera toxin would be necessary as a mucosal adjuvant for the whole pneumococcal vaccine. Finally, we used a systemic-infection model to test the protective effect of this vaccine when administered on the appropriate mucosal site.
MATERIALS AND METHODS
Bacteria.
A human isolate of S. pneumoniae serotype 4 was used for immunization and challenge. Heat-killed bacteria for immunization were prepared by culturing pneumococci in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) with 17% fetal calf serum (Gibco Laboratories, Life Technologies Ltd., Paisley, Scotland) for 18 h at 36°C in 5% CO2, after which they were centrifuged and washed three times in sterile pyrogen-free saline (3). The number of bacteria in the final suspension was determined by plating 10-fold serial dilutions onto horse blood agar plates. Heat inactivation was accomplished in a water bath at 56°C for 30 min. No live bacteria were detected after this suspension was plated onto agar plates.
Preparation of the bacterial inoculum for challenge was done as previously described (1). Briefly, small aliquots of pneumococci in mid-log growth phase were prepared by a standarized method and kept frozen at −70°C, ready for challenge experiments after thawing and appropriate dilution.
Animals.
Inbred female BALB/cABom mice, 7 to 9 weeks old, were obtained from Gl. Bomholtgård Ltd. Ry, Denmark. Outbred female HsdOla:NIHS mice, 6 to 8 weeks old, were obtained from Harlan Olac Ltd., Oxon, United Kingdom. They were all specific-pathogen-free mice and were housed in cages with six to eight mice each with Beekay GLP bedding (B & K Universal AS, Nittedal, Norway) under standard conditions with regulated day length, temperature, and humidity. Tap water and pelleted food (Ewos-Alab R3, rats and mice; Ewos AB, Södertälje, Sweden) were offered ad libitum. The experiments were performed in conformity with the laws and regulations controlling experiments with live animals in Norway and were approved by the local officer of the Experimental Animal Board under the Ministry of Agriculture of Norway.
Immunizations.
In the first experiment, groups of six BALB/c mice were immunized four times at weekly intervals, each group at a different mucosal site (intranasal, oral, intragastric, and colonic-rectal). No anesthetics were given in this first experiment. Each dose of antigen solution corresponded to a mixture of 25 μl of the heat-inactivated pneumococci (1010 CFU/ml before heat inactivation) and 5 μl (1 mg/ml) of CT (Calbiochem Corp., La Jolla, Calif.). The intranasal immunization was carried out with the mouse held in a supine position with the head down while 30 μl of the antigen solution was delivered slowly with a micropipette onto the nares so that the mouse could sniff it in. For oral immunizations, the antigen solution was given slowly with a micropipette so that the mouse could suck the fluid from the tip. The antigen solution to be given intragastrically was mixed with 150 μl of 0.1 M NaHCO3 (pH 8.1), making up 180 μl per dose, which was given intragastrically with a blunt steel feeding tube. For colonic-rectal immunization, 30 μl of the antigen solution was delivered by a feeding tube inserted via the anus with the tip approximately 3 cm from the anal opening.
In the second experiment, groups of NIHS mice were immunized intranasally four times at weekly intervals with 25 μl of heat-inactivated pneumococci, either mixed with or without added CT. These mice were briefly anesthetized intravenously with 0.01 ml (10 mg/ml) of propofol (Diprivan; Zeneca Ltd., Macclesfield Cheshire, United Kingdom) before intranasal immunization, which was performed as described for the first experiment. The mice recovered completely 1 to 2 min after anesthesia. Groups of mice given anesthesia and 25 μl of pyrogen-free saline intranasally served as controls.
Collection of samples for antibody determinations.
Blood was obtained from the lateral femoral vein in heparinized capillary tubes (Vitrex, Herlev, Denmark) and was separated and stored at −20°C until it was analyzed (4). Saliva and feces were collected, and extracts were made as described previously (17). Briefly, saliva was collected by inserting the tips of absorbent cylindrical wicks (2 by 25 mm) (Polyfiltronics Group Inc., Rockland, Mass.) into the mouths of mice immediately after salivation was induced by a single intraperitoneal injection of 0.1 mg of pilocarpin-HCl (Sigma Chemical Co., St. Louis, Mo.) in 100 μl of phosphate-buffered saline (PBS). The weight of the collected secretions was calculated as the difference between the weights of the wicks before and after collection. Two wicks saturated with saliva were obtained from each mouse, frozen at −20°C in 1.5-ml microcentrifuge tubes, and subsequently extracted with 500 μl of PBS containing 5% nonfat dry milk (Oxoid skim milk powder; Unipath Ltd., Basingstoke, Hampshire, England) and the following protease inhibitors: 0.2 mM 4-(2-aminoethyl)-benzenesulfonylfluoride (AEBSF) (Calbiochem), 1 μg of aprotinin/ml, 10 μM leupeptin (both from Sigma), and 3.25 μM bestatin (Boehringer Mannheim, Indianapolis, Ind.). After being vortexed twice for 15 s, the tubes were centrifuged at 16,000 × g for 2 min at 4°C to drive fluid out of the wicks.
Three to five pieces of freshly voided feces were collected into 1.5-ml microcentrifuge tubes, frozen at −20°C, and subsequently vacuum dried in a Speed Vac concentrator (Savant Instruments, Inc., Farmingdale, N.Y.). After the net dry weights were recorded, extracts were made by adding 20 μl of PBS, with dry milk and protease inhibitors, per mg of dry feces, followed by extensive vortexing and centrifugation at 16,000 × g for 2 min at 4°C (17). The clear supernatants were used for further analyses.
Immediately after the animals were killed, lung lavage fluid was obtained by a single injection into the trachea of 1.5 ml of PBS, followed by aspiration through a 25-G needle. Possible backflow of saline during this procedure was prevented by tying off the proximal part of the trachea.
In the first experiment, all samples except lung lavage fluids were collected before and 1 week after the fourth immunization. In the second experiment, samples were also collected just before the third immunization and on the day before bacterial challenge. Blood for bacterial counts was obtained 3, 12, 24, 48, and 72 h after challenge. Unless otherwise specified, all samples and extracts intended for antibody analyses were stored at −20°C until they were analyzed.
Quantitation of anti-pneumococcal antibodies.
Immunoglobulin M (IgM), IgG, and IgA antibodies to pneumococcal polysaccharide (anti-PPS) serotype 4 were determined by enzyme-linked immunosorbent assay (ELISA) with Nunc (Roskilde, Denmark) Immuno plates (no. 269787) as previously described (5). Briefly, the plates were coated by incubation with PPS serotype 4 (American Type Culture Collection, Rockville, Md.). Plasma samples were neutralized with pneumococcal C polysaccharide to remove activity against this pneumococcal antigen and tested at a dilution of 1:100 (5). Secretions were tested at a dilution of 1:50. A pool of sera from immunized mice was included on each plate as a positive control. Alkaline phosphatase (ALP)-conjugated goat anti-mouse IgM, IgG, or IgA (μ, γ, and α chain specific, respectively) (Sigma Chemical Company) was used as a conjugate.
IgM, IgG, and IgA antibodies to whole pneumococci serotype 4 were determined by ELISA with Nunc MaxisorpF96 plates. The plates were coated with heat-inactivated pneumococci (about 107 CFU/ml) for 30 min at room temperature before centrifugation for 15 min and incubation for 3 h at 37°C. Nonspecific protein binding sites were blocked with PBS containing 5% nonfat dry milk (Oxoid). Twofold dilutions of both test samples and standard solutions were made, and sample dilutions of 100 μl were applied to ELISA plates and incubated for 90 min at room temperature. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgA or IgG (Sigma) was added. In the last series of experiments, the same ALP conjugates used for determination of anti-PPS serotype 4 antibodies were used. After incubation for 1 h at room temperature, the plates were washed six times and the substrates o-phenylenediamine hydrochloride (Sigma) for HRP conjugates and paranitrophenylphosphate for ALP conjugates were added. Optical densities were read after incubation for 10 min when HRP conjugates were used or after 30 min with ALP conjugates. Standard curves were generated, and arbitrary units were determined based on a defined pool of sera.
Experimental infections.
In the second experiment, the mice were challenged by intraperitoneal injection of live pneumococci according to a previously described infection model (1). The mice were challenged 1 week after the last immunization, and a challenge dose 10 times the 50% lethal dose (LD50) for this serotype, i.e., approximately 20 to 30 CFU per mouse, was given. The number of bacteria in the inoculum was confirmed by plating 100 μl from serial 10-fold dilutions onto blood agar plates. The number of bacteria in the blood of infected animals was determined by applying 25-μl volumes of similar 10-fold dilutions of blood to the plates. Colonies of bacteria were counted after incubation at 36°C in a 5% CO2 atmosphere for 18 h. The challenged mice were observed twice daily by an experienced person, and signs of sickness and dead mice were recorded. Moribund animals, which it was assumed were going to die within a few hours, were sacrificed by cervical dislocation for humane reasons. Mice still alive after 14 days were considered to have survived the infection.
Statistical analysis.
Statistical analysis was performed with SigmaStat statistical software (version 3.01; Jandel Scientific, Erkrath, Germany). The nonparametric Mann-Whitney U test, the chi square test, and the Fischer exact test were used. The limit of statistical significance used was a P value of 0.05.
RESULTS
Nasal immunizations are most efficient for induction of systemic and mucosal antibodies.
To find the most efficient means of applying mucosal vaccines consisting of whole killed pneumococci, groups of mice were immunized via the nasal, oral, gastric, or rectal route. It was evident from the results of this experiment that the nasal route, with CT as a mucosal adjuvant, was by far superior to any other route for induction of serum IgG antibodies to whole pneumococci (Table 1). No such antibody response was elicited when the pneumococcal antigen was given via other routes, even when antigen was given directly into the stomach with bicarbonate to neutralize the gastric acid. However, all immunized mice belonging to any group developed strong serum IgG antibodies to CT (results not shown). In the second experiment, in which whole killed pneumococci were given intranasally with or without CT, levels of serum IgG and IgM antibodies to whole pneumococci were markedly higher in mice which were given pneumococci with CT than in those given pneumococci without CT (data not shown).
TABLE 1.
Concentration of IgG antibodies after immunization with heat-inactivated pneumococci plus CT by various mucosal routes
| Sample | Concn after immunization by indicated routea
|
||||
|---|---|---|---|---|---|
| Nasalb | Oralb | Gastricc | Rectalb | Controlsb | |
| Serum | 2,107*** (1,507–3,874) | 100 (100–383) | 127 (100–315) | 236 (100–370) | 100 (100–565) |
| Saliva | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0.1) | 0 (0–0) |
| Lung lavage fluid | 5.1* (3.4–9.3) | 0.07 (0–0.8) | 0.07 (0–0.7) | 0.4 (0.1–3.1) | 0.1 (0–0.1) |
| Extracts of feces | 3.3*** (1.8–6.1) | 0 (0–0) | 0 (0–0) | 1.6* (0–2.1) | 0 (0–1.1) |
Median concentrations (ranges) of IgG antibodies to whole pneumococci (kilounits per milliliter or kilounits per gram of dry feces); ***, P ≤ 0.005; *, 0.01 < P ≤ 0.05 (statistical significance versus the control group; Mann-Whitney U test).
n = 6.
n = 4.
The advantage of nasal immunization, compared to immunization via other mucosal routes, was likewise clearly evident for induction of IgA antibodies to whole pneumococci in serum, as well as in saliva, lung lavage fluid, and extracts of feces (Table 2). Presentation of the antigen into the lower part of the intestine via the rectal route, however, also induced consistent increases in fecal IgA antibodies to pneumococci (Table 2), and rectal, as well oral, immunizations led to systemic IgA antibody responses.
TABLE 2.
Concentration of IgA antibodies after immunization with heat-inactivated pneumococci plus CT by various mucosal routesa
| Sample | Concn after immunization by indicated routea
|
||||
|---|---|---|---|---|---|
| Nasalb | Oralb | Gastricc | Rectalb | Controlsb | |
| Serum | 103.3*** (9.9–757.7) | 31.3*** (7.2–130.4) | 2.9 (1.7–6.4) | 28.4** (5.0–140.4) | 1.5 (6.4–1.2) |
| Saliva | 5.5*** (3.8–83.7) | 2.3* (0.1–13.9) | 0.1 (0.1–0.2) | 0.1 (0.1–0.3) | 0.1 (0.1–0.1) |
| Lung lavage fluid | 0.8* (0.5–1.3) | 0.7* (0.1–3.2) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 0.1 (0.1–0.1) |
| Extracts of feces | 143.0*** (43.5–443.7) | 33.0 (10.0–193.5) | 10.0 (10.0–19.1) | 310.8*** (52.3–672.0) | 10.0 (10.0–28.0) |
Median concentrations (ranges) of IgA antibodies to whole pneumococci (kilounits per milliliter or kilounits per gram of dry feces); ***, P ≤ 0.005; **, 0.005 < P ≤ 0.01; *, 0.01 < P ≤ 0.05 (statistical significance versus the control group; Mann-Whitney U test).
n = 6.
n = 4.
IgA antibodies in saliva and IgG antibodies in serum correlate with the corresponding antibodies in pulmonary lavage fluid.
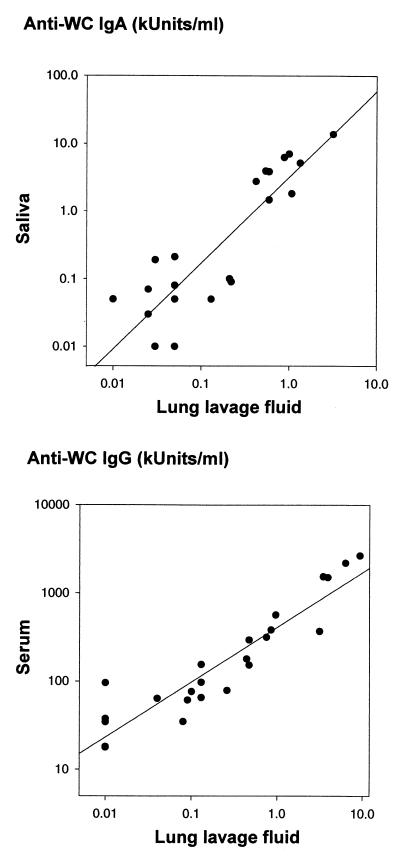
Only low concentrations of IgA antibodies to pneumococci were found in lung lavage fluid. Still, a significant increase in IgA antibodies was observed in the lung lavage fluid in the groups of mice which had been immunized via the nasal or oral route (Table 2). Moreover, the concentration of IgA antibodies in lung lavage fluid correlated well with the concentration of IgA antibodies in saliva (Fig. 1, upper panel) (r = 0.89; P < 0.0001) but with concentrations roughly 10 times less than those in saliva. On the other hand, IgA antibody concentrations in lung lavage fluid did not correlate significantly with serum IgA concentrations.
FIG. 1.
(Upper panel) Correlation between concentrations of IgA antibodies to whole pneumococci (WC) in lung lavage fluid and saliva from mice which had been immunized via different routes with heat-inactivated pneumococci type 4 plus CT (r = 0.89; P < 0.0001). (Lower panel) Correlation between concentrations of IgA antibodies to whole pneumococci type 4 in serum and lung lavage fluid from the same mice (r = 0.93; P < 0.0001).
Lung lavage fluid, as well as extracts of feces, contained relatively high concentrations of IgG antibodies, especially in the group of mice which had been immunized via the intranasal route (Table 1). IgG antibodies to pneumococci in lung lavage fluid correlated well with the corresponding serum IgG values (Fig. 1, lower panel) (r = 0.93; P < 0.0001), although the absolute concentrations in the pulmonary secretions were at least 100 times lower than those in the sera.
Nasal immunization with whole killed pneumococci can induce antibodies to PPSs.
In the first immunization experiment, with the use of CT as a mucosal adjuvant with BALB/c mice, IgG antibodies to PPS could not be detected in serum or secretions. However, significant increases in serum IgA antibodies to PPS could be demonstrated after nasal, oral, gastric, and rectal immunization (Table 3). On the other hand, extracts of feces were the only kind of sample representing secretions in which such IgA antibody responses could be demonstrated, and then only after nasal and rectal antigen deliveries (Table 3). Significant increases in serum IgM antibodies to PPS were detected after intranasal oral, gastric, and rectal immunization, but no IgM antibodies to PPS could be demonstrated in any secretion (results not given).
TABLE 3.
Concentrations of IgA antibodies after immunization with heat inactivated pneumococci plus CT by various mucosal routesa
| Sample | Concn after immunization by indicated routea
|
||||
|---|---|---|---|---|---|
| Nasalb | Oralb | Gastricc | Rectalb | Controlsb | |
| Serum | 0.34*** (0.29–0.80) | 0.30*** (0.26–0.33) | 0.34** (0.29–0.47) | 0.46** (0.38–0.55) | 0.12 (0.07–0.22) |
| Saliva | 0.26 (0.11–1.12) | 0.17 (0.15–0.18) | 0.17 (0.15–0.21) | 0.18 (0.15–0.31) | 0.15 (0.11–0.39) |
| Lung lavage fluid | 0.32 (0.24–0.33) | 0.28 (0.22–0.59) | 0.30 (0.27–0.32) | 0.29 (0.23–0.37) | 0.34 (0.32–0.38) |
| Extracts of feces | 0.56*** (0.25–1.65) | 0.11 (0.07–0.33) | 0.22 (0.08–0.36) | 0.28** (0.15–0.99) | 0.14 (0.11–0.19) |
Median concentrations (ranges) of IgA antibodies to pneumococcal polysaccharide type 4 (absorbance); ***, P ≤ 0.005; **, 0.005 < P ≤ 0.01 (statistical significance versus the control group; Mann-Whitney U test).
n = 6.
n = 4.
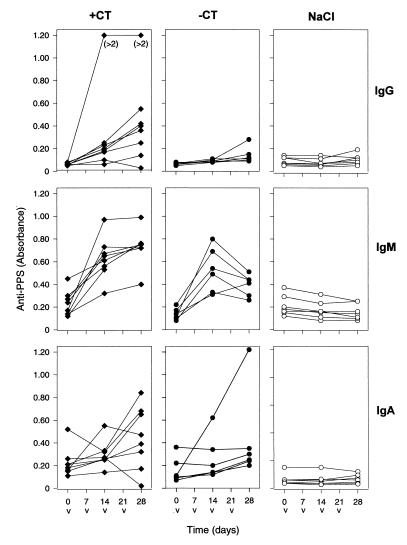
The second immunization experiment, in which whole killed pneumococci were given intranasally, with or without CT as a mucosal adjuvant, to NIHS mice, confirmed that serum anti-PPS antibodies could be elicited. In this experiment, animals responded with both IgG and IgM antibodies to PPS when CT was used (P ≤ 0.01 and P < 0.001, respectively) (Fig. 2). Some mice showed an increase in IgA antibody levels, but the increase was not statistically significant. When pneumococci alone were used for nasal immunizations, however, only IgM antibodies to PPS were increased (P < 0.001). Moreover, there was no significant difference in the IgM antibody levels whether or not CT was added to the antigen solution (Fig. 2).
FIG. 2.
Serum antibodies to PPS 4 in mice immunized intranasally with heat-inactivated pneumococci type 4, with CT (+CT; eight mice), or without CT (−CT; six mice). Immunizations (v) were carried out at weekly intervals (days 0, 7, 14, and 21), and blood samples were taken on days 0, 14, and 28. A control group (seven mice) was immunized intranasally with saline (NaCl). The symbols represent individual levels (absorbance) of IgG, IgM, and IgA antibodies.
Nasal immunizations with whole killed pneumococci can protect against systemic pneumococcal infection.
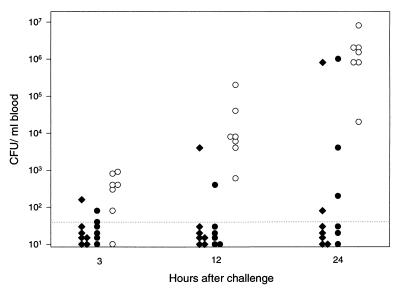
The groups of mice which had been immunized intranasally as part of the second experiment, and which were given lethal doses of viable pneumococci intraperitoneally, were examined for viable bacteria in the blood. As early as 3 h after the bacterial challenge, the animals which had been immunized with the pneumococcal preparation had significantly (P = 0.01) fewer bacteria than those which had been given only saline as a nasal placebo vaccine (Fig. 3). It made no difference whether CT had been given together with the killed pneumococci. This difference in bacterial counts between control animals and animals which had been given pneumococci with or without CT was even more pronounced at 12 h after the bacterial challenge (P = 0.001).
FIG. 3.
Bacteremia, expressed as number of CFU per milliliter of blood from individual mice after intraperitoneal challenge with 10 times the LD50 of live virulent pneumococci type 4 (∼20 CFU/mouse). Groups of mice were immunized intranasally either with whole heat-inactivated pneumococci type 4 mixed with CT (solid diamonds) or with the same antigen without CT (solid circles) or were not immunized (open circles). The dotted line indicates the limit of detection of bacteremia. The mice were challenged 1 week after the fourth nasal immunization dose, which corresponds to day 28 in Fig. 2.
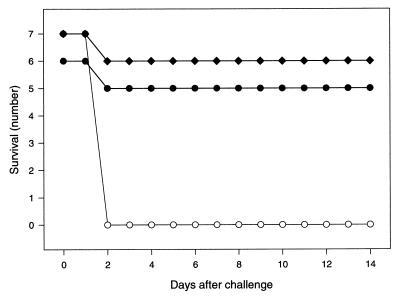
None of the mice which had been given saline as a placebo vaccine survived the first 2 days after challenge with viable bacteria (Fig. 4). On the other hand, all except one mouse in each of the two groups which had been given the killed pneumococcal vaccine intranasally survived the whole observation period of 2 weeks. The protective effect of this nonproliferating nasal vaccine has thus been confirmed to include severe life-threatening pneumococcal sepsis. Moreover, pneumococci alone were sufficient to attain this effect, i.e., it did not seem necessary to include the commonly used CT as a mucosal adjuvant.
FIG. 4.
Survival of mice immunized intranasally either with whole heat-inactivated pneumococci type 4 mixed with CT (solid diamonds), with the same antigen without CT (solid circles), or not immunized (open circles) after intraperitoneal challenge with 10 times the LD50 of live virulent pneumococci type 4 (∼20 CFU/mouse).
DISCUSSION
In this study, we have shown that whole heat-inactivated pneumococci can induce both systemic and mucosal antibodies when applied on various mucosal surfaces. Results of our first experiment indicate that intranasal application of this antigen, plus CT as a mucosal adjuvant, was superior to the oral, gastric, and rectal routes of antigen delivery (2). It was also evident that immunization via these alternative routes was not able to induce anti-pneumococcal IgG antibodies, which might be crucial for protection against systemic disease. However, IgG antibodies against CT were induced after immunization at all mucosal sites. The induction of systemic immunity to pneumococci via the nasal route suggests that the nasopharyngeal mucosa possesses the necessary structures to make mucosal immunizations a realistic alternative to the use of parenteral vaccines (11).
The superiority of nasal versus oral, gastric, and rectal routes of antigen presentation was confirmed by the demonstration of specific IgA antibodies in serum and samples representing secretions. The demonstration of IgA antibodies in secretions after the antigen was given orally, and not gastrically, might thus be explained by the antigen in the first case having reached the nasopharyngeal induction sites. Furthermore, only intranasal immunizations, in addition to rectal antigen delivery, induced significant increases in intestinal IgA antibodies, as reflected in extracts of feces. This was surprising, considering the fact that neither oral nor gastric immunizations with the same antigen were able to induce significant increases in such intestinal antibodies. The lack of intestinal antibodies after oral and gastric immunizations indicates that induction of intestinal antibodies after intranasal immunization was not due to swallowing or leakage of antigen from the nose into the intestines. The stimulus for antibodies to be produced locally in the gut is therefore suggestive of a cellular link between the nasal induction site and the intestinal effector site.
Our finding of IgG as well as IgA antibodies to pneumococci in lung lavage fluid, especially after nasal immunization, might indicate that both these antibodies have a barrier function against invasive pneumococci. The IgG antibodies in the pulmonary lavage fluids also seemed to mirror antibodies in serum, from which they are probably derived. Similarly, we have recently showed that IgG antibodies to B. pertussis in pulmonary secretions reflected the corresponding serum antibodies, which were initiated by nasal immunization (9). A protective effect of systemic antibodies against pulmonary infections might thus be conferred all the way from the tissue fluid to the mucosal surfaces.
The present finding that pulmonary IgA antibodies to pneumococci correlated with such antibodies in saliva indicates that at least some of the IgA is produced locally in the lungs to contribute to this presumed surface protection. It seems, therefore, that the IgA antibodies in saliva reflect the IgA antibodies in the lung secretions and that analyses of salivary IgA would be sufficient for evaluation of mucosal airway antibodies. Since saliva samples can be collected several times from the same animal, there is less need for collection of pulmonary secretions, and the number of mice used for experimental purposes can be reduced.
In the second experiment, significant increases of serum anti-polysaccharide IgG and IgM were induced in NIHS mice, whereas only serum IgM antibodies were induced in the first experiment with BALB/c mice. Parenteral immunization of BALB/c mice with PPS, conjugate vaccine, or heat-inactivated pneumococci also seems to induce serum IgM and no IgG antibodies (3, 5, 19). In other strains of mice, however, IgG antibodies can be induced after parenteral immunization with a pneumococcal conjugate vaccine (19), and low levels of IgG antibodies may even be induced in NIHS mice after immunization with polysaccharides alone (unpublished observations). The discrepancy in antibody responses in the first versus the second experiment may therefore be due to the use of different strains of mice.
Intranasal immunization with PPS type 3 containing liposomes has also been shown by others to induce IgA antibodies specific for type 3 polysaccharide in lung lavage fluid (6). In addition, we have now shown that both nasal and rectal immunizations induced intestinal IgA antibodies directed against the homologous type of polysaccharide. To some extent, however, these intestinal antibody responses to the polysaccharide antigens after mucosal immunization seemed to depend on the use of strong mucosal adjuvants, such as CT, and it remains to be shown whether polysaccharides as part of a mucosal vaccine induce protective immunity.
The intranasally immunized mice in the present study were well protected against lethal intraperitoneal challenge with live pneumococci. This was evident also with a vaccine consisting of only killed whole pneumococci, i.e., without additional mucosal adjuvants, despite the fact that some mice developed only low levels of antibody to polysaccharides in the serum. A similar discrepancy between low levels of ELISA antibodies specific for serotype 4 polysaccharide and protection against pneumococci of the same serotype has also been observed in other studies (4, 19). As opposed to antibodies against polysaccharides, in the present study, intranasal immunization induced strong antibody responses to whole pneumococci in all mice, even without CT. Antibodies to pneumococcal antigens other than polysaccharide antigens may therefore have contributed to protection. Since such antigens may be common to many pneumococcal serotypes, one could speculate whether a pneumococcal whole-cell vaccine given intranasally would protect against infections caused by several other serotypes.
The present finding that CT was not necessary for a nasal whole-cell vaccine to induce effective antibodies is in accordance with results of studies with outer membrane vesicles from meningococci (14). Recently, it has been found that CT actually inhibited the antibody responses to whole group B streptococci (18) and to B. pertussis (9) that had been given intranasally. It may thus be possible to create effective nonproliferating mucosal vaccines based on very simple formulations.
ACKNOWLEDGMENTS
We thank Else-Carin Groeng, Inger Lise Haugen, Rita Bente Leikvold, Gro Lermark, and Trude Olsen for invaluable help and excellent technical assistance and Morten Harboe for inspiring discussions.
REFERENCES
- 1.Aaberge I S, Eng J, Lermark G, Løvik M. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995;18:141–152. doi: 10.1016/s0882-4010(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 2.Aaberge I S, Groeng E-C, Haugen I L, Dalseg R, Løvik M, Haneberg B. Induction of systemic and mucosal antibodies to pneumococcal antigens by mucosal immunization. J Cell Biochem Suppl. 1995;19A:242. [Google Scholar]
- 3.Aaberge I S, Løvik M. The antibody response after immunization with pneumococcal polysaccharide vaccine in splenectomized mice: the effect of re-immunization with pneumococcal antigens. APMIS. 1996;104:307–317. doi: 10.1111/j.1699-0463.1996.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 4.Aaberge I S, Michaelsen T E, Rolstad A K, Groeng E-C, Solberg P, Løvik M. Scid-hu mice immunized with a pneumococcal vaccine produce specific human antibodies and show increased resistance to infection. Infect Immun. 1992;60:4146–4153. doi: 10.1128/iai.60.10.4146-4153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaberge I S, North R J, Groeng E-C, Løvik M. Antibody response to pneumococcal polysaccharide vaccine in young, adult and old mice. Scand J Immunol. 1993;38:17–30. doi: 10.1111/j.1365-3083.1993.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 6.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–468. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae. An overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berstad A K H, Holst J, Møgster B, Haugen I L, Haneberg B. A nasal whole-cell pertussis vaccine can induce strong systemic and mucosal antibody responses which are not enhanced by cholera toxin. Vaccine. 1997;15:1473–1478. doi: 10.1016/s0264-410x(97)00064-9. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. APMIS. 1995;103:1–19. doi: 10.1111/j.1699-0463.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 11.Brandtzaeg P, Haneberg B. Role of nasal-associated lymphoid tissue in the human mucosal immune system. Mucosal Immunol Update. 1997;5:4–8. [Google Scholar]
- 12.Butler J C, Breiman R F, Campbell J F, Lipman H B, Broome C V, Facklam R R. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 13.Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist. 1995;3:97–103. [Google Scholar]
- 14.Dalseg R, Wedege E, Holst J, Haugen I L, Høiby E A, Haneberg B. Outer membrane vesicles from group B meningococci are strongly immunogenic when given intranasally to mice. Vaccine. 1999;17:2336–2345. doi: 10.1016/s0264-410x(99)00046-8. [DOI] [PubMed] [Google Scholar]
- 15.Gwaltney J M, Jr, Sande M A, Austrian R, Hendley J O. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J Infect Dis. 1975;132:62–68. doi: 10.1093/infdis/132.1.62. [DOI] [PubMed] [Google Scholar]
- 16.Haneberg B, Dalseg R, Wedege E, Høiby E A, Haugen I L, Oftung F, Andersen S R, Næss L M, Aase A, Michaelsen T E, Holst J. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina, measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hordnes K, Tynning T, Brown T A, Haneberg B, Jonsson R. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 19.Hvalbye B, Aaberge I S, Peeters C, Løvik M. Pneumococcal polysaccharide protein-conjugate vaccine in a mouse model: immune response and protection. Scand J Immunol. 1995;41:622. [Google Scholar]
- 20.Koivula I, Stén M, Leinonen M, Mäkelä P H. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population based trial. Am J Med. 1997;103:281–290. doi: 10.1016/s0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee C J, Wang T R. Pneumococcal infection and immunization in children. Crit Rev Microbiol. 1994;20:1–12. doi: 10.3109/10408419409113544. [DOI] [PubMed] [Google Scholar]
- 22.Obaro S K, Monteil M A, Henderson D C. The pneumococcal problem. Brit Med J. 1996;312:1521–1525. doi: 10.1136/bmj.312.7045.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcells V, Margolis A, Adair R K, Clemens J D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Programme for the control of acute respiratory infections. Recent developments. Weekly Epidemiol Rec. 1993;68:353–360. [PubMed] [Google Scholar]
- 26.Wu H-Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H-Y, Nahm M H, Guo Y, Russel M W, Briles D E. Intranasal immunization of mice with PspA (Pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 28.Örtqvist Å, Hedlund J, Burman L-Å, Elbel E, Höfer M, Leinonen M, Lindblad I, Sundelöf B, Kalin M the Swedish Pneumococcal Vaccination Study Group. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399–403. doi: 10.1016/s0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]