Abstract
Background and Objective
Most guidelines in the UK, Europe and North America do not recommend organised population-wide screening for prostate cancer. Prostate-specific antigen-based screening can reduce prostate cancer-specific mortality, but there are concerns about overdiagnosis, overtreatment and economic value. The aim was therefore to assess the cost effectiveness of eight potential screening strategies in the UK.
Methods
We used a cost-utility analysis with an individual-based simulation model. The model was calibrated to data from the 10-year follow-up of the Cluster Randomised Trial of PSA Testing for Prostate Cancer (CAP). Treatment effects were modelled using data from the Prostate Testing for Cancer and Treatment (ProtecT) trial. The participants were a hypothetical population of 10 million men in the UK followed from age 30 years to death. The strategies were: no screening; five age-based screening strategies; adaptive screening, where men with an initial prostate-specific antigen level of < 1.5 ng/mL are screened every 6 years and those above this level are screened every 4 years; and two polygenic risk-stratified screening strategies. We assumed the use of pre-biopsy multi-parametric magnetic resonance imaging for men with prostate-specific antigen ≥ 3 ng/mL and combined transrectal ultrasound-guided and targeted biopsies. The main outcome measures were projected lifetime costs and quality-adjusted life-years from a National Health Service perspective.
Results
All screening strategies increased costs compared with no screening, with the majority also increasing quality-adjusted life-years. At willingness-to-pay thresholds of £20,000 or £30,000 per quality-adjusted life-year gained, a once-off screening at age 50 years was optimal, although this was sensitive to the utility estimates used. Although the polygenic risk-stratified screening strategies were not on the cost-effectiveness frontier, there was evidence to suggest that they were less cost ineffective than the alternative age-based strategies.
Conclusions
Of the prostate-specific antigen-based strategies compared, only a once-off screening at age 50 years was potentially cost effective at current UK willingness-to-pay thresholds. An additional follow-up of CAP to 15 years may reduce uncertainty about the cost effectiveness of the screening strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01191-1.
Key Points for Decision Makers
| Prostate cancer screening is associated with a risk of overdiagnosis of indolent disease and adverse effects associated with biopsy and overtreatment, leading to uncertainty around whether it can be cost effective. |
| Tailored screening according to a man’s predicted risks may improve the cost effectiveness of screening; however, robust evidence on the long-term effectiveness and cost effectiveness of these approaches is lacking. |
| This analysis has calibrated a natural history model using rich UK data from the Cluster Randomised Trial of PSA Testing for Prostate Cancer (CAP) and the Prostate Testing for Cancer and Treatment (ProtecT) trial to show that a once-off screen at age 50 years has the potential to be clinically effective and cost effective in a UK setting. |
Introduction
Most guidelines for early detection of prostate cancer in Europe and North America do not recommend formal population-wide screening, although opportunistic prostate-specific antigen (PSA) testing is common in many countries [1–5]. Trial evidence has varied. The European Randomised Study of Screening for Prostate Cancer (ERSPC) [6, 7] found that repeated PSA screening at 4-yearly intervals reduced prostate cancer mortality but the UK Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) trial [8] of a single PSA screen found no significant difference in prostate cancer mortality after a median follow-up of 10 years. Screening is associated with a risk of overdiagnosis of indolent disease [9] and adverse effects associated with biopsy [10] and overtreatment [11], leading to uncertainty around whether prostate cancer screening can be cost effective.
Tailored screening according to a man’s predicted risks may improve the cost effectiveness of screening [12, 13]. One measure would be to use polygenic risk scores, which indicate genetic susceptibility to disease [14]. Another is adaptive screening, where the interval between repeated screening is based on a man’s PSA level. This may reduce overdiagnosis if men with very low PSA levels are given a longer time to their next screen [15–17]. A third is the use of multiparametric magnetic resonance imaging (mpMRI), which is used as a pre-biopsy triage test post-PSA or another biomarker test and followed by combined systematic and magnetic resonance imaging (MRI)-targeted biopsies. This might allow men with no or likely indolent cancer to avoid an unnecessary biopsy and improve diagnostic accuracy for more aggressive disease [18–20]. However, robust evidence on the long-term effectiveness and cost effectiveness of these measures, which may be used in combination, is lacking.
Our aim was to assess the impact of various potential screening strategies—polygenic risk stratified, PSA based adaptive and age based—on the long-term costs, health outcomes and cost effectiveness of prostate cancer screening in the UK. We adapted the Prostata model [21] to the UK setting, including the use of data from the UK CAP trial (ISRCTN92187251) [8]. The CAP trial, which is the largest prostate cancer screening trial to date, compared a once-off PSA test (in men who consented after receiving information about PSA testing), with standard UK National Health Service care where PSA testing is offered opportunistically and advice about the potential benefits and harms of a PSA test is provided only on request. [8]
Methods
Overview
The Prostata model is a microsimulation model for the natural history of prostate cancer [21]. It is an open-source model, coded in the software R and C++, which allows for individual heterogeneity in natural history including disease onset, progression, diagnosis and death. The Prostata model was adapted from a US model [22–24] and allows for prostate cancer onset and progression to correlate with changes in PSA over time.
Prostate cancer onset is modelled via a time-dependent hazard (from age 35 years) following a Weibull distribution. The Gleason grade assigned at cancer onset (≤ 6, 7 or ≥ 8) is dependent on age, with older men more likely to have higher grade disease, and does not change over time. Transitions between disease states (T1–2, T3–4, M1) are possible and are dependent on a man’s age and his current PSA level. The transition from T1–T2 to T3–T4 is the same for all Gleason categories. Both preclinical and clinical states are modelled. Preclinical states are defined as men with prostate cancer that is asymptomatic but could potentially be detected by PSA screening. Clinical (as opposed to screening) detection is also modelled (see Fig. A5 of the Electronic Supplementary Material [ESM]).
The mechanism of benefit of prostate cancer screening is assumed to be the cancer stage shift, in that the benefit associated with screening is due to a shift to a less advanced cancer stage at diagnosis, resulting in earlier diagnosis and improved survival through potentially curative treatment. The target population was men in the UK from age 30 years. For a detailed description of the model, see Karlsson et al. [21].
To adapt the Prostata model to the UK context, we: extended the cancer onset sub-model to allow for a polygenic risk score; included detailed UK-specific parameters; calibrated the model to observed UK data; and validated the model predictions against mortality rate ratios from two large randomised screening trials [7, 8]. Finally, the calibrated model was used to project the lifetime cost effectiveness of eight candidate screening strategies.
Strategies Compared
The strategies compared in the base-case analysis reflected those identified as relevant by a panel of experts in a modified Delphi consensus process (see Table 1) [25].
Table 1.
Screening strategies compared
| Title | Starting age (years) | Stopping age (years) | Repeat screening interval | Comment |
|---|---|---|---|---|
| No screening | NA | NA | NA | |
| Screen 50 | 50 | NA | None. Once-off screen | |
| Screen 60 | 60 | NA | None. Once-off screen | |
| Screen 70 | 70 | NA | None. Once-off screen | |
| Repeat screen every 4 years | 50 | 70 | 4 yearly | |
| Repeat screen every 2 years | 50 | 70 | 2 yearly | |
| Risk stratified 4 yearly | Age at which a 10-year risk of developing prostate cancer is 7.5%, based on polygenic risk score | 70 | 4 yearly | Based on most cost-effective strategy identified in recent analysis by Callender et al. [13] |
| Risk stratified 2 yearly | Age at which a 10-year risk of developing prostate cancer is 7.5%, based on polygenic risk score | 70 | 2 yearly | To compare to age-based 2-yearly screening strategy |
| Adaptive screening | 50 | 70 | PSA level of < 1.5 ng/mL screened every 6 years, with value > 1.5 ng/mL resulting in 4-yearly screening | Based on ProScreen trial [15] |
NA Not Applicable, PSA prostate-specific antigen
As the Delphi consensus process did not reach a consensus on the exact age ranges to screen or specific screening intervals, scenario analyses were also conducted to observe the impact of using different ages to start and stop screening, screening intervals and risk thresholds. The scenario analyses tested are detailed in Table A7 of the ESM.
In the no screening strategy, we assumed no organised or opportunistic testing. In all other strategies, men with a PSA value ≥ 3 ng/mL received a pre-biopsy mpMRI and combined systematic biopsy and MRI-targeted biopsy if a Prostate Imaging-Reporting and Data System (PI-RADS) [26] value of 3–5 was found. This is in line with recent guidance from the UK National Institute for Health and Care Excellence (NICE) [27]. For each symptomatic diagnosis, we assumed no screening-focused PSA testing but an average of two diagnostic, 10–12 core, transrectal ultrasound-guided biopsies.
Model Parameters
Estimates of test accuracy were based on Hao et al. [28], including the specificity and sensitivity (Gleason ≤ 6, or Gleason ≥ 7) for: an mpMRI result; a systematic biopsy; and mpMRI-targeted biopsies given a positive pre-biopsy MRI (Table 2).
Table 2.
Model input parameters
| Parameter | Estimate | 95% CI | Distribution assumed in PSA | Source/notes |
|---|---|---|---|---|
| Specificity for mpMRI = Pr(mpMRI-|Healthy) | 0.548 | 0.435–0.657 | Normal | Hao et al. [28] |
| Sensitivity for mpMRI (G ≤ 6) | 0.715 | 0.614–0.798 | ||
| Sensitivity for mpMRI (G ≥7) | 0.931 | 0.893–0.956 | ||
| Sensitivity for standard biopsy (G ≤6) | 0.860 | 0.824–0.889 | ||
| Sensitivity for standard biopsy (G ≥7) | 0.897 | 0.809–0.947 | ||
| Sensitivity for mpMRI-targeted biopsy (G ≤ 6) | 0.753 | 0.568–0.875 | ||
| Sensitivity for mpMRI-targeted biopsy (G ≥ 7) | 0.934 | 0.889–0.962 | ||
| Slope of log odds of G7 at onset | 0.03905 | Variance: 1.686e−4 |
Multivariate normal Covariance: 1.079e−05 |
Calibration |
| Slope of log odds of G ≥ 8 at onset | 0.2453 | Variance: 1.78e−06 | ||
| Costs (£) | ||||
| PSA test | £21 | 17–25 | Gamma | NICE guideline [27]. Based on cost of a PSA test kit and nurse consultation |
| Polygenic risk stratification | £25 | 20–30 | Callender et al. [13] Estimated from costs charged to NHS hospitals for prostate cancer genome-wide association studies | |
| Biopsy (systematic/MRI targeted) | £581 | 465–697 | Callender et al. [13] Weighted average of cost of transrectal ultrasound-guided and perineal biopsy. Includes relevant histopathology, potential admission for sepsis and cost of a urological appointment | |
| Multiparametric MRI | £339 | 271–407 | NICE guideline. Includes time of two radiographers, an appointment with a consultant, and equipment, administration and consumable costs | |
| Assessing suspected prostate cancer | £545 | 436–654 | Callender et al. Includes an isotope bone scan, assessment by a urological multi-disciplinary team and a further outpatient urological appointment | |
| Prostatectomy | £9808 | 7846–11,770 | Callender et al. Includes an appointment with a urologist and a weighted average of the cost of major open, robotic and laparoscopic radical prostatectomies from NHS reference costs | |
| Radiation therapy | £6462 | 5170–7754 | Callender et al. Includes an appointment with a clinical oncologist, preparation for intensity modulated radiation therapy and outpatient delivery of treatment on a megavoltage machine | |
| Active surveillance (yearly) | £577 | 462–692 | Callender et al. Hao et al. Includes cost of 3 PSA tests and 2 urological appointments Assumes a third of men will need an annual mpMRI and biopsy | |
| Palliative care/terminal illness | £7383 | 5906–8860 | Round et al. [29] Model assumed terminal care for the 6 months prior to a death due to prostate cancer and palliative care for the 6–30 months prior | |
| Utility estimates (measured by EQ-5D) | ||||
| Biopsy | 0.90 | 0.87–0.94 | Normal | Hao et al. Decrement assumed for 3 weeks |
| Cancer diagnosis | 0.80 | 0.75–0.85 | Hao et al. Decrement assumed for 1 month | |
| Prostatectomy part 1 | 0.83 | 0.73–0.91 | Hao et al. Decrement assumed for 2 months | |
| Prostatectomy part 2 | 0.89 | 0.88–0.91 | Hao et al. Decrement assumed for 10 months | |
| Radiation therapy part 1 | 0.82 | 0.75–0.88 | Hao et al. Decrement assumed for 2 months | |
| Radiation therapy part 2 | 0.83 | 0.88–0.91 | Hao et al. Decrement assumed for 10 months | |
| Active surveillance | 0.90 | 0.85–0.95 | Hao et al. Decrement assumed for 7 years | |
| Post-recovery period | 0.86 | 0.84–0.88 | Hao et al. Decrement assumed for 9 years | |
| Palliative therapy | 0.62 | 0.58–0.66 | Hao et al. Decrement assumed for 1 year | |
| Terminal illness | 0.40 | Held constant | Hao et al. Decrement assumed for 6 months | |
| EQ-5D index population norms 18–24 | 0.934 | Held constant | Janssen and Szende [30] | |
| 25–34 | 0.922 | |||
| 35–44 | 0.905 | |||
| 45–54 | 0.849 | |||
| 55–64 | 0.804 | |||
| 65–74 | 0.785 | |||
| 75+ | 0.734 | |||
CI confidence interval, EQ-5D EuroQol 5D, G Gleason score, mpMRI multi-parametric magnetic resonance imaging, NICE National Institute for Health and Care Excellence, NHS National Health Service, PSA prostate-specific antigen
Management pathways in the model included treatment by conservative management, radical prostatectomy or radical radiotherapy, post-treatment follow-up, palliative therapy and terminal care. Probabilities for initial treatment assignment reflected the most recently available data from the National Cancer Registration and Analysis Service (Fig. A7 of the ESM) [31]. Background mortality used UK life tables [32]. The mortality hazard ratio assumed for prostate cancer-specific death in radical treatment over those initially assigned to active surveillance was 0.63 based on the ProtecT trial [33].
Costs and Health State Values
Costs and health state values were from the perspective of the UK National Health Service. Cost parameters used in the model are shown in Table 2. The cost of a PSA test was estimated from the recent NICE guideline on prostate cancer [27]. The costs of prostate biopsy, radical prostatectomy, radical radiotherapy and active surveillance were taken from Callender et al. [13] The cost of mpMRI was based on estimates from the 2019 guidelines on prostate cancer diagnosis and management [27]. Terminal care costs were based on model-based estimates [29]. Costs reported from earlier years were inflated to the 2020 price, UK£ sterling [34].
Health state values were sourced from Hao et al. [28] The health state values used are based on a meta-analysis of estimates measured using the EQ-5D instrument. Quality-adjusted life-year (QALY) norms for the UK were used to reflect background age-specific quality of life, which decreases naturally with age. All state QALYs were multiplied by their age-specific norms to reflect this natural reduction in quality of life. The values used are shown in Table 2[30]. Although Hao et al. also reported utilities based on the disease-specific Patient Oriented Prostate Utility Scale-Utility (PORPUS-U) [35] (see Table A5 of the ESM), we chose to use the EQ-5D health state values in the base case as they have the advantage of being general to any disease area and to correspond with the QALY norms used [28]. A sensitivity analysis using the PORPUS-U estimates was carried out.
Modelling Polygenic Risk
Men with a high polygenic risk score are known to be more susceptible to prostate cancer than men with a low risk. If the polygenic risk is known and varies across a population, then this offers the potential of providing risk-stratified screening programmes. It was assumed that known prostate cancer susceptibility genetic variability follows a log-normal distribution with a mean of −0.68/2 and variance of 0.68 on the natural logarithm scale, such that the frailty has a mean of 1 [13, 36, 37]. This is based on the 175 susceptibility loci for prostate cancer that have been identified in genome-wide association studies [38]. We additionally assumed that there was unmeasured genetic variability on a log-normal distribution with a mean of −1.14/2 and a variance of 1.14, where again the frailty has a mean of 1. This was based on evidence from Kiciński et al. who carried out a meta-analysis on familial clustering of prostate cancer [39]. The estimates of a 10-year absolute risk of a prostate cancer diagnosis for 1-year age groups were based on Callender et al. [40], from which we derived an age-specific rate ratio for the 10-year absolute prostate cancer risk of 7.5% compared with the population risk. Men with a genetic variability above the rate ratio were eligible for screening (Table A3 of the ESM).
Calibration and Validation
The model was calibrated to incidence rates from the Office for National Statistics [41] and data from the CAP trial [8] on prostate cancer incidence by age and Gleason score. On the basis of low levels of PSA testing in the UK, we assumed no PSA testing for both the UK and CAP simulations. The model was adapted to reflect repeat PSA testing in the UK [42]. The rate of prostate cancer onset in the model was first estimated by calibrating the onset parameters to 2017 data on prostate cancer incidence by age provided by the UK Office for National Statistics using a Poisson likelihood [43]. Fixing the estimated onset parameters, we calibrated the proportion of Gleason score 6, 7 and >7 cancers by age, study year and study arm at diagnosis to CAP data after 10 years of follow-up using a separate Poisson likelihood. Further details are given in Sect. 1 of the ESM.
To validate the natural history model, we compared prostate cancer mortality predictions to observed 10-year data from the CAP trial [8]. We also predicted prostate cancer mortality rate ratios comparing screening strategies from ERSPC (16 years follow-up for 4-yearly screening from either age 55 years or age 60 years) [7] and CAP (10 years follow-up for a single screen at age 50 years or aged 60 years) with no screening.
Reporting of Outcomes
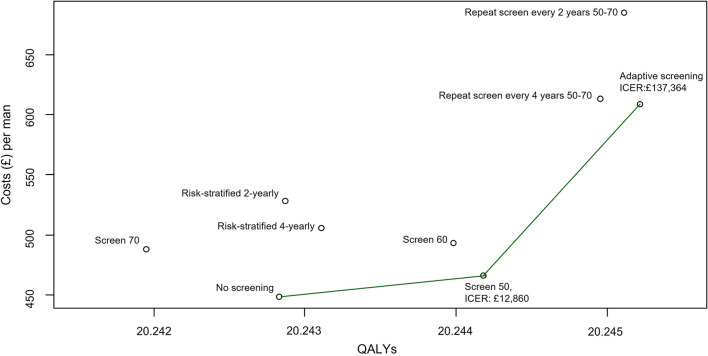
The life histories of 10 million men born in 1950 were simulated with outcomes compared under the different screening strategies. We assumed 85% of men would participate in the first round of screening and 85% biopsy compliance based on the ProtecT trial [8]. Re-screening participation was assumed to be higher at 95% for each screen. The outcomes reported include the difference in the number of pre-biopsy mpMRIs, prostate biopsies, prostate cancer incidence and prostate cancer deaths. The results were reported from age 30 years over a lifetime horizon. For the cost-effectiveness analysis, we reported costs and QALYs, and plotted the cost-effectiveness frontier, which indicates which strategy (or pair of strategies) has the lowest cost per QALY gained; strategies above this frontier should be rejected on cost-effectiveness grounds.
Incremental cost-effectiveness ratios, which are calculated by dividing incremental costs by incremental QALYs, are reported for all interventions compared with the next non-dominated intervention. An intervention is considered to be dominated if it provides less mean QALYs at a higher mean cost than another intervention, and extendedly dominated if it provides less mean QALYs at a higher mean cost than a weighted average of two alternative interventions. An incremental cost-effectiveness ratio (ICER) below £30,000 per QALY gained was considered cost effective in accordance with the NICE reference case [44]. We also reported net monetary benefits at thresholds of £20,000 and £30,000 per QALY gained. These were calculated by multiplying QALYs by the willingness-to-pay (WTP) threshold and subtracting costs [45]. We applied a discount rate of 3.5% to all future costs and QALYs. [44]
Sensitivity Analyses
One-way sensitivity analyses were carried out on key parameters including the unit costs and disutilities associated with a prostate biopsy, mpMRI and treatment. Scenario analyses were also carried out to test the impact of using different health state utility values and screening intervals, age ranges and risk thresholds.
A probabilistic sensitivity analysis was carried out using 1000 replicates for a population of one million men to address the impact of uncertainties in the test accuracies, costs, health state values and natural history parameters (Table 2). To assess the stability of the probabilistic sensitivity analyses, results were compared to those from 100 replicates for a population of 10 million men. Test accuracies and health state values were assumed to be normally distributed on the logit scale. We assumed that the test characteristics were independent. Costs were sampled from a gamma distribution with mean of 1 and a 95% confidence interval (CI) between 0.8 and 1.22. The natural history parameters were assumed to follow a multivariate normal distribution.
Results are presented using cost-effectiveness acceptability curves, which reflect the probability of a strategy being most cost effective at each WTP per QALY gained threshold. The incremental costs and QALYs compared to no screening estimated for each strategy at each iteration are also plotted on the cost-effectiveness plane. The probability of each strategy being optimal at £20,000 and £30,000 per QALY gained was calculated by counting the proportion of samples for which the expected net benefit was highest.
Results
Calibration and Validation
The calibration to incidence data from the UK Office for National Statistics provided a good fit on visual inspection (Fig. A1 of the ESM). The model succeeded in replicating the increase in prostate cancer incidence between the ages of 40 and 70 years with a subsequent levelling off in the older age groups. The calibration results to CAP data are also shown in Figs. A2 and A3 of the ESM. Although the calibrated model did not predict the attenuation of the screening effect on incidence in the later years of the trial, the majority of prostate cancers by age group and Gleason score were well predicted.
For the validation compared with ERSPC, we predicted mortality rate ratios over 16 years follow-up of 0.77 and 0.82 for 4-yearly screening from age 55 and age 60 years, respectively, compared with no screening. The observed mortality rate ratio from ERSPC was 0.80 (95% CI 0.72–0.89). Compared with CAP, we predicted a mortality rate ratio over 10 years follow-up of 0.91 for a single screen at age 50 and age 60 years compared with no screening. The adherence-adjusted mortality rate ratio from CAP was 0.93 (95% CI 0.67–1.29). The predicted prostate cancer mortality was slightly higher in the later years compared with the mortality rates from the CAP trial (Fig. A4 of the ESM).
Outcomes
Table 3 presents predicted outcomes per 10,000 men simulated over a lifetime from the age of 30 years. Compared with no screening, the screening strategies resulted in more biopsies, more prostate cancers diagnosed and fewer prostate cancer deaths. Comparing with 4-yearly age-based screening, risk-stratified screening with 4-yearly intervals was associated with 46% fewer overdiagnosed cancers, 66% fewer pre-biopsy mpMRIs and 63% fewer screen-initiated biopsies. Those reduced costs and harms were at the expense of 8% more prostate cancer deaths.
Table 3.
Predicted outcomes and health economic results from age 30 years by strategy
| No screening | Screen 50 | Screen 70 | Screen 60 | Risk-stratified 4 yearly to age 70 years | Risk stratified 2 yearly to age 70 years | Adaptive screening at age 50–70 years, interval of 6 years if PSA level < 1.5 ng/mL, 4 years if >1.5 ng/mL | Repeat screen every 4 years at age 50–70 years | Repeat screen every 2 years at age 50–70 years | |
|---|---|---|---|---|---|---|---|---|---|
| Outcomes per 10,000 mena | |||||||||
| Number of screens | 0 | 8285 | 7029 | 7978 | 6173 | 10,049 | 31,155 | 40,223 | 65,335 |
| Number of MRI events | 0 | 96 | 1022 | 627 | 834 | 1205 | 2458 | 2423 | 3519 |
| Number of screen-initiated biopsies | 0 | 64 | 631 | 400 | 538 | 750 | 1471 | 1452 | 2013 |
| Number of clinically initiated biopsies | 2817 | 2752 | 2474 | 2491 | 2390 | 2295 | 1925 | 1942 | 1826 |
| Diagnosed PCa | 1487 | 1488 | 1547 | 1509 | 1538 | 1555 | 1583 | 1582 | 1600 |
| Screen diagnosis | 0 | 36 | 245 | 195 | 278 | 345 | 569 | 558 | 636 |
| Metastatic cancer | 254 | 248 | 217 | 226 | 215 | 207 | 173 | 174 | 167 |
| Localised and G <6 | 702 | 705 | 757 | 731 | 757 | 774 | 805 | 803 | 823 |
| Localised and G = 7 | 292 | 296 | 313 | 308 | 313 | 318 | 336 | 336 | 339 |
| Localised and G >7 | 238 | 239 | 260 | 244 | 252 | 256 | 268 | 269 | 271 |
| Overdiagnosis | 0 | 2 | 60 | 22 | 51 | 68 | 97 | 95 | 113 |
| PCa death | 527 | 520 | 512 | 504 | 497 | 489 | 457 | 459 | 444 |
| Life-years, QALYs, costs, ICERs and NMB per man. Strategies sorted by increasing costsa | |||||||||
| Life years (undiscounted) | 51.5370 | 51.5506 | 51.5515 | 51.5671 | 51.5707 | 51.5785 | 51.6237 | 51.6212 | 51.6388 |
| Life-years, 3.5% discounted | 23.5624 | 23.5652 | 23.5646 | 23.5676 | 23.5680 | 23.5692 | 23.5775 | 23.5771 | 23.5801 |
| QALYs (undiscounted) | 42.518 | 42.527 | 42.519 | 42.534 | 42.532 | 42.535 | 42.560 | 42.559 | 42.567 |
| QALYs, 3.5% discounted | 20.2428 | 20.2442 | 20.2419 | 20.2440 | 20.2431 | 20.2429 | 20.2452 | 20.2450 | 20.2451 |
| NHS costs, undiscounted | 2013 | 2042 | 2152 | 2117 | 2161 | 2224 | 2414 | 2424 | 2610 |
| NHS costs, 3.5% discounted (£) | 449 | 466 | 488 | 493 | 506 | 528 | 609 | 613 | 685 |
| ICER | £12,860 | Dominated | Extendedly dominated | Extendedly dominated | Extendedly dominated | £137,364 | Dominated | Extendedly dominated | |
| Net monetary benefit (£20k)b | 404,408 | 404,417 | 404,351 | 404,386 | 404,356 | 404,329 | 404,296 | 404,286 | 404,217 |
| Net monetary benefit (£30k)b | 606,836 | 606,859 | 606,771 | 606,826 | 606,787 | 606,758 | 606,748 | 606,735 | 606,668 |
G Gleason score, ICER incremental cost-effectiveness ratio, MRI magnetic resonance imaging, NHS National Health Service, NMB net monetary benefit, PCa prostate cancer, QALYs quality-adjusted life-years
aBased on life histories of 10 million simulated men
bStrategy with the highest net monetary benefit highlighted in bold
Costs and QALYs
The strategies in Table 3 are sorted by the predicted mean costs. The lowest costs were found for no PSA screening and the highest for repeat PSA screening every 2 years for all men between the ages of 50 and 70 years (for detailed costs, see Table A4 of the ESM). All screening strategies other than a once-off screen at age 70 years resulted in a slight increase in QALYs compared with no screening. Compared with no screening, the mean net monetary benefit was lower for all screening strategies at both a £20,000 and £30,000 per QALY gained threshold, other than a once-off screen at age 50 years. Note that the risk-stratified strategies, although not cost effective compared to no screening, had higher net monetary benefits than their age-based equivalents.
The strategies on the cost-effectiveness frontier, as shown in Fig. 1, were no screening, a once-off screen at 50 and adaptive screening. The ICER of moving from a policy of no screening to a once-off screen at 50 is £12,860 per QALY gained, which is under the £20,000 per QALY gained threshold recommended by NICE and therefore considered to be cost effective. Adaptive screening, although on the frontier, would not be considered cost effective owing to the high ICER of moving to this strategy from a once-off screen at 50 (£137,364 per QALY gained).
Fig. 1.
Cost-effectiveness frontier. ICER stands for Incremental Cost-Effectiveness Ratio
Although risk-stratified screening is not on the frontier, it is interesting to note that the ICER of moving from a strategy of 4-yearly age-based screening to 4-yearly risk-stratified screening is £19,882 per QALY gained and from 2-yearly age based to 2-yearly risk stratified is £25,687 per QALY gained, suggesting an improvement in cost effectiveness for risk-stratified approaches compared with age based at current WTP thresholds.
Sensitivity Analyses
One-Way and Scenario Analyses
Figure A6 in the ESM plots the results of the sensitivity analysis using PORPUS-U utility estimates rather than EQ-5D on the cost-effectiveness frontier. Applying the PORPUS-U weights leads to a change in the optimal policy choice. A once-off screen at age 50 years is still found to be cost effective compared with no screening with an ICER of £8996 per QALY gained. However, in this sensitivity analysis, the ICER for the comparison of adaptive screening to a once-off screen at age 50 years is lower than the £20,000–£30,000 WTP threshold recommended by NICE at £16,236 per QALY gained and would therefore be recommended. Repeat screening every 2 years from age 50 to 70 years is also on the cost-effectiveness frontier with an ICER compared to adaptive screening of £58,116 per QALY gained.
The one-way sensitivity analyses further highlight the importance of the utility estimates used. The impact on the ICER comparing a once-off screen at age 50 years to no screening is shown in Fig. A8 of the ESM. Of the parameters tested, those with the biggest impact were the utilities associated with active surveillance and the post-recovery period.
Section 9 of the ESM details the results of the scenario analysis considering different start and stop ages, intervals and risk thresholds. Figure A10 of the ESM shows that most alternative strategies tested resulted in a negative expected net benefit compared with their respective base-case scenarios. The exceptions were the scenario analyses considering risk thresholds of 5% and 10% as opposed to 7.5% in the risk-stratified screening strategies. At a £30,000 WTP threshold, the strategies using risk thresholds of 5% resulted in an increased net benefit compared with those using a threshold of 7.5%. Conversely, at a £20,000 per QALY gained WTP threshold, the strategies using risk thresholds of 10% resulted in an increased net benefit relative to those using a 7.5% threshold. This suggests that further research around optimal risk thresholds is warranted. Figure A11 of the ESM demonstrates the impact of the alternative strategies on life-years gained. As expected, more intensive strategies result in increased life-years. Figure A12 and Table A8 of the ESM detail all predicted outcomes and health economic results from the alternative strategies. These show that the strategies on the frontier are unchanged from those considered in the base case.
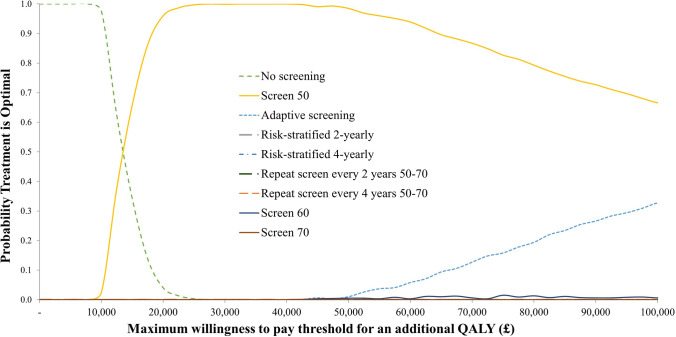
Probabilistic Sensitivity Analysis
The cost-effectiveness acceptability curves shown in Fig. 2 highlight the separation of strategies by the WTP threshold. At any WTP threshold above £15,000 per QALY gained, the strategy most likely to be optimal is a once-off screen at age 50 years. At a WTP threshold of £20,000 per QALY gained, a once-off screen at age 50 years has a 95.5% probability of being optimal with no screening having a 4.5% chance. At a threshold of £30,000 per QALY gained, the probability that a once-off screen at age 50 years is optimal increases to 99.9%.
Fig. 2.
Cost-effectiveness acceptability curves. QALY stands for Quality Adjusted Life Year
Figure A9 in the ESM shows the estimated costs and effects of each strategy at each PSA iteration plotted on the cost-effectiveness plane. Figure A13 of the ESM plots the CEAC using 100 replicates for 10 million men. This shows the results are very similar even with a much larger number of men simulated.
Discussion
In summary, the natural history model calibrated well to prostate cancer incidence in the UK and gave mortality rate ratios that were broadly consistent with the CAP and ERSPC trials. Of the strategies compared, a once-off PSA screen at age 50 years was the only strategy that would be considered cost effective at the threshold recommended by NICE. Although this strategy was estimated to avert only seven prostate cancer deaths per 10,000 men compared with no screening, it also resulted in only two over-diagnosed cancers per 10,000 men. Our analysis suggests that this improvement in quantity and quality of life is enough to justify the increased costs of screening at current WTP per QALY gained thresholds. The finding was sensitive to the health state utility values used with a sensitivity analysis finding adaptive screening to be potentially cost effective. There was evidence to suggest that the genetic risk-stratified screening strategies may be cost effective compared with their age-based alternatives, but not to the other strategies considered. There was uncertainty as to the optimal risk threshold of the risk-stratified screening strategies.
Comparison with Previous Studies
Two previous UK cost-effectiveness analyses have found that a PSA screening strategy with risk stratification by polygenic risk score was associated with an improvement in cost effectiveness compared with age-based screening [13, 40]. The studies by Callender and colleagues compared age-based 4-yearly PSA screening to risk-based 4-yearly PSA screening at different risk thresholds, with no comparison of once-off PSA screens or adaptive PSA screening. Making the same comparison in our analysis (risk based 4 yearly vs age based 4 yearly) gives an ICER of £19,882, also suggesting potential cost effectiveness, but not in comparison to less intensive screening strategies such as a once-off screen at age 50 years.
In both our study and those of Callender et al., risk-stratified screening was associated with fewer overdiagnoses and biopsies and lower costs than age-based screening but with more deaths from prostate cancer. The magnitude of expected differences between the strategies varied, with the most recent Callender et al. paper finding risk-stratified PSA screening with the use of mpMRI-targeted biopsy to be associated with 60% fewer over-diagnosed cancers at a 7.5% risk threshold compared with 46% in this study. Callender et al. predicted around 50% fewer pre-biopsy mpMRIs and prostate biopsies compared with age-based screening, compared with around 65% in this analysis. They found 12% more deaths from prostate cancer with risk-stratified PSA screening compared with our finding of 8%. These differences are likely due to the life table-based approach to modelling used in the Callender et al. papers where simplifying assumptions were made with regard to the effect of screening, for example, a 15% reduction in advanced cancer at diagnosis if screened.
Two previous studies were identified comparing the cost effectiveness of once-off and repeat universal screens to adaptive screening [46, 47]. One study [47] assumed that men with PSA levels above the median for their age are rescreened in 2 years and the rest return in 4 years. That study also found that adaptive screening was on the cost-effective frontier when considering cost per life-year gained but that there was a reduction in QALYs for all screening strategies compared with no screening [47]. Similar to our study, this result was very sensitive to the utility estimates used. The other study [46] compared universal screening to adaptive strategies where the screening interval was every 1 year if the PSA level was higher than 3.0 ng/mL and every 2 years otherwise, or the screening interval was every 2 years if the PSA level was higher than 1.0 ng/mL and every 4 years otherwise. They found that the latter strategy compared favourably to no screening, with an ICER under the acceptable threshold, although the best performing strategies were non-adaptive.
Heijnsdijk et al. used the MIcrosimulation SCreening Analysis (MISCAN) model to determine optimal prostate cancer screening intervals and ages based on data from ERSPC [48]. They found that screening strategies with intervals of 3 years are more cost effective than those using longer intervals whereas we found an interval of 4 years to be preferable to 3 years. Both studies agreed that a lower age to stop screening is preferable with our scenario analysis finding that stopping screening at age 70 years achieves a higher net benefit than stopping at age 74 years.
The most recent UK cost-effectiveness analysis comparing once-off and repeat age-based PSA screening strategies identified was that of Hummel and Chilcott in 2013 [49]. Hummel and Chilcott concluded that PSA screening was not effective compared with no screening. However, it is unclear whether their model was well calibrated to the 11-year prostate cancer mortality rate ratio from ERSPC [50].
Strengths and Limitations
One strength of this analysis is the detailed natural history model used to compare the strategies, which has been calibrated to rich UK data sources, including the large CAP trial. The natural history model predicted mortality rate ratios that were close to the point estimates from ERSPC and CAP and well within their 95% CIs. A further strength is the comparison of strategies in the base case that were chosen as relevant by a group of experts in a recent Delphi consensus process [25]. The strategies compared give an accurate picture of the potential impact of relevant screening strategies in the UK today.
A limitation is that our analysis of risk-stratified screening made several basic assumptions. One is that there will be no screening in lower risk groups with a common screening strategy for those at higher risk. An alternative strategy might involve different re-screening intervals for high-risk and low-risk groups [51]. Similar to the work of Callender and colleagues [13, 40], our analysis also assumed that a higher risk score impacts only on the risk of developing cancer, with no impact on progression, and that genetic samples for all men would be available at a low cost of acquisition (£25). In terms of risk thresholds, we have taken the threshold found to be optimal by Callender et al. [13] as our starting point and explored different thresholds around this. In reality, the optimal risk threshold, and that which would be deemed acceptable by men and their caregivers in terms of the trade-off between overdiagnosis and prostate cancer deaths averted, is still uncertain. Further research is needed in the area of polygenic risk scores and risk-stratified screening to more accurately model these options.
Recommendations for Future Research
Although the base-case analysis and scenario analyses have explored a wide range of screening strategies, there is scope for further exploration. As an absolute absence of PSA screening is unlikely to be achieved in countries where opportunistic screening is increasingly common, future research could consider comparing potential screening strategies to opportunistic screening, rather than no screening. This approach would require accurate data on PSA testing, pre-biopsy MRIs and prostate biopsies. Future research might also explore the efficiency of other low-intensity strategies such as screening twice over a lifetime, considering our finding that a once-off screen at age 50 years is optimal. Additional exploration of adaptive strategies with different PSA thresholds and intervals may also be useful.
The choice of screening model may affect the predicted cost effectiveness. A stage-shift model, which was used in this analysis, assumes that the benefit associated with screening is due to a shift to a less advanced stage at diagnosis. Cure models assume that a proportion of cancers detected earlier are cured [52]. Previous analyses have shown that predicted mortality reductions depend on the model used [53, 54]. Future research could explore the use of another mechanism of screening benefit with the UK data.
The sensitivity analyses suggested that the health state values assigned to prostate cancer health states are important parameters. Two sets of health state values were available. In comparison to the estimates when using the disease-specific PORPUS-U measure, the EQ-5D estimates assumed lower health-related quality of life associated with prostatectomy, radiation therapy, active surveillance, the post-recovery period and palliative therapy. This has the effect of making the diagnosis and treatment of prostate cancers less cost effective. The strategy that dominates is therefore that which diagnoses the least cancers—a once-off screen at age 50 years. Our sensitivity analysis using the PORPUS-U estimates showed that adaptive screening may be cost effective if the health-related quality of life associated with these states is higher. This suggests that up-to-date EQ-5D surveys of men with prostate cancer are warranted.
Clinical practice and diagnostic pathways for men at risk of prostate cancer continue to evolve, including the use of trans-perineal biopsy with image registration [55] and radio-labelled prostate-specific membrane, antigen positron emission tomography computed tomography [56, 57]. Our results should be interpreted cautiously in the light of better diagnostic and staging pathways that may impact on cost effectiveness. In addition, the calibration to CAP data was based on data available at 10-years follow-up. Additional follow-up of CAP to 15 years may reduce uncertainty about the cost effectiveness of the screening strategies.
Future cost-effectiveness research could conduct a value of information analysis using our model to formally identify parameters on which to focus further trials. Additionally, this model has assumed the use of pre-biopsy MRI in a screening setting, following NICE recommendations [27], but future work could explore the cost effectiveness of MRI in a UK setting. Finally, future models could build on this work to improve the analysis of risk-stratified screening and explore different risk thresholds, which our scenario analyses have shown are influential.
Conclusions
There is evidence that PSA testing is being undertaken opportunistically [42] As this is likely to continue, it is important for policymakers to consider the optimal approach for screening. This analysis has used data from the 10-year follow-up of the CAP trial to show that a once-off screen at age 50 years has the potential to be clinically effective and cost effective in a UK setting. Updating the model using data from the 15-year follow-up of the trial may reduce uncertainty about the cost effectiveness of the screening strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the contributions of all members of the CAP and ProtecT trial research groups, of all the CAP and ProtecT study participants and the CAP trial general practitioners and practice staff. We also acknowledge Ruth Etzioni, Shuang Hao and Andreas Karlsson for their contributions to the development of the simulation model.
Declarations
Funding
RMM is the lead principal investigator (and JLD, FCH and DEN co-PIs) of the CAP trial, funded by Cancer Research UK and the UK Department of Health (C11043/A4286, C18281/A8145, C18281/A11326, C18281/A15064 and C18281/A24432). RMM was supported by a Cancer Research UK (C18281/A29019) programme grant (the Integrative Cancer Epidemiology Programme). RMM is a National Institute for Health Research Senior Investigator (NIHR202411). RMM and HT were supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. The ProtecT trial is funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment Programme (NIHR HTA: projects 96/20/06, 96/20/99). RG was supported by the National Cancer Institute at the National Institutes of Health (U01 CA253915 and R50 CA221836). HT was supported by a MRC grant MR/S036709/1 for this research. MSC was supported by the Swedish Research Council (through the Swedish eScience Research Centre and project 2018-02526), Prostatacancerförbundet, Karolinska Institutet and Cancerfonden (project CAN 2018/539). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflicts of interest/competing interests
EK reports personal consulting fees unrelated to the present work from Pfizer, Roche, Novartis Pharma and BMS. SS reports a postdoctoral fellowship from the NIHR and support for travel from the University of Leeds. JD, RM, ET and EW report receiving grants to their institution from Cancer Research UK. RG reports receiving grants from the National Cancer Institute. HT reports receiving grants to his institution from the UK Medical Research Council, personal consulting fees unrelated to the present work from Pfizer, Roche, BMS, Eisai, Lundbeck, Merck, Novartis Pharma, and Janssen and owns stock in Clifton Insight (consulting company). MC reports receiving funding to his institution from the Swedish Research Council, the Swedish Cancer Society and the Swedish Prostate Cancer Federation.
Ethics approval
Ethical approval was not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Because of the nature of this research, participants of this study did not agree for their data to be shared publicly, thus supporting data are not available.
Code availability
Not applicable.
Author contributions
EK, SS, RMM, ELT, HT and MSC contributed to the study conception and design. MSC developed the model and assisted with the analyses. EK performed the analyses with guidance from SS, RMM, RG, FW, ELT, HT and MSC. ELW assisted with data provision. The first draft of the manuscript was written by EK and MSC and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Transparency declaration
EK affirms that this article is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Equal contribution by Sabina Sanghera, Richard M. Martin, Emma L. Turner and Howard Thom.
References
- 1.Parker C, Gillessen S, Heidenreich A, et al. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl_5):v69–77. doi: 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 2.Nam RK, Oliver TK, Vickers AJ, et al. Prostate-specific antigen test for prostate cancer screening: American Society of Clinical Oncology provisional clinical opinion. J Oncol Pract. 2012;8(5):315. doi: 10.1200/JOP.2012.000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 draft recommendation statement on screening for prostate cancer: an invitation to review and comment. JAMA. 2017;317(19):1949–1950. doi: 10.1001/jama.2017.4413. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67(2):100–121. doi: 10.3322/caac.21392. [DOI] [PubMed] [Google Scholar]
- 5.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 6.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugosson J, Roobol MJ, Mansson M, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur Urol. 2019;76(1):43–51. doi: 10.1016/j.eururo.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity psa-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883–895. doi: 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pashayan N, Duffy SW, Neal DE, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med. 2015;17(10):789–795. doi: 10.1038/gim.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callender T, Emberton M, Morris S, et al. Benefit, harm, and cost-effectiveness associated with magnetic resonance imaging before biopsy in age-based and risk-stratified screening for prostate cancer. JAMA Netw Open. 2021;4(3):e2037657. doi: 10.1001/jamanetworkopen.2020.37657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sipeky C, Talala KM, Tammela TLJ, et al. Prostate cancer risk prediction using a polygenic risk score. Sci Rep. 2020;10(1):17075. doi: 10.1038/s41598-020-74172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auvinen A, Rannikko A, Taari K, et al. A randomized trial of early detection of clinically significant prostate cancer (ProScreen): study design and rationale. Eur J Epidemiol. 2017;32(6):521–527. doi: 10.1007/s10654-017-0292-5. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Eastham JA, Scardino PT, et al. The Memorial Sloan Kettering Cancer Center recommendations for prostate cancer screening. Urology. 2016;91:12–18. doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 19.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eklund M, Jäderling F, Discacciati A, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med. 2021;85(10):908–920. doi: 10.1056/NEJMoa2100852. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson A, Jauhiainen A, Gulati R, et al. A natural history model for planning prostate cancer testing: calibration and validation using Swedish registry data. PLoS ONE. 2019;14(2):e0211918. doi: 10.1371/journal.pone.0211918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etzioni R, Gulati R, Falcon S, et al. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making. 2008;28(3):323–331. doi: 10.1177/0272989X07312719. [DOI] [PubMed] [Google Scholar]
- 23.Gulati R, Inoue L, Katcher J, et al. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11(4):707–719. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies. Ann Intern Med. 2013;158(3):145–153. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keeney E, Thom H, Turner E, et al. Using a modified Delphi approach to gain consensus on relevant comparators in a cost-effectiveness model: application to prostate cancer screening. Pharmacoeconomics. 2021;39(5):589–600. doi: 10.1007/s40273-021-01009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purysko AS, Rosenkrantz AB, Barentsz JO, et al. PI-RADS Version 2: a pictorial update. Radiographics. 2016;36(5):1354–1372. doi: 10.1148/rg.2016150234. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence. (2019). Prostate cancer: diagnosis and management [NICE Guideline No. 131]. https://www.nice.org.uk/guidance/ng131. Accessed 30 Sep 2022
- 28.Hao S, Karlsson A, Heintz E, et al. Cost-effectiveness of magnetic resonance imaging in prostate cancer screening: a microsimulation study. Value Health. 2021;24(12):1763–1772. doi: 10.1016/j.jval.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med. 2015;29(10):899–907. doi: 10.1177/0269216315595203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer, Netherlands; 2014. pp. 19–30. [Google Scholar]
- 31.National cancer registration and analysis service 2021. https://www.cancerdata.nhs.uk/getdataout/prostate. Accessed 13 May 2021.
- 32.Office for National Statistics. "National life tables: UK." (2020).
- 33.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 34.Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6(1):51–59. doi: 10.1332/174426410x482999. [DOI] [Google Scholar]
- 35.Krahn M, Ritvo P, Irvine J, et al. Construction of the Patient-Oriented Prostate Utility Scale (PORPUS): a multiattribute health state classification system for prostate cancer. J Clin Epidemiol. 2000;53(9):920–930. doi: 10.1016/S0895-4356(00)00211-0. [DOI] [PubMed] [Google Scholar]
- 36.Dadaev T, Saunders EJ, Newcombe PJ, et al. Fine-mapping of prostate cancer susceptibility loci in a large meta-analysis identifies candidate causal variants. Nat Commun. 2018;9(1):2256. doi: 10.1038/s41467-018-04109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. doi: 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matejcic M, Saunders EJ, Dadaev T, et al. Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-06863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiciński M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS ONE. 2011;6(10):e27130. doi: 10.1371/journal.pone.0027130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callender T, Emberton M, Morris S, et al. Polygenic risk-tailored screening for prostate cancer: a benefit–harm and cost-effectiveness modelling study. PLoS Med. 2019;16(12):e1002998. doi: 10.1371/journal.pmed.1002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Office for National Statistics. "Cancer survival in England: adults diagnosed between 2013 and 2017 and followed up to 2018." (2018).
- 42.Young GJ, Harrison S, Turner EL, et al. Prostate-specific antigen (PSA) testing of men in UK general practice: a 10-year longitudinal cohort study. BMJ Open. 2017;7(10):e017729. doi: 10.1136/bmjopen-2017-017729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caul S, Broggio J. Cancer registration statistics, England. Off Natl Stat. 2017;2019:1–16. [Google Scholar]
- 44.National Institute for Health and Care Excellence. (2013). Guide to the methods of technology appraisal 2013. [PubMed]
- 45.Paulden M. Calculating and interpreting ICERs and net benefit. Pharmacoeconomics. 2020;38(8):785–807. doi: 10.1007/s40273-020-00914-6. [DOI] [PubMed] [Google Scholar]
- 46.Roth JA, Gulati R, Gore JL, et al. Economic analysis of prostate-specific antigen screening and selective treatment strategies. JAMA Oncol. 2016;2:890. doi: 10.1001/jamaoncol.2015.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pataky R, Gulati R, Etzioni R, et al. Is prostate cancer screening cost-effective? A microsimulation model of prostate-specific antigen-based screening for British Columbia, Canada. Int J Cancer. 2014;135(4):939–947. doi: 10.1002/ijc.28732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heijnsdijk EAM, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst. 2014;107(1):366. doi: 10.1093/jnci/dju366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hummel S, Chilcott J. Option appraisal: screening for prostate cancer model update. ScHARR, Sheffield: University of Sheffield; 2013. [Google Scholar]
- 50.Roobol MJ, Kranse R, Bangma CH, et al. Screening for prostate cancer: results of the Rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol. 2013;64(4):530–539. doi: 10.1016/j.eururo.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 51.O’Mahony JF. Risk stratification in cost-effectiveness analyses of cancer screening: intervention eligibility, strategy choice, and optimality. Med Decis Making. 2021;42(4):513–523. doi: 10.1177/0272989X211050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wever EM, Hugosson J, Heijnsdijk EA, et al. To be screened or not to be screened? Modeling the consequences of PSA screening for the individual. Br J Cancer. 2012;107(5):778–784. doi: 10.1038/bjc.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wever EM, Draisma G, Heijnsdijk EA, et al. How does early detection by screening affect disease progression? Modeling estimated benefits in prostate cancer screening. Med Decis Making. 2011;31(4):550–558. doi: 10.1177/0272989x10396717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heijnsdijk EAM, Gulati R, Lange JM, et al. Evaluation of prostate cancer screening strategies in a low-resource, high-risk population in the Bahamas. JAMA Health Forum. 2022;3(5):e221116. doi: 10.1001/jamahealthforum.2022.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamid S, Donaldson IA, Hu Y, et al. The SmartTarget Biopsy Trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur Urol. 2019;75(5):733–740. doi: 10.1016/j.eururo.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murthy V, Sonni I, Jariwala N, et al. The role of PSMA PET/CT and PET/MRI in the initial staging of prostate cancer. Eur Urol Focus. 2021;7(2):258–266. doi: 10.1016/j.euf.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216. doi: 10.1016/s0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.