Abstract
Introduction
Chlormethine (CL) gel is a skin-directed therapy approved for treatment of stage IA/IB mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) in the USA. MF-CTCL has a chronic clinical course, requiring long-term maintenance therapy with one or more therapies. This analysis describes real-world patterns of maintenance therapy and use of concomitant therapy with CL gel among patients with stage IA/IB MF-CTCL.
Methods
In a US-based registry, MF-CTCL patients treated with CL gel were enrolled between 3/2015 and 10/2018 across 46 centers and followed for up to 2 years. Patient demographics, clinical characteristics, CL gel treatment patterns, concomitant treatments, clinical response, and adverse events (AEs) were collected from medical records. Descriptive statistics are reported.
Results
Of the 206 patients with stage IA/IB MF-CTCL, 58.7% were male, and average age was 60.7 years with 4.6 years since diagnosis. Topical steroids, phototherapy, and topical retinoids were used concomitantly with CL gel in 62.6%, 26.2%, and 6.3% of patients, respectively. Most concomitant therapies (up to 85%) were started before CL gel initiation and, in about half of the cases (up to 57%), were used concurrently for ≥ 12 months. Overall, 158 (76.7%) patients experienced partial response (PR) and 144 continued with maintenance therapy. After achieving PR, most patients (74.3%) kept the same maintenance therapy schedule, most commonly once daily. Of patients who had any skin-related AE (31.6%) or skin-related AEs associated with CL gel (28.2%), nearly half experienced CL gel treatment interruption and ~40% had a dosing reduction. The observed real-world treatment patterns were concordant with National Comprehensive Cancer Network (NCCN) guidelines.
Conclusion
The study results suggest that continuing CL gel maintenance therapy and combining treatments with CL gel are common practice in the real-world setting, with most maintained on a stable dosing schedule. Careful management of AEs may help patients maintain long-term optimal dosing with less treatment interruptions and dosing reductions.
Keywords: Chlormethine gel, Mechlorethamine gel, Mycosis fungoides-type cutaneous T-cell lymphoma, MF-CTCL, Registry, Real-world setting, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) has a chronic clinical course, requiring long-term maintenance therapy with one or more therapies. |
| Chlormethine (CL) gel is a skin-directed therapy approved for treatment of stage IA/IB MF-CTCL in the USA. |
| The objective of this study is to describe the real-world patterns of maintenance therapy and use of concomitant therapy with CL gel among patients with stage IA/IB MF-CTCL. |
| What was learned from the study? |
| Continuing CL gel maintenance therapy and combining treatments with CL gel are common practices in the real-world setting, with most patients maintained on a stable dosing schedule. |
| Deliberate management of adverse events may help patients maintain long-term optimal dosing with less treatment interruptions and dosing reductions. |
Introduction
Mycosis fungoides (MF) is a cutaneous T-cell form of non-Hodgkin lymphoma (MF-CTCL). Chlormethine (CL) gel (mechlorethamine) is a Food and Drug Administration (FDA)-approved skin-directed therapy for the treatment of stage IA and IB MF-CTCL in the USA [1]. The same formulation was approved by the European Medicines Agency in 2017 for treatment of adult patients with MF, and is now available commercially in a number of European countries [2]. CL gel is also approved in Israel and several other countries worldwide [3]. Patient experiences have been collected in a product-specific MF-CTCL registry called A PROspective, Observational, US-based Study Assessing Outcomes, Adverse Events, Treatment Patterns, and Quality of Life in Patients Diagnosed with MF-CTCL and Treated with Valchlor (PROVe) [4]. The study reported that CL gel contributed to reduced severity of MF-CTCL and improved health-related quality of life.
MF-CTCL has a slowly progressive, chronic clinical course, and patients at early stages may require long-term maintenance therapy as well as concomitant therapies. Analyses of the PROVe study data could provide better understanding of how maintenance therapy is used in a real-world setting. Prior evidence supports the need for this analysis, where concomitant use of topical corticosteroids was shown to improve management of CL-induced contact dermatitis [5]. The PROVe study reported that topical steroids and phototherapy were commonly used concomitantly with CL gel by MF-CTCL patients, but additional post hoc analyses are required to understand the use of these concomitant therapies in a real-world setting. Because MF-CTCL is a rare disease, many clinicians care for a small number of patients and do not have the opportunity to accumulate experience of a diverse patient population. Data from the collective experience of a registry are important to inform clinical decisions [6]. The objective of this analysis is to report real-world patterns of maintenance therapy and use of concomitant therapy with CL gel in patients with stage IA/IB MF-CTCL.
Methods
The PROVe study was a US-based prospective, observational, non-interventional study, assessing outcomes, adverse events, treatment patterns, and quality of life in patients diagnosed with MF-CTCL treated with CL gel [4]. Information on patient demographics, medical history, clinical characteristics, concurrent treatments for MF-CTCL, and response were collected for patients from 46 centers between March 2015 and October 2018. Patients (n = 298) across all cutaneous lymphoma stages were enrolled and prospectively followed for up to 2 years until either the end of the follow-up period, the end of CL gel use, or until withdrawal from the study. Reasons for study termination may include patient withdrew consent, patient loss to follow-up, adverse event, physician’s decision to withdraw, or termination of the study by the sponsor. The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines. The appropriateness of the study protocol and all risks and benefits to participants were approved by institutional review boards.
The current post hoc analysis focused on the 206 patients with stage IA/IB MF-CTCL at CL gel initiation out of the original 298 patients. MF-CTCL stage information prior to CL gel initiation was used if available, or stage information at enrollment was used. Among patients with unavailable stage information, those who have received prior systemic therapy were conservatively classified as having stage II or higher and were therefore not included in this analysis.
Treatment Response
Partial response (PR) was defined as ≥ 50% reduction in body surface area (BSA) from the time of CL gel initiation to the current visit BSA, or a reported “partial response” on the clinician assessment questions: “What is patient’s response at ongoing visits?” and “What is response post-CL gel treatment and before enrollment?”. Complete response (CR) was defined as BSA of 0 or a reported “complete response” after CL gel initiation on the basis of the clinical assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?”. For only patients who achieved PR, deepening response (DR) was defined as improvements by another 10% beyond the initial 50% BSA reduction. For subjects who did not have BSA information to evaluate DR, a reported “complete response” to clinician assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?” that followed a previous PR was considered as having achieved DR. Duration of PR/CR was assessed as the number of days between the date of first PR/CR to earlier of end of study or loss of response. Loss of response was defined for the patients after they achieved CR as two BSA values below 50% improvement or addition of systemic therapy or “relapse”/“progressive” reported on the basis of the clinical assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?”.
Concomitant and Maintenance Therapy
Treatment start and stop dates for topical steroids, phototherapy (PUVA, UVB, or nbUVB), and topical retinoids were analyzed in relation to CL gel therapy initiation to assess concomitant medication sequence and duration of overlap. Concomitant use was defined as use of CL gel and another skin-directed therapy during the study period, and overlap was defined as at least 1 day of concurrent use (i.e., overlap and concurrent use are interchangeable in this report). The relationship between concomitant/concurrent therapy use and treatment response (PR/CR) was evaluated. Maintenance therapy, defined as continuing treatment with CL gel after achieving PR, was also described. The association between CL gel treatment patterns (treatment schedules, interruption, discontinuation, and treatment duration) and treatment response (PR/DR) was evaluated.
Treatment Interruption and Discontinuation
Treatment interruption was defined as a gap of < 3 months between two CL gel treatment episodes, whereas discontinuation was a gap of ≥ 3 months between CL gel treatment episodes. Episodes were defined on the basis of CL gel start and stop dates.
Safety
Any reported adverse events (AEs) and, separately, skin-related AEs were described. The association between AEs and CL gel treatment interruption or dose reduction was evaluated. AEs were documented as related to CL gel treatment, as determined by study investigators.
Statistical Analysis
Descriptive analyses of patient demographics, clinical characteristics, concomitant treatment, treatment patterns, treatment response, and adverse events were conducted. Frequency distribution is reported for categorical variables. Mean, standard deviation, median, and first/third quartiles are reported for continuous variables. Bivariate analyses were conducted to explore association between variables.
Results
Patient Demographics and Clinical Characteristics
There were 206 patients with stage IA/IB MF-CTCL at study enrollment, of whom 58.7% were male, with mean age of 60.7 years (SD 13.9 years) and mean duration of time since MF-CTCL diagnosis of 4.6 years (SD 6.5 years) (Table 1). Most patients (99.5%) were existing CL gel users at registry enrollment, and nearly half (47.8%) had initiated CL gel more than 180 days prior to enrollment. The mean BSA at CL gel initiation (before or within 30 days) was 11.9% (SD 16.0%). Before CL gel initiation, three-quarters (78.2%) of patients had prior skin-directed treatment and one-quarter (25.2%) had prior systemic therapy (patients may have used one or both types of therapies) (Table 1).
Table 1.
Patient characteristics
| Characteristics | |
|---|---|
| Patients with stage IA/IBa, n (%) | 206 (100.0) |
| CL gel duration, days, mean (SD) | 650 (347) |
| Patients on CL gel prior to enrollment, n (%) | 205 (99.5) |
| Duration between enrollment and CL initiation, n (%) | |
| 1–30 days | 28 (13.7) |
| 31–90 days | 45 (22.0) |
| 91–180 days | 34 (16.6) |
| > 180 days | 98 (47.8) |
| Not applicable | 1 (0.5) |
| Age, mean (SD), years | 60.7 (13.9) |
| Age, median (IQR), years | 61 (54.0–71.0) |
| Female sex, n (%) | 85 (41.3) |
| Race/ethnicity, n (%) | |
| Asian | 9 (4.4) |
| Black | 30 (14.6) |
| Hispanic or Latino | 18 (8.7) |
| Native Hawaiian or other Pacific Islander | 1 (0.5) |
| White | 144 (70.0) |
| Unknown or two or more races/ethnicities | 4 (1.9) |
| Baseline duration of MF-CTCL at CL gel initiation, years | |
| Mean (SD) | 4.6 (6.5) |
| Median (Q1–Q3) | 2.0 (0.0–6.0) |
| BSA at enrollment of the study, m | |
| n | 173 |
| Mean (SD) | 9.1 (12.2) |
| Median (Q1–Q3) | 5.0 (2.0–10.0) |
| BSA before or within 30 days of CL gel initiation (used for PR/CR assessment), m | |
| n | 118 |
| Mean (SD) | 11.9 (16.0) |
| Median (Q1–Q3) | 6.0 (2.0–13.0) |
| Prior skin-directed treatment (at CL gel initiation), n (%) | 161 (78.2) |
| Prior systemic therapy (at CL gel initiation), n (%) | 52 (25.4) |
| Medical history, n (%) | |
| Non-melanoma skin cancer | 43 (20.9) |
| Prior viral infections | 20 (9.7) |
| Atopic disorder | 24 (11.7) |
| Psoriasis | 4 (1.9) |
| Urticaria | 8 (3.9) |
| Mental disorders | 8 (3.9) |
| Relevant family history (i.e., lymphoma) | 12 (5.8) |
| Secondary malignancies | 4 (1.9) |
| Other malignancies | 34 (16.5) |
CL chlormethine, SD standard deviation, IQR interquartile range, MF-CTCL mycosis fungoides-type cutaneous T-cell lymphoma, BSA body surface area
aDefinition of stage: if stage information prior to CL gel initiation was available, it was used. If not, stage information at enrollment was used. If patient was still defined as having unknown stage and patient had received prior systemic therapy, patient was classified as having stage II or higher
Use of Concomitant Therapy
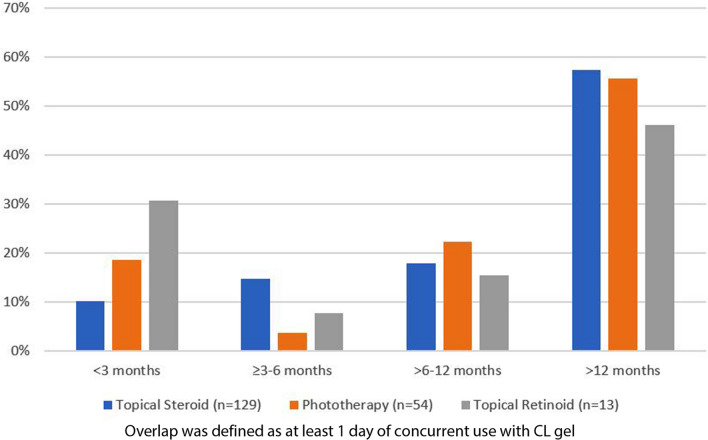
Over the entire follow-up period, 62.6% of patients used topical steroid and 26.2% of patients used phototherapy concurrently with CL gel. Only 6% of patients used topical retinoids concurrently with CL gel (Table 2). Most of these concomitant therapies (between 76% and 85%) were started prior to CL gel initiation (Table 2). About half of patients (between 46% and 57%) used these therapies concurrently for at least 12 months (Fig. 1). Patients using CL gel achieved a similar level of PR/CR response with or without concurrent topical steroid use (79.8% versus 88.2%, P = 0.185), phototherapy use (77.8% versus 84.6%, P = 0.299), or topical retinoid use (84.6% versus 83.0%, P = 0.884) (Table 2).
Table 2.
Use of concomitant therapies and outcomes
| Use of concomitant therapy | P valuec | P valuec | P valuec | |||
|---|---|---|---|---|---|---|
| Total, n (%) | 206 (100.0) | 206 (100.0) | 206 (100.0) | |||
| Treatment use, n (%) | Topical steroid | Phototherapya | Topical retinoids | |||
| No | 51 (24.8) | 91 (44.2) | 171 (83.0) | |||
| Yes | 155 (75.2) | 115 (55.8) | 35 (17.0) | |||
| Patients with overlapb with CL gel, n (%) | 129 (62.6) | 54 (26.2) | 13 (6.3) | |||
| Treatment initiation, n (%) | ||||||
| Prior to CL gel | 98 (76.0) | 45 (83.3) | 11 (84.6) | |||
| After CL gel initiation | 31 (24.0) | 9 (16.7) | 2 (15.4) | |||
| Patients with PR/CR, n (%) | ||||||
| Among patients who DID NOT use concomitant treatment | 45 (88.2) | 0.216 | 77 (84.6) | 0.482 | 142 (83.0) | 0.667 |
| Among patients who USED concomitant treatment | 125 (80.6) | 93 (80.9) | 28 (80.0) | |||
| Among patients who had concurrent use with CL gel | 103 (79.8) | 0.185d | 42 (77.8) | 0.299d | 11 (84.6) | 0.884d |
CL chlormethine, PR partial response, CR complete response
aPhototherapy included phototherapy PUVA, phototherapy UVB, and phototherapy nbUVB
bConcomitant use was defined as use of CL gel and another skin-directed therapy during the study period, and overlap was defined as at least 1 day of concurrent use
cP values were determined by chi-squared or Fischer exact test
dP value compared with those who DID NOT use concomitant treatment
Fig. 1.
CL gel duration of overlap with concurrent treatment
CL Gel Schedule
At CL gel initiation, most patients used the therapy daily (57.3%), while the remaining patients more commonly used an every second (25.7%) or every third day (11.2%) dosing schedule (Table 3). The mean duration of CL gel use was 650 days (Table 1). Most patients used CL gel for more than 12 months (77.7%) or for 6–12 months (13.1%) (Table 3). About 28% used CL gel every second day for more than 6 months. Approximately 32% of patients on CL gel experienced treatment interruption; however, very few patients (12.6%) experienced complete discontinuation of CL gel treatment (Table 3).
Table 3.
Relationship between CL gel treatment patterns and treatment response
| CL gel treatment pattern | Overall n (%) | Patients with partial response n (% of overall) |
P valueb | Patients with deepening response after PR n (% of PR) |
P valueb |
|---|---|---|---|---|---|
| Total | 206 (100.0) | 158 (76.7) | 46 (29.1) | ||
| CL gel schedule at initiation | |||||
| Daily | 118 (57.3) | 88 (74.6) | 24 (27.3) | ||
| Five times per week | 5 (2.4) | 4 (80.0) | 2 (50.0) | ||
| Every second day | 53 (25.7) | 42 (79.3) | 12 (28.6) | ||
| Every third day | 23 (11.2) | 19 (82.6) | 6 (31.6) | ||
| One time per week | 3 (1.5) | 3 (100.0) | 1 (33.3) | ||
| Less frequently/unknown | 4 (1.9) | 2 (50.0) | 1 (50.0) | ||
| CL gel treatment interruption (< 3 months) | |||||
| Yes | 66 (32.0) | 51 (77.3) | 0.894 | 17 (33.3) | 0.417 |
| No | 140 (68.0) | 107 (76.4) | 29 (27.1) | ||
| CL gel treatment discontinuation (≥ 3 months)a | |||||
| Yes | 26 (12.6) | 16 (61.5) | 0.050 | 4 (25.0) | 0.456 |
| No | 180 (87.4) | 142 (78.9) | 42 (29.6) | ||
| CL gel duration categories (during entire study period) | |||||
| 0–30 days | 1 (0.5) | 1 (100.0) | 0 (0.0) | ||
| 31–90 days | 8 (3.9) | 5 (62.5) | 3 (60.0) | ||
| 91–180 days | 10 (4.9) | 8 (80.0) | 2 (25.0) | ||
| 181–360 days | 27 (13.1) | 21 (77.8) | 8 (38.1) | ||
| > 360 days | 160 (77.7) | 123 (76.9) | 33 (26.8) | ||
CL chlormethine, PR partial response
aDiscontinuation and restarting CL gel treatment. This does not account for patients who discontinued and never started again
bP values were determined by chi-squared or Fischer exact test
Overall Treatment Response
Overall, 170 (82.5%) patients experienced either partial or complete response (Table 4). Most of these patients (158, 76.7%) achieved PR with CL gel by a median time of 240 days, and the median duration of response was 280 days (Table 4). Forty-six (29.1%) of these patients also experienced further deepening of response after PR over a median time of 405 days (Table 4). Complete response was achieved by 47 (22.8%) patients by a median time of 301 days (Table 4).
Table 4.
Overall treatment response
| Total, n (%) | 206 (100.0) |
| Response type | |
| Complete/partial response (includes patients who achieved PR, CR, or both PR and CR), n (%) | 170 (82.5) |
| Time to complete/partial response, days | |
| Mean (SD) | 309.2 (263.7) |
| Median (Q1–Q3) | 234 (94.0–493.0) |
| Duration of CR/PR, daysa (stop at earlier of end of study or loss of responsef) | |
| Mean (SD) | 357.9 (254.9) |
| Median (Q1–Q3) | 366 (153.0–513.0) |
| Partial response (PR)b, n (%) | 158 (76.7) |
| Time to partial response, days | |
| Mean (SD) | 312.2 (262.5) |
| Median (Q1–Q3) | 240 (97.0–499.0) |
| Duration of PR, daysc (stop at earlier of end of study or loss of responseg or CR) | |
| Mean (SD) | 310.2 (241.2) |
| Median (Q1–Q3) | 279.5 (119.0–399.0) |
| Achieved deepening response (DR)d, n (%) | 46 (29.1) |
| Time to first deepening response | |
| Mean (SD) | 459.11 (230.5) |
| Median (Q1–Q3) | 404.5 (273.0–644.0) |
| Complete response (CR)e, n (%) | 47 (22.8) |
| Time to complete response, days | |
| Mean (SD) | 369.0 (251.6) |
| Median (Q1–Q3) | 301 (172.0–551.0) |
PR partial response, CR complete response, DR deepening response
aDuration of PR/CR assessed as days between date of first PR/CR to earlier of end of study or loss of response
bPartial response defined as 50% improvement in BSA (e.g., 30–15%) from time of CL gel initiation to current visit BSA, or “partial response” reported based on the clinical assessment questions
cDuration of PR assessed as days between date of first PR to earlier of end of study, loss of response, or CR
dDeepening response defined for the patients after they achieved PR as improvements by ≥ 10% past 50% in BSA. For subjects who did not have BSA information to evaluate DR, a reported “complete response” per to clinician assessment questions: “What is patient’s response at ongoing visits=” or “What is response post-CL gel treatment and before enrollment?” that followed a previous PR was considered as having achieved DR
eComplete response was defined as BSA = 0 OR “complete response” after CL gel initiation reported based on the clinical assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?”
fLoss of response defined for the patients after they achieved CR as two BSA values below 50% improvement OR addition of systemic therapy, or “relapse” or “progressive” reported based on the clinical assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?”
gLoss of response defined for the patients after they achieved PR as two BSA values below 50% improvement, or addition of systemic therapy, or “relapse” or “progressive” reported based on the clinical assessment questions: “What is patient’s response at ongoing visits?” or “What is response post-CL gel treatment and before enrollment?”
Chlormethine Gel Use Prior to PR
Of the 158 patients who achieved PR; prior to PR, 93 (58.9%) patients used CL gel daily and most patients (77.2%) did not change their CL gel schedule prior to achieving response (Table 5). Among those who had a change or a switch (n = 36, 22.8%), a decrease (n = 26, 72.2%) in schedule was most commonly experienced (Table 5). Similarly, of 46 patients who achieved deepening response after PR, 56.5% (n = 26) had used CL gel daily and 80.4% (n = 37) maintained the same dosing schedule prior to response (Table 5).
Table 5.
CL gel dosing schedule changes pre- and post-PR and maintenance therapy
| Among patients with PRa | Patients with PR n (%) | Patients with DRb n (%) |
|---|---|---|
| Total | 158 (100.0) | 46 (100.0) |
| Schedule/intensity immediately pre-PRc | ||
| Daily | 93 (58.9) | 26 (56.5) |
| Five times per week | 5 (3.2) | 2 (4.4) |
| Every second day | 35 (22.2) | 11 (23.9) |
| Every third day | 18 (11.4) | 5 (10.9) |
| One time per week | 2 (1.3) | 1 (2.2) |
| Less frequently/unknown | 5 (3.2) | 1 (2.2) |
| Schedule changes Pre-PRc | ||
| Stable | 122 (77.2) | 37 (80.4) |
| Change/switch | 36 (22.8) | 9 (19.6) |
| Increased at least onced in pre-PR period | 12 (33.3) | 3 (33.3) |
| Decreased at least onced in pre-PR period | 26 (72.2) | 7 (77.8) |
| Maintenance schedule/intensity post-PRe | ||
| NA (PR achieved after end of CL gel therapy) | 14 (8.9) | 2 (4.4) |
| Patients who received maintenance therapy | 144 (91.1) | 44 (95.6) |
| Same schedule as Pre-PR | 107 (74.3) | 33 (75.0) |
| Increased at least onced from pre-PR | 17 (11.8) | 4 (9.1) |
| Decreased at least onced from pre-PR | 22 (15.3) | 8 (18.2) |
PR partial response, DR deepening response
aPartial response defined as 50% improvement in BSA (e.g., 30–15%) from time of CL gel initiation to current visit BSA, or “partial response” reported based on the clinical assessment questions
bDeepening response defined for the patients after they achieved PR as improvements by 10% past 50% in BSA, or “complete response” based on the clinical assessment questions
cPre-PR period is considered from CL gel initiation date to the day prior to date of PR. Patients may fall into more than one category; e.g., the same patient may have first increased and then decreased frequency pre-PR
dCategories “Increase at least once” and “Decrease at least once” are not mutually exclusive. A patient may have both increased and decreased the intensity in specific period
ePost-PR period is considered from date of PR to earlier of date of CR/date of loss of response/study end date. Patients may fall into more than one category; e.g., the same patient may have first increased and then decreased frequency post-PR
Maintenance Therapy after PR Achievement
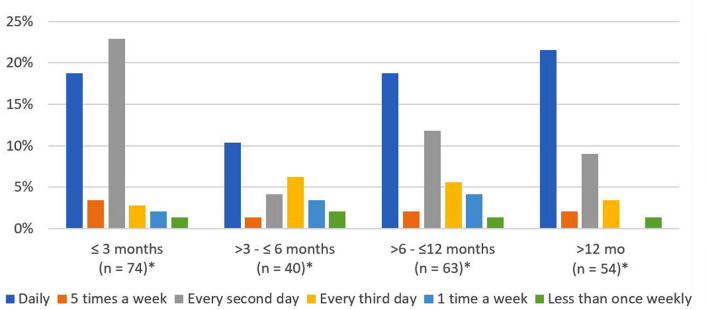
Among the 158 patients who achieved PR, 144 (91.1%) received CL gel maintenance therapy. Among those patients who achieved PR and received CL gel maintenance therapy, most (107, 74.3%) maintained the same schedule, which typically was once daily (Table 5). Approximately 40% and 21.5% used daily maintenance CL gel for at least 6 months and at least 12 months, respectively (Fig. 2). During the post-PR period, 17 patients (11.8%) increased while 22 (15.3%) decreased their CL schedule (Table 5). Among patients with PR, 46 further achieved DR, and 44 of them continued with maintenance therapy. Similar to the overall group of patients who achieved PR, more than half of the patients with DR had once daily dosing schedule immediately prior to PR. Of the patients who achieved DR, most (n = 33, 75%) used the same dosing schedule as prior to response (Table 5). Duration of maintenance use was long, with most patients (n = 30 for 6–12 months, n = 77 for > 12 months) maintaining on CL gel for longer than 6 months. For all durations of maintenance use, CL gel once daily use remained a common schedule after PR (Fig. 2).
Fig. 2.
Duration of CL gel maintenance use by dosing schedule. *The sum of the number of subjects across all duration categories exceeds 144 (100%) because subjects can be classified into multiple CL gel dosing schedules over time
Adverse Events (AEs)
Overall, 42.7% of patients experienced any AE and 30.1% had any AE related to CL gel (Table 6). Common AEs with incidence ≥ 5% included dermatitis, pruritus, and skin irritation (Table 6). Approximately 31.6% of patients had skin-related AE and 28.2% had skin-related AE associated with CL gel (Table 6). Among those who had any AE or a skin-related AE, nearly half experienced interruption or discontinuation of CL therapy, and approximately two out of five patients experienced a reduction in CL gel dosing schedule (Table 6). By comparison, patients without AEs experienced fewer treatment interruptions or discontinuation (20.3%) and dose reduction (22.0%) (results not shown).
Table 6.
Adverse events and CL gel treatment changes (any time during study period)
| Adverse events | n (%) |
|---|---|
| Total | 206 (100.0) |
| Any adverse event | |
| Overall | 88 (42.7) |
| CL gel related | 62 (30.1) |
| CL gel treatment interruption (< 3 or > 3 months) | |
| Overall | 42 (47.7) |
| CL gel related | 31 (50.0) |
| Any dose reduction in CL gel schedule | |
| Overall | 32 (36.4) |
| CL gel related | 26 (41.9) |
| Skin-related AEa | |
| Overall | 65 (31.6) |
| CL gel related | 58 (28.2) |
| CL gel treatment interruption (< 3 or > 3 months) | |
| Overall | 32 (49.2) |
| CL gel related | 28 (48.3) |
| Any dose reduction in CL gel schedule | |
| Overall | 26 (40.0) |
| CL gel related | 24 (41.4) |
| Frequency of any AE with ≥ 5% incidenceb | |
| Dermatitis | 33 (16.0) |
| Not assessed | 3 (9.1) |
| Mild | 13 (39.4) |
| Moderate | 12 (36.4) |
| Severe | 5 (15.2) |
| Pruritus | 22 (10.7) |
| Skin irritation | 16 (7.8) |
AE adverse event, CL chlormethine
aSkin-related AEs include “dermatitis,” “dry skin,” “erythema,” “pruritis,” “rash,” “skin burning sensation,” “skin erosion,” “skin hyperpigmentation,” “skin irritation,” “skin ulceration,” “blistering,” and “skin ulceration or blistering”
bThe same patient may have multiple AEs and was counted in each AE for the frequency of any AE analysis
Discussion
The current analysis of data from the PROVe registry shows that maintenance therapy with CL gel was commonly continued after achievement of PR. Of the 158 patients who achieved PR, most (n = 144 patients, 91.1%) continued with maintenance therapy and most (n = 107 patients, 74.3%) retained the same schedule as pre-PR (i.e., once daily). CL gel was often used in combination with other MF-CTCL treatments, most commonly with topical steroid (62.6%), followed by phototherapy (26.2%) and topical retinoid (6%). The AE patterns showed that patients who had any AEs experienced more occurrences of treatment interruption and dose reduction.
The findings of this analysis suggest good tolerability of topical CL gel as indicated by the wide adoption of daily treatment schedules with flexibility to vary dosing as needed. The majority of patients received the FDA-approved once daily frequency, while others initiated less than daily, but half of them subsequently increased frequency. While the majority remained on stable dosage, dosing was flexible and CL gel frequency can be tailored by physicians to best serve patients in a real-world setting. Real-world evidence from patients in the registry shows that maintenance therapy was received by a majority of patients, and about half of these patients received maintenance therapy for more than 1 year. This practice pattern is aligned with the current NCCN guidelines, which recommend using individualized regimens with good tolerability for long duration of therapy to be given in an ongoing or maintenance fashion [7].
A recent retrospective study conducted by researchers at the Thomas Jefferson University showed that patients of MF-CTCL of all stages who were receiving maintenance CL gel therapy demonstrated improvement in modified Severity Weighted Assessment Tool (mSWAT) and quality of life [8]. In addition, CL gel therapy may be an effective maintenance regimen as the patients in the study had progression-free survival for a median of 29.45 months. Our analysis provided further findings that CL gel maintenance therapy is routinely utilized in real-world practice settings, particularly among patients with stage IA/IB MF-CTCL.
Among the patients in the PROVe registry, CL gel was commonly used concomitantly with several other skin-directed therapy for MF-CTCL, including topical steroid, phototherapy, and topical retinoid. Most of the concurrent uses had a duration of 12 months or longer, indicating good tolerability. Additionally, approximately 75% of the patients received maintenance therapy with CL gel in combination with other therapies and continued with the same schedule after PR. The use of combination skin-directed therapies for stage IA/IB MF-CTCL is in accordance with the current NCCN guidelines [7].
The lower rates of skin-related AEs observed in the previously published PROVe analysis may be related to this flexible schedule approach [4]. Patients who had AEs experienced more treatment interruptions or dosing reductions but were able to continue CL therapy at a modified regimen. These observations further emphasize the importance of AE management, as suggested by Gilmore et al. that maximizing the tolerance of CL gel is an important way to strengthen the effectiveness of the therapy [5]. Therefore, appropriate management of AEs may allow patients to maintain long-term therapy at the optimal dosing schedules.
Limitations
Clinical care across patient populations is typically characterized as heterogeneous with respect to timing of healthcare, so patients may be seen at irregular intervals and across different durations of time; therefore, responses were not evaluated at predefined intervals. As this observational study was designed to understand clinical practice patterns in the real-world setting, the registry collected clinical data documenting relevant events, such as starting and stopping of therapy, dose changes, and use of other therapies, that were not negatively affected by varying time intervals or duration of follow-up.
Healthcare in the real-world setting is also characterized by heterogeneity in how clinicians identify patient outcomes, and therefore our analysis accounted for this heterogeneity. As proper assessment of an outcome depends on the completeness of data, we ensured that any relevant data in the medical record were appropriately used in categorizing an outcome. For example, response was evaluated based on clinician assessment and/or data on reported percentage BSA over time, as present in the medical record. Nearly half of patients started CL gel at least 6 months before study enrollment. This limited available data on BSA, PR, or CR from the period between CL gel initiation and enrollment, which could be useful to evaluate the extent that DR occurred before study enrollment; therefore, the true DR rate may be underestimated.
The changes in dosing schedules for concomitant medications were not collected systematically in the registry, thus the associations between these changes with CL gel schedule changes were not evaluated in this analysis. Future studies on this topic could further understanding of optimal multimodel therapy in MF-CTCL patients.
Conclusions
This study finds that continuing CL gel maintenance therapy was provided to a majority of patients with stage IA/IB MF-CTCL, with most patients maintained on a stable dosing schedule. These observations suggest that, in real-life settings, combination treatments with CL gel are common practice as physicians utilize long-term concurrent therapies. Deliberate management of adverse events may help patients maintain long-term optimal dosing with less treatment interruptions and dosing reductions.
Acknowledgements
Funding
This study was supported by Helsinn Therapeutics US, Inc., who were involved in the analysis plan and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript and the decision to submit it for publication. Helsinn Therapeutics US, Inc. also funded the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided by Genesis Research and funded by Helsinn Therapeutics US, Inc.
Author Contributions
Concept and design: CQ, DG, CLP, MT, JTA, LJG. Analysis and interpretation: CQ, WWN, DG, CLP, QVD, MT, JTA, LJG. Drafting of manuscript: CQ, WWN, DG, CLP, QVD, MT, JTA, LJG. Critical revision of the manuscript: CQ, WWN, DG, CLP, QVD, MT, JTA, LJG.
Prior Presentations
Parts of this study have been previously presented at the following scientific meetings: (1) poster presentation at the 2021 American Academy of Dermatology in San Francisco, USA (March 19–23, 2021) and (2) poster presentation at EORTC Cutaneous Lymphoma Group 20–21 Meeting in Marseille, France (October 14–16, 2021).
Disclosures
Christiane Querfeld has received a research grant from Celgene; acted as clinical investigator for Celgene, Trillium, miRagen, Bionez, Helsinn and Kyowa Kirin; and served on a steering committee or advisory board for Helsinn/Actelion, miRagen, Bioniz, Trillium, and Kyowa Kirin. Winnie W. Nelson and James T. Angello are employees of Helsinn Therapeutics US, Inc. Marco Turini is an employee of Helsinn Healthcare SA. Deval Gor and Quan V. Doan are consultants employed by Genesis Research, a healthcare consultancy that received funding from Helsinn Therapeutics to conduct this research. Chris L. Pashos is an advisor to Genesis Research. Larisa J. Geskin has received a research grant from Actelion; consulting fees or honorarium from Actelion, Helsinn, Mallinckrodt, Sanofi, and Regeneron; and served on a speakers’ bureau for Helsinn.
Compliance with Ethics Guidelines
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. The appropriateness of the study protocol and all risks and benefits to participants were approved by the Western Institutional Review Board (approval number: 11/18/2014). This is a post-hoc analysis of registry data.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Christiane Querfeld, Email: cquerfeld@coh.org.
Winnie W. Nelson, Email: Winnie.nelson@helsinn.com
Deval Gor, Email: deval@genesisrg.com.
Chris L. Pashos, Email: chris@genesisrg.com
Quan V. Doan, Email: quan@genesisrg.com
Marco Turini, Email: Marco.Turini@helsinn.com.
James T. Angello, Email: James.Angello@helsinn.com
Larisa J. Geskin, Email: ljg2145@cumc.columbia.edu
References
- 1.VALCHOR [package insert]. Helsinn Therapeutics US, Inc., 2020.
- 2.Ledaga. INN—chlormethine; Annex I, Summary of product characteristics. European Medicines Agency. [cited 2022 June 28]. https://www.ema.europa.eu/en/documents/product-information/ledaga-epar-product-information_en.pdf.
- 3.Geskin LJ, et al. Chlormethine gel for the treatment of skin lesions in all stages of mycosis fungoides cutaneous T-cell lymphoma: a narrative review and international experience. Dermatol Ther (Heidelb) 2021;11(4):1085–1106. doi: 10.1007/s13555-021-00539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EJ, et al. The PROVe study: US real-world experience with chlormethine/mechlorethamine gel in combination with other therapies for patients with mycosis fungoides cutaneous T-cell lymphoma. Am J Clin Dermatol. 2021;22(3):407–414. doi: 10.1007/s40257-021-00591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore ES, et al. Evaluation and management of patients with early-stage mycosis fungoides who interrupt or discontinue topical mechlorethamine gel because of dermatitis. JAAD Case Rep. 2020;6(9):878–881. doi: 10.1016/j.jdcr.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querfeld C, et al. A little experience goes a long way: chlormethine/mechlorethamine treatment duration as a function of clinician-level patient volume for mycosis fungoides cutaneous T-Cell lymphoma (MF-CTCL)—a retrospective cohort study. Front Med. 2021 doi: 10.3389/fmed.2021.679294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN. Clinical practice guidelines in oncology. Primary cutaneous lymphoma. Version 1.2022. 2022 April 2022]. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- 8.Correia E, et al. Response to chlormethine/mechlorethamine gel maintenance treatment regimen in patients with mycosis fungoides: a single-center retrospective study. Clin Lymphoma Myeloma Leuk. 2022;22:581–588. doi: 10.1016/j.clml.2022.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.