Abstract
Background
Osteoarthritis (OA) is a chronic disease that may cause articular cartilage degeneration, and synovial inflammation, resulting in considerable pain, poor quality of life, and functional limitations. Previous research has shown that ECM degradation and inflammation are involved in the progression of OA. Stevioside (STE), a naturally diterpenoid glycoside, is isolated from the Stevia rebaudiana (Bertoni), which has been exerted a variety of pharmacological activities, involving anti-inflammatory, anti-oxidative, and neuroprotective effects. However, STE's effects on OA and its mechanism still need further research.
Methods
In the present study, we examined the anti-inflammatory effects of STE (STE) in both mouse chondrocytes and OA model induced by destabilization of the medial meniscus (DMM). In vitro, the mouse chondrocytes were treated with STE (0, 10, 20, 40 M, 24 h) after stimulated with IL-1β (10 ng/mL, 24 h). The expression of ant-inflammation-relative mediators iNOS and Cox-2 were detected by Western blot and RT-PCR. The catabolic factors (MMP-13, ADAMTS-4) and cartilage matrix constituent (Aggrecan, Collagen II) were measured by Western blot and Immunofluorescence staining. The Nrf2/HO-1/NF-κB signaling molecules were detected by Western blot. In vivo, histological analysis was used to evaluate the severity of mouse OA models.
Results
STE remarkably inhibited the IL-1β-induced expression of iNOS and Cox-2, generation of MMP-13, ADAMTS-4 and degradation of Aggrecan and Collagen II. Furthermore, we found that the chondroprotective effect of STE via Nrf2/HO-1/NF-κB signaling pathway. In vivo, the cartilage treated with STE displayed attenuated degeneration, low OARIS scores compared with DMM group. In conclusion, we considered that STE might be a promising therapeutic agent for the treatment of OA in future.
Conclusions
Our findings indicated that STE can ameliorate the development of OA via inhibiting the inflammation. The underlying mechanism may be related to the Nrf2/HO-1/NF-κB signaling pathway. Moreover, the treatment of STE significantly relieves the progression in the mouse DMM model. All of the results demonstrated the therapeutic of STE in OA treatment.
The translational potential of this article
This study demonstrates a more efficient and safe application of STE in treating osteoarthritis, provide a new concept for the cartilage targeted application of natural compounds.
Keywords: Osteoarthritis, Stevioside, Anti-inflammation, Nrf2/HO-1/NF-κB signaling pathway, Potential agent
1. Introduction
Osteoarthritis (OA) is a chronic disease that may cause articular cartilage degeneration, subchondral bone thickening, and synovial inflammation, resulting in considerable pain, poor quality of life, and functional limitations [1,2]. So far, its clear cause is still unclear; however, Current research has identified inflammation as a key factor in the pathogenesis of OA [3,4]. A typical feature of OA is the increase in pro-inflammatory cytokines produced by articular cartilage cells, such as tumor necrosis factor (TNF-α) and interleukin-1β (IL-1 killing factor), which may lead to cartilage destruction [5]. IL-1β protein is produced highly in stimulated chondrocytes and activated synoviocytes in OA [6]. IL-1β involves in motivating enzymes related to cartilage degradation, then reduced the synthesis of primary cartilage components [7,8]. Besides IL-1β triggers the production of other destructive and inflammatory mediators of OA including prostaglandin-E2 and nitric oxide (NO) [9]. Therefore, inhibiting the IL-1β inhibitor-induced inflammatory response is a promising therapeutic goal for treating OA.
The NF-kB pathway has been uncovered to participate in osteoarthritis and exert a decisive effect [10,11]. The NF-kB signal pathway plays a significant role in regulating the inflammatory and immune systems to ameliorate injury. Stimulated via IL-1β, IKK (IκB kinase) was activated by a series of membrane-proximal events. Then, phosphorylated IκBs triggers NF-κB releases which led to activating gene transcription and nuclear translocation, finally resulting in a series of inflammatory responses [12]. Nrf2 can regulate the expression of metabolic and cytoprotective genes via inducing its downstream target genes [13]. Previous research has been demonstrated that Nrf2 could inhibit inflammation by attenuating the expression of the NF-κB pathway [[14], [15], [16]]. Therefore, Targeting Nrf2/NF-kB may have an effective therapeutic effect in OA.
Stevioside (STE), a major constituent of the herb Stevia rebaudiana, possesses a variety of pharmacological activities, involving anti-oxidative, anti-inflammatory anti-cancer, and neuroprotective effects [[16], [17], [18]]. It has been demonstrated that STE exerts the neuroprotective effect in CoCl2-induced hypoxianeutal cells via inhibiting MAPK and NF-kB pathways [19]. Some scholars have found that STE protects against lipopolysaccharide-induced intestinal mucosal damage through the NF-κB pathway in broiler chickens [20]. Besides, STE can alleviate mouse myocardial fibrosis induced by isoproterenol via the myocardial NF-κB/TGF-β1/Smad axis [21]. But whether STE has an anti-inflammatory effect on OA still needs further research. In our study, we designed in vivo and in vitro experiments to clarify its potential mode of action in the progress of OA.
2. Materials and methods
2.1. Experimental animals and ethics statement
The operating steps involving animal care procedures were performed strictly following the guidelines for Animal Care and Use outlined by the Committee of Wenzhou Medical University.
2.2. Chemicals and reagents
Stevioside (STE) (purity ≥98%) was obtained from MedChemExpress (Shanghai, China). Recombinant mouse IL-1β was acquired from Novoprotein (China). Safranin-O/Fast Green and collagenase-II were procured from Solarbio (Beijing, China). Antibodies of collagen II, aggrecan, Nrf2, ADAMTS-4, and Lamin B1 were purchased from Abcam (Cambridge, UK). GAPDH, p65, HO-1, IkBα, and iNOS were purchased from Boster Biological Technology (Wuhan, China). MMP-13 and COX-2 were obtained from Cell Signaling Technology (Danvers, MA, USA). Fetal bovine serum (FBS), Dulbecco's modified Eagles' medium (DMEM)/F12, and 0.25% trypsin-ethylenediaminetetraacetic acid (trypsin–EDTA) were acquired from Gibco (Grand Island, NY, USA). Bovine serum albumin (BSA) was procured from Beyotime Biotechnology (Shanghai, China). The second antibodies of Goat Anti-Mouse IgG, Goat Anti-Rabbit IgG, Alexa Fluor®594 and Alexa Fluor®488 labeled were purchased from Bioworld (OH, USA).
2.3. The isolation and cultivation of primary mouse chondrocyte
First, took the knee joint from the articular cartilage and femoral head of mice were rinsed with PBS more than 3 times. Next, digested the tissues and then centrifuged the digested chondrocytes at 37 °C. The chondrocytes were cultured in a 100 mm culture bottle. When the chondrocyte confluence rate reached 85%–90%, the chondrocytes were treated with STE and IL-1β, and then the cells were obtained with 0.25% trypsin–EDTA (Djibouti, Invitrogen). The medium was replaced every 2–3 days. When the confluence reached 85–90%, the chondrocytes were subcultured with 0.25% trypsin–EDTA solution.
2.4. Experimental design
In vitro studies, to determine the effect of concentration on the protective effect of STE on cartilage, we incubated mouse chondrocytes with diverse doses (0, 5, 10, 20, 40, 80,160 μM) of STE for 24 h. Then we stimulated the cells with IL-1β (10 ng mL−1) or with nothing. We only exchanged the medium in the control group. We cultured the cells for 24 h and then collected the cells. As for in vivo experiments, both the OA group and the STE group used surgery to change the stability of DMM of the right joint, while the left joint was the sham operation group. Then the experimental group was orally administered STE (50 mg kg−1 day−1) twice a day for 8 weeks. Mice in the vehicle group were given the same amount of saline. All the animals were reared under standard feeding and environmental conditions (temperature: 20 ± 2 °C, humidity: 50% ± 10%, day and night cycle for 12 h), and were given normal food and water. Eight weeks after the operation, the mouse was executed, and preserved joint tissue was for further analysis.
2.5. siRNA transfection
We obtained the Nrf2 small interfering RNA (siRNA) from Invitrogen (Carlsbad, California, USA). We seeded cells in 6-well plates and 24 h later were transfected with Nrf2-siRNA duplexes or negative controls using 50 nM Lipofectamine 2000 siRNA transfection reagent (Thermo Fisher, UT, USA) for 36 h. The sequence of the Nrf2-siRNA was: sense, 5′- UUGGGAUUCACGCAUAGGAGCACUG-3′; antisense, 5′-CAGUGCUCCUAUGCGUGAAUCCCAA-3′.
2.6. Immunofluorescence staining of chondrocytes
For staining of MMP-13 and type II collagen, we inoculated chondrocytes (5 × 104 cells per well), incubate them for 24 h with or without STE (40 μM), then add IL-1β protein (10 ng mL−1) for 2 h or add nothing. We incubated IL-1β (10 ng mL−1) For Nrf2 and p65 staining and the time was performed for 2 h. Then, we used PBS three times before fixing 4 %paraformaldehyde. Next, we washed the cells more than three times in PBS and 0.25% Triton X-100 was used at 25 °C for 10 min. Finally, bovine serum albumin was used to seal the cells and then we incubated the cells with primary antibodies against MMP-13 (1:100), collagen Ⅱ (1:100), Nrf2(1:100), and p65 (1:100) overnight at 4 °C. After that, we incubated the cells for 1 h with a FITC-conjugated secondary antibody (1:400) after washing three times. Finally, we used DAPI to stain the cell nuclei. Eventually, we chose five random visual fields to observe the result.
2.7. Animal models
Forty-five C57BL/6 male mice, which were wild-type (WT) and in the age of 10 weeks, were sourced from the Shanghai Animal Center of the Chinese Academy of Sciences. The experimental steps were followed the Laboratory Animal Use and Care Guidelines of the National Institutes of Health. We destabilized the medial meniscus (DMM) to make OA models [22]. The dose of 2% pentobarbital (40 mg kg−1) intraperitoneal injection was used to anesthetize the mice. We obtained the joint capsule and cut the tibial ligament of the medial meniscus in the right joint. Sham surgeries were applied on another side. Finally, we divided all the animals evenly into three groups (15 in each group): control group (sham operation), OA group (OA), and STE-treated OA group (STE).
2.8. Cell viability assay
The CCK-8 kit was used to evaluate the viability of chondrocytes. First, the passage 3 chondrocytes were seeded into a 96-well plate (4000 cells/well) and incubated for 24 h. We incubated cells for 24 h and 48 h under the concentration gradient of STE (5,10, 20, 40, 80 160 μM) when the cells reached 90%–95% confluence. Then, we incubated half of the cells in IL-1β (10 ng/mL) for 24 h. Finally, we added 10 mol/l CCK-8 solutions to each well and observed the optical density at 450 nm with a spectrophotometer (Thermo Fisher Scientific) 2 h later.
2.9. ELISA
We used the ELISA kits (R&D Systems, Minneapolis, MN USA) to evaluate the concentration of PGE2, TNF-a, IL-6 according to the manufacturer s instructions. And we used the Griess reaction to determine the expression of NO. All assays were repeated three times.
2.10. Real-time PCR
We exposed cells to diverse doses of STE and IL-1β (10 ng/mL), then we isolated total mouse chondrocyte RNA from cells in a 6 cm culture plate. cDNA was acquired from a total RNA 1000 ng (MBI Fermantas, Germany). Real-time quantitative PCR (qPCR) was performed via the CFX96 Real-Time PCR System (Bio-Rad Laboratories, California, USA). 10 μl of reaction volume was conducted in the qPCR in the conditions like 10 min 95 °C, followed by 40 cycles of 15 s 95 °C and 1 min 60 °C. We normalized the target mRNA level to the GAPDH level and matched it with the control group. We used NCBI Primer-Blast ToolPrimers to design the primers of iNOS, IL-6, COX-2, TNF-α, MMP-13, ADAMTS-4, Aggrecan, and Collagen II, and the sequences are listed below: iNOS (F) 5′-GACGAGACGGATAGGCAGAG-3′ (R) 5′-CACATGCAAGGAAGGGAACT-3'; COX-2 (F) 5′–TCCTCACATCCCTGAGAACC-3′ (R) 5′-GTCGCACACTCTGTTGTGCT-3′; IL-6 (F) 5′-CCGGAGAGGAGACTTCACAG-3′(R)5′-TCCACGATTTCCCAGAGAAC-3′; TNF-α(F)5′-ACGGCATGGATCTCAAAGAC-3′ (R) 5′-GTGGGTGAGGAG CACGTAGT-3′; MMP13 (F) 5′-CCA GAACTTCCCAACCAT-3′ (R) 5′-ACCCTCCATAATGTCATACC-3′; Aggrecan (F) 5′-AAGTGCTATGCTGG CTGGTT-3′ (R) 5′-GGTCTGGTTGGGGTAGAGGT-3′; Collagen II (F) 5′-CTCAAGTCGCTGAACAACCA-3′ (R) 5′-GTCTCCGCTCTTC CACTCTG-3′.
2.11. Western blotting
Total protein was obtained by lysing chondrocytes and centrifuged at 12 000 rpm for 15 min at 4 °C. We used a BCA protein detection kit to evaluate protein concentration. Then, SDS PAGE was used to electrophoresis the added 40 ng of protein and transfer the separated protein to a PVDF membrane. Then we blocked the membrane with 5% skim milk, and detected overnight at 4 °C with the following antibody: aggrecan (1: 2000), Nrf2(1: 2000), collagen II (1: 2000), HO-1 (1: 2000), iNOS (1: 2000), COX-2 (1: 2000), ADAMTS-4 (1: 2000), GAPDH (1: 3000), MMP-13 (1: 2000), Lamin B1 (1: 2000), IκBα (1: 2000) and p65 (1: 2000) overnight at 4 °C.
Then, we washed the membrane and then incubated it with the corresponding secondary antibody for 4 h at room temperature. We use an ECL plus reagent (Invitrogen) to visualize the blots. Image Lab 3.0 software (Bio-Rad, Hercules, CA, USA) was used to evaluate the intensity of proteins.
2.12. Histological analysis
In a short, the knee joints of each group were soaked in 4% paraformaldehyde for 24 h at 4 °C and decalcified in 10% EDTA solution for 4 weeks. The anterior part of the entire joint was sliced serially (6 μm thick), and Safranin-O-Fast green staining (S–O) was used to assess cartilage destruction. We used light microscopy to estimate the extent of cartilage degeneration in stained sections according to Osteoarthritis Research Society International (OARSI) scoring system, and synovitis scored, as mentioned before. For immunohistochemistry, we deparaffinized the sections (6 μm) with xylene and rehydrated the sections in a graded ethanol series. We washed the sections and then blocked them with 3% (v/v) H2O2 for 10 min. Then, we treated them with 0.4% pepsin (Sangon Biotech, Shanghai, China) in 5 mM HCl at 37 °C for 20 min, then blocked with 10% (w/v) bovine serum albumin phosphate-buffered saline at 37 °C for 30 min. Finally, we incubated the sections overnight at 4 °C with the antibody against Nrf2 and MMP-13. Next, sections were incubated with an HRP-conjugated secondary antibody. We calculated the obtained images via Image-Pro Plus software, version 6.0 (Media Cybernetics, Rockville, MD, USA).
2.13. Statistical analysis
All of the data were analyzed via SPSS statistical software program 18.0. The data were shown as mean ± SD. Differences among the groups were compared by t-test or one-way analysis of variance (ANOVA). P < 0.05 was considered significance.
3. Results
3.1. Potential cytotoxicity of STE on mouse chondrocytes
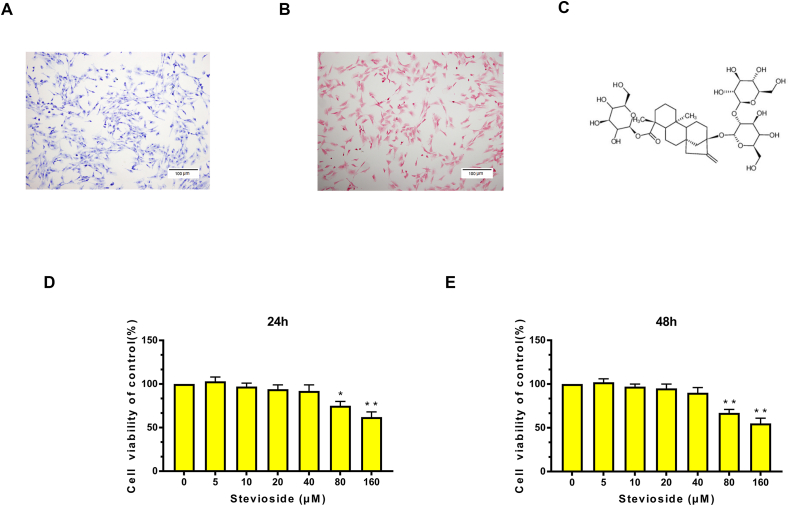
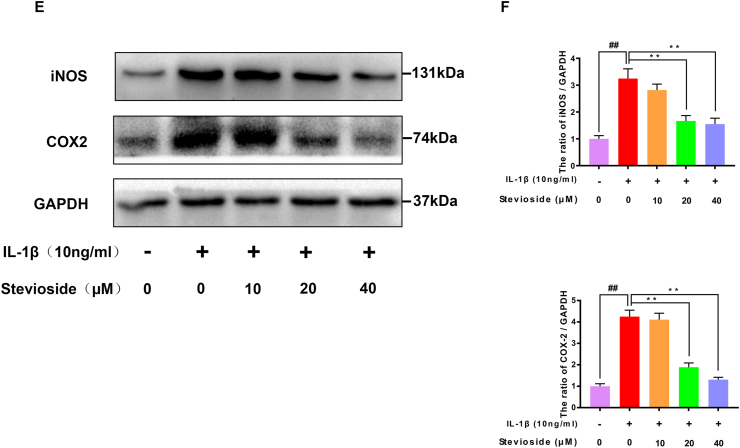
We made isolated mouse chondrocytes easy to visualize by toluidine blue staining and safranin O staining. Safranin O staining can stain chondrocytes red (Fig. 1A), and toluidine blue staining can stain proteoglycan in the chondrocyte cytoplasm purple, indicating that mouse primary chondrocytes are spindle-shaped (Fig. 1B). The structure of STE is indicated in Fig. 1C. To explore the cytotoxic effects of STE on chondrocytes, we conducted a CCK-8 assay. The chondrocytes were cultivated with different concentrations of STE (0, 5, 10, 20, 40, 80,160 μM) for 24 and 48 h. The result indicated that STE did not show an obvious cytotoxic effect on mouse chondrocytes at a concentration of 0–40 μM, while the cytotoxic effect was shown at 80 μM and higher concentration (Fig. 1D). As shown in Fig. 1E, the results of treatment for 48 h exhibited a similar phenomenon. Consequently, STE concentrations of 0–40 μM were used for the following research.
Figure 1.
Effect of STE on the cell viability of chondrocytes. Primary chondrocytes were stained by toluidine blue staining (A) and S–O staining (B) (scale bar: 100 μm). Chemical structure of STE (C). We incubated the cells with various concentrations of STE (0, 5, 10, 20, 40, 80, or 160 μM) for 24 (D) and 48 h (E). We calculated cell viability via a CCK-8 assay. Data were expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 (vs. con group, n = 3).
3.2. Effect of STE on the expressing level of PGE2, TNF-a, IL-6, NO, iNOS, and COX-2 in mouse OA chondrocytes
Next, we studied the effects of STE on the production of TNF-α, PGE2, IL-6, and NO in chondrocytes induced by IL-1β protein by western blotting analysis, RT-PCR, and ELISA kits. As pictured in Fig. 2A–B, IL-1β-induction up-regulated the expression of TNF-α, PGE2, IL-6, and NO compared with the sham group. However, STE down-regulated the level of IL-6, TNF-α, PGE2, and NO in a dose-dependent manner. Similarly, the results from RT-PCR exhibited that the mRNA level of IL-6 and TNF-α were consistent with these results (Fig. 2C–D). Furthermore, we investigated the effects of STE on the production of iNOS and COX-2 in chondrocytes induced by IL-1β protein by western blotting analysis and RT-PCR. Fig. 2E–F shows the results of western blotting and RT-PCR which indicated that STE significantly decrease the expression of iNOS and COX-2 in a dose-dependent manner compared to stimulation with IL-1β.
Figure 2.
Anti-inflammation effects of STE in mouse chondrocytes. The effects of STE on PGE2, NO, IL-6, and TNF-α in mouse chondrocytes induced by IL-1β were determined using Griess reaction or ELISA (A, B). The mRNA level of iNOS, IL-6, TNF-α, and COX-2 was determined via RT-qPCR (C, D). The expressing level of iNOS and COX-2 (E, F). Data were evaluated as mean ± SD. ##P < 0.01 compared to the control group; ∗∗P < 0.01, compared to the IL-1β alone stimulation group, n = 3.
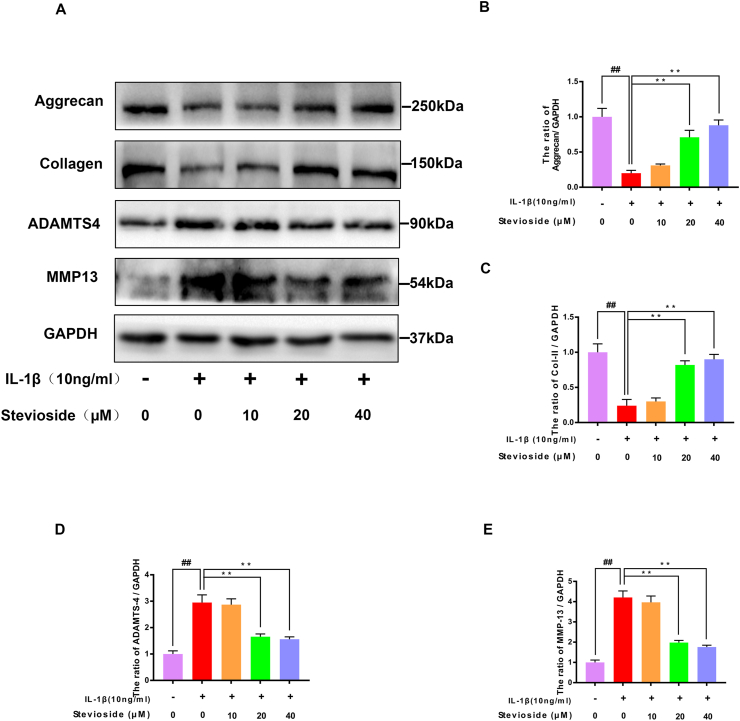
3.3. STE influenced ECM metabolism in mouse chondrocytes
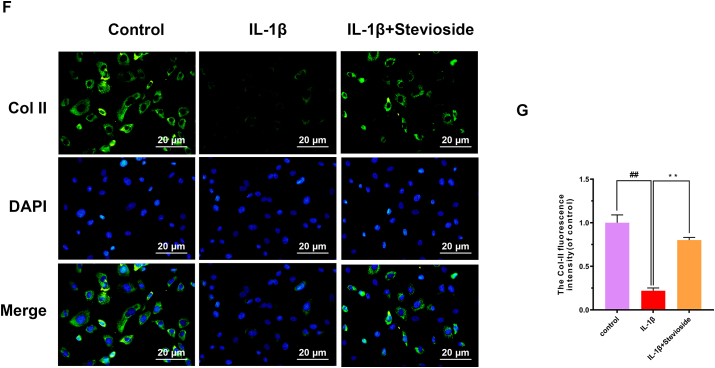
Western blotting analyses were performed to examine the expression of aggrecan, collagen type II, ADAMTS-4, and MMP-13 proteins in mouse cells. In Fig. 3A–E, the results of western blotting showed that IL-1β-induction decreased the expression of Aggrecan and Collagen II compared with the sham group. Instead, STE reversed the decreased expressions of Aggrecan and Collagen II which were associated with dose. IL-1β-induction boosted the expressing level of ADAMTS-4 and MMP-13 compared to the sham group. Instead, STE reversed the increased expressions of ADAMTS-4 and MMP-13 which were associated with dose. Similarly, the immunofluorescence result demonstrated that the level of collagen type II was similar to western blotting analysis (Fig. 3F–G).
Figure 3.
Influence of STE on the synthesis of ECM. The protein expression of collagen II, aggrecan, ADAMTS-4, and MMP-13 in the chondrocytes was detected by western blot analysis (A–E). Immunofluorescence for the assessment of Type II collagen (Col II) in mouse chondrocytes (F-G). Data were expressed as mean ± SD. ##P < 0.01 compared to the control group; ∗∗P < 0.01 compared to the IL-1β alone stimulation group, n = 3.
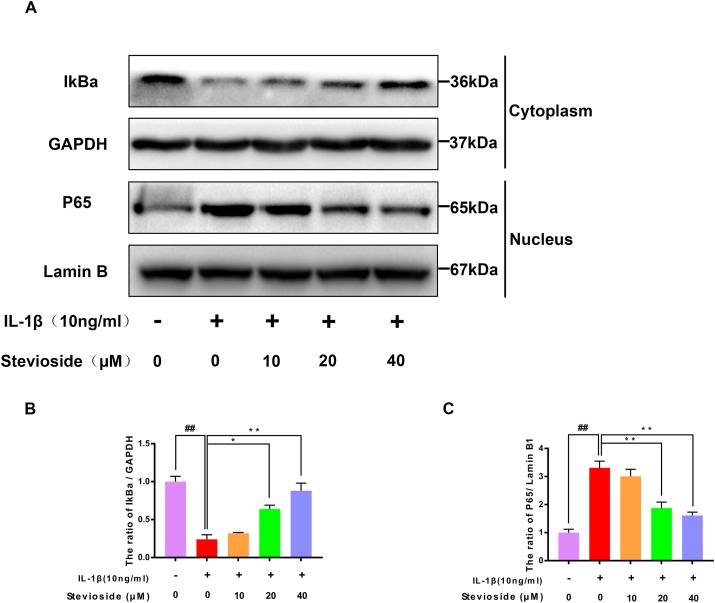
3.4. STE restrained activating NF-κB in mouse chondrocytes
The western blotting analysis was applied to investigate the influence of STE on the NF-kB signaling pathway. In Fig. 4A–C, compared to the sham group, the expressing level of IkBa protein in the IL-1β-induced group was decreased. However, STE reversed this phenomenon in an associated with dose. Besides, IL-1β stimulated p65 to translocate the nucleus compared with the sham group, which can be inhibited and associated with concentration by STE.
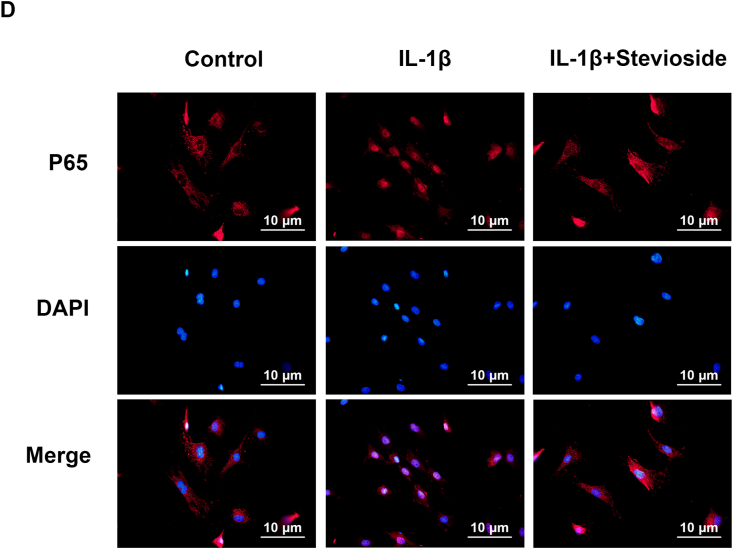
Figure 4.
Effects of STE on NF-κB pathway. The expression level of IκBα in the cytoplasm and p65 in the nuclei was determined via western blot analysis (A–C). Immunofluorescence and DAPI staining of nuclei to assess nuclear translocation of p65 (scale bar: 10 μm) (D). Data were expressed as mean ± SD. Significant differences between different groups are shown as ##P < 0.01, vs control group; ∗∗P < 0.01, vs IL-1β alone stimulation group, n = 3.
Moreover, we used immunofluorescence images to evaluate p65. In the control group, most of the p65 protein was localized in the cytoplasm of chondrocytes, but in the IL-1β-stimulated group, most of the p65 protein was localized in the nucleus of chondrocytes. However, STE reversed the translocation of p65 (Fig. 4D).
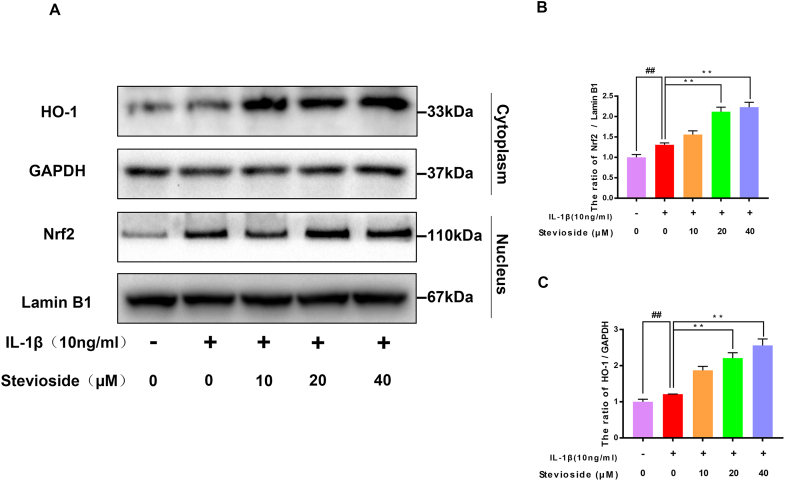
3.5. STE promoted Nrf2/HO-1 pathway
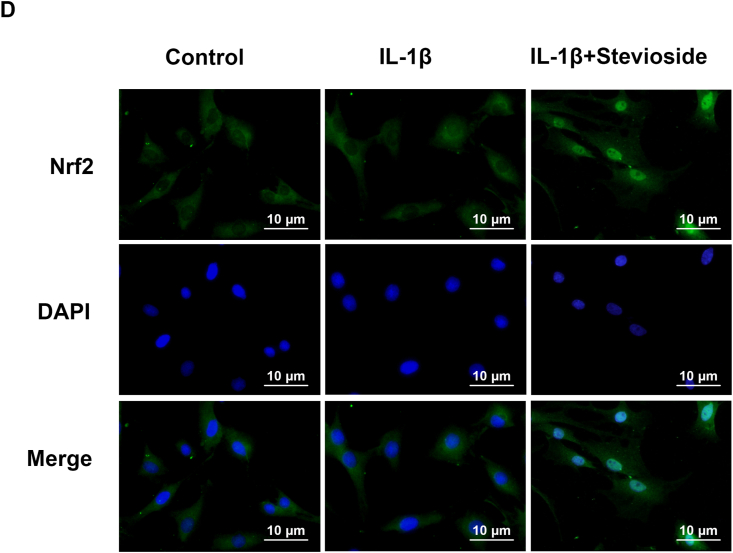
Researchers found that STE can promote the Nrf2/HO-1 pathway, but its Nrf2 activation in OA needs further study. The western blotting result was depicted in Fig. 5A–C. The expressing level of Nrf2 and HO-1 were not remarkably changed in the IL-1β treatment group compared to the control group. However, the expression level of HO-1 and Nrf2 in the STE group was remarkably enhanced compared with IL-1β-induced. Furthermore, the Nrf2 immunofluorescence results showed no difference between the IL-1β treatment group control group. After the use of sSTE, an increase in the intensity of Nrf2 in the nucleus could be observed (Fig. 5D). According to the result, we could suggest that STE could activate the Nrf2/HO-1 signal pathway in chondrocytes (see Fig. 6).
Figure 5.
Effects of STE on the Nrf2/HO-1 signal pathway. The expressing level of Nrf2 and HO-1 (A–C). We used immunofluorescence staining to evaluate the nuclear translocation of Nrf2 (scale bar: 10 μm) (D). Data were expressed as mean ± SD. ##P < 0.01 compared to the control group; ∗∗P < 0.01 compared to the IL-1β alone stimulation group, n = 3.
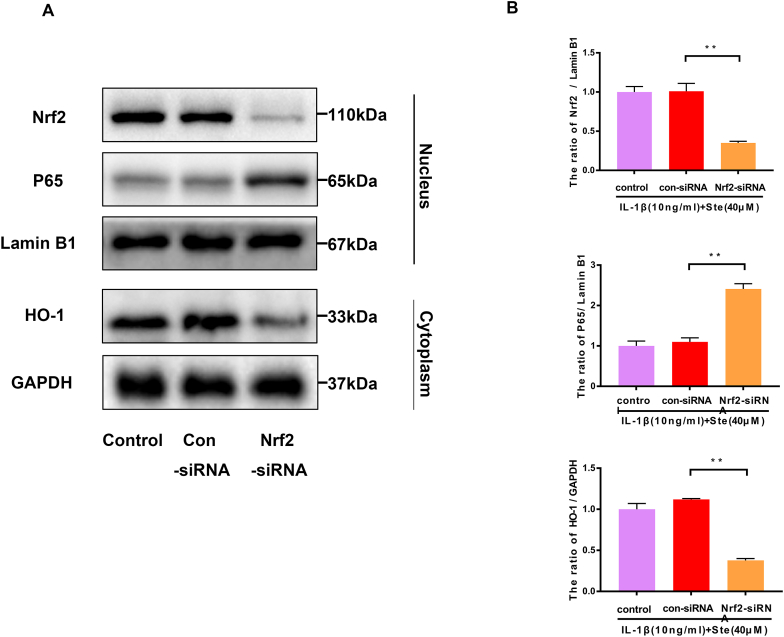
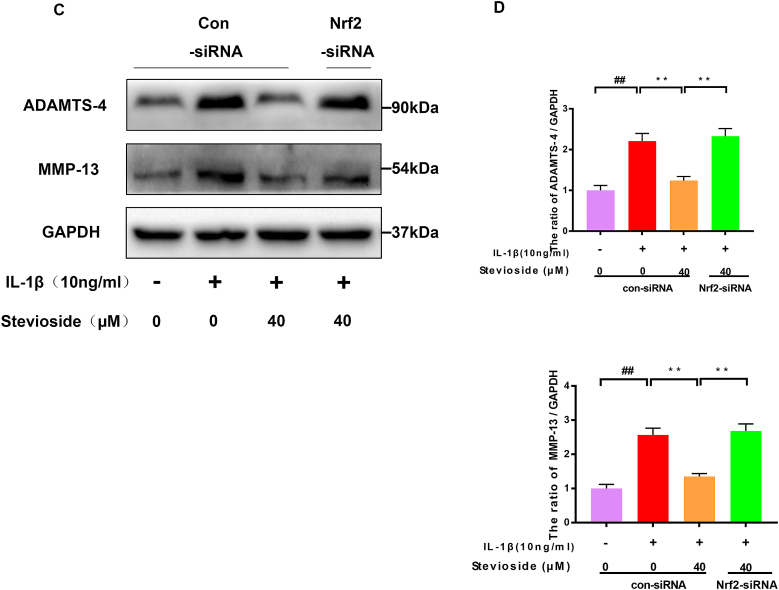
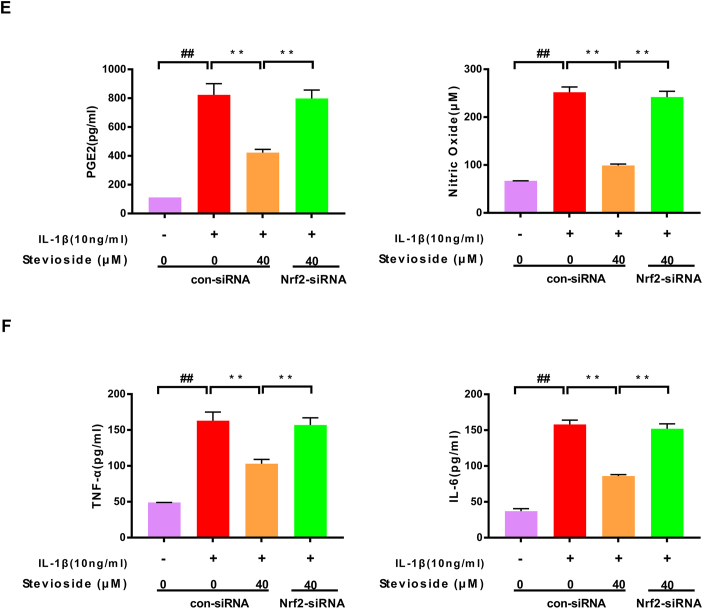
Figure 6.
Nrf2-siRNA blocked the protective effect of STE. The expressing levels of p65, Nrf2, and HO-1 were evaluated by western blotting (A) and quantified in (B) after Nrf2 knockdown. The expressing levels of MMP-13 and ADAMTS-4 (C, D). The expression level of NO, PGE2, IL-6, and TNF-α was determined via ELISA (E and F). Data were shown as mean ± SD. ##P < 0.01 compared to the control group; ∗∗P < 0.01 compared to the IL-1β alone stimulation group, n = 3.
3.6. STE exerted its anti-inflammatory effect in chondrocytes through Nrf2
We demonstrate by Nrf2 siRNA that STE restrains the NF-kB pathway by activating Nrf2. After Nrf2 siRNA knockdown, we found that the protein expression of P65 was increased in the Nrf2 siRNA group compared with the IL-1β+ STE group. The expression of HO-1 and IκBα were dramatically decreased in the Nrf2 siRNA group (Fig. 5A–B). The result shows that Nrf2 can induce the activation of the NF-κB signal pathway. Furthermore, we used ELISA and western blot to examine the effect of STE on the expressing level of PGE2, NO, TNF-α, IL-6, MMP-13, and ADAMTS-4 in IL-1β-induced chondrocytes (Fig. 5C–D). Unsurprisingly, STE can significantly down-regulate the expressing level of PGE2, NO, TNF-α, IL-6, MMP-13, ADAMTS-4, and Nrf2 siRNA can abolish the anti-inflammatory ability of STE (Fig. 5E–F). Taken together, we found that STE can inhibit ECM degradation while can be reversed by the Nrf2 siRNA treatment.
3.7. STE enhanced OA development in the DMM mouse model
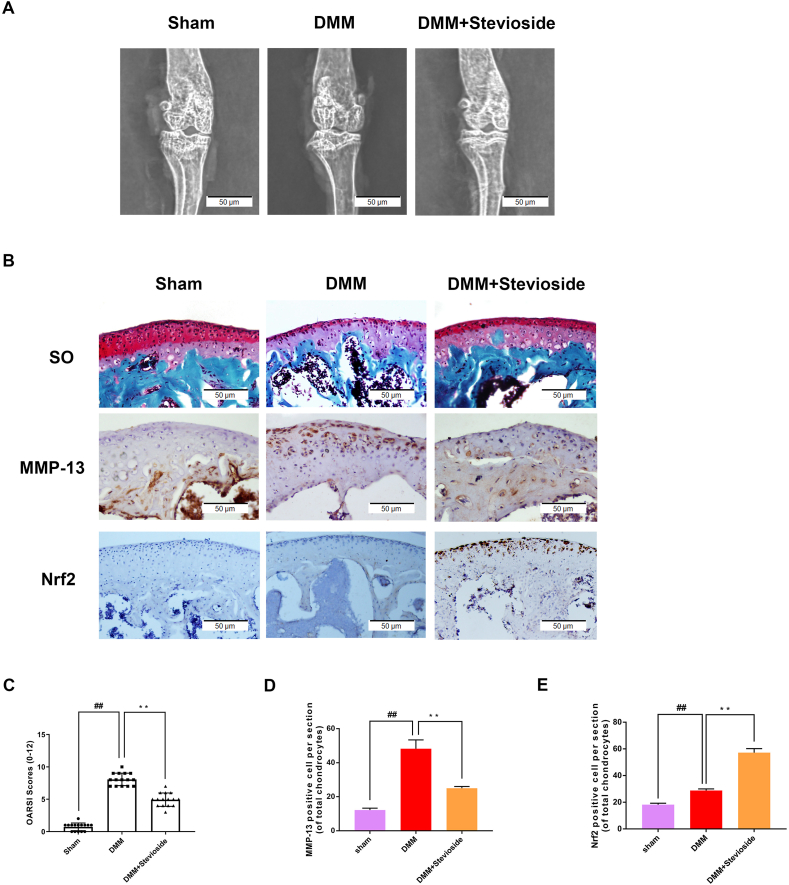
We investigated the chondroprotective influence of STE on OA via establishing a DMM mouse model. The results of X-ray showed that the joint space was severely narrowed and cartilage was obviously ossified in DMM mice, while the joint space narrowing and cartilage solidification were improved after STE treatment (Fig. 7A). The S–O staining result exhibited that compared with the sham operation group, the cartilage surface of the OA group had a more significant degree of damage and an obvious decrease of properly. The degradation of cartilage matrix in the STE group was decreased compared with the OA group and increased compared with the control group, which was effective in increasing the thickness of articular cartilage and restoring damaged cartilage (Fig. 7B). OARSI scores (Fig. 7C) were in keeping with the above-mentioned results. According to the result, the STE treatment group was remarkably different from the OA group, and the OARSI score of the OA group was elevated than that of the STE group. ALL in all, the above results showed that STE can exert the protective effect of osteoarthritis. To verify the effect of STE on Nrf2, we used immunohistochemical staining on Nrf2. The results showed that the expressing level between the two groups was similar, while the expressing level of Nrf2 was remarkably enhanced in the STE treatment group (Fig. 7B, E). Moreover, we used immunohistochemical staining on MMP-13, which is a major collagen lytic enzyme influenced by OA. In the DMM group, MMP-13 was significantly positive in the extracellular matrix and around chondrocytes. On the contrary, STE significantly reduced the expression of MMP-13 (Fig. 7B, D). These data indicated that STE may restrain the development of OA in mice.
Figure 7.
STE inhibits the OA process (A) The digital X-ray images of mouse knee joints (B) The Safranin O staining of cartilage shows STE alleviated the cartilage erosion and depletion of proteoglycans in mouse OA model at 8 weeks post-surgery (scale bar: 50 μm) (C) OARSI scores. The immunohistochemistry of Nrf2, MMP-13 and quantification of Nrf2-positive cells (B, D, E) (scale bar: 50 μm). Data were expressed as mean ± SD. ##P < 0.01, compared to the sham group; ∗∗P < 0.01 compared to the DMM group, n = 15.
4. Discussion
Osteoarthritis is a common chronic disease among older adults and a predisposing disease among working-ages, characterized by synovitis, cartilage degeneration, and osteophyte formation [22,23]. Current treatments for OA include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, intra-articular corticosteroids, walkers, and supportive insoles [24]. However, these only relieve joint pain and swelling, but can not cure the disease completely, and overuse can even lead to serious side effects. Artificial joint replacement is currently the only way to treat end-stage OA [25]. Consequently, we need an effective and safe drug that can alleviate OA. Recently, fruit-derived compounds attract researchers' attention for treating OA due to the lack of harmful side effects.
Stevioside (STE), a diterpene glycoside component of Stevia rebaudiana, possesses an anti-inflammation effect in LPS-induced acute lung injury through down-regulating the NF-κB pathway [19,26]. A previous study demonstrated that STE reduces inflammatory response, apoptosis, and oxidative stress in lipopolysaccharide-induced intestinal mucosal damage via restraining the activation of NF-κB signal pathways [20,27]. Researchers demonstrated that STE possesses the neuroprotective effect in hypoxia-induced mouse neuroblastoma N2a cells via inhibiting MAPK and NF-κB pathways [19]. Our research further proved that STE exerts a protective effect via Nrf2/NF-κB regulation in OA chondrocytes. In addition, our study proved STE OA progression in a DMM model of mice.
In OA, pro-inflammatory cytokines, IL-1β were produced through activated synoviocytes and articular chondrocytes produce, which promotes chondrocyte apoptosis and activates NF-κB pathways to trigger catabolism [28]. The IL-1β induces PGE2 and iNOS production in synovial fibroblasts, which is involved in osteoarthritis [29]. NO has long been thought to exert anti-anabolic and pro-catabolic effects on extracellular matrix homeostasis in cartilage, including inhibition of proteoglycan and collagen synthesis [30]. Matrix metalloproteinases (MMPs) family, induced by IL-1β in chondrocytes, are major extracellular proteolytic enzymes that lead to cartilage collagenolysis [31]. Matrix metalloproteinase 13 (MMP-13) is one of the major proteases that induce degrading extracellular cartilage matrix [32]. ADAMTS-4 has long been known to drive cleavage of aggrecan, playing a decisive role in cartilage degradation [33]. In our research, we proved that STE inhibited the overproduction of PGE2, NO, TNF-α, and IL-6 and the upregulation of iNOS and COX-2 by IL-1β at the protein and mRNA levels. Moreover, the result showed that in mouse OA chondrocytes, STE significantly inhibited the expressing level of MMP-13 and ADAMTS-4 induced via IL-1β protein at the protein and mRNA levels.
The NF-κB pathway is involved in the occurrence of osteoarthritis and exerts an important effect [34]. When IL-1β was used to induce the cells, phosphorylation of IKK (IκB kinase) is initiated, triggering NF-κB releases, and further regulating gene expression depends on NF-κB, then inducing the translocation of p65 [35]. In addition, NF-κB inhibitors reduced joint swelling in mice induced by collagen [36,37]. In our research, we proved that STE down-regulated IL-1β-induced NF-κB activation, nuclear translocation of p65, and IκBα degradation in chondrocytes. Studies have confirmed that the activation of Nrf2 has an anti-inflammatory effect [[38], [39], [40]]. The Nrf2 and NF-κB pathways are involved in regulating the transcription of downstream target proteins [41,42]. Sael et al. referred that STE can prevent liver damage via upregulating Nrf2 and immunomodulatory activity by blocking NF-κB signaling [43]. Therefore, we predicted that STE may inhibit the NF-κB pathway in OA via promoting the Nrf2/HO-1 axis. Our research proved that STE could significantly up-regulate Nrf2/HO-1 and down-regulated NF-κB in cells incubated with IL-1β. This confirms that Nrf2/HO-1 protects joints in STE-mediated OA.
OA is a highly prevalent, degenerative, progressive joint disorder characterized by osteophyte formation, cartilage degradation, and synovial membrane inflammation, subchondral bone sclerosis [44]. In vivo studies, the DMM mouse model is reliable, which has been widely accepted. Therefore, a mouse DMM model was established to explore the anti-inflammatory effects of STE in this experiment. Histological staining indicated that STE remarkably inhibited the progression of the disease. Besides, STE treatment down-regulated OARSI score. Moreover, the results of Immunohistochemistry for MMP13 indicated that STE relieved the ECM degradation. All in all, our study proved the value of STE in treating OA.
5. Conclusion
The result proved that STE suppresses the inflammatory which triggered via IL-1β stimulation, which inhibited the expressing level of MMP-13, iNOS, COX-2, and ADAMTS-4 through the Nrf2/NF-κB signaling pathway in mouse OA chondrocytes. Moreover, the treatment of STE significantly relieves the progression in the mouse DMM model. All of the results demonstrated the therapeutic of STE in OA treatment.
Credit author statement
Peng Luo: Funding acquisition, Study design, Study conduct, Revising manuscript. Jia Wu: Drafting manuscript, Data collection, Investigation. Haoliang Li: Investigation, Data Collection, Resources. Fei Hu:Data analysis, Data interpretation.
Funding
This research was assisted via the Wenzhou Municipal Science and Technology Brueau (2021Y1484), Wenzhou Municipal Science and Technology Bureau (Y20210086).
Availability of data and materials
The datasets in this study are available.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgement
We appreciate our members for their valuable efforts on this study.
References
- 1.Shadyab A.H., Terkeltaub R., Kooperberg C., Reiner A., Eaton C.B., Jackson R.D., et al. Prospective associations of C-reactive protein (CRP) levels and CRP genetic risk scores with risk of total knee and hip replacement for osteoarthritis in a diverse cohort. Osteoarthritis Cartilage. 2018;26:1038–1044. doi: 10.1016/j.joca.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shadyab A.H., Li W., Eaton C.B., LaCroix A.Z. General and abdominal obesity as risk factors for late-life mobility limitation after total knee or hip replacement for osteoarthritis among women. Arthritis Care Res. 2018;70:1030–1038. doi: 10.1002/acr.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frondoza C.G., Fortuno L.V., Grzanna M.W., Ownby S.L., Au A.Y., Rashmir-Raven A.M. Alpha-lipoic acid potentiates the anti-inflammatory activity of avocado/soybean unsaponifiables in chondrocyte cultures. Cartilage. 2018;9:304–312. doi: 10.1177/1947603516686146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu-Bryan R., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Andres M.C., Imagawa K., Hashimoto K., Gonzalez A., Goldring M.B., Roach H.I., et al. Suppressors of cytokine signalling (SOCS) are reduced in osteoarthritis. Biochem Biophys Res Commun. 2011;407:54–59. doi: 10.1016/j.bbrc.2011.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haneda M., Hayashi S., Matsumoto T., Hashimoto S., Takayama K., Chinzei N., et al. Depletion of aquaporin 1 decreased ADAMTS4 expression in human chondrocytes. Mol Med Rep. 2018;17:4874–4882. doi: 10.3892/mmr.2018.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelwick R., Desanlis I., Wheeler G.N., Edwards D.R. The ADAMTS (A disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M., McKelvey K., Shen K., Minhas N., March L., Park S.Y., et al. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology. 2014;53:2270–2279. doi: 10.1093/rheumatology/keu254. [DOI] [PubMed] [Google Scholar]
- 9.Colbath A.C., Dow S.W., Hopkins L.S., Phillips J.N., McIlwraith C.W., Goodrich L.R. Induction of synovitis using interleukin-1 beta: are there differences in the response of middle carpal joint compared to the tibiotarsal joint? Front Vet Sci. 2018;5:208. doi: 10.3389/fvets.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu K., Gao Y., Yang L., Liu Y., Wang C. Magnolin exhibits anti-inflammatory effects on chondrocytes via the NF-kappaB pathway for attenuating anterior cruciate ligament transection-induced osteoarthritis. Connect Tissue Res. 2021;62:475–484. doi: 10.1080/03008207.2020.1778679. [DOI] [PubMed] [Google Scholar]
- 11.Scanzello C.R. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. 2017;29:79–85. doi: 10.1097/BOR.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigoglou S., Papavassiliou A.G. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Sandberg M., Patil J., D'Angelo B., Weber S.G., Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren J., Su D., Li L., Cai H., Zhang M., Zhai J., et al. Anti-inflammatory effects of Aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-kappaB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways. Toxicol Appl Pharmacol. 2020;387:114846. doi: 10.1016/j.taap.2019.114846. [DOI] [PubMed] [Google Scholar]
- 15.Yang P.M., Wu Z.Z., Zhang Y.Q., Wung B.S. Lycopene inhibits ICAM-1 expression and NF-kappaB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016;155:94–101. doi: 10.1016/j.lfs.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Takasaki M., Konoshima T., Kozuka M., Tokuda H., Takayasu J., Nishino H., et al. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorg Med Chem. 2009;17:600–605. doi: 10.1016/j.bmc.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 17.Kaur T., Singh D., Singh A.P., Pathak D., Arora S., Singh B., et al. Stevioside protects against rhabdomyolysis-induced acute kidney injury through PPAR-gamma agonism in rats. Drug Dev Res. 2021;82:59–67. doi: 10.1002/ddr.21722. [DOI] [PubMed] [Google Scholar]
- 18.Shen W., Fan K., Zhao Y., Zhang J., Xie M. Stevioside inhibits unilateral ureteral obstruction-induced kidney fibrosis and upregulates renal PPARgamma expression in mice. J Food Biochem. 2020;44 doi: 10.1111/jfbc.13520. [DOI] [PubMed] [Google Scholar]
- 19.Zhong K.L., Lu M.Y., Liu F., Mei Y., Zhang X.J., Zhang H., et al. Isosteviol sodium protects neural cells against hypoxia-induced apoptosis through inhibiting MAPK and NF-kappaB pathways. J Stroke Cerebrovasc Dis. 2019;28:175–184. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J., Qi L., Lv Z., Jin S., Wei X., Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019;8 doi: 10.3390/antiox8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Shen W., Zhang J.Y., Jia C.H., Xie M.L. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-kappaB/TGF-beta1/Smad signaling pathway. Food Funct. 2019;10:1179–1190. doi: 10.1039/c8fo01663a. [DOI] [PubMed] [Google Scholar]
- 22.Thysen S., Luyten F.P., Lories R.J. Targets, models and challenges in osteoarthritis research. Dis Model Mech. 2015;8:17–30. doi: 10.1242/dmm.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa B.R., Pereira T.V., Saadat P., Rudnicki M., Iskander S.M., Bodmer N.S., et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375:n2321. doi: 10.1136/bmj.n2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed U., Thornalley P.J., Rabbani N. Possible role of methylglyoxal and glyoxalase in arthritis. Biochem Soc Trans. 2014;42:538–542. doi: 10.1042/BST20140024. [DOI] [PubMed] [Google Scholar]
- 25.Poulet B., Staines K.A. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi: 10.1016/j.coph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Shivanna N., Naika M., Khanum F., Kaul V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabet Complicat. 2013;27:103–113. doi: 10.1016/j.jdiacomp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Potocnjak I., Broznic D., Kindl M., Kropek M., Vladimir-Knezevic S., Domitrovic R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-kappaB activation. Food Chem Toxicol. 2017;107:215–225. doi: 10.1016/j.fct.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Daheshia M., Yao J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 29.Chabane N., Zayed N., Afif H., Mfuna-Endam L., Benderdour M., Boileau C., et al. Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis Cartilage. 2008;16:1267–1274. doi: 10.1016/j.joca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K., Hattori T., Fujisawa T., Takahashi K., Inoue H., Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem. 1998;123:431–439. doi: 10.1093/oxfordjournals.jbchem.a021955. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell P.G., Magna H.A., Reeves L.M., Lopresti-Morrow L.L., Yocum S.A., Rosner P.J., et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little C.B., Barai A., Burkhardt D., Smith S.M., Fosang A.J., Werb Z., et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T., He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Tak P.P., Firestein G.S. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mankan A.K., Lawless M.W., Gray S.G., Kelleher D., McManus R. NF-kappaB regulation: the nuclear response. J Cell Mol Med. 2009;13:631–643. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang L.J., Kwon E.S., Lee K.M., Cho C., Lee J.I., Ryu Y.B., et al. 3'-Sialyllactose as an inhibitor of p65 phosphorylation ameliorates the progression of experimental rheumatoid arthritis. Br J Pharmacol. 2018;175:4295–4309. doi: 10.1111/bph.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozawa M., Nishida K., Yoshida A., Saito T., Harada R., Machida T., et al. Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1beta production and subsequent NF-kappaB activation. Inflamm Res. 2015;64:243–252. doi: 10.1007/s00011-015-0804-2. [DOI] [PubMed] [Google Scholar]
- 38.Tang Q., Feng Z., Tong M., Xu J., Zheng G., Shen L., et al. Piceatannol inhibits the IL-1beta-induced inflammatory response in human osteoarthritic chondrocytes and ameliorates osteoarthritis in mice by activating Nrf2. Food Funct. 2017;8:3926–3937. doi: 10.1039/c7fo00822h. [DOI] [PubMed] [Google Scholar]
- 39.Liu G.H., Qu J., Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 41.Kim B.C., Jeon W.K., Hong H.Y., Jeon K.B., Hahn J.H., Kim Y.M., et al. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCdelta/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J Ethnopharmacol. 2007;113:240–247. doi: 10.1016/j.jep.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas-Grajales S., Ramos-Tovar E., Chavez-Estrada E., Alvarez-Suarez D., Hernandez-Aquino E., Reyes-Gordillo K., et al. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: in vivo, in vitro and in silico assays. Life Sci. 2019;224:187–196. doi: 10.1016/j.lfs.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu G., Zhao X., Wang C., Geng Y., Zhao J., Xu J., et al. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets in this study are available.