Abstract
INTRODUCTION:
This study aimed to identify the intra-arch risk factors for palatally displaced canine by comparing the maxillary transverse dimensions, palatal depth (PD), and arch length (AL) of the subjects with and without impacted canine using cone-beam computed tomography (CBCT).
METHODS:
In this prospective case–control study, 79 CBCT images of gender- and skeletal feature-matched subjects (25 cases and 54 controls) were compared. Based on the CBCT images, maxillary transverse widths at four levels (molar basal, molar alveolar, premolar basal, and premolar alveolar), maxillary PD, and maxillary AL were measured. Group comparisons were assessed using analysis of variance (ANOVA), followed by post-hoc Scheffe's test, and risk factors were identified using univariate and multivariate logistic regression.
RESULTS:
The impacted canine group showed significantly smaller molar alveolar width, premolar alveolar width, PD, and greater AL compared to the control group (P = 0.046, P < 0.001, P = 0.003, and P = 0.001, respectively). No significant difference was observed in the molar and premolar basal width measurements between the two groups. Multivariate analysis showed that impacted maxillary canine was influenced by premolar alveolar width (odds ratio (OR): 0.669), PD (OR: 0.532), and AL (OR: 1.739).
CONCLUSION:
Intra-arch risk factors, such as reduced maxillary premolar transverse alveolar width, PD, and greater AL, are associated with palatally displaced canine.
Keywords: Arch length, CBCT, palatal depth, transverse width
Maxillary canine impaction is a clinical condition frequently encountered in orthodontics, and the underlying etiological factors have been studied extensively.[1,2,3,4,5] Several morphological variations of the dentoskeletal structures of the maxilla are associated with the occurrence of impacted canine.[6,7,8]
The incidence of the upper canine impaction is 1–2.2%.[9] Compared to the mandibular canine, the maxillary canines are 10–20 times more commonly impacted.[10,11] Among the maxillary impacted canines, palatally displaced canines (PDC) are more prevalent than buccally impacted canines.[12,13] Two theories have been proposed to explain the occurrence of palatal displacement of maxillary canine: guidance theory[6] and genetic theory.[1]
However, the exact risk factors for canine impaction are not yet clear. Previous studies[2,9] have demonstrated that the presence of excess space in the maxillary arch could lead to palatal canine impaction, whereas buccal canine impaction is associated with the lack of space.[2]
Many studies[6,7,8,14] evaluated the skeletal features of the impacted canine but did not find any significant association between canine impaction and sagittal skeletal pattern,[6,15] whereas a few reported an increased prevalence of PDC in class I and class II division 2 malocclusions and buccally displaced canines in class III skeletal pattern.[7,8]
Regarding transverse dimension also there are differing views. Some studies identified an association between impaction and transverse maxillary deficiency,[3,16,17,18,19] whereas others observed an excessive maxillary width[7,13] in the impacted patients, and some others could not detect any correlation with maxillary width.[18,20] However, studies have been undertaken to alter the transverse dimension at an early age to intercept maxillary canine impaction.[21,22] Hence, the association of canine impaction and maxillary transverse dimension is yet controversial. In addition, palatal depth (PD), arch length (AL), and their roles in maxillary canine impaction have not been studied extensively.
Cone-beam computed tomography (CBCT) provides a precise method to study the intra-arch risk factors compared to two-dimensional (2D) radiographs.[23] Therefore, the objective of this prospective case–control study was to identify the intra-arch risk factors associated with palatally displaced maxillary canines compared to matched controls using CBCT.
Materials and Methods
The prospective case–control study was conducted in accordance with STROBE guidelines, approved by the Institutional Ethics Committee (IEC/M/19/2020/DCK). The sample size was calculated based on an expected minimum mean difference in the intermolar width of 3.9 mm between the bilateral impacted canine and the control groups with a level of significance of 0.05, as described previously.[24] Thus, a minimum of 11 bilateral PDC cases were required for the study.
Subjects aged between 15 and 40 years with unilateral or bilateral PDC, no history of previous orthodontic treatment, and anterior dental crowding ≤2 mm reported for the treatment and had a willingness to participate were included as cases in this study, whereas the control group comprised subjects without canine impaction necessitating CBCT scan for other purposes (impacted third molars and airway assessment). Patients with cleft lip and palate, other dentofacial anomalies, and dental agenesis other than third molars were excluded from both groups. Standard records, including photographs and study models were obtained from cases and controls. CBCT scans were acquired using a Planmeca Promax 3D machine (Helsinki, Finland). For cases wherein a large field of view scan was indicated, no additional lateral cephalogram or OPG (Orthopantomogram) was obtained to reduce the radiation dose, and later, a CBCT-synthesized lateral cephalogram was generated. For cases and controls wherein the scan was limited to the maxilla, an additional lateral cephalogram was either available or was obtained while reporting was utilized. The digital imaging and communication in medicine files of CBCT images were imported into Planmeca Romexis viewer software (version 4.6.2R, Helsinki, Finland) for evaluation.
The CBCT images of 25 patients with PDC (13 unilateral and 12 bilateral as cases) and 54 subjects without canine impaction (controls) were included (case: control = 1:2) over a period of 1.5 years. Two groups of cases (unilateral and bilateral) and controls were frequency-matched for gender and skeletal characteristics. Conventional or CBCT-synthesized lateral cephalograms were used to match the skeletal characteristics. The maxillary sagittal position was assessed using the sella-nasion-A point angle (SNA) as normal, retrusive, and protrusive and classified into class I, II, and III according to the A point-nasion-B point angle (ANB). The growth pattern was classified as normodivergent, hypodivergent, and hyperdivergent based on Frankfort-mandibular plane angle (FMA). Arch perimeter analysis was performed to assess the arch length (AL) tooth-size discrepancy. The transverse maxillary measurements on CBCT were based on the method described by Podesser et al.[25]
The transverse dimensions were measured at four levels in the coronal section of CBCT:first molar basal width (MBW), first molar alveolar width (MAW), first premolar basal width (PMBW), and first premolar alveolar width (PMAW) [Table 1]. The coronal slice of CBCT showing buccal root furcation with horizontal palatal plane was used for molar measurements. The line that connects the most inferior point on the right and left nasal floor was considered the reference plane for the measurement of the basal width of the molar [Figures 1a] and distance between the right and left tip of the molar alveolar process considered as the molar alveolar width [Figure 1b]. Similar to molar measurements, the premolar measurements were made on the coronal slice of CBCT, showing the center of the root canal along the reference line [Figures 1c and 1d].
Table 1.
Definitions of the Cone Beam Computed Tomography measurements used in the study
| Cbct measurements | |
|---|---|
| MBW | The maxillary first molar basal width dimension was measured as distance between the lateral limits of the right and left sides of the maxilla along the nasal floor reference plane. |
| MAW | The maxillary first molar alveolar width dimension was measured as the distance between the right and left tip of the maxillary first molar alveolar process on the coronal slice. |
| PMBW | The maxillary first premolar basal width dimension was measured as the distance between the lateral limits of the right and left sides of the maxilla along the nasal floor reference plane. |
| PMAW | The maxillary first premolar alveolar width dimension was measured as the distance between the right and left tip of the maxillary first molar alveolar process on the coronal slice. |
| PD | Palatal depth was measured on the axial view by locating the coronal plane on the sites of the upper first molars in both the left and right sides and ensuring that it will be passed through the left of the tooth. Then a line was drawn between the cemento-enamel junctions (C.E.J) of the two first molars. From the mid-point of this line, another perpendicular line was drawn up to the palate |
| AL | Maxillary arch length (AL) was measured on an axial plane as the distance from incisal foramina to the line that connects the distal ends of right and left first molars |
Figure 1.
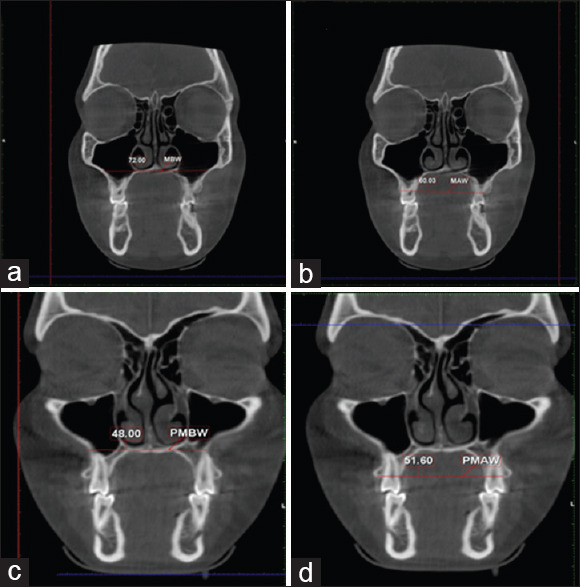
Maxillary width measurements: (a) MBW, first molar basal width; (b) MAW, first molar alveolar width; (c) PMBW, first premolar basal width; (d) PMAW, first premolar alveolar width.
PD was measured on the axial view by locating the coronal plane on the sites of the upper first molars on both the left and right sides and ensuring its passage through the center of the tooth [Figure 2a].
Figure 2.
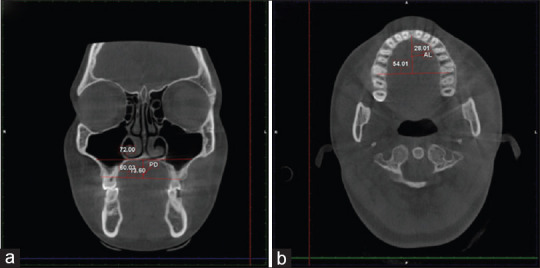
(a) PD, Palatal Depth; (b) AL, Arch length.
AL was measured as the distance from incisal foramina to the line that connects the distal ends of right and left first molars on an axial plane [Figure 2b].
Statistical analysis
Statistical analyses were performed using SPSS software for Windows (version 22; IBM, Armonk, NY, USA). The normality of the data was assessed using Shapiro–Wilk test. Descriptive statistics were represented as mean and standard deviation (SD). The intergroup comparison of gender and age was performed using Chi-square test and analysis of variance (ANOVA), respectively. The group comparisons for skeletal parameters, such as SNA, ANB, and FMA, were performed using ANOVA, followed by post-hoc Scheffe's test. The intergroup comparison of growth pattern (normodivergent, hypodivergent, and hyperdivergent), skeletal sagittal correlation (class I, class II, and class III), and maxillary sagittal position (normal, retrusive, and protrusive) was carried out using Chi-square tests. Similarly, the comparison of maxillary transverse dimensions (MAW, MBAW, PMBW, and PMAW), maxillary AL, and PD was performed with ANOVA and post-hoc Scheffe's test.
A univariate analysis followed by backward stepwise multivariate logistic regression model was used to determine the association of maxillary canine impaction with intra-arch risk variables. If the P value for the association variable was <0.1 based on univariate analysis, it was included in the multiple regression analysis. Statistical significance was set at P < 0.05 for all the tests.
Results
This study consisted of 39 males and 40 females, and all the groups were similar with respect to gender distribution [P = 0.072; Table 2]. The mean age of the participants in this study was 22.72 ± 8.68 years. A statistically significant difference was detected in age among the control, unilateral, and bilateral PDC groups (P = 0.007). Conversely, the skeletal characteristics were comparable in the case and control groups (P > 0.05), whereas no significant differences were observed among groups regarding skeletal parameters [Table 3], such as SNA (P = 0.518), ANB (P = 0.149), and FMA (P = 0.153).
Table 2.
Intergroup gender distributions and age comparison
| Control | Unilateral | Bilateral | Total | P | |
|---|---|---|---|---|---|
| Sex | 0.072 | ||||
| Male | 31 (79.5%) | 3 (7.7%) | 5 (12.8%) | 39 (100.0%) | |
| Female | 23 (57.5%) | 10 (25.0%) | 7 (17.5%) | 40 (100.0%) | |
| Age | 0.007 | ||||
| Mean (SD) | 24.74 (9.40) | 19.31 (5.266) | 17.33 (3.651) | 22.72±8.68 |
Chi-square test=5.27; df=2; P=0.072; ANOVA test. P=0.007. Statistically significant at P<0.05
Table 3.
Intergroup comparison of growth pattern, skeletal sagittal relationship, and maxillary sagittal position (Chi-square test)
| Control (n=54) | Unilateral (n=13) | Bilateral (n=12) | Total | P | |
|---|---|---|---|---|---|
| Maxillary sagittal position | 0.995 | ||||
| Normal | 30 (68.2%) | 7 (15.9%) | 7 (15.9%) | 44 (100.0%) | |
| Retrusion | 14 (70.0%) | 3 (15.0%) | 3 (15.0%) | 20 (100.0%) | |
| Protrusion | 10 (66.7%) | 3 (20.0%) | 2 (13.3%) | 15 (100.0%) | |
| Sagittal skeletal relationship | 0.697 | ||||
| CLASS I | 32 (74.4%) | 5 (11.6%) | 6 (14.0%) | 43 (100.0%) | |
| CLASS II | 13 (61.9%) | 5 (23.8%) | 3 (14.3%) | 21 (100.0%) | |
| CLASS III | 9 (60.0%) | 3 (20.0%) | 3 (20.0%) | 15 (100.0%) | |
| Growth pattern | 0.808 | ||||
| Normo-divergent | 31 (68.9%) | 7 (15.6%) | 7 (15.6%) | 45 (100.0%) | |
| Hyperdivergent | 14 (60.9%) | 5 (21.7%) | 4 (17.4%) | 23 (100.0%) | |
| Hypodivergent | 9 (81.8%) | 1 (9.1%) | 1 (9.1%) | 11 (100.0%) |
Statistically significant at P<0.05
Transverse maxillary measurements, MAW and PMAW were significantly greater in the control group compared to the unilateral and bilateral groups (P = 0.046 and P < 0.001, respectively). However, no significant difference was observed in MBW (P = 0.327) and PMBW (P = 0.145) measurements among the three groups [Table 4].
Table 4.
Intergroup comparisons of maxillary transverse width, palatal depth and arch length (ANOVA followed by post hoc Scheffe tests)
| Control (n=54) | Unilateral (n=13) | Bilateral (n=12) | P | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| MBW | 63.94 | 2.91 | 61.98 | 4.23 | 63.55 | 4.11 | 0.327 |
| MAW | 58.12A | 3.05 | 56.69 | 2.24 | 56.05B | 2.82 | 0.046 |
| PMBW | 37.25 | 6.61 | 33.43 | 5.17 | 36.93 | 5.49 | 0.145 |
| PMAW | 46.47A | 2.86 | 42.37B | 3.05 | 44.73 | 4.53 | 0.000 |
| PD | 12.80A | 2.04 | 10.69B | 2.70 | 10.91B | 2.94 | 0.003 |
| AL | 24.77A | 3.01 | 27.16B | 2.36 | 27.63B | 2.27 | 0.001 |
Different alphabets indicate statistically significant differences according to Post hoc Scheff’e test; statistically significant at P<0.05. MBW, Maxillary first molar basal width; MAW, maxillary first molar alveolar width; PMBW, maxillary first premolar basal width; PMAW, maxillary first premolar alveolar width; PD, palatal width; AL, arch length.
PD and AL measurements showed a significant difference in the control, unilateral, and bilateral groups [P = 0.003 and P = 0.001, respectively; Table 4].
Univariate logistic regressions showed that PDC was influenced by MAW, PMAW, PD, and AL. Furthermore, MAW, PMAW, and PD have a negative association with PDC (odds ratio (OR): 0.813, 0.751, and 0.674, respectively), whereas AL showed a positive association with PDC (OR: 1.405) [Table 5]. The stepwise backward multivariate logistic regression analyses showed that PDC was influenced by PMAW (OR: 0.669, CI: 0.498–0.899), PD (OR: 0.532, CI: 0.319–0.887), and AL (OR: 1.739, CI: 1.217–2.483) (R2 0.679 indicates that 67% of the data fit to the regression model) [Table 6].
Table 5.
Univariate logistic regression models for variables associated with maxillary canine impaction.
| Variables | S.E | P | Unadjusted Odds Ratio | Confidence Interval | |
|---|---|---|---|---|---|
|
| |||||
| Lower limit | Upper limit | ||||
| AGE | 0.050 | 0.004 | 0.867 | 0.786 | 0.956 |
| MBW | 0.059 | 0.240 | 0.933 | 0.834 | 1.048 |
| MAW | 0.089 | 0.020 | 0.813 | 0.682 | 0.968 |
| PMBW | 0.039 | 0.167 | 0.947 | 0.876 | 1.023 |
| PMAW | 0.092 | 0.002 | 0.751 | 0.628 | 0.899 |
| PD | 0.128 | 0.002 | 0.674 | 0.524 | 0.865 |
| AL | 0.105 | 0.001 | 1.405 | 1.143 | 1.727 |
| SNA | 0.075 | 0.259 | 0.919 | 0.794 | 1.064 |
| ANB | 0.107 | 0.574 | 1.062 | 0.861 | 1.311 |
| FMA | 0.056 | 0.252 | 1.066 | 0.956 | 1.190 |
| Maxilla- Normal | Reference | ||||
| Maxillary retrusion | 0.586 | 0.884 | 0.918 | 0.291 | 2.894 |
| Maxillary protrusion | 0.636 | 0.914 | 1.071 | 0.308 | 3.728 |
| Class I | Reference | ||||
| Class II | 0.632 | 0.295 | 0.516 | 0.149 | 1.781 |
| Class III | 0.693 | 0.908 | 0.923 | 0.238 | 3.587 |
| Normo- divergent | Reference | ||||
| Hyperdivergent | 0.536 | 0.509 | 1.423 | 0.499 | 4.062 |
| Hypodivergent | 0.845 | 0.402 | 0.492 | 0.094 | 2.580 |
Table 6.
Multivariate logistic regression model for variables associated with maxillary canine impaction
| Variables | S.E | P | Adjusted odds | Confidence Interval | |
|---|---|---|---|---|---|
|
| |||||
| Lower limit | Upper limit | ||||
| Age | 0.069 | 0.056 | 0.876 | 0.764 | 1.003 |
| PMAW | 0.151 | 0.008 | 0.669 | 0.498 | 0.899 |
| PD | 0.261 | 0.016 | 0.532 | 0.319 | 0.887 |
| AL | 0.182 | 0.002 | 1.739 | 1.217 | 2.483 |
| SNA | 0.292 | 0.066 | 0.585 | 0.330 | 1.036 |
| Normo- divergent | Reference | ||||
| Hyperdivergent | 1.251 | 0.037 | 0.074 | 0.006 | 0.854 |
| Hypodivergent | 1.348 | 0.316 | 0.259 | 0.018 | 3.632 |
PMAW, maxillary first premolar alveolar width; PD, palatal width; AL, arch length. R2-0.679
Discussion
This case–control study aimed to identify the intra-arch risk factors for PDC by comparing the maxillary transverse width at the molar and premolar regions at two levels, PD and AL in subjects with and without canine impactions. The CBCT scans of 79 participants were obtained, and they were matched and divided into case and control groups for assessment. The case–control studies are suitable to study the risk factors and the recommended case: control ratio is ≥1:2.[26]
The results of this study indicated a statistically significant difference in age between the three groups [Table 2]. The bilaterally impacted group was the youngest among the three with a mean age of 17.33 ± 3.6 years, which could be attributed to the fact that subjects with bilaterally impacted maxillary canines tend to seek treatment early because of grave concerns.[24] The mean age of the unilateral and control groups was 19.31 ± 5.2 and 24.74 ± 9.4 years, respectively. However, the differences in age between the groups did not influence the transverse measurements because these dimensions were defined before 13 years.[27]
Although there is a high prevalence among females for PDC,[6,9] this study did not show any significant gender difference, ensuring that the group was comparable for intra-arch risk factor assessment. A tertiary-level referral hospital-based convenient sampling might be the reason for the lack of a gender difference among samples.
Skeletal characteristics, such as maxillary sagittal position, sagittal skeletal relation, and growth pattern, were distributed similarly among the groups [Table 3]. The evaluation of cephalometric values did not show any significant difference between the groups (SNA, P = 0.518; ANB, P = 0.149; and FMA, P = 0.153). In addition, regression analyses did not show any significant influence on the transverse dimensions based on these skeletal parameters.
This study identified that transverse maxillary alveolar width in the molar (unilateral: 56.69 ± 2.24 mm, bilateral: 56.05 ± 2.82 mm) and premolar (unilateral: 42.37 ± 3.05 mm, bilateral: 44.73 ± 4.53 mm) regions was smaller in the impacted group than the control group (MAW: 58.12 ± 3.05 mm, PMAW: 46.47 ± 2.8 mm) [Table 4]. Previous studies reported similar findings using study casts and radiography and thus associated maxillary canine impaction with transverse deficiency.[3,16]
Furthermore, a statistically significant reduction was detected in the transverse dimension only at the alveolar region of molars and premolars (MAW, P = 0.046; PMAW, P < 0.001). The transverse width of the molars and premolars in the basal region (MBW, P = 0.327; PMBW, P = 0.145) did not show any significant difference between the impacted and control groups [Table 4]. However, in a similar but cross-sectional study, Arboleda-Ariza et al.[24] reported that both maxillary transverse basal and alveolar dimensions were smaller in the impacted group than in the control group.
The univariate analysis showed a significant association between transverse dimensions (MAW, OR: 0.813; PMAW, OR: 0.751) and canine impaction [Table 5]. The final multivariate logistic regression model could identify only PMAW as the intra-arch risk factor [OR: 0.669; Table 6].
In a split-mouth CBCT study, D’Oleo-Aracena et al.[19] reported that maxillary transverse deficiency was increased on the impacted side. Yan et al.[18] used CBCT and showed that buccal canine impaction was associated with maxillary transverse deficiency, whereas PDC was not associated with transverse deficiency. However, Kim et al.[17] demonstrated that the palatally impacted canine group showed a transverse discrepancy compared to the buccally impacted canine group.
In contrast to the findings of this study, some studies used dental casts and 2D radiography and reported that canine impaction was associated with greater maxillary transverse width,[9,13] whereas others could not demonstrate an association between maxillary canine impaction and transverse deficiency.[18,20] This difference in results might be because of the lack of true skeletal comparison between groups based on the CBCT images.
In the final regression model established in this study, MAW value did not show any significant influence on the canine impaction, indicating that only anterior maxillary transverse dentoalveolar deficiency is associated with canine impaction. Similarly, McConnel et al.[16] also demonstrated that canine impaction was associated with transverse anterior maxillary deficiency.
A previous study by Fattahi et al.[28] compared palatal height index, arch width, and AL in palatal and buccal canine impaction and matched control groups and found significant difference only in the AL. However, this study showed that PD and AL measurements have a significant difference among control, unilateral, and bilateral impaction groups [P = 0.003 and P = 0.001, respectively; Table 4]. Furthermore, univariate and multivariate regression analysis confirmed that PDC was influenced by PD and AL such that the impacted group was associated with decreased PD and increased AL (OR: 0.532 and 1.739, respectively) [Table 4]. Kim et al.[17] demonstrated that the palatal vault of the PDC group was narrower and deeper compared to the buccally impacted canine group.
Although the results showed an association between age and the occurrence of canine impaction, the final model revealed that age acts as a confounder. Some studies have reported that transverse maxillary deficiency is defined at an early age (between 8 and 10 years).[26,29] Furthermore, the mean age for the eruption of the maxillary canine is 10.5 years in girls and 11.5 years in boys (with an individual variation of 3–4 years).[30] Since the transverse width in the maxilla and canine impaction were established before 13 years of age, the difference in the groups with respect to age would not interfere with the results.
Several studies[3,16,24] demonstrated an association between the maxillary canine impaction and the transverse deficiency of the maxilla; thus, the early diagnosis of transverse deficiency would guide clinicians to perform interceptive procedures when necessary.[24] Therefore, maxillary expansion could be performed as an interceptive procedure to correct the transverse deficiency and decrease the probability of canine impaction.[21]
Furthermore, a randomized clinical trial (RCT) by Baccetti et al.[22] revealed that subjects treated with rapid maxillary expansion (RME) have a high rate of successful eruption of PDC (65.7%) and concluded that maxillary expansion is effective as an interceptive procedure to prevent impaction of maxillary canines with palatal displacement in the early mixed dentition. Because canine impaction was associated with a transverse deficiency at the alveolar level, the use of RME as an interceptive procedure for canine impaction is controversial. However, the improvement in the intraosseous position of PDC may be a justifiable reason for using RME. Pereira et al.[31] compared the effects of RME and slow maxillary expansion (SME) in the transverse maxillary expansion and concluded that only RME produced maxillary skeletal expansion, and both maxillary expansion modalities efficiently promoted the maxillary transverse dimension at the alveolar level.[31] Previous studies have also been in concordance with this finding.[32,33] However, Caprioglio et al.[34] found that RME treatment improved the canine position significantly compared to the SME.
Because this study showed that maxillary canine impaction was associated with transverse maxillary dentoalveolar deficiency and normal maxillary basal bone width, SME is adequate for the increase in alveolar transverse width and prevention of canine impaction. This phenomenon indicated that RME is not essential for the interceptive treatment of maxillary canine impaction. High-quality RCT using SME to intercept maxillary canine impaction is essential to address this issue.
Because the multifactorial etiology of canine impaction is yet unclear in the literature, additional studies are essential to elucidate the correlation between transverse deficiency of maxilla and canine impaction. It is hoped that this study provides a preliminary insight for future experimental studies.
Conclusions
Maxillary arch width at the alveolar level (not basal width) in subjects with unilateral and bilateral canine impactions is smaller compared to those of controls.
Subjects with unilateral and bilateral canine impaction have smaller PD compared to subjects without canine impaction.
Unilateral and bilateral canine impaction groups have greater AL compared to the control group.
Thus, a reduced maxillary arch width at the alveolar level, smaller PD, and increased AL were identified as risk factors leading to potential maxillary palatal canine impaction such that one unit increase in PMAW and PD reduced the risks of impaction by 33% and 46%, respectively, and one unit increase in AL increased the risk of impaction by 73%.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Peck S, Peck L, Kataja M. The palatally displaced canine as a dental anomaly of genetic origin. Angle Orthod. 1994;64:249–56. doi: 10.1043/0003-3219(1994)064<0249:WNID>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby H. The etiology of maxillary canine impactions. Am J Orthod. 1983;84:125–32. doi: 10.1016/0002-9416(83)90176-8. [DOI] [PubMed] [Google Scholar]
- 3.Schindel RH, Duffy SL. Maxillary transverse discrepancies and potentially impacted maxillary canines in mixed-dentition patients. Angle Orthod. 2007;77:430–5. doi: 10.2319/0003-3219(2007)077[0430:MTDAPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Thilander B, Jakobsson SO. Local factors in impaction of maxillary canines. Acta Odontol Scand. 1968;26:145–68. doi: 10.3109/00016356809004587. [DOI] [PubMed] [Google Scholar]
- 5.Becker A, Smith P, Behar R. The incidence of anomalous maxillary lateral incisors in relation to palatally-displaced cuspids. Angle Orthod. 1981;51:24–9. doi: 10.1043/0003-3219(1981)051<0024:TIOAML>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Sacerdoti R, Baccetti T. Dentoskeletal features associated with unilateral or bilateral palatal displacement of maxillary canines. Angle Orthod. 2004;74:725–32. doi: 10.1043/0003-3219(2004)074<0725:DFAWUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Cernochova P, Izakovicova-Holla L. Dentoskeletal characteristics in patients with palatally and buccally displaced maxillary permanent canines. Eur J Orthod. 2012;34:754–61. doi: 10.1093/ejo/cjr069. [DOI] [PubMed] [Google Scholar]
- 8.Basdra EK. Congenital tooth anomalies and malocclusions: A genetic link? Eur J Orthod. 2001;23:145–52. doi: 10.1093/ejo/23.2.145. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nimri K, Gharaibeh T. Space conditions and dental and occlusal features in patients with palatally impacted maxillary canines: An aetiological study. Eur J Orthod. 2005;27:461–5. doi: 10.1093/ejo/cji022. [DOI] [PubMed] [Google Scholar]
- 10.Kuftinec MM, Shapira Y. The impacted maxillary canine: I. Review of concepts. ASDC J Dent Child. 1995;62:317–24. [PubMed] [Google Scholar]
- 11.Mulick JF, James F., Dr. Mulick on impacted canines. J Clin Orthod JCO. 1979;13:824–34. [PubMed] [Google Scholar]
- 12.Shapira Y, Kuftinec MM. Early diagnosis and interception of potential maxillary canine impaction. J Am Dent Assoc 1939. 1998;129:1450–4. doi: 10.14219/jada.archive.1998.0080. [DOI] [PubMed] [Google Scholar]
- 13.Sambataro S, Baccetti T, Franchi L, Antonini F. Early predictive variables for upper canine impaction as derived from posteroanterior cephalograms. Angle Orthod. 2005;75:28–34. doi: 10.1043/0003-3219(2005)075<0028:EPVFUC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Mercuri E, Cassetta M, Cavallini C, Vicari D, Leonardi R, Barbato E. Skeletal features in patient affected by maxillary canine impaction. Med Oral Patol Oral Cir Bucal. 2013;18:e597–602. doi: 10.4317/medoral.18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Batham PR, Garg A, Virang B, Pereira Kalia UD. The effect of displaced canine on the dentoskeletal and soft tissue development of the face: A cephalometric study. Int J Orthod Rehabil. 2018;9:14–22. [Google Scholar]
- 16.McConnell TL, Hoffman DL, Forbes DP, Janzen EK, Weintraub NH. Maxillary canine impaction in patients with transverse maxillary deficiency. ASDC J Dent Child. 1996;63:190–5. [PubMed] [Google Scholar]
- 17.Kim Y, Hyun H, Jang K-T. The position of maxillary canine impactions and the influenced factors to adjacent root resorption in the Korean population. Eur J Orthod. 2011;34:302–6. doi: 10.1093/ejo/cjr002. [DOI] [PubMed] [Google Scholar]
- 18.Yan B, Sun Z, Fields H, Wang L, Luo L. Etiologic factors for buccal and palatal maxillary canine impaction: A perspective based on cone-beam computed tomography analyses. Am J Orthod Dentofac Orthop. 2013;143:527–34. doi: 10.1016/j.ajodo.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 19.D Oleo-Aracena MF, Arriola-Guillén LE, Rodríguez-Cárdenas YA, Ruíz-Mora GA. Skeletal and dentoalveolar bilateral dimensions in unilateral palatally impacted canine using cone beam computed tomography. Prog Orthod. 2017;18:7. doi: 10.1186/s40510-017-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saiar M, Rebellato J, Sheats RD. Palatal displacement of canines and maxillary skeletal width. Am J Orthod Dentofacial Orthop. 2006;129:511–9. doi: 10.1016/j.ajodo.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Baccetti T, Leonardi M, Armi P. A randomized clinical study of two interceptive approaches to palatally displaced canines. Eur J Orthod. 2008;30:381–5. doi: 10.1093/ejo/cjn023. [DOI] [PubMed] [Google Scholar]
- 22.Baccetti T, Mucedero M, Leonardi M, Cozza P. Interceptive treatment of palatal impaction of maxillary canines with rapid maxillary expansion: A randomized clinical trial. Am J Orthod Dentofac Orthop. 2009;136:657–61. doi: 10.1016/j.ajodo.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Almeida RC, Cevidanes LHS, Carvalho FA, Motta AT, Almeida MA, Styner M, et al. Soft tissue response to mandibular advancement using 3D CBCT scanning. Int J Oral Maxillofac Surg. 2011;40:353–9. doi: 10.1016/j.ijom.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arboleda-Ariza N, Schilling J, Arriola-Guillén LE, Ruíz-Mora GA, Rodríguez-Cárdenas YA, Aliaga-Del Castillo A. Maxillary transverse dimensions in subjects with and without impacted canines: A comparative cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2018;154:495–503. doi: 10.1016/j.ajodo.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Podesser B, Williams S, Bantleon HP, Imhof H. Quantitation of transverse maxillary dimensions using computed tomography: A methodological and reproducibility study. Eur J Orthod. 2004;26:209–15. doi: 10.1093/ejo/26.2.209. [DOI] [PubMed] [Google Scholar]
- 26.Celentano DD, Szuklo M, Gordis L, editors. Gordis Epidemiology. 6th ed. Philadelphia, PA: 2019. [Google Scholar]
- 27.Bishara SE, Jakobsen JR, Treder J, Nowak A. Arch width changes from 6 weeks to 45 years of age. Am J Orthod Dentofac Orthop. 1997;111:401–9. doi: 10.1016/s0889-5406(97)80022-4. [DOI] [PubMed] [Google Scholar]
- 28.Fattahi H, Ghaeed F, Alipour A. Association between maxillary canine impaction and arch dimensions. Aust Orthod J. 2012;28:57–62. [PubMed] [Google Scholar]
- 29.Moorrees CF, Gron AM, Lebret LM, Yen PK, Fröhlich FJ. Growth studies of the dentition: A review. Am J Orthod. 1969;55:600–16. doi: 10.1016/0002-9416(69)90037-2. [DOI] [PubMed] [Google Scholar]
- 30.Ericson S, Kurol J. Early treatment of palatally erupting maxillary canines by extraction of the primary canines. Eur J Orthod. 1988;10:283–95. doi: 10.1093/ejo/10.4.283. [DOI] [PubMed] [Google Scholar]
- 31.Pereira JDS, Jacob HB, Locks A, Brunetto M, Ribeiro GLU. Evaluation of the rapid and slow maxillary expansion using cone-beam computed tomography: A randomized clinical trial. Dental Press J Orthod. 2017;22:61–8. doi: 10.1590/2177-6709.22.2.061-068.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbridge JK, Campbell PM, Taylor R, Ceen RF, Buschang PH. Transverse dentoalveolar changes after slow maxillary expansion. Am J Orthod Dentofacial Orthop. 2011;140:317–25. doi: 10.1016/j.ajodo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Weissheimer A, de Menezes LM, Mezomo M, Dias DM, de Lima EM, Rizzatto SM. Immediate effects of rapid maxillary expansion with Haas-type and hyrax-type expanders: A randomized clinical trial. Am J Orthod Dentofacial Orthop. 2011;140:366–76. doi: 10.1016/j.ajodo.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Caprioglio A, Castiglioni F, Sambataro S, Giuntini V, Comaglio I, Lorvetti F, Fastuca R. Changes in canine inclination after rapid and slow maxillary expansion compared to untreated controls. Orthod Craniofac Res. 2020;23:351–6. doi: 10.1111/ocr.12377. [DOI] [PubMed] [Google Scholar]


