Abstract
OBJECTIVE:
Lower face soft tissue thickness and dentoskeletal features form the lower facial profile. Sagittal skeletal malocclusions with varying degrees of soft tissue thickness in males and females were herein examined using soft tissue cephalometric radiography.
METHODS:
Based on their dentoskeletal correlations, a total of 160 lateral cephalometric radiographs of adult males and females (n = 80) seeking orthodontic treatment were classified as class I (n = 40), class II division 1 (n = 40), class II division 2 (n = 40), or class III (n = 40). Holdaway analysis was used to assess soft tissue thickness in seven linear parameters.
RESULTS:
In class I, class II division 1, class II division 2, and class III dentoskeletal connections, males exhibited larger soft tissue thickness. They have an average lower lip thickness, chin depth H, and depth V for class I males. Males and females differed from one another when it came to the thicknesses of the upper and the lower lips. These lip thicknesses as well as the chin's width differed more between men and women in class II division 1. Except for upper lip strain, all measures in the class II division 2 sample males demonstrated a greater significance. In the class III sample, males also demonstrated more significance than females.
CONCLUSION:
Males with various sagittal skeletal malocclusions demonstrated a significant difference in lower soft tissue thickness (characterized as thicker lower facial soft tissue) compared to female patients in class I, class II division 1, class II division 2, and class III malocclusions.
Keywords: Gender differences, sagittal skeletal malocclusions, soft tissue cephalometric analysis, soft tissue thickness
Introduction
The soft tissue thickness is a crucial element to be considered during the orthodontic examination. Sometimes, the significant skeletal disparity is disguised by good soft tissue. Nature has a propensity of compensating. According to Arnett and Gunson,[1] when assessing the soft tissue profile, the person receiving orthodontic treatment should always be calm. To see how soft tissues and hard tissues interact, they recommended that the patient is placed in a relaxed lip position while examining his/her soft tissue profile.
However, in the case of soft tissue analysis, the results of a specific ethnic group's cephalometric measurements may not apply to others. Each population should have its own set of criteria. The features of various racial groupings must be considered when treating them. A variety of reasons can cause class II malocclusion, including soft tissues, the hypotonic upper lip or reclined lower incisors, and the hyperactive lower lip.[2,3]
For many years, the desire to improve one's facial appearance has been cited as the most compelling reason for undergoing orthodontic treatment. Understanding the link between the face bones and soft tissue is critical to facial esthetics. Until recently, it was believed that the skeletal structure predominantly influenced the soft tissue profile configuration. Because of its wide range of thickness, it is said that the soft tissue may work independently of the fundamental dentoskeletal basis and, thus, can be regarded as the primary component in determining an individual's ultimate facial profile.[4]
Even if a person's bones are perfectly aligned, the face soft tissues might grow out of proportion to them.[5] The soft tissues’ length, thickness, and tonicity may affect face shape and position, thereby affecting facial esthetics.[6,7] With these results in mind, the aim of this study was to discover how gender differences in soft tissue thickness vary among numerous different types of adult Iraqi samples from both genders.
Materials and Methods
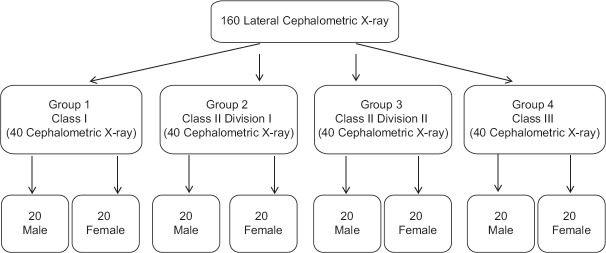
Hilla University College's Dentistry Department performed the cross-sectional, observational study. The sample size estimation was performed by using 80% power of the study using OpenEpi, Version 3, open-source calculator at an alpha of 0.05.[8] This yielded a sample size of 160 lateral cephalometric X-rays, divided into four main groups of 40 each according to their sagittal skeletal relationships [Figure 1][9]: (i) class I malocclusions in group 1 (A point, nasion, B point [ANB] angle between 0° and 4°), (ii) class II division 1 malocclusions in group 2 (ANB angle >4° with normal or proclined upper incisors), (iii) class II division 2 malocclusions in group 3 (ANB angle >4° with retroclined upper incisors), and (iv) class III malocclusions in group 4 (ANB angle <0°).
Figure 1.
Flowchart of the study
With regard to class II, the incisal connection distinguishes between two types of malocclusion. According to the British Standards Institute[10] Classification of Malocclusion for class II division 1, the lower incisor margins extend beyond the cingulum plateau of the upper central incisors, whereas for class II division 2, the lower incisor is touching the cingulum plateau of upper central incisor.
The inclusion criteria included subjects satisfying the following: (i) Iraqi adult sample aged between 18 and 45 years old, (ii) normal vertical skeletal relationship with a sella-nasion/mandibular plane (SN-MP) angle equal to 32° ± 2°[11], and (iii) all teeth are present with or without the presence of third molars. The exclusion criteria included subjects that met the following: (i) having previous orthodontic treatment or orthognathic surgery, (ii) having a systemic disease and a craniofacial anomaly, (iii) having traumatic injuries, and (iv) having received facial esthetic treatment, including Botox and fillers. Before beginning the study, ethical approval was obtained from Hilla University College's Ethics Board, Institutional Review Board (IRB).
Methods
All lateral cephalometric X-rays were taken by the same operator using the same machine (X-ray device, Kodak 9000 3D; Carestream Health, Inc., Rochester, NY, USA) and under the same technical conditions. The patient had to be positioned so that the Frankfort horizontal plane was parallel to the horizontal plane to match the X-ray path. The lips and teeth were kept relaxed, while centric occlusion was employed for the teeth. One hundred and sixty lateral cephalometric radiographs were traced and analyzed using the Adobe Photoshop CC program (2018; Version 20.0). Magnification was recorded for each cephalometric head film, and the readings were adjusted accordingly. Hard and soft tissue landmarks were defined [Table 1], and all cephalometric landmarks were determined according to the definition of Jacobson.[12] The tracings were completed, and the variables were measured in mm.
Table 1.
Hard and soft tissue landmarks[12]
| Point A (A) | The curve between the Anterior Nasal Spine ANS and prosthion at the deepest, most posterior midline position. |
| Point B (B) (supramentale) | Mandibular concavity between the infradental and the Pog is the deepest and most posterior midline point. |
| U1 | The most prominent labial point of the maxillary incisor. |
| L1 | The most prominent labial point of the mandibular incisor. |
| Pog | On the bony chin’s midsagittal plane, the most anterior point on its shape. |
| Me | The mandibular symphysis’s most inferior point. |
| Sn | The point of convergence of the nose and the upper lip. |
| A’ | The point of greatest concavity in the midline of the upper lip between Sn and Ls. |
| Ls | The mucocutaneous junction or midpoint of the upper vermilion line. |
| Li | The mucocutaneous junction or midpoint of the lower vermilion line. |
| B’ | Between Li and Pog of the soft tissues, where the lip has the highest concavity. |
| Pog’ | The soft tissue chin’s most conspicuous point is in the midsagittal plane. |
| Me’ | The lowest place on the chin’s soft tissue. |
U1=upper incisor, L1=lower incisor, Pog=pogonion, Me=menton, Sn=subnasale, A’ = soft tissue point A, Ls=labialesuperius, Li=labialeinferius, B’ = soft tissue point B, Pog’ = soft tissue pogonion, Me’ = soft tissue menton, ANS=Anterior Nasal Spine
Cephalometric measurements
The cephalometric X-rays were adjusted according to the natural head position by adding 5.6° to SN inclination [Figure 2].[13] Seven linear lower facial soft tissue thicknesses were assessed [Table 2 and Figure 3].
Figure 2.
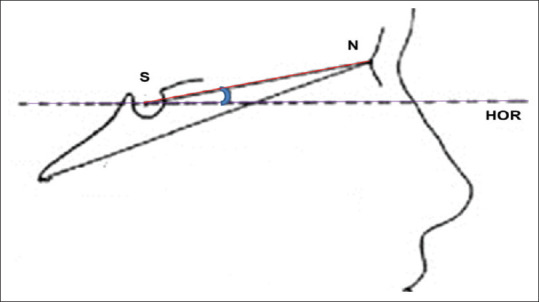
Head orientation procedure
Table 2.
Linear measurements (in mm)
| Lower facial soft tissue thickness [Figure 3][14] | |
|---|---|
| 1-Basic upper lip thickness | A straight line extending from Sn, 3 mm below A-point. |
| 2-Upper lip thickness | U1 labial tip to the labralsuperius of the maxillary incisor (Ls). |
| 3-Upper lip strain | There are two levels of upper lip thickness: basic and advanced. |
| 4-Lower lip thickness | The linear distance from the most prominent labial point of the mandibular incisor (L1) to labrale inferius (Li) |
| 5-Basic lower lip thickness | The labiodental fold’s deepest point is a straight line away from B-point. |
| 6-Chin thickness-H (horizontal) | How distant is the Pogonion from its sagittal projection (Pog–Pog’) concerning the rest of the skeleton? |
| 7-Chin thickness-V (vertical) | Menton’s vertical projection (Me–Me’) on soft tissue is separated from it by a distance of (linear distance). |
U1=upper incisor, L1=lower incisor, Pog=pogonion, Me=menton, Sn=subnasale, Ls=labialesuperius, Li=labialeinferius, Pog’ = soft tissue pogonion, Me’ = soft tissue menton
Figure 3.
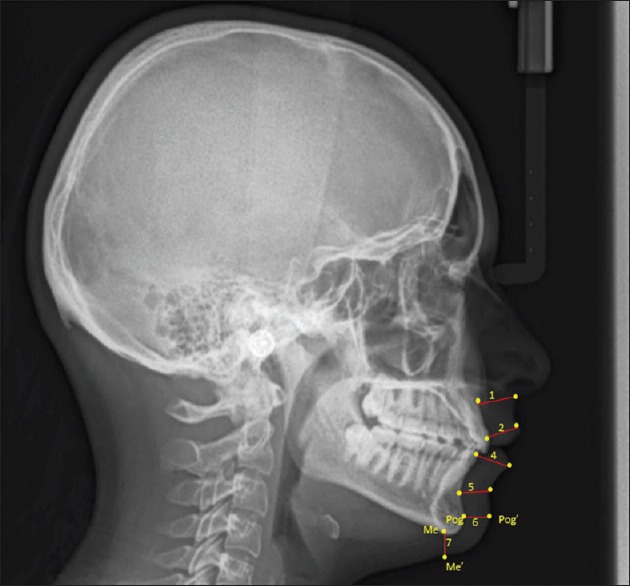
Statistical analyses
The SPSS for Windows software was used to conduct statistical analysis of the obtained data (Chicago, IL, USA, version 25.0). A P value < 0.05 was used as the cutoff point for statistical significance. The intra-class correlation coefficient (ICC) was used to test the repeatability of the measurements, twice, on a sample of 20 participants; the ICC was found to be 0.990, thereby indicating great repeatability. For the continuous variables, Kolmogorov–Smirnov tests were carried out to determine their normality distribution. Variables with a high degree of statistical significance were tested using parametric methods. We used Student's t-tests and Mann–Whitney tests to evaluate the soft tissue thickness characteristics between males and females for each sagittal skeletal malocclusion type.
Results
Hard and soft tissues parameters in males and females and in the whole sample [Tables 3–6] show the lower soft tissue thickness parameters’ lowest, maximum, mean, and standard deviation (SD) for the participants under the various classes.
Table 3.
Descriptive statistics of lower soft tissue thickness parameters and t-test assessment of the difference between males and females of class I
| Variable (mm) | Sex | n | Mean | SD | P |
|---|---|---|---|---|---|
| Basic upper lip thickness | Male | 20 | 16.05 | 1.89 | 0.000** |
| Female | 20 | 13.66 | 1.34 | ||
| Upper lip Thickness | Male | 20 | 13.57 | 2.24 | 0.001** |
| Female | 20 | 11.36 | 1.49 | ||
| Upper lip strain | Male | 20 | 2.48 | 1.53 | 0.692 |
| Female | 20 | 2.32 | 0.93 | ||
| Lower lip thickness | Male | 20 | 15.49 | 1.41 | 0.000** |
| Female | 20 | 12.78 | 1.48 | ||
| Basic lower lip thickness | Male | 20 | 11.90 | 1.41 | 0.012* |
| Female | 20 | 10.76 | 1.30 | ||
| Chin thickness H | Male | 20 | 13.27 | 2.60 | 0.001** |
| Female | 20 | 10.71 | 1.82 | ||
| Chin thickness V | Male | 20 | 9.16 | 2.27 | 0.001** |
| Female | 20 | 7.10 | 1.30 |
*Significant at P<0.05, **highly significant at P<0.001
Table 6.
Descriptive statistics of lower soft tissue thickness parameters and t-test assessment of the difference between males and females of class III
| Variable (mm) | Sex | n | Mean | SD | P |
|---|---|---|---|---|---|
| Basic upper lip thickness | Male | 20 | 16.580 | 1.7124 | 0.000** |
| Female | 20 | 14.110 | 1.6880 | ||
| Upper lip Thickness | Male | 20 | 13.770 | 1.8405 | 0.000** |
| Female | 20 | 11.370 | 1.6332 | ||
| Upper lip strain | Male | 20 | 2.810 | 1.8026 | 0.895 |
| Female | 20 | 2.745 | 1.2352 | ||
| Lower lip thickness | Male | 20 | 13.795 | 1.8712 | 0.000** |
| Female | 20 | 11.655 | 1.3117 | ||
| Basic lower lip thickness | Male | 20 | 11.740 | 1.6462 | 0.006* |
| Female | 20 | 10.405 | 1.2159 | ||
| Chin thickness H | Male | 20 | 12.995 | 2.1107 | 0.011* |
| Female | 20 | 11.340 | 1.7718 | ||
| Chin thickness V | Male | 20 | 9.335 | 1.4217 | 0.018* |
| Female | 20 | 8.110 | 1.7115 |
*Significant at P<0.05, **highly significant at P<0.001
Comparison of soft tissue thickness parameters between class I males and females [Table 3]
The basic upper lip thickness (P < 0.001), the basic lower lip thickness (P = 0.012), the upper lip thickness (P = 0.001), the lower lip thickness (P < 0.001), the chin thickness H (P = 0.001), and the chin thickness V (P = 0.001) were significantly higher in males compared to females with a class I angle. The difference was insignificant for the other soft tissue parameters (P > 0.05).
Comparison of soft tissue thickness parameters between class II division 1 males and females [Table 4]
Table 4.
Descriptive statistics of lower soft tissue thickness parameters and t-test assessment of the difference between males and females of class II division 1
| Variable (mm) | Sex | n | Mean | SD | P |
|---|---|---|---|---|---|
| Basic upper lip thickness | Male | 20 | 15.005 | 2.333 | 0.04* |
| Female | 20 | 13.615 | 1.772 | ||
| Upper lip Thickness | Male | 20 | 11.245 | 2.127 | 0.183 |
| Female | 20 | 10.445 | 1.563 | ||
| Upper lip strain | Male | 20 | 3.750 | 1.549 | 0.297 |
| Female | 20 | 3.220 | 1.622 | ||
| Lower lip thickness | Male | 20 | 13.645 | 1.794 | 0.559 |
| Female | 20 | 13.355 | 1.277 | ||
| Basic lower lip thickness | Male | 20 | 12.265 | 1.767 | 0.006* |
| Female | 20 | 10.850 | 1.253 | ||
| Chin thickness H | Male | 20 | 13.010 | 1.964 | 0.025* |
| Female | 20 | 11.625 | 1.797 | ||
| Chin thickness V | Male | 20 | 8.665 | 1.693 | 0.004* |
| Female | 20 | 7.125 | 1.444 |
*Significant at P<0.05, **highly significant at P<0.001
The basic upper lip thickness (P = 0.040), the basic lower lip thickness (P = 0.006), the chin thickness H (P = 0.025), and the chin thickness V (P = 0.004) were significantly higher in males compared to females under the class II division 1 classification. The difference was insignificant for the other parameters (P > 0.05).
Comparison of soft tissue thickness parameters between class II division 2 males and females [Table 5]
Table 5.
Descriptive statistics of lower soft tissue thickness parameters and t-test assessment of the difference between males and females of class II division 2
| Variable (mm) | Sex | n | Mean | SD | P |
|---|---|---|---|---|---|
| Basic upper lip thickness | Male | 20 | 15.910 | 2.097 | 0.000** |
| Female | 20 | 13.380 | 1.460 | ||
| Upper lip Thickness | Male | 20 | 14.510 | 1.734 | 0.001** |
| Female | 20 | 12.080 | 2.620 | ||
| Upper lip strain | Male | 20 | 1.370 | 1.712 | 0.901 |
| Female | 20 | 1.290 | 2.296 | ||
| Lower lip thickness | Male | 20 | 15.460 | 1.723 | 0.000** |
| Female | 20 | 12.875 | 1.658 | ||
| Basic lower lip thickness | Male | 20 | 10.775 | 1.604 | 0.073 |
| Female | 20 | 9.995 | 1.006 | ||
| Chin thickness H | Male | 20 | 13.025 | 1.919 | 0.003* |
| Female | 20 | 11.235 | 1.655 | ||
| Chin thickness V | Male | 20 | 9.175 | 1.866 | 0.004* |
| Female | 20 | 7.390 | 1.760 |
*Significant at P<0.05, **Highly significant at P<0.001
The basic upper lip thickness (P < 0.001), the basic lower lip thickness (P = 0.006), the upper lip thickness (P = 0.001), the lower lip thickness (P < 0.001), the chin thickness H (P = 0.003), and the chin thickness V (P = 0.004) were significantly elevated in males compared to females within the class II division 2 group. The difference was insignificant for the other soft tissue parameters (P > 0.05).
Comparison of soft tissue thickness parameters between class III males and females [Table 6]
The basic upper lip thickness (P < 0.001), the basic lower lip thickness (P = 0.0062), the upper lip thickness (P < 0.001), the lower lip thickness (P < 0.001), the chin thickness H (P = 0.011), and the chin thickness V (P = 0.018) were significantly elevated in males compared to females with class III. The difference was insignificant for the other soft tissue parameters (P > 0.05).
Discussion
In this comparative, descriptive cross-sectional research, all sagittal skeletal malocclusions were shown to be accompanied by a larger amount of soft tissue thickness in males than females [Tables 3–6]. There is a functional influence on the underlying dental structures of class I, class II division 1, class II division 2, and class III malocclusions [Tables 3–6]. This is because the soft tissues, such as the lips and the chin, are partially muscle based. For Subtelny,[5] the lips’ vermillion appearance has a strong postural link to the tissues that sustain it. Lips protruding (either one or both) appear to be associated with the most drastic alterations in facial appearance. Changes to the lips may significantly affect how they seem for a person's appearance. Because of the increased emphasis on cephalometric analysis, it is crucial to recognize the distinctive lip characteristics of various genders to make an accurate diagnosis.[5]
When evaluating how much incisor retraction is required to improve esthetics, it is important to consider gender differences in lip thickness. Table 3 indicates substantial differences in soft tissue measurements between the sexes (men and women). In terms of basic measurements, upper and lower lip thicknesses are higher in men than in women; this is also true for basic upper lip thickness, chin thickness H as well as V, and basic lower lip thickness. It appears that men's soft tissue structures are thicker than those of women.
The importance of the lips cannot be stressed if you are contemplating an incisor retraction or an invasion. If you are thinking about getting braces, remember that nature's way of fixing misalignment is soft tissue camouflage. During adolescence, adolescent gender differences can be seen in the form of the face. Because of the prominence of the forehead, nose, and chin on males and the larger curve of the jaw, their bone structure is bolder and more pronounced. Males are often bigger than females, as is the norm. To put it another way, males develop over a longer period of time than girls, thereby resulting in a greater ultimate dimension.[16]
Patients requiring orthognathic surgery in addition to orthodontic therapy may need to consider the age at which orthodontic treatment can begin based on gender variations in soft tissue measurements. According to the current study, lip thickness at points A and B grew more than the vermilion borders. Females showed no significant increase in lower lip thickness along the vermilion border. Males’ lips get longer and thicker as a result of these modifications.
Several research studies comparing male and female soft tissue measures were almost identical.[17,18] According to the results of this study, males had larger perioral soft tissue thickness than females when classified under class II division 1. Males in all study groups had considerably higher values for chin thickness H and V as well as for basic upper and lower lip thicknesses.
According to our data, the top and lower lip thicknesses were significantly different. Group II-L and II-lower H's lip thicknesses were significantly greater than those of group I in Lee et al.[18]; independently of ethnic background, a thicker lower lip skeletal class II division 1 soft tissue can be detected. Turkish malocclusion patients classified under a skeletal class II, regardless of gender, had the broadest lower lips.[11]
Soft tissue thickness in various races has also been investigated.[19,20] For example, the soft tissue thickness of African Americans has been shown to differ considerably from that of White Americans. According to another study, the soft tissue thickness of Saudi Arabians was shown to differ from that of White people.[21] Future studies should look at ethnic disparities when confirming our findings, as the thickness of this study is confined to only one geographic location (Iraq).
Researchers from Korea employed face CT scans to create an adult database for craniofacial reconstruction, and they found that men had greater values for most markers than women did, with lip landmarks showing the highest disparity of over 2 mm.
To cover up the upper and the lower incisor retrusion and dentoalveolar jaw segment posteriority, men in class II division 2 had thicker soft tissues, which was more significant than those of men in class I division 1.[22] Compared to females, males were shown to have a more efficient compensatory mechanism.
There were substantial disparities between males and females in the findings of a skeletal jaw connection in the class III soft tissue thickness measures. There were no significant gender differences in the soft tissue thickness of the chin region soft tissue thickness in class III patients, a fact that contradicts the findings of Jabbar et al.[23] This compensation for skeletal disharmony has been highlighted by several studies[11,24] that emphasize the reduced soft tissue thickness and a rise in the area of the upper lip and the upper lip sulcus.
Perović and Blažej have found no variation in soft tissue thickness between males and females in class III samples.[25] The racial discrepancies between the findings of this study and the findings of the other authors may be to blame for the discrepancy.
This study showed that men had thicker skin than women in all groups; a finding that may be because of the testosterone's role in promoting collagen production, which results in males having thicker skin. However, female hormones (such as estrogen) are known to boost hyaluronic acid production, thereby reducing collagen production and thinning a woman's skin.[26]
Conclusion
Compared to female patients in classes I, II division 1, II division 2, and III, males with various sagittal skeletal malocclusions were characterized by thicker lower face soft tissues.
Recommendation
More attention should be implemented regarding the importance of soft tissue characteristics in constructing optimal orthodontic diagnosis and treatment planning. Further studies that include other geographical areas with the use of Cone-beam computed tomography (CBCT) and the software Dolphin imaging are recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The present research work is supported and edited by Dr Apostolos Zarros, physician, MRes, PhD, PhD, FIBMS. Director, Pharmacological Research Observatory. Visiting Professor and BPS Ambassador, College of Pharmacy, University of Babylon. Honorary Research Fellow, Institute of Cancer Sciences, University of Glasgow.
References
- 1.Arnett GW, Gunson MJ. Facial planning for orthodontists and oral surgeons. Am J OrthodDentofacialOrthop. 2004;126:290–5. doi: 10.1016/j.ajodo.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Sarver DM, editor. Esthetic Orthodontics and Orthognathic Surgery. St Louis: CV Mosby; 1998. [Google Scholar]
- 3.Profit WR, Profit WR, White RP Jr, Sarver DM, editors. Contemporary treatment of dentofacial deformity. Louis: CV Mosby; 2003. [Google Scholar]
- 4.Albarakati SF. Soft-tissue facial profile of adult Saudis. Saudi Med J. 2011;32:836–42. [PubMed] [Google Scholar]
- 5.Subtelny JD. The soft tissue profile, growth, and treatment changes. Angle Orthod. 1961;31:105–22. [Google Scholar]
- 6.Burstone CJ. Lip posture and its significance in treatment planning. Am J Orthod. 1967;53:262–84. doi: 10.1016/0002-9416(67)90022-x. [DOI] [PubMed] [Google Scholar]
- 7.Jazmati HM, Ajaj MA, Hajeer MY. Assessment of facial soft tissue dimensions in adult patients with different sagittal skeletal classes using cone beam computed tomography. J Contemp Dent Pract. 2016;17:542–8. [PubMed] [Google Scholar]
- 8.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3. 2017. Available from: www.OpenEpi.com .
- 9.Steiner CC. Cephalometrics in clinical practice. Angle Orthod. 1959;29:8–29. [Google Scholar]
- 10.British Standards Institute. Glossary of Dental Terms. BS 4492. London, UK: BSI; 1983. [Google Scholar]
- 11.Kamak H, Celikoglu M. Facial soft tissue thickness among skeletal malocclusions: Is there a difference? Korean J Orthod. 2012;42:23–31. doi: 10.4041/kjod.2012.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson A, Jacobson RL, editors. Radiographic Cephalometry: From Basics to 3-D. Hanover Park, IL: Quintessence Publishing Co, Inc; 2006. [Google Scholar]
- 13.Lundström F, Lundström A. Natural head position as a basis for cephalometric analysis. Am J OrthodDentofacialOrthop. 1992;101:244–7. doi: 10.1016/0889-5406(92)70093-P. [DOI] [PubMed] [Google Scholar]
- 14.Holdaway RA. A soft-tissue cephalometric analysis and its use in orthodontic treatment planning. Part I. Am J Orthod. 1983;84:1–28. doi: 10.1016/0002-9416(83)90144-6. [DOI] [PubMed] [Google Scholar]
- 15.Alhumadi A, Osman E, Bouserhal J. Relationship between maxillary occlusal plane inclination and soft tissue harmony in adults with different sagittal skeletal malocclusions. Int J Dent OraHea. 2021;7:5. [Google Scholar]
- 16.Sinclair PM, Little RM. Dentofacial maturation of untreated normals. Am J Orthod. 1985;88:146–56. doi: 10.1016/0002-9416(85)90239-8. [DOI] [PubMed] [Google Scholar]
- 17.Sung JO, Kyung HM, Kwon OW, Sung JH. Cephalometric norms for orthognathic surgery. Korean J Orthod. 1989;19:169–85. [Google Scholar]
- 18.Lee Y, Park J, Cha J. Perioral soft tissue evaluation of skeletal Class II Division 1: Alateral cephalometric study. Am J OrthodDentofacialOrthop. 2015;148:405–13. doi: 10.1016/j.ajodo.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Phillips VM, Smuts NA. Facial reconstruction: Computerized tomography to measure facial tissue thickness in a mixed racial population. Forensic SciInt. 1996;83:51–9. doi: 10.1016/0379-0738(96)02010-5. [DOI] [PubMed] [Google Scholar]
- 20.Aulsebrook WA, Becker PJ, Iscan MY. Facial soft-tissue thicknesses in the adult male Zulu. Forensic SciInt. 1996;79:83–102. doi: 10.1016/0379-0738(96)01893-2. [DOI] [PubMed] [Google Scholar]
- 21.Hashim HA, AlBarakati SF. Cephalometric soft tissue profile analysis between two different ethnic groups: Acomparative study. J Contemp Dent Pract. 2003;4:60–73. [PubMed] [Google Scholar]
- 22.Tanić T, Blažej Z, Mitić V. Analysis of soft tissue thickness in persons with malocclusions of Class II Division 1 and Class II Division 2. SrpArhCelokLek. 2012;140:412–8. doi: 10.2298/sarh1208412t. [DOI] [PubMed] [Google Scholar]
- 23.Jabbar A, Zia AU, Shaikh IA, Channar KA, Memon AB, Jatoi N. Evaluation of soft tissue chin thickness in various skeletal malocclusions. Pak Orthod J. 2016;8:62–6. [Google Scholar]
- 24.Tanić T, Blažej Z, Mitić V. Soft tissue thickness of face profile conditioning by dento-skeletal anomalies. SrpArhCelokLek. 2011;139:439–45. doi: 10.2298/sarh1108439t. [DOI] [PubMed] [Google Scholar]
- 25.Perović T, Blažej Z. Male and female characteristics of facial soft tissue thickness in different orthodontic malocclusions evaluated by cephalometric radiography. Med SciMonit. 2018;24:3415–24. doi: 10.12659/MSM.907485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Mashhadany SM, Al-Chalabi HM, Nahidh M. Evaluation of facial soft tissue thickness in normal adults with different vertical discrepancies. Int J Sci Res. 2017;6:938–42. [Google Scholar]