Abstract
The purpose of this study was to clarify the glass-transition behavior of bacteria (Cronobacter sakazakii) as a function of water activity (aw). From the water sorption isotherm (298 K) for C. sakazakii, monolayer water content and monolayer aw were determined to be 0.0724 g/g-dry matter and 0.252, respectively. Mechanical relaxation was investigated at 298 K. In a higher aw range of over 0.529, the degree of mechanical relaxation increased with an increase in aw. From the effect of aw on the degree of mechanical relaxation, the mechanical awc (aw at which mechanical glass transition occurs at 298 K) was determined to be 0.667. Mean-square displacement of atoms in the bacteria was investigated by incoherent elastic neutron scattering. The mean-square displacement increased gradually with an increase in temperature depending on the aw of samples. From the linear fitting, two or three dynamical transition temperatures (low, middle, and high Tds) were determined at each aw. The low-Td values (142–158 K) were almost independent from aw. There was a minor effect of aw on the middle Td (214–234 K) except for the anhydrous sample (261 K). The high Td (252–322 K) largely increased with the decrease in aw. From the aw dependence of the high Td, the dynamical awc was determined to be 0.675, which was almost equivalent to the mechanical awc. The high Td was assumed to be the glass-transition temperature (Tg), and anhydrous Tg was estimated to be 409 K. In addition, molecular relaxation time (τ) of the bacteria was calculated as a function of aw. From the result, it is suggested that the progress of metabolism in the bacterial system requires a lower τ than approximately 6 × 10−5 s.
Graphical abstract

Significance
There is a phenomenological similarity between glass to rubber transition (glass transition) of polymers and bacterial inactive (no growing) to active (growing) transition. To understand the bacterial inactive-active transition based on the polymer-bacteria analogy, the isothermal mechanical relaxation and mean-squared displacement of bacteria (Cronobacter sakazakii) were investigated. The mechanical glass transition could correspond to dynamical transition with pronounced aw dependence. From the results, molecular relaxation time (τ) of the bacteria was calculated as a function of aw. The results suggest that the progress of metabolism in the bacterial system requires a lower τ than approximately 6 × 10−5 s.
Introduction
Water activity (aw) is defined as the water vapor pressure in a system relative to the vapor pressure of pure water at a given temperature (1). It is known that various types of degradation rates can be described as a function of aw independent from the system. For example, the growth rate of bacteria becomes negligible at an aw lower than approximately 0.85 (2). The bacterial activity, however, recovers at least partially when the aw increases. From a physical viewpoint, bacterial inactive-active transition is somewhat similar to glass-rubber transition (in short, glass transition). Glass transition is a physical phenomenon observed in amorphous materials. Amorphous materials turn into a glassy state at a lower temperature than the glass-transition temperature (Tg). Since the molecular mobility of a glassy material is sufficiently low, it is impossible to diffuse despite its liquid nature. When a glassy material is warmed at a higher temperature than the Tg, the amorphous material regains its molecular mobility for diffusion and behaves as a rubbery material. In the case of a hydrophilic amorphous material, glass transition occurs by dehydration and rehydration even at a constant temperature because the Tg increases and decreases with the decrease and increase in aw, respectively, due to the water plasticizing effect. The water content and aw at which the Tg becomes 298 K (typical room temperature) are described as the critical water content (wc) and critical aw (awc), respectively (3).
The Tg of amorphous materials is commonly determined using differential scanning calorimetry. Complex systems such as food products (4, 5, 6) and bacteria (7), however, show unclear glass transition behavior. In such cases, thermomechanical approaches such as thermal rheological analysis (TRA) are expected to be effective tools. In TRA measurement, the sample is initially compressed with a certain force at a temperature below the expected Tg, and then heat scanned. The Tg of the sample can be detected as a force drop (softening) induced by glass transition. The Tg determined by a thermomechanical approach is described as the mechanical Tg.
Bacterial control based on glass transition is a challenging concept for food preservation. In our previous studies, the glass-transition behavior of Salmonella enterica (8) and Cronobacter sakazakii (C. sakazakii) (9) was investigated using TRA, and the effect of aw on the mechanical Tg was reported. As an experimental issue for TRA studies, the effect of artifacts on the mechanical glass-transition behavior was pointed out. The force drop detected in the study showed great curvature and thus provided a degree of uncertainty with respect to the mechanical Tg.
To solve this problem and to clarify the glass-transition behavior of bacteria in more detail, two experimental approaches were undertaken in this study. One is isothermal mechanical relaxation measurement. This approach has been employed to validate the awc of complex systems such as carbohydrate mixtures (10,11), plant roots (12), food (6), and yeast (13). The samples with varying aw are compressed at 298 K and held for a given period. The force drop between the initial force and the force after the period (ΔF) is evaluated as the degree of isothermal mechanical relaxation under a given condition. A glassy sample shows a low constant ΔF independent of the aw, but a rubbery sample shows a larger ΔF depending on the aw. Thus, awc will be determined from the crossover point between the aw dependence of ΔF for glassy samples and that for rubbery samples.
The other approach is incoherent elastic neutron scattering. Incoherent elastic neutron scattering can detect molecular dynamics of atoms (mainly hydrogen) on a picosecond to nanosecond timescale and sub nano-meter spatial scale. Q (scattering vector) dependence of the incoherent elastic incoherent neutron scattering gives the atomic mean-square displacement (MSD) based on the Gaussian approximation (14,15). Because the incoherent scattering cross section of hydrogen is quite large compared with that of other atoms, the advantage of incoherent elastic neutron scattering is the selective observation of dynamics in biological systems in combination with selective deuteration. From the temperature dependence of the MSD of atoms, harmonic-anharmonic transition can be evaluated as “dynamical transition” (14). The dynamical transition temperature (Td) does not agree with Tg, but they are interrelated physical phenomena (16,17). In previous studies, the molecular dynamics of various biomaterials such as proteins (15,18, 19, 20, 21, 22), DNA (23), RNA (24), polysaccharides (25), and phospholipids (26) have been investigated using neutron scattering. In addition, water dynamics for bacteria such as Shewanella oneidensis (27) and Escherichia coli (28) have also been investigated using neutron scattering. However, there are no reports on the effect of aw on the Td of bacteria.
The purpose of this study was to understand the effect of aw on the isothermal mechanical glass-transition behavior and dynamical transition behavior of bacteria. As the bacterial sample, C. sakazakii, which is an opportunistic Gram-negative rod, was employed. It is known that C. sakazakii is resistant to desiccation and heating (29,30) and is a causative microorganism of neonatal infections (31).
Materials and methods
Sample preparation
A strain of C. sakazakii (JCM 1233T) was purchased from RIKEN BioResource Center (Ibaraki, Japan). The strain was preserved in 10% glycerol solution containing tryptic soy broth (TSB; Merck, Darmstadt, Germany). The preserved strain was incubated on tryptic soy ager (TSA; Merck) plates at 310 K for 24 h. Colonies were transferred into 5 mL TSB and then incubated for 24 h at 310 K. The cultured suspension was added into 400 mL TSB and incubated for 48 h at 310 K. The suspension was centrifuged (3,000 × g for 10 min), and the supernatant was removed. The precipitate was homogenized with 5 mL distilled water, then centrifuged, and the excess water was removed. This process was repeated twice in order to wash the precipitate of C. sakazakii. The sample was frozen at 193 K for 12 h in a freezer and freeze dried at 193 K for 24 h (FDU-2200; Tokyo Rikakikai, Tokyo, Japan). The freeze-dried sample was powdered manually and then hermetically sealed in a sterile tube.
Adjustment of aw and water sorption isotherm
The freeze-dried sample was vacuum dried at 310 K (stage temperature) for 6 h in order to remove as much residual moisture as possible. The fully dehydrated was equilibrated at 298 K in a desiccator at a range of relative humidity (RH) conditions for 7 days. The RH was adjusted using saturated salts: LiCl (RH = 11.3%), MgCl2 (RH = 32.8%), Mg(NO3)2 (RH = 52.9%), KI (RH = 68.9%), NaCl (RH = 75.3%), KCl (RH = 84.3%), and NH4H2PO4 (RH = 92.5%) (32). It was previously reported that the viable cells of freeze-dried samples decreased by three orders at RH = 43% and seven orders at RH = 87% during storage at 298 K for 7 days (9). The aw of the sample was assumed from the equilibrium RH (aw = RH/100).
For the incoherent elastic neutron scattering experiment, the sample was equilibrated using saturated salts in D2O to observe the dynamics of C. sakazakii. To promote H-D exchange, the dehydration-rehydration process was repeated two times as follows: the fully dehydrated sample was semi-equilibrated for 3 days, re-dehydrated, and equilibrated for 7 days.
The equilibrium water content (g/g-DM, dry matter) was measured gravimetrically by oven drying at 378 K for 16 h. The measurements were performed in triplicate, and the results were averaged.
The effect of aw on the water content was analyzed using the Guggenheim-Anderson-de Boer (GAB) model (Equation 1),
| (1) |
where W is the water content (g/g-DM), Wm is the monolayer water content (g/g-DM), C is a factor correcting the sorption properties of the monolayer with respect to the bulk liquid, and K is a factor correcting the sorption properties of the multilayer with respect to the bulk liquid.
Isothermal mechanical relaxation measurement
Isothermal mechanical relaxation measurement was carried out using a rheometer (CR-150; Sun Scientific, Tokyo, Japan) according to a previous study (13). The sample (approximately 50 mg) was placed in a stainless-steel sample holder (diameter = 7 mm), and transferred to the cup of the heat sink attached to the texture meter. The temperature of the cup was kept at 298 K using a water bath circulator (LTB-125; As One, Osaka, Japan). The sample was compressed using a plunger (diameter = 5 mm) at 80 N (approximately 2.1 MPa) and held for 3 min. The ΔF (the difference between the initial force and the force after 3 min) was evaluated as the degree of isothermal mechanical relaxation (6,10, 11, 12, 13). The measurements were carried out in triplicate, and the results were averaged.
Incoherent elastic neutron scattering experiment
The sample (approximately 150 mg) equilibrated in saturated salts prepared by D2O was wrapped with aluminum foil approximately 40 mm in height and hermetically sealed in a cylindrical aluminum sample holder (14 mm inner diameter, 0.25 mm thick) using indium wire. In order to prevent water sorption/desorption during preparation, this procedure was carried out under an RH-adjusted atmosphere in a glove box using saturated salt solutions as described above.
The neutron scattering experiment was carried out using the Biomolecular Dynamics Spectrometer (BL02-DNA) in the Material and Life Science Experimental Facility at Japan Proton Accelerator Research Complex, Tokai, Ibaraki (33). The energy resolution was 3.5 μeV using an Si (111) analyzer and pulse shaping chopper at 225 Hz operation, and the the covered Q range was from 0.1 to 1.86 Å−1. The measurement can detect molecular dynamics of atoms (mainly hydrogen) on approximately 190 psec. The fixed window scan was performed at the temperature range of 100–350 K at cooling and heating rates of 1 K/min and 2 K/min, respectively. The measurement was carried out once.
Under the assumption of harmonic oscillator approximation, the elastic intensity (I) at a given scattering vector (Q) and absolute temperature (T) is as follows:
| (2) |
where A is a constant and u2 is the MSD at T (K). The observed scattering intensity (including elastic scattering) in the system, C. sakazakii hydrated using D2O, was treated as incoherent scattering from hydrogen atoms in C. sakazakii due to the large incoherent scattering cross section of hydrogen. Thus, the molecular mobility in C. sakazakii at T was evaluated by the MSD obtained from the Q dependence of I (15). The data was analyzed using Igor Pro 9 (WaveMetrics, Lake Oswego, OR, USA).
Results and discussion
Water sorption isotherm
The water sorption isotherm (water content versus aw) for C. sakazakii is shown in Fig. 1. The water content of the sample sigmoidally increased with the increase of aw. A solid line was obtained by fitting the experimental data to the GAB equation. The GAB parameters Wm, C, and K were determined to be 0.0724 g/g-DM, 13.1, and 0.859, respectively. In addition, the Wm can be converted to monolayer aw through the water sorption isotherm. The monolayer aw for C. sakazakii, which can be converted from Wm and the GAB equation, was determined to be 0.252.
Figure 1.

Water sorption isotherm for C. sakazakii at 298 K. The values are expressed as mean ± SD (n = 3). The solid line was obtained by fitting to the GAB equation (Eq. 1).
Among the GAB parameters, Wm and/or monolayer aw are important parameters because the monolayer water molecules affect the storage stability of biomaterials (34). It is known that lipid oxidation shows a minimum rate at around the Wm (2). As a result, the biological activity of dry yeast decreases gradually during storage at a lower aw than the monolayer aw (13). The Wm values for bacteria (Streptococcus thermophilus strains) reported in a previous study (35) were in the range of 0.044–0.070 g/g-DM. In addition, the monolayer aw values were estimated to be 0.307–0.382 from the data. The values were relatively near to those of C. sakazakii. The major component of dehydrated bacteria is protein. It is known that 42%–60% protein (proteome) exists in the system, depending on the growth stage (36). Thus, Wm and monolayer aw are primarily corresponded to those of hydrated protein. The effect of monolayer aw on the mechanical properties and molecular dynamics of bacteria is discussed below.
Isothermal mechanical glass-transition behavior of C. sakazakii
Typical results of the isothermal mechanical relaxation behavior of C. sakazakii are shown in Fig. 2 a. The sample having aw = 0.328 showed a small mechanical relaxation, indicating high elasticity (low molecular mobility). The sample having aw = 0.843, on the other hand, exhibited a large mechanical relaxation, indicating low elasticity (high molecular mobility). From the isothermal mechanical relaxation behavior, ΔF between the initial force and the force after 3 min was evaluated as a typical parameter for isothermal mechanical relaxation.
Figure 2.
(a and b) Typical isothermal mechanical relaxation behavior for C. sakazakii samples at 298 K (a) and the effect of aw on ΔF for C. sakazakii samples at 298 K (b). The values are expressed as mean ± SD (n = 3).
The effect of aw on ΔF of C. sakazakii at 298 K is shown in Fig. 2 b. The ΔF value slightly increased but was almost constant in the low aw region (aw ≤ 0.529). In the higher aw range, the ΔF value increased gradually with an increase in aw. This suggests that the glass transition occurred at the turning point. The aw dependence of ΔF was described by two linear functions, and the crossover point was determined to be 0.667. The wc corresponding to aw was 0.160 g/g-DM through the GAB equation (Eq. 1). The water content and aw are described as “mechanical wc” and “mechanical awc,” respectively, hereafter.
In the many previous studies, wc is evaluated as an interpolated value from the effect of water content on the calorimetric Tg (3,37, 38, 39) or mechanical Tg (5,40). In addition, awc is converted from wc through the GAB equation. To the best of our knowledge, the approach of awc determination employed in this study is a first. In order to valid the mechanical awc, the awc values determined previously from the effect of water content on the calorimetric or mechanical Tg (6,10, 11, 12,41) were compared with the mechanical awc determined by the approach suggested in this study. There was good agreement among the values (R2 = 0.856), as seen in Fig. S1.
As mentioned above, the major component of dehydrated bacteria is protein (proteome). Thus, the origin of the mechanical glass-transition behavior for bacteria will be primarily discussed based on that of hydrated proteins. The wc for a globular protein (bovine serum albumin [BSA]) was estimated to be approximately 0.2 g/g-DM from the effect of water content on Tg (42,43). The reason C. sakazakii showed a much lower wc (0.16 g/g-DM) than BSA is that bacteria contain water-insoluble materials (e.g., phospholipids and cell wall components) in the system. The water-insoluble materials are not involved in glass transition but are included in the weight of the dry matter. Thus, Tg at each water content (g/g-DM) becomes lower through the existence of water-insoluble materials. That is, the wc decreases “apparently” with the increase in water-insoluble materials (11). Since aw is affected not by water-insoluble materials but by water-soluble materials, similar to Tg, awc is more suitable as a reference parameter. The awc for BSA can be estimated to be 0.71–0.75 from wc and the effect of aw on the water content (44,45). The awc for C. sakazakii (0.667) was slightly lower than that for BSA. This suggests that proteome is plasticized by other hydrophilic amorphous small molecules in the cell. The effect of aw on the Tg of C. sakazakii is further discussed in the context of the following incoherent elastic neutron scattering study.
Temperature dependence of MSD for C. sakazakii
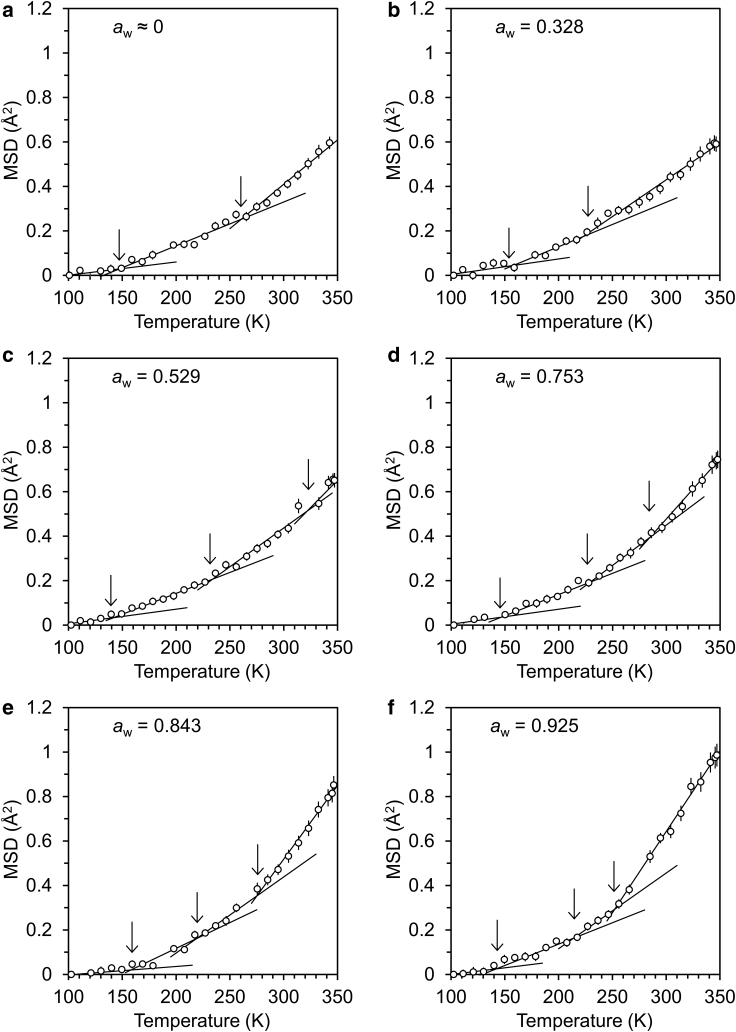
The effect of temperature on the MSD of C. sakazakii (heating measurement) at varying aw conditions is shown in Fig. 3 a–f. Strictly speaking, aw is a function of temperature, and thus aw decreases slightly with a decrease in temperature (32). For simplification, the aw provided at 298 K was treated as a typical aw value. The MSD can be mainly attributed to the molecular dynamics of C. sakazakii because there is a negligible contribution of D2O to the neutron scattering data due to the large incoherent scattering cross section of hydrogen (46). The MSD increased gradually with an increase in temperature depending on the aw of samples. For hydrated biomaterials such as proteins (15,18,21,22), saccharides and polysaccharides (25,47, 48, 49), and DNA (23), the MSD increases linearly with the increase in temperature, and the slope of the linear function becomes greater at a certain temperature. This means that the MSD of atoms changes in nature from harmonic (solid-like) motion to anharmonic (liquid-like) motion at that temperature. The turning point has been determined to be Td. In the case of C. sakazakii, there was unclear turning point in the temperature dependence of the MSD. This suggests that various motions activate continuously with the increase in temperature. The results were described by three (aw = 0 and 0.328) and four (aw = 0.529–0.925) linear functions, and Td values were determined from the crossover points. For the high aw samples (0.843 and 0.925), there is a possibility that hydration water crystalizes during the measurement (13). When water crystalized during the cooling, MSD drastically increases at the melting point of ice. It is noted that there was no experimental evidence indicating crystallization of water in Fig. 3.
Figure 3.
Temperature-dependence of MSD for C. sakazakii (heating measurement) at each aw. Arrows indicate Tds.
The effect of aw on the Td values for C. sakazakii is shown in Fig. 4. The vertical dashed and dotted lines are monolayer aw and mechanical awc, respectively. As mentioned above, the major component of bacteria is protein (proteome), and thus it will be effective to discuss the dynamical transition behavior of bacteria based on protein dynamics. However, the interpretation of protein dynamics is somewhat controversial (20,21,50,51). In addition, bacteria system is heterogeneous due to the presence of other components (e.g., cell membrane, DNA, and lipopolysaccharide), and the effects of the components should be taken into account.
Figure 4.

Effect of aw on the Tds for C. sakazakii. The vertical dashed and dotted lines are monolayer aw and mechanical awc, respectively. The horizontal dashed line is a reference temperature (298 K).
The Td observed at a low temperature (142–158 K) was denoted as low Td. It is noted that the low-Td values were almost independent from aw. It is reported that the first onset of anharmonic molecular dynamics appears at around 100 K regardless of hydration level in protein (lysozyme) system (51). This is thought to be originated from methyl group rotation. In the case of bacteria, phospholipid in cell membrane will also contribute to the methyl group rotation. The low Td of C. sakazakii was higher than the temperature of which methyl group rotation of hydrated protein activates. As mentioned above, it is suggested that various motions in the cell activate continuously with the increase in temperature because of its heterogeneous natures. In this case, the individually different motions in the cell interlock with each other; low-Td dynamics will shift to a higher temperature.
The Td observed at a middle temperature (214–261 K) was denoted as middle Td. The dynamical transition is reported at a similar temperature range (220–240 K) in the hydrated protein in the hydration range of 0.3–0.62 g/g-protein (14,22,52,53). Golub et al. (20) suggested that vibrational anharmonicity of protein is observed above 160 K and that softening of the protein matrix occurs at approximately 240 K. In addition, the Td value strongly depends on the solvent (21,54). Demmel et al. (55) suggested that glass transition of solvent is transmitted to polar side chains on the protein surface and to the main chain. On the other hand, the Tg values of 187–226 K for 0.12–0.43 g/g-protein (42), 188–216 K for 0.23–0.96 g/g-protein (56), and 148–248 K for 0.08–0.84 g/g-protein (57) were reported by adiabatic calorimetry. The origin of the Tg for hydrated proteins is suggested to be the local motion originated from the polar side chains of amino acids coupled with hydration water. Translational motion for polymeric main chain (polypeptide) will be freezing at this stage, as long-chain polymers commonly have a high Tg (16).
The middle Td slightly increased with a decrease in aw because the hydration water played the role of plasticizer for the bacteria (57). The middle Td for anhydrous C. sakazakii was slightly higher than that expected through linear regression of the middle Td for hydrated C. sakazakii (aw = 0.328–0.925). It is thought that the polar side chains are hydrated sufficiently at a higher aw than the monolayer aw (0.252) because they are mainly located in the surface of bacteria. A lack of hydration water (plasticizer) will cause greater elevation of the middle Td.
The Td observed at the highest temperature (high Td) was more sensitive to aw in the whole range. The high Td increased from 252 to 322 K with a decrease in aw (Fig. 4). According to the knowledge on the glass-transition behavior of hydrated proteins (16,42), it is thought that the high Td corresponds to the rearrangement of the polymeric main chain composed of amino acids (polypeptide). Other polymeric materials will also contribute to the motion in the temperature region. For example, it is known that DNA (polynucleotide) has slightly higher Tg than protein (58). In addition, Gram-negative bacteria have a large number of hydrophilic short chains (lipopolysaccharide) on the surface of cell (59). Tg of polysaccharide increases with an increase in molar mass (60).
The macroscopic molecular motion can directly correspond to mechanical relaxation behavior. From the linear regression in the measured range, the aw at which high Td becomes 298 K (dynamical awc) was estimated to be 0.675. The dynamical awc was almost equivalent to the mechanical awc (0.667). To evaluate the MSD of C. sakazakii, the hydrated sample was prepared not by H2O but by D2O. Since D2O is slightly slower mobility than H2O, the dynamical awc may have been evaluated as a slightly higher value than mechanical awc.
Predicted anhydrous Tg and molecular relaxation time of C. sakazakii
It is known that Tg is the temperature at which the viscosity (η) and molecular relaxation time (τ) become 1012 Pa s and 102 s, respectively (61). As discussed above, the high Td can be identified with the Tg because they are thought to be intrinsically same physical origin (rearrangement of polymeric main chain). As is well known, the effect of water content on the Tg can be analyzed by the Gordon-Taylor equation (Eq. 3),
| (3) |
where W1 and W2 are the mass fraction of the bacteria and water, respectively, Tg1 and Tg2 are Tg for anhydrous bacteria and water, respectively, and k is a constant. The Tg2 was set as 136 K from the literature (62,63). The Tg values were replaced by the determined high-Td values at each water content, and then Tg1 and k were determined as fitting parameters. The fitting result is shown as a solid line in Fig. 5 a.
Figure 5.
Effect of aw on the Tg for C. sakazakii predicted by the Gordon-Taylor and GAB equations (a). The high Td was identified with the Tg. The vertical and horizontal dashed lines indicate dynamical awc and a reference temperature (298 K), respectively. The effect of aw on the τ for C. sakazakii predicted by the Williams-Landel-Ferry equation (b). The vertical dashed and dotted lines indicate dynamical awc and the typical aw (0.85) for inactive-active transition for bacteria, respectively.
The anhydrous Tg (Tg1) and k for C. sakazakii were estimated to be 409 K and 4.17, respectively. The anhydrous Tg for C. sakazakii was slightly lower than that (approximately 420 K) for globular proteins (42). As stated above, this suggests that proteome is plasticized by other hydrophilic amorphous small molecules in the cell. It is confirmed that the glass-transition behavior of C. sakazakii can be assumed to be a typical amorphous biopolymer.
As suggested above, bacteria have similar glass-transition behavior as biopolymers. The effect of temperature on the η of polymers can be characterized as a function of Tg using the Williams-Landel-Ferry equation (Eq. 4),
| (4) |
where ηT and ηTg are the viscosities at T and Tg, respectively. The C1 and C2 are set as 17.4 and 51.6, respectively, as universal constants (64). As stated above, Tg corresponds to the temperature at which η and τ become approximately 1012 Pa s and 102 s, respectively (61). In addition, η and τ at an infinite temperature can be set to 10−5 Pa s and 10−14 s, respectively. It is known that the η is an interconversion to τ. According to the linear proportion between log η and log τ, η can be converted to τ as follows:
| (5) |
The effect of aw on the expected log τ at T = 298 K for C. sakazakii is shown in Fig. 5 b. The vertical dashed and dotted lines are the mechanical awc and the typical aw (0.85) required for the growth of bacteria, respectively. As mentioned above, bacterial inactive-active transition generally occurs at around aw = 0.85 (2). The aw is slightly higher than the mechanical awc point, and thus bacteria are suggested to be in a rubbery state at the given condition. As a matter of course, the bacterial inactive-reactive transition requires molecular mobility at least for the progress of metabolism in the system. From Fig. 5 b, the τ for C. sakazakii was estimated to be 6 × 10−5 s at aw = 0.85. It is known that this time narrowly allows for DNA synthesis (helix-coli transition) and chemical reactions such as hydrolysis (65).
Conclusion
This study demonstrated that the mechanical awc value of C. sakazakii could be evaluated from isothermal mechanical relaxation behavior. In addition, neutron scattering was applied to clarify the temperature dependence of the MSD for C. sakazakii, and three different dynamical transition processes were established. Furthermore, aw dependences of Tg and τ were estimated from the results obtained via analytical models established in polymer science. This is fundamental knowledge regarding amorphous polymers but represents novel data regarding bacteria. Given the fact that metabolism is a result of multiple and continuous chemical reactions, it is thought that the inactive-active transition of bacteria will be affected by the molecular dynamics in bacteria. The glass-transition behavior of other bacteria (e.g., aw sensitive or resistant) is an interesting topic for future research.
Author contributions
Conceptualization, T.S., H.N., T.Y., S.K., and K.K.; formal analysis, T.S., H.N., and T.Y.; investigation, T.S., H.N., S.K., and K.K.; writing – original draft preparation, T.S.; project administration, H.N., T.Y., S.K., and K.K.; investigation, T.Y. and S.K.; funding acquisition, S.K.; supervision, K.K.; writing – review & editing, K.K.
Acknowledgments
This work was supported by KAKENHI (Grant-in-Aid for Scientific Research (A); 21H04710) from the Japan Society for the Promotion of Science. The neutron experiment at the Materials and Life Science Experimental Facility of J-PARC was performed under a user program (proposal no. 2019B0102).
Declaration of interests
The authors declare no conflicts of interest associated with this manuscript.
Editor: Frank Gabel.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.09.001.
Supporting material
References
- 1.Labuza T.P. The properties of water in relationship to water binding in foods: a review. J. Food Process. Preserv. 1977;1:167–190. doi: 10.1111/j.1745-4549.1977.tb00321.x. [DOI] [Google Scholar]
- 2.Rahman M.S. Food stability beyond water activity and glass transtion: macro-micro region concept in the state diagram. Int. J. Food Prop. 2009;12:726–740. doi: 10.1080/10942910802628107. [DOI] [Google Scholar]
- 3.Roos Y. Characterization of food polymers using state diagrams. J. Food Eng. 1995;24:339–360. doi: 10.1016/0260-8774(95)90050-L. [DOI] [Google Scholar]
- 4.Kawai K., Toh M., Hagura Y. Effect of sugar composition on the water sorption and softening properties of cookie. Food Chem. 2014;145:772–776. doi: 10.1016/j.foodchem.2013.08.127. [DOI] [PubMed] [Google Scholar]
- 5.Sogabe T., Kawai K., et al. Hagura Y. Effects of porous structure and water plasticization on the mechanical glass transition temperature and textural properties of freeze-dried trehalose solid and cookie. J. Food Eng. 2018;217:101–107. doi: 10.1016/j.jfoodeng.2017.08.027. [DOI] [Google Scholar]
- 6.Jothi J.S., Ebara T., et al. Kawai K. Effect of water sorption on the glass transition temperature and texture of deep-fried models. J. Food Eng. 2018;237:1–8. doi: 10.1016/j.jfoodeng.2018.05.014. [DOI] [Google Scholar]
- 7.Fonseca F., Obert J.P., et al. Marin M. State diagrams and sorption isotherms of bacterial suspensions and fermented medium. Thermochim. Acta. 2001;366:167–182. doi: 10.1016/S0040-6031(00)00725-5. [DOI] [Google Scholar]
- 8.Lee K., Shoda M., et al. Koseki S. Relationship between glass transition temperature, and desiccation and heat tolerance in Salmonella enterica. PLoS One. 2020;15:e0233638. doi: 10.1371/journal.pone.0233638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K., Koyama K., et al. Koseki S. Why does Cronobacter sakazakii survive for a long time in dry environments? Contribution of the glass transition of dried bacterial cells. Microbiol. Spectr. 2021;9:e0138421. doi: 10.1128/SPECTRUM.01384-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvino Granados A.E., Mochizuki T., Kawai K. Effect of glass transition temperature range on the caking behavior of freeze-dried carbohydrate blend powders. Food Eng. Rev. 2021;13:204–214. doi: 10.1007/s12393-020-09226-z. [DOI] [Google Scholar]
- 11.Alvino Granados A.E., Kawai K. Effect of cellulose powder content on the water sorption, glass transition, mechanical relaxation, and caking of freeze-dried carbohydrate blend and food powders. Lebensm. Wiss. Technol. 2021;148:111798. doi: 10.1016/j.lwt.2021.111798. [DOI] [Google Scholar]
- 12.Alvino Granados A.E., Fongin S., et al. Kawai K. Continuously distributed glass transition of maca (Lepidium meyenii Walpers) powder and impact on caking properties. Food Biophys. 2019;14:437–445. doi: 10.1007/s11483-019-09593-z. [DOI] [Google Scholar]
- 13.Kawai K., Sato K., et al. Koseki S. Effects of glass transition and hydration on the biological stability of dry yeast. J. Food Sci. 2021;86:1343–1353. doi: 10.1111/1750-3841.15663. [DOI] [PubMed] [Google Scholar]
- 14.Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989;337:754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- 15.Doster W. The dynamical transition of proteins, concepts and misconceptions. Eur. Biophys. J. 2008;37:591–602. doi: 10.1007/s00249-008-0274-3. [DOI] [PubMed] [Google Scholar]
- 16.Green J.L., Fan J., Angell C.A. The protein-glass analogy: some insights from homopeptide comparisons. J. Phys. Chem. A. 1994;98:13780–13790. doi: 10.1021/J100102A052. [DOI] [Google Scholar]
- 17.Angell C.A. The old problems of glass and the glass transition, and the many new twists. Proc. Natl. Acad. Sci. USA. 1995;92:6675–6682. doi: 10.1073/pnas.92.15.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paciaroni A., Cinelli S., Onori G. Effect of the environment on the protein dynamical transition: a neutron scattering study. Biophys. J. 2002;83:1157–1164. doi: 10.1016/S0006-3495(02)75239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitter J., Lechner R.E., Dencher N.A. Picosecond molecular motions in bacteriorhodopsin from neutron scattering. Biophys. J. 1997;73:2126–2137. doi: 10.1016/S0006-3495(97)78243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golub M., Rusevich L., et al. Pieper J. Rigid versus flexible protein matrix: light-harvesting complex II exhibits a temperature-dependent phonon spectral density. J. Phys. Chem. B. 2018;122:7111–7121. doi: 10.1021/ACS.JPCB.8B02948/ASSET/IMAGES/LARGE/JP-2018-02948B_0008.JPEG. [DOI] [PubMed] [Google Scholar]
- 21.Capaccioli S., Ngai K.L., et al. Paciaroni A. Evidence of coexistence of change of caged dynamics at Tg and the dynamic transition at Td in solvated proteins. J. Phys. Chem. B. 2012;116:1745–1757. doi: 10.1021/jp2057892. [DOI] [PubMed] [Google Scholar]
- 22.Zanotti J.-M., Gibrat G., Bellissent-Funel M.-C. Hydration water rotational motion as a source of configurational entropy driving protein dynamics. Crossovers at 150 and 220 K. Phys. Chem. Chem. Phys. 2008;10:4865–4870. doi: 10.1039/b808217k. [DOI] [PubMed] [Google Scholar]
- 23.Cornicchi E., Capponi S., et al. Paciaroni A. Temperature dependence of fast fluctuations in single- and double-stranded DNA molecules: a neutron scattering investigation. Phil. Mag. 2007;87:509–515. doi: 10.1080/14786430600909022. [DOI] [Google Scholar]
- 24.Caliskan G., Briber R.M., et al. Sokolov A.P. Dynamic transition in tRNA is solvent induced. J. Am. Chem. Soc. 2006;128:32–33. doi: 10.1021/JA056444I. [DOI] [PubMed] [Google Scholar]
- 25.Di Bari M., Deriu A., et al. Cavatorta F. Dynamics of hydrated starch saccharides. Chem. Phys. 2003;292:333–339. doi: 10.1016/S0301-0104(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 26.Sharma V.K., Mamontov E., Anunciado D.B., Oneill H., Urban V. Nanoscopic dynamics of phospholipid in unilamellar vesicles: effect of gel to fluid phase transition. J. Phys. Chem. B. 2015;119:4460–4470. doi: 10.1021/ACS.JPCB.5B00220. [DOI] [PubMed] [Google Scholar]
- 27.Foglia F., Hazael R., et al. Mcmillan P.F. Water dynamics in Shewanella oneidensis at ambient and high pressure using quasi-elastic neutron scattering. Sci. Rep. 2016;61:18862–18879. doi: 10.1038/srep18862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jasnin M., Moulin M., et al. Tehei M. Down to atomic-scale intracellular water dynamics. EMBO Rep. 2008;9:543–547. doi: 10.1038/embor.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breeuwer P., Lardeau A., et al. Joosten H.M. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 2003;95:967–973. doi: 10.1046/j.1365-2672.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- 30.Arku B., Mullane N., et al. Jordan K. Enterobacter sakazakii survives spray drying. Int. J. Dairy Technol. 2008;61:102–108. doi: 10.1111/j.1471-0307.2008.00375.x. [DOI] [Google Scholar]
- 31.Beuchat L.R., Kim H., et al. Richards G.M. Cronobacter sakazakii in foods and factors affecting its survival, growth, and inactivation. Int. J. Food Microbiol. 2009;136:204–213. doi: 10.1016/j.ijfoodmicro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977;81:89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- 33.Shibata K., Takahashi N., et al. Arai M. Proc. 2nd Int. Symp. Sci. J-PARC — Unlocking Myster. Life, Matter Universe —. 2015. The performance of TOF near backscattering spectrometer DNA in MLF, J-PARC. [DOI] [Google Scholar]
- 34.Passot S., Cenard S., et al. Fonseca F. Critical water activity and amorphous state for optimal preservation of lyophilised lactic acid bacteria. Food Chem. X. 2012;132:1699–1705. doi: 10.1016/j.foodchem.2011.06.012. [DOI] [Google Scholar]
- 35.Nachtigall C., Vogel C., et al. Jaros D. How capsular exopolysaccharides affect cell surface properties of lactic acid bacteria. Microorganisms. 2020;8:1904. doi: 10.3390/microorganisms8121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremer H., Dennis P.P. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. EcoSal Plus. 2008;3 doi: 10.1128/ECOSAL.5.2.3/ASSET/2161FEE9-57BC-43B0-97B1-E90AF882F322/ASSETS/GRAPHIC/FIG3.GIF. [DOI] [PubMed] [Google Scholar]
- 37.Fabra M.J., Márquez E., et al. Chiralt A. Effect of maltodextrins in the water-content-water activity-glass transition relationships of noni (Morinda citrifolia L.) pulp powder. J. Food Eng. 2011;103:47–51. doi: 10.1016/j.jfoodeng.2010.09.018. [DOI] [Google Scholar]
- 38.Jouppila K., Roos Y.H. Glass transitions and crystallization in milk powders. J. Dairy Sci. 1994;77:2907–2915. doi: 10.3168/jds.S0022-0302(94)77231-3. [DOI] [Google Scholar]
- 39.Al-Ghamdi S., Hong Y.K., et al. Sablani S.S. State diagram, water sorption isotherms and color stability of pumpkin (Cucurbita pepo L.) J. Food Eng. 2020;273:109820. doi: 10.1016/j.jfoodeng.2019.109820. [DOI] [Google Scholar]
- 40.Ebara T., Hagura Y., Kawai K. Effect of water content on the glass transition and textural properties of hazelnut. J. Therm. Anal. Calorim. 2019;135:2629–2634. doi: 10.1007/s10973-018-7672-2. [DOI] [Google Scholar]
- 41.Jothi J.S., Le T.N.D., Kawai K. Effects of trehalose and corn starch on the mechanical glass transition temperature and texture properties of deep-fried food with varying water and oil contents. J. Food Eng. 2020;267:109731. doi: 10.1016/j.jfoodeng.2019.109731. [DOI] [Google Scholar]
- 42.Watanabe K., Kawai K., et al. Oguni M. Multiple glass-transitions of globular protein BSA aqueous solutions depending on the hydration degree. Bull. Chem. Soc. Jpn. 2019;92:729–737. doi: 10.1246/bcsj.20180295. [DOI] [Google Scholar]
- 43.Farahnaky A., Badii F., et al. Hill S.E. Enthalpy relaxation of bovine serum albumin and implications for its storage in the glassy state. Biopolymers. 2005;78:69–77. doi: 10.1002/BIP.20265. [DOI] [PubMed] [Google Scholar]
- 44.Imamura K., Suzuki T., et al. Okazaki M. Water sorption & glass transition of amophous sugar containing BSA. J. Chem. Eng. Jpn. 2000;33:657–660. [Google Scholar]
- 45.Panagopoulou A., Kyritsis A., et al. Pissis P. Glass transition and dynamics in BSA–water mixtures over wide ranges of composition studied by thermal and dielectric techniques. Biochim. Biophys. Acta. 2011;1814:1984–1996. doi: 10.1016/j.bbapap.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa H., Kataoka M. How can we derive hydration water dynamics with incoherent neutron scattering and molecular dynamics simulation? Biophys. Physicobiol. 2019;16:213–219. doi: 10.2142/biophysico.16.0_213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Bari M., Cavatorta F., et al. Albanese G. Mean square fluctuations of hydrogen atoms and water-biopolymer interactions in hydrated saccharides. Biophys. J. 2001;81:1190–1194. doi: 10.1016/S0006-3495(01)75776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonvico F., Teresa Di Bari M., et al. Albanese G. Mean square hydrogen fluctuations in chitosan/lecithin nanoparticles from elastic neutron scattering experiments. Phys. B Condens. Matter. 2006;385:725–727. doi: 10.1016/J.PHYSB.2006.06.034. [DOI] [Google Scholar]
- 49.Bari M., Albanese G., et al. Deriu A. Dynamics of hydrated saccharides of interest in food science. Phys. B Condens. Matter. 2000;276:256–257. doi: 10.1016/S0921-4526(99)01477-5. [DOI] [Google Scholar]
- 50.Doster W. The two-step scenario of the protein dynamical transition. J. Non-Cryst. Solids. 2011;357:622–628. doi: 10.1016/j.jnoncrysol.2010.08.002. [DOI] [Google Scholar]
- 51.Roh J.H., Novikov V.N., et al. Sokolov A.P. Onsets of anharmonicity in protein dynamics. Phys. Rev. Lett. 2005;95:038101. doi: 10.1103/PhysRevLett.95.038101. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto N., Kofu M., et al. Shibayama N. Freezable and unfreezable hydration water: Distinct contributions to protein dynamics revealed by neutron scattering. J. Phys. Chem. Lett. 2021;12:2172–2176. doi: 10.1021/acs.jpclett.0c03786. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa H., Kataoka M. Percolation of hydration water as a control of protein dynamics. J. Physical Soc. Japan. 2010;79:083801. doi: 10.1143/JPSJ.79.083801. [DOI] [Google Scholar]
- 54.Cornicchi E., Marconi M., et al. Paciaroni A. Controlling the protein dynamical transition with sugar-based bioprotectant matrices: a neutron scattering study. Biophys. J. 2006;91:289–297. doi: 10.1529/BIOPHYSJ.106.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demmel F., Doster W., et al. Schulte A. Vibrational frequency shifts as a probe of hydrogen bonds: thermal expansion and glass transition of myoglobin in mixed solvents. Eur. Biophys. J. 1997;26:327–335. doi: 10.1007/s002490050087. [DOI] [PubMed] [Google Scholar]
- 56.Miyazaki Y., Matsuo T., Suga H. Glass transition of myoglobin crystal. Chem. Phys. Lett. 1993;213:303–308. doi: 10.1016/0009-2614(93)85136-C. [DOI] [Google Scholar]
- 57.Miyazaki Y., Matsuo T., Suga H. Low-temperature heat capacity and glassy behavior of lysozyme crystal. J. Phys. Chem. B. 2000;104:8044–8052. doi: 10.1021/jp0007686. [DOI] [Google Scholar]
- 58.Tsereteli G.I., Belopolskaya T.V., et al. Vaveliouk O.L. Calorimetric study of the glass transition process in humid proteins and DNA. J. Therm. Anal. Calorim. 2000;62:89–99. doi: 10.1023/A:1010110727782. [DOI] [Google Scholar]
- 59.Mazgaeen L., Gurung P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020;21:379. doi: 10.3390/IJMS21020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawai K., Fukami K., et al. Kajiwara K. Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym. 2011;83:934–939. doi: 10.1016/j.carbpol.2010.09.001. [DOI] [Google Scholar]
- 61.Angell C.A., Bressel R.D., et al. Sare E.J. Liquid fragility and the glass transition in water and aqueous solutions. J. Food Eng. 1994;22:115–142. doi: 10.1016/0260-8774(94)90028-0. [DOI] [Google Scholar]
- 62.Johari G.P., Hallbrucker A., Mayer E. The glass-liquid transition of hyperquenched water. Nature. 1987;330:552–553. doi: 10.1038/330552a0. [DOI] [Google Scholar]
- 63.Sastry S. Going strong or falling apart? Nature. 1999;398:467–469. doi: 10.1038/18982. [DOI] [Google Scholar]
- 64.Williams M.L., Landel R.F., Ferry J.D. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J. Am. Chem. Soc. 1955;77:3701–3707. doi: 10.1021/ja01619a008. [DOI] [Google Scholar]
- 65.Eigen M. In: Claesson S., editor. 333–369. Almqvist & Wiksell/Stockholm; 1967. Fast reactions and primary processes in chemical kinetics; pp. 477–484. (Proceedings of the Fifth Nobel symposium). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.