Abstract
Intracellular transport of fatty acids involves binding of ligands to their carrier fatty acid binding proteins (FABPs) and interactions of ligand-free and -bound FABPs with membranes. Previous studies focused on ligand-free FABPs. Here, our amide hydrogen exchange data showed that oleic acid binding to human intestinal FABP (hIFABP) stabilizes the protein, most likely through enhancing the hydrogen-bonding network, and induces rearrangement of sidechains even far away from the ligand binding site. Using NMR relaxation techniques, we found that the ligand binding affects not only conformational exchanges between major and minor states but also the affinity of hIFABP to nanodiscs. Analyses of the relaxation and amide exchange data suggested that two minor native-like states existing in both ligand-free and -bound hIFABPs originate from global “breathing” motions, while one minor native-like state comes from local motions. The amide hydrogen exchange data also indicated that helix αII undergoes local unfolding through which ligands can exit from the binding cavity.
Significance
Interactions between long-chain fatty acids and fatty acid binding protein (FABP) as well as between the protein and membranes are indispensable for intracellular transport of fatty acids. Although binding of fatty acids to the protein does not significantly alter backbone structure, we found that the binding strengthens the hydrogen-bonding network of FABP and induces rearrangement of sidechains even far away from the ligand binding site. Such changes likely lead to higher protein stability and lower affinity to membranes. We further revealed that minor native-like states can be caused by global protein “breathing” motions or local motions. Moreover, local unfolding of the second helix in FABP is likely a common mechanism for ligands to exit from the binding cavity.
Introduction
Fatty acids play important roles in many biological processes, such as cell membrane synthesis, energy supply, metabolic regulation, and hormone action regulation(1). Due to poor solubility of long-chain fatty acids in water, their diffusion is extremely slow in cytoplasm. Thus, unlike water-soluble small molecules, whose cytoplasmic transport occurs mainly through diffusion along concentration gradients, fatty acids require water-soluble chaperones for intracellular transport (2). Members of the fatty acid binding protein (FABP) family serve as chaperones or aqueous carriers. Through binding and releasing of ligands and interactions with membranes (3, 4, 5, 6), FABPs may actively assist to transport fatty acids to specific compartments in the cell for storage, signaling, membrane synthesis, breakdown, and lipid-mediated transcriptional regulation (7).
FABPs exist in nine isoforms in humans, which are differentially expressed in various tissues (8). They each contain about 130 amino acids and adopt a conserved 3D structure with a slightly elliptical β barrel comprising 10 antiparallel β strands, a cap consisting of two short α helices, and a large cavity (Fig. 1) (8,9). Except liver FABP, which binds to two fatty acids in its interior cavity, all other FABPs each bind to only one ligand (Fig. 1). In crystal state, protein structures of ligand-free and -bound forms are nearly identical and there are no obvious openings as ligand entry sites (10, 11, 12). In solution, however, previous studies on rat intestinal FABP (IFABP) found that the portal region including helix αII, the linker between αII and βB, and the turn between β strands C and D is more disordered than other regions and that the binding of fatty acid leads to an ordering of helix II by stabilizing a series of long-range cooperative interactions (13,14). The disorder in the portal region was hypothesized to permit the entry of ligands. However, such disorder and a transition from a less ordered to a more ordered helix II are not so obvious for other FABPs in solution (15, 16, 17, 18, 19). In addition, hydrogen exchange studies carried out in solution showed that the hydrogen-bond network of protein backbone amides is essentially the same in the ligand-free and -bound forms, although ligand binding stabilizes some residues more than others (20). All these suggest that ligand binding does not affect the protein structure significantly regardless of whether the protein is in solution or crystal state and the ligand entry mechanism cannot be revealed from the structures alone.
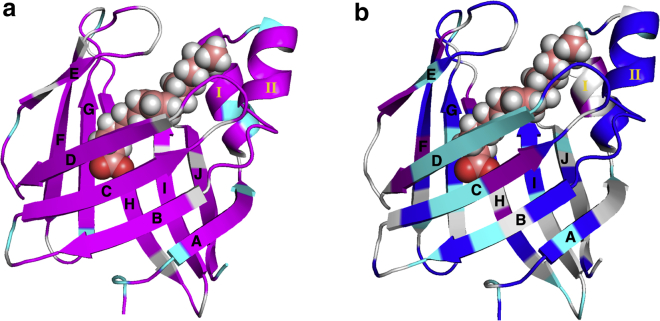
Figure 1.
Ribbon diagram of Holo-hIFABP structure (PDB: 2MO5) in complex with oleate. Oleate is indicated in a space-filled model. (a) Residues are colored according to the ratio of kobs(Apo)/kobs(Holo): magenta, kobs(Apo)/kobs(Holo) > 1.5; cyan, kobs(Apo)/kobs(Holo)≤1.5; gray, unavailable data. (b) Residues are colored according to backbone 15N chemical shift differences between native state N and ND-bound state: blue, |Δδ| ≥ 1.0 ppm; purple, 0.5 ≤ |Δδ| < 1.0 ppm; cyan, |Δδ| < 0.5 ppm; and gray, unavailable data. The chemical shift differences were derived from analysis of relaxation data. To see this figure in color, go online.
Heteronuclear 1H-15N nuclear Overhauser effect (hnNOE) data, which represent fast dynamics, revealed that the portal region of ligand-free FABP is only slightly more flexible than other regions on picosecond-nanosecond timescale (14,15,20, 21, 22, 23). Moreover, the flexibility on the picosecond-nanosecond timescale is nearly not affected by ligand binding (14,18,20, 21, 22). This indicates that the fast dynamics on picosecond-nanosecond is irrelevant to the ligand entry process that occurs on a millisecond timescale (24,25) since high flexibility of the ligand entry site is expected. Amide hydrogen exchange experiments, which probe slow dynamics over a wide range of timescales from microseconds to hours, found that the portal region is much more dynamic than all β strands and helix I for both ligand-free and -bound forms (14,20,25) and that ligand binding reduces the slow dynamics (14,20). This suggests that the slow dynamics of the portal region is likely associated with the ligand entry process. NMR relaxation studies further revealed that residues located in all secondary structure elements have slow dynamics on the millisecond timescale, while only helix II has slow dynamics on the microsecond timescale (14,20,25). The slow dynamics vary from one type of FABP to another (14,18,20,22) and are influenced more significantly by fatty acid binding than by bile acid binding (20), suggesting that the dynamic behavior of FABPs is ligand specific as well as protein specific. Our recent studies on ligand-free human IFABP (hIFABP) showed that a major native state is in dynamic equilibria with multiple minor states on microsecond to millisecond timescales in solution (25,26). These studies demonstrated that one of the minor states (<2%) is locally disordered in helix II and interconverts with the major state on the microsecond timescale (25), while other minor states are native-like (25,27), explaining that no obvious disorder is observable for helix II in the experimentally determined structures. This locally disordered state contains an opening through which ligands can enter the cavity for binding. The locally disordered minor state very likely acts as an intermediate state in the ligand binding pathway for all ligand-free FABPs. Until now, it has been unclear whether this locally disordered state may also exist in ligand-bound FABPs to allow ligands exit the binding cavity. Moreover, for the native-like minor states, we still do not know their origin and functional relevance.
To address the origin of native-like minor states and how ligands exit the protein cavity, here we performed NMR relaxation experiments on Holo-hIFABP, which is the complex of hIFABP and oleic acid. To answer whether ligand binding influences FABP-membrane interactions, we also carried out relaxation experiments on Holo-hIFABP in the presence of nanodiscs (NDs). Our data indicated that the ligand binding affects not only conformational exchanges between major and minor states but also the affinity of hIFABP to nanodiscs. Our data analysis suggests both global “breathing” and local motions can induce native-like minor states.
Materials and methods
1,2-Ditetradecanoyl-sn-glycero-3-phosphocholine (DMPC 14:0) and 1,2-ditetradecanoyl-sn-glycero-3- phospho-(1′-rac-glycerol) (DMPG 14:0) were purchased from Avanti Polar Lipids. Peptide 22A (PVLDLFRELLNELLEALKQKLK) was purchased from Bio Basic Asia Pacific. DMPC and DMPC/DMPG (7:3) NDs were prepared as described previously (6).
Wild-type hIFABP was expressed, purified, and delipidated using the protocols described previously (23). The pH of the delipidated protein solution was raised to 9.0 and then mixed with oleic acid in a molar ratio of 1.2:1.0 (oleic acid:protein). Finally, the excess of oleic acid was removed, and the pH of the Holo-hIFABP sample was lowered to 7.1 by buffer exchange. For the samples with NDs, the Holo-hIFABP solution was added to purified ND solution in a protein:lipid molar ratio of 1:5 or protein:ND molar ratio of 1:∼0.04. The final sample used for NMR experiments contained 0.5 mM 15N-labeled Holo-hIFABP, 20 mM sodium phosphate (pH 7.1), 50 mM NaCl, 1 mM EDTA, and 0.05% NaN3. Amide hydrogen exchange experiments were done at 30°C. All other NMR experiments were performed at 25°C on a Bruker 800 MHz instrument equipped with a cryoprobe.
For slow-exchange amides, hydrogen-deuterium exchange rates were measured by measuring the dependence of 1H-15N heteronuclear single quantum coherence (HSQC) peak intensities on time after dissolving a lyophilized sample in 100% D2O. Each HSQC spectrum was acquired with a total time of 93 s using the so-fast HSQC scheme (28). For fast exchange amides, the exchange rates were measured on a 15N-labeled sample containing 5% D2O and 95% H2O with a radiation-damping-based water inversion scheme described previously (29) using an inter-scan delay of 2 s and 16 mixing times (20–300 ms).
15N relaxation dispersion (RD) data were acquired with a continuous wave (CW) decoupling and phase-cycled Carr-Purcell-Meiboom-Gill (CPMG) method (30, 31, 32) using a constant time relaxation delay of 40 ms and inter-scan delay of 2 s. Sixteen RD data points were collected by varying the separation of CPMG pulses to achieve a series of CPMG field strengths from 25 to 1000 Hz. To estimate uncertainties in the apparent relaxation rates, the measurements at a CPMG field of 50 Hz were repeated three times.
15N chemical exchange saturation transfer (CEST) profiles were acquired at two different weak saturation fields with a saturation time of 0.5 s and inter-scan delay of 1.5 s (33,34). For the higher saturation field strength (12.5 Hz), 48 HSQC-based spectra were acquired using a series of 15N carrier frequencies ranging from 107 to 109 and 113 to 131 ppm at a spacing of 0.5 ppm, and additional frequencies at 106, 110, 111, 112, 132, and 133 ppm. For the lower saturation field strength (6.25 Hz), 76 HSQC-based spectra were recorded at carrier frequencies from 107.2 to 109.6 ppm and 115 to 129.1 ppm at a spacing of 0.3 ppm, from 110 to 114.5 and 129.5 to 131 ppm at a spacing of 0.5 ppm, plus 105, 106, 106.7, 132, and 133 ppm. Reference spectra were also recorded with similar parameters except a saturation time of zero. The uncertainties of the data points were estimated from the standard deviation of the points over a region far away from CEST dips.
Dark-state exchange saturation transfer (DEST) profiles were recorded at two different weak saturation fields (150 and 300 Hz) with a saturation time of 0.5 s and inter-scan delay of 1.5 s (35). For each saturation field, 14 HSQC-based spectra were acquired at a series of 15N carrier frequencies: −229, 0, 56, 76, 91, 106, 121, 136, 151, 166, 186, 246, 346, and 471 ppm. The uncertainties of the data points were estimated from the standard deviation of the points at 246, 346, and 471 ppm.
15N relaxation rates in the rotating-frame (R1ρ) and longitudinal relaxation rates (R1) were obtained as described previously (36,37). Six data points with relaxation delays of 10, 150, 300, 450, 700, and 1000 ms were acquired for determination of R1 values. R1ρ values were measured by acquiring six points with delays of 1, 15, 30, 45, 60, and 81 ms using a spin-lock field strength of 1600 Hz. Transverse relaxation rates R2 were calculated from R1 and R1ρ values as described previously (38). Except for residues 59–61, the contribution from exchange to relaxation (Rex) was suppressed to a level of <0.5 s−1 by spin-lock in the measurement of R1ρ and thus the R2 values obtained here were nearly free of Rex.
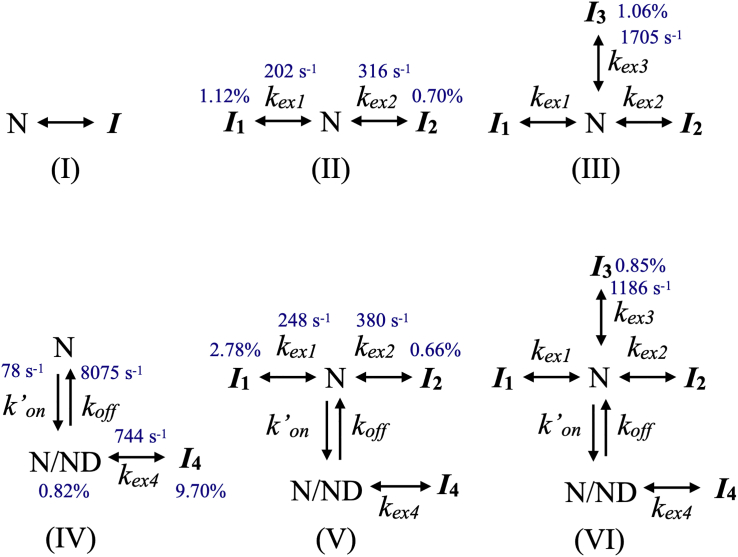
NMR relaxation data (RD, CEST, DEST, and R1ρ) were analyzed using exchange models shown in Fig. 2. In the absence of NDs, model I was a test model, while models II and III were used to extract kinetic parameters and chemical shifts of minor states from RD and CEST data as described previously (26). Although there are more possible exchange models, we showed that models N—I1—I2 and N—I2—I1 could be rejected based on total fitting residuals and reduced fitting residuals in our previous study on Apo-hIFABP (26). In the presence of NDs, models IV–VI were used to analyze RD, CEST, DEST, and R1ρ data in a combined way by following the method described previously (6). To ensure a global minimum is identified in global fitting, we first did a grid search. For model II, kex1 was set to a range from 50 to 400 s−1 in step size 50 s−1, kex2 was 100–900 s−1 in step size 100 s−1, PI1 and PI2 ranged 0.5%–3.5% in step size 0.2%, and residue-specific fitting parameters such as relaxation rates and chemical shifts were fixed at the values obtained from individual fitting. For model IV, k’on + koff was set to 10–1010 s−1 in step size 100 s−1 and 1010–10,010 s−1 in step size 1000 s−1, kex4 ranged 100–1000 s−1 in step size 100 s−1, PN/ND was 0.2%–1.6% in step size 0.2%, and PI4 was 3.0%–12.0% in step size 1%. Next, the obtained global parameters (kex and P) that gave rise to the lowest fitting residuals in the grid search were used as the initial values in the subsequent least-squares nonlinear minimization by a MATLAB function (LSQNONLIN). Model III was built from model II, model V was constructed from models IV and III, and model VI was derived from V. The two additional global parameters for each of these models were found by grid search and then nonlinear minimization.
Figure 2.
Conformational exchange models of Holo-hIFABP in the absence (models I–III) and presence (IV–VI) of NDs. N represents native state, while Ij represents the jth minor state. N/ND represent the ND-bound state or ND-protein complex. k’on is the apparent association rate of N to ND, while koff is the dissociation rate of N from N/ND complex. kex1 (kex2, kex3) is the total exchange rate between state N and state I1 (I2, I3), and kex4 is the total exchange rate between states N/ND and I4. The rates, which were obtained from fitting relaxation data of selected residues to each model, are indicated. The populations of minor states (in percentage) are also indicated. To see this figure in color, go online.
Results and discussion
Effect of ligand binding on amide hydrogen exchange rates of hIFABP
For slow exchange amide hydrogen (<0.01 s−1), the hydrogen exchange rates were measured by hydrogen-deuterium exchange experiments. For fast exchange labile hydrogen (≥0.3 s−1), the rates were measured by amide-water exchange experiments. All the slow exchange amides are located in αI and βA–βJ (Table S1). For both Apo- and Holo-hIFABP, all the backbone amides in αII had fast exchange rates (≥0.3 s−1) and small protection factors (Table S1, and Table S1 in (26)). The amide hydrogen atoms of F17–M21 in αI and K29–H33 in αII all are involved in hydrogen bonding and are not accessible to water based on the 3D structure of Holo-hIFABP (PDB: 2MO5) and solvent-accessible surface area (SASA) calculation (39), but the exchange rates of amides in K29-H33 are at least ∼2000 times larger than those in F17–M21 (Table S1). The result suggests that local unfolding of αII occurs for both Apo- and Holo-hIFABP. The local unfolding of αII in an Apo-hIFABP variant was also demonstrated previously by 1H RD experiments (25) and was found to be the key step for ligands to enter the cavity of hIFABP (25,27). This local unfolding should be also necessary for ligands to exit from the cavity of Holo-hIFABP because Apo- and Holo-FABPs are nearly identical in protein structure (10, 11, 12). Therefore, local unfolding of αII is likely a generic mechanism for ligands to enter and exit the cavities of FABPs. Although no local unfolding occurs for extracellular fatty acid binding proteins (40, 41, 42) and periplasmic lipid transport systems (43) in ligand uptake and release, conformational changes are common.
hIFABP contains two tryptophan residues, W6 and W82, of which the sidechains are located inside the cavity. Upon binding to oleic acid, the sidechain of W6 is relocated from a solvent-exposed conformation (kobs = 2.1 s−1) to a buried conformation (kobs = 8.9 × 10−4 s−1), while the sidechain of W82 is rearranged from a partially solvent-exposed conformation (3.7 × 10−3 s−1) to a more solvent-exposed conformation (kobs = 0.01–0.3 s−1). Because oleic acid has no direct contacts with the sidechains of W6 or W82, their conformational changes should be caused by the allosteric effect. Therefore, oleic acid binding induces significant changes in sidechain conformations, although the binding does not significantly disturb the backbone structures (13,44). On the contrary, conformational rearrangements in backbones occur upon ligand binding for extracellular lipid binding proteins, such as lactoglobulin (40), odorant binding protein (41), tear lipocalin (42), and engineered lipocalin FluA (45), which all adopt an eight-stranded antiparallel β barrel structure. Upon binding to oleic acid, the hydrogen exchange rates of most backbone amides in hIFABP decreased by more than 1.5 times (Table S1; Fig. 1 a), suggesting that local stabilities of individual residues far away from the binding site can be enhanced by ligand binding through the allosteric effect since only a small portion of residues are involved in direct contacts with oleic acid through sidechains (13,44).
Coexistence of multiple conformational states of Holo-hIFABP and origin of minor states
In Apo-hIFABP, three minor native-like states coexist with one major native state (26). Until now, the origin of the minor state was still unknown. To examine whether the minor states are influenced by protein stability, we probed conformational exchanges of Holo-hIFABP since it is more stable than Apo-hIFABP. NMR RD and CEST experiments were used to detect conformational exchanges of both Apo- and Holo-hIFABP at the same buffer and temperature. Table 1 summarizes the results. Overall, the number of residues undergoing conformational exchanges is reduced significantly by oleic acid binding, and Rex is substantially smaller in Holo-hIFABP than in Apo-hIFABP (Figs. 3 and S1). A reduction in conformational exchanges was also detected upon complex formation of lipocalin FluA with its ligands (45).
Table 1.
Numbers of residues with RD and two or more CEST dips in Apo- and Holo-hIFABP
| Number of residues resolved in HSQC | Number of residues with Rex≥1.5 s−1 | Number of residues with Rex≥3.0 s−1 | Number of residues with Rex≥5.0 s−1 | Number of residues with 2 CEST Dips | Number of residues with 3 CEST Dips | |
|---|---|---|---|---|---|---|
| Apo-hIFABP | 116 | 69 | 43 | 30 | 32 | 1 |
| Holo-hIFABP | 124 | 45 | 15 | 7 | 21 | 0 |
Figure 3.
Comparison of Apo- and Holo-hIFABP in CEST (a, c, e, and g) and RD (b. d, f, and h) profiles. The experimental CEST data at lower (6.25 Hz) and higher (12.5 Hz) rf fields are indicated by x and o respectively. The locations (or chemical shifts) of states N, I1, and I2 in the CEST profiles are indicated by arrows. The solid lines are the best fits to model II. To see this figure in color, go online.
To investigate the effect of oleic acid binding on the number of conformational states and kinetic parameters for exchanges between different states, we analyzed the RD and CEST data of Holo-hIFABP as described previously (26). For Holo-hIFABP, no residues displayed more than two CEST dips. Thus, we tried to fit the RD and CEST data of the residues located outside F47-T67 (βC and βD region) to model I (two state, Fig. 2). Eighteen residues in this region, which displayed two CEST dips and had Rex > 1.5 s−1, were used to obtain kinetic parameters. The data (140 × 18 points) did not fit well to model I with two global fitting parameters and 4 × 18 residue-specific parameters in a global way, yielding kex = 308 s−1, PI = 0.91%, and χ2 = 2716. On the other hand, the same set of data could be globally fitted very well to model II (three state, Figs. 2 and 3) with four global fitting parameters and 5 × 18 residue-specific parameters, yielding PI1 = 1.12% ± 0.02%, PI2 = 0.70% ± 0.01%, kex1 = 202 ± 17 s−1, kex2 = 316 ± 8 s−1, and χ2 = 1275. F-test analysis shows that model I can be rejected at a confidence interval of >99.999%. Model II is also suitable for Apo-hIFABP(26). Therefore, ligand binding does not change the number of conformational state, and the model used to describe Apo-hIFABP can also be applied to Holo-hIFABP. Based on this conclusion, four-state model III (Fig. 2), which is suitable for F47-T67 region of Apo-hIFABP, was used to fit the data of F47–T67 in Holo-hIFABP. Fixing kex1, kex2, PI1, and PI2 at the values obtained from model II, we obtained population of state I3 (PI3 = 1.06% ± 0.02%) and exchange rate (kex3 = 1705 ± 25 s−1) through global fitting of seven residues in F47–T67 region to model III. For other residues that had Rex > 1.0 s−1 and were not used in the global fitting, their 15N chemical shifts in the minor states were extracted by individual fitting by fixing global exchange rates and populations. All the 15N chemical shifts at major and minor states are listed in Table S2. To obtain structural information about the minor states, we compared their chemical shifts with those of the native and unfolded (U) states. In terms of 15N chemical shifts, minor states I1, I2, and I3 are very similar to state N, but very different from state U (Table S1), indicating that all the minor states of Holo-hIFABP are native-like, the same as the conclusion drawn from Apo-hIFABP (26). The local unfolding of αII implicated from our amide hydrogen exchange data was not detected in our 15N RD and CEST experiments, suggesting that the local unfolding process of Holo-hIFABP is on a microsecond timescale instead of a millisecond timescale. For Apo-hIFABP, the local unfolding has been shown to be on a microsecond timescale using 1H RD experiment(25).
Comparing the minor state populations of Holo-hIFABP (PI1 = 1.12% ± 0.02%, PI2 = 0.70% ± 0.01%, PI3 = 1.06% ± 0.02%) and Apo-hIFABP (PI1 = 4.20% ± 0.50%, PI2 = 1.95% ± 0.04%, PI3 = 1.10% ± 0.09%), we see that oleic acid binding reduces the populations of states I1 and I2 greatly. According to the distributions of residues containing minor states I1 and I2 (Table S2 and Table S1 in (26)), all the secondary structure elements (αI, αII, βA-βJ) of hIFABP undergo two types of conformational exchanges: between states N and I1 and between state N and I2. This suggests that states I1 and I2 are generated by global structural changes instead of local structural changes. The global structural changes may be caused by breathing motion; i.e., slight rearrangement of different secondary structure elements or structural deformation in the β barrel (46,47). Because states I1 and I2 are native-like, the breathing motion does not alter the overall structure of hIFABP. Oleic acid binding strengthens the hydrogen-bond networks as demonstrated by our amide exchange data. As a result, the deformation in the β barrel should be more difficult for the Holo-form than for the Apo- form. This explains well why the populations of states I1 and I2 are reduced by ligand binding.
The population of state I3 is nearly not affected by oleic acid binding and the conformation of state I3 is also native-like. In addition, the conformational exchange between states N and I3 involves only the βC and βD region (F47–T76). Therefore, state I3 may be generated by local motion around the βC-βD turn rather than by the global breathing motion discussed above.
Comparing the exchange rates for Holo-hIFABP (kex1 = 202 ± 17 s−1, kex2 = 316 ± 8 s−1, and kex3 = 1705 ± 25 s−1) and Apo-hIFABP (kex1 = 27 ± 3 s−1, kex2 = 589 ± 8 s−1, and kex3 = 2803 ± 106 s−1), we see that oleic acid binding dramatically reduces the total conversion rates between state I2/I3 and state N, and significantly enhances the total conversion rate between state I1 and state N. The result indicates that ligand binding differentially influences the stabilities of different transition states between the major state and minor state .
Effect of oleic acid binding on interactions between nanodiscs and hIFABP
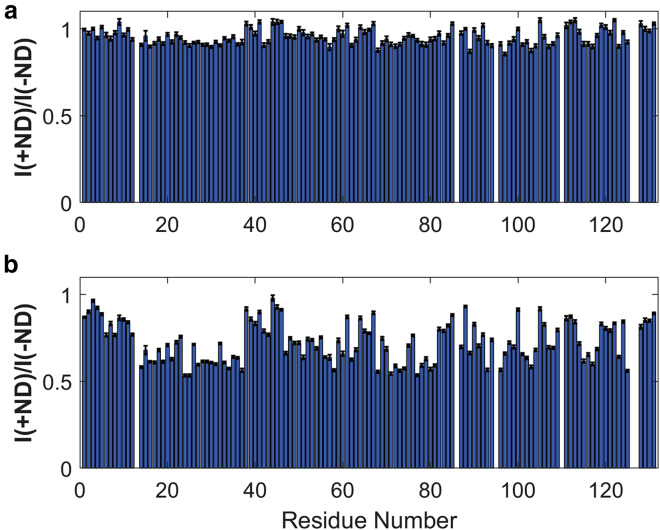
To examine whether Holo-hIFABP interacts with membrane, we measured 15N-1H correlation peak intensities in the absence and presence of DMPC and DMPC/DMPG NDs, respectively. DMPC NDs reduced the peak intensities by about 4% (Fig. 4 a). Different from DMPC NDs, DMPC/DMPG NDs had a significant impact on the peak intensities, with an average decrease of ∼27% (Fig. 4 b), but did not change the peak positions (Fig. S2). The results indicate that interactions exist between Holo-hIFABP and NDs, and the interactions are much stronger for DMPC/DMPG NDs than for DMPC NDs. As the difference between DMPC and DMPG lies in the net charges of DMPC (0) and DMPG (−1), the formation of protein-ND complex is regulated mainly by charge-charge interactions. The conclusion is the same as that drawn from the study on Apo-hIFABP (6).
Figure 4.
Intensity ratios of HSQC peaks in the presence of NDs to those in the absence of NDs for hIFABP in DMPC NDs (a) and in DMPC/DMPG NDs (b). The errors are indicated by error bars (black). To see this figure in color, go online.
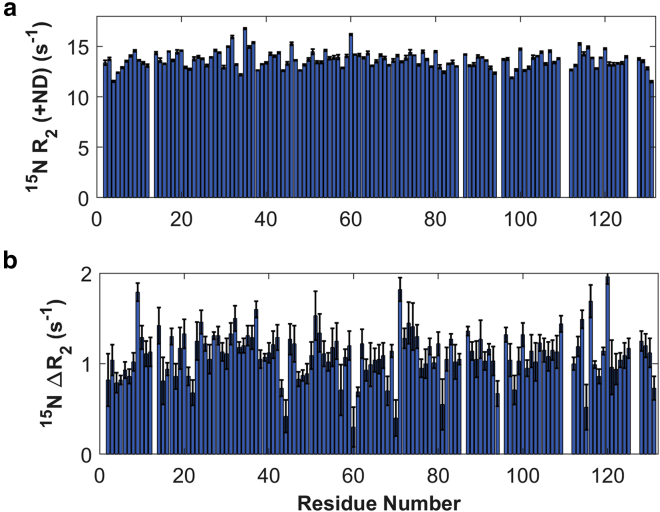
The intensity reduction varied significantly from one residue to another (Fig. 4 b). Similar to what was demonstrated previously on Apo-hIFABP (6), the significant variation in intensity reduction is caused by low affinity of Holo-hIFABP for DMPC/DMPG NDs. To know whether the affinity of hIFABP to NDs is influenced by ligand binding, we measured 15N transverse relaxation rates in the rotating frame (R1ρ) in the presence and absence of NDs. 15N transverse relaxation rates (R2) of Holo-hIFABP, which were derived from R1ρ, were larger in the presence of DMPC/DMPG NDs than in the absence of the NDs (Fig. 5). The rate difference (ΔR2) is caused by the presence of ND-bound hIFABP that is in fast exchange with the ND-free hIFABP. The magnitude of ΔR2 increases with the population of the ND-bound form (or protein-ND complex). The average ΔR2 value for Holo-hIFABP (∼1.2 s−1; Fig. 5 b) is significantly smaller than that for Apo-hIFABP (∼2.0 s−1) (6) at the same lipid:protein ratio, suggesting that oleic acid binding reduces the affinity of hIFABP for NDs.
Figure 5.
Transverse relaxation rates of backbone 15N in the presence of DMPC/DMPG NDs, R2(+ND). (a) Relaxation rate differences, ΔR2 = R2 (+ND) − R2 (−ND), where R2(−ND) is the relaxation rate in the absence of DMPC/DMPG NDs. The errors are indicated by error bars (black). To see this figure in color, go online.
To quantify the affinity of Holo-hIFABP for DMPC/DMPG NDs, we collected RD, CEST, and DEST data in the presence of NDs. For most residues, the RDs were significantly larger in the presence of DPMC/DMPG NDs than in the absence of NDs (Fig. S3 b, d, f, and h). Moreover, an additional CEST dip was observed clearly for several residues in the presence of NDs compared with those in the absence of NDs (Fig. S3 a, c, e, and g), indicating a new conformational state (I4) is induced by NDs. This result is similar to that for Apo-hIFABP. Therefore, NDs have similar effects on both Apo- and Holo-hIFABP, and the exchange models previously used to analyze the Apo-hIFABP data (6) can be applied to the Holo-hIFABP data in the presence of DMPC/DMPG NDs.
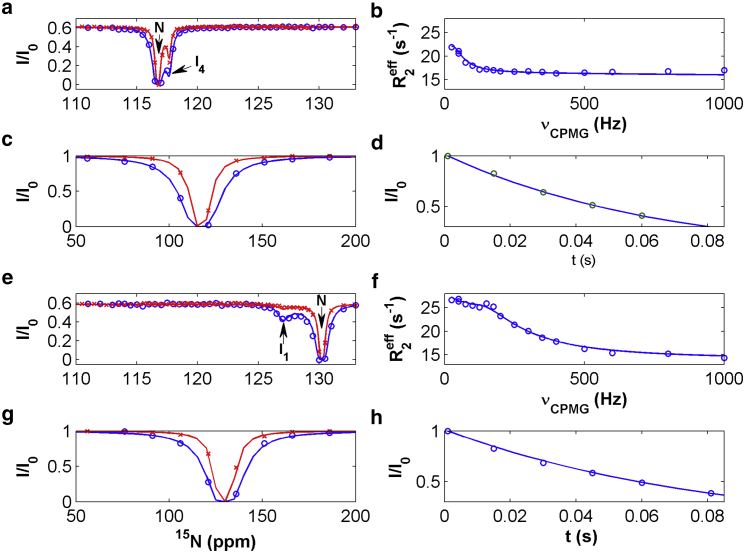
Following the method described previously (6), we analyzed RD, CEST, DEST, and R1ρ data in a combined way. In total, five residues (V23, R28, A32, L72, R79) exhibited no conformational exchanges without RD or CEST minor dips in the absence of NDs, but displayed one CEST minor dip and large RD (≥4 s−1) in the presence of NDs. The data of these residues were fitted globally to model IV (Fig. 2). From the best fit (Fig. 6 a–d), we obtained populations of the ND-bound form (PN/D = Pb = 0.82% ± 0.21%) and state I4 (PI4 = 9.70% ± 0.15%), the exchange rate between the ND-bound and -free forms (kex = kon’+koff = 8153 ± 970 s−1), and the exchange rate between the ND-bound form and state I4 (kex4 = 744 ± 150 s−1). Subsequently, we fitted the residues outside the βC and βD region, which had Rex > 2.5 s−1 and two CEST dips, to model V (Fig. 2) in a global way by fixing kex, kex4, Pb, and PI4 at the values derived from model IV. From the fitting of 12 residues, we obtained the populations of minor states I1 and I2 (PI1 = 2.78% ± 0.10%, PI2 = 0.66% ± 0.03%) and their exchange rates with the major state N (kex1 = 248 ± 11 s−1, kex2 = 380 ± 30 s−1). Finally, we fitted the residues inside the βC and βD region with Rex > 5 s−1 and two CEST dips to model VI (Fig. 2). Fixing kex, kex1, kex2, kex4, Pb, PI1, PI2, and PI4 at the values derived from models IV and V, we obtained the global exchange rate (kex3 = 1186 ± 59 s−1) and population of state I3 (PI3 = 0.85% ± 0.04%) by global fitting of seven residues (Fig. 6 e–h). For other residues with Rex > 1.5 s−1, which were not used in the global fitting, their 15N chemical shifts in the minor states were extracted by individual fitting by fixing global exchange rates and populations. Table S2 lists the chemical shifts.
Figure 6.
Representative CEST (a and e), RD (b and f), DEST (c and g), and R1ρ (d and h) profiles. The experimental CEST data at lower (6.25 Hz) and higher (12.5 Hz) rf fields are indicated by x and o respectively, so are the DEST data at lower (150 Hz) and higher (300 Hz) rf field strengths. The red and blue solid lines in (a), (c), (e), and (g) are best fits obtained with model II at lower and higher rf field strengths, respectively. The locations (or chemical shifts) of states N, I1, and I4 in the CEST profiles are indicated by arrows. (a–d) for residues A32, while (e–h) are for E51. To see this figure in color, go online.
The 1HN and 15N chemical shifts of major state N are not affected by NDs (Fig. S2). In addition, the 15N chemical shifts of minor states I1, I2, and I3 are similar in the absence and presence of NDs (Table S2). The data indicate that the structures of the major and minor states are not disturbed significantly by the presence of NDs in the sample. The population of state I1 in the presence of NDs (2.78%) is much larger than that in the absence of NDs (1.12%), while populations of states I2 and I3 are similar in the absence and presence of NDs. The result suggests that NDs have similar effects on the stability of states N, I2, and I3 and seem to stabilize state I1 more than other states of Holo-hIFABP. The differential effects on state I1 and others were not observed for Apo-hIFABP, in which NDs affect all the states similarly (6). The exchange rates between state N and minor states I1 and I2 (kex1 and kex2) were enhanced by NDs from 208 and 316 s−1 to 248 and 380 s−1 respectively, while the rate between state N and I3 were reduced from 1705 to 1186 s−1. Thus, the stability of transition states between major and minor states is also affected by NDs.
From the population of ND-bound form and its exchange rate with the ND-free form, the apparent association rate of Holo-hIFABP to DMPC/DMPG NDs (kon’ = kex × Pb/(Pb + PN)) was estimated to be ∼78 s−1, the dissociation rate (koff = kex × PN/(Pb + PN)) was ∼8075 s−1, and the association rate or on-rate (kon = kon’/[ND], where [ND] is the concentration of protein-free NDs and was estimated as described previously(6)) was 5.2 × 106 M−1s−1. On the basis of koff and kon values, the binding affinity (Kd = koff/kon) was estimated to be 1564 μM. In comparison with Apo-hIFABP (kon = 9.8 × 106 M−1s−1, Kd = 487 μM), we found that ligand binding reduces the affinity by about three times and association rate by two times. The result suggests that Holo-hIFABP interacts with DMPC/DMPG NDs more weakly than Apo-hIFABP. This is consistent with the previous study on the interactions of Drosophila FABP with membranes (48). As oleic acid binds to the internal cavity of FABP, the structures of Apo- and Holo-IFABPs are nearly identical (13,44), and NDs interact directly with the external surface of FABP, reduction of FABP-ND interactions by oleic acid binding may result from conformational changes at the sidechains, especially charged sidechains on the protein surface.
The ND-bound state and major state N are similar in 15N chemical shifts (Table S2), suggesting that the ND-bound state is native-like. This is consistent with a weak binding mode in an aqueous environment. This weak binding mode is determined by the structural features of hIFABP and ND/membrane: charged and hydrophilic protein surface and headgroups of membrane phospholipids. Different from FABPs, β barrel membrane proteins have highly hydrophobic outer surfaces and thus strongly associate with the hydrophobic tail groups of phospholipids and are stable in membrane environments instead of aqueous environments (49). According to significant chemical shift difference between the ND-bound and native states (Fig. 1 b; Table S2), the first protein-ND interaction site is likely located at helix II with three positively charged residues (K27, R28, and K29), and the second interaction site is probably located around βF and βG containing a positively charged cluster of R79, K92, and K94. The binding sites are the same as those for the Apo-hIFABP (6). Therefore, ligand binding does not change the protein-membrane interaction mode but reduces the affinity, likely by altering protein sidechain conformations.
Conclusion
Our results show that ligand binding stabilizes hIFABP and induces structural changes of mainly sidechains located both inside and outside the cavity. Due to the structural changes mainly in sidechains, the ligand-bound hIFABP (Holo-hIFABP) has significantly lower affinity to NDs than the ligand-free hIFABP (Apo-hIFABP), although Holo- and Apo-hIFABPs have nearly identical backbone structures. Besides the impact on the structure, ligand binding also influences the conformational exchange processes between the major and minor conformational states through affecting populations of the minor states and interconversion rates. Comparisons of Apo- and Holo-hIFABP in amide exchange rates and minor state populations reveal that minor states I1 and I2 originate from global breathing motion, while minor state I3 originates from local motion. Our amide exchange data suggest that ligands very likely exit from Holo-hIFABP via the local unfolding of αII.
Author contributions
D.Y. conceived the project and performed data analysis. Y.L. performed experiments and data analysis. G.Z.Y. did data analysis. D.Y. wrote the manuscript with input from Y.L. and G.Z.Y.
Acknowledgments
This research was supported by grants from Synthetic Biology Research & Development Programme (SBP) of National Research Foundation Singapore (SBP-P4, R154000A71592) and Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2017-T2-1-125).
Declaration of interests
The authors declare no competing interests.
Editor: Charles Deber.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.09.043.
Supporting material
References
- 1.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.McArthur M.J., Atshaves B.P., et al. Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 3.Storch J., McDermott L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009;50:S126–S131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H.K., Storch J. Mechanism of free fatty acid transfer from rat heart fatty acid-binding protein to phospholipid membranes. Evidence for a collisional process. J. Biol. Chem. 1992;267:20051–20056. [PubMed] [Google Scholar]
- 5.Hsu K.-T., Storch J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J. Biol. Chem. 1996;271:13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y., Yang D. Conformational exchange of fatty acid binding protein induced by protein-nanodisc interactions. Biophys. J. 2021;120:4672–4681. doi: 10.1016/j.bpj.2021.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman A.W., Veerkamp J.H. New insights into the structure and function of fatty acid-binding proteins. Cell. Mol. Life Sci. 2002;59:1096–1116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertzel A.V., Bernlohr D.A. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol. Metabol. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil G.S., Bernlohr D.A. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015;11:592–605. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacchettini J.C., Gordon J.I., Banaszak L.J. Crystal structure of rat intestinal fatty-acid-binding protein. Refinement and analysis of the Escherichia coli-derived protein with bound palmitate. J. Mol. Biol. 1989;208:327–339. doi: 10.1016/0022-2836(89)90392-6. [DOI] [PubMed] [Google Scholar]
- 11.Thompson J., Winter N., et al. Banaszak L. The crystal structure of the liver fatty acid-binding protein. A complex with two bound oleates. J. Biol. Chem. 1997;272:7140–7150. doi: 10.1074/jbc.272.11.7140. [DOI] [PubMed] [Google Scholar]
- 12.Sacchettini J.C., Gordon J.I., Banaszak L.J. Refined apoprotein structure of rat intestinal fatty acid binding protein produced in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1989;86:7736–7740. doi: 10.1073/pnas.86.20.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodsdon M.E., Cistola D.P. Discrete backbone disorder in the nuclear magnetic resonance structure of Apo intestinal fatty acid-binding protein: implications for the mechanism of ligand entry. Biochemistry. 1997;36:1450–1460. doi: 10.1021/bi961890r. [DOI] [PubMed] [Google Scholar]
- 14.Hodsdon M.E., Cistola D.P. Ligand binding alters the backbone mobility of intestinal fatty acid-binding protein as monitored by 15N NMR relaxation and 1H exchange. Biochemistry. 1997;36:2278–2290. doi: 10.1021/bi962018l. [DOI] [PubMed] [Google Scholar]
- 15.Cai J., Lücke C., et al. Hamilton J.A. Solution structure and backbone dynamics of human liver fatty acid binding protein: fatty acid binding revisited. Biophys. J. 2012;102:2585–2594. doi: 10.1016/j.bpj.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F., Lücke C., et al. Hamilton J.A. Solution structure of human intestinal fatty acid binding protein: implications for ligand entry and exit. J. Biomol. NMR. 1997;9:213–228. doi: 10.1023/a:1018666522787. [DOI] [PubMed] [Google Scholar]
- 17.He Y., Yang X., et al. Stark R.E. Solution-state molecular structure of Apo and oleate-liganded liver fatty acid-binding protein. Biochemistry. 2007;46:12543–12556. doi: 10.1021/bi701092r. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez-González L.H., Ludwig C., et al. Lücke C. Solution structure and backbone dynamics of human epidermal-type fatty acid-binding protein (E-FABP) Biochem. J. 2002;364:725–737. doi: 10.1042/BJ20020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lücke C., Rademacher M., et al. Rüterjans H. Spin-system heterogeneities indicate a selected-fit mechanism in fatty acid binding to heart-type fatty acid-binding protein (H FABP) Biochem. J. 2001;354:259–266. doi: 10.1042/0264-6021:3540259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favretto F., Assfalg M., et al. Molinari H. Ligand binding promiscuity of human liver fatty acid binding protein: structural and dynamic insights from an interaction study with glycocholate and oleate. Chembiochem. 2013;14:1807–1819. doi: 10.1002/cbic.201300156. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Sui X., Yang D. Probing methyl dynamics from 13C autocorrelated and cross-correlated relaxation. J. Am. Chem. Soc. 2006;128:5073–5081. doi: 10.1021/ja057579r. [DOI] [PubMed] [Google Scholar]
- 22.Lücke C., Fushman D., et al. Rüterjans H. A comparative study of the backbone dynamics of two closely related lipid binding proteins: bovine heart fatty acid binding protein and porcine ileal lipid binding protein. Mol. Cell. Biochem. 1999;192:109–121. [PubMed] [Google Scholar]
- 23.Long D., Yang D. Buffer interference with protein dynamics: a case study on human liver fatty acid binding protein. Biophys. J. 2009;96:1482–1488. doi: 10.1016/j.bpj.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cistola D.P., Kim K., et al. Frieden C. Fatty acid interactions with a helix-less variant of intestinal fatty acid-binding protein. Biochemistry. 1996;35:7559–7565. doi: 10.1021/bi952912x. [DOI] [PubMed] [Google Scholar]
- 25.Xiao T., Fan J.S., et al. Yang D. Local unfolding of fatty acid binding protein to allow ligand entry for binding. Angew. Chem., Int. Ed. Engl. 2016;55:6869–6872. doi: 10.1002/anie.201601326. [DOI] [PubMed] [Google Scholar]
- 26.Yu B., Yang D. Coexistence of multiple minor states of fatty acid binding protein and their functional relevance. Sci. Rep. 2016;6:34171. doi: 10.1038/srep34171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao T., Lu Y., et al. Yang D. Ligand entry into fatty acid binding protein via local unfolding instead of gap widening. Biophys. J. 2020;118:396–402. doi: 10.1016/j.bpj.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schanda P., Kupce E., Brutscher B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J. Biomol. NMR. 2005;33:199–211. doi: 10.1007/s10858-005-4425-x. [DOI] [PubMed] [Google Scholar]
- 29.Fan J.S., Lim J., et al. Yang D. Measurement of amide hydrogen exchange rates with the use of radiation damping. J. Biomol. NMR. 2011;51:151–162. doi: 10.1007/s10858-011-9549-6. [DOI] [PubMed] [Google Scholar]
- 30.Jiang B., Yu B., et al. Yang D. A (15)N CPMG relaxation dispersion experiment more resistant to resonance offset and pulse imperfection. J. Magn. Reson. 2015;257:1–7. doi: 10.1016/j.jmr.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Long D., Liu M., Yang D. Accurately probing slow motions on millisecond timescales with a robust NMR relaxation experiment. J. Am. Chem. Soc. 2008;130:2432–2433. doi: 10.1021/ja710477h. [DOI] [PubMed] [Google Scholar]
- 32.Hansen D.F., Vallurupalli P., Kay L.E. An improved 15N relaxation dispersion experiment for the measurement of millisecond time-scale dynamics in proteins. J. Phys. Chem. B. 2008;112:5898–5904. doi: 10.1021/jp074793o. [DOI] [PubMed] [Google Scholar]
- 33.Lim J., Xiao T., et al. Yang D. An off-pathway folding intermediate of an acyl carrier protein domain coexists with the folded and unfolded states under native conditions. Angew. Chem., Int. Ed. Engl. 2014;53:2358–2361. doi: 10.1002/anie.201308512. [DOI] [PubMed] [Google Scholar]
- 34.Vallurupalli P., Bouvignies G., Kay L.E. Studying "invisible" excited protein states in slow exchange with a major state conformation. J. Am. Chem. Soc. 2012;134:8148–8161. doi: 10.1021/ja3001419. [DOI] [PubMed] [Google Scholar]
- 35.Fawzi N.L., Ying J., et al. Clore G.M. Atomic-resolution dynamics on the surface of amyloid-β protofibrils probed by solution NMR. Nature. 2011;480:268–272. doi: 10.1038/nature10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korzhnev D.M., Skrynnikov N.R., et al. Kay L.E. An NMR experiment for the accurate measurement of heteronuclear spin-lock relaxation rates. J. Am. Chem. Soc. 2002;124:10743–10753. doi: 10.1021/ja0204776. [DOI] [PubMed] [Google Scholar]
- 37.Farrow N.A., Muhandiram R., et al. Kay L.E. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- 38.Ran X., Miao H.H., et al. Yang D. Structural and dynamic characterization of a neuron-specific protein kinase C substrate, neurogranin. Biochemistry. 2003;42:5143–5150. doi: 10.1021/bi0271751. [DOI] [PubMed] [Google Scholar]
- 39.Koradi R., Billeter M., Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 40.Ragona L., Fogolari F., et al. Molinari H. EF loop conformational change triggers ligand binding in beta-lactoglobulins. J. Biol. Chem. 2003;278:38840–38846. doi: 10.1074/jbc.M306269200. [DOI] [PubMed] [Google Scholar]
- 41.Nespoulous C., Briand L., et al. Pernollet J.C. Odorant binding and conformational changes of a rat odorant-binding protein. Chem. Senses. 2004;29:189–198. doi: 10.1093/chemse/bjh017. [DOI] [PubMed] [Google Scholar]
- 42.Gasymov O.K., Abduragimov A.R., Glasgow B.J. pH-Dependent conformational changes in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2010;49:582–590. doi: 10.1021/bi901435q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X., Chang S., et al. Dong H. Cryo-EM structures of lipopolysaccharide transporter LptB2FGC in lipopolysaccharide or AMP-PNP-bound states reveal its transport mechanism. Nat. Commun. 2019;10:4175. doi: 10.1038/s41467-019-11977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacchettini J.C., Scapin G., et al. Gordon J.I. Refinement of the structure of Escherichia coli-derived rat intestinal fatty acid binding protein with bound oleate to 1.75-A resolution. Correlation with the structures of the apoprotein and the protein with bound palmitate. J. Biol. Chem. 1992;267:23534–23545. [PubMed] [Google Scholar]
- 45.Mills J.L., Liu G., et al. Szyperski T. NMR structure and dynamics of the engineered fluorescein-binding lipocalin FluA reveal rigidification of beta-barrel and variable loops upon enthalpy-driven ligand binding. Biochemistry. 2009;48:7411–7419. doi: 10.1021/bi900535j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y., Kim J.G., et al. Ihee H. Ultrafast coherent motion and helix rearrangement of homodimeric hemoglobin visualized with femtosecond X-ray solution scattering. Nat. Commun. 2021;12:3677. doi: 10.1038/s41467-021-23947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita A., Sato T., et al. Adachi S.I. Visualizing breathing motion of internal cavities in concert with ligand migration in myoglobin. Proc. Natl. Acad. Sci. USA. 2009;106:2612–2616. doi: 10.1073/pnas.0807774106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Y.Y., Huang Y.F., et al. Lyu P.C. The ligand-mediated affinity of brain-type fatty acid-binding protein for membranes determines the directionality of lipophilic cargo transport. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2019;1864:158506. doi: 10.1016/j.bbalip.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Hermansen S., Linke D., Leo J.C. Transmembrane beta-barrel proteins of bacteria: from structure to function. Adv. Protein Chem. Struct. Biol. 2022;128:113–161. doi: 10.1016/bs.apcsb.2021.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.