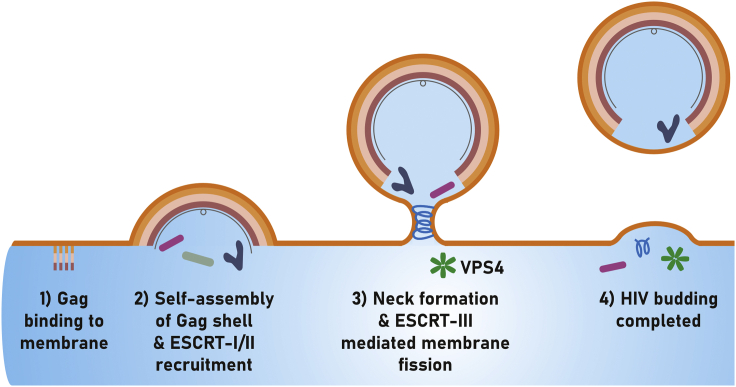
Retrovirus assembly is coordinated by the viral polyprotein Gag, and after assembly, the particles are released from the host cell in a process called budding. Assembly of one of the best-studied retroviruses, human immunodeficiency virus-1 (HIV-1), occurs at the plasma membrane of infected cells (Fig. 1). Driven by Gag, an almost complete viral shell is formed when the particle buds from the cell via membrane fission (2). As other retroviruses, HIV-1 hijacks the host endosomal complex required for transport (ESCRT) machinery for budding. The ESCRT system is involved in a variety of cellular processes, including multivesicular body morphogenesis, exosome formation, and cytokinesis (3). ESCRT proteins can drive membrane fission from within narrow membrane necks, budding away from the cytoplasm. Thereby, it makes it an ideal machinery to support viral egress. The human ESCRT system consists of >30 proteins, and while parts are described in great detail, there are also still many open questions. ESCRT-III, denoting a subset of the ESCRT complex, typically plays a role in the late events of the fission process, and this is also the case for HIV-1 budding. One of the last events in scission is dependent on vacuolar protein sorting-associated protein 4 (VPS4). VPS4 is a hexameric AAA+ ATPase (ATPase associated with various activities). Human cells express two paralogs of this protein, VPS4A and VPS4B (4); however, there is little known about the different roles (or the absence of difference) of VPS4A and VPS4B in HIV-1 egress. After VPS4 hexamerizes, it functions by threading ESCRT-III filaments through the central hole in the hexamer. Hereby, it dissociates the polymer while consuming ATP. While the molecular components of this process are increasingly well described, it remains unclear how it leads to fission and HIV-1 release. In order to address this question, detailed kinetic data of budding are needed.
Figure 1.
Schematic of HIV-1 budding. Gag binds to the cytoplasmic membrane of the host cell (1) and initiates self-assembly, whereas concomitantly initial ESCRT recruitment occurs (2). The budding neck is formed, and the activity of ESCRT-III and VPS4 (3) leads to membrane fission and virus release (4). Modified with permission from ref. (1).
In this issue of Biophysical Journal, Itay Rousso and co-workers generate such data by filming HIV-1 budding using high-speed atomic force microscopy (HS-AFM) (5). HS-AFM is a technique in which a small needle scans over the surface, making topographic images of the surface at ∼second rates and ∼nanometer resolution (6). HS-AFM has been used before to study VPS4 activity (7,8); however, those experiments were performed using purified ESCRT proteins. Harel et al. now use live cells to study the dynamics of budding and the influence of VPS4 on this process (5). Previous experiments on retroviral budding from the same lab using conventional AFM revealed that the temporal resolution of ∼4 min/frame of normal AFM was not enough to study HIV-1 egress (9,10). Therefore, they turned to HS-AFM—incidentally called fast AFM by the authors—to reach imaging velocities up to 4 s/frame. The imaging speed turned out to be sufficient to observe the dynamics of HIV-1 budding. Realizing imaging the growth of these ∼100 nm particles on top of living cells is a major achievement and yielded unexpected new insights.
In particular, they were able to follow the particles from the first bulge in the membrane, where self-assembly had started, all the way until particle release. A minority of the events reached a maximum height above the surface of less than 50 nm, and these particles were labeled as short particles. The others, representing the majority of events, were labeled as tall particles. A large spread in budding times was observed, with a mean budding time of ∼9 min; however, budding could be as fast as 2.5 min. These data fit with fluorescence microscopy results (11). The tall particles in the HS-AFM experiments could be subdivided in two groups, showing slow and fast budding kinetics. The slow pathway differed primarily from the fast pathway in that it exhibited a prolonged stationary phase during the budding process. As this phase was not during growth, it is likely not related to reduced accessibility to cytosolic Gag. The slow and fast pathways were characterized by a similar growth rate, and the stationary phase typically occurred after the particle finished growing. This indicates that the slow pathway is associated with retarding events during the final fission steps in the budding membrane neck.
Attempts to knock out both VPS4 isoforms did not yield any cells, indicating that at least one isoform is needed for cell viability. Therefore, the researchers continued with cells where either VPS4A or VPS4B was deleted. While they recorded a reduction in virus production for the knockout cells, a much larger reduction was observed after additionally introducing small interfering RNA (siRNA). Using siRNA, also called silencing RNA, translation of mRNA is hampered, thereby largely reducing the production of the targeted protein. In particular, the VPS4 isoform that was not already knocked out was silenced by siRNA, resulting in very low levels of VPS4 in the cell. The HS-AFM experiments revealed that under these conditions, the predominant activity was the formation of short particles. As Western blot analysis showed that virus production was almost halted, it was concluded that this pathway does not lead to particle release but rather to reabsorption of the particle into the cell. Surprisingly, a clear difference between the VPS4A and VPS4B knockout mutants was observed, and the results lead the authors to conclude that VPS4B is more important for HIV-1 budding than VPS4A. VPS4B activity in HIV-1 egress has been studied before (12), and the new HS-AFM experiments elegantly reveal the difference in activity with respect to the other isoform. Interestingly, the observation that VPS4 deletion leads to a large decrease in particle height suggests that this protein not only plays a role in late events in budding (i.e., membrane scission) but also in earlier phases where the Gag-driven self-assembly occurs. Together with the observed difference between the two VPS4 isoforms, this finding is likely to trigger further research in order to better understand the role of VPS4 in ESCRT-driven processes in general and HIV-1 budding in particular. All in all, the imaging of viral budding at unprecedented ∼seconds temporal and ∼nanometer spatial resolution has led to new insights not only on the dynamics of this process but also on the role of the VPS4 isoforms. It is expected that the HS-AFM approach to follow HIV-1 budding will inspire others to study the dynamics of viral egress at this resolution.
Acknowledgments
Declaration of interests
The author declares no competing interests.
Editor: Anne Kenworthy.
References
- 1.Hurley J.H., Cada A.K. Inside job: how the ESCRTs release HIV-1 from infected cells. Biochem. Soc. Trans. 2018;46:1029–1036. doi: 10.1042/BST20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundquist W.I., Kräusslich H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012;2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remec Pavlin M., Hurley J.H. The ESCRTs – converging on mechanism. J. Cell Sci. 2020;133:jcs240333. doi: 10.1242/jcs.240333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caillat C., Maity S., et al. Weissenhorn W. The role of VPS4 in ESCRT-III polymer remodeling. Biochem. Soc. Trans. 2019;47:441–448. doi: 10.1042/BST20180026. [DOI] [PubMed] [Google Scholar]
- 5.Harel S., Altaras Y., et al. Rousso I. Analysis of individual HIV-1 budding event using fast AFM reveals a multiplexed role for VPS4. Biophys. J. 2022;121 doi: 10.1016/j.bpj.2022.08.035. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando T. High-speed atomic force microscopy. Curr. Opin. Chem. Biol. 2019;51:105–112. doi: 10.1016/j.cbpa.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Maity S., Caillat C., et al. Weissenhorn W. VPS4 triggers constriction and cleavage of ESCRT-III helical filaments. Sci. Adv. 2019;5:eaau7198. doi: 10.1126/sciadv.aau7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mierzwa B.E., Chiaruttini N., et al. Gerlich D.W. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 2017;19:787–798. doi: 10.1038/ncb3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladnikoff M., Rousso I. Directly monitoring individual retrovirus budding events using atomic force microscopy. Biophys. J. 2008;94:320–326. doi: 10.1529/biophysj.107.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladnikoff M., Shimoni E., et al. Rousso I. Retroviral assembly and budding occur through an actin-driven mechanism. Biophys. J. 2009;97:2419–2428. doi: 10.1016/j.bpj.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouvenet N., Bieniasz P.D., Simon S.M. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature. 2008;454:236–240. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieffer C., Skalicky J.J., et al. Sundquist W.I. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev. Cell. 2008;15:62–73. doi: 10.1016/j.devcel.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]