Abstract
It is not known whether obesity has a differential effect on allogeneic HCT outcomes with alternative donor types. We reported the results of a retrospective registry study examining the effect of obesity (body mass index [BMI] >30) on outcomes with alternative donors (haploidentical related donor with ≥2 mismatches and receiving post-transplant cyclophosphamide [haplo] and cord blood [CBU]) vs. matched unrelated donor (MUD). Adult patients receiving HCT for hematologic malignancy (2013-2017) (N=16,182) using MUD (n=11,801), haplo (n=2894) and CBU (n=1487) were included. The primary outcome was non-relapse mortality (NRM). The analysis demonstrated a significant, non-linear interaction between pre-transplant BMI and the 3 donor groups for NRM: NRM risk was significantly higher with CBU compared to haplo at BMI 25-30 (HR 1.66-1.71, p<0.05) and MUD transplants at BMI 25-45 (HR, 1.61-3.47, p<0.05). The results demonstrated that NRM and survival outcomes are worse in overweight and obese transplant recipients (BMI ≥25) with one alternative donor type over MUD, although obesity does not appear to confer a uniform differential mortality risk with one donor type over the other. BMI may serve as a criterion for selecting a donor among the 3 (MUD, haplo and CBU) options, if matched sibling donor is not available.
Keywords: allogeneic cell transplant, non-relapse mortality, obesity, alternative donor types, haploidentical, cord blood units
INTRODUCTION
Obesity is a worsening global public health problem1. In the United States, the age-adjusted prevalence of obesity in adults was approximately 42% in 2017-20182,3. The impact of obesity on transplant outcomes has been the subject of much investigation4. Obesity is defined in several ways, but one commonly accepted definition is body mass index (BMI) of 30 kg/m2 and above. It is often associated with other comorbid conditions, such as diabetes, cardiovascular diseases, and is generally associated with increased mortality. In the context of hematopoietic cell transplantation (HCT), obesity can be a barrier to a successful procedure. Published data suggest that obesity impacts non-relapse mortality (NRM), overall survival (OS) and disease-free survival (DFS) in both allogeneic HCT5–8 and autologous HCT settings9–11. A Center for International Blood and Marrow Transplant Research (CIBMTR) observational study examined the transplant outcomes in over 4000 AML patients undergoing myeloablative conditioning (MAC) and reported no significant effect of obesity (BMI >30 kg/m2) on NRM and OS, although underweight (BMI <18 kg/m2) recipients of related donor allogeneic HCT were found to have decreased OS compared to patients within the normal BMI (18-25 kg/m2)12. Another CIBMTR study examined the outcomes in children with severe aplastic anemia and demonstrated worse OS among overweight (BMI >95th percentile adjusted for age) patients compared to children with lower BMI (59% vs >70% at 2 years)13. Among 3827 unrelated donor transplant recipients in the Japanese transplant registry, a higher BMI was associated with more acute graft-versus-host disease (GVHD) and infections, but with similar rates of relapse and OS14. HCT-Comorbidity Index (HCT-CI), a commonly used validated risk-assessment tool developed as a scoring system for patients undergoing allogeneic HCT15 includes obesity, defined as body mass index [BMI] over 35 kg/m2, as an independent risk factor for NRM.
While there is significant data evaluating the impact of obesity on posttransplant outcomes, there is paucity of contemporary data to confirm differential effect of obesity on transplant outcomes with one donor type over the other. It is not known if a particular donor type affects transplant outcomes in obese patients more than or differently from non-obese patients. A single-center matched case-control study (n=322) showed an association between obesity (weight over 120% ideal body weight) and worse survival outcomes after HCT in patients with HLA-matched donors, but not with mismatched donors6. However, this study had several caveats including small sample size of HLA-matched obese patients, short follow up, and use of serologic HLA typing. Another single-center study published in 1995 examined the impact of patient weight on NRM after marrow transplant (n=2238) and showed no significant impact of obesity (weight over 145% ideal body weight) on outcomes in any subgroup of patients16.
With the availability of alternative donor options including haploidentical related donor, and umbilical cord blood units17 in addition to unrelated donors for patients lacking HLA-identical siblings, understanding the interaction between obesity and donor type may be relevant to pre-transplant evaluation of, including donor selection for, obese patients. We, therefore, conducted a retrospective analysis from the observational database of the CIBMTR to examine the effect of obesity on outcomes after transplant with alternative donor types (of haploidentical-related and umbilical cord blood units) vs. matched unrelated donor (MUD). We hypothesized that obesity has a differential effect on transplant outcomes with one donor type over the other: the study hypothesis was that patients with alternative donor types have worse NRM and other survival outcomes with increasing BMI when compared to MUD.
PATIENTS AND METHODS
Data sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program, which consists of a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplantations to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information issued in the performance of such research is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
Patients
Adult patients who received first allogeneic HCT for any hematologic malignancy between 2013 and 2017, using the following donors: MUD, defined as 8/8-HLA match in A-, B-, C-, and DRB1-; haploidentical related donor (haplo), defined as related donor with ≥2 mismatches and umbilical cord blood units (CBU). Recipients of matched sibling donor and mismatched unrelated donor (defined as <8/8-HLA match) allogeneic HCT, haplo-HCT recipients not receiving posttransplant cyclophosphamide (ptCy), and patients undergoing ex vivo T-cell depleted and CD34+ selected HCT were excluded. Patients with BMI of <15 and >50 (n=120) were also excluded.
Objective, Endpoints, and Definitions
The study hypothesis was that patients’ BMI has a differential effect on outcomes after HCT using one donor type over the other. The primary outcome studied was NRM, defined as time to death from any cause without disease relapse or progression. NRM was summarized as cumulative incidence estimate with disease relapse/progression as competing risk. The secondary outcomes studied were OS, DFS, and disease relapse. OS was defined as time to death from any cause. Patients who were alive were censored at the time of last contact. DFS was defined as time to disease relapse/progression or death from any cause. Patients who were alive were censored at the time of last follow-up. For relapse, NRM was a competing risk. Obesity was defined as BMI ≥30 according to consensus developed by the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC)18,19.
Statistical Analysis
This is a retrospective comparative cohort study comparing outcomes after allogeneic HCT using 3 donor types for patients with hematologic malignancies. The objective of this analysis was to compare the NRM and other survival outcomes in the three donor groups. The primary endpoint of the study was NRM, while relapse, DFS and OS were secondary endpoints. Patient-, disease-, and transplant-related characteristics were compared among the three donor groups using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Univariate analysis of outcomes by BMI group was performed for each of the 3 donor groups. Probabilities of NRM and relapse were calculated by cumulative incidence function accounting for competing risks of relapse and death, respectively. Survival probabilities of DFS and OS were calculated using the Kaplan-Meier estimator and compared using the log-rank test. Comparison of survival curves and cumulative incidence curves was done with the log-rank test and Gray’s test respectively, along with point-wise comparisons at day +100, 6 months, 1 and 2 years post-HCT. Multivariable analysis of NRM, relapse, DFS, and OS were performed using Cox proportional hazards regression models. All variables were assessed for proportional hazards using graphical and/or time-dependent approaches. Two sets of models were included, treating BMI either as categorical variable based on traditional cutoffs, or treating BMI as a continuous variable (ranging from 20 through 45, in increments of 5) through the use of splines (e.g., BMI 20 (+/−2.5)+, 25 (+/− 2.5), etc.). We used cubic basis splines (on BMI) defined with 3 equally spaced knots at 24, 33, and 41. Stepwise model building with a significance level of 0.05 was used to identify variables to be included in the multivariable models. However, interaction terms were kept in the model if their level of significance was less than 0.0025. Main effects of donor and BMI were added to the models after the model building steps were completed. Interactions between donor group and the BMI variable were assessed to examine whether the impact of BMI is consistent or differential across donor types, and if there is a significant interaction, the BMI effects were described in the three donor groups. In addition, three-way interactions between donor, conditioning intensity, and BMI, and two-way interactions between donor and conditioning intensity, and BMI and conditioning intensity were examined. Secondary post hoc subgroup analyses were performed to compare outcomes between double cord blood unit (dCBU) transplant recipients and other donor groups, and haplo vs. MUD recipients of peripheral blood (PB) stem cell graft source. A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS v9.4 (Cary, NC).
RESULTS
Patient Characteristics
Patient and transplant characteristics are shown in Table 1. The study cohort included a total of 16,182 patients receiving allogeneic HCT (MUD, n=11,801; haplo, n=2894; CBU, n=1487). There were significant differences in the following baseline characteristics among the donor groups. African-Americans comprised 17% of haplo and 13% of CBU, compared to 2% of MUD group. Myeloablative conditioning (MAC) was more frequently used in MUD and CBU (51% and 49%, respectively) than in haplo group (39%), whereas non-myeloablative conditioning (NMA) was more common in haplo (32%) vs. MUD (14%) and CBU (18%) groups. The graft source utilized was peripheral blood in a greater proportion of MUD (86%) vs. haplo group (66%). Majority of CBU transplant patients received dCBU at transplant (82%). GVHD prophylaxis was tacrolimus-based in 80% and cyclosporin-based in 13% of MUD patients, while 43% of CBU group received tacrolimus-based prophylaxis and 51% received cyclosporin-based prophylaxis. CD34+ cell doses in the grafts were missing for a large number of haplo transplant recipients (41%) vs. 8% of MUD and 16% of CBU groups. In vivo T cell depletion using anti-thymocyte globulin was uncommon in the haplo group (2%), compared to MUD (35%) and CBU (20%) groups. Supplemental Table 1 shows the distribution of patients by BMI group (<30, 30-34.9 and ≥35) in the three donor groups. Only 15% (n=63) of the obese CBU recipients received single CBU (Supplemental Table 2). The median follow-up of survivors was 25, 36 and 36 months in the haplo, MUD and CBU groups, respectively.
Table 1.
Characteristics of study population by donor type
| Characteristic | Haploidentical | Matched Unrelated | Cord Blood | P Value |
|---|---|---|---|---|
| No. of patients | 2894 | 11801 | 1487 | |
| No. of centers | 149 | 227 | 121 | |
| Age, median (range) | 55 (18-88) | 58 (18-83) | 50 (18-75) | <.01a |
| Sex (%) | <.01b | |||
| Male | 1722 (60) | 6832 (58) | 778 (52) | |
| Race (%) | <.01b | |||
| White | 2004 (69) | 10496 (89) | 1019 (69) | |
| Black or African American | 479 (17) | 253 (2) | 192 (13) | |
| Asian | 165 (6) | 307 (3) | 118 (8) | |
| Native Hawaiian/Pacific Islander | 15 (1) | 19 (0) | 13 (1) | |
| American Indian or Alaska Native | 10 (0) | 38 (0) | 11 (1) | |
| More than one race | 12 (0) | 27 (0) | 14 (1) | |
| Ethnicity (%) | <.01b | |||
| Hispanic or Latino | 356 (12) | 620 (5) | 214 (14) | |
| Non-Hispanic or non-Latino | 2211 (76) | 10133 (86) | 1126 (76) | |
| Non-resident of the U.S. | 269 (9) | 802 (7) | 112 (8) | |
| Karnofsky performance score <90 (%) | 1228 (42) | 5047 (43) | 511 (34) | <.01b |
| HCT-CI without obesity (%) | 0.01b | |||
| 0 | 678 (23) | 2504 (21) | 352 (24) | |
| 1 | 433 (15) | 1590 (13) | 226 (15) | |
| 2 | 416 (14) | 1838 (16) | 222 (15) | |
| 3 | 515 (18) | 2237 (19) | 277 (19) | |
| 4 | 369 (13) | 1517 (13) | 177 (12) | |
| 5+ | 478 (17) | 2102 (18) | 230 (15) | |
| Body Mass Index (BMI) (%) | <.01 | |||
| Median (range) | 28 (16-50) | 27 (16-50) | 26 (15-48) | |
| <18.5 | 45 (2) | 177 (1) | 31 (2) | |
| 18.5-24.9 | 875 (30) | 3732 (32) | 534 (36) | |
| 25-29.9 | 952 (33) | 4183 (35) | 506 (34) | |
| 30-34.9 | 629 (22) | 2273 (19) | 263 (18) | |
| 35+ | 393 (14) | 1436 (12) | 153 (10) | |
| Refined-Disease Risk Index (%) | <.01b | |||
| Low | 316 (11) | 1078 (9) | 134 (9) | |
| Intermediate | 1464 (51) | 6317 (54) | 851 (57) | |
| High | 752 (26) | 3029 (26) | 331 (22) | |
| Very high | 141 (5) | 416 (4) | 50 (3) | |
| Disease (%) | <.01b | |||
| AML | 1197 (41) | 5003 (42) | 732 (49) | |
| ALL | 433 (15) | 1508 (13) | 292 (20) | |
| CML | 119 (4) | 390 (3) | 39 (3) | |
| Other Leukemias | 108 (4) | 399 (3) | 39 (3) | |
| MDS | 447 (15) | 2358 (20) | 178 (12) | |
| MPN | 83 (3) | 590 (5) | 14 (1) | |
| NHL | 319 (11) | 1114 (9) | 148 (10) | |
| HD | 117 (4) | 189 (2) | 33 (2) | |
| MM | 62 (2) | 221 (2) | 9 (1) | |
| Non-MM PCD | 9 (0) | 29 (0) | 3 (0) | |
| Prior autologous transplant (%) | 303 (10) | 973 (8) | 102 (7) | <.01b |
| Graft source (%) | <.01b | |||
| Bone marrow | 974 (34) | 1606 (14) | 0 (0) | |
| Peripheral blood | 1920 (66) | 10195 (86) | 0 (0) | |
| Umbilical cord blood | 0 (0) | 0 (0) | 1487 (100) | |
| Number of cord blood units (%) | <.01b | |||
| 1 | 0 (0) | 0 (0) | 269 (18) | |
| 2 | 0 (0) | 0 (0) | 1218 (82) | |
| NA | 2894 (100) | 11801 (100) | 0 (0) | |
| TBI used in conditioning regimen (%) | 2100 (73) | 2680 (23) | 1245 (84) | <.01b |
| Conditioning Intensity (%) | <.01b | |||
| MAC | 1142 (39) | 6054 (51) | 724 (49) | |
| RIC | 823 (28) | 4102 (35) | 489 (33) | |
| NMA | 929 (32) | 1645 (14) | 274 (18) | |
| Donor/recipient sex match (%) | <.01b | |||
| M-M | 1092 (38) | 5169 (44) | 71 (5) | |
| M-F | 679 (23) | 3251 (28) | 71 (5) | |
| F-M | 630 (22) | 1644 (14) | 59 (4) | |
| F-F | 493 (17) | 1700 (14) | 55 (4) | |
| Double cord - recipient M | 0 (0) | 0 (0) | 642 (43) | |
| Double cord - recipient F | 0 (0) | 0 (0) | 576 (39) | |
| Double cord - Missing | 0 (0) | 0 (0) | 13 (1) | |
| Missing | 0 (0) | 37 (0) | 0 (0) | |
| Donor/recipient CMV serostatus (%) | <.01b | |||
| +/+ | 1300 (45) | 3289 (28) | 53 (4) | |
| +/− | 241 (8) | 1287 (11) | 32 (2) | |
| −/+ | 721 (25) | 3889 (33) | 64 (4) | |
| −/− | 615 (21) | 3277 (28) | 38 (3) | |
| Double cord - recipient + | 0 (0) | 0 (0) | 854 (57) | |
| Double cord - recipient − | 0 (0) | 0 (0) | 353 (24) | |
| Double cord - recipient CMV unknown | 0 (0) | 0 (0) | 12 (1) | |
| Missing | 17 (1) | 59 (0) | 81 (5) | |
| CD34+ dose, x 106/kg (BM only) (%) | <.01b | |||
| Median (25th-75th quartile) | 2.67 (1.75, 3.88) | 2.65 (1.75, 3.91) | ||
| 0-1.9 | 194 (7) | 453 (4) | ||
| 2-3.9 | 258 (9) | 612 (5) | ||
| 4-7.9 | 123 (4) | 304 (3) | ||
| ≥8 | 17 (1) | 37 (0) | ||
| NA (PBSC graft) | 1920 (66) | 10195 (86) | ||
| Missing | 382 (13) | 200 (2) | ||
| CD34+ dose, x 106/kg (PBSC) (%) | <.01b | |||
| Median (25th-75th quartile) | 5.12 (4.33, 7.68) | 6.39 (4.88, 8.64) | ||
| 0-1.9 | 77 (3) | 449 (4) | ||
| 2-3.9 | 156 (5) | 958 (8) | ||
| 4-7.9 | 645 (22) | 5057 (43) | ||
| ≥8 | 250 (9) | 2951 (25) | ||
| NA (BM graft) | 974 (34) | 1606 (14) | ||
| Missing | 792 (27) | 780 (7) | ||
| CD34+ dose, x 105/kg (CBU) (%) | ||||
| Single | ||||
| Median (IQR) | 2.31 (1.33, 4.34) | |||
| 0-1.9 | 84 (6) | |||
| 2-3.9 | 58 (4) | |||
| 4-7.9 | 30 (2) | |||
| ≥8 | 21 (1) | |||
| Missing | 76 (5) | |||
| Double | ||||
| Median (IQR) | 2.24 (1.45, 3.51) | |||
| 0-1.9 | 456 (31) | |||
| 2-3.9 | 378 (25) | |||
| 4-7.9 | 152 (10) | |||
| ≥8 | 63 (4) | |||
| Missing | 169 (11) | |||
| GVHD Prophylaxis (%) | <.01b | |||
| TAC + MMF ± others | 0 | 1381 (12) | 508 (34) | |
| TAC + MTX ± others (not MMF) | 0 | 6841 (58) | 26 (2) | |
| TAC ± others (not MMF, MTX) | 0 | 1214 (10) | 109 (7) | |
| CSA + MMF ± others (not TAC) | 0 | 650 (6) | 736 (50) | |
| CSA + MTX ± others (not MMF,TAC) | 0 | 853 (7) | 6 (<1) | |
| CSA ± others (not TAC, MMF, MTX) | 0 | 91 (<1) | 11 (<1) | |
| Other GVHD Prophylaxisc | 0 | 125 (1) | 79 (5) | |
| Posttransplant Cyclophosphamide ± others | 2844 (100) | 579 (5) | 7 (1) | |
| ATG/Alemtuzumab (%) | <.01b | |||
| ATG + Alemtuzumab | 54 (2) | 4086 (35) | 279 (19) | |
| ATG alone | 0 (0) | 1 (0) | 0 (0) | |
| Alemtuzumab alone | 2812 (97) | 7052 (60) | 1202 (81) | |
| Missing | 28 (1) | 662 (6) | 6 (0) | |
| Year of transplant (%) | <.01b | |||
| 2013 | 104 (4) | 1146 (10) | 231 (16) | |
| 2014 | 369 (13) | 2558 (22) | 375 (25) | |
| 2015 | 615 (21) | 2585 (22) | 343 (23) | |
| 2016 | 835 (29) | 2744 (23) | 302 (20) | |
| 2017 | 971 (34) | 2768 (23) | 236 (16) |
HCT-CI, hematopoietic cell transplantation-comorbidity index; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; HD, Hodgkin lymphoma; MM, multiple myeloma; N, number; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; NMA, nonmyeloablative conditioning; GVHD, graft-versus-host disease; CMV, cytomegalovirus; CBU, cord blood unit; MMF, mycophenolate mofetil; MTX, methotrexate; CSA, cyclosporine; TAC, tacrolimus; ATG, anti-thymocyte globulin; PBSC, peripheral blood stem cells; BM, bone marrow; NA, not applicable; IQR, interquartile range.
Hypothesis testing:
Kruskal-Wallis test
Pearson chi-square test
Other GVHD Prophylaxis: MMF or MTX + siro: n=84; Missing: n=120
Results of the Primary Analysis
Multivariable analysis (after adjusting for age, Karnofsky Performance Score (KPS), race, HCT-CI, disease and disease risk, donor-recipient CMV sero-status, GVHD prophylaxis, conditioning intensity, in vivo T cell depletion and using BMI as a continuous variable) showed a significant, but non-linear interaction between BMI and donor groups for NRM (p<0.0001), DFS (p=0.0002) and OS (p<0.0001), but not for disease relapse (Supplemental Tables 3–6).
Non-Relapse Mortality
NRM at 2 years after haplo, MUD and CBU transplants, regardless of BMI, was 19% (95% confidence interval [95CI], 18-21%), 19% (95CI, 18-20%) and 29% (95CI, 27-31%), respectively (p<0.001) (Supplemental Table 7). For patients with BMI 30-35, 2-year NRM was 19% (95CI, 16-23%), 18% (95CI, 17-20%) and 29% (95CI, 23-35%) for haplo, MUD and CBU, respectively (p=0.003) (Supplemental Table 8), for BMI >35, 2-year NRM was 19% (95CI, 15-23%), 21% (95CI, 19-24%) and 30% (95CI, 23-38%), respectively (p=0.03) (Supplemental Table 9). Compared to MUD recipients, CBU transplant patients had a significantly increased risk of NRM at BMI of 25 (hazard ratio [HR], 2.26, p<0.0001, BMI 30 (HR, 2.28, p<0.0001, BMI 35 (HR, 1.61, p=0.02) and BMI 45 (HR, 3.47, p=0.02). Compared to haplo, CBU had an increased risk of NRM at BMI 25 (HR, 1.71, p=0.02), and BMI 30 (HR, 1.66, p=0.04). There was no statistically significant difference in NRM between haplo and MUD at any BMI (20-45) (Table 2, Figure 1A). Supplemental Table 3 shows the additional variables significant for non-relapse mortality in the multivariable analysis: patients 50 years and older, HCT-CI ≥3, KPS <90, high Refined-Disease Risk Index, chronic myeloid leukemia/ myelodysplasia/ myeloproliferative neoplasm, cytomegalovirus (CMV)-seropositive donor/recipient, calcineurin inhibitor plus mycophenolate mofetil-based GVHD prophylaxis and MAC regimen were associated with higher NRM risk.
Table 2.
Multivariable analysis: Interaction between BMI as a continuous variable and donor type*
| Label | Hazard Ratio | 95% Hazard Ratio Confidence Limits | Adjusted P-value‡ | |
|---|---|---|---|---|
| NRM | 1.000 | <.0001 | ||
| Haplo vs MUD at BMI = 20 | 1.250 | 0.882 | 1.770 | 0.4923 |
| Haplo vs MUD at BMI = 25 | 1.318 | 1.015 | 1.710 | 0.1557 |
| Haplo vs MUD at BMI = 30 | 1.375 | 1.065 | 1.775 | 0.0724 |
| Haplo vs MUD at BMI = 35 | 1.224 | 0.920 | 1.628 | 0.4632 |
| Haplo vs MUD at BMI = 40 | 1.082 | 0.723 | 1.618 | 0.7314 |
| Haplo vs MUD at BMI = 45 | 1.279 | 0.638 | 2.567 | 0.7314 |
| CBU vs Haplo at BMI = 20 | 1.152 | 0.711 | 1.868 | 0.5652 |
| CBU vs Haplo at BMI = 25 | 1.712 | 1.175 | 2.494 | 0.0250 |
| CBU vs Haplo at BMI = 30 | 1.657 | 1.135 | 2.418 | 0.0375 |
| CBU vs Haplo at BMI = 35 | 1.319 | 0.862 | 2.020 | 0.3711 |
| CBU vs Haplo at BMI = 40 | 1.514 | 0.836 | 2.742 | 0.3711 |
| CBU vs Haplo at BMI = 45 | 2.709 | 0.971 | 7.561 | 0.1852 |
| CBU vs MUD at BMI = 20 | 1.440 | 0.991 | 2.092 | 0.0882 |
| CBU vs MUD at BMI = 25 | 2.256 | 1.699 | 2.995 | <.0001 |
| CBU vs MUD at BMI = 30 | 2.278 | 1.705 | 3.043 | <.0001 |
| CBU vs MUD at BMI = 35 | 1.615 | 1.155 | 2.258 | 0.0166 |
| CBU vs MUD at BMI = 40 | 1.638 | 1.010 | 2.656 | 0.0882 |
| CBU vs MUD at BMI = 45 | 3.466 | 1.470 | 8.175 | 0.0166 |
| OS | 1.000 | 0.0002 | ||
| Haplo vs MUD at BMI = 20 | 1.231 | 0.985 | 1.538 | 0.1857 |
| Haplo vs MUD at BMI = 25 | 1.267 | 1.069 | 1.503 | 0.0334 |
| Haplo vs MUD at BMI = 30 | 1.263 | 1.068 | 1.494 | 0.0334 |
| Haplo vs MUD at BMI = 35 | 1.215 | 1.008 | 1.465 | 0.1397 |
| Haplo vs MUD at BMI = 40 | 1.133 | 0.869 | 1.477 | 0.5802 |
| Haplo vs MUD at BMI = 45 | 1.154 | 0.735 | 1.812 | 0.5802 |
| CBU vs Haplo at BMI = 20 | 0.856 | 0.611 | 1.201 | 0.6337 |
| CBU vs Haplo at BMI = 25 | 1.446 | 1.105 | 1.891 | 0.0350 |
| CBU vs Haplo at BMI = 30 | 1.389 | 1.056 | 1.828 | 0.0762 |
| CBU vs Haplo at BMI = 35 | 1.176 | 0.864 | 1.600 | 0.6337 |
| CBU vs Haplo at BMI = 40 | 1.357 | 0.891 | 2.067 | 0.4364 |
| CBU vs Haplo at BMI = 45 | 1.512 | 0.662 | 3.450 | 0.6337 |
| CBU vs MUD at BMI = 20 | 1.054 | 0.800 | 1.389 | 0.7090 |
| CBU vs MUD at BMI = 25 | 1.832 | 1.478 | 2.271 | <.0001 |
| CBU vs MUD at BMI = 30 | 1.755 | 1.403 | 2.195 | <.0001 |
| CBU vs MUD at BMI = 35 | 1.429 | 1.105 | 1.847 | 0.0235 |
| CBU vs MUD at BMI = 40 | 1.537 | 1.075 | 2.197 | 0.0537 |
| CBU vs MUD at BMI = 45 | 1.744 | 0.826 | 3.683 | 0.2630 |
| DFS | 1.000 | 0.0002 | ||
| Haplo vs MUD at BMI = 20 | 1.130 | 0.933 | 1.369 | 0.5003 |
| Haplo vs MUD at BMI = 25 | 1.191 | 1.029 | 1.377 | 0.0927 |
| Haplo vs MUD at BMI = 30 | 1.172 | 1.015 | 1.353 | 0.1238 |
| Haplo vs MUD at BMI = 35 | 1.144 | 0.974 | 1.343 | 0.3134 |
| Haplo vs MUD at BMI = 40 | 1.065 | 0.846 | 1.341 | 0.8300 |
| Haplo vs MUD at BMI = 45 | 1.022 | 0.680 | 1.536 | 0.9184 |
| CBU vs Haplo at BMI = 20 | 0.832 | 0.613 | 1.129 | 0.6028 |
| CBU vs Haplo at BMI = 25 | 1.187 | 0.927 | 1.519 | 0.5361 |
| CBU vs Haplo at BMI = 30 | 1.211 | 0.942 | 1.557 | 0.4742 |
| CBU vs Haplo at BMI = 35 | 1.023 | 0.772 | 1.356 | 0.8748 |
| CBU vs Haplo at BMI = 40 | 1.115 | 0.754 | 1.649 | 0.7980 |
| CBU vs Haplo at BMI = 45 | 1.343 | 0.623 | 2.894 | 0.7980 |
| CBU vs MUD at BMI = 20 | 0.940 | 0.727 | 1.215 | 0.6835 |
| CBU vs MUD at BMI = 25 | 1.413 | 1.150 | 1.735 | 0.0054 |
| CBU vs MUD at BMI = 30 | 1.419 | 1.147 | 1.755 | 0.0057 |
| CBU vs MUD at BMI = 35 | 1.170 | 0.919 | 1.490 | 0.5420 |
| CBU vs MUD at BMI = 40 | 1.188 | 0.844 | 1.671 | 0.6835 |
| CBU vs MUD at BMI = 45 | 1.372 | 0.681 | 2.764 | 0.6835 |
NRM, non-relapse mortality; BMI, body mass index; haplo, haploidentical related donor transplant; MUD, matched unrelated donor transplant; CBU, cord blood unit; OS, overall survival; DFS, disease-free survival.
adjusting for age, KPS, HCT-CI, disease and disease risk, donor-recipient CMV sero-status, GVHD prophylaxis, conditioning intensity, in vivo T cell depletion.
Figure 1A.
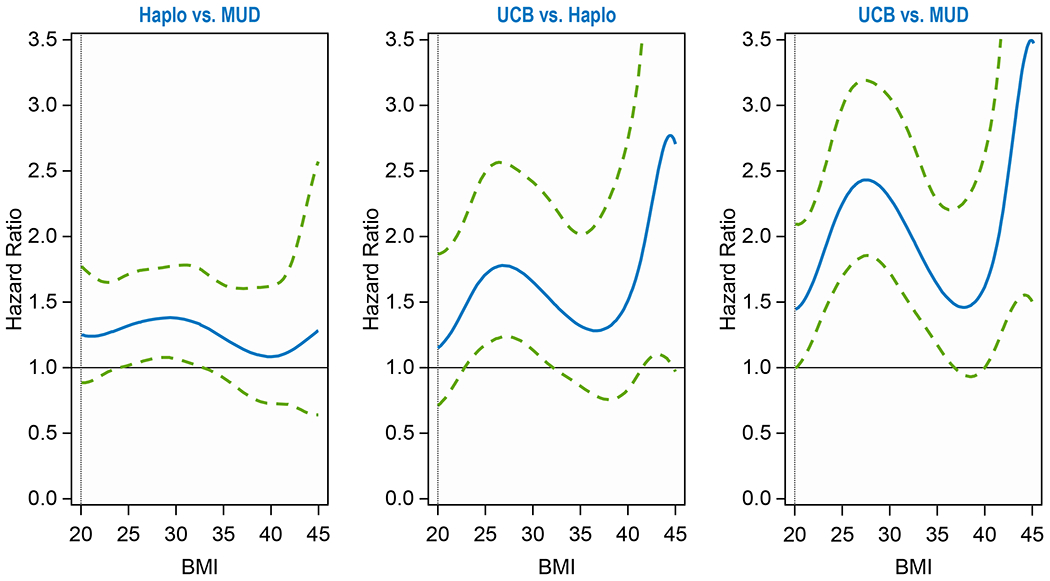
Non-Relapse Mortality of Donor Groups
Overall Survival
Two-year OS probability was 57% (95CI, 55-59%), 58% (95CI, 57-59%) and 49% (95CI, 46-51%) for haplo, MUD and CBU groups, respectively (p<0.001) regardless of BMI (Supplemental Table 7). Compared to MUD, CB had a statistically increased mortality risk at BMI 25-35 (HR = 1.4-1.8) (Table 2). Compared to haplo, CB had an increased mortality risk at BMI of 25 (HR = 1.45). Compared to MUD, haplo had an increased mortality risk at BMI of 25-30 (HR = 1.3). There were no statistically significant differences at other BMIs (Table 2, Figure 1B).
Figure 1B.
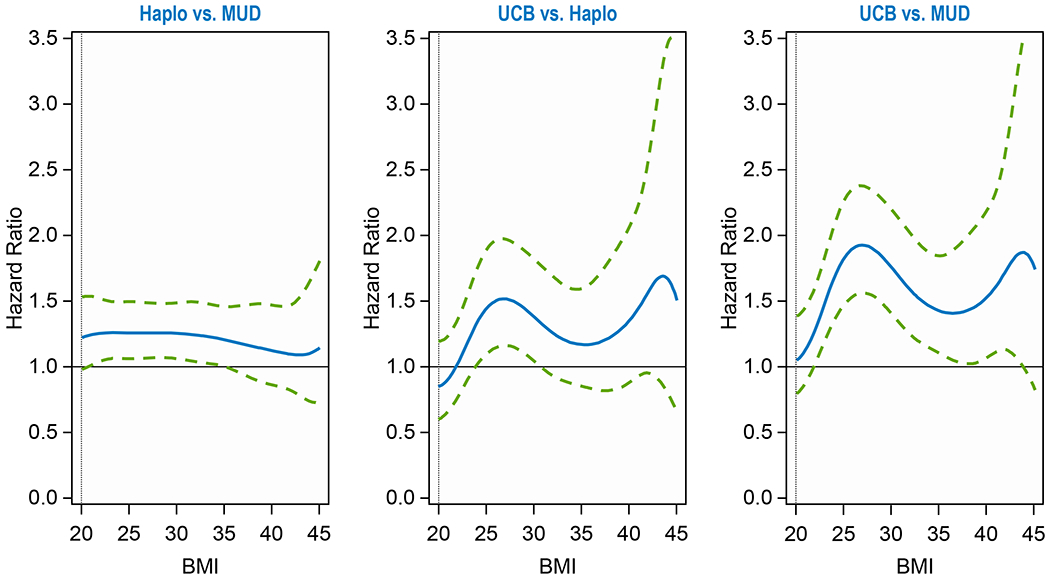
Overall Survival by Donor Groups
Disease-Free Survival
The probability of DFS at 2 years was 43% (95CI, 41-45%), 47% (95CI, 46-48%) and 42% (95CI, 40-45%) for haplo, MUD and CBU transplants, respectively (p<0.001), regardless of BMI (Table 2). Compared to MUD, CBU was associated with approximately 41% increased risk of treatment failure at BMI 25 (HR, 1.41, p=0.005) and BMI 30 (HR, 1.42, p=0.006) (Table 2, Figure 1C). There were no significant differences at other BMIs, nor between the other donor groups.
Figure 1C.
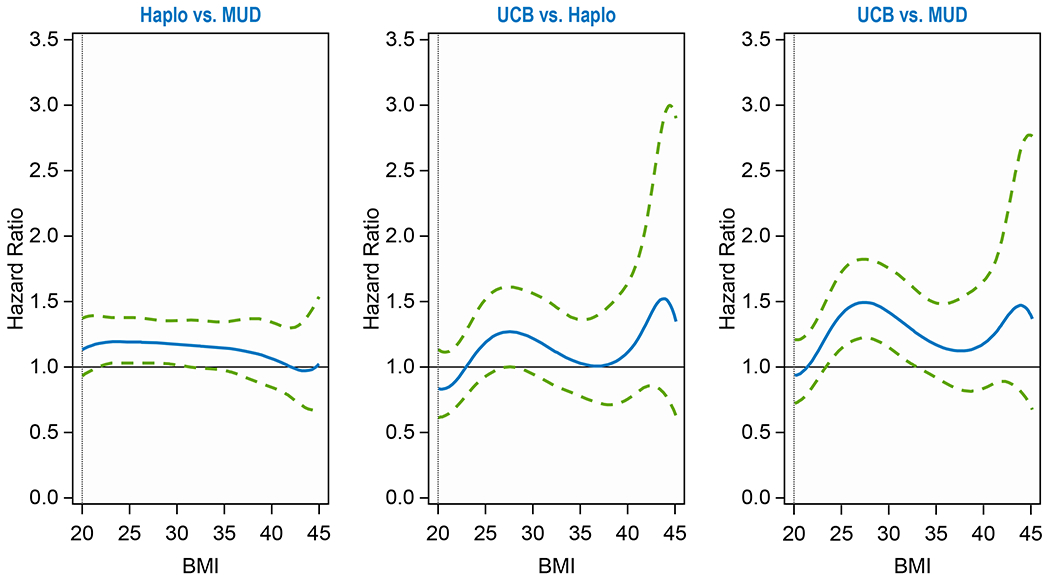
Disease-Free Survival by Donor Groups
Relapse
The cumulative incidence of disease relapse at 2 years was 38% (95CI, 37-40%), 34% (95CI, 33-35%) and 29% (95CI, 27-31%) for the haplo, MUD and CBU groups, respectively (p<0.001), regardless of BMI (Supplemental Table 7). While there was no interaction found between BMI and donor source for relapse, a significant interaction was observed between BMI and conditioning intensity for relapse (p<0.0001) (Supplemental Table 10, Supplemental Figure 1). RIC had a significantly higher risk of relapse (vs. MAC) at BMI of 25 (HR, 1.14, p=0.04) and 40 (HR, 1.38, p=0.03), and NMA had a higher risk of relapse vs. MAC at BMI 25 to 40 (HR ranging from 1.22-1.71, p<0.05) and vs. RIC at BMI 25, 30 and 35 (HR ranging from 1.18-1.35, p<0.05).
Secondary Analyses
A post hoc subgroup analysis was conducted after excluding patients receiving single CBU to identify if the outcomes with dCBU were differently influenced by recipient BMI, adjusting for the center effect for each outcome. This multivariable analysis demonstrated a significant interaction between BMI and donor group for NRM (p=0.0005), OS (p=0.0007) and DFS (p=0.002): the outcomes after dCBU (vs. haplo and vs. MUD) were no longer significantly different at any BMI, and haplo recipients had a significantly higher risk of NRM (vs. MUD) at BMI of 25-30 (HR, 1.35-1.49, p<0.05), and had significantly worse OS at BMI 20-35 (HR, 1.26-1.34, p<0.05) (Supplemental Table 11). In addition, we performed a subgroup analysis comparing the outcomes between patients receiving PB stem cell graft for MUD vs. haplo transplants and demonstrated no interaction between BMI and donor type for NRM and OS. For all BMIs tested, HR for death (in multivariable analysis) for NRM [1.05-1.50] and OS [1.02-1.27]) was numerically higher for haplo (contrasting with HR values for haplo vs. MUD in the primary analysis: 1.08-1.37 for NRM and 1.13-1.27 for OS) but did not meet statistical significance (Supplemental Table 12).
DISCUSSION
In this large registry-based retrospective analysis of adult recipients of allogeneic HCT using one of the three donor types (haplo, MUD and CBU) for treatment of hematologic malignancies, we have demonstrated that obesity in transplant recipients, as defined by BMI at the time of transplant, has a differential effect on mortality among recipients of CBU and haplo transplants, although level of significance was met only for certain BMI ranges. Moreover, it appears that obesity does not have a consistent or uniform effect on mortality risk with CBU and haplo (vs. MUD). We observed a non-linear pattern (“saddle-shaped” or “N-shaped”) of hazard for NRM with haplo (vs. MUD) and CBU (vs. haplo and vs. MUD): in general, with increasing BMI, we observed a trend for increasing mortality risk at BMI 25-30, followed by a plateauing or declining trend with higher BMI, and then further increase in risk toward the end of BMI spectrum of the study. The secondary subgroup analysis displayed no statistically significant difference in survival outcomes between dCBU vs. haplo and dCBU vs. MUD groups at any BMI, even though the hazards of death with dCBU were numerically similar to the overall CBU group in the primary analysis. For non-obese patients (BMI 20-25), there was no significant difference in outcomes between any two donor types in any of the analyses.
It is important to clarify that the study did not attempt to identify the impact of BMI on outcomes after allogeneic HCT using a particular donor, but hypothesized the presence of an interaction between pre-transplant BMI and donor type for NRM and other survival outcomes. Since the study did not compare outcomes in HCT recipients with different BMI within a donor group, we cannot comment on the effect of BMI on mortality risk after MUD transplant or alternative donors of haplo or CBU individually.
This is the first contemporary study to examine the presence of differential effect of obesity on transplant outcomes after alternative donor type compared to MUD. It was anticipated that the differential effect of obesity on outcomes after one of the alternative donors, if present, will provide new insight into donor selection process for obese patients. The results suggest that BMI, in fact, has a differential effect on outcomes after alternative donor transplant. We observed significantly, but not uniformly, worse NRM and survival outcomes in overweight and obese transplant recipients with one alternative donor type over MUD. The analysis, therefore, suggests that for obese patients, or overweight patients (BMI 25-29.9) for that matter, with no HLA-matched sibling available, MUD may be the preferred choice.
The secondary analysis showed no significantly worse NRM in dCBU patients, when compared to MUD and haplo recipients. We do acknowledge that improved outcomes with dCBU have been demonstrated in prior studies20,21. These results might suggest that the significantly higher mortality in the CBU cohort was due to the inclusion of single units in the CBU group, and may be partially explained by the low CD34+/total nucleated cell (TNC) dose received by adult transplant recipients with higher BMI. However, the fact that the mortality risk was still the same for dCBU as in primary analysis, as evidenced by the HR values, suggests that the removal of single CBU for the cohort may have resulted in loss of power to demonstrate statistical significance. The delayed neutrophil recovery in CBU subgroups with BMI 30-35 and >35 (28-day recovery in 78% and 69% patients, respectively, compared to 92% and 88%, respectvely, in haplo and 97% in MUD group; Supplemental Table 8 and 9) may also be suggestive of the effect of low CD34+/TNC dose in obese CBU recipients.
The study has a few limitations, including its retrospective nature, the potential for unrecognized biases and residual confounding despite a carefully conducted multivariable analysis adjusting for several variables. The registry does not capture the reason for using haplo vs. CBU as donor. Some institutions/physicians possibly preferred an alternative donor type, over other options including MUD. It is also possible that BMI itself factored into donor selection, which could also have confounded the results. The only possible way to circumvent these biases is to conduct a prospective randomized clinical trial. Another limitation is that conditioning dose adjustments for higher BMIs used by transplant centers could not be ascertained from the registry database. The study results cannot inform whether the outcomes were worse in haplo and cord blood recipients with higher BMIs due to lower CD34+ cell dose in the respective grafts. CD34+ cell doses infused during transplant were not available for majority of haplo patients. In addition, the post hoc subgroup analyses demonstrating that dUCB patients had numerically equivalent risks of NRM as the overall UCB cohort (based on HR values, though not meeting the significance threshold), and haplo recipients of PB stem cell graft had worse NRM and OS compared to MUD patients receiving PB graft (numerically higher HRs, though not statistically significant) suggest that differences in CD34+ cell doses are likely not the primary reason for differences observed in the outcomes. The decrease in sample size for the subgroup analyses may have resulted in loss of statistical significance.
Conditioning regimen dosing in the study may have been based on actual body weight or adjusted ideal body weight22 and as a result, the outcomes may have been confounded by chemotherapy dose adjustments made for obese patients. In addition, obesity may have affected the choice of conditioning intensity in that obese patients may have been more likely to receive reduced intensity or non-myeloablative conditioning, thereby affecting relapse risk. Another caveat is using BMI as a surrogate for obesity, which is not the most accurate measure. Calculating BMI to assess body fat indirectly23, while convenient, has flaws- a key limitation being that BMI cannot differentiate between bone density, muscle mass, and body fat. Furthermore, BMI may not necessarily reflect age-related changes of increase in body fat, with decrease in muscle mass. The sensitivity and specificity of BMI are low23. In addition, the correlation between BMI and body fat percentage is non-linear and is different in men and women. The study did not examine the effect of obesity of the incidence and severity of GVHD and infections with the three donor types, and that can be construed as a limitation of the study. Children and adolescents (<18 years) were not included in this study, and so the results are not applicable to this patient population.
It is difficult to provide a simple explanation for the “saddle-“ or “N-shaped” survival curves with varying degrees of slopes displaying BMI-donor type interactions. We speculate that more careful patient selection may be the reason for the characteristic mortality trend in patients with BMI>35, despite having similar HCT-CI scores, but with BMI approaching the upper limit of the spectrum (45-50), the presence of comorbidities accompanying morbid obesity may have resulted in higher mortality risk. It is worth noting that mismatched unrelated donors (MMUD) were not included in the study given the traditionally inferior survival in MMUD transplant recipients with conventional GVHD prophylaxis and the small number of patients with ptCy-based GVHD prophylaxis after MMUD transplant. Matched siblings are, by far, the preferred donors, and if one is not available, then the donor is usually selected from the available unrelated and alternative donor options. So in order to reduce the bias in the study, it was logical to use MUD instead of matched sibling donor as a control group for analysis with the 2 alternative donor groups. The great majority of MUD patients received calcineurin inhibitor-based GVHD prophylaxis, and therefore, we cannot comment on the effect of obesity on MUD recipients with ptCy-based prophylaxis. We also evaluated the effect of race on outcomes after transplants using the 3 donor types: race was forced into the multivariable models, and was not found to be a significant covariate (Supplemental Table 13).
In summary, this registry study showed differential effect of obesity on survival outcomes after allogeneic HCT among haplo and CBU, as compared to MUD. It is apt to state that increased BMI does confer a greater mortality risk, albeit in a non-uniform manner, after allogeneic HCT with an alternative donor over MUD. In the absence of prospective clinical trial data, the study results support including patients’ BMI in donor selection process in patients without matched sibling donor and if the available options are matched unrelated, haploidentical related and umbilical cord blood. The choice of donor for obese patients is less clear if it is between MUD and haplo.
Supplementary Material
HIGHLIGHTS.
Increasing body mass index ≥25 (overweight and obesity) of allogeneic transplant patients adversely impacts mortality risk with umbilical cord blood and T-cell replete haploidentical related donor compared with matched unrelated donor.
Body mass index may serve as a criterion for donor selection. For patients with BMI ≥25 with no HLA-matched sibling available, matched unrelated donor may be the preferred choice, followed by haploidentical related donor.
ACKNOWLEDGMENTS (other members of the Writing Committee)
Hisham Abdel-Azim, Muhammad Bilal Abid, Vaibhav Agrawal, Mahmoud Aljurf, Medhat Askar, Joseph Bubalo, Jan Cerny, Miguel Angel Diaz, César Freytes, Robert Peter Gale, Siddhartha Ganguly, Usama Gergis, Hasan Hashem, Gerhard Hildebrandt, Mark Litzow, Hemant Murthy, Sunita Nathan, Roomi Nusrat, Attaphol Pawarode, Marjolein van der Poel, David Rizzieri, Sachiko Seo, Amir Steinberg, John L. Wagner, Basem M. William, Baldeep Work
Disclosure of Conflicts of Interest:
Dr. Metheny reports grants or contracts from Pfizer; Consulting fees from Gamida Cell; Payment or honoraria from Incyte, Takeda, Tiaho; and Participation on a Data Safety Monitoring Board or Advisory Board from Gamida Cell.
Dr. de Lima reports grants from Pfizer, grants from Celgene, personal fees from Kadmon, personal fees from Pfizer, personal fees from Incyte, personal fees from BMS, outside the submitted work.
Dr. Sharma reports grants or contracts from CRISPR therapeutics, Consulting fees from Spotlight Therapeutics and Medexus, Inc., Payment or Honoraria from Vindico Medical Education, and Clinical trial site PI, support paid to the institution from Novartis, CRISPR Therapeutics, and Vertex Pharmaceuticals.
Dr. Nishihori reports research support for clinical trial to the institution from Novartis, research support (drug supply) for clinical trial to the institution from Karyopharm; both outside of the current work.
Dr. Chhabra reports payment or honoraria from GSK, and research support for clinical trial to the institution from Amgen, Janssen and Sanofi.
REFERENCES
- 1.Health Effects of Overweight and Obesity in 195 Countries over 25 Years. New England Journal of Medicine 377:13–27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, et al. : Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 315:2284–2291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, et al. : Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief:1-8, 2020 [PubMed]
- 4.Weiss BM, Vogl DT, Berger NA, et al. : Trimming the fat: obesity and hematopoietic cell transplantation. Bone Marrow Transplant 48:1152–60, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Doney K, McMillen K, Buono L, et al. : Impact of Body Mass Index on Outcomes of Hematopoietic Stem Cell Transplantation in Adults. Biol Blood Marrow Transplant 25:613–620, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Fleming DR, Rayens MK, Garrison J: Impact of obesity on allogeneic stem cell transplant patients: a matched case-controlled study. Am J Med 102:265–8, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Lin S, Luo Y, et al. : Obesity is correlated with poor outcome after allogeneic hematopoietic stem cell transplantation in patients with acute leukemia. Jpn J Clin Oncol 50:889–896, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Voshtina E, Szabo A, Hamadani M, et al. : Impact of Obesity on Clinical Outcomes of Elderly Patients Undergoing Allogeneic Hematopoietic Cell Transplantation for Myeloid Malignancies. Biology of Blood and Marrow Transplantation 25:e33–e38, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Scheich S, Enßle JC, Mücke VT, et al. : Obesity is associated with an impaired survival in lymphoma patients undergoing autologous stem cell transplantation. PLoS One 14:e0225035, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meloni G, Proia A, Capria S, et al. : Obesity and autologous stem cell transplantation in acute myeloid leukemia. Bone Marrow Transplantation 28:365–367, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Tarella C, Caracciolo D, Gavarotti P, et al. : Overweight as an adverse prognostic factor for non-Hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft. Bone Marrow Transplantation 26:1185–1191, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Navarro WH, Agovi M-A, Logan BR, et al. : Obesity Does Not Preclude Safe and Effective Myeloablative Hematopoietic Cell Transplantation (HCT) for Acute Myelogenous Leukemia (AML) in Adults. Biology of Blood and Marrow Transplantation 16:1442–1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker CC, Agovi MA, Logan B, et al. : Childhood obesity and outcomes after bone marrow transplantation for patients with severe aplastic anemia. Biol Blood Marrow Transplant 17:737–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuji S, Kim SW, Yoshimura K, et al. : Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant 15:73–82, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–2919, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeg HJ, Seidel K, Bruemmer B, et al. : Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant 15:461–8, 1995 [PubMed] [Google Scholar]
- 17.D’Souza A, Fretham C, Lee SJ, et al. : Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 26:e177–e182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894:i–xii, 1-253, 2000 [PubMed] [Google Scholar]
- 19. [Accessed July 26, 2021]. https://www.cdc.gov/obesity/adult/defining.html.
- 20.Scaradavou A, Brunstein CG, Eapen M, et al. : Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood 121:752–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri A, Sanz G, Bittencourt H, et al. : Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia 28:779–86, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Bubalo J, Carpenter PA, Majhail N, et al. : Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant 20:600–16, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Rothman KJ: BMI-related errors in the measurement of obesity. Int J Obes (Lond) 32 Suppl 3:S56–9, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Westfall PH, and Young SS. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. New York: John Wiley & Sons, 1993. [Google Scholar]
- 25.Westfall PH. Multiple Testing of General Contrasts Using Logical Constraints and Correlations. Journal of the American Statistical Association 92:299–306, 1997 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


