Abstract
Increasing proton concentration in the environment represents a potentially lethal stress for single-celled microorganisms. To survive in an acidifying environment, the foodborne pathogen Listeria monocytogenes quickly activates the alternative sigma factor B (σB), resulting in upregulation of the general stress response (GSR) regulon. Activation of σB is regulated by the stressosome, a multi-protein sensory complex involved in stress detection and signal transduction. In this study, we used L. monocytogenes strains harbouring two stressosome mutants to investigate the role of this complex in triggering expression of known amino acid-based resistance mechanisms in response to low pH. We found that expression of glutamate decarboxylase (gadD3) and arginine and agmatine deiminases (arcA and aguA1, respectively) were upregulated upon acid shock (pH 5 for 15 min) in a stressosome-dependent manner. In contrast, transcription of the arg operons (argGH and argCJBDF), which encode enzymes for the l-arginine biosynthesis pathway, were upregulated upon acid shock in a stressosome-independent manner. Finally, we found that transcription of argR, which encodes a transcriptional regulator of the arc and arg operons, was largely unaffected by acidic shock. Thus, our findings suggest that the stressosome plays a role in activating amino acid-based pH homeostatic mechanisms in L. monocytogenes . Additionally, we show that genes encoding the l-arginine biosynthesis pathway are highly upregulated under acidic conditions, suggesting that intracellular arginine can help withstand environmental acidification in this pathogen.
Keywords: acid adaptation, general stress response, kinase RsbT, Listeria monocytogenes, RsbR1, sigB, stressosome
Introduction
The foodborne pathogen Listeria monocytogenes , the aetiological agent of listeriosis, is a robust bacterium capable of surviving in harsh environments including the extremely low pH of the human stomach [1, 2]. L. monocytogenes senses acidification of the environment through a multi-protein complex designated as the stressosome, composed of putative sensory proteins RsbR1 and its four paralogues, the scaffold protein RsbS and the serine–threonine kinase RsbT, which is responsible for the phosphorylation of RsbR1 and RsbS [3, 4]. Under stressful conditions, the stressosome activates a signal cascade that ultimately releases the alternative sigma factor B (σB) from an anti-sigma factor, culminating in the upregulation of approximately 300 genes that comprise the general stress response (GSR) regulon. A range of homeostatic and protective mechanisms are encoded by the GSR regulon that are responsible for enhancing resistance to lethal stresses, including extreme acidic conditions [5–8]. The σB regulon also encompasses some virulence factors of L. monocytogenes, such as the internalins inlA and inlB [9–12], and mutants lacking σB (ΔsigB) exhibit attenuated virulence in intragastrically inoculated guinea pigs [12, 13].
It is well known that L. monocytogenes can increase acid tolerance in response to sub-lethal acid exposure, a response known as the adaptive acid tolerance response (ATR) [14, 15]. σB probably contributes to this response since it is activated at the same low pH values that trigger the ATR, although some researchers have suggested that it is not the main regulator controlling the response [16]. In a recent study, we demonstrated the pivotal role of the stressosome in the sensing of low pH and the subsequent activation of σB [3]. Pre-treating mid-log phase cultures at pH 5 for 15 min increased the transcription of highly σB-dependent genes lmo2230 and lmo0596, and enhanced L. monocytogenes acid tolerance in a stressosome-dependent manner. The genes lmo2230 and lmo0596 encode a putative arsenate reductase and a transmembrane protein with unknown function, respectively [3]. It is currently unknown whether the stressosome is required for upregulation of the amino acid-based acid resistance mechanisms in response to acidification of the medium, although σB is known to play a role in regulating some elements of this system, including the glutamate decarboxylase (GAD) system [17] and the arginine deiminase (ADI) system [18] (Fig. 1).
Fig. 1.
σB and ArgR regulatory network over the GAD, ADI and AgDI systems. Schematic representation of the regulatory functions of the stressosome, σB and the transcriptional regulator ArgR, the metabolic pathways glutamate decarboxylase, arginine and agmatine deiminase systems and l-arginine biosynthesis pathway in L. monocytogenes . The enzymes depicted in green (GadD3, ArcA and AguA1) correspond to the genes gadD3, arcA and aguA1, respectively, analysed in this study by RT-qPCR. Red and blue arrows represent gene upregulation while dashed blue arrows represent gene repression in stationary phase grown cells identified in other studies. *The newly formed ammonia (NH3) reacts with protons (H+) forming ammonium (NH4 +), which neutralizes the cytosolic pH.
The L. monocytogenes GAD system plays a critical role in acid tolerance by consuming protons (H+) through the decarboxylation of l-glutamate into γ-aminobutyrate (GABA) [19–23]. This system comprises two glutamate/GABA antiporters, GadT1 (Lmo0448) and GadT2 (Lmo2362), and three glutamate decarboxylases, GadD1 (Lmo0448), GadD2 (Lmo2363) and GadD3 (Lmo2434), of which only GadD3 is known to be σB-dependent [24]. Similarly, the ADI system contributes to L. monocytogenes acid tolerance by metabolizing l-arginine into citrulline and ammonia (NH3), which serves to buffer the cytoplasmic pH [21, 25, 26]. Citrulline is further metabolized to ornithine and carbamoyl-phosphate and the latter is subsequently metabolized to ATP, CO2 and NH3. The ADI system is also induced by σB and comprises the arginine/ornithine antiporter ArcD (Lmo0037, also known as AguD), catabolic ornithine carbamoyltransferase ArcB (Lmo0036, also known as AguB), the carbamate kinase ArcC (Lmo0039, also known as AguC) and the arginine deiminase ArcA (Lmo0043) [18]. An additional acid tolerance mechanism, the agmatine deiminase (AgDI) system, was identified in L. monocytogenes and like the GAD and ADI systems, AgDI plays a role in acid tolerance [21, 25, 27–30] and is upregulated at pH 5 [18, 28]. Except for the agmatine deiminase AguA1 (Lmo0038), which metabolizes agmatine into carbamoyl-putrescine, the components of ADI are shared with AgDI, known as either arc or agu genes [29]. While the stressosome is known to transduce acid signals [3], thus far its role in regulating these amino acid-based resistance mechanisms has not been studied.
In addition to σB, the ADI system is regulated by the transcriptional regulator ArgR [18, 29]. ArgR (homologous to AhrC in Bacillus subtilis ) consists of a DNA binding transcriptional activator of the ADI system and a repressor of l-arginine biosynthesis in several bacterial species [31–36]. This regulator is implicated in the acid tolerance of L. monocytogenes [18, 29]. In the presence of l-arginine, ArgR suppresses the transcription of two operons encoding the arginine biosynthesis pathway, the argCJBDF operon (lmo1591–lmo1587, respectively) and argGH (lmo2090 and lmo2091, respectively) [18, 29]. Interestingly, Cheng and colleagues demonstrated that ArgR also binds to the promoter region of rsbV at the rsbVW-sigB-rsbX operon and suppresses the transcription of sigB in the absence of arginine [29]. However, this regulatory effect is likely to be minor, with the partner-switching pathway regulated by the stressosome playing the dominant role in controlling activity of σB. ArgR is implicated in acid tolerance as an ΔargR strain exhibits increased acid tolerance 90 min after the onset of stress (pH 3.5) [29]. In addition, argR is upregulated by σB in cultures grown to stationary phase [18]. To our knowledge, it is currently unknown whether σB influences the transcription of argR under conditions of mild acid stress.
In this study, we aimed to extend current knowledge of the role of the stressosome in regulating expression of the acid tolerance mechanisms such as GAD, ADI and AgDI in L. monocytogenes . Here, we analysed the transcriptional response to acidification of the medium of the genes gadD3, arcA and aguA1, integral components of the GAD, ADI and AgDI systems, as well as the argR, argC and argG genes. We found that a σB knockout deletion strain (ΔsigB) and RsbTN49A, a stressosome inactive strain unable to phosphorylate RsbR1 and RsbS, were unable to upregulate gadD3, arcA or aguA1. Our data show a critical role for the stressosome in regulation of the amino acid-based pH homeostatic mechanisms employed by L. monocytogenes to withstand the detrimental effects of acidification of the environment.
Methods
Bacterial strains and primers
L. monocytogenes EGD-e (serovar 1/2 a), isogenic mutant strains and primers used in this study are listed in Table 1. Strains were grown in BHI broth (LabM) at 37 °C with constant shaking at 150 rpm at initial neutral pH of ~7.4.
Table 1.
Strains and primers used in this study
|
Strain/mutant |
Source |
|---|---|
|
Listeria monocytogenes EGD-e |
K. Boor |
|
L. monocytogenes EGD-e ΔsigB |
[47] |
|
L. monocytogenes EGD-e RsbTN49A |
[4] |
|
L. monocytogenes EGD-e RsbLC56A; ΔrsbR2; ΔrsbR3; ΔrsbR4 |
[4] |
|
Primer (5′−3′) |
Target |
|
TGGGGAGCAAACAGGATTAG |
16S_F |
|
TAAGGTTCTTCGCGTTGCTT |
16S_R |
|
GAAACGCTCGAGAAAAATGC |
gadD3_F |
|
AGTTTGGTCGTTTTGCCTGT |
gadD3_R |
|
GGTCGCAAATTAGAAGTGCATAA |
aguA1_F |
|
GGATCCCCAAATAGCGGAAAA |
aguA1_R |
|
GGCGGAGAAGATGTAATTGTTTC |
arcA_F |
|
CCCGCACTTCTTAACAGATCG |
arcA_R |
|
CCCACATCAAAAACTAAAACGCG |
argR_F |
|
GGCCAGTCCAAGTTATCGATTAA |
argR_R |
|
CCTTTGTTCGTGAAGTGGCA |
argG_F |
|
CCTTTAAATAATTTGACGCGGATGG |
argG_R |
|
CGCCCCTTTGACTAAATTATCAAT |
argC_F |
|
CCGAATCCAACCAGAGAATGTATA |
argC_R |
GAD, glutamate decarboxylase.
Acid shock treatment in L. monocytogenes
L. monocytogenes strains were grown to stationary phase cultures at 37 °C for 16 h followed by dilution to an initial OD600 nm of 0.05 in fresh BHI. Cultures were allowed to grow at 37 °C to the mid-log phase (OD600 nm of 0.4). Acid shock-treated cultures were made by adding 5 M HCl until pH 5 was reached. Treated (+) and untreated (-) cultures were incubated for a further 15 min at 37 °C. Three independent biological replicates were made.
RNA extraction and RT-qPCR
To stop transcription, cultures were diluted in RNAlater (Sigma) at a 1 : 5 ratio. The total RNA was extracted using an RNeasy Minikit (Qiagen) according to the manufacturer’s recommendations. Cells were disrupted by bead beating twice using the FastPrep-24 (MP Biomedicals) at a speed of 6 m s−1 for 40 s. DNA was digested with Turbo DNA-free (Invitrogen) according to the manufacturer’s recommendations. RNA integrity was verified by electrophoresis in 0.7 % (w/v) agarose gels. Synthesis of cDNA was performed with a SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s recommendations. cDNA was quantified using a NanoDrop 2000c (Thermo Scientific) and diluted to a final concentration of 7 ng ml−1. Real-time quantitative PCR (RT-qPCR) was performed using the QuantiTect SYBR Green PCR kit (Qiagen) and pair of primers for the target genes (Table 1). Primer efficiency for 16S, gadD3, arcA, aguA1, argR, argG and argC were previously determined using cDNA [3]. Samples were analysed on the LightCycler 480 system (Roche) with the following parameters: 95 °C for 15 min; 45 cycles of 15 s at 95 °C, 15 s at 53 °C and 30 s at 72 °C; a melting curve drawn for 5 s at 95 °C and 1 min at 55 °C, followed by increases of 0.11 °C s−1 until 95 °C was reached; and cooling for 30 s at 40 °C. Cycle quantification values were calculated by using LightCycler 480 software version 1.5.1 (Roche) and the Pfaffl relative expression formula [37, 38]. Expression of 16S rRNA was used as a reference gene. Expression of the 16S rRNA gene remained stable and unresponsive towards the acid shock treatment in all strains and biological replicates. Results are expressed as Log2 relative expression ratios normalized against average expression of the L. monocytogenes wild-type (WT) strain in the absence of stress.
Statistical analysis
All statistical analyses were performed by conducting unpaired Student’s t-tests with GraphPad Prism 8. All analyses were made by comparing each strain with the untreated L. monocytogenes WT strain. P values of <0.05 (*), <0.01 (**) and <0.001 (***) were considered statistically significant.
Results and discussion
Expression of gadD3, arcA and aguA1 is stressosome-dependent under mild acidic conditions
In this study, we aimed to assess the impact of the stressosome on the regulation of amino acid-based acid tolerance mechanisms in L. monocytogenes . First, we analysed transcription of three genes (gadD3, arcA and aguA1) which are integral parts of the GAD, ADI and AgDI systems, respectively (Fig. 1), in several L. monocytogenes mutant strains grown to mid-log phase and then exposed to mild acidic conditions (see Methods). One strain, designated ‘RsbR1-only’, possesses only RsbR1 while the remaining RsbR paralogues were genetically deleted or inactivated [4]. Strain RsbTN49A harbours a single codon substitution in rsbT that inactivates its kinase activity [4]. Transcription of gadD3, arcA and aguA1 genes was upregulated (~5.6 log2- and ~6.1 log2-fold increase for gadD3 and arcA, respectively, and ~3.4 log2-fold increase for aguA1, P<0.05) after the acid shock treatment in both the WT and the RsbR1-only strains (Fig. 2a–c). In the ΔsigB and RsbTN49A strains, transcript levels of gadD3 and aguA1 were not increased in response to the acid pretreatment in comparison with the treated WT strain (P<0.001). However, a small but significant increase was observed for the arcA transcript in response to acid in the RsbTN49A strain (P<0.05), albeit still well below the level detected in the WT strain. The diminished transcriptional activation of these genes was correlated with the inability to activate σB via the stressosome, demonstrating that acidic conditions promote the upregulation of gadD3, arcA and aguA1 in a stressosome-dependent manner. σB is crucial for the survival of this bacterium in acidic environments such as the extremely low pH of the human stomach [39–41]. Previous studies found that transcription of gadD3 and arcA is upregulated under mild acidic pH [11, 18]. In addition, Ryan and colleagues identified putative σB promoters upstream of several genes that comprise the ADI system [18]. However, little was known about the stressosome-mediated activation of σB and its influence over the transcription of the ADI, AgDI and GAD systems under the same conditions. Our results demonstrate the crucial role of the putative acid sensor RsbR1 and the kinase RsbT, components of the stressosome, in the regulation of these pH homeostatic mechanisms in L. monocytogenes . It seems plausible to speculate that this transcriptional upregulation may also increase activity of the GAD, ADI and AgDI systems, but future studies are needed to corroborate these assumptions.
Fig. 2.
The GAD, ADI and AgDI systems are upregulated by the stressosome and σB under low pH stress. Mid-log phase cultures (OD600 nm of 0.4) grown at 37°C of L. monocytogenes EGD-e wild type, ΔsigB, RsbR1-only and RsbTN49A were non-treated (-) and treated (+) at pH 5 for 15 min and the expression of (a) gadD3, (b) arcA and (c) aguA1 was measured by RT-qPCR. Three independent biological replicates were made. Transcript levels shown for each gene are expressed relative to the average of those detected in the untreated wild-type strain. Error bars represent sd. Statistical analysis was performed using an unpaired Student t-test. Coloured asterisks represent differences relative to the wild-type untreated (-). Black asterisks represent the indicated paired comparisons (*P<0.05; **, P<0.01; ***P<0.001).
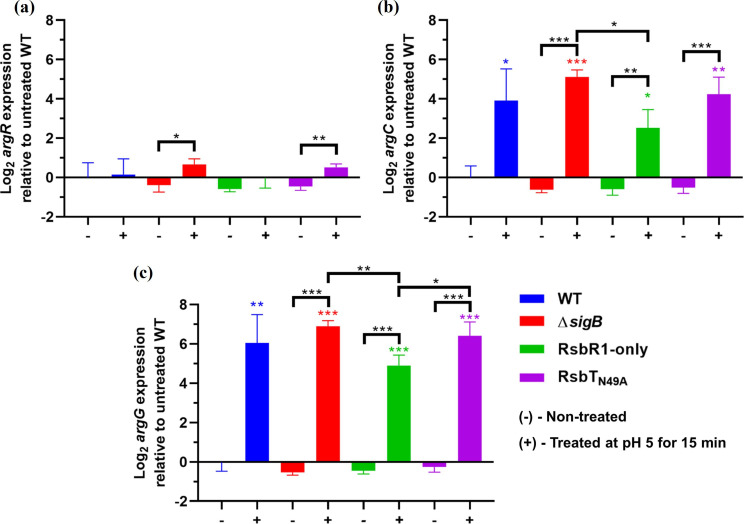
Transcription of argR is unaffected by acid stress in mid-log phase culture
Similar to σB, ArgR is also required for expression of the ADI system at both neutral and acidic conditions and is strongly upregulated at low pH (5.0–5.5), under anaerobic conditions and in stationary phase grown cells [18, 29]. Furthermore, Ryan and colleagues identified a putative σB promoter upstream of argR [18]. In this study, we aimed to further assess the influence of the stressosome on the transcription of ArgR in mildly acidic conditions. Our results showed no substantial changes in argR transcription in mid-log phase cultures treated with acidic shock in either WT or RsbR1-only strains (Fig. 3a). However, a small but significant increase (~0.6 log2-fold increase, P<0.05) was detected in both ΔsigB and RsbTN49A strains (Fig. 3a). Although increased argR expression in these two mutant strains was unexpected, it is perhaps not surprising that regulators, other than σB, control the transcription of argR during the mid-log phase under stressful conditions and that this control can occur in the absence of σB. Interestingly, anaerobic growth conditions increase the transcription of argR [18, 42], which contrasts with the aerobic growth conditions used in our study and conceivably explains the absence of upregulation of the argR under acidic conditions. ArgR and σB may work in concert to upregulate the ADI and AgDI systems, as Ryan and colleagues observed a downregulation of arcA in both ΔsigB and ΔargR strains [18].
Fig. 3.
The argC and argG genes are upregulated independently of the stressosome and σB under low pH stress. Mid-log phase cultures (OD600 nm of 0.4) grown at 37°C of L. monocytogenes EGD-e wild type, ΔsigB, RsbR1-only and RsbTN49A were non-treated (-) and treated (+) at pH 5 for 15 min and the expression of (a) argR, (b) argC and (c) argG was measured by RT-qPCR. Three independent biological replicates were made. Transcript levels shown for each gene are expressed relative to the average of those detected in the untreated wild-type strain. Error bars represent sd. Statistical analysis was performed using an unpaired Student t-test. Coloured asterisks represent differences relative to the wild-type untreated (-). Black asterisks represent the indicated paired comparisons (*P<0.05; **P<0.01; ***P<0.001).
The l-arginine biosynthesis genes argC and argG are upregulated under mild acid stress independently of the stressosome
In this study, we aimed to evaluate the role of the stressosome under mild acidic conditions on transcription of the first genes of the arg biosynthetic operons, argC and argG. We found that transcription of both genes was highly upregulated (~3.9 log2- and ~6.1 log2-fold increase, respectively) with acid shock treatment (Fig. 3b, c). The RsbR1-only strain showed slightly lower argC and argG transcription following acidification (2.5 log2- and 5.0 log2-fold increase in argC and argG, respectively) compared with the other strains, and these differences were significant when compared to the ΔsigB (P<0.05 in argC and P<0.01 in argG) and the RsbTN49A (P<0.05 in argG) strains. Ryan and colleagues observed increased transcription of argG in stationary phase cells treated at pH 5, although an increase in transcription of the negative regulator argR was also observed under the same conditions [18]. As ArgR activity is post-translationally regulated by l-arginine, it has been suggested that the repressing action of ArgR is possibly removed following acidification through depletion of the cytoplasmic l-arginine pool [43]. Whether the upregulation of argC and argG contributes to the acid tolerance in L. monocytogenes is still unknown and future studies are needed to assess this question. In Escherichia coli , the importation of extracellular arginine contributes to extreme acid tolerance by providing l-arginine for the synthesis of agmatine (reviewed in [44]). In L. monocytogenes , the arginine ABC-transporter gene, arpJ [45] (encoded by lmo2250), was transcriptionally upregulated under acidic conditions [11], suggesting increased import of l-arginine in response to acidification of the medium. Additionally, the acid inducible arginine decarboxylase AdiA, responsible for the conversion of arginine to agmatine, is essential for arginine-dependent acid resistance in E. coli [46]. L. monocytogenes possesses a putative adiA homologue (encoded by lmo2694), but its regulation and function have not been characterized in this bacterium. Together, our results show that expression of the argC and argG genes is upregulated in response to acidification of the medium and independently of the stressosome in mid-log phase grown cells.
Overall, our results show that sensing of pH-related signals by the stressosome is required for upregulation of the GAD, ADI and AgDI systems under mild acidic pH (Fig. 1). We found that for upregulation of gadD3, arcA and aguA1 to occur, a functional stressosome with at least RsbR1 is necessary and that the acid-induction of these genes is highly σB-dependent. The increased expression of these systems is probably followed by the increased consumption of protons at the expense of amino acids, culminating in an enhanced tolerance of L. monocytogenes to extremely low pH. The arginine biosynthetic genes argC and argG were upregulated upon acid shock in a stressosome-independent manner, but their role in the adaptability of L. monocytogenes to acid remains unknown. Additionally, the role of ArgR in the upregulation of these genes is unclear; whether an alleviation of the repression over argC and argG due to a decreased level of l-arginine or perhaps due to a decrease in the cytosolic pH remains to be elucidated. Future studies will be required to assess the impact of the stressosome sensing function on the activity of GAD, ADI and AgDI as well as l-arginine biosynthesis, in post-stress environments by assessing the pools of l-arginine in the cells under mild pH stress.
Funding information
This work was supported by funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 721456 and by the Irish Higher Education Authority under the COVID-19 Costed Extension Fund.
Acknowledgements
We are grateful to colleagues on the PATHSENSE Training Network (www.pathsense.eu/) and the EuroMicropH COST Action (CA18113) for helpful discussions during preparation of the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ADI, arginine deiminase; AgDI, agmatine deiminase; ATR, acid tolerance response; GABA, gamma aminobutyric acid; GAD, glutamate decarboxylase; GSR, general stress response.
References
- 1.Barbuddhe SB, Chakraborty T. Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol. 2009;337:173–195. doi: 10.1007/978-3-642-01846-6_6. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Suárez JV, Ortiz S, López-Alonso V. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front Microbiol. 2016;7:638. doi: 10.3389/fmicb.2016.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerreiro DN, Pucciarelli MG, Tiensuu T, Gudynaite D, Boyd A, et al. Acid stress signals are integrated into the σB-dependent general stress response pathway via the stressosome in the food-borne pathogen Listeria monocytogenes . PLOS Pathog. 2022;18:e1010213. doi: 10.1371/journal.ppat.1010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dessaux C, Guerreiro DN, Pucciarelli MG, O’Byrne CP, García-Del Portillo F. Impact of osmotic stress on the phosphorylation and subcellular location of Listeria monocytogenes stressosome proteins. Sci Rep. 2020;10:20837. doi: 10.1038/s41598-020-77738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arcari T, Feger ML, Guerreiro DN, Wu J, O’Byrne CP. Comparative review of the responses of Listeria monocytogenes and Escherichia coli to low pH stress. Genes. 2020;11:1330. doi: 10.3390/genes11111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerreiro DN, Arcari T, O’Byrne CP. The σB-mediated general stress response of listeria monocytogenes: life and death decision making in a pathogen. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund PA, De Biase D, Liran O, Scheler O, Mira NP, et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front Microbiol. 2020;11:556140. doi: 10.3389/fmicb.2020.556140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakicevic BZ, Den Besten HMW, De Biase D. Landscape of stress response and virulence genes among Listeria monocytogenes strains. Front Microbiol. 2021;12:738470. doi: 10.3389/fmicb.2021.738470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturongakul S, Raengpradub S, Palmer ME, Bergholz TM, Orsi RH, et al. Transcriptomic and phenotypic analyses identify coregulated, overlapping regulons among PrfA, CtsR, HrcA, and the alternative sigma factors sigmaB, sigmaC, sigmaH, and sigmaL in Listeria monocytogenes . Appl Environ Microbiol. 2011;77:187–200. doi: 10.1128/AEM.00952-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte MP, Petrone G, Di Biase AM, Ammendolia MG, Superti F, et al. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb Pathog. 2000;29:137–144. doi: 10.1006/mpat.2000.0379. [DOI] [PubMed] [Google Scholar]
- 11.Neuhaus K, Satorhelyi P, Schauer K, Scherer S, Fuchs TM. Acid shock of Listeria monocytogenes at low environmental temperatures induces prfA, epithelial cell invasion, and lethality towards Caenorhabditis elegans. BMC Genomics. 2013;14:285. doi: 10.1186/1471-2164-14-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver HF, Orsi RH, Wiedmann M, Boor KJ. Listeria monocytogenes {sigma}B has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl Environ Microbiol. 2010;76:4216–4232. doi: 10.1128/AEM.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner MR, Njaa BL, Wiedmann M, Boor KJ. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect Immun. 2006;74:876–886. doi: 10.1128/IAI.74.2.876-886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MJ, Coote PJ, O’Byrne CP. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology. 1996;142:2975–2982. doi: 10.1099/13500872-142-10-2975. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll B, Gahan CG, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira A, Sue D, O’Byrne CP, Boor KJ. Role of Listeria monocytogenes sigma(B) in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl Environ Microbiol. 2003;69:2692–2698. doi: 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wemekamp-Kamphuis HH, Wouters JA, de Leeuw PPLA, Hain T, Chakraborty T, et al. Identification of sigma factor sigma B-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl Environ Microbiol. 2004;70:3457–3466. doi: 10.1128/AEM.70.6.3457-3466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan S, Begley M, Gahan CGM, Hill C. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol. 2009;11:432–445. doi: 10.1111/j.1462-2920.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 19.Cotter PD, Gahan CGM, Hill C. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol Microbiol. 2001;40:465–475. doi: 10.1046/j.1365-2958.2001.02398.x. [DOI] [PubMed] [Google Scholar]
- 20.Cotter PD, Ryan S, Gahan CGM, Hill C. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl Environ Microbiol. 2005;71:2832–2839. doi: 10.1128/AEM.71.6.2832-2839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang C, Fang X, Chen X, Wang C, Liang X, et al. Evaluating the contribution of acid resistance systems and probing the different roles of the glutamate decarboxylases of listeria monocytogenes under acidic conditions. Kafkas Univ Vet Fak Derg. 2020;26:231–238. [Google Scholar]
- 22.Karatzas K-AG, Brennan O, Heavin S, Morrissey J, O’Byrne CP. Intracellular accumulation of high levels of gamma-aminobutyrate by Listeria monocytogenes 10403S in response to low pH: uncoupling of gamma-aminobutyrate synthesis from efflux in a chemically defined medium. Appl Environ Microbiol. 2010;76:3529–3537. doi: 10.1128/AEM.03063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karatzas K-AG, Suur L, O’Byrne CP. Characterization of the intracellular glutamate decarboxylase system: analysis of its function, transcription, and role in the acid resistance of various strains of Listeria monocytogenes . Appl Environ Microbiol. 2012;78:3571–3579. doi: 10.1128/AEM.00227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. Listeria monocytogenes sigma B regulates stress response and virulence functions. J Bacteriol. 2003;185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C, Chen J, Shan Y, Fang C, Liu Y, et al. Listeria monocytogenes ArcA contributes to acid tolerance. J Med Microbiol. 2013;62:813–821. doi: 10.1099/jmm.0.055145-0. [DOI] [PubMed] [Google Scholar]
- 26.Lund P, Tramonti A, De Biase D. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev. 2014;38:1091–1125. doi: 10.1111/1574-6976.12076. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Cheng C, Xia Y, Zhao H, Fang C, et al. Lmo0036, an ornithine and putrescine carbamoyltransferase in Listeria monocytogenes, participates in arginine deiminase and agmatine deiminase pathways and mediates acid tolerance. Microbiology. 2014;157:3150–3161. doi: 10.1099/mic.0.049619-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, Chen J, Fang C, Xia Y, Shan Y, et al. Listeria monocytogenes aguA1, but Not aguA2, Encodes a Functional Agmatine Deiminase. J Biol Chem. 2013;288:26606–26615. doi: 10.1074/jbc.M113.477380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng C, Dong Z, Han X, Sun J, Wang H, et al. Listeria monocytogenes 10403S arginine repressor argR finely tunes arginine metabolism regulation under acidic conditions. Front Microbiol. 2017;8:145. doi: 10.3389/fmicb.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares CA, Knuckley B. Mechanistic studies of the agmatine deiminase from Listeria monocytogenes . Biochem J. 2016;473:1553–1561. doi: 10.1042/BCJ20160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czaplewski LG, North AK, Smith MCM, Baumberg S, Stockley PG. Purification and initial characterization of AhrC: the regulator of arginine metabolism genes in Bacillus subtilis . Mol Microbiol. 1992;6:267–275. doi: 10.1111/j.1365-2958.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 32.Fulde M, Willenborg J, de Greeff A, Benga L, Smith HE, et al. ArgR is an essential local transcriptional regulator of the arcABC operon in sStreptococcus suis and is crucial for biological fitness in an acidic environment. Microbiology. 2011;157:572–582. doi: 10.1099/mic.0.043067-0. [DOI] [PubMed] [Google Scholar]
- 33.Larsen R, Buist G, Kuipers OP, Kok J. ArgR and AhrC are both required for regulation of arginine metabolism in Lactococcus lactis . J Bacteriol. 2004;186:1147–1157. doi: 10.1128/JB.186.4.1147-1157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim DB, Oppenheim JD, Eckhardt T, Maas WK. Nucleotide sequence of the argR gene of Escherichia coli K-12 and isolation of its product, the arginine repressor. Proc Natl Acad Sci. 1987;84:6697–6701. doi: 10.1073/pnas.84.19.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CD, Winteler H, Abdelal A, Haas D. The ArgR regulatory protein, a helper to the anaerobic regulator ANR during transcriptional activation of the arcD promoter in Pseudomonas aeruginosa. J Bacteriol. 1999;181:2459–2464. doi: 10.1128/JB.181.8.2459-2464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maghnouj A, de Sousa Cabral TF, Stalon V, Vander Wauven C. The arcABDC gene cluster, encoding the arginine deiminase pathway of Bacillus licheniformis, and its activation by the arginine repressor argR. J Bacteriol. 1998;180:6468–6475. doi: 10.1128/JB.180.24.6468-6475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaffl MW, Georgieva TM, Georgiev IP, Ontsouka E, Hageleit M, et al. Real-time RT-PCR quantification of insulin-like growth factor (IGF)-1, IGF-1 receptor, IGF-2, IGF-2 receptor, insulin receptor, growth hormone receptor, IGF-binding proteins 1, 2 and 3 in the bovine species. Domest Anim Endocrinol. 2002;22:91–102. doi: 10.1016/s0739-7240(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 39.Davis ML, Ricke SC, Donaldson JR. Establishment of Listeria monocytogenes in the Gastrointestinal Tract. Microorganisms. 2019;7:E75. doi: 10.3390/microorganisms7030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi M, Chikindas ML. Listeria: A foodborne pathogen that knows how to survive. Int J Food Microbiol. 2007;113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. General stress transcription factor sigmaB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/JB.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowman JP, Hages E, Nilsson RE, Kocharunchitt C, Ross T. Investigation of the Listeria monocytogenes scott A acid tolerance response and associated physiological and phenotypic features via whole proteome analysis. J Proteome Res. 2012;11:2409–2426. doi: 10.1021/pr201137c. [DOI] [PubMed] [Google Scholar]
- 43.Garnett JA, Baumberg S, Stockley PG, Phillips SEV. A high-resolution structure of the DNA-binding domain of AhrC, the arginine repressor/activator protein from bacillus subtilis. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:914–917. doi: 10.1107/S1744309107048166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlier D, Bervoets I. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids. 2019;51:1103–1127. doi: 10.1007/s00726-019-02757-8. [DOI] [PubMed] [Google Scholar]
- 45.Klarsfeld AD, Goossens PL, Cossart P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 46.Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW. Control of acid resistance in Escherichia coli . J Bacteriol. 1999;181:3525–3535. doi: 10.1128/JB.181.11.3525-3535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerreiro DN, Wu J, Dessaux C, Oliveira AH, Tiensuu T, et al. Mild stress conditions during laboratory culture promote the proliferation of mutations that negatively affect sigma B activity in Listeria monocytogenes . J Bacteriol. 2020;202:e00751-19. doi: 10.1128/JB.00751-19. [DOI] [PMC free article] [PubMed] [Google Scholar]