Abstract
Objectives
To estimate the basic reproductive number (Ro) to help us understand and control the spread of monkeypox in immunologically naive populations.
Methods
Using three highest incidence populations including England, Portugal, and Spain as examples as of 18 June 2022, we employed the branching process with a Poisson likelihood and gamma-distributed serial interval to fit daily reported case data of monkeypox to estimate Ro. Sensitivity analyses were performed by varying mean serial interval from 6.8 to 12.8 days.
Results
The median posterior estimates of Ro for monkeypox in the three study populations were statistically >1 (England: Ro = 1.60 [95% (credible interval) CrI, 1.50–1.70]; Portugal: Ro = 1.40 [95% CrI, 1.20–1.60]; Spain: Ro = 1.80 [95% CrI, 1.70–2.00]). Ro estimates varied over 1.30 to 2.10, depending on the serial interval.
Discussion
The updated Ro estimates across different populations will inform policy makers' plans for public health control measures. Currently, monkeypox has a sustainable outbreak potential and may challenge healthcare systems, mainly due to declines in the population level immunity to Orthopoxviruses since the cessation of routine smallpox vaccination. Smallpox vaccination has been shown to be effective in protecting (≤85% effectiveness) against monkeypox infection in earlier times. So early postexposure vaccination is currently being offered in an attempt to control its spread.
Keywords: Basic reproductive number, Branching process, Epidemic, Monkeypox, Outbreak
Introduction
Monkeypox is caused by the monkeypox virus, a member of the Orthopoxvirus genus, in the Poxvirus family of enveloped, double-stranded DNA viruses (genome size ∼200 kilobases). Other members of this family include smallpox, camelpox, and cowpox. After an incubation period of 10 to 14 days (range, 5–21), a 1 to 3 day flu-like prodrome occurs, consisting of fever, lethargy, headache, backache, sore throat, before the appearance of the rash, which is centrifugal, affecting the face and peripheries (including palms and soles) more than the trunk. All of the lesions progress at the same time through the various stages (macules, papules, vesicles, pustules, and finally scabs). Infectiousness should be assumed throughout the rash phase, which lasts 2 to 4 weeks [[1], [2], [3]].
Since the eradication of smallpox in 1980 and the cessation of routine smallpox vaccination thereafter, the potential for an increase in monkeypox cases has been monitored. Two distinct clades (strains or lineages) have since emerged—a milder West African (WA) and a more severe Central African (CA) strain [2,4].
The first reports of monkeypox cases outside endemic areas were in children and adults in the United States during 2003, originating from pet prairie dogs which had been housed with imported infected Gambian giant rats from Ghana [2,[4], [5], [6]]. More recently, an outbreak of monkeypox in Nigeria in 2017 [7] led to the importation of human monkeypox cases into various countries around the world, including the United Kingdom, Israel, and Singapore [8]. In the United Kingdom, two epidemiologically unrelated cases were imported into Cornwall and Blackpool [9], with a secondary case in a nurse that attended the Blackpool patient [10]. A few years later in 2021, a family cluster in the United Kingdom was identified—also related to the ongoing Nigerian 2017 outbreak [9,11]. All of these nonendemic cases were clinically mild, with no fatalities. The current growth in global monkeypox cases in 2022 now appears to be spreading locally in multiple countries, with most cases presenting with various combinations of the rash, lymphadenopathy, and fever, without any history of travel to any monkeypox-endemic areas themselves [12].
Here, we estimate the growth of these cases in immunologically naïve populations, where the most comprehensive, publicly available datasets are accessible.
Methods
We extracted the incidence data of monkeypox of all the affected populations from the publicly available dashboard [12]. The cut-off date for data extraction was 18 June 2022. Using the branching process with a Poisson likelihood [13,14] and γ-distributed serial interval (mean = 9.8 days and SD = 9.1 days) to fit the daily number of reported cases of top three incidence populations, median posterior estimates of basic reproductive number (Ro) with its 95% credible interval (CrI) were derived. This serial interval value was obtained by the UK Health Security Agency from 17 infector-infectee pairs with known symptom onset dates in early outbreak investigations of the WA strain using a double truncation with interval censoring [15]. Sensitivity analyses to test the impact of a range of serial interval values (within ±3 days of the baseline serial interval value) on the Ro estimation were conducted. All of the analyses were conducted by R (v4.0.5; The R Foundation for Statistical Computing, Vienna, Austria).
Results
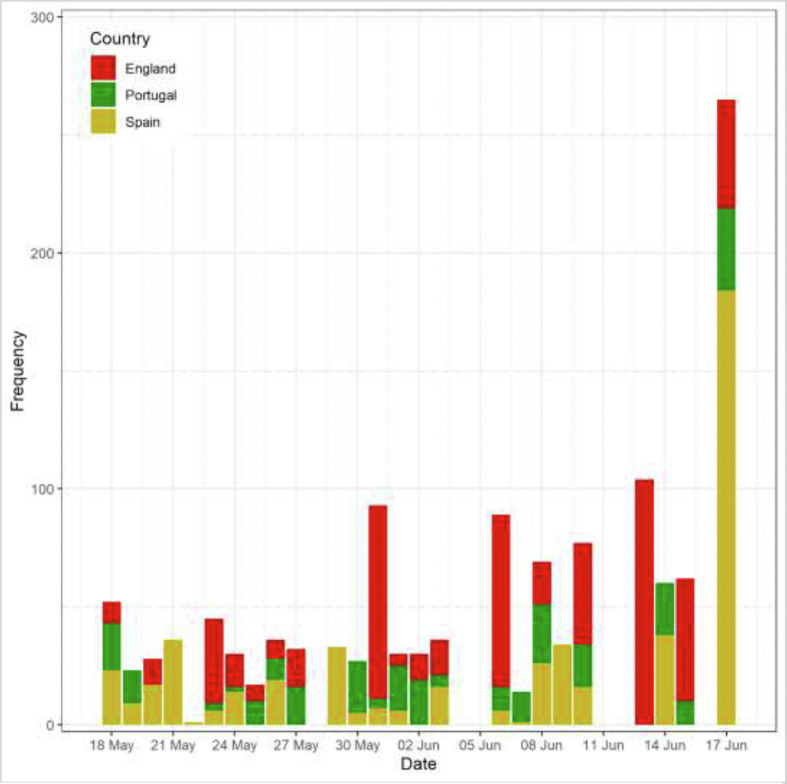
As of 18 June 2022, there were 2551 confirmed cases from 56 populations [12]. The three populations with the highest cumulative number of confirmed cases were in England (n = 550) followed by Spain (n = 497) and Portugal (n = 276) (Fig. 1 ). The Ro estimates for three study populations from 18 May and 18 June 2022 were statistically >1 (the condition that an epidemic outbreak occurs), with the estimate for Spain statistically higher than those for England and Portugal (England: Ro = 1.60 (95% CrI, 1.50–1.70); Portugal: Ro = 1.40 (1.20–1.60); Spain: Ro = 1.80 (1.70–2.00)).
Fig. 1.
Monkeypox epidemic curve of England (Red), Portugal (Green) and Spain (Yellow) as of 18 June 2022.
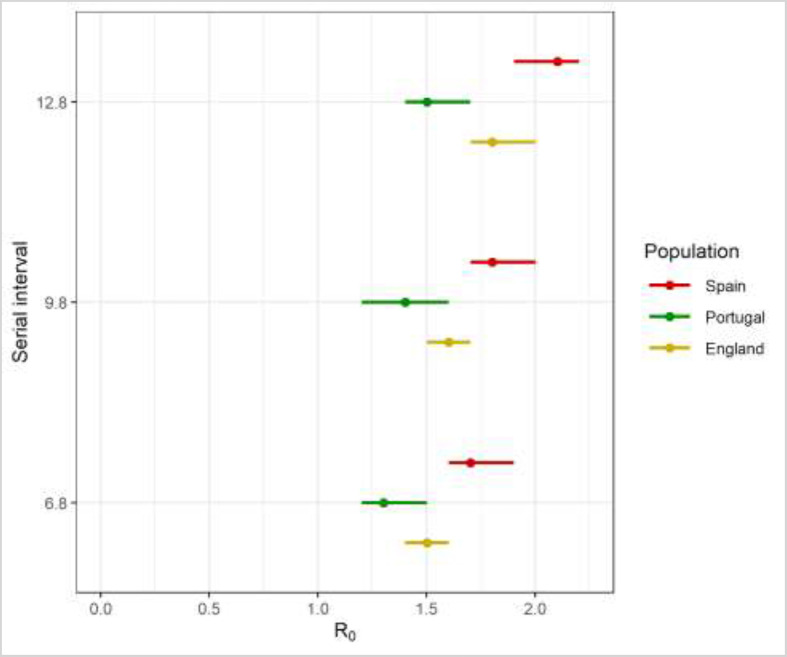
The serial interval was found to be positively correlated with the Ro estimation (Fig. 2 ). Lower estimates of Ro were obtained (England: Ro = 1.50 [95% CrI, 1.40–1.60]; Portugal: Ro = 1.30 [95% CrI, 1.20–1.50]; Spain: Ro = 1.70 [95% CrI, 1.60–1.90]) if a shorter serial interval of 6.8 days was assumed. In contrast, a longer serial interval of 12.8 days gave higher Ro estimates across these 3 study populations of: 1.8 (95% CrI, 1.70–2.00) in England; 1.50 (95% CrI, 1.40–1.70) in Portugal; and 2.10 (95% CrI, 1.90–2.20) in Spain.
Fig. 2.
Estimates of Ro for England (Yellow), Portugal (Green) and Spain (Red) as serial interval varies. Dots, median of posterior estimate; line, 95% credible interval.
Discussion
Monkeypox is assumed to be transmitted by several routes—direct contact with infected secretions and body fluids (including via intimate contact during sex), via fomites contaminated with such secretions when in contact with mucous membranes or breaks in the skin, and also by ‘respiratory droplets’ by inhalation at short-range [2], which is effectively an aerosol transmission route [1]. All of these transmission routes may be present in combination, so it is difficult to distinguish between them in any given individual exposure or outbreak scenario.
This study has provided an early estimation of disease transmissibility within mostly men who have sex with men (MSM) populations which will guide ongoing public health control measures. Our Ro estimates ranging from 1.4 to 1.8 for the current monkeypox epidemic in these three study populations were smaller than earlier estimates of 2.13 from the Democratic Republic of the Congo (CA strain) 1980-1984 epidemic [16] and the estimate of 3 to 6 for smallpox from an analysis of previous outbreaks [17]. This latter endemic Democratic Republic of the Congo monkeypox Ro estimate did not affect predominantly MSM cases, unlike the current global outbreaks, although it is known that various inter-related factors can drive rapid spread of infections in such populations [18].
There were several limitations to this Ro estimation. First, any super-spreading individual or event may skew these estimates, where early spillover infections to the at-risk populations may be different from the ongoing transmission risk to more typical at-risk populations. Second, our parsimonious Ro estimate based on homogeneous social mixing assumption may not be generalizable to all MSM populations as it depends on the level of age-specific immunity induced by previous smallpox infection or vaccination (e.g. any pre-existing immunity in older age groups). Notably, most cases reported in the current global outbreaks are <50 years of age [12]. Third, in the absence of any detailed earlier data for the serial interval for the WA monkeypox strain, we have used that estimated from an earlier outbreak report of the CA strain—although we do not expect these to differ much. Monkeypox therefore has a sustainable outbreak potential and may challenge healthcare systems, mainly due to declines in the population level immunity to Orthopoxviruses since the cessation of routine smallpox vaccination. Smallpox vaccination has been shown to be effective in protecting (up to 85% effectiveness) [19] against monkeypox infection in earlier times. So early postexposure vaccination is currently being offered in an attempt to control its spread.
Transparency declaration
None of the authors has any conflicts of interest to declare. KOK acknowledges support from Health and Medical Research Fund (reference numbers: INF-CUHK-1, 17160302, 18170312, COVID1903008, CID-CUHK-A), General Research Fund (reference numbers: 14112818, 24104920), Wellcome Trust Fund (United Kingdom, 200861/Z/16/Z), Direct Grant for Research (reference number: 2019.020) and Group Research Scheme of The Chinese University of Hong Kong.
Author's contributions
Conceptualization: KOK and JT conceptualized this research note and wrote the original draft. Formal analysis: KOK, AT, and WWI conducted formal analysis. Methodology: KOK, WWI, SYSW, and AT are responsible for the methodology. KOK and WWI collected the data. Writing - review & editing: KOK, WWI, JT, SYSW, and AT reviewed and edited the research note. SYSW and JT supervised. All authors critically assessed the final version of the submitted manuscript.
Acknowledgements
K.O. Kwok acknowledges support from Health and Medical Research Fund (reference numbers: INF-CUHK-1, 17160302, 18170312), General Research Fund (reference numbers: 14112818, 24104920), Wellcome Trust Fund (United Kingdom, 200861/Z/16/Z), and Group Research Scheme of The Chinese University of Hong Kong.
Editor: L. Leibovici
References
- 1.Nalca A., Rimoin A.W., Bavari S., Whitehouse C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 2.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2022. Monkeypox - fact sheets.https://www.who.int/news-room/fact-sheets/detail/monkeypox [updated 19 May 2022] [Google Scholar]
- 4.Berthet N., Descorps-Declere S., Besombes C., Curaudeau M., Nkili Meyong A.A., Selekon B., et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease C, Prevention Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:537–540. [PubMed] [Google Scholar]
- 6.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 7.Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S., et al. Reemergence of human monkeypox in Nigeria. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauldin M.R., McCollum A.M., Nakazawa Y.J., Mandra A., Whitehouse E.R., Davidson W., et al. Exportation of monkeypox virus from the African continent. J Infect Dis. 2022;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunasekaran M. Report of monkeypox cases in 2018 in the United Kingdom. Glob Biosecur. 2019;1 doi: 10.31646/gbio.22. [Accessed 21 May 2022] [DOI] [Google Scholar]
- 11.Hobson G., Adamson J., Adler H., Firth R., Gould S., Houlihan C., et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonio Caramia. Covid19 (UK & Scotland) | MonkeyPox data visualization by @antonio_caramia. https://www.ilpandacentrostudio.it/uk.html [Accessed 20 June 2022].
- 13.Kwok K.O., Wan In W., Huang Y., Wong A., Tang A., Wong S.Y.S. Estimation of early phase local-to-local transmissibility and importation hazard of Coronavirus Disease 2019 (COVID-19) epidemic under assorted containment measures in Hong Kong. Travel Med Infect Dis. 2022;45 doi: 10.1016/j.tmaid.2021.102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jombart T., Cori A., Nouvellet P., Skarp J. Welcome to the earlyR package! https://www.repidemicsconsortium.org/earlyR/
- 15.UK Health Security Agency . 2022. Investigation into monkeypox outbreak in England: technical briefing 1.https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1 [Accessed 20 June 2022] [Google Scholar]
- 16.Grant R., Nguyen L.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gani R., Leach S. Transmission potential of smallpox in contemporary populations. Nature. 2001;414:748–751. doi: 10.1038/414748a. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary D. The syndemic of AIDS and STDS among MSM. Linacre Q. 2014;81:12–37. doi: 10.1179/2050854913Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . 2019. Monkeypox and smallpox vaccine guidance.https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html#:∼:text=pre%2Devent%20setting.-,Vaccine%20Effectiveness,85%25%20effective%20in%20preventing%20monkeypox [updated 2019 Dec 2] [Google Scholar]