Abstract
Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a massive impact on the health sector, especially in patients with pre-existing comorbidities. This study aims to define the predictor factors for worse outcomes in kidney transplant patients infected with SARS-CoV-2 and affected by coronavirus disease 2019 (COVID-19). We have analyzed in these patients their prior medical history, their clinical symptoms, and their laboratory results.
Method
We assessed outcomes of kidney transplant patients with confirmed COVID-19 until July 2021 from PubMed, Medline, Science Direct, Cochrane databases, EMBASE, Scopus, and EBSCO. We performed meta-analyses of nine published studies to estimate predictor factors. The analysis was analyzed by the Newcastle-Ottawa Scale (NOS) and then using the Review Manager 5.4 software.
Result
Our analysis demonstrated that the most significant risk factors for the worse COVID-19 outcomes for kidney transplant patients included: age of 60 and older [MD 9.31(95% CI, 6.31–12.30), p < 0.0001, I2 = 76%], diabetic nephropathy [OR 2.13 (95% CI, 1.49–3.04), p < 0.0001, I2 = 76%], dyspnea [OR 4.53, (95% CI, 2.22–9.22), p < 0.0001, I2 = 76%], acute kidney injury (AKI) [OR 4.53 (95% CI, 1.10–5.21), p = 0.03, I2 = 58%], and some laboratory markers. Many patients had two or multiple risk factors in combination.
Conclusion
Age and several comorbidities were the most significant factors for COVID-19 outcomes for kidney transplant recipients.
Keywords: Kidney transplant, COVID-19, Risk factor, Outcome, Systematic review, Meta-analysis
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the associated coronavirus disease 2019 (COVID-19) pandemic, has been responsible for massive changes in various sectors, but especially in the health sector. SARS-CoV-2 has spread worldwide rapidly [1]. Patients with pre-existing comorbidity have higher mortality due to more severe symptoms of COVID-19 developed. Kidney transplant recipients became the risk of suffering from severe manifestations of COVID-19 [2,3].
Studies have shown that the mortality rate of kidney transplant recipients infected with COVID-19 was unusually high. This rate fluctuated between 20% and 28%, compared to 1% to 5% in the general population of COVID-19. It was explained that kidney transplant recipients are included in the immune-compromised individuals who are more susceptible to viral infections and at greater risk for severe infection due to their impaired immune responses. Poor outcome in patients with COVID-19 was also associated with other factors such as gender, age, lifestyle, and cardio-metabolic symptoms. In previous studies the poor outcome or higher mortality rate was usually shown as severe conditions and comorbidities of patients [4,5] However, there are still limited studies on risk factors that predict poor outcomes in kidney transplant patients with COVID-19. Therefore, this meta-analysis aims to determine whether predictors such as demographics, comorbidities, clinical symptoms, and/or laboratory evaluations may lead to poor outcomes in kidney transplant patients with COVID-19.
2. Methods
2.1. Search strategies
We performed literature searching using the keywords “kidney transplant” and “COVID-19” through electronic databases. There were 878 kinds of literature found in PubMed and 341 kinds of literature found in other sources (Medline, Science Direct, Cochrane Databases, EMBASE, Scopus, EBSCO), published until July 2021. We searched for the population of kidney transplant patients with a history of confirmed COVID-19 without any concern in vaccination status and antibody markers.
We defined the more severe conditions or death, in-hospital patients, as the worse outcome. While both outcomes are attached, severe conditions are chosen. From that, we analyzed the factors which possibly contributed to the outcomes of the patients and defined them as the predictor factor.
2.2. Study selection and eligibility criteria
We conducted the study selection using the PRISMA guideline [6]. The inclusion criteria of this study are patients with a history of kidney transplantation and confirmed with COVID-19. The literature selected was written in English in all sources. We excluded the study that performed transplantation during the recent pandemic time. The kind of literature, including epidemiological studies, article reviews, systematic reviews, meta-analysis studies, and case reports, were excluded.
2.3. Data extraction and management
The primary data were extracted from the studies and concluded in Table 1 . The data information includes the first author, publication year, study location, study design, total patients, patient characteristics, kidney transplant profile, comorbidities, and Newcastle – Ottawa Scale (NOS) [7].
Table 1.
Characteristics Study and Profile Patient of The Included Studies.
| Author | Location | Study Design | Total patients | Age (year) | Sex (Gender, %) | Race |
|---|---|---|---|---|---|---|
| Craverdi, 2020 | North America and Europe | Retrospective Cohort | 144 | 62 (52–69) | Male, 65.3 Female, 34.7 |
Hispanic Caucasian African American Others |
| Crespo, 2020 | Spain | Prospective Cohort | 414 | 62 (52–71) | Male, 64 Female, 36 |
N/A |
| Azzi, 2020 | USA | Prospective Cohort | 229 | 59 (49–68) | Male, 62 Female, 38 |
Hispanic African American Others |
| Perez-Saez, 2020 | Spain | Prospective Cohort | 80 | 59.3 ± 11.7 | Male, 67.5 Female, 32.5 |
Caucasian |
| Benotmane, 2020 | France | Retrospective Cohort | 40 | 63.8 (54.6–68.2) | Male, 77.5 Female, 22.5 |
N/A |
| Kute, 2021 | India | Retrospective Cohort | 250 | 43 (35–51) | Male, 86 Female, 14 |
N/A |
| Rahbar, 2020 | Iran | Prospective Cohort | 19 | 47.6 ± 12.4 | Male, 68.4 Female, 31.6 |
N/A |
| Cailard, 2020 | France | Prospective Cohort | 243 | 61.6 (50.8–69) | Male, 66.7 Female, 33.3 |
N/A |
| Santeusanio, 2020 | USA | Retrospective Cohort | 38 | 53.8 ± 13.6 | Male, 65.8 Female, 34.2 |
Hispanic European African American Asia/Pacific Islander |
Abbreviations: N/A, not available. Data are expressed as mean ± standard deviation or median (min-max) or count %, as appropriate.
2.4. Data synthesis
The meta-analysis was done by Review Manager 5.4 software. The variables were compared through mean difference (MD) or odd ratio (OR) with a 95% confidence interval (CI). The outcomes were presented in two groups, worse or good outcomes. The heterogeneity of the studies was measured using I2 with a value of >50% is seen as considerable heterogeneity. The significance of the analysis is measured by a p-value <0.05 is considered significant. All the results were presented using a forest plot. This meta-analysis study did not need ethical clearance from the local ethical committee.
3. Results
By the study selection flow, excluding the duplicates, we found 924 potential studies. Of 924 studies, 171 publications were excluded due to inappropriate criteria during abstract and title reviews. The main criteria for exclusion were the type of publication, opinion, perspective, commentaries on COVID-19, and articles which not discuss the outcome of COVID-19 patients. As a result, 57 full-text review articles were conducted, and 171 were excluded for reasons shown in Fig. 1 . Since Benotmane et al. [12] presented a similar sample in two studies, we excluded one of these studies. Finally, we performed meta-analyses of 9 published studies to derive a pooled estimate of predictor factors of worse outcomes in kidney transplant patients with positive for the SARS-CoV- 2 test.
Fig. 1.
The Flowchart of Literature Research.
In total, four studies (44.4%) were conducted in Europe, two studies (22.2%) in the United States, two studies (22.2%) in Asia or the Pacific and one study (11.2%) merged data from the United States and Europe [[8], [9], [10], [11], [12], [13], [14], [15], [16]]. The characteristics of these nine studies are summarized in Table 1, Table 2, Table 3 where their quality was evaluated by the NOS scale. The scoring system assigns stars (up to nine stars) based on the specific quality metrics relating to cohort selection, outcome, and comparability. Three studies achieved nine stars. Four other studies got eight stars, and two studies got seven stars.
Table 2.
Patient's Comorbid, Kidney Profile, and Outcome of The Included Studies.
| Author | Type of Donor (%) | Time from Transplant to COVID-19 (month) | BMI | Comorbid | Mortality Rate (%) |
|---|---|---|---|---|---|
| Craverdi, 2020 | Deceased, 77.8 | 60 (24–111) | N/A | Hypertension Diabetes Obesity Hearth Disease Lung Disease Cancer Smoker (current/past) HIV/AIDS |
31.94 |
| Crespo, 2020 | N/A | 72 (31–145) | N/A | 26.33 | |
| Azzi, 2020 | Deceased, 73 | 58.2 (25.4–127.6) | 28.5 (24.2–32.6) | Hypertension Diabetes mellitus Heart disease Lung disease Cancer History of smoking |
20.52 |
| Perez-Saez, 2020 | N/A | 72 (16.5–165) | N/A | Hypertension Diabetes mellitus Obesity (BMI > 30) Ischaemic heart disease Lung disease Cancer Smoking |
32.5 |
| Benotmane, 2020 | N/A | 79.2 (33.6–175.2) | 29.5 (24–33) | Obesity (BMI >30) Hypertension Diabetes Obstructive sleep apnea Cardiovascular disease Respiratory disease Active cancer |
N/A |
| Kute, 2021 | Deceased, 9.6 | 42 (21.6–74.4) | N/A | Obesity (BMI >30) Hypertension Diabetes Heart disease Virus Infection (CMV/HCV/HBV) Prior Allograft dysfunction |
11.6 |
| Rahbar, 2020 | Decease, 42.1 | 105.3 ± 75.4 | N/A | Diabetes Hypertension |
47.37 |
| Cailard, 2020 | N/A | 74.1 (27.6–138.7) | N/A | Hypertension Diabetes Cardiovascular disease Respiratory disease Cancer Smoking |
17.70 |
| Santeusanio, 2020 | Deceased, 55.3 | 69.6 (16.1–111.8) | 28.3 ± 6.2 | Hypertension Diabetes HIV/AIDS Interstitial lung disease Malignancy within 1 year Prior infection within 1 year Prior transplantation |
28.95 |
N/A, not available; BMI, Body Mass Index. Data are expressed as mean ± standard deviation or median (min-max) or count %, as appropriate.
Table 3.
Clinical Symptoms, Drugs, and Laboratory of The Included Studies.
| Author | Pre-Medication | Clinical Symptoms | Management | Laboratory and Imaging |
|---|---|---|---|---|
| Craverdi, 2020 | T cell depletion Tacrolimus MMF Everolimus Prednisone ARB ACE-I Flu vaccination |
Fever Myalgia Dyspnea Diarrhea Tachypnea AKI |
Intubation Increased steroids hydroxychloroquine antibiotics tocilizumab remdesivir lopinavir-ritonavir darunavir-ritonacir darunavir-cobicistat Tacrolimus withdrawal MMF or everolimus withdrawal |
White blood cell count, Lymphocyte count Hemoglobin Platelet count Baseline creatinine eGFR Aspartate transaminase Alanine aminotransferase Lactate dehydrogenase Creatine phosphokinase C-reactive protein Serum ferritin D-dimer Interleukin - 6 Procalcitonin |
| Crespo, 2020 | Tacrolimus Mycophenolate Prednisone ARB ACE-I mTOR inhibitors |
Fever Cough, expectoration and/or rhinorrhea Dyspnea Gastrointestinal symptoms Pneumonia |
Ventilator support Hospitalization ICU admission Glucocorticoids Hydroxychloroquine Azithromycin Tocilizumab Lopinavir/ritonavir |
N/A |
| Azzi, 2020 | ACE-I or ARB Influenza vaccination Statin |
Bacterial pneumonia AKI Bacteremia Urinary tract infection Fungal infection Cytomegalovirus viremia Deep venous thrombosis Cerebrovascular accident |
Intubation Steroid Hydroxychloroquine Antibiotics Tocilizumab Remdesivir Caclneurin Inhibitors withdrawal Antimetabolite withdrawal Plasma Convalescent IVIG Anakira |
White Blood Cell count Lymphocytes Hemoglobin Platelets Baseline serum creatinine Serum creatinine Lactate dehydrogenase Creatine kinase C-reactive protein Ferritin D-dimer Interleukin - 6 Procalcitonin Fibrinogen CD3 cell count CD4 cell count CD8 cell count Pro- B-type Natriuretic Peptide |
| Perez-Saez, 2020 | Mycophenolate Prednisone ACE-i or ARB mTOR-I Calcineurin inhibitor Thymoglobulin induction |
Fever Respiratory symptoms Dyspnea Gastrointestinal symptoms AKI with dialysis need |
Endotracheal intubation Non-invasive mechanical ventilation ICU admission Steroids Hydroxychloroquine Azithromycin Other antibiotic Ritonavir/lopinavir/remdesivir Both Calcineurin Inhibitors and MMF or imTOR withdrawal Only Calcineurin Inhibitors withdrawal Only MMF or imTOR withdrawal IVIG Interferon Anakinra |
Pneumonia by X-ray PaO2/FiO2 White blood cells Lymphocytes Neutrophils Lactate dehydrogenase C-reactive protein Ferritin D-dimer Interleukin - 6 Procalcitonin Log Interleukin - 6 |
| Benotmane, 2020 | Tacrolimus MMF, MPA Steroids ARB ACE-I mTOR-I Ciclosporin Azathioprine Eclizumab Belatacept Anti-thymocyte globulin Anti-CD25 |
Fever myalgia Dyspnea cough Diarrhea Headache Vomiting Anosmia/ageusia Neurological Manifestations |
High dose corticosteroid Hydroxychloroquine Azitromycin Other antibiotics Tocilizumab Lopinavir/ritonavir Caclineurin Inhibitors withdrawal MMF/MPA withdrawal mTOR inhibitor withdrawal Delayed Belatacept administration Antifungal azole |
N/A |
| Kute, 2021 | Thymoglobulin induction Antirejection Therapy Basiliximab ACE-i or ARB Flu vaccination Antimetabolite Calcineurin inhibitor Prednisolone Sirolimus/Everolimus |
Fever Myalgia Dyspnea Diarrhea Cough Sputum production AKI |
Intubation ICU admission Increased Steroid Hydroxychloroquine Azithromycin Tocilizumab Remdesivir Calcineurin inhibitors Discontinued/ reduced Antimetabolites Discontiued Favipiravir Convalescent plasma Cytosorb filter IVIG |
Chest X-ray Chest CT scan White blood cell Lymphocyte Neutrophil Hemoglobin Platelet Serum Creatinine Blood urea Alanine aminotransferase Lactate dehydrogenase C-reactive protein Feritin D-dimer Interleukin-6 Procalcitonin Albumin |
| Rahbar, 2020 | Tacrolimus MMF/MPA Sirolimus Cyclosporin ARB |
Fever Myalgia Dyspnea Dry cough AKI Hypoxemia (Spo2 < 93%) |
Hydroxychloroquine Lopinavir/ritonavir Oseltamivir Calcineurin Inhibitors discontinued / Reduced IVIG Ribavirin |
White Blood Cell Lymphocyte count Neutrophil count Eosinophil count Neutrophil/Lymphocyte ratio Platelet count Platelet/ lymphocyte ratio eGFR Aspartate transaminase Alanine aminotransferase Lactate dehydrogenase Creatine phosphokinase C-Reactive Protein Neutrophil/Lymphocyte ratio × C-Reactive Protein Ferritin Interleukin - 6 Albumin Hypoalbuminemia Troponin, Fibrinogen, PT, PTT, INR, PH |
| Cailard, 2020 | Mycophenolate RAS blockers Steroids mTOR-I Azathioprine Belatacept Calcineurin Inhibitors |
Fever Headache Dyspnea Anosmia Rhinitis Cough Diarrhea |
Hydoxychloroquine Azithromycin Other antibiotics Tocilizumab Remdesivir Lopinavir/ritonavir Oseltamivir Belatacept withdrawal mTOR inhibitor withdrawal Calcineurin Inhibitors withdrawal Antimetabolite withdrawal Antifungal drugs |
Lymphocyte count, Platelet count Creatinine C-reactive protein Procalcitonin |
| Santeusanio, 2020 | Lymphocyte depleting Interleukin - 2 inhibitor ACE-I or ARB Tacrolimus Corticosteroid Tacrolimus + mycophenolate Tacrolimus + mycophenolate + corticosteroid Tacrolimus + corticosteroid mTOR-i + mycophenolate + corticosteroid Belatacept + mycophenolate + corticosteroid Corticosteroid + IVIG |
Fevers Gastrointestinal (diarrhea/vomiting) Respiratory (cough/dyspnea) AKI or dialysis requirement qSOFA score ≥ 2 at admission |
Hydroxychloroquine Azithromycin Remdesivir Convalescent plasma Interleukin - 6 or Interleukin - 1 inhibitor Anticoagulation therapy Prednison Mycophenolate discontinued/reduced Tacrolimus Discontinued/reduced |
Chest X-ray White Blood Cell count Absolute lymphocyte count C-Reactive Protein Ferritin D-dimer Procalcitonin Microbiologically bacterial, viral, or fungal co-infection |
MMF, Mycophenolate mofetil; ACE-I, Angiotensin Converting Enzyme Inhibitor; ARB, angiotensin II receptor blocker; MPA, Methiopropamine; mTOR, Mechanistic target of rapamycin inhibitor; IVIG, Intravenous immunoglobulin; eGFR, Estimated Glomerular Filtration Rate
Data are expressed as mean [standard deviation] or median [interquartile range] or count (%), as appropriate.
3.1. Patients characteristics
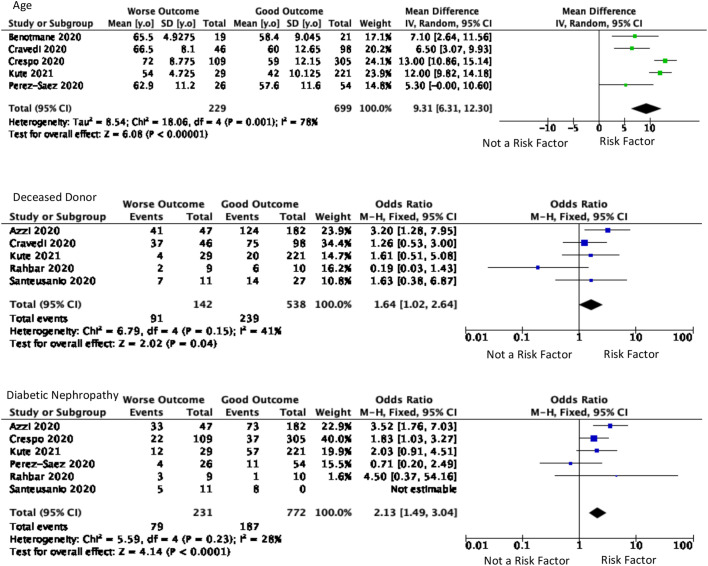
Patient characteristics involved age, gender, and race. Age was a significant predictor factor for worsening outcomes in kidney transplant recipients (p < 0.001) with an I2 value was 78%. The outcome severity of kidney transplants was increasingly associated with the older mean age of included patients. There were 928 patients included in five studies which showed that the mean difference of patients with poorer outcomes was 9.31 years older (95% CI, 6.31–12.30), as presented in Fig. 2 . There were also 514 patients from four studies which showed that patients aged >60 years old, geriatric patients, were 3.78 times more likely to have poorer outcomes than kidney transplant patients aged 60 years old or less (95% CI, 2.23–6.38). A total of nine studies discussed gender included with 1457 total patients. However, the differences in kidney transplant outcomes based on gender (p = 0.33) were insignificant. This study analyzed three races: Hispanic, Caucasian, and African American. In the Hispanic race, three studies with 182 patients were included. Based on the Caucasian race, three studies were included with 262 patients. There were three studies with 411 patients included based on African American race. The race was not a significant predictor for worse outcomes in kidney transplant recipients (p > 0.05).
Fig. 2.
Older Age showed significance as the predictor values of worse outcomes. Deceased Donor and Diabetic Nephropathy are also significant as the predictor values.
3.2. Kidney profile
The kidney profile was assessed with the type of kidney donor and the time from the transplantation. The type of kidney donors was deceased (five studies with 680 patients) and living (three studies with 517 patients), showed in Fig. 2. The deceased kidney donor tends to cause worse outcomes than a living kidney donor. Deceased kidney donor type was 1.64 times more likely to cause worse outcomes [(95% CI, 1.02–2.64), p = 0.04, I2 = 41%]. Patients who had kidney transplantation less than one year before the outcome were assessed from five studies with 1280 patients. The duration of kidney transplantation has no significant effect on kidney transplant patients' outcomes. Of the four diseases that underlie the patient's kidney damage that causes undergoing a kidney transplant, Diabetic nephropathy is one of the basic causes of kidney damage that is significant in predicting the prognosis of patients. Based on 1003 patients from six studies, patients with these causes were 2.13 times [(95% CI, 1.49–3.04), p < 0.0001, I2 = 28%] more likely to have a poor prognosis.
3.3. Pre-existing condition
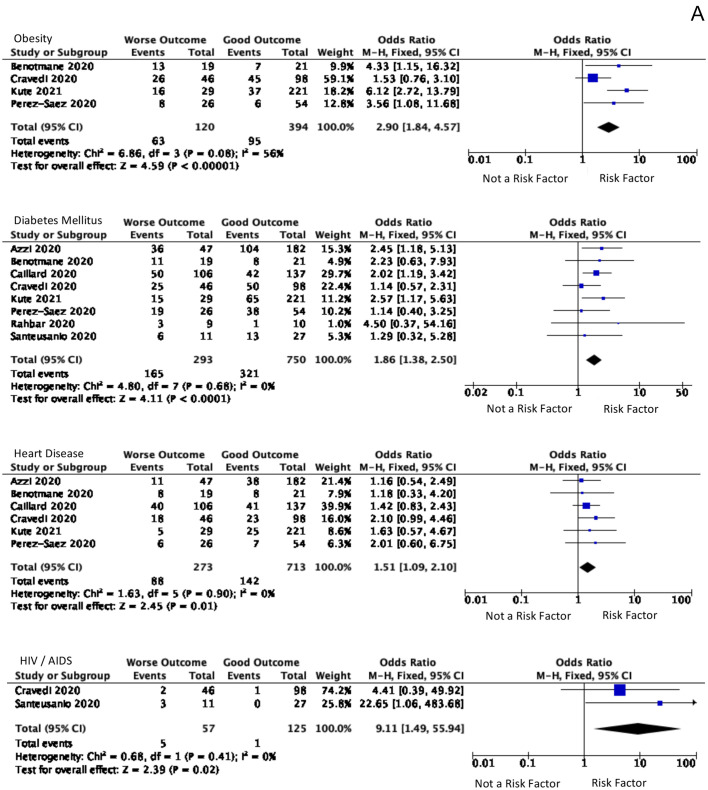
The pre-existing conditions included the patient's comorbidities and prior medications. A total of 12 prior medications have been analyzed, but none showed significant results. However, the patient's comorbidities showed to be statistically significant, summarized in Fig. 3A. Analytical for BMI (kg/m2) has been done by two methods. The first method was comparing the total BMI, which is not significant. The following methods are the number of patients with BMI >30 statistically significant. The risk of worse prognosis was increased to 2.90 times [(95% CI, 1.84–4.57), p < 0.00001, I2 = 56%] in 514 patients from four studies with a BMI > 30 kg/m2.
Fig. 3.
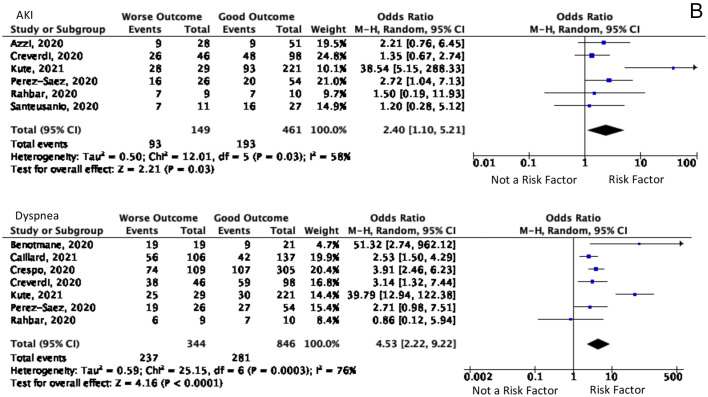
Comorbid and Symptoms that significant A. Diabetes, hypertension, heart disease, and HIV are comorbid and significant for predicting the worsening symptoms B. AKI and dyspnea were the significant symptoms as the predictor values of worse outcomes.
Another significant comorbidity is Diabetes Mellitus. 1143 with comorbid DM from eight studies showed 1.86 times [(95% CI, 1.38–2.50), p < 0.0001, I2 = 0%] tended to have a poor prognosis. According to 986 patients from six studies, a history of heart disease showed 1.51 times [(95% CI, 1.09–2.10), p = 0.01, I2 = 0%] more risk of a poor outcome. The analysis of patients with comorbid cancer had a poor prognosis of 1.58 times (95% CI, 1.01–2.48). Moreover, HIV patients had the highest risk, 182 patients in two studies, which showed 9.11 times [(95% CI, 1.49–55.94) p = 0.02, I2 = 0%] of having a poor prognosis.
3.4. Symptoms
In the analysis of the symptoms shown in Fig. 3B, patients experienced nine symptoms when they were exposed to COVID-19. The development of acute kidney injury (AKI) and the onset until the patient's admission to the hospital were also analyzed. The analysis showed significant results in symptoms of dyspnea, diarrhea, gastrointestinal symptoms except for diarrhea, and progression of symptoms to AKI. Based on an analysis of 610 patients in six studies, AKI symptoms developed 2.4 times [(95% CI, 1.10–5.21), p = 0.03, I2 = 58%] higher in patients with worse outcomes, compared to others. Dyspnea was a symptom that has 4.53 times [(95% CI, 2.22–9.22), p < 0.0001, I2 = 76%] more frequently complained of by patients with poor outcomes from seven studies in 1190 patients. Even though diarrhea and other gastrointestinal symptoms were significant, it had obtained a significant inversely result and the impact did not analyze further.
3.5. Laboratory results
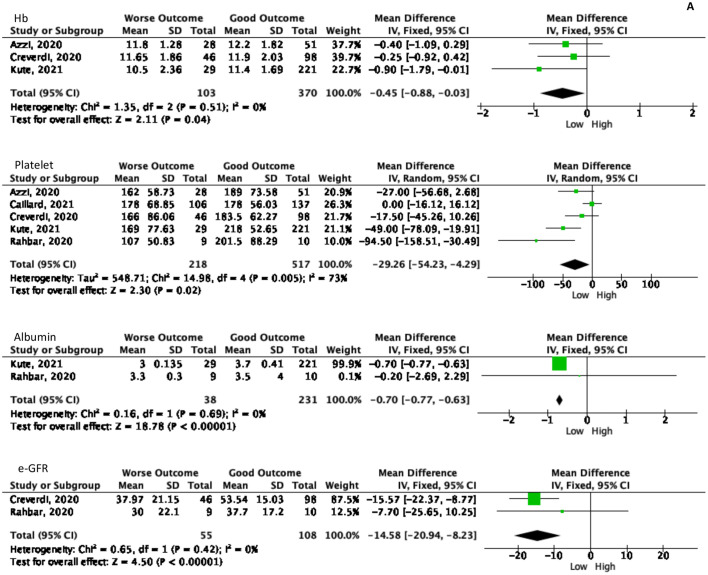
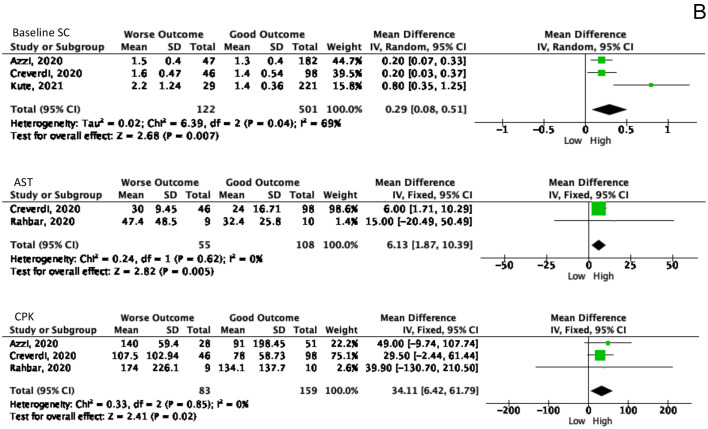
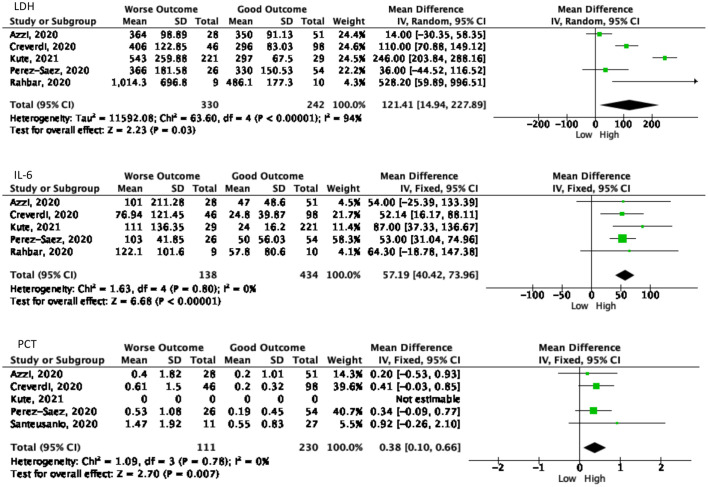
Laboratory results also become one of the predictor values to determine the patient's outcome. Out of 18 laboratory markers from nine studies ten markers had a significant Forrest plot result as a predictor for the worsening process as summarized in Fig. 4 . The following marker levels correlated with the worse COVID-19 outcomes, namely: hemoglobin (Hb), platelet, albumin, creatinine (SC), creatinine phosphokinase (CPK), estimated glomerular filtration rate (e-GFR), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), IL-6, and procalcitonin (PCT).
Fig. 4.
The significant values of laboratory examination as the predictor values of worsening symptoms. A. Laboratory values in poor outcome patients showed lower than another group. B. The elevated laboratory values showed in worse outcome patients.
A total of 473 patients from three studies was examined for Hb levels, showing that patients with poor outcomes had 0.45 g/dL [(95% CI, 3% - 88%), p = 0.04, I2 = 0%] lower Hb levels than patients with better outcomes. This result agrees with the results of platelet counts in 735 patients from five studies, with a lower result of 29.26 × 10^9 /L [(95% CI, 4.29–54.23), p = 0.02, I2 = 73%] than the control group. In addition, albumin examination in 269 patients from two studies, showed that patients with a poor prognosis had 0.7 g/dL [(95 CI, 0.63–0.77), p < 0.0001, I2 = 0%] lower than others.
The baseline SC was carried out in the laboratory examination before being infected with COVID-19 and after being infected with COVID-19. Results of baseline SC in 623 patients in three studies found that baseline SC in patients with poor outcomes was 0.29 mg/dL [(95% CI, 0.08–0.51), p = 0.007, I2 = 69%]. In addition, CPK was performed in 242 patients from three studies which showed that patients with poor prognosis had 34.11 U/L [(95% CI, 6.42–61.79), p = 0.02, I2 = 0%] higher than patients with a good prognosis. The e-GFR examination is also one of the significant examination results, in which patients with poor outcomes obtained a lower e-GFR of 14.58 L/Min [(95% CI 8.23–20.94), p < 0.0001, I2 = 0%] compared to other groups. In addition to kidney function tests, liver function tests are also necessary to evaluate. Liver function examination showed significant results on AST but not on ALT. On AST examination in 163 patients, the result was 6.13 U/L [(95% CI, 1.87–10.39), p = 0.005, I2 = 0%] higher in a poor outcome.
The LDH test can be used as a marker to determine the process of tissue damage that occurs. In 330 patients with poor outcomes, the result was 121.41 U/L [(95% CI, 14.94–227.89), p = 0.03, I2 = 94%] higher than 242 patients with a good outcome. Furthermore, the results of IL-6 as a marker of the inflammatory process showed 57.19 pg/mL [(95% CI, 40.42–73.96), p < 0.00001, I2 = 0%] higher in 138 patients with poor prognosis than 572 patients who underwent IL-6 examination. PCT is also an important marker in determining the urgency of antibiotic use. In total, 341 patients from five studies had been examined, showing 0.38 ng/mL [(95% CI, 0.10–0.66), p = 0.007, I2 = 0%] greater than in patients with a good prognosis.
4. Discussions
The present analysis provides an update and additional detail laboratory markers evaluations. On one hand, the meta-analysis study conducted by Raja et al. analyzed the most common symptoms and the treatment frequently used in solid organ transplant patients [17]. The meta-analysis conducted by Kremer et al. carried out an approach by comparing the proportion of mortality against age and time from transplant to be diagnosed with COVID-19 [3]. On the other hand, a systematic review conducted by Bansal et al. focused on patient comorbidities on outcomes in kidney transplant patients with COVID-19 [18]. A meta-analysis conducted by Phanish et al. in Southwest London also showed similar things, namely the case fatality ratio and AKI [19]. Recent research, conducted by Duarsa et al. with the univariate meta-regression method, evaluated multiple variables such as age, gender, and time on the kidney transplant outcomes [20]. However, in this study, the authors analyzed all variables as predictor values in estimating the occurrence of worsening conditions. Our study carried an update on included studies that focused on differentiating between good and poor outcomes: the variables included patient characteristics, kidney profile, pre-existing conditions, symptoms, and laboratory values.
Kidney transplantation is recognized as an optimal management for end-stage renal disease patients. In the current COVID-19 pandemic, the mortality rate among kidney transplant recipients was higher. Several factors clearly contributed to the worse outcome of kidney transplantation in patients infected by COVID-19.
In our study, older age contributed as a significant factor to worse outcomes in kidney transplant recipients infected by COVID-19. A multivariable analysis by Grasselli et al. showed that a 10-year increase in age was significantly associated with mortality in a kidney transplant patient with COVID-19 in ICUs [21]. Older age contributed as a risk factor for severity stages and progression of COVID-19. Older age could affect the immune system, enhancing viral replication. Higher BMI values also increased the risk for COVID-19 [22,23]. Recipients with a deceased donor kidney were more likely to have a higher mortality rate than those receiving a living donor kidney. A study in Korea also found that living-donor kidney transplants showed significantly higher rejection-free survival rates when compared to deceased-donor kidney transplants [24,25].
In our analysis, comorbidities such as diabetes mellitus, cardiovascular disease, cancer, and HIV infection in patients undergoing kidney transplants significantly affected the outcome. Kidney transplant patients with cardiovascular disease tend to have higher mortality compared to the general population. The most common cause of mortality in kidney transplant recipients is cardiovascular disease [26]. It becomes the leading cause of reduced early renal graft loss and results in significant morbidity [23] Therefore, cardiovascular management in kidney transplant recipients is essential to prolong the lives of kidney transplant patients, especially the ones infected with COVID-19.
Infections were the second leading cause of mortality in kidney transplant recipients. After kidney transplantation, the risk of infection is significantly higher than in the general population, which leads to higher mortality and morbidity rates. This is related to our study where COVID-19 infection increased the mortality in kidney transplant recipients. HIV infections among kidney transplant recipients also tend to have worse outcomes. Proper screening and early diagnosis for infections among kidney transplant recipients are important to prolong patients' survival [26,27]. Malignancy is another comorbidity as a leading cause of mortality in kidney transplant recipients. The incidence of malignancy in kidney transplant recipients is three to five times higher than in the general population [26,28].
Diabetes mellitus was associated with the severity and aggravation of COVID-19 symptoms in many studies. Hyperglycemic conditions in diabetes impaired the immune system including neutrophils, antioxidants, and humoral immunity. Diabetes patients are more vulnerable to infections, especially when accompanied by kidney transplantation [29]. Diabetes mellitus became the second most prevalent pre-existing condition among chronic kidney disease patients with COVID-19. It was also concluded that frequent co-existence of comorbidities increased the risk of poorer outcomes [30]. In our study, kidney transplant recipients infected by COVID-19 with diabetic nephropathy had a greater risk for worse outcomes. COVID-19 pneumonia patients with diabetic nephropathy had a higher case-fatality rate and nearly a twofold probability of intubation than patients with only chronic kidney disease. Kidney disease may cause significant reductions in quality and length of life, and diabetic patients significantly reduce lung capacities including forced vital capacity and forced expiratory volume. Therefore, kidney transplant patients with diabetic nephropathy will have a worse prognosis when infected with COVID-19 [31,32].
COVID-19 has a variety of symptoms that typically emerge symptoms like fever, cough, nasal congestion, anosmia, malaise, and dyspnea within 4–7 days [33,34]. Other types of clinical presentation include sore throat, muscle weakness, myalgia, headache, diarrhea, and other gastrointestinal symptoms. The radiologic findings might develop in COVID-19 patients, clinical severity depends on the degree of hypoxemia conditions. It could be ground glass appearance, alveolar exudates, patchy consolidation bilateral, and interlobular involvement [34]. According to the analysis and the pathologic process, dyspnea was the only significant predictor value for the symptoms of the worsening process in kidney transplant patients.
According to Tian et al., the most common symptom in adults was anorexia (39.9% - 50.2%), while vomiting was more common in children (6.5% - 66.7%). Diarrhea was the second, both in adults and children (2% - 49.5%) [35]. However, diarrhea and gastrointestinal symptoms significantly experienced by kidney transplant patients had a good outcome, although no further analysis was discussed regarding the extent of protective factors from diarrhea and other gastrointestinal symptoms [36].
In worsening patients, severe acute respiratory syndrome and multiorgan failure might be present. Both developments can lead to critical illness conditions and death [34,37]. The worsening symptoms are associated with the process of cytokine storm as a response to COVID-19, in which unscaled and disproportionally high immune response. The cytokine storm can result in the sequence and severity of organ dysfunction in multiple organ dysfunction syndromes (MODS), including lungs and renal [37,38]. The cytokine storm directly damages the pulmonary capillary mucosa and alveolar edema is the consequence of cytokine storms in pulmonary systems, thus resulting in changes in alveolar structure and pulmonary ventilation dysfunction [37]. Moreover, cytokine storms in the kidney can lead to AKI, a significant predictor value of the worsening process in patients.
Predictor values in kidney transplant patients with COVID-19 can be seen with the patient's laboratory values. A cohort study by Du et al. in 85 patients showed high frequencies of lymphopenia (77.6%) and thrombopenia (41.2%) found more in non-survivors of COVID-19 patients [39]. Increased levels of D-dimers, thrombocytopenia, and defective coagulation function, mainly caused by cytokines storm, developed worse outcomes and might be developed to multiple organ failures or death in kidney transplant patients infected by COVID-19. Another prognostic factor in severe cases is prominent lymphopenia, which indicates an immune system impairment [40].
A meta-analysis study by Kumar et al. reported comparing liver function abnormalities, including Gamma-Glutamyl Transferase, aminotransferase hypoalbuminemia, and bilirubin elevations were found more frequently in patients with severe disease. Even though in the overall COVID-19 cases, the AST and ALT elevations' frequency was similar, the elevation of AST was seen more in severe COVID-19 patients than in ALT, a consistent result in our meta-analysis [41].
Inflammation markers, particularly CRP and IL-6, might become monitoring tools for the COVID-19 severity stages. Enhancement production of cytokines potentially developed glucose variability due to acute increase of glycemia, an effect to avoid in COVID-19 [42]. PCT responses are inhibited by IFN-γ, in vitro studies, which hypothesize that secondary infection in SARS-CoV-2 is inhibitory of a PCT response, but the initial or routine PCT test is not recommended because the function remains unclear [43].
In the end, markers of renal function will be important in determining the patient's prognosis. The study by Caillard et al. demonstrated that CRP >60 mg/L and serum creatinine >115 mol/L were risk factors for severe COVID-19. Moreover, mortality in patients with serum creatinine >115 mol/L was 2.32 higher compared to another group [44]. Besides, e-GFR < 30 mL/min showed a significant risk, 2.5 times developing severe COVID-19 [45]. This presentation supported our results that an increase in serum creatinine and a decrease in e-GFR can be a predictor values for the worsening process.
The other kidney function tests to support COVID-19 progression were proteinuria and hematuria. The significance of those tests on mortality has not cleared yet [46]. The study by Karras et al. showed that an increased urine protein-creatinine ratio and a urine retinol binding protein-creatinine ratio > 0.03 mg/mmol increased the risk of worsening the condition to AKI or requiring ICU treatment due to tubular damage [47]. However, this study did not discuss proteinuria and hematuria as predictor values in more detail.
We found that studies related to COVID-19, especially among kidney transplant recipients, are still scarce. Therefore, there were no randomized-controlled trial studies included in this meta-analysis. The high heterogeneity analyses among the included studies, patient characteristics, sample size, and laboratory follow-up highlight the cautiousness of applying these results to specific populations. We recommend that all academics conduct random-controlled trials relating to the prevention and management of COVID-19 in kidney transplant recipients, including a multicenter clinical trial to gain even more heterogenic samples.
5. Conclusions
Our meta-analyses demonstrated that the most significant risk factors for the worse COVID-19 outcomes for kidney transplant patients are age (p < 0.0001), diabetic nephropathy (p < 0.0001), dyspnea (p < 0.0001), acute kidney injury (p = 0.03), and several laboratory markers. Overall, many patients had two or multiple risk factors in combination.
Author contributions
Gede Wirya Kusuma Duarsa
-
•
Participated in research design
-
•
Participated in the writing of the paper
-
•
Participated in the performance of the research
-
•
Participated in data-analysis.
Ronald Sugianto
-
•
Participated in research design
-
•
Participated in the writing of the paper
-
•
Participated in the performance of the research
-
•
Participated in data-analysis.
I Gusti Agung Ayu Andra Yusari
-
•
Participated in research design
-
•
Participated in the writing of the paper
-
•
Participated in the performance of the research
-
•
Participated in data-analysis.
Pande Made Wisnu Tirtayasa
-
•
Participated in research design
-
•
Participated in the performance of the research
-
•
Participated in data-analysis.
Gerhard Reinaldi Situmorang
-
•
Participated in research design
-
•
Participated in the performance of the research.
Nur Rasyid
-
•
Participated in research design
-
•
Participated in the performance of the research.
Arry Rodjani
-
•
Participated in research design
-
•
Participated in the performance of the research.
Besut Daryanto
-
•
Participated in the performance of the research.
Kurnia Penta Seputra
-
•
Participated in the performance of the research.
Paksi Satyagraha
-
•
Participated in the performance of the research.
Funding statement
None.
Declaration of Competing Interest
The author declares that he has no conflict of interest.
Acknowledgements
None.
Data availability
I have shared the raw and supplementary as an additional paper in "Data in Brief" and "Methodsx."
References
- 1.Ahn C., Amer H., Anglicheau D., Ascher N.L., Baan C.C., Battsetset G., et al. Global transplantation COVID report march 2020. Transplantation. 2020 Oct;104(10):1974–1983. doi: 10.1097/TP.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelico R., Trapani S., Manzia T.M., Lombardini L., Tisone G., Cardillo M. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am. J. Transplant. 2020 Jul;20(7):1780–1784. doi: 10.1111/ajt.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer D., Pieters T.T., Verhaar M.C., Berger S.P., Bakker S.J.L., Zuilen A.D., et al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: lessons to be learned. Am. J. Transplant. 2021 Dec;21(12):3936–3945. doi: 10.1111/ajt.16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias M., Pievani D., Randoux C., Louis K., Denis B., Delion A., et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J. Am. Soc. Nephrol. 2020 Oct;31(10):2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal A., Kumar A., Bansal R., Maheshwari R., Chaturvedi S. The impact of comorbidities on clinical course and outcome, in kidney transplant recipients with COVID-19: a systematic review and analysis. Indian J. Transplant. 2020;14(4):275. [Google Scholar]
- 6.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A., et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am. J. Transplant. 2020 Jul;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Program E based S. Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness. :113. [PubMed]
- 8.Cravedi P., Mothi S.S., Azzi Y., Haverly M., Farouk S.S., Pérez-Sáez M.J., et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am. J. Transplant. 2020 Nov;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo M., Mazuecos A., Rodrigo E., Gavela E., Villanego F., Sánchez-Alvarez E., et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020 Nov;104(11):2225–2233. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 10.Azzi Y., Parides M., Alani O., Loarte-Campos P., Bartash R., Forest S., et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020 Dec;98(6):1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Sáez M.J., Blasco M., Redondo-Pachón D., Ventura-Aguiar P., Bada-Bosch T., Pérez-Flores I., et al. Use of tocilizumab in kidney transplant recipients with COVID-19. Am. J. Transplant. 2020 Nov;20(11):3182–3190. doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I., Gautier-Vargas G., Wendling M., Perrin P., Velay A., Bassand X., et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am. J. Transplant. 2020 Nov;20(11):3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kute V.B., Bhalla A.K., Guleria S., Ray D.S., Bahadur M.M., Shingare A., et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021 Apr;105(4):851–860. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaffari Rahbar M., Nafar M., Khoshdel A., Dalili N., Abrishami A., Firouzan A., et al. Low rate of COVID-19 pneumonia in kidney transplant recipients—A battle between infection and immune response? Transpl. Infect. Dis. [Internet]. 2020 Oct;22(5) doi: 10.1111/tid.13406. https://onlinelibrary.wiley.com/doi/10.1111/tid.13406 [cited 2022 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caillard S., Anglicheau D., Matignon M., Durrbach A., Greze C., Frimat L., et al. To COVID-19 in recipients of kidney transplants. Clin. Investig. 2020;11 doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santeusanio A.D., Menon M.C., Liu C., Bhansali A., Patel N., Mahir F., et al. Influence of patient characteristics and immunosuppressant management on mortality in kidney transplant recipients hospitalized with coronavirus disease 2019 (COVID-19) Clin. Transplant [Internet]. 2021 Apr;35(4) doi: 10.1111/ctr.14221. https://onlinelibrary.wiley.com/doi/10.1111/ctr.14221 [cited 2022 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raja M.A., Mendoza M.A., Villavicencio A., Anjan S., Reynolds J.M., Kittipibul V., et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant. Rev. 2021 Jan;35(1) doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal A., Kumar A., Bansal R.M., Maheshwari R., Chaturvedi S. The impact of comorbidities on clinical course and outcome, in kidney transplant recipients with COVID-19: a systematic review and analysis. Indian J. Transplant. 2020;14(4):275–282. [Google Scholar]
- 19.Phanish M., Ster I.C., Ghazanfar A., Cole N., Quan V., Hull R., et al. Systematic review and meta-analysis of COVID-19 and kidney transplant recipients, the south West London kidney transplant network experience. Kidney Int. Rep. 2021 Mar;6(3):574–585. doi: 10.1016/j.ekir.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duarsa M.D.V.I., Kandarini Y., Yogiswara N., Kloping Y.P. Survival and clinical outcomes of kidney transplant recipients with coronavirus disease infection: an updated systematic review and meta-analysis. Turk. J. Urol. 2022;48(1):17–29. doi: 10.5152/tud.2022.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020 Oct 1;180(10):1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.R., Nam S.H., Kim Y.R. Risk factors on the progression to clinical outcomes of COVID-19 patients in South Korea: using national data. Int. J. Environ. Res. Public Health. 2020 Nov 28;17(23):8847. doi: 10.3390/ijerph17238847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thongprayoon C., Hansrivijit P., Leeaphorn N., Acharya P., Torres-Ortiz A., Kaewput W., et al. Recent advances and clinical outcomes of kidney transplantation. J. Clin. Med. 2020 Apr 22;9(4):1193. doi: 10.3390/jcm9041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H.S., Kang M., Kim B., Park Y. Outcomes of kidney transplantation over a 16-year period in Korea: an analysis of the National Health Information Database. Stepkowski S, editor. PLoS One. 2021 Feb 19;16(2) doi: 10.1371/journal.pone.0247449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Htay H., Pascoe E.M., Hawley C.M., Campbell S.B., Chapman J., Cho Y., et al. Patient and center characteristics associated with kidney transplant outcomes: a binational registry analysis. Transpl. Int. 2020 Dec;33(12):1667–1680. doi: 10.1111/tri.13681. [DOI] [PubMed] [Google Scholar]
- 26.Parajuli S., Mandelbrot D.A., Aziz F., Garg N., Muth B., Mohamed M., et al. Characteristics and outcomes of kidney transplant recipients with a functioning graft for more than 25 years. Kidney Dis. 2018;4(4):255–261. doi: 10.1159/000491575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karuthu S., Blumberg E.A. Common infections in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2012 Dec;7(12):2058–2070. doi: 10.2215/CJN.04410512. [DOI] [PubMed] [Google Scholar]
- 28.Parajuli S., Clark D.F., Djamali A. Is kidney transplantation a better state of CKD? Impact on diagnosis and management. Adv. Chronic Kidney Dis. 2016 Sep;23(5):287–294. doi: 10.1053/j.ackd.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Jie Guan W., Hua Liang W., Zhao Y., Rui Liang H., Sheng Chen Z., Min Li Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020 May;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang M.C., Park Y.K., Kim B.O., Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect. Dis. 2020 Dec;20(1):445. doi: 10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon-Abarca J.A., Memon R.S., Rehan B., Iftikhar M., Chatterjee A. The impact of COVID-19 in diabetic kidney disease and chronic kidney disease: a population-based study [internet] Nephrology. 2020 Sep doi: 10.23750/abm.v91i4.10380. http://medrxiv.org/lookup/doi/10.1101/2020.09.12.20193235 [cited 2022 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020 Jul;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 May;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boban M. Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments. Int. J. Clin. Pract. [Internet]. 2021 Apr;75(4) doi: 10.1111/ijcp.13868. https://onlinelibrary.wiley.com/doi/10.1111/ijcp.13868 [cited 2022 Feb 8]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020 May;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhar J., Samanta J., Kochhar R. Corona virus Disease-19 pandemic: the gastroenterologists’ perspective. Indian J. Gastroenterol. 2020 Jun;39(3):220–231. doi: 10.1007/s12664-020-01075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020 Nov 30;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amirfakhryan H., safari F. Outbreak of SARS-CoV2: pathogenesis of infection and cardiovascular involvement. Hell. J. Cardiol. 2021 Jan;62(1):13–23. doi: 10.1016/j.hjc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020 Jun 1;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anka A.U., Tahir M.I., Abubakar S.D., Alsabbagh M., Zian Z., Hamedifar H., et al. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand J. Immunol. [Internet]. 2021 Apr;93(4) doi: 10.1111/sji.12998. https://onlinelibrary.wiley.com/doi/10.1111/sji.12998 [cited 2022 Feb 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar-M P., Mishra S., Jha D.K., Shukla J., Choudhury A., Mohindra R., et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol. Int. 2020 Sep;14(5):711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceriello A., Standl E., Catrinoiu D., Itzhak B., Lalic N.M., Rahelic D., et al. Issues for the management of people with diabetes and COVID-19 in ICU. Cardiovasc. Diabetol. 2020 Dec;19(1):114. doi: 10.1186/s12933-020-01089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen Jeschke K., Bonnesen B., Hansen E.F., Jensen J.U.S., Lapperre T.S., Weinreich U.M., et al. Guideline for the management of COVID-19 patients during hospital admission in a non-intensive care setting. Eur. Clin. Respir. J. 2020 Jan 1;7(1):1761677. doi: 10.1080/20018525.2020.1761677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caillard S., Chavarot N., Francois H., Matignon M., Greze C., Kamar N., et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug 20;584:431–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassanein M., Radhakrisnan Y., Sedor J., Vachhrajani T., Vachhrajani V.T., Augustine J., et al. COVID-19 and the kidney. Cleve. Clin. J. Med. 2020 Oct;87(10):619–631. doi: 10.3949/ccjm.87a.20072. [DOI] [PubMed] [Google Scholar]
- 47.Karras A., Livrozet M., Lazareth H., Benichou N., Hulot J.S., Fayol A., et al. Proteinuria and clinical outcomes in hospitalized COVID-19 patients. CJASN. 2021;16:514–521. doi: 10.2215/CJN.09130620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I have shared the raw and supplementary as an additional paper in "Data in Brief" and "Methodsx."