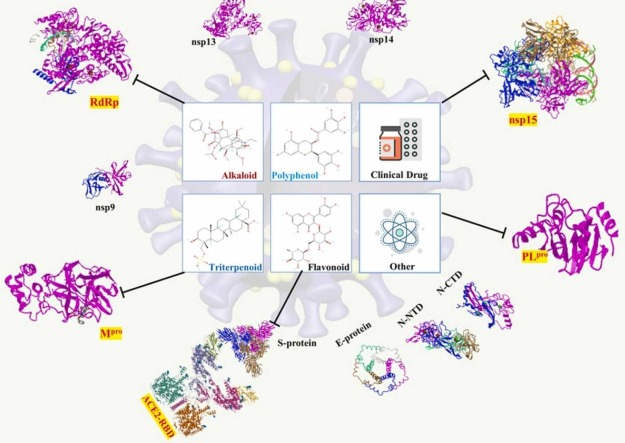
Abstract
Background
The novel coronavirus disease-2019 (COVID-19) that emerged in China, is an extremely contagious and pathogenic viral infection caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that has sparked a global pandemic. The few and limited availability of approved therapeutic agents or vaccines is of great concern. Urgently, Remdesivir, Nirmatrelvir, Molnupiravir, and some phytochemicals including polyphenol, flavonoid, alkaloid, and triterpenoid are applied to develop as repurposing drugs against the SARS-CoV-2 invasion.
Methods
This study was conducted to perform molecular docking and absorption, distribution, metabolism, excretion and toxicity (ADMET) analysis of the potential phytocompounds and repurposing drugs against three targets of SARS-CoV-2 proteins (RNA dependent RNA polymerase, RdRp, Endoribonclease, S-protein of ACE2-RBD).
Results
The docking data illustrated Arachidonic acid, Rutin, Quercetin, and Curcumin were highly bound with coronavirus polyprotein replicase and Ebolavirus envelope protein. Furthermore, anti- Ebolavirus molecule Remedesivir, anti-HIV molecule Chloroquine, and Darunavir were repurposed with coronavirus polyprotein replicase as well as Ebolavirus envelope protein. The strongest binding interaction of each targets are Rutin with RdRp, Endoribonclease with Amentoflavone, and ACE2-RBD with Epigallocatechin gallate.
Conclusions
Taken altogether, these results shed a light on that phytocompounds have a therapeutic potential for the treatment of anti-SARS-CoV-2 may base on multi-target effects or cocktail formulation for blocking viral infection through invasion/activation, transcription/reproduction, and posttranslational cleavage to battle COVID-19 pandemic.
Keywords: SARS-CoV-2, Remedesivir, Virus entry, Viral proliferation, Immune evasion, Phytocompounds
Graphical Abstract
Introduction
The recent COVID-19 outbreak caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing pandemic and presents a public health crisis. Once SARS-CoV-2 infects the host particular human being by firstly binding to the ectoenzyme Angiotensin Converting Enzyme 2 (ACE2), a serine protease acting as the receptor, while another serine protease-transmembrane serine protease 2 (TMPRSS2) is indispensable for priming the viral spike protein requisite for entering the cells. Two phases of COVID-19 pathophysiology are being marked 1. increased SARS-CoV-2 virus transmission and infection rates due to the wide expression of the main infection-related ACE2, TMPRSS2 and CTSB/L human genes in tissues of the respiratory and gastrointestinal tract as well as 2. an overwhelming inflammatory response due to cytokine storm, the development of acute respiratory distress syndrome (ARDS), severe thrombotic events containing pulmonary embolism, deep vein thrombosis, and microthrombi emerged as additional symptoms, multiple organ damages, and systemic failure likely because of imbalanced ACE/ANGII/AT1R and ACE2/ANG[1], [2], [3], [4], [5], [6], [7]/MASR axes signalling [1], [2]. The virus genome would be reproduced by host cell growth, and finally virus is egotistic to prevent other virus to concurrently infect the same host cell [3]. In addition, SARS-CoV-2 has an unique machinery proofreading appliance to preserve the genome maintenance in the virus world and their transaction are complicatedly affected by multiple factors [4]. Owing to living in intermediate host and unique machinery proofreading, coronavirus could adapt the series of environmental stress subsequently to infect the human [5]. Infection with SARS-CoV-2 leads to COVID-19 pandemics, the severity of which originates from the host’s immune response, particularly the release of a storm of pro-inflammatory cytokines.
The mutation rate of SARS-CoV-2 is very fast and there have been found several major variants (i.e. variant bata, delta, omicron) that caused at least 5 surges global spread associated with 7 waves of death peaks under the global circumstance (https://COVID19.who.int/). Unfortunately, the effective drug has not been officially licensed or approved to suppress the epidemic prevalence due to non-specific treatment even vaccination currently available. Presently, several vaccines (Oxford AstraZeneca, Pfizer–BioNTech, Moderna, SINOVAC etc.) and drugs (Remdesivir, Molnupiravir, and Paxlovid) are being used for COVID-19 treatment under emergency use authorization (EUA) by approval of FDA or WHO. Even peoples have received 3 or 4 dose vaccinations with the EUA approval by WHO, but are still predisposed to new variants of SARS-CoV-2 ( i.e. omicron, delta variant) infection [6], [7], [8] and some of COVID-19 patients may endure serious symptoms and lead to death [9], [10]. The repurposing of drugs including antiviral agents (Chloroquine, Ivermectin, Nitazoxanide, Hydroxychloroquine, Lopinavir, Remdesivir, Tocilizumab), supporting agents (Azithromycin, Corticosteroids, Vitamin C, Vitamin D) and favourable investigational vaccines are tried to meet this urgent demand against COVID-19 pandemic [11]. Remdesivir is an inactive form prodrug of nucleotide can be converted into its active form metabolite as an adenosine nucleoside triphosphate analog to inhibit the viral RNA polymerase activity [12]. Therefore, the emerging researches repurposed the nucleoside analogue, Remdesivir (anti-Ebola) could suppress human and zoonotic coronavirus at the cell culture level and mouse model [12], [13]. Interestingly, previous anti-Ebola prodrug Remdesivir was demonstrated as a RNA-dependent RNA polymerase (RdRp) inhibitory regime in COVID-19 pandemics by co-crystallized structure of Remdesivir with CoV-2 RdRp [14]. This evidence shown not only the active metabolite from Remdesivir can act on RdRp of SARS-CoV-2 to interrupt viral replication, but this prodrug may direct bind and interact with RdRp of SARS-CoV-2 to alter enzyme activity.
In terms of transcription and replication of SARS-CoV-2, main protease (Mpro) and papain-like protease (PLpro) play a main function to cleave the polyprotein replicase into non-structure protein. A previous literature elicited that Mpro could attach and cleave the C-terminus of polyprotein replicase at 11 protease digestion sites [15] to construct nsp4∼nsp16 [16]; in contrast PLpro should adhere on the N-terminus of polyprotein replicase to form nsp1∼nsp3 as well [17]. Subsequently, RdRp of SARS-CoV-2 consists of one catalytic subunit (nsp12) and two non-structure proteins (nsp7 and nsp8) has been illustrated in the replication of viral RNA. Taken a deep insight of COVID-19 immune evasion, uridylate-specific endoribonuclease (nsp15) will assist the SARS-CoV-2 virus escape from the detection of host cell dsRNA sensors as well as endoribonuclease is simultaneously active during the replication of the poly(A) region of viral genomic and subgenomic RNAs to degrade the 5'-polyuridines of the anti-genome [18], [19]. With respect to virus entry, the human ACE2 transmembrane receptor is firstly anchored by spike protein (S-protein) of SARS-CoV-2. Hereafter, the S-protein attached cells can form an endosome which will lead virus encapsulated to facilitate the virus entering the human host cell [14].
There potential phytochemical candidates include Curcumin, Cinnamaldehyde, Quercetin, Lactoferrin, Probiotics, Selenium, Zn, Vitamin D, Vitamin C, etc. [20]. Notably, according to accumulative evidence reveal that the phytocompounds have been classified into at least four categories including polyphenol, flavonoid, alkaloid, and triterpenoid [21]. The biological and pharmacological activities of medicinal phytochemicals such as anti-oxidation, anti-inflammation, anti-tumor and anti-virus have been well investigated [22]. Furthermore, numerous studies have illustrated that some natural products i.e. flavonoid could be an inhibitor to interact with SARS-CoV-2 3CL protease (3CLpro) by computational analysis [23], [24], [25]. As an alternative therapeutic strategy, flavonoids should be considered into one of potential anti-virus agents. Thereby, those phytomedicines are evidenced to hinder SARS-CoV-2 activity via exploring the mechanisms of PLpro, 3CLpro, and NTPase/helicase [26]. Epigallocatechin gallate (EGCG) is one of polyphenols, which has been suggested to prevent and treat COVID-19 via suppressing Mpro and PLpro to interrupt viral non-structure protein reproduction [27]. In terms of alkaloid, Berberine was isolated from a plenty of traditional Chinese formulas; and moreover has been investigated to reduce virus replication and targets specific interactions between the virus and host cells [28]. As for the development of innovative drug, a major effort has been directed to the discovery of therapeutic antibodies targeting S-protein and inhibitors of 3CLpro as well as RdRp of SARS-CoV-2. In the investigations of repurposed or prodrug from natural compounds to fight COVID-19 pandemics become an urgent demand. Henceforth, this study attempted to search some phytocompounds according to their anti-viral and anti-inflammatory properties and subsequently docked according to three major aspects of SARS-Cov-2 infection such as virus-entry, viral replication, and immune evasion related proteins as targets for abrogating the virus expansion via in silico to shorten the exploration time of new phytomedicine discovery and development. Moreover, this study might provide a favourable perspective for evaluating the efficacy of a single antiviral drug candidate as well as afford an integrated on various levels of SARS-CoV-2 infection from viral infection, viral replication, and immune evasion to accomplish the COVID-19 prevention or treatment strategies.
Materials and methods
Molecule docking
Program, protein module, and chemical structure preparation
Molecular docking was performed by CCDC Gold Suite V5.3 (Cambridge Crystallographic Data Centre, Cambridge, UK) [29]. 3-D chemical structure of small molecules were retracted from PubChem (https://pubchem.ncbi.nlm.nih.gov) (Supplementary document). Replicase polyprotein of coronavirus SARS: 5N19 [30]; NL63: 5NH0 [30], 229E: 2ZU2 [31] ; COVID-19: 6M2Q [32] , and 6WX4 [33] as well as Ebolavirus envelope protein: 6G9I [34] were retrieved from PDB database of National Center for Biotechnology Information (NCBI, https://www.rcsb.org). The preparation of protein module/structure was executed by the Macromolecule preparation protocol in Discovery Studio (BIOVIA, San Diego, CA, USA). The key residues of putative binding pocket are: 229E 3CL protease (2ZU2): His41, Asn141, Ala143, Cys144, His162; SARS main protease (5N19): His41, Phe140, Gly143, Ser144, Cys145, Met165, Glu166, Pro168, Asp187, Arg188, Gln189; NL63 3CL protease (5NH0): His41, Phe139, Cys144, Gly142, Ala143, Glu166, Gly168, Asp187, Gln188, Pro189, Ser190, Leu191; COVID-19 main protease (6M2Q): Pro39, His41, Asn142, Gly146, Leu167, Pro168, Thr169, Gly170, Gln189, Thr190, Ala191; COVID-19 PL protease (6WX4): Trp106, Asn109 Cys111, Tyr112, Leu162, Gly163, Asp164, Pro247, Pro248, Tyr264, Tyr268, Gln269, Cys270, Gly271, His272, Tyr273; COVID-19 RdRp (7AAP): Lys545, Arg555, Val557, Cys622, Asp623, Ser682, Thr687, Ala688, Asn691, Asp760, Ser814; COVID-19 Endoribonuclease (6WXC): Ser294, Cys293, His250, Val292, Lys290, Gln245, Lys345, Tyr343; COVID-19 ACE2-RBD (6M17): Gln24, Thr27, Phe28, Asp30, Lys31, His34, Glu35, Glu37, Asp38, Tyr41, Gln42, Leu79, Met82, Tyr83, Gln325, Glu329, Asn330, Lys353, Gly354, Asp355, Arg357, Arg393, Lys417, Gly446, Tyr449, Tyr453, Leu455, Phe456, Ala475, Phe486, Asn487, Tyr489, Gln493, Gly496, Gln498, Thr500, Asn501, Gly502, Tyr505; Ebolavirus envelope protein (6G9I): Leu59, Leu63, Arg64, Phe88, Lys95. Finally, the full energy minimization was carried out in the presence of bound ligand by the steepest descent (SD) method with the selection of AMBER force field.
Docking procedure
The setup of the docking platform was as follows: GA run 10, and GA search efficiency 200%. The binding pocket was identified by a two dimensional molecular mapping technique provided by LigPlot+ (European Molecular Biology Laboratory (EMBL), the Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 1 SD, UK); furthermore, the presumed binding pockets were defined as a supplementary material. In addition, the binding score ChemPLP, which adopts ChemScore to gauge the hydrogen bonding and multiple linear potentials to model van der Waals and repulsive terms, was used. The three dimensional docking structures were analyzed by Discovery Studio. Furthermore, the interacting amino acid residues of docking results were visualized and elucidated their interactions of 2-dimensional and 3-dimensional conformation using Discovery studio 3.5 and PyMOL (Schrödinger, New York, USA). The chemical structures calculation were executed by Online SMILES Translator and Structure File Generator website (https://cactus.nci.nih.gov/translate/) as well as bioactivity capability information of top five potential compounds were analyzed and collected from Pre AMEDT (https://preadmet.bmdrc.kr/). In terms of chemical and physical properties, the LogP value is applied for predicting the lipophilicity of chemicals as well as presuming solubility, permeability plasma binding, and adsorption of biological membrane. Furthermore, in order to collect more LogP value of a plethora of chemicals; in previous study, researcher developed an additive model (XLogP3) to predict the presumed LogP value of unknown compounds [35]. Additionally, topological molecular polar surface area (TPSA) is an essential descriptor that could indicate the transport efficiency of chemical compounds [36].
Results
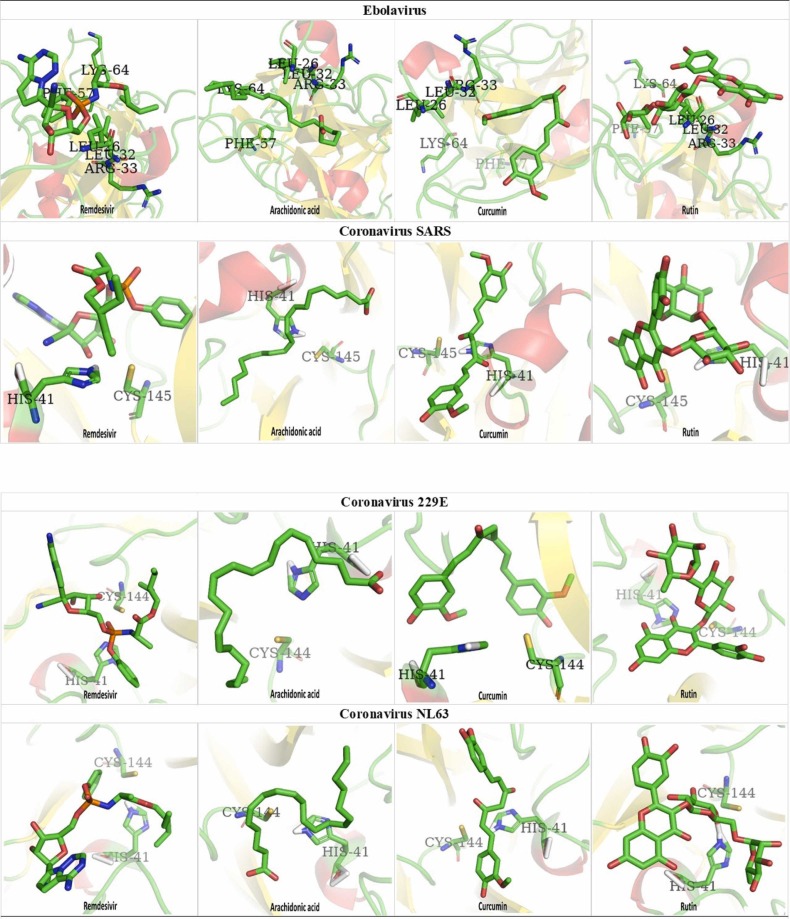
To search potential compounds for battling certain virus, the top 10 candidates in docking potency of clinic drugs and small molecules with Ebolavirus envelope protein are shown in Fig. S1. Unexpectedly, unsaturated fatty acid Arachidonic acid, Rutin, and Curcumin were highly bound with Ebolavirus envelope protein as well as anti-Ebolavirus molecule Remedesvir did. As evidence showed that unique amino acids of binding site for four potential molecules are located at Leu 57, Leu 63, Arg 64, Phe 88, and Lys 95 (Fig. S1). The viral 3CLpro enzyme controls coronavirus replication and is crucial for its life cycle. 3CLpro and PLpro are two imperative viral proteases liable for proteolysis, infection, and replication of the virus. Additionally, when small molecules and clinic drug were docked with coronavirus replicase polyprotein (3CLpro and PLpro) which is the main proteinase (Mpro) for cleaving the C-terminus of replicase polyprotein and the docking potency of top 10 candidates is represented in Fig. S2. Moreover, the Mpro of virus is a curtail polyprotein for assembly of the replicase-transcriptase complex and disruption of host responses; was also conducted to dock with tested compounds and the docking results of top 10 inhibitory candidate compounds were also depicted in Fig. S2. From Isophylogenetic tree analysis view, phytochemicals Curcumin, Rutin, and Arachidonic acid exhibited the same binding affinity profile as Ebolavirus envelope protein did (Fig. S2). As well as some clinical drugs such as Arbidol (Umifenovir), Darunavir, and Elvitegravir which have been applied to treat the acquired immune deficiency syndrome (AIDS), also could see those drugs performed good binding as a reference drug for anti-coronavirus by the suppression of viral activities as well.
Next to explore the basis of binding affinity of tested chemicals docked with selected viral protein molecules, the 3D dock-mapping of clinic drug and chemical compounds at Ebolavirus envelope protein and the replicase of coronavirus SARS, 229E, and NL63 are demonstrated in Fig. 1. From the results are shown, those of natural products such as Arachidonic acid, Rutin, and Curcumin had high ChemPLP docking score (SARS: Arachidonic acid 67.74, Rutin 69.62, Curcumin 64.14, Remedesvir 77.21; NL63: Arachidonic acid 67.19, Rutin 67.01, Curcumin 64.07, Remedesvir 72.90; 229E: Arachidonic acid 69.99, Rutin 73.51, Curcumin 65.56, Remedesvir 74.09; Ebola: Arachidonic acid 68.40, Rutin 61.08, Curcsumin 61.05, Remedesvir 62.92) with the specifically binding site, respectively. The computer analysis (Fig. 1, Table S1) revealed that Arachidonic acid, Rutin, Curcumin, and some anti-virus molecules Remdesivir (Ebola), Tenofovir alafenamide (HIV), Darunavir (HIV), Arbidol (HIV) etc. might affect the replicase of coronavirus furthermore inhibiting virus reproduction.
Fig. 1.
The 3D dock-mapping of clinic drug and chemicals at Ebolavirus envelope protein and the replicase of Coronavirus.
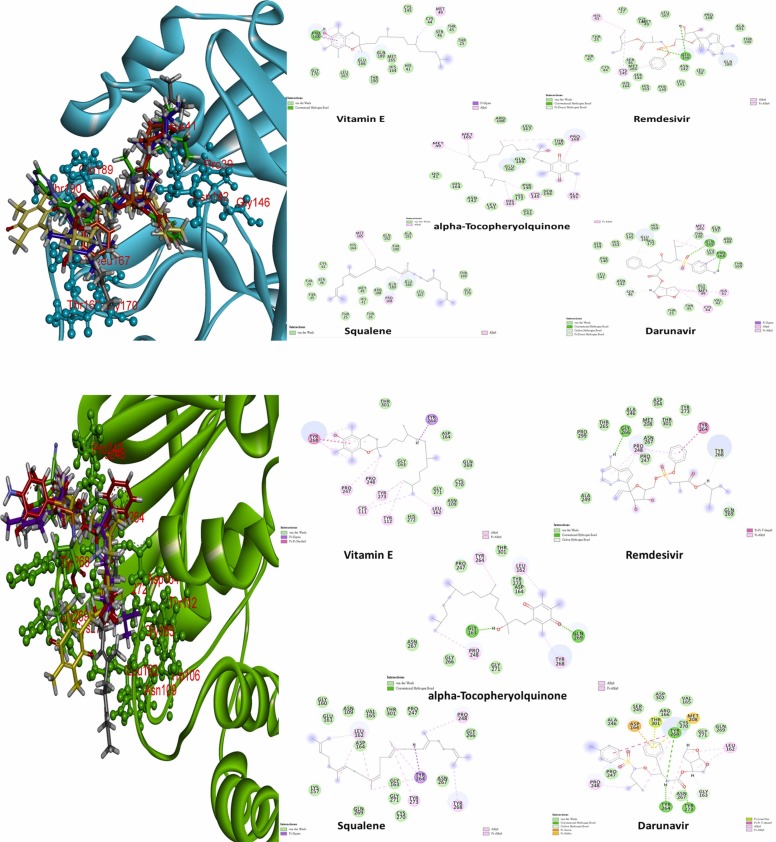
Strikingly, for a better understanding the interaction of potential candidates with putative binding pocket, the binding results are shown in Fig. 2. It portrays that the binding interaction with top five potential molecules as well as the 3D conformation of each compound at the putative binding pocket with Mpro and PLpro of SARS-CoV-2. In 3D mapping, the inhibitory compounds were decorated in five different colors (Squalene/black; α-tocopheryolquinone/yellow; Darunavir/red; Vitamin E/purple; Remdesivir/green). Form the figures, there are some hydrogen bonds between ligands and Mpro as well as those bonds showed in green dotted lines (Pro168/Vitamin E; Glu166/Remdesivir; Pro168 and Gln189/Darunavir). On the other hand, Gly163 of PLpro with α-tocopheryolquinone and Gly266 with Remdesivir molecules form sole one H-bond, respectively, compared to Darunavir with PLpro which composed two H-bonds at Tyr246 and Tyr268.
Fig. 2.
The 2D images of ligands-target protein interaction and 3D dock- superimpose mapping of candidate molecules with main(cyan) protease and COVID-19 PL (green) (Black: Squalene; Yellow: alpha-Tocopheryolquinone; Red: Darunavir; Purple: Vitamin E; Green: Remdesivir).
It is worth noting that this study provided three therapeutic strategies of anti-COVID-19 drug development via virus entry, virus proliferation, and immune evasion. The binding affinity between RdRp and natural compounds is shown in Table 1; For binding affinity of RdRp: Vitamin K> Rutin> Vitamin E > Arachidonic acid> Remdesivir> Amentoflavone> Vitamin D3 > Triterpenoid> Vitamin B2. In addition, there were only two compounds excessed 60 in the scoring function of Vitamin K and Rutin were 65.01 and 60.07, respectively. Furthermore, the scores of Vitamin E and Arachidonic acid were 59.57 and 56.47, respectively, and both of them were higher than Remdesivir was 53.47. With depicts in Table 2, the interaction group between EGCG and RdRp formed six hydrogen bonds; while four were found at Remdesivir and Rutin as well as three at Triterpenoid and Indirubin, separately, and one at Vitamin K. In Table 3, the overall scoring function of endoribonuclease was relevant higher than those of RdRp and ACE2-RBD groups. Especially, the scoring of Vitamin K and Amentoflavone approximately reached to 71.5 by contrast 64.23 of Remdesivir. There were some scores of natural products close to the score of Remdesivir such as Rutin, Vitamin E, Aconitine, and Arachidonic acid which were 65.67, 65.75, 62.34, and 61.15, respectively. The data revealed that there were five hydrogen bonds between EGCG and Endoribonuclease and four at Amentoflavone (Table 2). For the binding affinity of Endoribonclease: Amentoflavone> Vitamin K> Vitamin E > Rutin> Remdesivir> Aconitine> Arachidonic acid> EGCG> Nirmatrelvi> Chlorogenic acid> Vitamin B2 > Luteolin> Vitamin D3 > Mangiferin> Molnupiravir> Sapogenins> Quercetin> Indirubin> Catechin> Kaempferol> Baicalein> Vitamin A. It is worth mentioned that there was a common amino acid residue Gln245 appeared at the interaction groups of Remdesivir, Indirubin, and Arachidonic acid as well. Additionally, there were three hydrogen bonds were formed in each category. With respect to virus entry, the interaction scoring between co-crystallized ACE2-RBD structure and phytomedicinal compounds were represented in Table 4. For binding affinity of ACE-RBD: Arachidonic acid> EGCG> Vitamin E > Vitamin B2 > Amentoflavone> Catechin> Remdesivir> Rutin> Cucurbitacin E. Of note, the scoring functions of EGCG, Catechin, Amentoflavone, Aarachidonic acid, Vitamin E, Vitamin B2 were higher than Remdesivir which was 52.47. In accordance with Table 2, there was only one hydrogen bond was formed between Remdesivir and ACE2-RBD complex; in contrary to six at EGCG, three at Amentoflavone, two at Cucurbitacin E and Vitamin K, and one at Aconitine.
Table 1.
The binding affinity of phytocompounds compared to clinical drugs docked with RNA dependent RNA polymerase.
| Target | Clinical drug | Polyphenol | Triterpenoid | Flavonoid | Alkaloid | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RdRp PDB:7AAP | 121304016 | 53.47 | 65064 | 49.44 | 451674 | 52.00 | 5280805 | 60.07 | 10177 | 47.14 | 5280483 | 65.01 |
| Remdesivir | Epigallocatechin gallate | Triterpenoid | Rutin | Indirubin | Vitamin K | |||||||
| 155903259 | 45.97 | 5281647 | 44.33 | 44559416 | 47.63 | 5281600 | 52.79 | 245005 | 43.18 | 14985 | 59.57 | |
| Nirmatrelvi | Mangiferin | Cucurbitacine | Amentoflavone | Aconitine | Vitamin E | |||||||
| 145996610 | 41.93 | 1794427 | 41.66 | 5281319 | 44.56 | 5280863 | 47.31 | 165549 | 37.43 | 444899 | 56.47 | |
| Molnupiravir | Chlorogenic acid | Cucurbitacin E | Kaempferol | Sophoridine | Arachidonic acid | |||||||
| 6323266 | 35.72 | 1203 | 40.28 | 179651 | 37.73 | 5280343 | 46.29 | 114850 | 35.27 | 5280795 | 52.04 | |
| Tipiracil | Catechin | Limonin | Quercetin | Oxymatrine | Vitamin D3 | |||||||
| 370 | 33.37 | 21139920 | 37.42 | 5281605 | 43.22 | 24721085 | 35.12 | 493570 | 51.60 | |||
| Gallic Acid | Sapogenins | Baicalein | Sophocarpidine | Vitamin B2 | ||||||||
| 5280445 | 41.13 | 91466 | 34.98 | 445354 | 44.79 | |||||||
| Luteolin | Matrine | Vitamin A | ||||||||||
| 72378 | 33.28 | 171548 | 38.69 | |||||||||
| Lycorine | Biotin | |||||||||||
| 189377 | 31.05 | 8655 | 36.20 | |||||||||
| Alexine | Syringaldehyde | |||||||||||
| 29435 | 30.81 | 3314 | 33.90 | |||||||||
| 1-Deoxynojirimycin | Eugenol | |||||||||||
| 54670067 | 31.00 | |||||||||||
| Vitamin C | ||||||||||||
Table 2.
The intermolecular interaction between three COVID-19 infectious protein and phytomedicine.
| Genre |
H bond |
van der Waals |
|
|---|---|---|---|
|
COVID19 RdRp (PDB: 7AAP) |
Clinical Drug Remdesivir |
Ser549, Agr555, Asp760, Ser814 | Ile548, Lys551, Asp618, Asn691, Leu758, Glu811 |
|
Polyphenol Epigallocatechin gallate |
Asp618, Tyr619, Cys622, Asp623, Asp760, Asp761 | Lys621, Leu758, Ser759, Lys798, Trp800, Gluu811, Cys813, Ser814 | |
|
Triterpenoid Triterpenoid |
Ser682, Thr687, Ala688 | Lys545, Val557, Tyr619, Asp618, Cys622, Gly683, Asp684, Leu758, Ser,759, Asp760, Asp761, Cys813, Ser814 | |
|
Flavonoid Rutin |
Arg553, Thr556, Cys622, Asp623 | Val545, Arg555, Val557, Tyr619, Pro620, Lys621, Arg624, Ser682, Leu758, Ser759, Asp760, Asp761, Lys798, Glu811, Cys913, Ser814 | |
|
Alkaloid Indirubin |
Tyr619, Asp623, Asp760 | Trp617, Pro620, Asp761, Ala762 Lys798 | |
|
Other Vitamin K |
Lys621 | Trp617, Asp618, Tyr619, Asp623, Ser759, Asp760, Asp761, Ser814, Gln815 | |
|
Endoribonuclease (6WXC) |
Genre | H bond | van der Waals |
|
Clinical Drug Remdesivir |
Gln245, Gly248, Val292 | Asp240, His250, Lys290, Cys293, Ser294, Lys345 | |
|
Polyphenol Epigallocatechin gallate |
Gly248, His250, Ser294,Tyr343, Lys345 | Leu246, Gly247, Asn278, Cys293, Met331, Trp333, Thr341, Pro344, Leu346 | |
|
Triterpenoid Sapogenins |
– | Gln245, Gly247, Gly248, Asn278, Cys293, S294, Trp333, L345 | |
|
Flavonoid Amentoflavone |
Val292, Ser294, Pro343, Pro344 | Glu245, His250,Asn278, Lys290, Cys291, Cys293, Thr341, Leu346 | |
|
Alkaloid Indirubin |
Gln245, Gln248, His250 | His235, Leu246, Gly247, Ser294, Trp333, Glu340, Trp341, Lys345 | |
|
Other Arachidonic acid |
Gln245, Cys291, Val292 | Ser244, Leu246, Gly248, Ser289, Cys293, Ser294, Glu340, Thr341, Pro344, Lys345 | |
|
ACE2-RBD (6M17) |
Genre | H bond | van der Waals |
|
Clinical Drug Remdesivir |
Gln42 | Glu35, Asp38, Leu39, Glu75, Gln76, Leu452, Phe490, Leu492, Gln493, Ser494 | |
|
Polyphenol Epigallocatechin gallate |
Lys31, Asp38, Gln42,Tyr449, Leu492, Gln493 | Leu39, Asn450, Tyr451, Leu452, Phe490, Ser494 | |
|
Triterpenoid Cucurbitacin E |
Gln42, Tyr449 | Glu35, Asp38, Asn64, Asp67, Lys68, Ala71, Gln76 | |
|
Flavonoid Amentoflavone |
Gln35, Asp38, Tyr449 | His34, Leu39, Gln42, Asn450, Glu484, Leu492, Gln493, Ser494 | |
|
Alkaloid Aconitine |
Glu35 | Gln42, Leu452, Phe490, Leu492, Ser494 | |
|
Other Vitamin K |
Gly354, Arg393 | Glu37, Ser43, Ser44, Ser47, Trp349, Asp350, Leu351, Gly352, Phe356, Ala386, Phe390, Asn394 |
Table 3.
The binding affinity of phytocompounds compared to clinical drugs docked with endoribonclease.
| Target | Clinical Drug | Polyphenol | Triterpenoid | Flavonoid | Alkaloid | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endoribonuclease PDB:6WXC | 121304016 | 64.23 | 65064 | 57.76 | 21139920 | 54.00 | 5281600 | 71.81 | 245005 | 62.34 | 5280483 | 71.58 |
| Remdesivir | Epigallocatechin gallate | Sapogenins | Amentoflavone | Aconitine | Vitamin K | |||||||
| 155903259 | 57.60 | 1794427 | 56.84 | 451674 | 49.17 | 5280805 | 65.67 | 10177 | 53.80 | 14985 | 65.75 | |
| Nirmatrelvi | Chlorogenic acid | Triterpenoid | Rutin | Indirubin | Vitamin E | |||||||
| 145996610 | 54.21 | 5281647 | 54.56 | 44559416 | 48.62 | 5280445 | 55.96 | 165549 | 47.24 | 444899 | 61.15 | |
| Molnupiravir | Mangiferin | Cucurbitacine | Luteolin | Sophoridine | Arachidonic acid | |||||||
| 6323266 | 47.12 | 1203 | 53.42 | 179651 | 46.04 | 5280343 | 53.93 | 72378 | 47.08 | 493570 | 56.23 | |
| Tipiracil | Catechin | Limonin | Quercetin | Lycorine | Vitamin B2 | |||||||
| 370 | 44.35 | 5281319 | 42.54 | 5280863 | 52.84 | 114850 | 45.54 | 5280795 | 55.62 | |||
| Gallic Acid | Cucurbitacin E | Kaempferol | Oxymatrine | Vitamin D3 | ||||||||
| 5281605 | 52.43 | 24721085 | 45.11 | 445354 | 51.13 | |||||||
| Baicalein | Sophocarpidine | Vitamin A | ||||||||||
| 189377 | 44.68 | 171548 | 49.59 | |||||||||
| Alexine | Biotin | |||||||||||
| 91466 | 44.09 | 54670067 | 44.98 | |||||||||
| Matrine | Vitamin C | |||||||||||
| 29435 | 40.50 | 8655 | 42.89 | |||||||||
| 1-Deoxynojirimycin | Syringaldehyde | |||||||||||
| 3314 | 42.65 | |||||||||||
| Eugenol | ||||||||||||
Table 4.
The binding affinity of phytocompounds compared to clinical drugs docked with ACE2-RBD of SARS CoV-2.
| Target | Clinical Drug | Polyphenol | Triterpenoid | Flavonoid | Alkaloid | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE2-RBD PDB:6M17 | 121304016 | 52.47 | 65064 | 56.33 | 5281319 | 50.14 | 5281600 | 54.70 | 245005 | 47.46 | 444899 | 60.90 |
| Remdesivir | Epigallocatechin gallate | Cucurbitacin E | Amentoflavone | Aconitine | Arachidonic acid | |||||||
| 145996610 | 42.81 | 1203 | 53.42 | 44559416 | 45.92 | 5280805 | 50.42 | 72378 | 47.09 | 14985 | 55.76 | |
| Molnupiravir | Catechin | Cucurbitacine | Rutin | Lycorine | Vitamin E | |||||||
| 155903259 | 37.86 | 1794427 | 48.72 | 451674 | 43.73 | 5280445 | 47.87 | 10177 | 38.78 | 493570 | 55.37 | |
| Nirmatrelvi | Chlorogenic acid | Triterpenoid | Luteolin | Indirubin | Vitamin B2 | |||||||
| 6323266 | 36.05 | 5281647 | 48.43 | 179651 | 30.50 | 5280343 | 46.57 | 29435 | 37.79 | 54670067 | 45.78 | |
| Tipiracil | Mangiferin | Limonin | Quercetin | 1-Deoxynojirimycin | Vitamin C | |||||||
| 370 | 38.18 | 21139920 | – | 5280863 | 43.76 | 189377 | 37.59 | 3314 | 41.63 | |||
| Gallic Acid | Sapogenins | Kaempferol | Alexine | Eugenol | ||||||||
| 5281605 | 41.02 | 114850 | – | 171548 | 34.67 | |||||||
| Baicalein | Oxymatrine | Biotin | ||||||||||
| 91466 | – | 8655 | 31.22 | |||||||||
| Matrine | Syringaldehyde | |||||||||||
| 24721085 | – | 445354 | – | |||||||||
| Sophocarpidine | Vitamin A | |||||||||||
| 165549 | – | 5280795 | – | |||||||||
| Sophoridine | Vitamin D3 | |||||||||||
| 5280483 | – | |||||||||||
| Vitamin K | ||||||||||||
In terms of bioactivities, the collected ADMET information is exemplified in Table S2. All tested compounds were passed through the ADME filter. All tested compounds showed the considerable aqueous solubility and lipophilicity. Depending on molecular properties such as the molecular weight (MW), the number of hydrogen bond acceptors, donors, and partition coefficient (LogP) have been formulated. As far as chemical and physical properties is concerned, the top five hit molecules had been analysed by Lipinski's rule of five (RO5); moreover, the methodology of RO5 is considered by MW values (MW<500), numbers of hydrogen bond acceptors (HBA ≤10) and donors (HBD ≤5), and LogP value (LogP<5). Based on the principle of RO5, the data are shown in Table S3, the range of MW values was from 400 to 605 g/mol as well as the TPSA values of compounds was observed to be in the range from 0 to 203.57 Å2. All tested compounds obeyed RO5 and do not show any violation. Therefore, tested compounds were predicted to have a good bioavailability. In addition, the numbers of hydrogen bond acceptors and donors of Squalene, α-tocopheryolquinone, Darunavir, and Vitamin E located at a suitable range of RO5. The data exposed that those five candidate chemicals located into the appropriate range as well as illustrated the prospective for further drug discovery. Furthermore, tested compounds do not show any violations in Ghose, Veber, Egan, and Muegge filter. Besides, pharmacokinetic behaviours such as GI absorption and blood-brain barrier (BBB) permeate were also calculated. Likewise, tested compounds showed high GI absorption, suggesting both as better absorption quality on oral administration. It also showed that all tested compounds cannot penetrate the BBB, therefore it has less possibility to produce neurotoxicity. Regarding metabolism predictions, the program analyses if the molecules are P-glycoprotein (P-gp) substrates. P-gp substrate plays a role to protect the brain from toxic substances [37]. Fascinatingly, all tested compounds resulted “yes” for P-gp substrate. The webserver also predicts whether a compound can be capable of inhibiting 5 CYP isoenzymes or not [38]. Surprisingly, all tested compounds resulted “no” for 5 CYP isoenzymes inhibition. That means all tested compounds will not produce any toxic or not all possible adverse effects of such compounds. This online server also allows us to identify potentially problematic fragments such as PAINS (Panassay interference compounds) that are responsible for the false positive results in high-throughput screens [39]. All tested compounds did not contain any type of problematic fragments. That means proposed compound did not tend to non-specifically react with numerous biological targets rather than specifically affecting one desired target. Thus, considering all ADMET parameters together, we can conclude that all selected compounds would be useful for discovery and development of new purpose or repurpose drug in further study.
Discussion
Molecule docking is a primary medicinal screening approach based on bioinformatics and drug design assistance for exploring potential candidate drugs. The mainstream purpose of molecule docking is to envisage the interaction of small molecules with the known three dimensional (3D) structure of target proteins. In the computer analysis, the molecule docking simulation could be applied to virtually screen the binding affinity of chemical ligands interacted with template proteins, rank the binding score, and assume the inhibition efficacy of small molecules with target proteins [40]. In previous literature, the virtual screening of small molecules selected some natural products like flavonoids have been reported to find the inhibitors of SARS-CoV Mpro [41] for restraining coronavirus reproduction [23]. At present, there are several agents such as Remdesivir, Nirmatrelvir, and Molnupiravir are now used for the treatment of SARS-CoV-2 infection, however effective treatment options remain very limited.
Apparently, this study presented that some natural compounds i.e. polyphenol and flavonoid are categorized as anti-inflammatory substances which could inhibit inflammation by different acting mechanisms [42], [43]. It is worth perceiving, those compounds have not only anti-inflammatory properties but also other biological activities such as antibacterial, antiviral, antioxidant, and inhibition of enzyme [44]. In the light of high-risk epidemic virus impact on human society, neither unique vaccine nor specific treatment to be used so far, thus some scientists delicate in exploring the potential drug against virus infection. Interestingly, combinations of some natural compounds performed in enzymatic assay have been reported to observe the unexpected and effective results in previous studies [45], [46]. Furthermore, the investigation showed that anti-SARS-CoV-2 treatment could propose separately from two optional sites, one is from Host-based side ACE2 and the other one is Virus-based side (targets as shown in this study). The first line emphases when host was infected by virus, the immune cells would attack the pathogens to destroy them and produce the equivalent antibody for defending and protecting the host. In the second step, applicable drug would help the host to prevent or cease virus invasion via interfering virus cell cycle or thwart the S-protein attached the host cell membrane by inhibiting interaction between the virus and the host cell receptor [47]. Therefore, some antiviral drugs were available to suppress the coronavirus reproduction i.e. Remdesivir which is an adenosine analogue can insert the viral RNA and lead to stopping viral mutation. Besides, the Chloroquine also has revealed that it could interfere the glycosylation of SARS-CoV-2 and block the virus’ membrane to fuse into host cell [48]. Remarkably, some clinical drugs such as protease inhibitor (Paxlovid) [49], adenosine analogue (Remdesivir) [50], and antiretroviral drug (Darunavir) [51] were used in this study as the positive control to do the comparisons with tested natural compounds for partial validation of virtual screen. Additionally, RdRp also plays the other core character at SARS-CoV-2 replication; thereby, a plethora of researches was dedicated in inhibiting the activity of RNA-directed 5'− 3' polymerase [52]. Due to time consuming of new drug discovery and development, the current anti-virus drugs reposition were considered at the beginning of COVID-19 pandemic for an urgent need. Hence, nucleotide inhibitors such as Sofosbuvir, Ribavirin, Galidesivir, Remdesivir, Favipiravir, Cefuroxime, Tenofovir, and Hydroxychloroquine have been demonstrated at in silico study against RdRp to restrict COVID-19 replication [53]. Unfortunately, based on the adverse or side effects from the repurposed drugs, phytomedicines such as natural products, essential oil, and extraction from herbal plants might play a vital role to moderate those effects and conduct the therapeutic regimes due to less adverse effect of phytomolecules. In computational screen, accumulative evidence demonstrated that Gingerol, Rutin, Luteolin, Aalvianolic acid A, rosmarinic acid, and p-coumaric acid exerted an unexpected stronger binding affinity with RdRp when compared with repurposed anti-virus drugs [54], [55]. It is worth to mention that essential oil compounds were reported as an anti-COVID-19 RdRp treatment via molecular docking aspect [56]. Especially, the previously research portrayed in molecular docking perspective that is small interfering RNA (siRNA) also was applied in silencing the post-transcriptional gene of COVID-19 by encoding RdRp gene sequence and turning off gene expression [57].
Taken a deep insight of drug development, anti-COVID-19 regime could be divided into three strategies such as virus entry, virus proliferation, and immune evasion which could attribute to interact with ACE2-RBD, proteases as well as RdRp, and endoribonuclease. Thereby, according to National Institutes of Health COVID-19 treatment guidelines, a plenty of antiviral drugs are approved by EAU or under evaluated for combating with COVID-19 such as Remdesivir, Chloroquine, Hydroxychloroquine, Azithromycin, Ivermectin, HIV protease inhibitors, and Nitazoxanide etc.; moreover, Zanamivir, Indinavir, Saquinavir, and Remdesivir are among the exciting hits on the Mpro proteinase [58]. Unfortunately, those drugs still bring some undesirable adverse effects to patients. Taking Remdesivir for an instance, it can cause the gastrointestinal symptoms (disorder), elevated transaminase levels as well as prothrombin time, and hypersensitivity reactions [59], [60]; moreover, if taking Chloroquine or Hydroxychloroquine for the treatment of COVID-19 patients that would induce some cardiovascular problems such as QTc prolongation, Torsades de Pointes, ventricular arrytmia, and cardiac deaths [61]. Clinically, the adverse effects of Ivermectin would be dizziness, pruritis, nausea, diarrhoea, and neurological side effects [62]; additionally, the HIV protease inhibitors would induce nausea, vomiting, and diarrhoea [63]. Therefore, in previous research indicated that a traditional Chinese medicine formula could disrupt the progression of COVID-19 [64]; and this formula was composed of several bioactive compounds i.e. alkaloids, flavones, and flavonoids as well as those phytochemicals have been studied in numerous pharmaceutical activities such as anti-virus, anti-inflammatory, antioxidant etc.[65], [66], [67], [68], [69], [70], [71]. Remarkably, the investigation showed that 3CLpro and PLpro are the crucial targets to discover and develop the anti-coronavirus (SARS-CoV, MERS-CoV) drug design [72]. Furthermore, many studies have demonstrated that the bioactivities of several chemicals such as polyphenols (Catechin gallate, EGCG, Gallocatechin gallate (GCG), 4'-O-methylbavachalcone, Resveratrol, Tannic acid, 3-isotheaflavin-3-gallate (TF2B); flavonoids (Herbacetin, Bavachinin, Neobavaisoflavone, Apigenin, Luteolin, Myricetin, Scutellarein, Sinigrin, Hesperetin, Quercetin); benzopyrans (Psoralidin, Corylifol A, Pectolinarin); protease (Tomentin); chalcones (Isobavachalcone); indoles (Indigo); saccharides (Rhoifolin), tetra-O-galloyl-beta-D-glucose (TGG); Glucosinolates (sinigrin); and Anthraquinones (aloe emodin) have been suggested to inhibit Mpro and PLpro which are an essential role to impact the coronavirus cell cycle by in vitro and in silico study [72], [73], [74], [75], [76], [77], [78], [79]. Particularly, flavonoids (Herbacetin, Rhoifolin, and Pectolinarin) were found to efficiently block the enzymatic activity of SARS-CoV Mpro [23]; additionally, Kaempferol, Quercetin, and Rutin have the potential to be developed as novel inhibitors of SARS-CoV-2 Mpro with a comparable/higher potency as that of Remdesivir [80]. Furthermore, four HCV protease inhibitor drugs, Simeprevir, Vaniprevir, Paritaprevir, and Grazoprevir with Remdesivir inhibit the SARS CoV-2 PLpro [81]. One study showed that Amentoflavone, Rubranoside B, Savinin, Psoralidin, Hirsutenone, and Papyriflavonol A are good drug candidate for the search of antibiotics against PLpro and Mpro [82]. Nine natural biflavones were also confirmed to be effective PLpro inhibitors with IC50 values ranging from 9.5 to 43.2 μM via anti-proteolytic activity determination [83].
Considering first location of SARS-CoV-2 infection occurred in the airway mucosa or lung of most patients, and most oral drugs can’t attain the sufficient anti-viral concentration. The in situ (local) drug administration by inhalation spray can afford a locally effective dose to solve some of liabilities such as instability, poor solubility, low bioavailability, poor selectivity, and multiple modes of assay interference particular in curcuminoids. Fascinatingly, a curcumin-based spray formulation for anti-SARS-CoV-2 treatment was developed and has finished phase 2 clinical trial [84], and delivers a very important application value in drug formulation and treated strategy designs for the anti-SARS-CoV-2 candidate that targeting on viral S-protein, especially infected prevention.
Of note, PLpro of SARS-CoV-2 is not only a core character in virus translation/replication but also is a serious issue to explore from immune evasion standpoint. Thereby, PLpro of SARS-CoV-2 could reverse the post-translational modification of cellular proteins conjugated with ubiquitin or (Ub) or Ub-like interferon-stimulated gene product 15 (ISG15) via deubiquitination and deISGylation process [85]. Additionally, Pro-ISG15 cleavage assays further demonstrated that several biflavones exhibited potent inhibitory effects against PLpro-mediated deISGylation, a key process involved in viral immune evasion [83]. Furthermore, ORF8-IRF3 (open-reading frame 8-interferon regulatory factor 3) complex could induce the endoplasmic reticulum stress that COVID-19 virus should evade from host immune response [86]. Moreover, a computational molecular study found that the potent inhibitors (Quercetin, 3-O-(6″-galloyl)-beta-D-galactopyranosid, Tribuloside, and Rutin) could prevent the immune evasion of Coivd-19 [87]. And NSP10-NSP16 were demonstrated that they could avoid SARS-CoV-2 from detecting by immune recognition of host cell, a pervious study suggested that Mitocurcumin (MC) could potentially inhibit the activity of NSP3 [88]. Taken a deep insight into long COVID and multi-system inflammatory syndrome in Children (MIS-C) has been realized a critical and emerging issue for society health. Clinically, the common diagnosis of MSI-C contains fever, severe illness, and the involvement of two or more organ systems failure; while some clinical characters of MIS-C resemble Kawasaki Disease, toxic shock syndrome (TSS), and secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome [89].
Clinically, dexamethasone (a synthetic corticosteroid) would limit the production of and damaging effect of the cytokines and may be useful for the short-term in severe, intubated, COVID-19 patients, but will also inhibit the protective function of T cells and block B cells from making antibodies, potentially leading to increased plasma viral load that will persist after a patient survives SARS. These corticosteroids could be given together with the natural flavonoid quercetin and luteolin because of theirs antiviral and anti-inflammatory properties, especially its ability to inhibit mast cells, which are the main source of cytokines in the lungs [90]. Moreover, phytocompounds flavonoids exhibit a high safety profile, suitable bioavailability, and no significant adverse effects [91]. Notably, there severe inflammation and cytokine storms are the most fatal etiology of COVID-19. There are several scientific reports of phytocompounds can bind and affect COVID-19 relative cytokines pathways, such as curcumin, quercetin, kaempferol, and luteolin can form binding interaction with TNF, IL-6, and nuclear factor kappa-light chain enhancer of activated B cells (NF-κB) [92], [93], [94]. As anti-inflammatory role of dexamethasone, phytocompounds can also interfere cytokine production, affect inflammatory response and attenuate immunity with less intense of adverse effect when compared with dexamethasone.
The adjuvant co-supplementation of 168 mg curcumin, 260 mg quercetin, and 9 µg (360 IU) of cholecalciferol (CQC) may possibly have a therapeutic role in the early stage of COVID-19 infection including resolution of acute symptoms, and modulation of the hyperinflammatory response to moderate symptoms of COVID-19 [95]. Focused on flavonoids, especially epicatechin, EGCG , hesperidin, naringenin, quercetin, rutin, luteolin, baicalin, diosmin, genistein, biochanin A, and silymarin, which can counteract the virus-mediated elevated levels of inflammatory cytokines leading to multiple organ failure in silico, in vitro, and in vivo findings [96]. Flavonoids such as quercetin, genistein, apigenin, kaempferol, and epigallocatechin 3-gallate modulate the expression and activation of cytokines such as IL-1β, TNF-α, IL-6, and IL-8; regulate the gene expression of many pro-inflammatory molecules such as NF-κB, activator protein-1 (AP-1), intercellular adhesion molecule-1 (ICAM), vascular cell adhesion molecule-1 (VCAM), and E-selectins; and also inhibits inducible nitric oxide (NO) synthase, cyclooxygenase-2 (COX-2), and lipoxygenase, which are pro-inflammatory enzymes [97]. Based on the observations, taken 1000 mg quercetin daily plus the antiviral drugs Remdesivir or Favipiravir is safe and effective in lowering the serum levels of ALP, q-CRP, and LDH as critical markers involved in COVID-19 severity [98]. There is evidence that vitamin C and quercetin co-administration exerts a synergistic antiviral action due to overlapping antiviral and immunomodulatory properties and the capacity of ascorbate to recycle quercetin, increasing its efficacy as an adjunct to Remdesivir [99]. Scantly, squalene has been applied as an adjuvant of COVID-19 vaccine to augment protective immunity [100]. There are several clinical trials of phytocompounds used for the COVID-19 treatments within three years, include quercetin, isoquercetin, and curcumin. The treated dose of quercetin between 400 and 1000 mg/daily (NCT04578158, NCT04377789, NCT04861298), isoquercetin between 500 and 100 mg/daily (NCT04622865, NCT04536090), and curcumin between 160 and 500 mg/daily [94]. Some of those clinical trial of all three phytocompounds have been entered phase 2 or phase 3, indicating those phytocompounds have the potential to be the candidate drugs for COVID-19 treatment. Of note, the most use dosage of phytocompounds is higher due to lower bioavailability, less toxicity, and few adverse events. Moreover, herb-herb interactions or herb-drug interactions need to be taken into deep insight.
Unfortunately, there are not any appropriate approach as well as precision medicine to combat SARS-CoV-2 infection. Hereafter, this study was tried to find the candidate phytomedicines for battling the COVID-19 pandemic crisis. Henceforth, the search of anti-COVID from natural compounds (phytochemicals) as phytomedicines according to anti-inflammation, or underwent anti-virus drugs to investigate the potential hits against the epidemic virus particular SARS-CoV-2 for discovery and development of new drug. Notably, compared with in silico results, the reconfirmation of Vitamin K, Amentoflavone, EGCG, Sapogenins, Aconitine, Curcumin, Arachidonic acid, and Rutin have highly prospective to become a hit of natural products against the epidemic virus particular SARS-CoV-2 (COVID-19) based on multi-targets effects for blocking viral infection through invasion/activation, transcription/reproduction, and posttranslational cleavage.
Until now, in the face of the rapid mutation of SARS-CoV-2 and the continuous emergence of new mutant variants, based on the single targeting vaccination or treated strategies to fight with COVID-19 can only acquire a limited result. It is important and urgent to formulate the more comprehensively integrated therapeutic or preventive strategies for SARS-CoV-2 infection, replication, immune evasion and host immune response. Conclusively, this study provides a new or novel in silico evidence-based therapeutic perspective for developing the multi-target or cocktail therapies as HIV for combating the COVID-19 pandemics.
Sources of support
None.
Funding
The financial support received from Xiamen Medical College (K2019-03).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.11.022.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Trougakos I.P., Stamatelopoulos K., Terpos E., Tsitsilonis O.E., Aivalioti E., Paraskevis D., et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci. 2021;28:9. doi: 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohlfing A.K., Rath D., Geisler T., Gawaz M. Platelets and COVID-19. Hamostaseologie. 2021;41:379–385. doi: 10.1055/a-1581-4355. [DOI] [PubMed] [Google Scholar]
- 3.Elena S.F. Evolutionary transitions during RNA virus experimental evolution. Philos Trans R Soc Lond B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sola I., Almazan F., Zuniga S., Enjuanes L. Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu Rev Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai J., Zhang H., Zhang Y., Lin K., Zhang Y., Wu J., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Li Q., Liang Z., Li T., Liu S., Cui Q., et al. The significant immune escape of pseudotyped SARS-CoV-2 variant omicron. Emerg Microbes Infect. 2022;11:1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukaina M., Hasan M.M., Essar M.Y. Emergence of omicron BA.1 and BA.2 variants and concern over vaccine breakthrough infection. Ann Med Surg (Lond) 2022;79 doi: 10.1016/j.amsu.2022.103941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tekin A.B., Yassa M., Birol Ilter P., Yavuz E., Onden B., Usta C., et al. COVID-19 related maternal mortality cases in associated with Delta and Omicron waves and the role of lung ultrasound. Turk J Obstet Gynecol. 2022;19:88–97. doi: 10.4274/tjod.galenos.2022.36937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar-Debbiny R., Gronich N., Weber G., Khoury J., Amar M., Stein N., et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Santos W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharm. 2020;129 doi: 10.1016/j.biopha.2020.110493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allam A.E., Amen Y., Ashour A., Assaf H.K., Hassan H.A., Abdel-Rahman I.M., et al. In silico study of natural compounds from sesame against COVID-19 by targeting M(pro), PL(pro) and RdRp. RSC Adv. 2021;11:22398–22408. doi: 10.1039/d1ra03937g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezaei N., Ashkevarian S., Fathi M.K., Hanaei S., Kolahchi Z., Ladi Seyedian S.S., et al. Introduction on coronavirus disease (COVID-19) pandemic: the global challenge. Adv Exp Med Biol. 2021;1318:1–22. doi: 10.1007/978-3-030-63761-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajad N.G., Rayala S., Gutti G., Sharma A., Singh M., Kumar A., et al. Systematic review on role of structure based drug design (SBDD) in the identification of anti-viral leads against SARS-Cov-2. Curr Res Pharm Drug Disco. 2021;2 doi: 10.1016/j.crphar.2021.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillon M.C., Frazier M.N., Dillard L.B., Williams J.G., Kocaman S., Krahn J.M., et al. Cryo-EM structures of the SARS-CoV-2 endoribonuclease Nsp15 reveal insight into nuclease specificity and dynamics. Nat Commun. 2021;12:636. doi: 10.1038/s41467-020-20608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y., Wower J., Maltseva N., Chang C., Jedrzejczak R., Wilamowski M., et al. Tipiracil binds to uridine site and inhibits Nsp15 endoribonuclease NendoU from SARS-CoV-2. Commun Biol. 2021;4:193. doi: 10.1038/s42003-021-01735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P.M., Ravindra P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel J.R., Tripathi P., Sharma V., Chauhan N.S., Dixit V.K. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol. 2011;138:286–313. doi: 10.1016/j.jep.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Liu X., Qiu Z., Zhao G. Microbial synthesis of plant polyphenols. Sheng Wu Gong Cheng Xue Bao. 2021;37:2050–2076. doi: 10.13345/j.cjb.200747. [DOI] [PubMed] [Google Scholar]
- 23.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzym Inhib Med Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuman B.W., Buchmeier M.J. Supramolecular architecture of the coronavirus particle. Adv Virus Res. 2016;96:1–27. doi: 10.1016/bs.aivir.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoids against coronavirus infection. Chem Biol Inter. 2020;328 doi: 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Zhang X., Bi K., He Y., Yan W., Yang C.S., et al. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci Technol. 2021;114:11–24. doi: 10.1016/j.tifs.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warowicka A., Nawrot R., Gozdzicka-Jozefiak A. Antiviral activity of berberine. Arch Virol. 2020;165:1935–1945. doi: 10.1007/s00705-020-04706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones G., Willett P., Glen R.C., Leach A.R., Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Lin D., Kusov Y., Nian Y., Ma Q., Wang J., et al. alpha-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63:4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.C., Kuo C.J., Ko T.P., Hsu M.F., Tsui Y.C., Chang S.C., et al. Structural basis of inhibition specificities of 3C and 3C-like proteases by zinc-coordinating and peptidomimetic compounds. J Biol Chem. 2009;284:7646–7655. doi: 10.1074/jbc.M807947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharm Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rut W., Lv Z., Zmudzinski M., Patchett S., Nayak D., Snipas S.J., et al. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: a framework for anti-COVID-19 drug design. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y., Ren J., Fry E.E., Xiao J., Townsend A.R., Stuart D.I. Structures of ebola virus glycoprotein complexes with tricyclic antidepressant and antipsychotic drugs. J Med Chem. 2018;61:4938–4945. doi: 10.1021/acs.jmedchem.8b00350. [DOI] [PubMed] [Google Scholar]
- 35.Cheng T., Zhao Y., Li X., Lin F., Xu Y., Zhang X., et al. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J Chem Inf Model. 2007;47:2140–2148. doi: 10.1021/ci700257y. [DOI] [PubMed] [Google Scholar]
- 36.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 37.Elsinga P.H., Hendrikse N.H., Bart J., Vaalburg W., van Waarde A. PET Studies on P-glycoprotein function in the blood-brain barrier: how it affects uptake and binding of drugs within the CNS. Curr Pharm Des. 2004;10:1493–1503. doi: 10.2174/1381612043384736. [DOI] [PubMed] [Google Scholar]
- 38.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 40.Morris G.M., Lim-Wilby M. Molecular docking. Methods Mol Biol. 2008;443:365–382. doi: 10.1007/978-1-59745-177-2_19. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Bao B.B., Song G.Q., Chen C., Zhang X.M., Lu W., et al. Discovery of unsymmetrical aromatic disulfides as novel inhibitors of SARS-CoV main protease: Chemical synthesis, biological evaluation, molecular docking and 3D-QSAR study. Eur J Med Chem. 2017;137:450–461. doi: 10.1016/j.ejmech.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanput W., Krueyos N., Ritthiruangdej P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int Immunopharmacol. 2016;40:170–175. doi: 10.1016/j.intimp.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Van Hoorn D.E., Nijveldt R.J., Van Leeuwen P.A., Hofman Z., M'Rabet L., De Bont D.B., et al. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur J Pharm. 2002;451:111–118. doi: 10.1016/s0014-2999(02)02192-1. [DOI] [PubMed] [Google Scholar]
- 44.Cos P., Ying L., Calomme M., Hu J.P., Cimanga K., Van Poel B., et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–76. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 45.Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.W., Cho J.K., et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzym Inhib Med Chem. 2014;29:59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 46.Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., et al. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg Med Chem. 2013;21:3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Disco. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reina J., Iglesias C. Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination. Rev Esp Quim. 2022;35:236–240. doi: 10.37201/req/002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aleissa M.M., Silverman E.A., Paredes Acosta L.M., Nutt C.T., Richterman A., Marty F.M. New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother. 2020;65 doi: 10.1128/AAC.01814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina J.-M., Squires K., Sax P.E., Cahn P., Lombaard J., DeJesus E., et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 96-week results of a randomised, double-blind, non-inferiority, phase 3 trial. Lancet HIV. 2020;7:e16–e26. doi: 10.1016/S2352-3018(19)30336-4. [DOI] [PubMed] [Google Scholar]
- 52.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;117592 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn. 2021;39:3204–3212. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selvaraj J., Rekha U.V., Jh S.F., Sivabalan V., Ponnulakshmi R., Vishnupriya V., et al. Molecular docking analysis of SARS-CoV-2 linked RNA dependent RNA polymerase (RdRp) with compounds from Plectranthus amboinicus. Bioinformation. 2021;17:167–170. doi: 10.6026/97320630017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar Verma A., Kumar V., Singh S., Goswami B.C., Camps I., Sekar A., et al. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed Pharm. 2021;137 doi: 10.1016/j.biopha.2021.111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva J., Figueiredo P.L.B., Byler K.G., Setzer W.N. Essential oils as antiviral agents. potential of essential oils to treat SARS-CoV-2 infection: an in-silico investigation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shawan M., Sharma A.R., Bhattacharya M., Mallik B., Akhter F., Shakil M.S., et al. Designing an effective therapeutic siRNA to silence RdRp gene of SARS-CoV-2. Infect Genet Evol. 2021;93 doi: 10.1016/j.meegid.2021.104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall D.C., Jr., Ji H.F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopkins B.J., Prokesch B.C. Anaphylaxis due to remdesivir. Antimicrob Agents Chemother. 2021 doi: 10.1128/AAC.00233-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veronin M.A., Lang A., Reinert J.P. Remdesivir and coronavirus disease 2019 (COVID-19): essential questions and answers for pharmacists and pharmacy technicians. J Pharm Technol. 2020;37:62–74. doi: 10.1177/8755122520967634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen L.S., Dolladille C., Drici M.D., Fenioux C., Alexandre J., Mira J.P., et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the world health organization pharmacovigilance database. Circulation. 2020;142:303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandler R.E. Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98:382–388. doi: 10.4269/ajtmh.17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai K.C., Huang Y.C., Liaw C.C., Tsai C.I., Chiou C.T., Lin C.J., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed Pharm. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo K., Tong C., Fu Q., Xu J., Shi S., Xiao Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by HPLCDADQTOF-MS/MS. J Pharm Biomed Anal. 2019;170:153–160. doi: 10.1016/j.jpba.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 66.Batsukh Z., Toume K., Javzan B., Kazuma K., Cai S.Q., Hayashi S., et al. Characterization of metabolites in Saposhnikovia divaricata root from Mongolia. J Nat Med. 2021;75:11–27. doi: 10.1007/s11418-020-01430-9. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H.Q., Liu P., Duan J.A., Dong L., Shang E.X., Qian D.W., et al. Hierarchical extraction and simultaneous determination of flavones and triterpenes in different parts of Trichosanthes kirilowii Maxim. by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal. 2019;167:114–122. doi: 10.1016/j.jpba.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Wang B.S., Huang G.J., Tai H.M., Huang M.H. Antioxidant and anti-inflammatory activities of aqueous extracts of Schizonepeta tenuifolia Briq. Food Chem Toxicol. 2012;50:526–531. doi: 10.1016/j.fct.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Li T., Hua S., Ma J., Dong L., Xu F., Fu X. Spectrum-Effect Relationships of Flavonoids in Glycyrrhiza uralensis Fisch. J Anal Methods Chem. 2020;2020:8838290. doi: 10.1155/2020/8838290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Q., Wei R., Wang Z., Liu W., Sang Z., Li Y., et al. Bioactive alkaloids from the aerial parts of Houttuynia cordata. J Ethnopharmacol. 2017;195:166–172. doi: 10.1016/j.jep.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. 2018;56:465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park J.Y., Ko J.A., Kim D.W., Kim Y.M., Kwon H.J., Jeong H.J., et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzym Inhib Med Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 73.Chen C.N., Lin C.P., Huang K.K., Chen W.C., Hsieh H.P., Liang P.H., et al. Inhibition of SARS-CoV 3C-like Protease Activity by Theaflavin-3,3'-digallate (TF3) Evid Based Complement Altern Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen T.T., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W., et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roh C. A facile inhibitor screening of SARS coronavirus N protein using nanoparticle-based RNA oligonucleotide. Int J Nanomed. 2012;7:2173–2179. doi: 10.2147/IJN.S31379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.Y., Kim D., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rehman M.T., AlAjmi M.F., Hussain A. Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): a molecular docking and simulation approach to combat COVID-19. Curr Pharm Des. 2021;27:3577–3589. doi: 10.2174/1381612826999201116195851. [DOI] [PubMed] [Google Scholar]
- 81.Bafna K., White K., Harish B., Rosales R., Ramelot T.A., Acton T.B., et al. Hepatitis C virus drugs that inhibit SARS-CoV-2 papain-like protease synergize with remdesivir to suppress viral replication in cell culture. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.da Cunha L., Tizziani T., Souza G.B., Moreira M.A., Neto J.S.S., Dos Santos C.V.D., et al. Natural products with tandem anti-inflammatory, immunomodulatory and Anti-SARS-CoV/2 effects: a drug discovery perspective against SARS-CoV-2. Curr Med Chem. 2021 doi: 10.2174/0929867328666210726094955. [DOI] [PubMed] [Google Scholar]
- 83.Li L., Ma L., Hu Y., Li X., Yu M., Shang H., et al. Natural biflavones are potent inhibitors against SARS-CoV-2 papain-like protease. Phytochemistry. 2021;193 doi: 10.1016/j.phytochem.2021.112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hellou E., Mohsin J., Elemy A., Hakim F., Mustafa-Hellou M., Hamoud S. Effect of Artemic in patients with COVID-19: A Phase II prospective study. J Cell Mol Med. 2022;26:3281–3289. doi: 10.1111/jcmm.17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roy R., Jonniya N.A., Poddar S., Sk M.F., Kar P. Unraveling the molecular mechanism of recognition of human interferon-stimulated gene product 15 by coronavirus papain-like proteases: a multiscale simulation study. J Chem Inf Model. 2021;61:6038–6052. doi: 10.1021/acs.jcim.1c00918. [DOI] [PubMed] [Google Scholar]
- 86.Rashid F., Suleman M., Shah A., Dzakah E.E., Wang H., Chen S., et al. Mutations in SARS-CoV-2 ORF8 altered the bonding network with interferon regulatory factor 3 to evade host immune system. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albutti A. Rescuing the host immune system by targeting the immune evasion complex ORF8-IRF3 in SARS-CoV-2 infection with natural products using molecular modeling approaches. Int J Environ Res Public Health. 2021;19 doi: 10.3390/ijerph19010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pal D., Checker R., Kutala V.K., Sandur S.K. Molecular dynamic simulations reveal anti-SARS-CoV-2 activity of mitocurcumin by potentially blocking innate immune evasion proteins NSP3 and NSP16. Mol Divers. 2022 doi: 10.1007/s11030-022-10443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Child (Basel) 2020;7 doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Theoharides T.C., Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents. 2020;34:1241–1243. doi: 10.23812/20-EDITORIAL_1-5. [DOI] [PubMed] [Google Scholar]
- 91.Alzaabi M.M., Hamdy R., Ashmawy N.S., Hamoda A.M., Alkhayat F., Khademi N.N., et al. Flavonoids are promising safe therapy against COVID-19. Phytochem Rev. 2022;21:291–312. doi: 10.1007/s11101-021-09759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiong H., Dong Z., Lou G., Gan Q., Wang J., Huang Q. Analysis of the mechanism of Shufeng Jiedu capsule prevention and treatment for COVID-19 by network pharmacology tools. Eur J Integr Med. 2020;40 doi: 10.1016/j.eujim.2020.101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L., Wang Y., Yang W., He X., Xu S., Liu X., et al. Network pharmacology and molecular docking analysis on mechanisms of Tibetan Hongjingtian (Rhodiola crenulata) in the treatment of COVID-19. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu Y.S., Ho W.Y., Kang N., Tsai M.J., Wu J., Huang L., et al. Pharmaceutical prospects of curcuminoids for the remedy of COVID-19: truth or myth. Front Pharm. 2022;13 doi: 10.3389/fphar.2022.863082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan A., Iqtadar S., Mumtaz S.U., Heinrich M., Pascual-Figal D.A., Livingstone S., et al. Oral co-supplementation of curcumin, quercetin, and vitamin d3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial. Front Pharm. 2022;13 doi: 10.3389/fphar.2022.898062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gour A., Manhas D., Bag S., Gorain B., Nandi U. Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2. Phytother Res. 2021;35:4258–4283. doi: 10.1002/ptr.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Khayri J.M., Sahana G.R., Nagella P., Joseph B.V., Alessa F.M., Al-Mssallem M.Q. Flavonoids as potential anti-inflammatory molecules. A Rev Mol. 2022;27 doi: 10.3390/molecules27092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shohan M., Nashibi R., Mahmoudian-Sani M.R., Abolnezhadian F., Ghafourian M., Alavi S.M., et al. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: a randomized controlled trial. Eur J Pharm. 2022;914 doi: 10.1016/j.ejphar.2021.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arunachalam P.S., Walls A.C., Golden N., Atyeo C., Fischinger S., Li C., et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594:253–258. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material