Abstract
Background
Lower bone mineral density (BMD) increases the risk of osteoporosis in individuals with eating disorders (EDs), particularly women with anorexia nervosa (AN), making them susceptible to pain and fractures throughout adulthood. In AN, low weight, hypothalamic amenorrhoea, and longer illness duration are established risk factors for low BMD, and in people with other EDs a history of AN seems to be an important risk factor for low BMD.
Purpose
To conduct a systematic review and meta-analysis of BMD in individuals with EDs, including AN, bulimia nervosa (BN), binge-eating disorder (BED) and other specified feeding or eating disorders (OSFED) compared to healthy controls (HC).
Methods
Following PRISMA guidelines, electronic databases were reviewed and supplemented with a literature search until 2/2022 of publications measuring BMD (dual-energy X-ray absorptiometry or dual photon absorptiometry) in females with any current ED diagnosis and a HC group. Primary outcomes were spine, hip, femur and total body BMD. Explanatory variables were fat mass, lean mass and ED clinical characteristics (age, illness duration, body mass index (BMI), amenorrhoea occurrence and duration, and oral contraceptives use).
Results
Forty-three studies were identified (N = 4163 women, mean age 23.4 years, min: 14.0, max: 37.4). No study with individuals with BED met the inclusion criteria. BMD in individuals with AN (total body, spine, hip, and femur), with BN (total body and spine) and with OSFED (spine) was lower than in HC. Meta-regression analyses of women with any ED (AN, BN or OSFED) (N = 2058) showed low BMI, low fat mass, low lean mass and being amenorrhoeic significantly associated with lower total body and spine BMD. In AN, only low fat mass was significantly associated with low total body BMD.
Conclusion
Predictors of low BMD were low BMI, low fat mass, low lean mass and amenorrhoea, but not age or illness duration. In people with EDs, body composition measurement and menstrual status, in addition to BMI, are likely to provide a more accurate assessment of individual risk to low BMD and osteoporosis.
Keywords: Eating disorder, Anorexia nervosa, Bulimia nervosa, Bone mineral density, Osteoporosis, Body composition, Fat mass, Lean body mass, Amenorrhoea
Plain English summary
Individuals with eating disorders (EDs) have an increased risk for developing osteoporosis and suffering fractures. To better understand this problem, we conducted a systematic review and meta-analysis comparing bone mineral density (BMD) of females with EDs with that of healthy people without an ED. We also tried to identify key factors linked with reduced bone mass in EDs. We included studies reporting BMD of individuals with anorexia nervosa (AN), bulimia nervosa (BN), binge-eating (BED) or other non-specified ED (OSFED), and of healthy controls. We found that people with AN had overall lower BMD than controls and also in the spine, hip, and femur. In people with BN, there was lower BMD overall and in the spine, but that must be only in those who previously had AN. In people with OSFED, BMD was lower in the spine. Having a low BMI, low fat mass, low lean mass and not having menstrual periods seem to negatively affect BMD. Therefore, this systematic review supports the idea that people with current or past AN, irrespective of their current ED diagnosis, should have their bone health assessed. For early identification of those most at risk, body composition measurements, current menstrual status, duration of amenorrhoea and presence or absence of a history of AN should be considered in clinical practice.
Background
Eating disorders (EDs) are characterised by aberrant eating patterns, significant psychopathology, distress and/or impairment [1]. Anorexia nervosa (AN) is associated with low weight, fear of gaining weight and over influence of weight or shape on self-judgment. Two subtypes of AN are distinguished: a restricting type (AN-R) and a binge-eating/purging type (AN-BP). Bulimia nervosa (BN) is characterised by dieting, binge-eating and compensatory behaviours (i.e. self-induced vomiting and laxatives or diuretics misuse), while in binge-eating disorder (BED), distressing episodes of loss-of-control eating are not followed by compensatory behaviours [1]. Other specified feeding or eating disorder (OSFED), previously called eating disorder not otherwise specified (EDNOS), is a diagnostic ‘hold-all’ category for all other EDs which do not meet the full diagnostic criteria for AN, BN or BED [1].
Eating behaviours and symptoms affect individuals’ growth, development, metabolism, and body composition (fat mass and fat-free mass) [2]. Decreased leptin secretion by a diminished adipose tissue inhibits the hypothalamic–pituitary–gonadal axis (HPG). This leads to low levels of oestrogen and testosterone, increased bone resorption, and decreased bone formation [3]. Osteoporosis and osteopenia are silent conditions, characterised by decreased bone mineral density (BMD), increasing the risk of fractures [4]. Women with EDs have higher risk of developing osteopenia [5], osteoporosis, and bone fractures [6, 7]. In a study with 130 women with AN, 54% had osteopenia and 38% osteoporosis [8]. A large Swedish study by Axelsson et al. [9] with over 9000 patients with EDs of both male and female sex, found risk of fractures increased irrespective of age and sex. Longitudinal studies of patients with AN by Lucas et al. [10] and Frølich et al. [11] show that the fracture risk remains elevated several decades after initial diagnosis and even after remission compared to that in healthy controls (HC).
In AN, low nutrient intake, excessive physical activity and purging behaviours are associated with BMD loss [12, 13]. Known risk factors are low weight, hypothalamic amenorrhoea, and longer illness duration [6, 14, 15], with low weight being one important predictor of osteoporosis and risk of fracture [12]. A previous meta-analysis showed lower BMD in individuals with BN than HC but only for those with a history of AN [14]. There is some temporal fluidity between these diagnoses, e.g. in one study 50–64% of women with AN experienced bulimic symptoms, and one-third of them crossed over from AN to BN when followed for 7 years [16]. However, the relationship between BMD in BED and OSFED/EDNOS, is less clear than in AN due to the paucity of studies [1, 6, 14].
Osteopenia and osteoporosis in EDs are difficult to treat [17], with limited pharmacological treatments [18]. Thus, early identification of those most at risk and targeted interventions are imperative for reducing the problem [18]. Previous systematic reviews and meta-analyses have shown lower BMD in individuals with AN than HC [6, 14, 19]. They also showed that lower BMD in AN was associated with lower fat-mass, fat-free mass [19] and longer duration of amenorrhoea [6]. Since the publication of DSM-5, in which amenorrhoea is no longer a criterion for AN diagnosis [1], multiple studies assessing BMD in EDs have been published. Therefore, there is a need to re-assess the literature, in relation to the potential role of amenorrhoea (presence and duration) and changes in body composition in the lowering of BMD in females with EDs, not only AN.
The aim of this study was to conduct a systematic review and meta-analysis of BMD by dual-energy X-ray absorptiometry (DXA) or dual photon absorptiometry (DPA) across commonly measured anatomical sites (total body, spine, hip, and femur) in females with any EDs (AN, both AN-R and AN-BP subtypes, BN, BN with a history of AN, BED, and OSFED/EDNOS) in comparison to HC. Secondly, we wanted to identify predictors of the difference in BMD between EDs and HC by means of meta-regression analyses involving age, illness duration, body mass index (BMI), fat mass, lean mass, occurrence and duration of amenorrhoea, and oral contraceptive pill (OCP) use.
Materials and methods
Study selection
This is a systematic review of the international literature based on searches of MedLine, EMBASE, and PsychoInfo following the PRISMA guidelines [20] and registered in PROSPERO (CRD42019122053). Amendments were made in the registered protocol in order to conduct a meta-analysis of BMD, assessed by DXA scan in individuals with EDs (inclusion of BED and OSFED/EDNOS diagnoses and adolescents) and to identify predictors of BMD (secondary outcomes were limited to fat mass, lean mass, illness duration, BMI, amenorrhoea occurrence, duration of amenorrhoea and oral contraceptive use). Manual searches were conducted, and reference lists were searched for relevant articles. The following indexed descriptors were used and their combination for EDs (Anorexia nervosa*, Bulimia nervosa*, Eating disorders*, Binge-eating disorder*, EDNOS*, OSFED*) and bone health (Bone density*, Bone mineral density*, Bone mineral content*, Bone mass*, Fracture* and Osteoporosis*).
Inclusion and exclusion criteria
Publications in English were included if they were studies of adolescent (> 12 years) and adult females with a current ED diagnosis (AN, BN, BED or OSFED/EDNOS) and a HC group, and measured BMD using DXA or DPA. The BN and OSFED/EDNOS groups included individuals with or without a history of AN. Studies with participants recruited from the same population, institution and/or period had an independent sample selected only. Selection criteria were, in order: larger sample size, most recent publication date, and most recent ED diagnostic criteria adhered to upon recruitment. Study design (i.e. longitudinal, cross-sectional) was not used as an inclusion criterion, but only baseline measures of BMD were included.
Studies using other methods for bone and body composition assessment, not including BMD, such as skinfold measurement, bioelectrical impedance analysis (BIA) and computerised tomography (CT) scan, magnetic resonance imaging (MRI) were not included. The final search was performed on 2/2022. Following exclusion of duplicates, articles were screened based on title and abstract and were excluded if they were reviews or did not match inclusion criteria. Original manuscripts were assessed by two independent reviewers (MPL and LR): disagreements were resolved by consensus or with a third co-author.
Data extraction
Information extracted from each included paper consisted of: (1) article reference (study name, authors, year of publication, and country); (2) characteristics of participants with EDs and control group (number of participants, age, mean BMI, ED diagnosis, method of diagnosis, number of participants with amenorrhoea, duration of amenorrhoea, numbers using OCP, illness duration; sample source); (3) outcome measures of interest: DXA or DPA scanning methods, BMD data (total body, spine, hip, and femur), fat mass and lean mass where available.
Quality assessment
The Newcastle–Ottawa scale (NOS) [21] was used for risk of bias assessment. This allows quality evaluation of non-randomised studies for meta-analyses based on: selection of the study group, group comparability, and ascertainment of exposure of interest for case–control studies. It includes the definition of cases and controls, method of control selection, representativeness of cases, and comparability of cases and controls. Exposure of interest is assessed as the diagnosis of the ED and the sample bias through non-response rate. For case–control studies, a NOS score below 5 suggests a high risk of bias (maximum of 9) [22].
Statistical analysis
Statistical analyses were conducted on STATA SE software, version 16 [23]. Meta-analyses were performed using ‘meta set’ and ‘meta-summarize’ commands with random-effect models of total body, spine, femur, and hip BMD (where available) of ED groups and HC due to suspected heterogeneity. Standardised mean difference (SMD) was the primary outcome of the meta-analyses, generated by the ‘meta summarize’ command. Forest plots were generated using the ‘meta forest’ and funnel plots using the ‘meta funnel’ commands.
Meta-regression analyses were performed using the ‘metareg’ command to investigate the association between age, BMI, fat mass, lean mass, percentage of participants with amenorrhoea, percentage using OCP, and illness duration and the SMD of total body, spine, femur, and hip BMD (in the whole ED group and in the AN group).
Due to the limited reporting of AN subtypes, different methods of determining ED diagnoses, and cross-over between ED diagnoses, heterogeneity was suspected. Heterogeneity was assessed using the Higgins I2 test, with > 25% as low, > 50% as moderate, and > 75% as high. Trim and fill correction was used to investigate potential publication bias [24]. An Egger test was applied to investigate small-study effects on the spine, femur, hip, and total body BMD [25].
Results
Search results
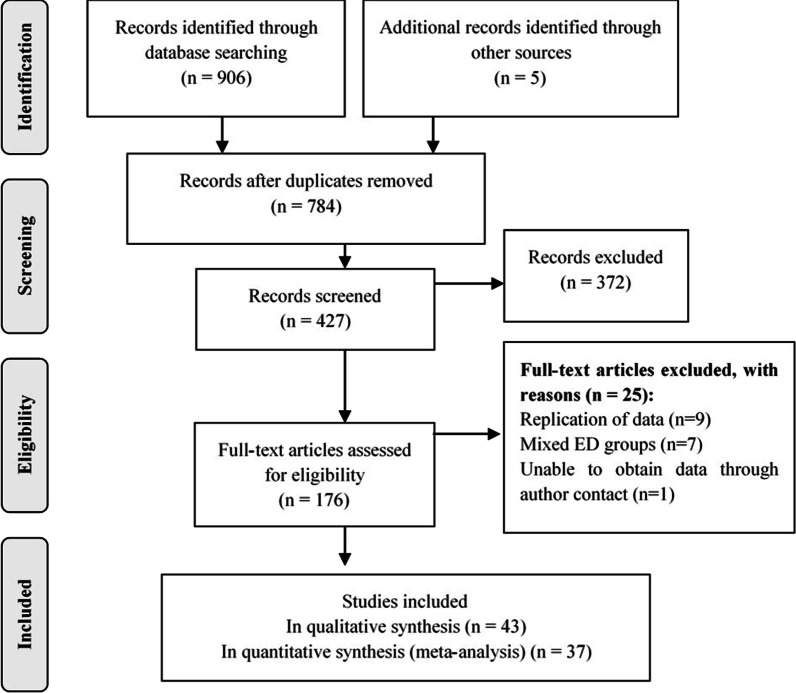
In the electronic database search, 911 full-text articles were found, resulting in 784 original articles after removal of duplicates. Following screening of titles and abstracts, 372 studies were excluded according to the following criteria: review or book, participants being male, no control group, females with athlete’s triad syndrome and exercisers, other diseases or non-DXA or DPA assessment or being a case-report. 176 full-text articles were reviewed and 43 original articles were included in the qualitative review and 37 in at least one of four meta-analyses (Fig. 1).
Fig. 1.
Flow diagram of studies included in the systematic review and meta-analysis
Study characteristics
Publication dates ranged from 1989 to 2022. Literature sources were: USA (42.9%); Europe (40.5.5%), Australia and New Zeland (7.1%) and Asia (9.5%).
In total, 43 papers were included with 4163 participants from 61 different groups with AN, BN or OSFED/EDNOS, and 43 healthy control groups. Participants mainly had AN diagnoses (N = 45 studies, 73.8% of the ED sample), with the remainder having BN (N = 15 studies, 24.6% of the ED sample) or OSFED/EDNOS diagnoses (N = 2 studies, 1.6% of the ED sample). No study with individuals with BED compared either to HC or to individuals with BED and a previous history of AN met the inclusion criteria. Of the 61 EDs groups included in this review, 50.8% (31/61) were described as ‘AN’ and 13.1% (8/61) as ‘BN’. In relation to AN subtypes, 12.7% (8/61) of the groups were of the ‘AN-Restrictive subtype (AN-R)’ and 1.6% (1/61) the ‘AN-Binge Purge subtype (AN-BP)’. In 6.5% (4/61) of them, ‘BN with no previous AN history (BN-NPAN)’ and in 3.3% (2/61) ‘BN with previous AN (BN-PAN)’ were reported. The diagnosis of ‘AN with an additional diagnosis of BN (according to DSM-III-R) (AN-BN)’’ was reported in 3.3% (2/61) of the groups. ‘OSFED/EDNOS’ and ‘AN-Atypical’ corresponded to 1.6% (1/61) each of the groups included.
From the 61 EDs groups, data on BMD were available for the total body (N = 35 groups), spine (N = 54 groups), hip (N = 19 groups) and femur (N = 24 groups) in AN, BN and OSFED groups. Due to the limited BMD measures in BN and OSFED groups for the hip (N = 2), whole ED group (AN, BN and OSFED) analyses were conducted for the total body, spinal and femur regions, and for the hip (N = 19) with AN groups only.
AN
Participants with AN had a mean age of 22.4 years [14.6–34.7], a BMI of 16.3 kg/m2 [14.4–19.1], an illness duration of 4.7 years [0.7–13.0] and 17.4 months of amenorrhoea [1.5–46.5]: 62.21% were amenorrhoeic and 24.4% used OCP. The majority of the studies (48.9%) used DSM-IV for diagnosis, followed by DSM-III-R (26.7%), DSM-5 (11.1%), ICD-10 (6.7%), both DSM-IV/5 (4.4%) and one study did not report it. The number of groups in the studies with investigation of the different parameters were: total body BMD (n = 26, 57.8%), spine (n = 38, 84.4%), femur (n = 21, 46.7%), hip (n = 19, 42.2%), fat mass (n = 28, 62.2%) and lean mass (n = 27, 60.0%).
BN
The BN group had a mean age of 25.6 years [20.7–30.7], a BMI of 21.5 kg/m2 [19.0–22.3], an illness duration of 8.1 years [2.8–16.6], and a duration of amenorrhoea (reported in three studies only and in one of them with participants with a previous diagnosis of AN) of 62.4 months [19.1–132.0]: 35.7% were amenorrhoeic and 35.7% were using OCP for contraception or for any other reason. Most studies (57.1%) used DSM-IV for diagnosis, followed by DSM-III-R (28.6%) and DSM-5 (14.3%). Body composition measurements investigated in the BN groups were: total body BMD (n = 7, 50.0%), spine (n = 14, 100.0%) femur (n = 3, 21.4%), hip (n = 2, 14.3%), fat mass (n = 7, 50.0%) and lean mass (n = 2, 14.3%).
OSFED/EDNOS
Only two studies included individuals with OSFED (EDNOS or atypical AN), with a mean age of 21.0 years [14.5–27.6] and a BMI of 16.9 kg/m2 [15.6–18.3] [26, 27]. In the study by Bratland-Sanda et al. [27], 36% of the individuals reported a history of AN. Duration of illness and amenorrhea were reported by one of these studies (Table 1). One paper used DSM-IV [27] for diagnosis and the other [26] the DSM-5 criteria. Body composition measurements assessed were: total body BMD (n = 2), spine (n = 2), femur (n = 1), hip (n = 0), fat mass (n = 1), and lean mass (n = 1). The study by Bacopoulou et al. [26] did not present standard deviations from BMD measures, therefore was not included in the whole ED meta-analysis and meta-regressions.
Table 1.
Summary of study characteristics (n = 43)
| Author | Country | ED | Sample size (N) | BMI (SD) (kg/m2) or % of IBW | Amenorrhoea (%) | OCP use (%) | Diagnostic method | Design | ED population | Controls | Scanning method | Bone density outcome measures | Body composition outcome measures | NOS-Scale stars of 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al. [28] | USA | AN-R | 28 | 68.1 (14.2) % of IBW | Yes | Unknown | DSM-IV | Case–control | Hospital outpatients |
Similar age and white controls Referred to radiology for non-orthopaedic, non-eating disorder problems to ‘rule out’ osteoporosis |
DXA | Spine BMD | 5 | |
| Unknown % | ||||||||||||||
| AN-BP | 36 | 73.3 (11.7) % of IBW | Unknown | Unknown | ||||||||||
| BN-PAN | 18 | 111.3 (27.8) % of IBW | Unknown | Unknown | ||||||||||
| BN-NPAN | 14 | 109.4 (11.3) % of IBW | Unknown | Unknown | ||||||||||
| HC | 15 | Unknown | Unknown | Unknown | ||||||||||
| Bachmann et al. [29] | USA | AN | 65 | 15.9 | Yes | Yes | DSM-5 | Case–control | Premenopausal women who had undergone quantitative computed tomography | Premenopausal women who had undergone quantitative computed tomography |
DXA QCT |
Spine BMD |
Fat mass Lean mass |
5 |
| Unknown % | Unknown % | |||||||||||||
| HC | 45 | 19.8 | ||||||||||||
| Bacopoulou et al. [26] | GRE | AN | 17 | 17.0 (1.1) | Yes | Unknown | DSM-5 | Case–control | Adolescent medicine clinic |
Healthy controls Age-matched girls presented for annual health examination in an adolescent medicine clinic |
DXA |
Total body BMD Spine aBMD |
5 | |
| 100% | ||||||||||||||
| AN-Atypical | 5 | 18.3 (16.0–22.9) | Yes | Unknown | DSM-5 | |||||||||
| 60% | ||||||||||||||
| HC | 15 | 22.1 (1.7) | ||||||||||||
| Bratland-Sanda et al. [27] | NOR | AN | 8 | 15.6 (1.4) | Unknown | Unknown | DSM- IV | Case–control | ED hospitalised patients |
Healthy controls Age-matched Randomly selected from a national pool |
DXA |
Total body BMD Spine BMD Femoral neck BMD |
Fat mass Lean mass |
8 |
| BN | 29 | 22.3 (3.5) | Unknown | Unknown | DSM- IV | |||||||||
| EDNOS | 22 | 21.0 (3.2) | Unknown | Unknown | DSM- IV | |||||||||
| HC | 53 | 25.3 (4.8) | ||||||||||||
| Bredella et al. [30] | USA | AN | 10 | 18.4 (1.0) | Yes | Yes | DSM-IV | Case–control | ED clinics |
Healthy controls Recruited through clinic advertisements |
DXA |
Total body BMD Spine BMD Femoral BMD Hip BMD |
Fat mass Lean mass |
7 |
| 100% | 20% | |||||||||||||
| HC | 10 | 20.5 (2.6) | ||||||||||||
| Bredella et al. [31] | USA | AN | 10 | 17.6 (1.0) | Unknown | Unknown | ‘Psychiatric diagnostic criteria for AN’ | Case–control | Clinic referrals |
Healthy controls Recruited through community advertisements |
DXA MRI |
Total body BMD Spine BMD Hip BMD |
5 | |
| HC | 10 | 21.9 (1.7) | ||||||||||||
| Bredella et al. [32] | USA | AN | 5 | 18.3 (0.9) | Unknown | No | DSM-IV | Case–control | Clinic referrals |
Healthy Controls Recruited through community advertisements |
DXA Fluorodeoxyglucose- PET CT |
Total body BMD Spine BMD Femoral neck BMD Hip BMD Lateral spine BMD |
Fat mass Lean mass |
5 |
| HC | 5 | 21.9 (1.7) | ||||||||||||
| Čagalová et al. [33] | SLO | AN | 63 | 14.5 (1.8) | Yes | No | DSM-5 | Case–control | Hospital patients of the Department of Paediatrics | General paediatric practice | DXA |
Total body BMD Spine BMD Hip BMD |
5 | |
| 81.4% | ||||||||||||||
| HC | 20 | 20.2 (1.7) | ||||||||||||
| Davies et al. [34] | USA | AN | 26 | Unknown | Yes | Yes | DSM-III-R | Case–control | Clinic records of eating disorder patients from medical centre |
Healthy controls Select for fitness and diet study or for a DXA and DPA compar ison group |
DPA |
Spine BMD Forearm BMD |
4 | |
| 80.8% | 15.4% | |||||||||||||
| AN-BN | 26 | Unknown | Yes | Yes | DSM-III | |||||||||
| 61.5% | 23.1% | |||||||||||||
| BN | 11 | Unknown | Yes | Yes | ||||||||||
| 18.2% | 9.1% | |||||||||||||
| HC | 211 | Unknown | ||||||||||||
| Estour et al. [35] | FRA | AN-R | 40 | 16.0 (0.8) | Yes | No | DSM-IV | Case–control | Hospital outpatients | Healthy controls ranging 18.6–25.0 kg/m2 BMI | DXA |
Spine BMD Femoral BMD |
Fat mass Lean mass |
5 |
| 100% | ||||||||||||||
| HC | 54 | 20.9 (2.2) | ||||||||||||
| Faje et al. [36] | USA | AN | 21 | 17.8 (0.2) | Unknown | Unknown | DSM-IV | Cohort | Hospital outpatients | Healthy controls with normal weight | DXA |
Total body aBMD Spine aBMD Hip aBMD Distal radius aBMD |
Fat mass Lean mass |
6 |
| HC | 23 | 22.4 (0.5) | ||||||||||||
| Fazeli, Klibanski [37] | USA | AN | 26 | 16.7 (0.5) | Yes | No | DSM-IV | Cohort | ED referrals |
Healthy controls Recruited through community advertisements |
DXA MRI |
Spine BMD Femoral BMD Hip BMD |
5 | |
| HC | 20 | 22.6 (0.3) | ||||||||||||
| Fernández-Soto et al. [38] | SPA | AN-R | 16 | 16.8 (1.5) | Yes | No | DSM-IV | Cohort | Clinic outpatients |
Healthy controls Caucasian women |
DXA MRI |
Total body BMD Spine BMD |
Fat mass Lean mass |
5 |
| AN-R | 31 | 19.1 (2.0) | Yes | No | DSM-IV | |||||||||
| HC | 25 | 21.8 (0.9) | ||||||||||||
| Frølich et al. [39] | DEN | AN | 25 | 16.2 (1.3) | Yes | Yes | ICD-10 | Case–control | ED unit outpatients |
Healthy controls Age and height-matched females Randomly selected from a national pool |
DXA |
Spine BMD Femoral BMD Hip BMD |
Fat mass Lean mass |
7 |
| 100% | ||||||||||||||
| HC | 25 | 22.8 (2.7) | ||||||||||||
| Guo et al. [40] | CHI | AN | 26 | 16.3 (0.6) | Yes | DSM-IV | Cohort | Clinical psychiatrist referrals |
Healthy controls Age-matched |
DXA |
Total body BMD Spine BMD Hip BMD |
Fat mass Lean mass |
5 | |
| 100% | ||||||||||||||
| HC | 24 | 20.8 (0.7) | ||||||||||||
| Haas et al. [41] | GER | AN | 103 | 16.5 (1.6) | Yes | Yes | DSM-IV | Longitudinal observational | Patients of adolescent and youth medicine clinic |
Normal weight controls Recruited from a local high school |
DXA | Total body BMD |
Fat mass Lean mass |
5 |
| 94.2% | 5.6% | |||||||||||||
| HC | 51 | 20.8 (1.8) | ||||||||||||
| Iketani et al. [42] | JAP | AN | 22 | 14.4 (2.3) | Yes | No | DSM-III-R | Cohort | Inpatients and outpatients |
Healthy controls Age-matched healthy females |
DPA |
Total body BMD Spine BMD |
5 | |
| Unknown % | ||||||||||||||
| AN-BN | 23 | 14.4 (1.6) | Yes | No | DSM-III-R | |||||||||
| Unknown % | ||||||||||||||
| BN-PAN | 10 | 19.0 (2.6) | Yes | No | DSM-III-R | |||||||||
| 40% | ||||||||||||||
| HC | 10 | 19.5 (0.8) | ||||||||||||
| Kandemir et al. [43] | USA | AN | 262 | 17.2 (0.1) | Yes | DSM-IV / DSM-5 | Case–control | AN participants from Childhood Study | Healthy controls participants from Childhood Study | DXA |
Total body BMD Spine BMD Hip BMD |
5 | ||
| 85.9% | ||||||||||||||
| HC | 90 | 21.0 (0.2) | ||||||||||||
| Karlsson et al. [44] | AUS | AN | 77 | 15.6 (0.2) | Unknown | Unknown | ICD-10 | Case–control | AN patients untreated with oestrogen therapy | Healthy controls | DXA |
Spine BMD Femoral neck BMD |
Fat mass Lean mass |
5 |
| HC | 205 | 23.1 (0.3) | ||||||||||||
| Kooh et al. [45] | JAP | AN | 22 | 15.9 (2.2) | Yes | Yes | DSM-III-R | Cohort |
Clinic referrals: Adolescent medicine clinic |
Healthy controls School and university students, no oral contraceptives |
DXA |
Spine BMD Femoral neck BMD |
Fat mass Lean mass |
5 |
| 72.3% | 4.50% | |||||||||||||
| HC | 24 | 21.6 (2.2) | ||||||||||||
| Maïmoun et al. [46] | FRA | AN-R | 206 | 15.8 (1.7) | Yes | No | DSM-IV | Case–control | Hospital outpatients |
Healthy controls Recruited through community advertisement |
DXA |
Total body aBMD Spine aBMD Hip BMD Proximal femur aBMD |
Fat mass Lean mass |
6 |
| 100% | ||||||||||||||
| AN-R | 99 | 16.3 (1.4) | No | Yes | DSM-IV | |||||||||
| 100% | ||||||||||||||
| HC | 121 | 21.6 (2.3) | ||||||||||||
| Masala et al. [47] | ITA | AN | 17 | 17.5 (1.6) | Unknown | Unknown | ICD-10 | Cohort | Patients in weight gain programme |
Healthy controls Exclusion included medication or illness to affect bone |
DXA QCT |
Spine BMD | 4 | |
| HC | 27 | 24.3 (4.8) | ||||||||||||
| Mathisen et al. [48] | SWE | BN-NPAN | 100 | Unknown | Unknown | DSM-5 | Cross-sectional | Patients from a randomised controlled trial | No healthy control group | DXA |
Total body BMD Spine BMD |
Fat mass Lean mass |
6 | |
| BN-PAN | 37 | Unknown | Unknown | DSM-5 | ||||||||||
| Misra et al. [49] | USA | AN | 23 | 16.7 (1.2) | Unknown | Unknown | DSM-IV | Cohort | Clinic referrals |
Healthy controls Age-matched and bone age- matched Recruited through adverts through healthcare providers and newspapers |
DXA |
Spine BMD Hip BMD |
Fat mass Lean mass |
6 |
| HC | 21 | 21.7 (3.7) | ||||||||||||
| Misra et al. [50] | USA | AN | 17 | 16.7 (1.3) | Unknown | Unknown | DSM-IV | Cohort | Paediatrician referrals |
Healthy controls Age-matched and bone age- matched Recruited through mailings to paediatricians |
DXA |
Total body BMD Spine BMD Femoral neck BMD Hip BMD |
Fat mass Lean mass |
6 |
| HC | 19 | 21.8 (3.4) | ||||||||||||
| Misra et al. [51] | EUA | AN | 110 | 17.4 (0.1) | Yes | No | DSM-IV | RCT | Hospital outpatient treatment programme |
Healthy controls Recruited through mailings to paediatricians |
DXA |
Spine BMD Hip BMD |
Fat mass Lean mass |
7 |
| 100% | ||||||||||||||
| HC | 40 | 21.4 (0.5) | ||||||||||||
| Morris et al. [52] | UK | AN | 51 | 15.1 (1.9) | Yes | Yes | DSM-IV | Cohort | ED specialist referrals | Control group data from department of medical physics |
DXA X-Ray |
Total body BMD Spine BMD |
Fat mass | 5 |
| 100% | ||||||||||||||
| BN | 47 | 21.9 ( 3.4) | Yes | DSM-IV | ||||||||||
| BN-NPAN | 26 | 22.3 (2.7) | Yes | DSM-IV | ||||||||||
| BN-PAN | 21 | 21.4 (3.0) | Yes | DSM-IV | ||||||||||
| HC | 40 | 23.3 (4.4) | ||||||||||||
| Naessén et al. [53] | SWE | BN | 77 | 22 | Yes | No | DSM-IV | Cohort | Recruited from hospital advertisements |
Healthy controls Hospital advertising: hospital staff and students, no current diseases or medication prior to 3 months before the study |
DXA |
Total body BMD Spine BMD Leg BMD |
Fat mass Lean mass |
8 |
| 7.8% | ||||||||||||||
| BN-NPAN | 59 | DSM-IV | ||||||||||||
| BN-PAN | 18 | DSM-IV | ||||||||||||
| HC | 56 | 22.2 | ||||||||||||
| Newman, Halmi [54] | USA | AN | 18 | 73.2 (8.4) | Yes | No | DSM-III-R | Cross-sectional | Inpatients from an ED unit | Normal weight women | DXA |
Spine BMD Femoral BMD |
4 | |
| 100% | ||||||||||||||
| BN | 12 | Yes | Yes | DSM-III-R | ||||||||||
| 66.7% | 8.4% | |||||||||||||
| HC | 12 | |||||||||||||
| Newton et al. [55] | AUS/UK | BN | 20 | 21.8 (3.5) | Yes | Yes | DSM-III-R | Cohort | ED outpatient treatment programme |
Healthy controls Age and sex-matched controls from hospital staff notice boards |
DXA | Spine BMD | 7 | |
| 20% | ||||||||||||||
| HC | 16 | 21.9 (1.8) | ||||||||||||
| Olmos et al. [56] | SPA | AN | 51 | 17.3 (2.4) | Yes | DSM-IV | Prospective longitudinal cohort study | ED unit outpatients |
Healthy controls Hospital advertisements |
DXA |
Spine BMD Femoral neck BMD Hip BMD |
5 | ||
| 100% | ||||||||||||||
| HC | 40 | 21.8 (2.7) | ||||||||||||
| Poet et al. [57] | FRA | AN | 18 | Unknown | Yes | No | DSM-III-R | Cohort | Hospital outpatients |
Healthy controls Volunteers |
DXA | Spine BMD | 5 | |
| 100% | ||||||||||||||
| HC | 36 | Unknown | ||||||||||||
| Resch et al. [58] | AU | AN | 20 | 16.0 (13–18) | Unknown | Unknown | DSM-III-R | Cohort | Hospital outpatients |
Healthy controls Age-matched nursing school students |
DXA |
Spine BMD Hip BMD |
5 | |
| HC | 20 | 23.0 (19–29) | ||||||||||||
| Schorr et al. [59] | USA | AN | 46 | 16.7 (1.8) | Yes | Yes | DSM-5 | Cross-sectional | Patients of National Institutes Health trials |
Lean healthy controls From the National Institutes Health trial |
DXA HRpQCT |
Spine BMD Femoral BMD Hip BMD |
6 | |
| 60.9% | 15.3% | |||||||||||||
| HC | 29 | 22.6 (1.4) | ||||||||||||
| Seeman et al. [60] | AUS | AN | 12 | 15.9 (1.3) | Yes | No | DSM-III-R | Cohort | Patients with AN |
Healthy controls Volunteers with no illness that affects the bone, no drugs, no medication |
DXA |
Total body BMD Spine BMD Proximal femur Femoral neck BMD Ward’s triangle BMD Trochanter BMD |
Fat mass Lean mass |
4 |
| 100% | ||||||||||||||
| AN | 37 | 16.6 (0.4) | Yes | No | DSM-III-R | |||||||||
| 100% | ||||||||||||||
| AN OCP | 16 | 17.2 (0.8) | Yes | DSM-III-R | ||||||||||
| 100% | ||||||||||||||
| HC | 52 | 23.3 (0.5) | ||||||||||||
| Singhal, Bredella [61] | USA | AN | 55 | 18.7 (0.2) | Yes | No | DSM-IV / DSM-5 | Cross-sectional | Ongoing studies assessing bone outcomes |
Normal-weight controls From ongoing studies assesing bone outcomes |
DXA HRpQCT |
Total body BMD Spine BMD Femoral BMD Hip BMD |
Fat mass Lean mass |
6 |
| 27.3% | ||||||||||||||
| HC | 48 | 21.7 (0.3) | ||||||||||||
| Soyka et al. [62] | USA | AN | 19 | 16.5 (0.4) | Yes | No | DSM-IV | Cohort | Healthcare provider referrals |
Healthy controls Recruited through advertisement in primary care providers and newspapers, BMI 25th-90th percentile, one participant in pre- menarche |
DXA |
Total body BMD Spine BMD Lateral spine BMD |
Fat mass Lean mass |
6 |
| 73.7% | ||||||||||||||
| HC | 19 | 21.8 (0.4) | ||||||||||||
| Strumila et al. [63] | FRA | BN-NPAN | 50 | 21.9 (2.6) | Irregular menstrual cycle | Unknown | DSM-5 | Cross-sectional | ED unit | No | DXA |
Spine BMD Hip BMD |
Fat mass | 5 |
| 35% | ||||||||||||||
| BN-PAN | 35 | 21.9 (2.9) | Irregular menstrual cycles | Unknown | DSM-5 | |||||||||
| 46.7% | ||||||||||||||
| Sundgot-Borgen et al. [64] | NOR | AN | 13 | 15.8 (0.9) | Yes | No | DSM-IV | Case–control | Clinic referrals |
Healthy controls University information board recruitment Comprehensive inclusion criteria for dietary, exercise and ED symptoms |
DXA |
Total body BMD Spine BMD Femoral neck BMD Leg BMD Arm BMD |
Fat mass | 7 |
| 100% | ||||||||||||||
| BN | 43 | 20.7 (2.0) | Yes | No | DSM-IV | |||||||||
| 32.6% | ||||||||||||||
| HC | 17 | 22.0 (2.4) | ||||||||||||
| van Marken Lichtenbelt et al. [65] | NL | AN-R | 12 | 16.5 (1.7) | Yes | Yes | DSM-III-R | Cohort | Non-hospitalised outpatients |
Healthy controls Normal weight participating from a study on energy expenditure |
DXA | Total body BMD |
Fat mass Lean mass |
3 |
| 58.3% | 33.3% | |||||||||||||
| HC | 16 | 23.7 (2.1) | ||||||||||||
| Walsh et al. [66] | USA | AN | 8 | 17.4 (1.4) | Unknown | Unknown | DSM-IV | Cohort | Hospital outpatients |
Healthy controls 90–100% ideal weight for age |
DXA CT |
Spine BMD Femoral neck BMD Hip BMD |
Fat mass Lean mass |
4 |
| HC | 6 | 24.4 (2.5) | ||||||||||||
| Wojcik et al. [67] | USA | AN | 15 | 17.6 (0.2) | Unknown | No | DSM-IV | Cohort | Healthcare referrals and community adverts |
Healthy controls Recruitment through community advertisement |
DXA |
Total body BMD Spine BMD Femoral neck BMD Hip BMD |
Fat mass Lean mass |
4 |
| HC | 16 | 23.5 (0.6) | ||||||||||||
| Wu et al. [68] | CHI | AN | 25 | 17.5 (1.3) | Yes | Unknown | DSM-5 | Case–control | ED providers referrals |
Healthy controls Recruited through mailings to paediatricians |
DXA |
Total body BMD Spine BMD Femoral neck BMD |
Fat mass Lean mass |
4 |
| 100% | ||||||||||||||
| HC | 31 | 20.2 (1.3) |
ED eating disorder, HC healthy controls, BMI body mass index, IBW ideal body weight, SD standard deviation, AN anorexia nervosa, BN bulimia nervosa, AN-R AN-restrictive subtype, BN-NPAN BN with no previous AN history, BN-PAN BN with previous AN, AN-BN AN with an additional diagnosis of BN (according to DSM-III-R), AN-PB AN-binge purging subtype, EDNOS ED not otherwise specified, OCP oral contraceptive pill, DSM Diagnostic and Statistical Manual of Mental Disorders, CID International Classification of Diseases, DXA dual-energy X-ray absorptiometry, DPA dual photon absorptiometry, CT computerised tomography, MRI magnetic resonance imaging, PET positron emission tomography, BMD bone mineral density
HC
The HC group had a mean age of 23.7 years [14.0–37.4] and a BMI of 21.9 kg/m2 [19.5–24.4]. They were females, with no history of ED, from the same community as the cases (n = 30) [27, 30–37, 39–41, 44–47, 49–53, 55–59, 61, 62, 64, 65, 67] or hospital controls (n = 6) [26, 28, 43, 48, 63, 68]. Six studies did not provide information on selection of controls [29, 38, 42, 54, 60, 66]. HC were matched for age (n = 15) [26–29, 31, 33, 37, 41, 42, 46, 47, 55, 56, 58, 64, 68], age and BMI with BN participants [48, 53], age and bone age (n = 1) [49], age and fat mass (n = 1) [40], age and ethnicity (n = 1) [45] and age, lean mass and body fat (n = 1) [44].
Of the included studies, 14 adjusted their analysis for at least one of the following: age, race, BMI, bone age, height, weight, normal weight, age at menarche, fat and fat-free mass or sexual maturity [30, 36, 38, 39, 43, 50–52, 59–62, 66, 67]. Seven studies did not mention any adjustments for the control group [32, 34, 35, 54, 57, 63, 65].
Quality assessment
Quality assessment of the studies is presented in Table 1. NOS scale scores ranged from 3 to 8 out of 9, with a mean of 5.3. Across all 43 studies, eight had a high risk of bias (score < 5) [34, 47, 54, 60, 65–68]. All of these had a cohort design or a publication date before the 1990’s. However, selection of participants was valid and adequate. Exposure assessment revealed only a few studies with the same methodology for ascertainment of ED and non-ED diagnosis, and none of the studies reported a non-response rate. The NOS score was not used to exclude articles from this review.
Meta-analysis results
BMD in EDs
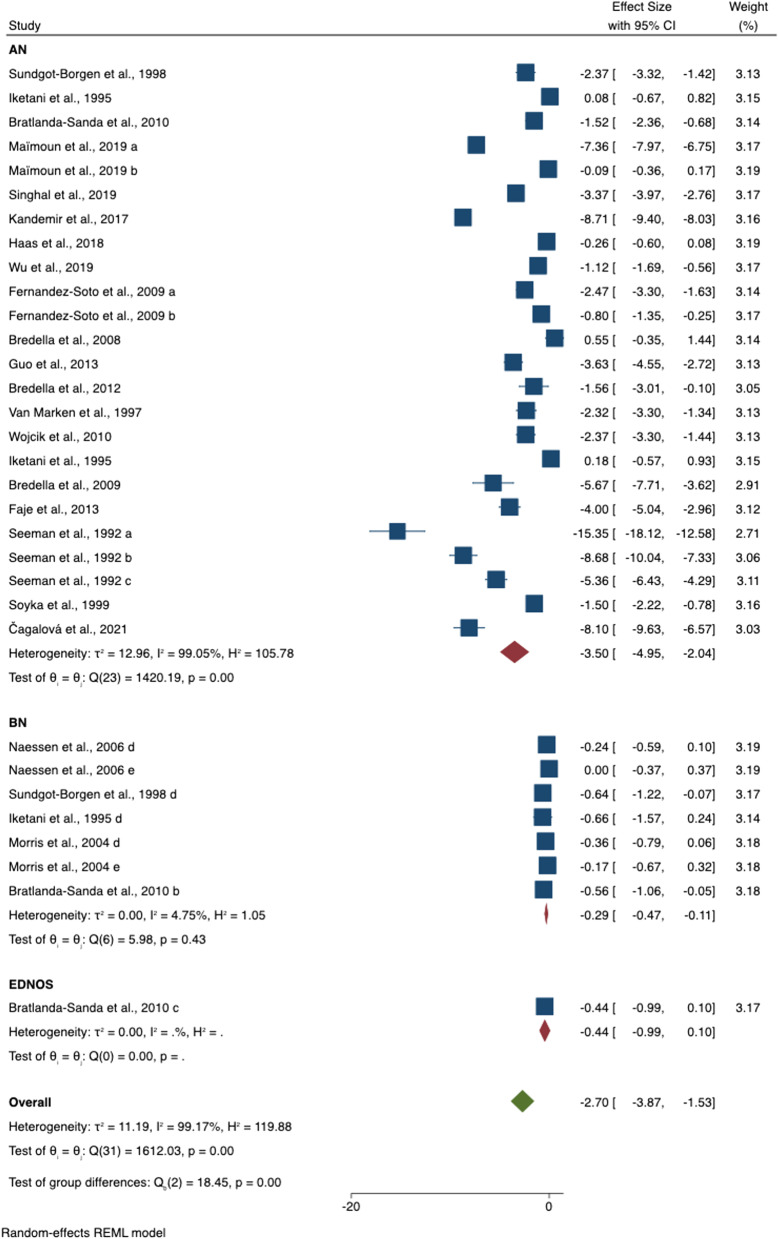
In the whole group analyses of AN, BN and OSFED/EDNOS (compared to HC), BMD was lower at three sites, total body BMD (SMD = − 2.62 [− 3.39 to − 1.84], p < 0.001), spine BMD (SMD = − 3.31 [− 3.98 to − 2.63], p < 0.001) and femur (SMD = − 3.08 [− 4.33 to − 1.83], p < 0.001). Hip BMD was only measured in studies of AN groups and is reported in the next section (Table 2 and Figs. 2, 3).
Table 2.
Meta-analysis results of females with eating disorders (EDs) versus healthy control (HC) groups
| Anatomical site | Groups in studies (N) | ED (N) | HC (N) | SMD | L 95% CI | U 95% CI | Z | p | I2 (%) | Egger | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All studies | |||||||||||
| Total body BMD | 32 | 996 | 963 | − 2.62 | − 3.39 | − 1.84 | 6.59 | < 0.001 | 99.2 | − 5.76 | < 0.001 |
| Spine BMD | 49 | 1941 | 2299 | − 3.31 | − 3.98 | − 2.63 | 9.61 | < 0.001 | 99.4 | − 6.87 | < 0.001 |
| Femur BMD | 24 | 894 | 1051 | − 3.08 | − 4.33 | − 1.83 | 4.82 | < 0.001 | 98.7 | − 5.03 | < 0.001 |
| AN | |||||||||||
| Total body BMD | 24 | 683 | 676 | − 3.45 | − 4.57 | − 2.33 | 6.04 | < 0.001 | 99.1 | ||
| Spine BMD | 36 | 1553 | 1743 | − 4.34 | − 5.27 | − 3.40 | 9.11 | < 0.001 | 99.4 | ||
| Femur BMD | 20 | 788 | 954 | − 3.38 | − 5.30 | − 2.22 | 4.78 | < 0.001 | 98.8 | ||
| Hip BMD | 19 | 1078 | 688 | − 4.95 | − 6.78 | − 3.12 | 5.30 | < 0.001 | 98.9 | − 2.52 | 0.012 |
| BN | |||||||||||
| Total body BMD | 7 | 291 | 253 | − 0.29 | − 0.46 | − 0.12 | 3.27 | 0.001 | 4.8 | ||
| Spine BMD | 12 | 366 | 522 | − 0.49 | − 0.77 | − 0.21 | 3.45 | 0.001 | 63.7 | ||
| Femur BMD | 3 | 84 | 63 | − 0.19 | − 0.80 | − 0.43 | 0.60 | 0.552 | 66.6 | ||
| BN with history of AN | |||||||||||
| Spine BMD | 4 | 67 | 21 | − 0.74 | − 1.06 | − 0.43 | 4.58 | < 0.001 | 0 | ||
| BN without history of AN | |||||||||||
| Spine BMD | 2 | 85 | 96 | − 0.07 | − 1.05 | − 0.90 | 0.14 | 0.885 | 89.7 | ||
| OSFED/EDNOS only | |||||||||||
| Total body BMD | 1 | 22 | 34 | − 0.04 | − 0.99 | 0.10 | 1.60 | 0.109 | |||
| Spine BMD | 1 | 22 | 34 | − 2.44 | − 3.15 | − 1.73 | 6.77 | < 0.001 | |||
| Femur BMD | 1 | 22 | 34 | − 0.24 | − 0.76 | 0.31 | 0.82 | 0.415 | |||
P-value in bold indicates statistical significance (p < 0.05)
N sample number, ED eating disorder, HC healthy control, SMD standardised mean difference, L lower, U upper, CI confidence interval, Z z-scores, p p value, BMD bone mineral density, AN anorexia nervosa, BN bulimia nervosa, OSFED other specified feeding or eating disorder, EDNOS Eating disorder not otherwise specified, I2 Higgins I2 test
Fig. 2.
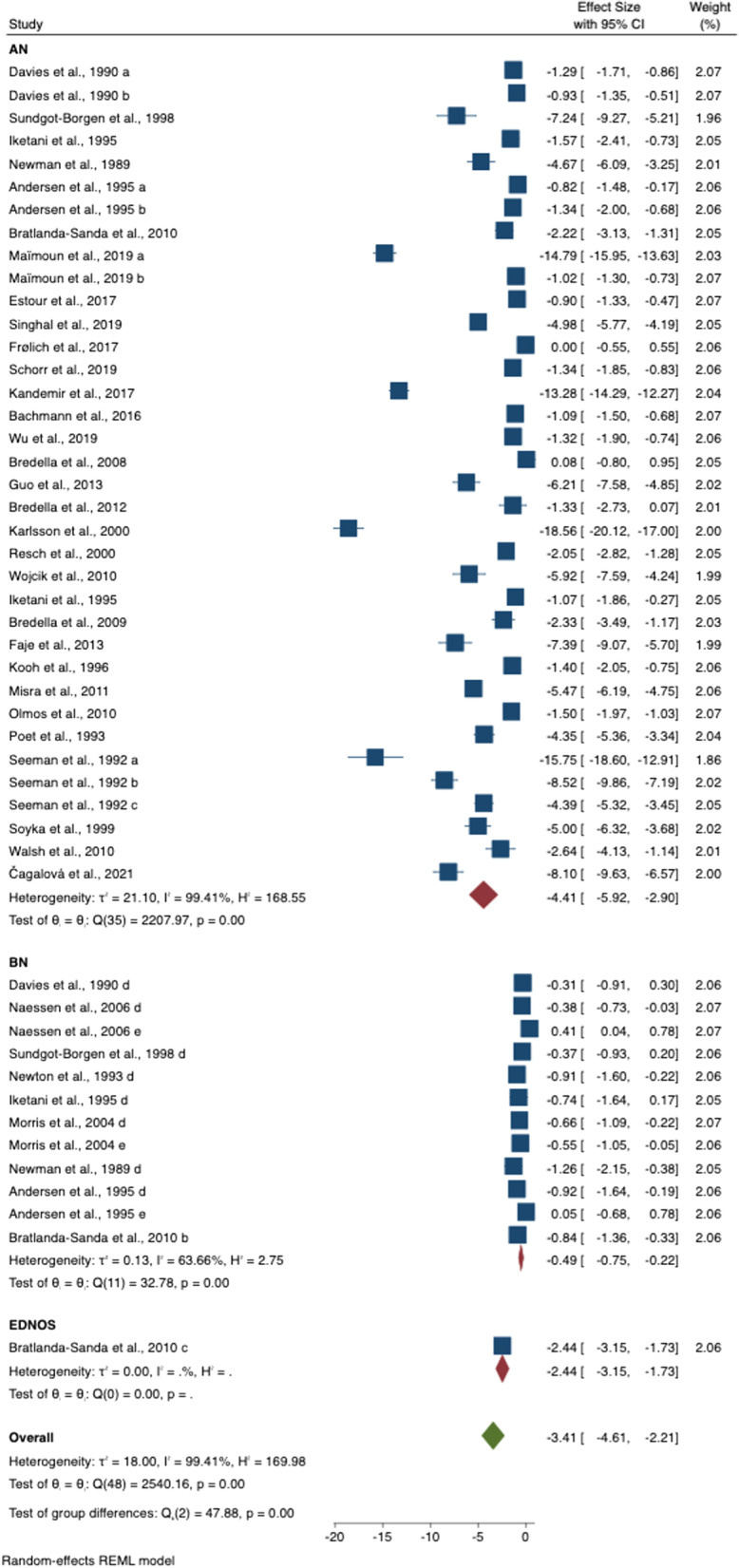
Meta-analysis results of spine bone mineral density in eating disorder (EDs) versus healthy controls (HC)
Fig. 3.
Meta-analysis results of total body bone mineral density in eating disorder (EDs) versus healthy controls (HC)
BMD in AN
The AN group had lower BMD than the HC in all the anatomical sites i.e. total body BMD (SMD = − 3.45 [− 4.57 to − 2.33], p < 0.001), spine BMD (SMD = − 4.34 [− 5.27 to − 3.40], p < 0.001), femur BMD (SMD = − 3.38 [− 5.29 to − 2.21], p < 0.001) and hip BMD (SMD = − 4.95 [− 6.78 to − 3.12], p < 0.001).
BMD in BN
The BN group had lower total body (SMD = − 0.29 [− 0.46 to − 0.12], p = 0.001)] and spine BMD (SMD = − 0.49 [− 0.75 to − 0.21], p = 0.001) than the HC group, respectively. However, the three studies included, found no differences at the femur (SMD = − 0.19 [− 0.80 to − 0.43], p = 0.552). Among those with BN (with or without previous history of AN), when compared to HC, only the group with a previous diagnosis of AN had significantly lower spine BMD (SMD = − 0.74 [− 1.06 to − 0.43], p < 0.001)].
BMD in OSFED/EDNOS
In the only study included, the OSFED/EDNOS group had lower spine BMD than HC (SMD = − 2.44 [− 3.15 to − 1.73], p < 0.001)]. No differences were found at total body BMD (SMD = − 0.04 [− 0.99 to 0.09], p = 0.109) and femur BMD (SMD = − 0.24 [− 0.76 to 0.31], p = 0.415).
Meta-regression results
Analysis of the whole EDs group (Table 3), showed that fat mass and lean mass were significantly predictive of a difference in total body BMD (β = 0.24; 95% CI 0.09–0.39; p = 0.002 and β = 0.41; 95% CI 0.037–0.78; p = 0.03, respectively). Both BMI (β = 0.74; 95% CI 0.11–1.38; p = 0.02) and fat mass (β = 0.26; 95% CI 0.04–0.48; p = 0.02) were positively associated with higher spine BMD but not lean mass. Presence of amenorrhoea was negatively associated with total body BMD (β = − 0.04; 95% CI − 0.07 to 0.01; p = 0.01) and spine BMD (β = − 0.04; 95% CI − 0.07 to − 0.01; p = 0.01).
Table 3.
Meta-regression results of covariables effect on total, spine, femur and hip bone mineral density
| Covariate | Total body BMD | Spine | Femur | Hip | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | L 95% CI | U 95% CI | p | β | L 95% CI | U 95% CI | p | β | L 95% CI | U 95% CI | p | β | L 95% CI | U 95% CI | p | |
| All studies | ||||||||||||||||
| Age | 0.08 | − 0.15 | 0.32 | 0.485 | 0.27 | 0.00 | 0.55 | 0.050 | 0.19 | − 0.30 | 0.69 | 0.443 | ||||
| Illness duration (yr) | 0.16 | − 0.26 | 0.58 | 0.460 | 0.30 | − 0.14 | 0.75 | 0.178 | − 0.03 | − 0.53 | 1.06 | 0.513 | ||||
| BMI (kg/m2) | 0.50 | − 0.11 | 1.01 | 0.055 | 0.74 | 0.11 | 1.38 | 0.022 | 0.66 | − 0.40 | 1.72 | 0.220 | ||||
| Fat mass (kg) | 0.24 | 0.09 | 0.39 | 0.002 | 0.26 | 0.04 | 0.48 | 0.022 | 0.07 | − 0.21 | 0.35 | 0.632 | ||||
| Lean mass (kg) | 0.41 | 0.04 | 0.78 | 0.031 | 0.45 | − 0.10 | 1.01 | 0.110 | 0.30 | − 0.30 | 0.91 | 0.322 | ||||
| Amenorrhea (%) | − 0.04 | − 0.08 | − 0.01 | 0.010 | − 0.04 | − 0.07 | − 0.01 | 0.011 | − 0.03 | − 0.08 | 0.02 | 0.214 | ||||
| Amenorrhea duration (mo) | 0.03 | − 0.03 | 0.09 | 0.380 | 0.04 | − 0.04 | 0.11 | 0.347 | − 0.99 | − 2.15 | 0.17 | 0.095 | ||||
| OCP use (%) | 0.01 | − 0.05 | 0.07 | 0.711 | 0.03 | − 0.03 | 0.08 | 0.376 | 0.04 | − 0.04 | 0.13 | 0.324 | ||||
| AN only | ||||||||||||||||
| Age | − 0.06 | − 0.36 | 0.24 | 0.698 | 0.19 | − 0.17 | 0.55 | 0.309 | 0.16 | − 0.46 | 0.78 | 0.610 | 0.10 | − 0.37 | 0.56 | 0.684 |
| Illness duration (yr) | 0.15 | − 1.03 | 1.34 | 0.804 | ||||||||||||
| BMI (Kg/m2) | 0.17 | − 1.13 | 1.47 | 0.797 | 0.61 | − 1.19 | 2.40 | 0.506 | 1.46 | − 0.75 | 3.67 | 0.196 | 0.55 | − 1.92 | 3.02 | 0.661 |
| Fat mass (kg) | 0.32 | 0.05 | 0.60 | 0.021 | 0.22 | − 0.19 | 0.64 | 0.291 | − 0.07 | − 0.50 | 0.37 | 0.768 | − 0.24 | − 0.85 | 0.38 | 0.445 |
| Lean mass (kg) | 0.38 | − 0.13 | 0.89 | 0.141 | 0.37 | − 0.38 | 1.12 | 0.336 | 0.21 | − 0.60 | 1.03 | 0.610 | 0.48 | − 0.59 | 1.55 | 0.379 |
| Amenorrhea (%) | 0.01 | − 0.09 | 0.11 | 0.860 | ||||||||||||
| Amenorrhea duration (mo) | − 0.17 | − 0.46 | 0.12 | 0.250 | − 0.07 | − 0.50 | 0.36 | 0.747 | ||||||||
| OCP use (%) | − 0.04 | − 0.16 | 0.10 | 0.564 | ||||||||||||
P-value in bold indicates statistical significance (p < 0.05)
BMD bone mineral density, β coefficient, L lower, U upper, CI confidence interval, p p-value, AN anorexia nervosa, yr years, mo months, OCP oral contraceptive pill
In individuals with AN, fat mass was significantly associated with total body BMD (β = 0.32; 95% CI 0.04–0.60; p = 0.02), but not with spine, femur, and hip BMD. Meta-regression analyses were not conducted on the BN and OSFED/EDNOS groups due to the limited number of studies measuring the predictors of interest.
Sensitivity analyses
The Higgins I2 test of total body BMD meta-analyses indicates high heterogeneity of the total ED group (99.2%) and the AN group (99.1%) but not of the BN group (4.8%). Heterogeneity of spine analyses was high for all EDs (I2 = 99.4%), AN (I2 = 99.4%) and BN (I2 = 63.7%) groups. Funnel plots (Fig. 4) and Egger’s test (t = − 6.87, p = < 0.001) suggest publication bias in meta-analyses on spine BMD.
Fig. 4.
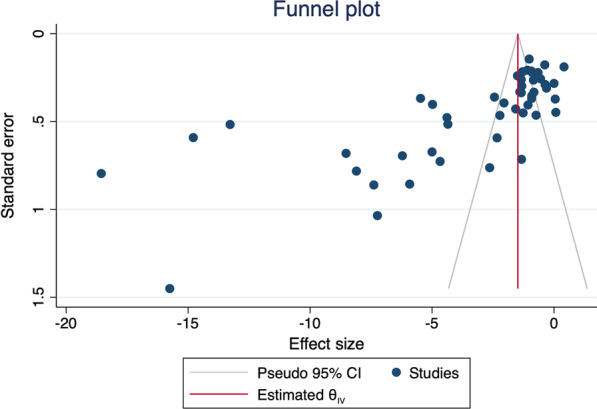
Funnel plot of spine bone mineral density studies included in the meta-analysis
Discussion
Summary of findings
We examined spine, hip, femur, and total body BMD data and identified key body composition and menstrual status predictors of the difference between EDs and HC. Due to the limited number of studies, absence of papers and/or measurements of predictors of interest, our meta-analyses and meta-regressions of total, spine and femur BMD included individuals with AN, BN and OSFED/EDNOS and that of the hip BMD included individuals with AN only. The quality of the studies included was good with the majority of studies controlling or adjusting comparisons with HC for age.
Whilst in people with AN lower BMD than HC at all sites is consistent with previous reviews [6, 14, 15], what we have found is that lower total body, spine and femur BMD is not limited to AN. Specifically, a history of AN and having amenorrhoea may increase the risk of low BMD in individuals with BN and OSFED.
Our study helps clarify the somewhat inconsistent literature on BMD in women with BN in cross-sectional studies [53, 64] and previous meta-analyses [6, 14]. In individuals with BN, BMD was lower than in HC, at total body and in the spine. However, in a subgroup analysis of studies reporting the presence or absence of a history of AN, only those with a history of AN had lower spine BMD than HC. This is in accord with previous reports [14]. Only five of the studies with BN assessed whether there was a history of AN and 36% of the individuals included in the OSFED/EDNOS group reported a history of AN. This raises the question whether individuals with BN/OSFED without a history of AN are at risk for impaired bone health [14]. There is evidence from the ALSPAC study suggesting that not only a lifetime history of AN but also having ED behaviours (i.e. fasting and food restriction) are associated with a reduction in BMD by middle-adulthood [69]. Therefore, measurement of BMD should not be limited to those with a current AN diagnosis, but also be made in individuals with a history of AN, irrespective of their current ED diagnosis.
When AN, BN and OSFED/EDNOS groups were assessed as one ‘ED’ group, the greatest magnitude of the difference in BMD was at the spine. This is likely to be driven by the AN and OSFED/EDNOS groups (both with a mean BMI < 18.5 kg/m2), i.e. there were no significant differences at the femur when BN and HC groups were assessed alone. In the AN group compared to HC, SMD at the hip and spine were greater than at the femur and total body. In the BN group versus HC, BMD was lower at the spine than in the total body. However, the majority of studies reported spine BMD, with fewer measuring total body BMD and, thus these two findings cannot be objectively compared. Spine BMD was lower than in HC in OSFED/EDNOS participants in the single study included with this diagnosis. While BMD at the hip was lowest in the AN group compared to HC, ~ 30% of studies involving participants with AN reported this measure. Oestrogen deficiency is more likely to affect trabecular bone, which can be detected by loss of spine BMD [70], while aging and peak mass accrual would more significantly affect cortical bone and be detected by hip and femur BMDs [71, 72]. In people with EDs, there is a need for a more consistent assessment of bone anatomical sites to understand the pathophysiology and the specific risk of fractures and pain at different parts of the skeleton.
Associations between body composition, menstrual health and BMD in EDs
Low BMI was a predictor of lower BMD in the whole group. Calorie restriction results in extensive weight loss in AN [73] and associations between low BMI and lower BMD, reported previously [14, 15, 74], are supported by our study.
Calorie restriction in people with EDs could be a potential predictor of BMD loss. Energy deficits lead to hormonal changes, including decreased insulin-like growth factor-1 (IGF-1), growth hormone (GH) resistance, amenorrhoea, increased cortisol levels, and changes in hunger and satiety signalling (due to decreased levels of leptin, increased peptide YY, and ghrelin resistance) [75–78]. In vitro and in vivo studies in EDs suggest an ‘uncoupling’ of bone turnover, with increased osteoclastic bone reabsorption activity in comparison to bone formation activity by osteoblasts [79]. In addition, calorie restriction or starvation in animal studies and in individuals with AN has been shown to increase marrow adipose tissue (MAT) in the bone [80, 81]. As MAT cells derive from the same lineage as osteoblast precursors cells [82, 83], it is speculated that MAT expansion in individuals with AN could potentially act as an ‘emergency storage’ of adipocytes to facilitate survival during starvation but leading to bone weakness [84].
Although caloric restriction is an essential feature for a diagnosis of AN, the study of Elran-Barak et al. [85] showed that individuals with AN and BN did not differ in terms of fasting, number of meals per day, very small meals and low-calorie meals. The authors suggest that a subset of people with BN (who are able to maintain dietary restriction, low frequency of binge eating and ingesting high calorie foods only during binge episodes) are prone to inadequate nutrition. In our study, total and spine BMD were lower in the BN group than in HC, i.e. behaviour resulting in calorie restriction in people with BN may be associated with loss of BMD.
Low fat mass was a predictor of lower BMD in the whole group and in the AN group. This could be related to decreased plasma leptin levels [86, 87], especially because women with AN tend to lose more peripheral (subcutaneous, extremity) than central (visceral, trunk, android) fat [88, 89]. However, low weight does not necessarily indicate low fat mass in all AN patients as it is also due to decreased muscle, organs and bone mass [90]. Lean mass was a predictor for lower BMD in individuals with EDs. However, we did not confirm previous evidence of a lower lean mass in AN being associated with decreased BMD [15]. Physical exercise (especially load-bearing) is important for gaining lean mass, and for achieving and maintaining peak bone mass in adults. However, the protective or detrimental effects of physical activity on BMD remain controversial in the ED field, where individuals are at a higher risk of excessive exercise [91]. People at very low weight, and with amenorrhoea, may be advised to limit physical activity and avoid high-impact activities that increase the chance of falls and injuries [18].
Our findings provide support for the proposal that body composition and history of lowest-ever and highest-ever BMI, in addition to BMI, should be used to determine individuals’ risk for low BMD and osteoporosis [92]. This could be particularly important for individuals with a history of AN, whose current body composition does not necessarily suggest that they have lowered BMD. Non-invasive, easy-to-operate, and reasonably accurate methods such as BIA could be used repeatedly to track individuals' changes in fat and lean mass before, during, and after ED treatment.
Despite previous evidence, we found that duration of amenorrhoea, age and illness duration were not significant predictors of BMD in our meta-analysis. This may reflect the fact that menstrual function and ED history were not well reported in many studies, and the lack of a standardised definition for estimating illness duration. Of the 61 groups with EDs included in the review, duration of amenorrhoea was reported in only 19 (31%) of them. As these were predominantly younger participants, the duration of both illness (M = 5.8 years, SD = 3.7) and amenorrhea (M = 23.9 months, SD = 28.7) were shorter, and for this reason they did not significantly associate with BMD. However, the percentage of participants with amenorrhoea was negatively related to total body and spine BMD, which was reported by part of the sample in 5 of the 12 studies with BN participants.
Any interruption of menstruation for a prolonged period results in bone loss and is the main determinant of osteoporosis risk in women [93]. The teenage years have the highest incidence of EDs [1] and are a critical growth period, when oestrogen has a role in the closure of epiphyseal growth plates [94]. Although resumption of menses in adult women with AN was a predictor of BMD recovery [76], the evidence of reduced BMD in both females with eumenorrhoea and amenorrhoea with a similar BMI [95] shows that low levels of oestrogen cannot solely explain the severity of bone loss in AN [76]. Therefore, longitudinal investigation of the relationship between ED onset and severity, menstrual function, and bone mass accrual is necessary to identify to what extent the ED impairs BMD and whether this can be reversed after ED recovery and throughout life.
In addition, there are not sufficient data to draw conclusions of how the exclusion of amenorrhoea from diagnostic criteria for AN in DSM-5 has affected differences in BMD of individuals with AN in comparison to HC. DSM-IV was the main method for AN diagnosis in the studies included and only 4 of them used DSM-5 [29, 33, 59, 68]. In these studies, participants with AN had mean a BMI varying from 14.4 to 17.5 kg/m2 [29, 33, 59, 68] but in the study by Wu et al. [68] all participants were amenorrheic. OCP use was reported by 15.2% of participants in the study by Schorr et al. [59] only. Although there is consensus that the use of OCP is not indicated for the purpose of preventing bone loss in EDs [96], the lack of studies reporting it did not allow us to systematically assess it as a predictor of BMD in this ED group.
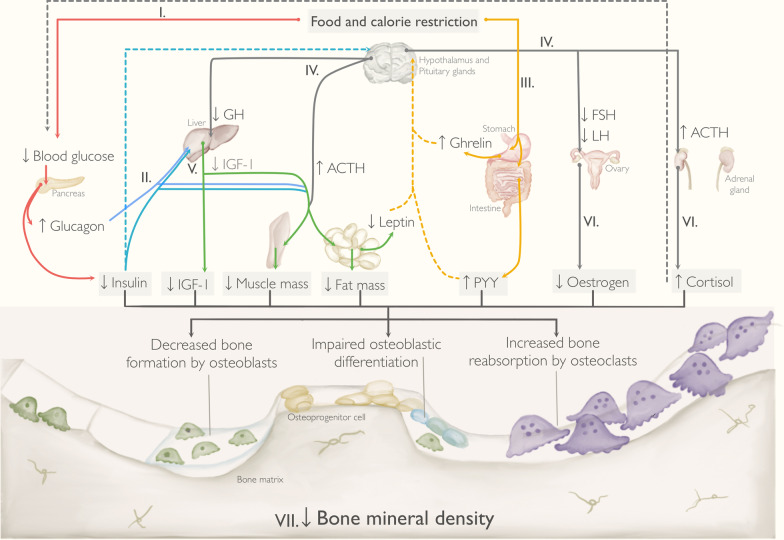
Figure 5 proposes an explanatory model of the possible interactions between body composition, hormonal changes and bone remodelling in people with EDs in which dietary restriction leads to a negative energy balance.
Fig. 5.
Model showing interactions between body composition, hormonal changes and decreased BMD in individuals with EDs, where dietary restriction leads to a negative energy balance. Legend: This figure illustrates potential mechanisms for interactions between body composition, hormonal changes and BMD loss in females with EDs, where dietary restriction leads to a negative energy balance. (I) Food and calorie restriction leads to lower levels of glucose in the blood, reducing insulin and increasing glucagon secretion by the pancreas. (II) Changes in insulin and glucagon hormones enhance lipolysis, and reduce glycogen synthesis and glucose uptake by the muscle. (III) In EDs, altered hunger and satiety signalling is marked by reduced levels of leptin (due to a diminished adipose tissue), increased peptide YY (PYY), and ghrelin resistance. (IV) All of this, in addition to the lower levels of insulin, inhibit the hypothalamic–pituitary–gonadal axis and lead to reduced levels of growth hormone (GH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and increased levels of adrenocorticotropic hormone (ACTH). (V) As a consequence, low GH has catabolic effects on muscle mass, fat mass and bone mineral density, via insulin-like growth factor-1 (IGF-1). (VI) Lower FSH and LH levels reduce oestrogen production by the ovaries and ACTH stimulates cortisol production by the adrenal glands. (VII) Decreased insulin, IGF-1, muscle mass, fat mass, oestrogen, and increased PYY and cortisol negatively affect the rate of bone formation and reabsorption by osteoblasts and osteoclasts, respectively
Osteopenia and osteoporosis in people with EDs are difficult to treat. Bone loss may not be completely reversible even after ED recovery, and nutritional supplements and oral contraceptives do not significantly increase BMD [17]. For people with long-term low body weight and low BMD, NICE guidelines consider the use of transdermal 17-β-oestradiol with cyclic progesterone (by young women between 13 and 17 years with a bone age lower than 15 years) and of bisphosphonates (by adult women 18 + years after discussing benefits and risks, i.e. teratogenic effects) [18]. Weight gain and menses resumption remain the first line of treatment for decreased BMD in individuals with EDs [17].
Repeated DXA scans (no more than once per year) are recommended for individuals with ongoing and persistent underweight (after 1 year in children and adolescents, 2 years in adults or earlier if bone pain and/or recurrent fractures are observed) [18]. The International Osteoporosis Foundation recommends that the skeletal assessment should include a comprehensive history and complete physical examination [97]. Therefore, understanding the interplay between body weight, fat mass, lean mass, menstrual function, and BMD changes across the various ED diagnoses can help identify those most at risk for osteoporosis and provide targeted and early intervention. Bone studies in EDs should (when possible) assess menstrual function and history, providing relevant information including number of participants with amenorrhoea, menarchal age, date of last menstrual period, current and history of OCP/other hormonal therapy use.
Strengths and limitations
We systematically reviewed the current literature assessing BMD in individuals with EDs (AN, BN, BED and EDNOS/OSFED) versus HC. To the best of our knowledge, this is the first review where age, illness duration, amenorrhoea, OCP use, BMI, fat mass, lean mass, and history of AN were investigated in the same meta-analysis as predictors for BMD difference for all EDs group. Studies with one or more eligible samples for the same ED diagnosis had the characteristics of each group preserved (ED subtype, menstrual status, OCP use, history of AN, diagnosis method) and outcome measures were included independently. Our findings provide strong evidence for conducting bone health assessments in individuals with a history of AN, irrespective of current ED diagnosis.
However, there was a limited number of papers with outcomes of interest for OSFED/EDNOS and BED derived from this review, and the only study focused on EDNOS did not allow a comparative analysis of BMD in those with or without a history of AN. Therefore, independent of the anatomical site, differences in BMD between EDs group and HC in OSFED/EDNOS were mostly driven by the AN group. Studies with BED did not meet the criteria for inclusion in this systematic review. The majority of studies did not use a structural clinical interview for distinguishing controls from individuals with EDs and bias in the selection of control may have influenced the accuracy of results. The results of our analyses were not controlled for race/ethnicity, symptom severity and variation in DXA machines. Therefore, the results of this present meta-analysis cannot be generalised beyond the populations studied within this review.
Our review includes studies with publication dates from 1989 to 2022. During this period several editions of DSM were published, and ED criteria changed between versions. However, except for amenorrhoea (which was removed in DSM-5 from the AN criteria) all the main elements of the diagnosis of an ED that might potentially affect BMD, remained relatively consistent across the editions (low weight/weight loss, food restriction, fear of weight gain, compensatory and purging behaviours).
Participants could not be classified and analysed separately according to menstrual status or illness duration in this meta-analysis. Lastly, physical activity data were not included in our review: as this is linked to protective and risk factors in relation to BMD, it could have helped clarify the effects of lean mass on BMD.
Conclusions
This review found lower BMD (total body, spine, and femur) in individuals with EDs (AN, BN and OSFED/EDNOS). In those with a diagnosis of BN, the lower BMD (especially in the spine), may be due to a history of AN. Secondly, individuals with AN are at more risk of diminished bone health in their spine and hip rather than in other regions. Ideally, assessing BMD in the four main anatomical sites will provide a more global picture of the effect of an ED on vulnerabilities in different bone regions and to what extent changes in BMD in different regions are/can be reversed following recovery.
Meta-regression analyses showed that low BMI, low fat mass, low lean mass and being amenorrhoeic are predictors of lower BMD in people with an ED. In those with AN, low fat mass was the only predictor of low spine BMD. Therefore, individuals with current or past AN should have their bone health assessed. To make more accurate assessments of individual risk of low BMD and osteoporosis, investigations should include measures that help predict body composition, menstrual function and history, physical exercise, and energy metabolism hormones.
Acknowledgements
São Paulo Research Foundation (FAPESP), National Institute of Health Research (NIHR) Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the FAPESP, NHS, the NIHR or the Department of Health.
Abbreviations
- AN
Anorexia nervosa
- AN-BP
AN-Binge Purge subtype
- AN-BN
AN with an additional diagnosis of BN (according to DSM-III-R)
- AN-R
AN-Restrictive subtype
- BED
Binge eating disorder
- BIA
Bioelectrical impedance analysis
- BMD
Bone mineral density
- BMI
Body mass index
- BN
Bulimia nervosa
- BN-NPAN
BN with no previous AN history
- BN-PAN
BN with previous AN
- CT
Computerised tomography
- DPA
Dual photon absorptiometry
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DXA
Dual-energy X-ray absorptiometry
- EDNOS
Eating disorder not otherwise specified
- EDs
Eating disorders
- GH
Growth hormone
- HC
Healthy controls
- HPG
Hypothalamic–pituitary–gonadal axis
- ICD
International Classification of Diseases
- IGF-1
Insulin-like growth factor-1
- MAT
Marrow adipose tissue
- MRI
Magnetic resonance imaging
- NOS
Newcastle–Ottawa scale
- OCP
Oral contraceptive pill
- OSFED
Other specified feeding or eating disorder
- PYY
Peptide YY
- SMD
Standardised mean difference
Author contributions
MPL conducted the review and was a major contributor in writing the manuscript; LR conducted statistical analysis, contributed in writing the manuscript and provided qualitative expertise during analysis. BS contributed with data analysis. MSA, LAM, ICC and US devised the project, supervised, and contributed in writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by São Paulo Research Foundation (FAPESP) [grant numbers 2016/25751-7, 2019 and 2019/22533-7]. LR, ICC and US receive salary support from the National Institute of Health Research (NIHR) Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.APA APA. Fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5). 2013;21.
- 2.Herpertz-Dahlmann B. Adolescent eating disorders: update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2015;24(1):177–196. doi: 10.1016/j.chc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Misra M, Klibanski A. Anorexia nervosa and its associated endocrinopathy in young people. Horm Res Paediatr. 2016;85(3):147–157. doi: 10.1159/000443735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escalante Boleas M, Franco Vicario R, Bustamante Murga V, Miguel de la Villa F. Bone metabolism and bone mass loss in eating disorders. An Med Interna. 2002;19(3):143–150. [PubMed] [Google Scholar]
- 6.Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2016;133(5):341–351. doi: 10.1111/acps.12556. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P, Emborg C, Støving RK, Hagen C, Mosekilde L, Brixen K. Patients with eating disorders. A high-risk group for fractures. Orthop Nurs. 2003;22(5):325–331. doi: 10.1097/00006416-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133(10):790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelsson KF, Woessner MN, Litsne H, Wheeler M, Flehr A, King AJ, et al. Eating disorders are associated with increased risk of fall injury and fracture in Swedish men and women. Osteoporos Int: J Establ Result Cooper Between Eur Found Osteoporos Natl Osteoporos Found USA. 2022;33(6):1347–1355. doi: 10.1007/s00198-022-06312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74(10):972–977. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 11.Frølich J, Winkler LA, Abrahamsen B, Bilenberg N, Hermann AP, Støving RK. Fractures in women with eating disorders—incidence, predictive factors, and the impact of disease remission: cohort study with background population controls. Int J Eat Disord. 2020;53(7):1080–1087. doi: 10.1002/eat.23223. [DOI] [PubMed] [Google Scholar]
- 12.Steinman J, Shibli-Rahhal A. Anorexia nervosa and osteoporosis: pathophysiology and treatment. J Bone Metab. 2019;26(3):133–143. doi: 10.11005/jbm.2019.26.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson EA, Fazeli PK, Calder G, Putnam H, Misra M, Meenaghan E, et al. Plasma sodium level is associated with bone loss severity in women with anorexia nervosa: a cross-sectional study. J Clin Psychiatry. 2012;73(11):e1379–e1383. doi: 10.4088/JCP.12m07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson L, Aldridge V, Clark EM, Misra M, Micali N. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos Int. 2016;27(6):1953–1966. doi: 10.1007/s00198-015-3468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra M, Golden NH, Katzman DK. State of the art systematic review of bone disease in anorexia nervosa. Int J Eat Disord. 2016;49(3):276–292. doi: 10.1002/eat.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165(2):245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson L, Micali N, Misra M. Eating disorders and bone metabolism in women. Curr Opin Pediatr. 2017;29(4):488–496. doi: 10.1097/mop.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NICE NIfH-CE. Eating disorders: recognition and treatment. National Institute for Health and Care Excellence (NICE); 2017. [PubMed]
- 19.Hübel C, Yilmaz Z, Schaumberg KE, Breithaupt L, Hunjan A, Horne E, et al. Body composition in anorexia nervosa: meta-analysis and meta-regression of cross-sectional and longitudinal studies. Int J Eat Disord. 2019;52(11):1205–1223. doi: 10.1002/eat.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 21.Wells G, Shea B. O′ Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012.
- 22.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5(4):80–84. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 23.Press S. Stata statistical software: release 16. StataCorp LLC. 2019.
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacopoulou F, Lambrou GI, Rodanaki ME, Stergioti E, Efthymiou V, Deligeoroglou E, et al. Serum kisspeptin concentrations are negatively correlated with body mass index in adolescents with anorexia nervosa and amenorrhea. Hormones (Athens) 2017;16(1):33–41. doi: 10.14310/horm.2002.1717. [DOI] [PubMed] [Google Scholar]
- 27.Bratland-Sanda S, Sundgot-Borgen J, Rosenvinge JH, Rø Ø, Hoffart A, Martinsen EW. Physical fitness, bone mineral density and associations with physical activity in females with longstanding eating disorders and non-clinical controls. J Sports Med Phys Fit. 2010;50(3):303–310. [PubMed] [Google Scholar]
- 28.Andersen AE, Woodward PJ, LaFrance N. Bone mineral density of eating disorder subgroups. Int J Eat Disord. 1995;18(4):335–342. doi: 10.1002/1098-108x(199512)18:4<335::aid-eat2260180406>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann KN, Bruno AG, Bredella MA, Schorr M, Lawson EA, Gill CM, et al. Vertebral strength and estimated fracture risk across the BMI spectrum in women. J Bone Miner Res. 2016;31(2):281–288. doi: 10.1002/jbmr.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, et al. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008;249(3):938–946. doi: 10.1148/radiol.2492080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97(4):E584–E590. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Čagalová A, Tichá Ľ, Gaál Kovalčíková A, Šebeková K, Podracká Ľ. Bone mineral density and oxidative stress in adolescent girls with anorexia nervosa. Eur J Pediatr. 2022;181(1):311–321. doi: 10.1007/s00431-021-04199-5. [DOI] [PubMed] [Google Scholar]
- 34.Davies KM, Pearson PH, Huseman CA, Greger NG, Kimmel DK, Recker RR. Reduced bone mineral in patients with eating disorders. Bone. 1990;11(3):143–147. doi: 10.1016/8756-3282(90)90207-f. [DOI] [PubMed] [Google Scholar]
- 35.Estour B, Marouani N, Sigaud T, Lang F, Fakra E, Ling Y, et al. Differentiating constitutional thinness from anorexia nervosa in DSM 5 era. Psychoneuroendocrinology. 2017;84:94–100. doi: 10.1016/j.psyneuen.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923–1929. doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazeli PK, Klibanski A. The paradox of marrow adipose tissue in anorexia nervosa. Bone. 2019;118:47–52. doi: 10.1016/j.bone.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Soto ML, González-Jiménez A, Chamorro-Fernández M, Leyva-Martínez S. Clinical and hormonal variables related to bone mass loss in anorexia nervosa patients. Vitam Horm. 2013;92:259–269. doi: 10.1016/b978-0-12-410473-0.00010-6. [DOI] [PubMed] [Google Scholar]
- 39.Frølich J, Hansen S, Winkler LA, Andresen AK, Hermann AP, Støving RK. The role of body weight on bone in anorexia nervosa: a HR-pQCT study. Calcif Tissue Int. 2017;101(1):24–33. doi: 10.1007/s00223-017-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo LJ, Jiang TJ, Liao L, Liu H, He HB. Relationship between serum omentin-1 level and bone mineral density in girls with anorexia nervosa. J Endocrinol Invest. 2013;36(3):190–194. doi: 10.3275/8458. [DOI] [PubMed] [Google Scholar]
- 41.Haas V, Kent D, Kohn MR, Madden S, Clarke S, Briody J, et al. Incomplete total body protein recovery in adolescent patients with anorexia nervosa. Am J Clin Nutr. 2018;107(3):303–312. doi: 10.1093/ajcn/nqx061. [DOI] [PubMed] [Google Scholar]
- 42.Iketani T, Kiriike N, Nakanishi S, Nakasuji T. Effects of weight gain and resumption of menses on reduced bone density in patients with anorexia nervosa. Biol Psychiatry. 1995;37(8):521–527. doi: 10.1016/0006-3223(94)00182-3. [DOI] [PubMed] [Google Scholar]
- 43.Kandemir N, Becker K, Slattery M, Tulsiani S, Singhal V, Thomas JJ, et al. Impact of low-weight severity and menstrual status on bone in adolescent girls with anorexia nervosa. Int J Eat Disord. 2017;50(4):359–369. doi: 10.1002/eat.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsson MK, Weigall SJ, Duan Y, Seeman E. Bone size and volumetric density in women with anorexia nervosa receiving estrogen replacement therapy and in women recovered from anorexia nervosa. J Clin Endocrinol Metab. 2000;85(9):3177–3182. doi: 10.1210/jcem.85.9.6796. [DOI] [PubMed] [Google Scholar]
- 45.Kooh SW, Noriega E, Leslie K, Müller C, Harrison JE. Bone mass and soft tissue composition in adolescents with anorexia nervosa. Bone. 1996;19(2):181–188. doi: 10.1016/8756-3282(96)00162-7. [DOI] [PubMed] [Google Scholar]
- 46.Maïmoun L, Renard E, Lefebvre P, Bertet H, Philibert P, Seneque M, et al. Oral contraceptives partially protect from bone loss in young women with anorexia nervosa. Fertil Steril. 2019;111(5):1020–9.e2. doi: 10.1016/j.fertnstert.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Masala S, Jacoangeli F, Fiori R, Staar Mezzasalma F, Marinetti A, Simonetti G, et al. Densitometric evaluation in women with anorexia nervosa. Acta Diabetol. 2003;40(Suppl 1):S177–S179. doi: 10.1007/s00592-003-0059-1. [DOI] [PubMed] [Google Scholar]
- 48.Mathisen TF, Rosenvinge JH, Pettersen G, Friborg O, Vrabel K, Bratland-Sanda S, et al. The PED-t trial protocol: the effect of physical exercise -and dietary therapy compared with cognitive behavior therapy in treatment of bulimia nervosa and binge eating disorder. BMC Psychiatry. 2017;17(1):180. doi: 10.1186/s12888-017-1312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra M, Miller KK, Stewart V, Hunter E, Kuo K, Herzog DB, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90(9):5082–5087. doi: 10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 50.Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92(6):2046–2052. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 51.Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris J, Tothill P, Gard M, McPhail K, Hannan J, Cowen S, et al. Reduced bone mineral density in bulimia as well as anorexia nervosa. Eur Eat Disord Rev: Prof J Eat Disord Assoc. 2004;12(2):71–78. doi: 10.1002/erv.561. [DOI] [Google Scholar]
- 53.Naessén S, Carlström K, Glant R, Jacobsson H, Hirschberg AL. Bone mineral density in bulimic women—influence of endocrine factors and previous anorexia. Eur J Endocrinol. 2006;155(2):245–251. doi: 10.1530/eje.1.02202. [DOI] [PubMed] [Google Scholar]
- 54.Newman MM, Halmi KA. Relationship of bone density to estradiol and cortisol in anorexia nervosa and bulimia. Psychiatry Res. 1989;29(1):105–112. doi: 10.1016/0165-1781(89)90190-x. [DOI] [PubMed] [Google Scholar]
- 55.Newton JR, Freeman CP, Hannan WJ, Cowen S. Osteoporosis and normal weight bulimia nervosa—which patients are at risk? J Psychosom Res. 1993;37(3):239–247. doi: 10.1016/0022-3999(93)90032-b. [DOI] [PubMed] [Google Scholar]
- 56.Olmos JM, Valero C, del Barrio AG, Amado JA, Hernández JL, Menéndez-Arango J, et al. Time course of bone loss in patients with anorexia nervosa. Int J Eat Disord. 2010;43(6):537–542. doi: 10.1002/eat.20731. [DOI] [PubMed] [Google Scholar]
- 57.Poet JL, Galinier Pujol A, Tonolli Serabian I, Conte Devolx B, Roux H. Lumbar bone mineral density in anorexia nervosa. Clin Rheumatol. 1993;12(2):236–239. doi: 10.1007/bf02231534. [DOI] [PubMed] [Google Scholar]
- 58.Resch H, Newrkla S, Grampp S, Resch A, Zapf S, Piringer S, et al. Ultrasound and X-ray-based bone densitometry in patients with anorexia nervosa. Calcif Tissue Int. 2000;66(5):338–341. doi: 10.1007/s002230010070. [DOI] [PubMed] [Google Scholar]
- 59.Schorr M, Fazeli PK, Bachmann KN, Faje AT, Meenaghan E, Kimball A, et al. Differences in trabecular plate and rod structure in premenopausal women across the weight spectrum. J Clin Endocrinol Metab. 2019;104(10):4501–4510. doi: 10.1210/jc.2019-00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seeman E, Szmukler GI, Formica C, Tsalamandris C, Mestrovic R. Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise. J Bone Miner Res. 1992;7(12):1467–1474. doi: 10.1002/jbmr.5650071215. [DOI] [PubMed] [Google Scholar]
- 61.Singhal V, Bredella MA. Marrow adipose tissue imaging in humans. Bone. 2019;118:69–76. doi: 10.1016/j.bone.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 63.Strumila R, Nobile B, Maimoun L, Jaussent I, Seneque M, Thiebaut S, et al. The implications of previous history of anorexia nervosa in patients with current bulimia nervosa: alterations in daily functioning, decision-making, and bone status. Eur Eat Disord Rev. 2020;28(1):34–45. doi: 10.1002/erv.2712. [DOI] [PubMed] [Google Scholar]
- 64.Sundgot-Borgen J, Bahr R, Falch JA, Schneider LS. Normal bone mass in bulimic women. J Clin Endocrinol Metab. 1998;83(9):3144–3149. doi: 10.1210/jcem.83.9.5085. [DOI] [PubMed] [Google Scholar]
- 65.van Marken Lichtenbelt WD, Heidendal GA, Westerterp KR. Energy expenditure and physical activity in relation to bone mineral density in women with anorexia nervosa. Eur J Clin Nutr. 1997;51(12):826–830. doi: 10.1038/sj.ejcn.1600492. [DOI] [PubMed] [Google Scholar]
- 66.Walsh CJ, Phan CM, Misra M, Bredella MA, Miller KK, Fazeli PK, et al. Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology. 2010;257(1):167–174. doi: 10.1148/radiol.10100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone. 2010;46(3):796–800. doi: 10.1016/j.bone.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Qu J, Li H, Yuan H, Guo Q, Ouyang Z, et al. Relationship between serum level of growth differentiation factors 8, 11 and bone mineral density in girls with anorexia nervosa. Clin Endocrinol (Oxf) 2019;90(1):88–93. doi: 10.1111/cen.13871. [DOI] [PubMed] [Google Scholar]
- 69.Robinson L, Aldridge VK, Clark EM, Misra M, Micali N. Bone health in adult women with ED: a longitudinal community-based study. J Psychosom Res. 2019;116:115–122. doi: 10.1016/j.jpsychores.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown SA, Rosen CJ. Osteoporosis. Med Clin N Am. 2003;87(5):1039–1063. doi: 10.1016/s0025-7125(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 71.Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014;27(2):252–267. doi: 10.1017/s0954422414000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010;25(9):1948–1957. doi: 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Misra M, Klibanski A. Anorexia nervosa and bone. J Endocrinol. 2014;221(3):R163–R176. doi: 10.1530/joe-14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuckerman-Levin N, Hochberg Z, Latzer Y. Bone health in eating disorders. Obes Rev. 2014;15(3):215–223. doi: 10.1111/obr.12117. [DOI] [PubMed] [Google Scholar]
- 75.Hansen JT, Koeppen BM. Netter's atlas of human physiology. London: ICON; 2002. [Google Scholar]
- 76.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29(5):535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89(3):755–765. doi: 10.3945/ajcn.2008.27056. [DOI] [PubMed] [Google Scholar]
- 78.Naessén S, Carlström K, Holst JJ, Hellström PM, Hirschberg AL. Women with bulimia nervosa exhibit attenuated secretion of glucagon-like peptide 1, pancreatic polypeptide, and insulin in response to a meal. Am J Clin Nutr. 2011;94(4):967–972. doi: 10.3945/ajcn.111.014837. [DOI] [PubMed] [Google Scholar]
- 79.Howgate DJ, Graham SM, Leonidou A, Korres N, Tsiridis E, Tsapakis E. Bone metabolism in anorexia nervosa: molecular pathways and current treatment modalities. Osteoporos Int. 2013;24(2):407–421. doi: 10.1007/s00198-012-2095-6. [DOI] [PubMed] [Google Scholar]
- 80.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 84.Suchacki KJ, Roberts F, Lovdel A, Farquharson C, Morton NM, MacRae VE, et al. Skeletal energy homeostasis: a paradigm of endocrine discovery. J Endocrinol. 2017;234(1):R67–r79. doi: 10.1530/joe-17-0147. [DOI] [PubMed] [Google Scholar]
- 85.Elran-Barak R, Sztainer M, Goldschmidt AB, Crow SJ, Peterson CB, Hill LL, et al. Dietary restriction behaviors and binge eating in anorexia nervosa, bulimia nervosa and binge eating disorder: trans-diagnostic examination of the restraint model. Eat Behav. 2015;18:192–196. doi: 10.1016/j.eatbeh.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64(1):105–113. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85(12):4511–4514. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 88.El Ghoch M, Calugi S, Lamburghini S, Dalle GR. Anorexia nervosa and body fat distribution: a systematic review. Nutrients. 2014;6(9):3895–3912. doi: 10.3390/nu6093895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 90.Lackner S, Mörkl S, Müller W, Fürhapter-Rieger A, Oberascher A, Lehofer M, et al. Novel approaches for the assessment of relative body weight and body fat in diagnosis and treatment of anorexia nervosa: a cross-sectional study. Clin Nutr. 2019;38(6):2913–2921. doi: 10.1016/j.clnu.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 91.Legroux I, Cortet B. Factors influencing bone loss in anorexia nervosa: assessment and therapeutic options. RMD Open. 2019;5(2):e001009. doi: 10.1136/rmdopen-2019-001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Surgenor LJ, Maguire S. Assessment of anorexia nervosa: an overview of universal issues and contextual challenges. J Eat Disord. 2013;1:29. doi: 10.1186/2050-2974-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galusca B, Bossu C, Germain N, Kadem M, Frere D, Lafage-Proust MH, et al. Age-related differences in hormonal and nutritional impact on lean anorexia nervosa bone turnover uncoupling. Osteoporos Int. 2006;17(6):888–896. doi: 10.1007/s00198-005-0063-0. [DOI] [PubMed] [Google Scholar]
- 94.Väänänen HK, Härkönen PL. Estrogen and bone metabolism. Maturitas. 1996;23(Suppl):S65–S69. doi: 10.1016/0378-5122(96)01015-8. [DOI] [PubMed] [Google Scholar]
- 95.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165(5):561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 96.Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–1439. doi: 10.1210/jc.2017-00131. [DOI] [PubMed] [Google Scholar]
- 97.Osteoporosis International. Anorexia Nervosa and Osteoporosis. 2022. https://www.osteoporosis.foundation/sites/iofbonehealth/files/2019-06/2018_Anorexia_FactSheet_English.pdf. Accessed on 27 Aug 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.