Abstract
Background
Falls and fall-related injuries are a major public health problem. Data on falls in older persons with cancer is limited and robust data on falls within those with a frailty profile are missing. The aim of this study is to investigate the incidence and predictive factors for falls and fall-related injuries in frail older persons with cancer.
Methods
This study is a secondary data analysis from data previously collected in a large prospective multicenter observational cohort study in older persons with cancer in 22 Belgian hospitals (November 2012–February 2015). Patients ≥70 years with a malignant tumor and a frailty profile based on an abnormal G8 score were included upon treatment decision and evaluated with a Geriatric Assessment (GA). At follow-up, data on falls and fall-related injuries were documented.
Results
At baseline 2141 (37.2%) of 5759 included patients reported at least one fall in the past 12 months, 1427 patients (66.7%) sustained an injury. Fall-related data of 3681 patients were available at follow-up and at least one fall was reported by 769 patients (20.9%) at follow-up, of whom 289 (37.6%) fell more than once and a fall-related injury was reported by 484 patients (62.9%). Fear of falling was reported in 47.4% of the patients at baseline and in 55.6% of the patients at follow-up. In multivariable analysis, sex and falls history in the past 12 months were predictive factors for both falls and fall-related injuries at follow-up. Other predictive factors for falls, were risk for depression, cognitive impairment, dependency in activities of daily living, fear of falling, and use of professional home care.
Conclusion
Given the high number of falls and fall-related injuries and high prevalence of fear of falling, multifactorial falls risk assessment and management programs should be integrated in the care of frail older persons with cancer. Further studies with long-term follow-up, subsequent impact on cancer treatment and interventions for fall prevention, and integration of other important topics like medication and circumstances of a fall, are warranted.
Trial registration
B322201215495.
Keywords: Older persons, Cancer, Frailty, Falls, Fall-related injuries, Geriatric assessment
Introduction
Falls are a major problem among the aging population. A fall is defined by ProFouND (The Prevention of Falls Network for Dissemination) as “an unexpected event that causes the person to fall to the ground, floor or a lower level” [1, 2]. According to the World Health Organization (WHO), 28–35% of people over the age of 65 fall at least once a year. This number rises to 32–42% in people over the age of 70 [3]. Approximately half of these persons fall more than once a year [4].
The etiology of falls is complex because biological, behavioral, environmental, and socioeconomic factors play an important role [3]. Consequences of fall incidents can occur on a physical, psychosocial, and financial level. For example, 5–10% of fall incidents lead to serious injury including fractures, tissue damage, or head trauma [5]. On a psychosocial level, fear of falling, reduced social interaction, and a decrease in the quality of life can occur. Fall and fall-related injuries also have financial consequences. In 2015, the direct medical costs for fatal and non-fatal falls are estimated to be $50 billion in the USA. Almost 99% of these costs are attributable to non-fatal falls [6]. In addition, the economic burden of falls seems to be sex dependent, with older females requiring greater healthcare use than older males after a fall.
Little research on a large scale has been done on fall problems in older persons with cancer, even though cancer is a disease of aging. Due to the aging of the population, a 67% increase in cancer incidence for older patients is expected in the USA [7]. Worldwide, 26.4 million new cancer diagnoses are expected every year [8]. In the literature the incidence of self-reported falls in this population varies from 17.6 to 35.8%, depending on the (sub) population of patients with cancer studied and the period of follow-up [9–11]. Although there is inconsistency whether falls are more common in older adults with cancer than without, two studies showed that older persons with cancer are 16–17% more likely to have a fall incident compared to those without cancer [12, 13].
The etiology of falls in older persons with cancer is similar to that of the general older population. In addition, there are specific disease-related risk factors including fatigue, depression, pain, malnutrition, anemia, metastases, and certain chemotherapeutic agents [14].
Within the population of older persons with cancer, a further distinction can be made between older persons with or without a frailty profile. For the concept of frailty, however, there is no consensus yet on a clear definition. In the literature, frailty is often described as an abnormal physiological condition that makes a person more sensitive to stressors and increases the risk of negative health outcomes [15, 16]. In previous research a cancer history and frailty were independently associated, for the most part resulting from high prevalence of geriatric syndromes like falls [12, 17]. Cancer treatments like surgery, systemic therapy, and radiotherapy are possible stressors that can cause the transition from a robust state to a frail state of older patients [18, 19].
The physical consequences of falls are significantly higher in older persons with cancer than in the general older population. For example, 29 to 74% of fall incidents in older persons with cancer result in serious injuries. According to Mohile et al., this can be explained by increased frailty [17]. A fall in older persons with cancer can cause delays or complications in treatment, and can have an impact on the course of the disease, care planning, and prognosis [20, 21].
Current literature regarding falls in older patients with cancer relies on a patient population that integrates patients with and without a frailty profile or doesn’t report any data related to a frailty profile. Robust data regarding falls in older patients with cancer and a frailty profile are missing. However, knowledge about this problem is important because this frailty, in combination with cancer, entails additional risks such as an increased risk for hospitalization and/or mortality, which should be taken into account in the older population [12].
This study aims to investigate the incidence of falls and fall-related injuries in older persons with cancer and a frailty profile and to investigate the predictive factors of these fall incidents and fall-related injuries.
Patients and methods
This secondary data analysis, focusing on falls and fall-related injuries at baseline and approximately 3 months follow-up, uses data previously collected in a large prospective multicenter observational cohort study in older persons with cancer [22].
Study design and population
The population of older persons with cancer (both in- and outpatients) was approached between November 2012 and February 2015, spread over 22 Belgian hospitals (8 academic and 14 non-academic hospitals). The inclusion criteria were 70 years or older and the presence of a malignancy (solid tumor or hematologic malignancy). Patients were included at diagnosis or at disease progression / relapse (when a change in therapeutic strategy was considered). Patients underwent a frailty screening using the G8 screening tool followed by a geriatric assessment (GA) if the G8 score was ≤14 out of 17 indicating the presence of a frailty profile [22, 23]. Follow-up was foreseen at approximately 3 months (further described as ‘at follow-up’) [22]. The study was approved by the ethics committee of all participating centers (B322201215495).
Patient, socio-demographic and clinical characteristics
The following patient, socio-demographic and clinical characteristics were collected: age, sex, social data (e.g. living situation, professional home care), tumor-specific data (e.g. new diagnosis vs. progression / relapse; solid tumor vs. hematological malignancy), comorbidities using the Charlson Comorbidity index (CCI) (no comorbidities vs. comorbidities score ≥ 1/37) [24], polypharmacy by the number of drugs taken the week before inclusion (number of drugs < 5 vs. ≥5) [25], and the Eastern Cooperative Oncology Group - Performance Status (ECOG-PS) [26].
Geriatric assessment
The following geriatric domains were assessed within the GA: functional status (FS) by activities of daily living (ADL) (independent score 6 vs. dependent score ≥ 7) [27] and instrumental activities of daily living (iADL) (male: independent score 5 vs. dependent score < 5; female: independent score 8 vs. dependent score < 8 )[28], the presence of pain and fatigue using a visual analogue score (VAS) (no pain versus presence of pain VAS ≥1/10 and no fatigue vs. presence of fatigue VAS ≥1/10) [29, 30], cognition by mini mental state examination (MMSE) (normal cognition score ≥ 24/30 vs. cognitive impairment score < 24/30) [31], mood status using the geriatric depression scale (GDS-15) (no risk for depression score < 5/15 vs. risk for depression score ≥ 5/15) [32], and nutritional status using the mini nutritional assessment – short form (MNA-SF) (no risk of malnutrition (score ≥ 12) vs. risk of malnutrition (score 8–11) vs. malnourished (score ≤ 7)) [33, 34].
Falls, fall-related injuries and fear of falling
At baseline all included patients were asked whether they had fallen in the past 12 months and whether they had fall-related injuries (minor / major) [22]. We subsequently divided patients into two groups, namely non-fallers (no falls) and fallers (presence of ≥1 fall). We further divided the fallers into single fallers (=1 fall) or recurrent fallers (≥2 falls) [35]. In addition, fall-related injuries were documented and categorized in minor and major injuries. Minor injuries were scrapes and scratches, bruises, and superficial wounds that required no or minimal medical assistance. Major injuries were sprains, severe soft-tissue bruises, severe head injuries, distortion or dislocation of the joints, contusions, lacerations, loss of consciousness, and fractures [36, 37].
At approximately 3 months follow-up, falls and fall-related injuries were recorded again by asking the patients whether they had fallen and had a fall-related injury as a consequence during the follow-up period.
Finally, fear of falling (i.e. never, sometimes, often, always) was assessed at both time points [38, 39].
Statistical analysis
We used descriptive statistics (mean, median, standard deviation, range for continues data, and frequency for categorical data) to describe patient characteristics and calculated the 95% confidence intervals. Proportions were compared using a Chi square test. Statistical techniques for handling missing data were not used. Statistical significance was considered at p ≤ 0.05.
Univariable logistic regressions were conducted with non-fallers versus fallers (≥1 fall), fallers without injuries versus fallers with injuries (minor and major combined) as the dependent variables. The independent baseline variables were: age, sex, characteristics of the tumor (solid vs. hematologic malignancy; new diagnosis vs. relapse/progression), CCI, polypharmacy, ECOG-PS, living situation, professional home care, ADL, IADL, falls history in the past 12 months, fear of falling, VAS for fatigue, VAS for pain, MMSE, GDS, and MNA-SF. These variables were dichotomized, except for the variable ‘age’, which was divided into four categories (i.e. 70–74, 75–79, 80–84, ≥85).
After univariable analyses, we conducted multivariable logistic regressions in order to explore the relationship between the dependent variables (non-fallers versus fallers, and fallers without injuries versus fallers with injuries), and patients’ baseline significant (p ≤ 0.05) characteristics identified in the univariable analyses.
Multivariable logistic regressions were conducted both without selection and with stepwise selection. Data of the regressions with selection are shown. The p-values to enter and stay in the model were 0.05. In case of only two significant variables, the multivariable logistic regression was done without selection.
Multicollinearity of the independent variables was investigated with the variance inflation factors (VIF). If the VIF was < 3, absence of multicollinearity was concluded. All statistical analyses were performed with SPSS 25.0 or with SAS v.9.4 software.
Results
Patient, socio-demographic and clinical characteristics
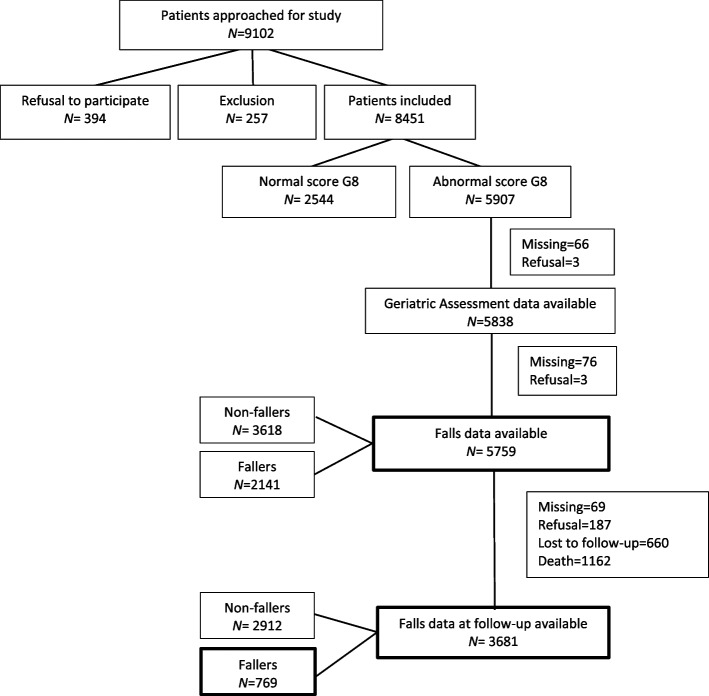
The patient flow-chart is presented in Fig. 1. We approached 9102 patients to participate in this study. Of these patients, 394 refused to participate and 257 did not meet the inclusion criteria resulting in 8451 included patients. We further selected patients based on an abnormal G8 score and the availability of GA data including fall-related data. This resulted in 5759 patients whose baseline falls history data were available. After approximately 3 months follow-up, fall-related data of 3681 patients were available.
Fig. 1.
Flow-chart of patient selection
Patient, socio-demographic and clinical characteristics are listed in Table 1. Of the included patients, 54.5% was female (n = 3133), and the median age was 80 years old (range 70–101). Most of the patients (77.9%) had a new diagnosis at inclusion, and 90.6% had a solid tumor. Comorbidity was present in 74.0%, and polypharmacy in 63.6% of the patients. Most patients lived at home, either alone (36.0%) or with a partner/family (55.2%) and 53.2% of the patients had professional home care.
Table 1.
Baseline patient, socio-demographic and clinical characteristics and geriatric assessment data
| Patients with data on falls at baseline available (n = 5759) | Patients with data on falls at follow-up available (n = 3681) | ||||
|---|---|---|---|---|---|
| Variable | Operationalization | N (%) | 95% CIa | N (%) | 95% CIa |
| Age (years) | 70–74 | 1212 (21.0) | 19.99–22.10 | 815 (22.1) | 20.80–23.48 |
| 75–79 | 1556 (27.0) | 25.87–28.17 | 1007 (27.4) | 25.92–28.80 | |
| 80–84 | 1668 (29.0) | 27.79–30.14 | 1038 (28.2) | 26.74–29.65 | |
| ≥85 | 1323 (23.0) | 21.87–24.04 | 821 (22.3) | 20.96–23.65 | |
| Median | 80.0 | 80.0 | |||
| Range | 70–101 | 70–100 | |||
| Sex | Male | 2626 (45.6) | 44.31–46.88 | 1601 (43.5) | 41.89–45.10 |
| Female | 3133 (54.4) | 53.12–55.69 | 2080 (56.5) | 54.90–58.11 | |
| Diagnosis general | Solid tumor/Carcinoma | 5218 (90.6) | 89.85–91.36 | 3352 (91.1) | 90.14–91.98 |
| Hematologic malignancy | 541 (9.4) | 8.64–10.15 | 329 (8.9) | 8.02–9.86 | |
| Diagnosis specific | New diagnosis | 4488 (77.9) | 76.86–79.00 | 2907 (79.0) | 77.66–80.29 |
| Relapse/ Progression | 1271 (22.1) | 21.00–23.14 | 774 (21.0) | 19.71–22.34 | |
| CCIa | Score 0 | 1486 (26.0) | 24.90–27.18 | 989 (27.0) | 25.58–28.46 |
| Score ≥ 1 | 4220 (74.0) | 72.82–75.10 | 2671 (73.0) | 71.54–74.42 | |
| Missing | 53 | 21 | |||
| Polypharmacya | Number 0–4 | 2052 (36.4) | 35.12–37.63 | 1355 (37.3) | 35.71–38.86 |
| Number ≥ 5 | 3589 (63.6) | 62.37–64.88 | 2279 (62.7) | 61.14–64.29 | |
| Missing | 118 | 47 | |||
| ECOG-PS | Score 0–1 | 2915 (50.6) | 49.32–51.91 | 2098 (57.0) | 55.40–58.60 |
| Score 2–4 | 2844 (49.4) | 49.32–51.91 | 1583 (43.0) | 41.40–44.60 | |
| Living situationa | Living alone | 2070 (36.0) | 34.70–37.18 | 1307 (35.5) | 33.96–37.05 |
| Living with others | 3179 (55.2) | 53.92–56.49 | 2080 (56.5) | 54.90–58.11 | |
| Other | 508 (8.8) | 8.12–9.59 | 294 (8.0) | 7.11–8.86 | |
| Missing | 2 | 0 | |||
| Professional home care a | No | 2694 (46.8) | 45.55–48.13 | 1696 (46.1) | 44.51–47.72 |
| Yes | 3057 (53.2) | 51.87–54.45 | 1982 (53.9) | 52.28–55.50 | |
| Missing | 8 | 3 | |||
| Geriatric domain | |||||
| FS: ADL (6–24) | Independent: score 6 | 2329 (40.5) | 39.1–41.7 | 1623 (44.1) | 42.5–45.7 |
| Dependent: score ≥ 7 | 3430 (59.5) | 58.0–61.0 | 2058 (55.9) | 54.0–58.0 | |
| FS: IADL a (0–5 (male)/8(female)) | Independent: score 8 (female) or 5 (male) | 1817 (31.8) | 30.3–32.7 | 1267 (34.4) | 33.0–36.0 |
| Dependent: score < 8 or 5 | 3902 (68.2) | 67.0–69.0 | 2401 (65.6) | 64.0–67.0 | |
| Missing | 40 | 13 | |||
| Pain (VAS) (0–10) a | No pain (score 0) | 2725 (48.3) | 47.0–49.6 | 1810 (50.1) | 48.4–51.7 |
| Mild pain (score 0.5–3) | 1086 (19.3) | 18.2–20.3 | 717 (19.8) | 18.5–21.1 | |
| Severe (score 3.5–10) | 1828 (32.4) | 31.2–33.6 | 1087 (30.1) | 28.6–31.6 | |
| Missing | 120 | 67 | |||
| Fatigue (VAS) (0–10) a | No fatigue (score 0) | 1261 (22.7) | 21.6–23.8 | 937 (26.3) | 24.8–27.7 |
| Presence of fatigue (score 0.5–10) | 4299 (77.3) | 76.0–78.0 | 2632 (73.5) | 72.0–75.0 | |
| Missing | 199 | 112 | |||
| Cognition (MMSE) (0–30) a | Score ≥ 24 = normal cognition | 3942 (76.9) | 75.7–78.1 | 2686 (80.1) | 78.8–81.5 |
| Score 18–23 = mild cognitive impairment | 831 (16.2) | 15.2–17.2 | 495 (14.8) | 13.6–16.0 | |
| Score ≤ 17 = severe cognitive impairment | 353 (6.9) | 6.2–7.6 | 170 (5.1) | 4.3–5.8 | |
| Missing | 633 | 330 | |||
| Depression (GDS) (0–15) a | Score 0–4 = not at risk for depression | 3301(62.8) | 61.5–64.1 | 2297 (66.8) | 65.3–68.4 |
| Score 5–15 = at risk for depression | 1954 (37.2) | 36.0–38.0 | 1140 (33.2) | 32.0–35.0 | |
| Missing | 504 | 244 | |||
| Nutrition (MNA-SF) (0–14) a | Normal nutritional status: score 12–14 | 1028 (17.9) | 16.9–18.9 | 760 (20.7) | 19.4–22.0 |
| Risk of malnutrition: score 8–11 | 2986 (52.0) | 50.7–53.3 | 2022 (55.1) | 53.4–56.6 | |
| Malnourished: score 0–7 | 1728 (30.1) | 28.9–31.3 | 891 (24.2) | 22.9–25.6 | |
| Missing | 17 | 8 | |||
aIn case of missings, calculation of the percentages and 95% CI were made by subtracting the missings from the denominator
Abbreviations: CCI Charlson Comorbidity Index, ECOG-PS Eastern Cooperative Oncology Group – Performance Status, FS functional status, ADL Activities of Daily Living, IADL Instrumental Activities of Daily Living, VAS Visual Analogue Scale, MMSE Mini Mental State Examination, GDS Geriatric Depression Scale, MNA-SF Mini Nutritional Assessment – Short Form
Geriatric assessment
Results of the GA are shown in Table 1. More than half of the patients showed a functional dependency on ADL (59.5%) and IADL (68.2%). The greatest clinical problems were fatigue (77.3%), and being at risk of malnutrition or malnourished (82%). A mild to severe pain was reported by 51.6% of the patients. Cognitive impairment was detected in 23.1% of the patients and 37.2% of the patients were at risk for depression.
Incidence of falls, fall-related injuries and fear of falling at baseline and at follow-up
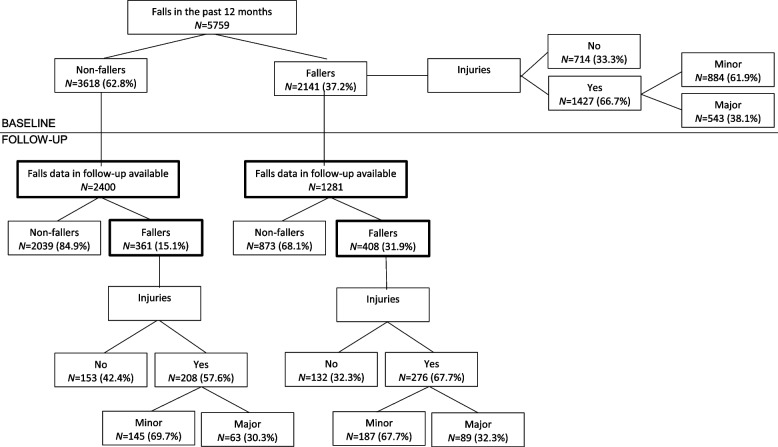
At baseline, 2141 (37.2%) patients reported at least one fall in the past 12 months before inclusion in the study, and 1427 patients (66.7%) sustained an injury (minor (61.9%); major (38.1%)) (see Table 2; Fig. 2).
Table 2.
Results of fall-related data at baseline and follow-up
| Patients with data on falls history available (related to 1 year before inclusion) N = 5759a |
Patients with data on falls in follow-up period available N = 3681a |
||||||
|---|---|---|---|---|---|---|---|
| Baseline data (related to 1 year before inclusion) | Follow-up data (related to follow-up period) | ||||||
| N (%) | CI 95% | N (%) | CI 95% | N (%) | CI 95% | ||
| Falls | Non-fallers | 3618 (62.8) | 61.6–64.1 | 2400 (65.2) | 63.7–66.7 | 2912 (79.1) | 77.8–80.4 |
| Fallers | 2141 (37.2) | 35.9–38.4 | 1281 (34.8) | 33.3–36.3 | 769 (20.9) | 19.6–22.2 | |
| • Single fallers | 1117 (19.4) | 18.4–20.4 | 686 (18.6) | 17.4–19.9 | 465 (12.6) | 11.6–13.7 | |
| • Recurrent fallers | 911 (15.8) | 14.9–16.8 | 537 (14.6) | 13.4–15.7 | 289 (7.9) | 6.9–8.6 | |
| • Unknownb | 113 (2.0) | 1.6–2.3 | 58 (1.6) | 1.2–2.0 | 15 (0.4) | 0.2–0.6 | |
| Fall-related injuriesc | Non-fallers | 3618 (62.8) | 61.6–64.1 | 2400 (65.2) | 63.7–66.7 | 2912 (79.1) | 77.8–80.4 |
| Fallers without injuries | 714 (12.4) | 11.5–13.2 | 419 (11.4) | 10.4–12.4 | 285 (7.7) | 6.9–8.6 | |
| Fallers with injuries | 1427 (24.8) | 23.7–25.9 | 862 (23.4) | 22.0–24.8 | 484 (13.1) | 12.1–14.2 | |
| Fallers with minor injuries | 884 (15.3) | 14.4–16.3 | 536 (14.6) | 13.4–15.7 | 332 (9.0) | 8.1–9.9 | |
| Fallers with major injuries | 543 (9.4) | 8.7–10.2 | 326 (8.9) | 7.9–9.8 | 152 (4.1) | 3.5–4.8 | |
| Falls with fall-related injuries | Non-fallers | 3618 (62.8) | 61.6–64.1 | 2400 (65.2) | 63.7–66.7 | 2912 (79.1) | 77.8–80.4 |
| Single fallers without injuries | 395 (6.9) | 6.2–7.5 | 241 (6.5) | 5.7–7.3 | 179 (4.9) | 4.2–5.6 | |
| Single fallers with minor injuries | 431 (7.5) | 6.8–8.2 | 265 (7.2) | 6.4–8.0 | 195 (5.3) | 4.6–6.0 | |
| Single fallers with major injuries | 291 (5.1) | 4.5–5.6 | 180 (4.9) | 4.2–5.6 | 91 (2.5) | 2.0–3.0 | |
| Recurrent fallers without injuries | 263 (4.6) | 4.0–5.1 | 150 (4.1) | 3.4–4.7 | 98 (2.7) | 2.1–3.2 | |
| Recurrent fallers with minor injuries | 415 (7.2) | 6.5–7.9 | 250 (6.8) | 6.0–7.6 | 134 (3.6) | 3.0–4.2 | |
| Recurrent fallers with major injuries | 233 (4.0) | 3.5–4.6 | 137 (3.7) | 3.1–4.3 | 57 (1.5) | 1.1–1.9 | |
| Unknownb | 113 (2.0) | 1.6–2.3 | 58 (1.6) | 1.2–2.0 | 15 (0.4) | 0.2–0.6 | |
| Fear of falling | Never | 3015 (52.6) | 51.3–53.9 | 2027 (55.3) | 53.7–56.9 | 1631 (44.4) | 42.8–46.0 |
| Sometimes | 1256 (21.9) | 20.8–23.0 | 773 (21.1) | 19.8–22.4 | 1170 (31.9) | 30.3–33.4 | |
| Often | 650 (11.3) | 10.5–12.2 | 404 (11.0) | 10.0–12.0 | 418 (11.4) | 10.4–12.4 | |
| Always | 810 (14.1) | 13.2–15.0 | 460 (12.6) | 11.5–13.6 | 454 (12.4) | 11.3–13.4 | |
| Missing | 28 | 17 | 8 | ||||
aIn case of missings, calculation of the percentages and the 95% CI were made by subtracting the missings from the denominator
bUnknown: patients that experienced a fall but not known if it was a single fall or recurrent falls
cMinor injuries were scrapes and scratches, bruises, and superficial wounds that required no or minimal medical assistance. Major injuries were sprains, severe soft-tissue bruises, severe head injuries, distortion or dislocation of the joints, contusions, lacerations, loss of consciousness, and fractures
Abbreviations: CI confidence interval
Fig. 2.
Overview of falls with fall-related injuries (minor + major). Minor injuries were scrapes and scratches, bruises, and superficial wounds that required no or minimal medical assistance. Major injuries were sprains, severe soft-tissue bruises, severe head injuries, distortion or dislocation of the joints, contusions, lacerations, loss of consciousness, and fractures
The follow-up period of approximately 3 months had an average of 89 days with a standard deviation of 20 days.
During this follow-up period, 769 patients (20.9%) reported a fall of whom 289 (37.6%) fell more than once. The fall risk during follow-up was significantly higher in patients with falls history in the past 12 months compared to those without falls history (31.9% versus 15.1% respectively, p < 0.0001) (see Table 2; Fig. 2).
A fall-related injury was reported by 484 patients (62.9% of the fallers) of which 332 patients (68.6%) reported a minor injury and 152 patients (31.4%) reported a major injury (see Fig. 2).
Fear of falling was reported in 47.4% of the patients at baseline and in 55.6% of the patients at follow-up (see Table 2).
Univariable and multivariable baseline predictors of falls at follow-up
Univariable predictive baseline factors for falls during the follow-up period were: age (p-value = 0.005), sex (p-value = 0.036), CCI (p-value = 0.028), polypharmacy (p-value = <.0001), ECOG-PS (p-value = <.0001), professional home care (p-value = 0.001), functional status (FS) measured by ADL (p-value = <.0001) and IADL (p-value = <.0001), falls history in the past 12 months (p-value = <.0001), fear of falling (p-value = <.0001), MMSE (p-value = <.0001), GDS (p-value = 0.001) and MNA-SF (p-value = 0.023) (see Table 3).
Table 3.
Univariable baseline predictors of falls at follow-up
| P-value | |||||
|---|---|---|---|---|---|
| Covariate | Patients with data on falls in follow-up period available (n = 3681) | Non-fallers (n = 2912) | Fallers (n = 769) | Non- fallers vs. fallers | |
| Age | 70–74 | 815 (22.1) | 676 (23.2) | 139 (18.1) | 0.005 |
| 75–79 | 1007 (27.4) | 784 (26.9) | 223 (29.0) | ||
| 80–84 | 1038 (28.2) | 827 (28.4) | 211 (24.4) | ||
| ≥85 | 821 (22.3) | 625 (21.5) | 196 (25.5) | ||
| Sex | Female | 2080 (56.5) | 1672 (57,4) | 408 (53.1) | 0.036 |
| Male | 1601 (43.5) | 1240 (42, 5) | 361 (46.9) | ||
| Diagnosis General | Solid tumor | 3352 (91.1) | 2665 (91.5) | 687 (89.3) | 0.065 |
| Hematologic malignancy | 329 (8.9) | 247 (8.5) | 82 (10.7) | ||
| Diagnosis Specific | New diagnosis | 2907 (79.0) | 2303 (79.1) | 604 (78.5) | 0.743 |
| Relapse/Progression | 774 (21.0) | 609 (20.9) | 165 (21.5) | ||
| CCIa | No comorbidities (0) | 989 (27.0) | 806 (27.8) | 183 (23.9) | 0.028 |
| Comorbidities (≥1) | 2671 (73.0) | 2089 (72.2) | 582 (76.1) | ||
| Polypharmacya | No polypharmacy (0–4) | 1355 (37.3) | 1124 (39.1) | 231 (30.3) | < 0.0001 |
| Polypharmacy (≥5) | 2279 (62.7) | 1748 (60.9) | 531 (69.7) | ||
| ECOG-PS | Score 0–1 | 2098 (57.0) | 1732 (59.5) | 366 (47.6) | < 0.0001 |
| Score 2–4 | 1583 (43.0) | 1180 (40.5) | 403 (52.4) | ||
| Living situation | Not living alone | 2374 (64.5) | 1889 (64.9) | 485 (63.1) | 0.354 |
| Living alone | 1307 (35.5) | 1023 (35.1) | 284 (36.9) | ||
| Professional home carea | No | 1696 (46.1) | 1382 (47.5) | 314 (40.9) | 0.001 |
| Yes | 1982 (53.9) | 1528 (52.5) | 454 (59.1) | ||
| FS: ADL | Independent (6) | 1623 (44.1) | 1368 (47.0) | 255 (33.2) | < 0.0001 |
| Dependent (> 6) | 2058 (55.9) | 1544 (53.0) | 514 (66.8) | ||
| FS: IADLa | Independent (5(male)/8(female)) | 1267 (34.5) | 1072 (36.9) | 195 (25.5) | < 0.0001 |
| Dependent (< 5(male)/8(female) | 2401 (65.5) | 1831 (63.1) | 570 (74.5) | ||
| Falls history in the past 12 months | No falls | 2400 (65.2) | 2039 (70.0) | 361 (46.9) | < 0.0001 |
| Falls | 1281 (34.8) | 873 (30.0) | 408 (53.1) | ||
| Fear of fallinga | Never | 2027 (55.3) | 1664 (57.4) | 363 (47.3) | < 0.0001 |
| Sometimes / often / always | 1637 (44.7) | 1233 (42.6) | 404 (52.7) | ||
| VAS for fatiguea | No fatigue (0) | 937 (26.3) | 756 (26.7) | 181 (24.4) | 0.193 |
| Presence of fatigue (≥1) | 2632 (73.7) | 2071 (73.3) | 561 (75.6) | ||
| VAS for paina | No pain (0) | 1810 (50.1) | 1453 (50.8) | 357 (47.4) | 0.099 |
| Presence of pain (≥1) | 1804 (49.9) | 1408 (49.2) | 396 (52.6) | ||
| MMSEa | Normal cognition (≥24) | 2686 (80.2) | 2184 (82.1) | 502 (72.5) | < 0.0001 |
| Cognitive impairment (< 24) | 665 (19.8) | 475 (17.9) | 190 (27.5) | ||
| GDSa | Not at risk for depression (< 5) | 2297 (66.8) | 1859 (68.2) | 438 (61.4) | 0.001 |
| At risk for depression (≥5) | 1140 (33.2) | 865 (31.8) | 275 (38.6) | ||
| MNA-SFa | Normal nutritional status (≥12) | 760 (20.7) | 624 (21.5) | 136 (17.8) | 0.023 |
| Risk of malnutrition/ malnourished (< 12) | 2913 (79.3) | 2284 (78.5) | 629 (82.2) |
aIn case of missings, calculation of the percentages was made by subtracting the missings from the denominator
Abbreviations: FS functional status, ADL Activities of Daily Living, IADL Instrumental Activities of Daily Living, VAS Visual Analogue Scale, MMSE Mini Mental State Examination, GDS Geriatric Depression Scale, MNA-SF Mini Nutritional Assessment – Short Form, CCI Charlson Comorbidity Index, ECOG-PS Eastern Cooperative Oncology Group – Performance Status
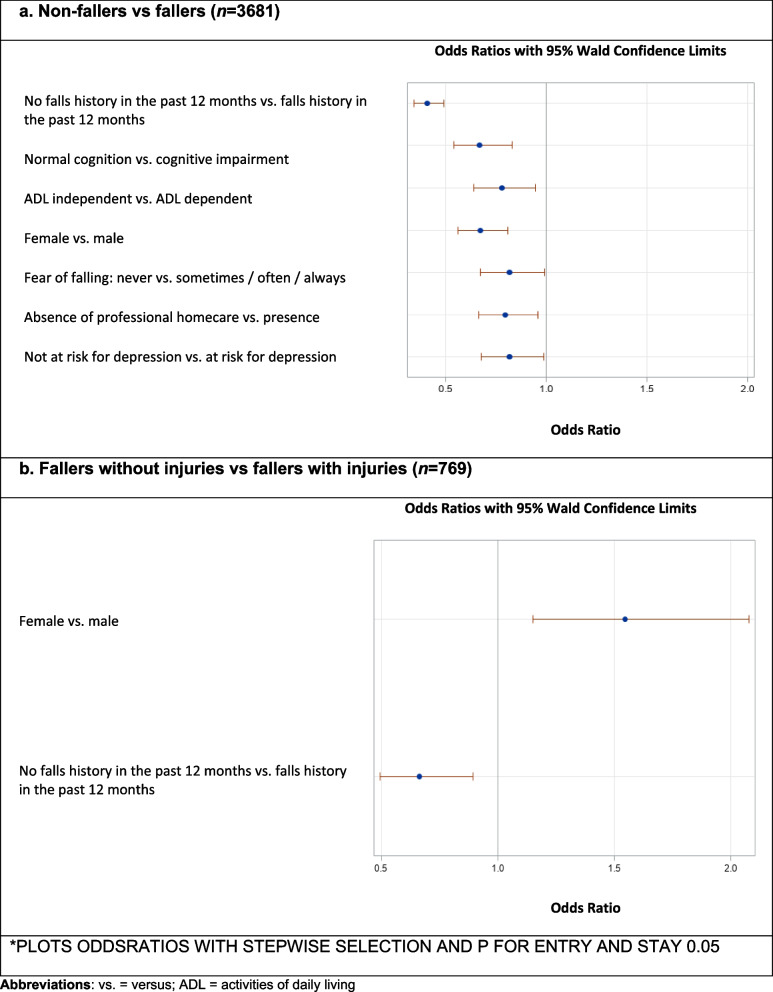
In multivariable regression analysis, falls during the follow-up period can be predicted significantly by presence of falls history in the past 12 months (OR: 0.41; 95%CI: 0.340–0.490), cognitive impairment measured by MMSE (OR: 0.70; 95%CI: 0.540–.0830), functional dependency measured by ADL (OR: 0.78; 95%CI: 0.639–.0947), male sex (OR: 0.67; 95%CI: 0.558–0.808), fear of falling (OR: 0.82; 95%CI: 0.673–0.989), use of professional homecare (OR: 0.80; 95%CI: 0.662–0.957), and a risk for depression measured by GDS-15 (OR: 0.82; 95%CI: 0.676–0.988) (see Fig. 3a).
Fig. 3.
Multivariable baseline predictors for falls (≥1 fall) and fall-related injuries at follow-up
There was no multicollinearity between the independent variables.
Univariable and multivariable baseline predictors of fall-related injuries at follow-up
Both univariable and multivariable predictive factors for fall-related injuries at follow-up were female sex (p-value = 0.003; OR: 1.52; 95% CI: 1.095–2.097) and presence of a falls history in the past 12 months (p-value = 0.004; OR: 0.66; 95% CI: 0.474–0.908) (see Table 4; Fig. 3b).
Table 4.
Univariable baseline predictors of fall-related injuries at follow-up
| Covariate | Fallers (n = 769) n (%) |
Fallers without injury (ies) (n = 285) n (%) |
Fallers with injury (ies) (n = 484) n (%) |
Fallers without injuries vs fallers with injury (ies) p-value |
|
|---|---|---|---|---|---|
| Age | 70–74 | 139 (18.1) | 57 (20.0) | 82 (16.9) | 0.612 |
| 75–79 | 223 (29.0) | 81 (28.4) | 142 (29.3) | ||
| 80–84 | 211 (24.4) | 80 (28.1) | 131 (27.1) | ||
| ≥85 | 196 (25.5) | 67 (23.5) | 129 (26.7) | ||
| Sex | Female | 408 (53.1) | 131 (46.0) | 277 (57.2) | 0.003 |
| Male | 361 (46.9) | 154 (54.0) | 207 (42.8) | ||
| Diagnosis General | Solid tumor | 687 (89.3) | 256 (89.8) | 431 (89.0) | 0.762 |
| Hematologic malignancy | 82 (10.7) | 29 (10.2) | 53 (11.0) | ||
| Diagnosis Specific | New diagnosis | 604 (78.5) | 226 (79.3) | 378 (78.1) | 0.772 |
| Progression / Relapse | 165 (21.5) | 59 (20.7) | 106 (21.9) | ||
| CCIa | No comorbidities (0) | 183 (23.9) | 68 (24.2) | 115 (23.8) | 0.950 |
| Comorbidities (≥1) | 582 (76.1) | 213 (75.8) | 369 (76.2) | ||
| Polypharmacya | No polypharmacy (0–4) | 231 (30.3) | 88 (31.0) | 143 (29.9) | 0.715 |
| Polypharmacy (≥5) | 531 (69.7) | 196 (69.0) | 336 (70.1) | ||
| ECOG-PS | Score 0–1 | 366 (47.6) | 137 (48.1) | 229 (47.3) | 0.709 |
| Score 2–4 | 403 (52.4) | 148 (51.9) | 255 (52.7) | ||
| Living situation | Not living alone | 485 (63.1) | 187 (65.6) | 298 (61.6) | 0.248 |
| Living alone | 284 (36.9) | 98 (34.4) | 186 (38.4) | ||
| Professional home carea | No | 314 (40.9) | 124 (43.5) | 190 (39.3) | 0.239 |
| Yes | 454 (59.1) | 161 (56.5) | 293 (60.7) | ||
| FS: ADL | Independent (6) | 255 (33.2) | 101 (35.4) | 154 (31.8) | 0.241 |
| Dependent (> 6) | 514 (66.8) | 184 (64.6) | 330 (68.2) | ||
| FS: IADLa | Independent (5(male)/8(female)) | 195 (25.5) | 73 (25.7) | 122 (25.4) | 0.801 |
| Dependent (< 5(male)/8(female) | 570 (74.5) | 211 (74.3) | 359 (74.6) | ||
| Falls history in the past 12 months | No falls | 361 (46.9) | 153 (53.7) | 208 (43.0) | 0.004 |
| Falls | 408 (53.1) | 132 (46.3) | 276 (57.0) | ||
| Fear of fallinga | Never | 363 (47.3) | 132 (46.6) | 231 (47.7) | 0.772 |
| Sometimes / often / always | 404 (52.7) | 151 (53.4) | 253 (52.3) | ||
| VAS for fatiguea | No fatigue (0) | 181 (24.4) | 69 (25.1) | 112 (24.0) | 0.742 |
| Presence of fatigue (≥1) | 561 (75.6) | 206 (74.9) | 355 (76.0) | ||
| VAS for paina | No pain (0) | 357 (47.4) | 131 (46.6) | 226 (47.9) | 0.724 |
| Pain (≥1) | 396 (52.6) | 150 (53.4) | 246 (52.1) | ||
| MMSEa | Normal cognition (≥24) | 502 (72.5) | 183 (72.0) | 319 (72.8) | 0.997 |
| Cognitive impairment (< 24) | 190 (27.5) | 71 (28.0) | 119 (27.2) | ||
| GDSa | Not at risk for depression (< 5) | 438 (61.4) | 167 (63.3) | 271 (60.4) | 0.433 |
| At risk for depression (≥5) | 275 (38.6) | 97 (36.7) | 178 (39.6) | ||
| MNA-SFa | Normal nutritional status (≥12) | 136 (17.8) | 46 (16.3) | 90 (18.6) | 0.435 |
| Risk of malnutrition/ malnourished (< 12) | 629 (82.2) | 236 (83.7) | 393 (81.4) |
aIn case of missings, calculation of the percentages was made by subtracting the missings from the denominator
Abbreviations: ADL Activities of Daily Living, IADL Instrumental Activities of Daily Living, CCI Charlson Comorbidity Index, VAS Visual Analogue Scale, MMSE Mini Mental State Examination, GDS Geriatric Depression Scale, MNA-SF Mini Nutritional Assessment-Short Form, ECOG-PS Eastern Cooperative Oncology Group Performance
Discussion
This study focused on the incidence and predictive factors for falls and fall-related injuries in older patients with cancer with a frailty profile, based on abnormal score on the G8 screening tool. Almost 4 out of 10 in the past 12 months and 1 out of 5 at approximately 3 months of follow-up had at least one fall, respectively. Almost 7 out of 10 fallers experienced fall-related injuries and more than half suffered from fear of falling at follow-up. The following predictive factors seem to play an important role for both falls and fall-related injuries in this population: sex and falls history in the past 12 months. Male patients had a higher risk for falls during follow-up and in contrary the risk for fall-related injuries was higher in female patients. Several other components were also predictive factors for falls: risk for depression, cognitive impairment, dependency in ADL, fear of falling and use of professional home care.
The amount of frail older patients with cancer experiencing a fall history seems to be congruent with figures found by Zhang et al. showing a fall rate of 38.5% in the past 6 months before inclusion in their study. In this study 53% of the included patients had a frailty profile, based on Fried’s criteria [11].
The fall incidence of 20.9% at follow-up in this study is somewhat higher compared with other studies in older patients with cancer. Vande Walle and Puts conclude a fall rate of 17.6% in a follow-up period of two to 3 months [9], and 18.7% in a follow up period of 6 months [40]; respectively. Both studies did not exclusively focus on patients with a frailty profile. The study of Vande Walle et al. described the presence of a frailty profile based on an abnormal result on the G8 screening as in our study, present in 74.4% of the patients, whereas the study of Puts et al. didn’t define if the patient had a frailty profile or not. Frailty indeed influences these figures; i.e. in an unplanned post hoc analysis of the current study we found a much lower incidence of 8.8% for falls at follow-up for non-frail patients (G8 score > 14; patients not included in this study because no baseline geriatric assessment data available). Furthermore, a study of Stone et al. concluded a much higher fall rate of 50.3% during a follow-up period of 6 months [41]. This might be due to the inclusion of people with metastatic or locoregionally advanced cancer and as a result negatively influencing the fall incidence.
Literature shows that the population of older persons with cancer has a higher risk of injuries which can be due to the characteristics that come with cancer, such as cancer treatment (e.g. chemotherapy), cancer stage, comorbidity, and osteoporosis [20, 42]. The number of injuries found in our study are somewhat comparable to those reported in the study of Vande Walle et al. (i.e. 62.0% experiencing fall-related injuries at two to 3 months follow-up) [9]. Two other studies in a population of older persons with cancer concluded an injury rate of 42 to 45%, but both studies didn’t report data related on a frailty profile [41, 43]. Thus, it seems that older persons with cancer and frailty profile have a higher risk of sustaining an injury after a fall than older persons of the general population with cancer.
Fear of falling is very common in older patients with cancer, as shown by the current study. Data on fear of falling in the older population with cancer is scarce and only a few studies reported some information on this topic [44]. One study of Sattar et al. (2019) reported a prevalence rate of fear of falling of 55% which is comparable with our results, but again data on a frailty profile are not available [38]. Prevalence rates in the general older population are varying a lot (e.g. between 3 and 85%) and this might be due to different methods and tools of measurements [45].
Falls history is described in the literature as the main predictive factor for falls and fall-related injuries, both in the general older population and the population of older patients with cancer [9, 11, 41]. This was confirmed in the current study. Overall, a higher risk for falls in females is reported more often in the general older population [46, 47]. However, our study shows that both male and female sex play an important role as predictive factor; e.g. males having a higher risk for falls than females during follow-up but females having a higher risk for fall-related injuries. Although more research is needed to explain this finding, behavioral (e.g. males taking more risk behavior and overestimating their true ability compared to females) and biological differences (e.g. females being more at risk for osteoporosis) might play a role in this [48]. Being at risk for depression and the presence of cognitive impairment are also predictive factors for falls in this study. Regarding the risk for depression, other research shows that depression increases fear of falling (high incidence in our study), which in turn increases the risk of a fall [11]. In addition our study shows that 23.1% of the older patients experience cognitive impairment. This is a high number compared to a percentage of 10.6%, found in another study [49], and may be due to the fact that this study includes patients with a frailty profile. Therefore, this high percentage of cognitive impairment could explain the high number of falls and fall-related injuries during follow-up. Indeed, cognitive impairment is a well-known fall risk factor [50]. ADL dependence is another predictor for falls during follow-up. A systematic review by Wildes et al. reported that an association exists between functional dependence measured by ADL and risk for falls in the community-dwelling population, and that this association remains present in older persons with cancer [14]. Another predictive factor for falls during follow-up in our study is fear of falling. Based on the literature in the general older population we know that previous falls can induce or increase fear of falling leading to reduced activities in daily living [51]. Decreased functionality can lead to an increased risk for falls, which was one of the main predictive factors for falls during follow-up in this study. Finally, the last predictive factor for falls during follow-up in this study was the presence of professional home care. This can possibly be explained by the fact that this study focused on older patients with a frailty profile who are possible more in need of professional homecare and therefore have a higher risk for falls.
Age, functional status measured by IADL, comorbidities, polypharmacy, ECOG-PS and nutritional status measured by MNA-SF were significant predictors in univariate analysis, but not significant in the multivariable analysis in this study. It is clear that several variables are in some way interconnected, and some studies withheld other significant variables after multivariable analysis [10, 11, 14, 20, 40]. Therefore, it is important for healthcare providers to be attentive to these problems also, as they play an important role in the overall health status of older persons with cancer.
A strength of this study is that the data set was a very large one, that included a representative picture on nearly all solid tumors and hematological malignancies seen in daily oncology practice. Another strength of this study is that it provides new information about the incidence of falls and fall-related injuries and associated predictive factors for falls and fall-related injuries in older persons with cancer and a frailty profile. The G8 tool was used to determine frailty in our study. This is a highly sensitive tool for the population of older persons with cancer [23]. The G8 tool provides a fast and reliable method to detect the persons who would benefit from more extensive GA.
Limitations
The period of follow-up is approximately 3 months. This is a short period and more research with a longer follow-up period is needed. The patients in this study were asked about falls and fall-related injuries in the past 12 months and during the follow-up period. Using self-report for collecting these data without verification from electronic patient charts incorporates always a chance on recall bias. Especially since the percentage of patients with cognitive impairment is high, although the majority of these patients had a mild cognitive impairment. Integrating the caregiver of the patient can be helpful to address this concern in future research.
Secondly, although fear of falling was not a primary outcome parameter within this study, the integration of a validated instrument like the Falls Efficacy Scale – International (FES-I) is recommended to evaluate fear of falling in future research [39, 52].
Finally, this was a preplanned secondary data analysis, and variables shown to be significant in previous studies, such as type of medication (e.g. benzodiazepines, antidepressants, and anti-psychotics) [53, 54] were not included in the data base of this study. Other variables that were not included were the circumstances of a fall, time point of a fall, impact of the incidence of falls and fall-related injuries on subsequent cancer treatment and efficacious interventions for fall prevention [21]. Further research on these aspects is therefore highly recommended.
Conclusion
This study shows that falls and fall-related injuries occur frequently in older persons with cancer and a frailty profile and that fear of falling is common in this patient population. Systematic fall screening when taking care for frail older patients with cancer in daily oncology practice is needed and needs to be integrated within the GA. Sex and falls history are common predictors for both falls and fall-related injuries. Other predictive factors for falls were risk for depression, cognitive impairment, dependency in ADL, fear of falling and use of professional home care. Designing and integrating GA-tailored falls prevention interventions in daily care of frail older patients with cancer are highly recommended.
Acknowledgements
We thank all the physicians who were involved in this study, particularly dr. S. Luce, dr. G. Debugne, dr. J.-C. Goeminne, dr. K. Geboers, dr. R. Van Rijswijk, dr. K. Vandenborre, dr. Bénédicte Petit, which were responsible for trial coordination in their institution and our Master in Science of Nursing students, Linde Peters and Laïlani Olkuski. We wish to thank all patients, physicians and healthcare professionals in all collaborating centers that took part in this study.
Abbreviations
- ADL
Activities of Daily Living
- CCI
Charlson Comorbidity index
- ECOG-PS
Eastern Cooperative Oncology Group - Performance Status
- FS
Functional status
- GA
Geriatric assessment
- GDS
Geriatric depression scale
- IADL
Instrumental Activities of Daily Living
- MMSE
Mini mental state examination
- MNA-SF
Mini nutritional assessment – short form
- ProFouND
The Prevention of Falls Network for Dissemination
- USA
United States of America
- VAS
Visual analogue scale
- VIF
Variance inflation factors
- WHO
World health organization
Authors’ contributions
CK, LD, JF, JPL, HW and KM conceptualized and designed the study. CK, LD, JF, PD, IDG, CF, FC, VV, CB, DB, HVdB, DS, CL, PS, GJ, J-PP, JDC, J-PL, HW and KM were involved in the acquisition of the data. CK, LD, JPL, HW and KM defined the analytical strategy and did the data interpretation. JPL performed the statistical analysis. CK and LD wrote the manuscript and designed the tables and figures with the substantive contributions of JPL, HW, KM. CK, LD, JF, PD, IDG, CF, FC, VV, CB, DB, HVdB, DS, CL, PS, GJ, J-PP, JDC, J-PL, HW and KM provided critical feedback and were actively involved in the writing process of the manuscript. CK, LD, JF, PD, IDG, CF, FC, VV, CB, DB, HVdB, DS, CL, PS, GJ, J-PP, JDC, J-PL, HW and KM read and approved the final manuscript.
Funding
This study was funded by the Cancer Plan 2012–2015, a grant provided by the Federal Public Service of Health, Food Chain Safety and Environment, Belgium (KPC_24_A_025).
HW is a recipient of a grant of the ‘Fonds Voor Wetenschappelijk Onderzoek Vlaanderen’ (1802211 N).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of all participating centers (B322201215495). All participants provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cindy Kenis and Lore Decoster are joint first author
Hans Wildiers and Koen Milisen are joint last author
Contributor Information
Cindy Kenis, Email: cindy.kenis@uzleuven.be.
Lore Decoster, Email: lore.decoster@uzbrussel.be.
Johan Flamaing, Email: johan.flamaing@uzleuven.be.
Philip R. Debruyne, Email: PHILIP.DEBRUYNE@azgroeninge.be
Inge De Groof, Email: inge.degroof@gza.be.
Christian Focan, Email: CHRISTIAN.FOCAN@chc.be.
Frank Cornélis, Email: frank.cornelis@uclouvain.be.
Vincent Verschaeve, Email: vincent.verschaeve@ghdc.be.
Christian Bachmann, Email: christian.bachmann@azstlucas.be.
Dominique Bron, Email: dominique.bron@bordet.be.
Heidi Van den Bulck, Email: Heidi.Van.Den.Bulck@imelda.be.
Dirk Schrijvers, Email: dirk.schrijvers@zna.be.
Christine Langenaeken, Email: Christine.Langenaeken@klina.be.
Pol Specenier, Email: pol.specenier@uza.be.
Guy Jerusalem, Email: g.jerusalem@chu.ulg.ac.be.
Jean-Philippe Praet, Email: jean-philippe.praet@stpierre-bru.be.
Jessie De Cock, Email: jessie.decock@uzbrussel.be.
Jean-Pierre Lobelle, Email: jeanpierre.lobelle@gmail.be.
Hans Wildiers, Email: hans.wildiers@uzleuven.be.
Koen Milisen, Email: koen.milisen@kuleuven.be.
References
- 1.Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 2.Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing. 2006;35(1):5–10. doi: 10.1093/ageing/afi218. [DOI] [PubMed] [Google Scholar]
- 3.WHO Global Report on Falls Prevention in Older Age. https://www.who.int/ageing/publications/Falls_prevention7March.pdf?ua=1. Accessed 23 Apr 2021.
- 4.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35 Suppl 2:ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 6.Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693–698. doi: 10.1111/jgs.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Report: Cancer Research for Cancer Prevention. https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020. Accessed 23 Apr 2021.
- 9.Vande Walle N, Kenis C, Heeren P, et al. Fall predictors in older cancer patients: a multicenter prospective study. BMC Geriatr. 2014;14:135. doi: 10.1186/1471-2318-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildes TM, Maggiore RJ, Tew WP, et al. Factors associated with falls in older adults with cancer: a validated model from the Cancer and aging research group. Support Care Cancer. 2018;26(10):3563–3570. doi: 10.1007/s00520-018-4212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Sun M, Liu S, et al. Risk factors for falls in older patients with cancer. BMJ Support Palliat Care. 2018;8(1):34–37. doi: 10.1136/bmjspcare-2017-001388. [DOI] [PubMed] [Google Scholar]
- 12.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spoelstra SL, Given BA, Schutte DL, Sikorskii A, You M, Given CW. Do older adults with cancer fall more often? A comparative analysis of falls in those with and without cancer. Oncol Nurs Forum. 2013;40(2):E69–E78. doi: 10.1188/13.ONF.E69-E78. [DOI] [PubMed] [Google Scholar]
- 14.Wildes TM, Dua P, Fowler SA, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6(1):70–83. doi: 10.1016/j.jgo.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 19.Reiser E, Pötsch N, Seebacher V, et al. Impact of frailty on the management of patients with gynecological cancer aged 80 years and older. Arch Gynecol Obstet. 2021;303(2):557–563. doi: 10.1007/s00404-020-05807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattar S, Alibhai SM, Spoelstra SL, Fazelzad R, Puts MT. Falls in older adults with cancer: a systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer. 2016;24(10):4459–4469. doi: 10.1007/s00520-016-3342-8. [DOI] [PubMed] [Google Scholar]
- 21.Sattar S, Haase K, Kuster S, et al. Falls in older adults with cancer: an updated systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer. 2021;29(1):21–33. doi: 10.1007/s00520-020-05619-2. [DOI] [PubMed] [Google Scholar]
- 22.Kenis C, Decoster L, Flamaing J, et al. Adherence to geriatric assessment-based recommendations in older patients with cancer: a multicenter prospective cohort study in Belgium. Ann Oncol. 2018;29(9):1987–1994. doi: 10.1093/annonc/mdy210. [DOI] [PubMed] [Google Scholar]
- 23.Liuu E, Canouï-Poitrine F, Tournigand C, et al. Accuracy of the G-8 geriatric-oncology screening tool for identifying vulnerable elderly patients with cancer according to tumour site: the ELCAPA-02 study. J Geriatr Oncol. 2014;5(1):11–19. doi: 10.1016/j.jgo.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346–353. doi: 10.1016/j.jgo.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 27.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 29.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 33.Guigoz Y, Vellas B. The Mini nutritional assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3–11. doi: 10.1159/000062967. [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56(6):M366–M372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 35.O'Halloran AM, Pénard N, Galli A, Fan CW, Robertson IH, Kenny RA. Falls and falls efficacy: the role of sustained attention in older adults. BMC Geriatr. 2011;11:85. doi: 10.1186/1471-2318-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milisen K, Coussement J, Flamaing J, et al. Fall prediction according to nurses' clinical judgment: differences between medical, surgical, and geriatric wards. J Am Geriatr Soc. 2012;60(6):1115–1121. doi: 10.1111/j.1532-5415.2012.03957.x. [DOI] [PubMed] [Google Scholar]
- 37.Milisen K, Staelens N, Schwendimann R, et al. Fall prediction in inpatients by bedside nurses using the St. Thomas's risk assessment tool in falling elderly inpatients (STRATIFY) instrument: a multicenter study. J Am Geriatr Soc. 2007;55(5):725–733. doi: 10.1111/j.1532-5415.2007.01151.x. [DOI] [PubMed] [Google Scholar]
- 38.Sattar S, Spoelstra SL, Alibhai SMH, Puts MTE. Circumstances of falls and fear of falling in community-dwelling older adults with cancer: results from a mixed-methods study. J Geriatr Oncol. 2019;10(1):105–111. doi: 10.1016/j.jgo.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Sammels M, Vandesande J, Vlaeyen E, Peerlinck K, Milisen K. Falling and fall risk factors in adults with haemophilia: an exploratory study. Haemophilia. 2014;20(6):836–845. doi: 10.1111/hae.12512. [DOI] [PubMed] [Google Scholar]
- 40.Puts MT, Monette J, Girre V, et al. The fall rate of older community-dwelling cancer patients. Support Care Cancer. 2013;21(3):775–783. doi: 10.1007/s00520-012-1579-4. [DOI] [PubMed] [Google Scholar]
- 41.Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128–2133. doi: 10.1200/JCO.2011.40.7791. [DOI] [PubMed] [Google Scholar]
- 42.Edwards BJ, Sun M, Zhang X, et al. Fractures frequently occur in older cancer patients: the MD Anderson Cancer Center experience. Support Care Cancer. 2018;26(5):1561–1568. doi: 10.1007/s00520-017-3962-7. [DOI] [PubMed] [Google Scholar]
- 43.Sattar S, Alibhai SMH, Spoelstra SL, Puts MTE. The assessment, management, and reporting of falls, and the impact of falls on cancer treatment in community-dwelling older patients receiving cancer treatment: results from a mixed-methods study. J Geriatr Oncol. 2019;10(1):98–104. doi: 10.1016/j.jgo.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Aburub AS, Phillips SP, Curcio CL, Guerra RO, Auais M. Fear of falling in community-dwelling older adults diagnosed with cancer: a report from the international mobility in aging study (IMIAS) J Geriatr Oncol. 2020;11(4):603–609. doi: 10.1016/j.jgo.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37(1):19–24. doi: 10.1093/ageing/afm169. [DOI] [PubMed] [Google Scholar]
- 46.Campbell AJ, Spears GF, Borrie MJ. Examination by logistic regression modelling of the variables which increase the relative risk of elderly women falling compared to elderly men. J Clin Epidemiol. 1990;43(12):1415–1420. doi: 10.1016/0895-4356(90)90110-b. [DOI] [PubMed] [Google Scholar]
- 47.Ward PR, Wong MD, Moore R, Naeim A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: a retrospective cohort study. J Geriatr Oncol. 2014;5(1):57–64. doi: 10.1016/j.jgo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Jehu DA, Davis JC, Barha CK, et al. Sex differences in subsequent falls and falls risk: a prospective cohort study in older adults. Gerontology. 2022;68(3):272–279. doi: 10.1159/000516260. [DOI] [PubMed] [Google Scholar]
- 49.Kenis C, Decoster L, Van Puyvelde K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32(1):19–26. doi: 10.1200/JCO.2013.51.1345. [DOI] [PubMed] [Google Scholar]
- 50.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012;41(3):299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- 51.Murphy SL, Dubin JA, Gill TM. The development of fear of falling among community-living older women: predisposing factors and subsequent fall events. J Gerontol A Biol Sci Med Sci. 2003;58(10):M943–M947. doi: 10.1093/gerona/58.10.m943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the falls efficacy scale-international (FES-I) Age Ageing. 2005;34(6):614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 53.Turner JP, Shakib S, Singhal N, et al. Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer. 2014;22(7):1727–1734. doi: 10.1007/s00520-014-2171-x. [DOI] [PubMed] [Google Scholar]
- 54.de Jong MR, Van der Elst M, Hartholt KA. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther Adv Drug Saf. 2013;4(4):147–154. doi: 10.1177/2042098613486829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.