Abstract
Background
Umbilical cord blood transplantation (UCBT) from unrelated donors is one of the successful treatments for acute leukemia in childhood. The most frequent side effect of UCBT is peri-engraftment syndrome (PES), which is directly associated with the greater prevalence of acute and chronic graft-versus-host-disease (aGvHD and cGvHD). In haploidentical stem cell transplantation, posttransplant cyclophosphamide (PTCY) has been demonstrated to be an effective method against GvHD. However, the effects of PTCY as a GvHD prophylactic in UCBT had not been investigated. This study aimed to evaluate the effects of PTCY on the outcomes of UCBT for pediatric acute leukemia.
Methods
This retrospective study included 52 children with acute leukemia who underwent unrelated single-unit UCBT after myeloablative conditioning regimens. The results from the PTCY and non-PTCY groups were compared.
Results
The incidence of transplantation-related mortality in non-PTCY and PTCY were 5% and 10% (p = 0.525), respectively. The incidence of relapse in non-PTCY and PTCY were 5% and 23% (p = 0.095), respectively. Second complete remission status (CR2) was an independent risk factor for relapse-free survival (hazard ratio = 9.782, p = 0.001). The odds ratio for sepsis or bacteremia incidence was significantly greater in the PTCY group (9.524, p = 0.017). PTCY group had increased rates of cytomegalovirus activity and fungal infection. The incidence of PES, aGvHD, cGvHD, and hemorrhagic cystitis in the PTCY group was lower than that in the non-PTCY group, although it was not significantly different. Additionally, higher doses of PTCY (29 mg/kg and 40 mg/kg) were associated with lower incidences of aGvHD and severe GvHD (65% and 29%, respectively) than lower doses (93% and 57%, respectively). Engraftment time and graft failure incidence were similar across groups.
Conclusion
The results support the safety and efficiency of PTCY as part of PES controlling and GvHD prophylaxis in single-unit UCBT for children with acute leukemia. A PTCY dosage of 29 mg/kg to 40 mg/kg appears to be more effective in GvHD prophylaxis for UCBT patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-10309-9.
Keywords: Umbilical cord blood transplantation, Acute leukemia, Posttransplant cyclophosphamide
Introduction
Unrelated umbilical cord blood transplantation (UCBT) is one of the effective treatments for hematological malignant diseases, and it has an advantage for those who need urgent transplantation because of its immediate availability from cord blood (CB) banks. Peri-engraftment syndrome (PES) [1–4], especially severe PES, is associated with the higher incidence of acute graft-versus-host-disease (aGvHD) and chronic graft-versus-host-disease (cGvHD) [1, 3, 5–7]. Severe aGvHD is associated with high overall mortality and NRM in pediatric single UCBT [8]. However, mild PES reduces the relapse rate in acute myeloid leukemia (AML) after UCBT [9]. Therefore, to be able to moderate the severity of PES has been an important issue in the UCBT study.
The incidence of PES can be decreased by early immunosuppression after UCBT [10–12], as well as by tocilizumab, the interleukin-6 (IL-6) antibody [13], which needs further clinical trial and long-term observation for outcomes. In previous studies on UCBT [1, 14, 15], GvHD prophylaxis consisted of cyclosporine A (CsA) or tacrolimus (Tac) in combination with methotrexate (MTX) or corticosteroid has been used in children [16]. MTX has been reported to decrease the incidence and severity of PES. However, the optimal MTX dosage in UCBT remains not available [10]. Another treatment, anti-thymocyte globulin (ATG), has been used in UCBT for children with leukemia. However, ATG often results in delayed and poor T-cell reconstitution, which leads to high incidences of infection and related mortality [17]. Therefore, it is necessary to explore new immunosuppression strategies for PES control and GVHD prophylaxis in UCBT.
Recently, posttransplant cyclophosphamide (PTCY) is one of the most widely used regimens for GvHD prophylaxis in Haplo-HSCT [18, 19], and has the treatment not increase the incidence of graft failure (GF) and relapse. Compared with standard double doses of PTCY (50 mg/kg on day + 3 and day + 4), a single dose of PTCY (50 mg/kg on day + 3) had a similar effect in preventing aGvHD for Haplo-PBSCT patients [18]. Furthermore, PTCY presents lower incidences of viral and fungal infection than ATG-based regimens [20], making PTCY a potentially better candidate for PES control and GvHD prophylaxis in UCBT.
However, the impact of PTCY in UCBT for acute leukemia had not been studied. Therefore, we investigated PTCY as a GvHD prophylaxis in UCBT patients. By retrospectively analyzing the clinical data of UCBT in children with acute leukemia, this report evaluated the efficacy and safety of the PTCY in UCBT.
Subjects and methods
Patients and donors
Fifty-two patients who received UCBT in the Department of Pediatrics of Sun Yat-sen Memorial Hospital between August 2018 and February 2021 were included in this study. These patients are with AML, acute lymphoblastic leukemia (ALL), or mixed lineage leukemia (MLL). The median follow-up was 21.6 months (range, 1.8 to 38.2 months). The latest follow-up was on March 1, 2022. The characteristics of patients are summarized in Table 1. Between August 2018 and August 2019, 21 patients received non-PTCY prophylaxis against GvHD using either CsA or Tac in combination with Mycophenolate Mofetil (MMF) from day + 1 (non-PTCY group). Between August 2019 and February 2021, 31 patients received CsA or Tac with PTCY on day + 3 and day + 5 (PTCY group).
Table 1.
Patients’ characteristics and the comparison for characteristics and outcomes in PTCY and non-PTCY groups
| Total (n = 52) |
Group | p value | |||
|---|---|---|---|---|---|
| PTCY (n = 31) | non-PTCY (n = 21) | ||||
| Age, years | 5.8 (1.1,13.5) | 6.04 (SD = 3.13) | 6.15 (SD = 3.47) | 0.913 | |
| Sex, n | male | 37 | 23 | 14 | 0.557 |
| female | 15 | 8 | 7 | ||
| Weight, kg | 17.6 (9.0, 42.4) | 19.35 (SD = 7.47) | 18.60 (SD = 6.35) | 0.707 | |
| Primary diagnosis, n | AML | 27 | 19 | 8 | 0.259 |
| ALL | 23 | 11 | 12 | ||
| others | 2 | 1 | 1 | ||
| Remission status, n | CR1 | 47 | 27 | 20 | 0.329 |
| CR2 | 5 | 4 | 1 | ||
| HLA matching, n | 7/10 | 4 | 3 | 1 | 0.704 |
| 8/10 | 22 | 14 | 8 | ||
| 9/10 | 17 | 10 | 7 | ||
| 10/10 | 9 | 4 | 5 | ||
| HLA matching 2, n | 7/10 or 8/10 | 26 | 17 | 9 | 0.329 |
| 9/10 or 10/10 | 26 | 14 | 12 | ||
| TNC, 107/kg (range) | 6.15 (3.3, 15.3) | 6.78 (5.5, 8.06) | 6.31 (3.29, 11.15) | 0.801 | |
| CD34+, 105/kg (range) | 2.61 (0.72, 11.6) | 3.25 (1.26, 10.7) | 1.97 (0.72, 11.6) | 0.003 | |
| Follow-up, months (range) | 21.6 (1.8, 38.2) | 14.4 (1.8, 30.0) | 34.3 (2.2, 38.1) | < 0.001 | |
| Neutrophil engraftment, days (range) | 14 (11, 22) | 15.8 (14.6, 16.9) | 14.5 (11,19) | 0.160 | |
| PLT engraftment, days (range) | 32 (12, 61) | 31.9 (27.8, 35.9) | 34.5 (13, 52) | 0.233 | |
| Graft failure, n (%) | 2 (4) | 1 (3) | 1 (5) | 0.777 | |
| PES, n (%) | 45 (87) | 25 (81) | 20 (95) | 0.130 | |
| Corticosteroid responding PES, n (%) | 38 (73) | 17 (71) | 19 (95) | 0.054 | |
| Acute GvHD, n (%) | 42 (81) | 24 (77) | 18 (86) | 0.456 | |
| Grade 3 and 4 Acute GvHD, n (%) | 20 (38) | 13 (42) | 7 (33) | 0.532 | |
| HC, n (%) | 14 (27) | 7 (23) | 7 (33) | 0.431 | |
| Chronic GvHD, n (%) | 24 (46) | 13 (42) | 11 (52) | 0.458 | |
| EBV activity, n (%) | 1(2) | 0 (0) | 1 (5) | 0.404 | |
| CMV activity, n (%) | 6 (12) | 5 (16) | 1 (5) | 0.211 | |
| pneumonia, n (%) | 17 (33) | 10 (32) | 7 (33) | 0.584 | |
| Fungal infection, n (%) | 8 (15) | 7 (23) | 1 (5) | 0.081 | |
| Sepsis or bacteremia, n (%) | 11 (21) | 10 (32) | 1 (5) | 0.017 | |
| TRM, n (%) | 4 (8) | 3 (10) | 1 (5) | 0.514 | |
| Relapse, n (%) | 7 (13) | 6 (19) | 1 (5) | 0.130 | |
| Event-free survival, n (%) | 41 (79) | 22 (71) | 19 (91) | 0.091 | |
PTCY posttransplant cyclophosphamide, SD standard deviation, AML acute myeloid leukemia, ALL acute lymphoblastic leukemia; others, mixed lineage leukemia, CR1 first complete remission, CR2 second complete remission, HLA human lymphocyte antigen, TNC total nucleated cell, CD34+ CD 34+ cell counts, PLT Platelet, GvHD Graft-versus-host-disease, HC Hemorrhagic cystitis, EBV Epstein-Barr virus, CMV Cytomegalovirus, PES peri-engraftment syndrome, TRM Transplantation related mortality
Donors were unrelated CB from public cord blood banks in mainland China. When searching for unrelated CB, complete human leukocyte antigen (HLA) matches between 10 loci of the ‐A, ‐B, ‐C, ‐DR, and ‐DQ alleles were required, with at least 7 out of 10 loci matching at high-resolution level. Two mismatch loci were not permitted to be located at the same allele.
Conditioning regimens and GvHD prophylaxis
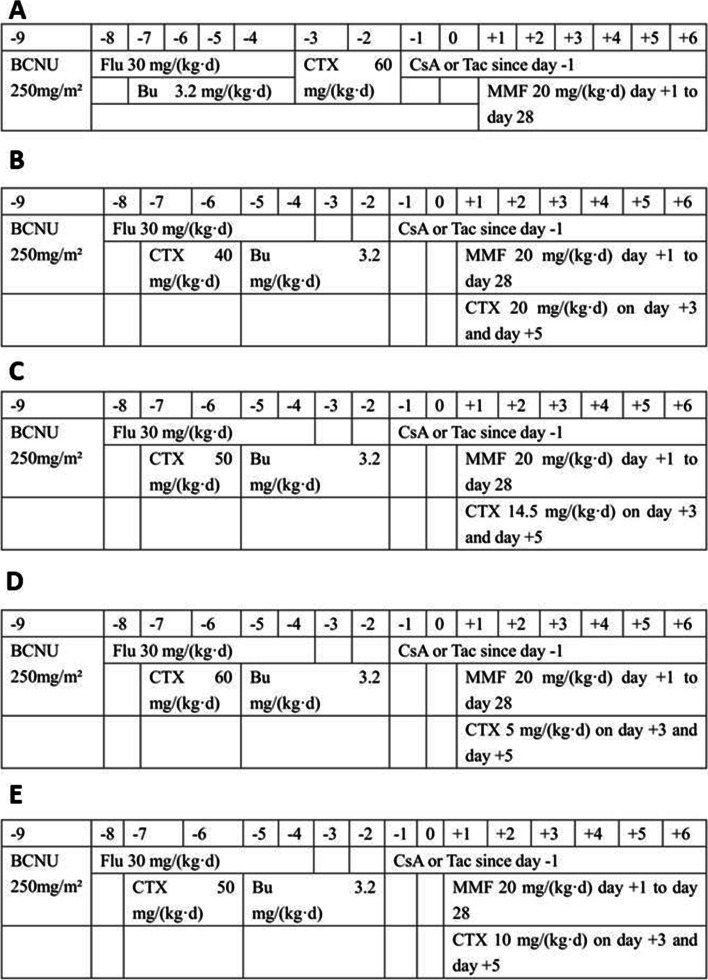
The conditioning regimen of the non-PTCY group consisted of cyclophosphamide (CY) at 60 mg/kg for 2 days, BU at 3.2 mg/kg for 4 days, and fludarabine (Flu) at 30 mg/m2 for 5 days (Fig. 1-A), with or without 250 mg/m2 semustine for one day. The conditioning regimen of the PTCY group consisted of CY at 40–60 mg/kg for 2 days before transplantation, BU at 3.2 mg/kg for 4 days, and Flu at 30 mg/m2 for 5 days (Fig. 1-B to E), with (n = 19) or without (n = 33) semustine at 250 mg/m2 for one day. Semustine was included in patients who were high-risk ALL and who had a history of central nervous system leukemia (CNSL).
Fig. 1.
Conditioning regimens. A Conditioning regimen of non-PTCY group. B-E Conditioning regimens of PTCY group
All patients received GvHD prophylaxis of CsA at 3 mg/kg/day (n = 37) or Tac at 0.02 mg/kg/day (n = 15) as continuous infusion from day -1 in combination with MMF at 20 mg/kg/day from day + 1 to day + 28. CsA blood level was maintained at 150–250 ng/mL, and Tac blood level was maintained at 8–12 ng/mL. The 31 patients in the PTCY group received CY at 5–20 mg/kg/day on day + 3 and day + 5 (Fig. 1-B to E), among which, 7 patients received 20 mg/kg/day, 10 received 14.5 mg/kg/day, 3 received 10 mg/kg/day and 11 received 5 mg/kg/day on day + 3 and day + 5.
Supportive care
Antibacterial prophylaxis was performed with intravenous piperacillin-sulbactam and antifungal prophylaxis was performed with intravenous micafungin, caspofungin, or oral posaconazole. Antiviral prophylaxis was performed with intravenous acyclovir from day -9 to day + 14, intravenous ganciclovir from day + 14 to day + 28, and oral valaciclovir from day + 28 to day + 120. Intravenous Immunoglobulin (IVIg) was given at 200–300 mg/kg every 14 days. Granulocyte colony-stimulating factor (G-CSF) was given intravenously at 5–10 mg/kg since day + 6. Oral mucositis was treated with local human recombinant interleukin-11 and parental nutrition. PES and aGvHD was treated with methylprednisolone. Cytomegalovirus (CMV) infection was treated with ganciclovir.
Definitions
PES was diagnosed at the duration between the onset of clinical symptoms and neutrophil recovery according to the Spitzer Criteria (Supplementary Information 1). The grading of PES was done in accordance with previous literature [13].
CMV infection was diagnosed positive when blood CMV DNA copies exceeded 103 copies/ml. Epstein-Barr virus (EBV) was diagnosed positive when blood EBV DNA copies exceeded 103 copies/ml.
The modified Glucksberg grading of aGvHD [21] was applied in the diagnosis. The onset of aGvHD from preceding PES was defined as the day of neutrophil engraftment. Skin biopsies were not performed on any of the patients for diagnosis of PES or skin GvHD. The 2014 National Institutes of Health (NIH) consensus criteria [22] were used to diagnose and grade cGvHD.
No donor hematopoiesis beyond day + 28 was regarded as graft failure. Neutrophil engraftment and platelet engraftment were in accordance with the literature [18, 19]. Quantitative chimerism monitoring was performed by short-tandem repeat (STR)-based PCR techniques [23].
The safety end points of this study included transplantation-related mortality (TRM), overall survival (OS), event-free survival (EFS), relapse-free survival (RFS), and the incidence of relapse after UCBT. The efficiency end-points included the incidence of PES, aGvHD, cGvHD, and infections after UCBT.
Events included death, disease relapse, graft failure, cGvHD, and secondary malignancy. EFS was defined as the duration between UCBT and observation of events/the last contact. OS was defined as the duration between transplantation and death/the last contact. RFS was defined as the duration between UCBT and relapse, death, or last contact. The definition of TRM was death after UCBT except for death from disease relapse. Morphologic evidence of disease was defined as relapse.
Immune cell recovery
Cells were analyzed as previously described in previous literature [24]. Fluorescence-conjugated monoclonal antibodies (BD multitest 6-color TBNK, San Jose, CA, USA) were added to mononuclear cells. Samples were analyzed on a Beckman navios cytometer (Beckman Coulter Life Science) and then analyzed using Navios tetra Software (Beckman Coulter Life Science). The lymphocyte subpopulation was gated and used as a reference for the determination of natural killer (NK) cell, total T-cell, CD3+CD4+ helper T-cell, CD3+CD8+cytolytic T-cell, and B-cell subsets between day + 28 and day + 35.
Statistical analysis
All patient follow-up was done by outpatient service and telephone. Follow-up was updated on February 28, 2022. Patients without outpatient records within one month before the end of the study were confirmed by telephone follow-up. Loss of contact for over one month after transplantation was defined as lost contact. RFS and OS were calculated from the date of infusion of CB to the date of the first event. If no event was reported, the observation time would be recorded at the last follow-up. RFS and OS curves were estimated according to Kaplan–Meier with Greenwood’s standard error (SE) and they were compared by the two-tailed log-rank test. The Cox proportional-hazards regression model was used for multivariate analysis and Hazard Ratio (HR). Risk factors with a p-value < 0.1 in each univariate analysis were included in the multivariate analysis. The Mann–Whitney U-test was used for continuous variables from unpaired samples. The Chi-square test and Fisher’s exact probability test were used for the correlation and Odds Ratio (OR) analysis between two groups of data. Statistical analysis was carried out by using SPSS 23.0 (SPSS Institute, Cary, NC) and R. p-values < 0.05 were considered significant.
Results
Survival-related variants analysis
In this study, the OS and the RFS were 79% and 78%, respectively. Univariate analyses showed that HLA matching, with or without the use of PTCY, and remission status before transplantation were potential key factors for both OS and RFS, as displayed in Table 2. Further multivariate analysis found that remission status before transplantation was an independent risk factor for RFS, as displayed in Table 3.
Table 2.
Univariate analysis of OS and RFS
| Variables | OS, % | p | RFS, % | p | ||
|---|---|---|---|---|---|---|
| Diseases, mean(95%CI) | ||||||
| AML | 84.8 (71.1, 98.5) | 0.771 | 85.2 (71.9, 98.5) | 0.358 | ||
| ALL | 72.6(51.4, 93.8) | 67.4(47.2, 87.6) | ||||
| HLA matching, mean(95%CI) | ||||||
| 10/10 and 9/10 | 95.7(87.3, 100) | 0.009 | 96.2(88.8, 100) | 0.002 | ||
| 8/10 and 7/10 | 70.7(52.1, 89.3) | 59.3(39.5, 79.1) | ||||
| PTCY, mean(95%CI) | ||||||
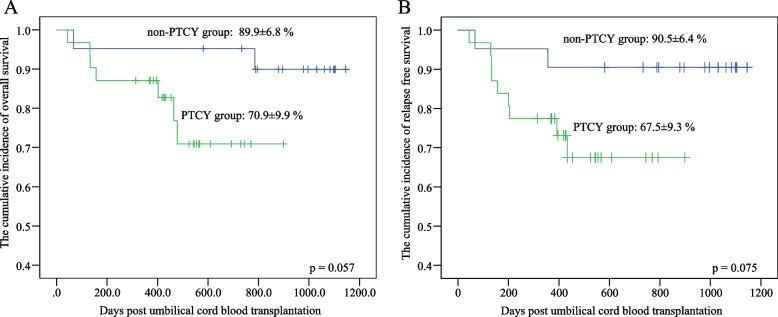
| Yes | 70.9(51.5, 90.3) | 0.057 | 67.5(49.3, 85.7) | 0.075 | ||
| No | 89.9(76.6, 100) | 90.5(77.5, 100) | ||||
| Remission, mean(95%CI) | ||||||
| CR1 | 86.0(75.4, 96.6) | 0.011 | 86.6(96.8, 76.4) | < 0.001 | ||
| CR2 | 60.0(17.1, 100) | 0 | ||||
AML acute myeloid leukemia, ALL acute lymphoblastic leukemia; others, mixed lineage leukemia, CR1 first complete remission, CR2 second complete remission, HLA human lymphocyte antigen, PTCY posttransplant cyclophosphamide, GvHD graft-versus-host-disease, PES peri-engraftment syndrome, OS overall survival, RFS Relapse-free survival, SE Standard error, 95%CI 95% confidence interval
Table 3.
Multivariate analysis of OS and RFS
| Variables | HR for OS (95%CI) | p | HR for RFS (95%CI) | p |
|---|---|---|---|---|
| HLA matching | ||||
| 10/10 and 9/10 | 1 | 0.071 | 1 | 0.417 |
| 8/10 and 7/10 | 7.286(0.842, 63.044) | 2.706(0.244, 29.967) | ||
| PTCY | ||||
| No | 1 | 0.153 | 1 | 0.192 |
| Yes | 4.342(0.581, 32.473) | 4.178(0.488, 35.801) | ||
| Remission | ||||
| CR1 | 1 | 0.360 | 1 | 0.002 |
| CR2 | 1.986(0.457, 8.624) | 21.042(3.158, 140.210) | ||
HR hazard ratio, 95% CI 95% confidence interval, CR1 first complete remission, CR2 second complete remission, HLA human lymphocyte antigen, PTCY posttransplant cyclophosphamide, GvHD graft-versus-host-disease, OS overall survival, RFS relapse-free survival
Comparison between the PTCY group and non-PTCY group
To explore the impact of PTCY in UCBT, we compared the baseline and outcomes data in the PTCY and non-PTCY groups (Table 1). Even though the transfused CD34+ cell counts in both groups were significantly different (p = 0.003), the cell count was not the risk factor affecting RFS (p = 0.674). The HLA matching point counts of both groups were similar (p = 0.704). Neutrophil engraftment time, platelet engraftment time, and graft failure incidence were similar across groups. Sepsis or bacteremia incidence in the PTCY group was significantly higher than in the non-PTCY group (OR = 9.524, 95% confidence interval (95%CI) (1.115–81.345), p = 0.017). The rates of CMV activity and fungal infection were higher in the PTCY group (Table 1). The incidences of PES, aGvHD, cGvHD, and hemorrhagic cystitis (HC) in the PTCY group were lower than that in the non-PTCY group, although there was no significant difference (Table 1). The gradings of PES (p = 0.638), HC (p = 0.407), liver aGvHD (p = 0.316), intestinal aGvHD (p = 0.178), skin aGvHD (p = 0.410), aGvHD (p = 0.871) were similar between both groups. Survival curves for OS and RFS are provided in Fig. 2. The incidence of OS and RFS of both groups are displayed in Table 2. In the proportional hazards model for the sub-distribution of a competing risk, the TRMs of non-PTCY and PTCY were 5% (95% CI (4%, 5%)) and 10% (95% CI (9%, 10%)) (p = 0.525), respectively, while the relapse rates were5% (95% CI (4%, 5%) and 23% (95% CI (21%, 24%)) (p = 0.095), respectively. There were four deaths, one in non-PTCY group and three in PTCY group.
Fig. 2.
Comparison of Overall Survival and Relapse-Free Survival between PTCY group and non-PTCY group. A The cumulative incidence of overall survival of the PTCY and non-PTCY groups. B The cumulative incidence of relapse-free survival of PTCY and non-PTCY groups
There were six cases of relapse in the PTCY group, among which, four patients were ALL. However, in the non-PTCY group, only one patient with ALL experienced relapse of CNSL. The median time of relapse of ALL was 204 days. The relapse rate of ALL was 22% (n = 5), while it was 7% in AML (n = 2) (p = 0.285). The four cases of relapsed ALL used PTCY at the total dosage of 40 mg/kg. The incidence of relapsed ALL is significantly higher at a dosage of 40 mg/kg PTCY (4/7) than at other kinds of doses (p = 0.004). In multivariate analysis, the risks of events in RFS for patients in the second complete remission (CR2) was 9.78 (95% CI (2.45, 39.12), p = 0.001) after adjustment for HLA matching and PTCY usage. So, it remained to be determined whether the dose of PTCY affects the prognosis.
Comparison between high-dose PTCY group and low-dose PTCY group
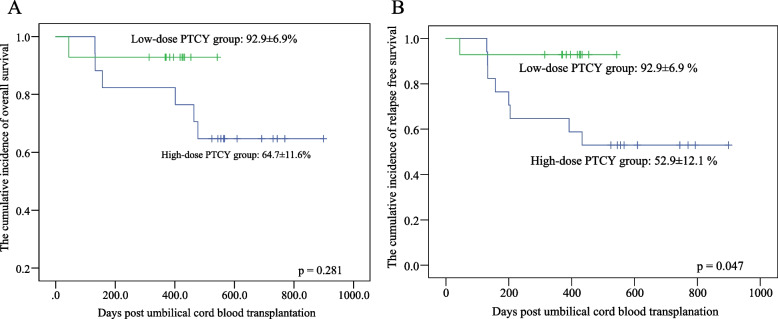
To further explore the impact of the dosage of PTCY in UCBT, we compared the baseline data and outcomes data between a high-dose PTCY group (40 mg/kg or 29 mg/kg) and a low-dose PTCY group (20 mg/kg or 10 mg/kg), as shown in Table 4. Interestingly, the incidences of aGvHD and severe GvHD in the high-dose PTCY group (65% and 29%, respectively) were both lower than the low-dose PTCY group (93% and 57%, respectively). However, the incidences of PES were not different across groups. No difference was found in complications, including CMV activation, EBV activation, fungal infection, and pneumonia. Incidence of sepsis or bacteremia was higher in the high-dose PTCY group, in which, 8/17 patients suffered from sepsis or bacteremia without leading to TRM. Increased relapse rate and decreased EFS were found in the high-dose PTCY group. There were six cases of relapse from the high-dose PTCY group. The relapse rate in the 40 mg/kg sub-group was 4/7 which contributed to 2/3 of the relapse cases. The other two cases of relapse were in the 29 mg/kg sub-group. No relapse was reported in the low-dose PTCY group. Survival curves for OS and RFS are provided in Fig. 3. The incidence of OS and RFS of both groups are displayed in Table 4. The incidence of TRM in the high-dose and low-dose groups were 13% and 7% (p = 0.515), respectively. The incidence of relapse in high-dose PTCY and low-dose PTCY groups were 39% and 0 (p = 0.034), respectively. In the multivariate analysis for RFS, the pre-transplantation remission status of CR2 was the independent risk factor (hazard ratio = 5.22, 95% CI (1.14, 23.87), p = 0.033).
Table 4.
Comparison for characteristics and outcomes of high-dose PTCY group and low-dose PTCY group
| high-dose PTCY group (n = 17) | low-dose PTCY group (n = 14) | p value | ||
|---|---|---|---|---|
| Age, years | 6.12 (SD = 3.66) | 5.96 (SD = 2.48) | 0.890 | |
| sex, n | male | 12 | 11 | 0.613 |
| female | 5 | 3 | ||
| Weight, kg | 19.7(SD = 9.0) | 18.9 (SD = 5.3) | 0.759 | |
| Primary diagnosis, n | AML | 11 | 8 | 0.436 |
| ALL | 6 | 5 | ||
| others | 0 | 1 | ||
| Remission status, n | CR1 | 14 | 13 | 0.385 |
| CR2 | 3 | 1 | ||
| HLA matching 2, n | 7/10 or 8/10 | 11 | 6 | 0.224 |
| 9/10 or 10/10 | 6 | 8 | ||
| TNC,107/kg (range) | 7.1 (3.39, 13.9) | 6.8 (1.0, 15.3) | 0.795 | |
| CD34+, 105/kg (range) | 3.4 (1.26, 10.72) | 3.6 (2.26, 6.61) | 0.767 | |
| Median follow-up, months (range) | 18.5 (4.4, 30.0) | 13.0 (1.8, 18.1) | 0.032 | |
| PTCY dose | ||||
| 40 mg/kg, n | 7 | |||
| 29 mg/kg, n | 10 | |||
| 20 mg/kg, n | 3 | |||
| 10 mg/kg, n | 11 | |||
| Neutrophil engraftment, days (range) | 18(13, 38) | 14 (12,33) | 0.251 | |
| PLT engraftment, days (range) | 32 (12, 61) | 31(14, 45) | 0.735 | |
| Graft failure, n (%) | 1 (6) | 0 (0) | 0.356 | |
| PES, n (%) | 13 (77) | 12 (86) | 0.517 | |
| Acute GvHD, n (%) | 11 (65) | 13 (93) | 0.062 | |
| Grade 3 to 4 Acute GvHD, n (%) | 5 (29) | 8 (57) | 0.119 | |
| HC, n (%) | 2 (12) | 5 (36) | 0.134 | |
| Chronic GvHD, n (%) | 5 (29) | 8 (57) | 0.119 | |
| CMV activity, n (%) | 3 (18) | 2 (14) | 0.597 | |
| pneumonia, n (%) | 6 (35) | 4 (29) | 0.497 | |
| Fungal infection, n (%) | 3 (18) | 4 (29) | 0.383 | |
| Sepsis or bacteremia, n (%) | 8 (47) | 2 (14) | 0.058 | |
| TRM, n (%) | 2 (12) | 1 (7) | 0.665 | |
| Relapse, n (%) | 6 (35) | 0 (0) | 0.013 | |
| Event-free survival, n (%) | 9 (53) | 13 (93) | 0.015 | |
| RFS, mean (95%CI) | 52.9 (29.18, 76.61) | 92.9 (79.38, 100) | 0.047 | |
| OS, mean (95%CI) | 64.7 (41.96, 87.43) | 92.9 (79.38, 100) | 0.281 | |
PTCY posttransplant cyclophosphamide, SD Standard deviation, AML Acute myeloid leukemia, ALL acute lymphoblastic leukemia; others, mixed lineage leukemia, CR1 first complete remission, CR2 second complete remission, HLA human lymphocyte antigen, TNC total nucleated cell, CD34+ CD 34+ cell counts, PLT platelet, GvHD graft-versus-host-disease, HC hemorrhagic cystitis, CMV Cytomegalovirus, PES peri-engraftment syndrome, TRM transplantation related mortality, OS overall survival, RFS relapse-free survival, SE Standard error, 95% CI 95% confidence interval
Fig. 3.
Comparison of Overall Survival and Relapse-Free Survival between high-dose PTCY group and low-dose PTCY group. A The cumulative incidence of overall survival of high-dose and low-dose groups. B The cumulative incidence of relapse-free survival of high-dose and low-dose groups
Immune cell recovery
Immunophenotypic analysis of T-lymphocyte (CD3+, CD3+CD4+, CD3+CD8+), B lymphocyte (CD19+) and NK cell (CD3−CD56+) reconstitution between days + 28 and + 35 was illustrated by percentages of immune cell counts in total white blood cell counts. At this period, CD3+CD4+-cell level was significantly lower in the high-dose PTCY group than in the low-dose group (20%, 95%CI (12%-27%) vs. 40%, 95%CI (31%-49%), p = 0.001). The median CD4+ to CD8+ ratio level was significantly lower in the high dose PTCY group than in the low dose PTCY group (0.62, range (0.04–6.46) vs. 2.04, range (0.29–5.05), p = 0.047). The total T-cell (p = 0.098), and CD3+CD8+-cell (p = 0.737) levels were not significantly different between the dosage groups. Additionally, the total T-cell (p = 0.925), CD3+CD4+-cell (p = 0.595), CD3+CD8+-cell (p = 0.539) levels, and the CD4+ to CD8+ ratio (p = 0.905) was not significantly different between the PTCY and non-PTCY groups. The differences of transfused NC (p = 0.812) and CD34+ (p = 0.361) were also not significant between the high-dose and low-dose PTCY groups.
Discussion
In this study, we evaluated the safety and efficiency of PTCY as GvHD prophylaxis in UCBT for pediatric acute leukemia. The most important result of this study is that PTCY, at a dose of no more than 40 mg/kg, is a safe and effective strategy against PES and aGVHD in UCBT. This finding, discovered in UCBT data, differs from findings regarding haploidentical donor SCT and previous research about UCBT GvHD prophylaxis.
There are a few options for GvHD prophylaxis in UCBT. MMF has been suggested as a first-line choice [25, 26]. However, according to the results of this study, when MMF was used, the incidence of GvHD and PES were both high in our center though good survival has been presented. Therefore, an extra immunosuppression commencement was considered for the control of PES and PES proceeding aGvHD. PTCY is effective in haplo-HSCT and haplo-HSCT plus CB transplantation [18]. In this present study, PTCY is associated with lower incidences of PES, aGvHD, cGvHD, and HC in PTCY group, although the differences were not significant. In a haplo-HSCT study, both PTCY and ATG decreased GvHD and were shown effective in aGvHD prophylaxis [27]. However, the activation of viruses after ATG has been a concern in UCBT, which was why ATG was not the first choice when it comes to UCBT [17, 28, 29]. In another study, GvHD prophylaxis using MTX (10 mg/m2 for day + 1 and day + 3) after UCBT decreased the incidence of PES and severe aGvHD [1, 11]. However, the neutrophil engraftment rate was 84%, while the platelet engraftment rate was 81%. The engraftment rates in that study were so low that it might lead to TRM. Based on these findings, we adapted the PTCY treatment in combination with CsA/Tac and MMF as an innovative GvHD prophylaxis. OS and RFS were lower when PTCY was used. According to the multivariate analysis, the use of PTCY or not is not an independent risk factor for OS or RFS. In addition, PTCY did not significantly affect neutrophil and platelet engraftment time or graft failure incidence. Regarding infections, we observed increased sepsis or bacteremia incidence in the PTCY group, as well as the elevation of the rates of CMV activity and fungal infection. However, the infections did not contribute to an increased TRM. Therefore, PTCY, at a dosage of not more than 40 mg/kg, was shown to be safe in UCBT for pediatric acute leukemia.
Regarding the dosage of PTCY, according to previous studies, a single dose of PTCY (50 mg/kg on day + 3) was effective in preventing aGVHD [18], indicating that there may be room for further reduction in the dosage of PTCY [19]. In this study, we lowered the total PTCY dose to no more than 40 mg/kg. Since PTCY in this study belonged to low-dose category, CsA/ Tac and MMF were used as before. OS and RFS were lower in our high-dose PTCY group. Decrement in RFS was mostly attributed to the pre-transplantation remission status of CR2 according to the result of multivariate analysis. TRM, which was similar in the different doses of PTCY, was mostly attributed to graft failure. High-dose PTCY delayed neutrophil engraftment for up to 3 days. Patients with high-dose PTCY were more likely to get sepsis or bacteremia and pneumonia, but not CMV activity and fungal infection. Further evaluation of the efficiency of PTCY at different dosages was done by comparison in the immune and inflammatory reaction-related manifestations. High doses of PTCY decreased the incidences of PES, aGvHD, severe aGvHD, cGvHD, and HC. According to the results of the lymphocyte subset study, higher doses of PTCY (40 mg/kg or 29 mg/kg) presented a significantly lower CD4+ percentage, which was in accordance with previous report on the mechanism of PTCY inhibiting GvHD by limiting CD4+ subset proliferation in the early period of transplantation [30]. We assumed that PTCY of both 40 mg/kg and 29 mg/kg would be effective in inhibiting GvHD, but the effects on relapse merit future studies. Furthermore, the effects on severe infections of PTCY should be cautiously evaluated in upcoming studies. The impact of 20 mg/kg PTCY should be evaluated in the future as well.
There are several limitations to the present study. The inclusion periods for patients in the non-PTCY group and the PTCY group were different, which made the follow-up time for the PTCY group shorter. However, both groups had completed first-year evaluations, which may have mitigated the impact on the results reported. This was not a prospective randomized control study. However, the baseline characteristics were comparable between groups, except for CD 34+ cell counts of grafts. The most important bias may be the primary diseases. AML and ALL post-transplantation achieved different prognoses, which made it difficult to determine whether PTCY was beneficial in acute leukemia. CR2 status was shown to be the independent risk factor for relapse post-UCBT. CR2 patients are less likely to benefit from PTCY than CR1 patients. However, due to the limited number of CR2 patients included, it is difficult to determine whether CR2 patients (especially CR2 ALL) benefited from PTCY in UCBT. It would be worthwhile to conduct randomized controlled studies on different PTCY dosages in UCBT for different types of acute leukemia. Due to the small sample size and different doses in PTCY, we should further analyze and discuss the impact of the dose of PTCY in the future studies.
Conclusion
The results of this study supported the safety and efficiency of PTCY as part of PES controlling and GvHD prophylaxis in single-unit UCBT for children with acute leukemia. The dose of 29 mg/kg or 40 mg/kg of PTCY presented more potential in GvHD prophylaxis in UCBT. Further study on the appropriate dose of PTCY in UCBT for acute leukemia is recommended.
Supplementary Information
Acknowledgements
The authors would like to thank all the patients and their guardians for providing important information and the out-patient assistant from the follow-up center of Sun Yat-sen Memorial Hospital, who contributed to data collection.
Authors’ contributions
XYL, LPZ, DDL, XWH, and HC contributed equally to the manuscript. SLH, KH, JPF, and HGX designed the study. XYL analyzed the data and wrote the manuscript. LPZ, DDL, XWH, and HC were responsible for extracting and analyzing data. ZZW, YW, LPQ, XJW, SL, and KMW contributed to the retrieval of essential data. All authors contributed to the charts, critical revision, and final approval of the manuscript.
Funding
This study was supported by grants from Guangdong Basic and Applied Basic Research Foundation (Grant No. 2020A1515010175, No.2021A1515010240), grants from the Bethune Medicine Scientific Research Fund Project (No. SCE111DS), grants from National Natural Science Foundation of China (Grant No. 81370603) and grants for a Clinical Key Discipline (The Subtropical Disease Center for Thalassemia) from the Chinese Ministry of Health (NO. 1311200006107).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutions’ Ethical Committee, and informed consent was obtained from the patients’ parents according to the Declaration of Helsinki in Sun Yat-sen Memorial Hospital of Sun Yat-sen University. The research protocol and informed consent were approved by the ethics committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Consent to participate was obtained from the participants and the parents/guardians of the children under the age of 18.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin-Yu Li, Li-Ping Zhan, Dian-Dian Liu, Xia-Wei Han, and Han Chen contributed equally to this work.
Contributor Information
Jian-Pei Fang, Email: fangjpei@mail.sysu.edu.cn.
Ke Huang, Email: hke@mail.sysu.edu.cn.
Hong-Gui Xu, Email: xuhgui@mail.sysu.edu.cn.
References
- 1.Iguchi A, Terashita Y, Sugiyama M, et al. Graft-versus-host disease (GVHD) prophylaxis by using methotrexate decreases pre-engraftment syndrome and severe acute GVHD, and accelerates engraftment after cord blood transplantation. Pediatr Transplant. 2016;20(1):114–119. doi: 10.1111/petr.12621. [DOI] [PubMed] [Google Scholar]
- 2.Kishi Y, Kami M, Miyakoshi S, et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation. 2005;80(1):34–40. doi: 10.1097/01.TP.0000163289.20406.86. [DOI] [PubMed] [Google Scholar]
- 3.Park M, Lee SH, Lee YH, et al. Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(4):640–646. doi: 10.1016/j.bbmt.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Liu H, Li L, et al. Pre-engraftment syndrome after unrelated donor umbilical cord blood transplantation in patients with hematologic malignancies. Eur J Haematol. 2012;88(1):39–45. doi: 10.1111/j.1600-0609.2011.01709.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Lim YJ, Kim JY, et al. Pre-engraftment syndrome in hematopoietic stem cell transplantation. J Korean Med Sci. 2008;23(1):98–103. doi: 10.3346/jkms.2008.23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel KJ, Rice RD, Hawke R, et al. Pre-engraftment syndrome after double-unit cord blood transplantation: a distinct syndrome not associated with acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(3):435–440. doi: 10.1016/j.bbmt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangoul H, Wang L, Harrell FE, Jr, et al. Preengraftment syndrome after unrelated cord blood transplant is a strong predictor of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(11):1485–1488. doi: 10.1016/j.bbmt.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Kanda J, Umeda K, Kato K, et al. Effect of graft-versus-host disease on outcomes after pediatric single cord blood transplantation. Bone Marrow Transplant. 2020;55(7):1430–1437. doi: 10.1038/s41409-020-0853-1. [DOI] [PubMed] [Google Scholar]
- 9.Isobe M, Konuma T, Kato S, et al. Development of Pre-Engraftment Syndrome, but Not Acute Graft-versus-Host Disease, Reduces Relapse Rate of Acute Myelogenous Leukemia after Single Cord Blood Transplantation. Biol Blood Marrow Transplant. 2019;25(6):1187–1196. doi: 10.1016/j.bbmt.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Adachi Y, Ozeki K, Ukai S, et al. Optimal dosage of methotrexate for GVHD prophylaxis in umbilical cord blood transplantation. Int J Hematol. 2019;109(4):440–450. doi: 10.1007/s12185-019-02598-x. [DOI] [PubMed] [Google Scholar]
- 11.Shiratori S, Ohigashi H, Takahashi S, et al. Reduced dose of MTX for GVHD prophylaxis promotes engraftment and decreases non-relapse mortality in umbilical cord blood transplantation. Ann Hematol. 2020;99(3):591–598. doi: 10.1007/s00277-020-03937-3. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N, Saito B, Matsui T, et al. Comparative Study of Tacrolimus and Short-Term Methotrexate: 2-Day versus 3-Day Methotrexate as Graft-versus-Host-Disease Prophylaxis after Umbilical Cord Blood Transplantation in Adults. Biol Blood Marrow Transplant. 2020;26(2):367–372. doi: 10.1016/j.bbmt.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Jin L, Sun Z, Liu H, et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat Commun. 2021;12(1):4137. doi: 10.1038/s41467-021-24412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 15.Tavares RCB, Bonfim CS, Seber A, et al. Hematopoietic cell transplantation in pediatric patients with acute leukemias or myelodysplastic syndrome using unrelated adult or umbilical cord blood donors in Brazil. Pediatr Transplant. 2020;24(7):e13789. doi: 10.1111/petr.13789. [DOI] [PubMed] [Google Scholar]
- 16.Narimatsu H, Terakura S, Matsuo K, et al. Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant. 2007;39(1):31–39. doi: 10.1038/sj.bmt.1705539. [DOI] [PubMed] [Google Scholar]
- 17.de Koning C, Admiraal R, Nierkens S, et al. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017;4:38. doi: 10.21037/sci.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Jiang J, Cai Y, et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant. 2019;54(7):1049–1057. doi: 10.1038/s41409-018-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugita J, Kamimura T, Ishikawa T, et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. 2021;56(3):596–604. doi: 10.1038/s41409-020-01065-0. [DOI] [PubMed] [Google Scholar]
- 20.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 22.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Wan LP, Qin YW, et al. Chimerism status is correlated to acute graft-versus-host disease after allogeneic stem cell transplantation. Int J Hematol. 2014;99(3):323–328. doi: 10.1007/s12185-014-1510-5. [DOI] [PubMed] [Google Scholar]
- 24.Ayello J, van de Ven C, Fortino W, et al. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transplant. 2006;12(6):608–622. doi: 10.1016/j.bbmt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Muranushi H, Kanda J, Arai Y, et al. Drug monitoring for mycophenolic acid in graft-vs-host disease prophylaxis in cord blood transplantation. Br J Clin Pharmacol. 2020;86(12):2464–2472. doi: 10.1111/bcp.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bejanyan N, Rogosheske J, DeFor TE, et al. Sirolimus and Mycophenolate Mofetil as Calcineurin Inhibitor-Free Graft-versus-Host Disease Prophylaxis for Reduced-Intensity Conditioning Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2016;22(11):2025–2030. doi: 10.1016/j.bbmt.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nykolyszyn C, Granata A, Pagliardini T, et al. Posttransplantation cyclophosphamide vs. antithymocyte globulin as GVHD prophylaxis for mismatched unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(2):349–355. doi: 10.1038/s41409-019-0682-2. [DOI] [PubMed] [Google Scholar]
- 28.Politikos I, Lavery JA, Hilden P, et al. Robust CD4+ T-cell recovery in adults transplanted with cord blood and no antithymocyte globulin. Blood Adv. 2020;4(1):191–202. doi: 10.1182/bloodadvances.2019000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo N, Garcia-Cadenas I, Barba P, et al. Early and Long-Term Impaired T Lymphocyte Immune Reconstitution after Cord Blood Transplantation with Antithymocyte Globulin. Biol Blood Marrow Transplant. 2017;23(3):491–497. doi: 10.1016/j.bbmt.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Nunes NS, Kanakry CG. Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front Immunol. 2019;10:2668. doi: 10.3389/fimmu.2019.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.