Abstract
Background
The primary antibody (Ab) response to Plasmodium falciparum is a critical step in developing immunity to malaria. Information on the initial Ab responses of babies in malaria-endemic areas is incomplete, in part, because babies receive maternal IgG via transplacental-transfer and usually become infected before maternal IgG wanes. The study aimed to identify the primary IgM and IgG Ab responses to malarial antigens in Cameroonian babies.
Methods
Infants (n = 70) living in a high malaria transmission area were followed from birth throughout the first year of life (mean 341 ± 42 days, an average of 8.5 time points per infant). Malaria infection was assessed by microscopy and PCR, and IgM and IgG antibodies (Abs) were measured using a multiplex immunoassay to AMA1, EBA-175, MSP1-42, MSP2, MSP3, RESA, LSA1, and CSP.
Results
The half-life of maternal IgG varied among the antigens, ranging from 0.7 to 2.5 months. The first infection of 41% of the babies was sub-microscopic and only 11 to 44% of the babies produced IgM to the above antigens; however, when the first infection was detected by microscopy, 59–82% of the infants made IgM Abs to the antigens. Infants were able to produce IgM even when maternal IgG was present, suggesting maternal Abs did not suppress the baby’s initial Ab response. Using longitudinal regression models that incorporated time-varying covariates, infants were found to produce IgG Ab to only AMA-1 when the first infection was sub-microscopic, but they produced IgG Abs to MSP1-42 (3D7, FVO), AMA1 (3D7, FVO) MSP2-FC27, MSP3, RESA, and LSA1, but not MSP 2-3D7, EBA-175, and CSP during their first slide-positive infection. Notably, the primary and secondary IgG responses were short-lived with little evidence of boosting.
Conclusions
The primary Ab response of babies who had maternal IgG was similar to that reported for primary infections in malaria-naïve adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04360-x.
Keywords: Malaria, Plasmodium falciparum, First antibody response, Babies, IgM, IgG
Background
Antibodies (Abs) play an important role in protection from falciparum malaria. The primary antibody (Ab) response is a critical step in the development of immunity as it ‘sets the stage’ for subsequent events in the maturation process. The primary anti-malarial IgG Ab responses of babies residing in high-transmission settings have been difficult to unravel for several reasons.
First, babies are born with anti-malarial IgG Abs acquired in utero from their mothers that wane during the first few months of life. Unfortunately, it is impossible to distinguish between maternal IgG and IgG produced by the baby. Longitudinal and cross-sectional studies have reported the decline of maternal IgG in groups of infants and the subsequent increase in IgG Ab as babies become infected [1–12]. Antigens (Ags) that have been studied include exoerythrocytic-stage antigens (CSP, LSA1, TRAP) [3, 4, 6, 11], merozoite antigens (MSP1, MSP2, MSP3, AMA1, EBA-175 [3, 5, 6, 8, 10–12], as well as PfEMP1, RESA, GLURP, and PfSEA-1 [2, 4, 7–9]. These data provide a generalized picture of the change in IgG for these malaria-specific antigens, but they do not fully characterize the primary response in individual babies. Thus, many questions remain: (i) about the level of parasite burden needed to stimulate an IgG response; (ii) if the neonatal immune system can recognize malarial antigens in the presence of maternal IgG (i.e., do maternal Abs inhibit the neonatal response); (iii) if class-switching from IgM to IgG occurs during primary infection; and, iv) if Abs are produced against all malarial antigens or if the initial response is restricted to immunodominant antigens or epitopes.
Secondly, primary Plasmodium falciparum infections in babies may be asymptomatic with very low or sub-microscopic infections [13] that are transient and cleared without treatment [14, 15]. It is currently unclear if these early, sub-patent infections are adequate to stimulate an Ab response. Previous studies have examined the Ab response during the first clinical episode of malaria; however, babies in endemic areas rarely develop clinical disease before 5–6 months of age [14–17]. Thus, the Ab response associated with a clinical infection may not be the primary response, but rather reflect the summation of prior exposures. Third, prior studies that measured Abs to one or a few antigens provide information about specific antigen(s), but they do not give an overview of the response to antigens with different immunogenicities (e.g., immunodominant vs cryptic). Finally, first infections may be missed if blood samples are not collected frequently or assessed by PCR. Surprisingly, only a few studies have used PCR to detect parasitaemia in infants during the first year of life [13, 18–20].
The current study sought to characterize the primary Ab response of 70 newborns residing in the rural village of Ngali II, Cameroon, where P. falciparum transmission is perennial and individuals receive ~ 257 infectious mosquito bites annually [21]. Infections were detected by PCR and microscopy, and IgM and IgG Abs to 8 P. falciparum antigens were measured. The dataset was used to help answer relevant questions, including: (i) do early sub-microscopic infections induce IgM and IgG Ab to the 8 antigens or is parasitaemia detected by microscopy required to induce a response?; (ii) do babies produce both IgM and IgG Abs upon primary infection?; (iii) do infants produce IgM and/or IgG Ab in the presence of maternal IgG Abs?; and, (iv) is the Ab repertoire produced during the initial response the same as that produced during the first clinical malaria episode? Overall, the dataset helped fill some of the gaps in our knowledge about the primary anti-P. falciparum Ab response of infants residing in high transmission settings.
Methods
Study design
The study was performed as previously described in the rural community of Ngali II, near Yaoundé, Cameroon, where individuals receive an estimated 257 infectious mosquito bites annually [19, 21]. From May 2001 to November 2004, pregnant women were informed about the project and the newborns of women who signed informed consent forms were included as study participants. At enrolment, delivery information was recorded and maternal venous and placental blood samples were collected. After birth, infants were monitored regularly during programmed visits at 9 time points: 7 days, 6 weeks, 3, 4, 5, 6, 8, 10 and 12 months of life [19]. At each visit, infant peripheral blood samples were collected by finger prick and used for parasitological and Ab studies. Samples collected from 70 infants who were not lost from follow-up before the 8th-month visit were included in the current study.
Parasitological studies
Thick- and thin-blood smears of maternal venous and baby finger prick blood were prepared, stained with Diff-Quick, and examined for parasites by microscopy. Total WBC/µl were determined from the corresponding blood samples and results were calculated as number of infected erythrocytes (IE)/µl of blood. In addition, placental intervillous space (IVS) blood samples were collected and examined for parasites by microscopy, as well as, histological sections of placental biopsies, stored in 10% buffered formalin, were prepared, stained with Hematoxylin–Eosin and examination by microscopy. Placental malaria (PM) was defined as the presence of P. falciparum IE in IVS blood smears and/or in histological sections.
Detection of infection by PCR
Plasmodium falciparum infections were also detected in peripheral blood samples of infants by PCR during the first year of life. PCR was performed by amplifying the 18S ribosomal subunit gene of P. falciparum using primers and protocols that have been described previously [22, 23].
Antibody studies
Abs to 8 P. falciparum antigens were measured, including 5 P. falciparum merozoite-stage Ags (MSP1 [3D7, FVO], MSP2 [FC27], MSP3, AMA1 [3D7], EBA175), one ring-stage erythrocyte surface Ag (RESA), and 2 exo-erythrocytic-stage Ags (CSP and LSA1) using a multiplex Luminex assay. The description of each Ag, including the amount of Ag used for coupling, is provided in Additional file 7: Table S1. A mixture of beads was prepared, containing 100 beads/µl of each antigen coupled to a different spectral address, and 50 µl of the mixture in PBS-1% BSA was combined with 50 µl of a 1:100 dilution of plasma (total dilution 1:200) in filter plate wells (Multiscreen BV, Millipore). After one hour of incubation in the dark on a 500-rpm shaker at RT, the wells were washed 5 times with PBS-0.05% Tween 20. Then, 100 µl of secondary Ab, either 0.1 mg of R-phycoerythrin conjugated to the F(ab’) 2 fragment of goat anti-human IgG or to the F(ab’) 2 fragment of donkey anti-human IgM (Jackson Immunoresearch) was added to each well. After 30 min of incubation, the plates were washed again 5 times and the beads were resuspended in 100 μl PBS-1% BSA. The plates were then read in the Liquichip M100 reader (Qiagen) that detected the spectral address of each bead type (hence corresponding Ag) and the median fluorescence intensity (MFI) of phycoerythrin (i.e., amount of Ab bound). Pooled positive and negative control plasma samples were included on each plate. The cut-off for waning maternal IgG Ab was the mean + 3 SD of the lowest MFI each baby had prior to a rise due to Ab production by the baby. The cut-off for IgM Abs was the mean MFI ± 3SD at the first visit (mean 9 ± 2 days), after deleting outliers that were 1 SD above the combined mean. These cut-off values were selected rather than the traditional MFI from naïve adult controls (e.g., plasma from Americans), since these cut-offs reflect MFI in young babies in rural setting who were becoming infected with a variety of pathogens.
Statistics
Results from the 70 infants were summarized using descriptive statistics, either as means ± standard deviations (SD) or median ± interquartile range (IQR) based on distribution of the data. The rate of antibody decline was determined using a longitudinal linear mixed-effects model. The model accounts for repeated measurements from an infant and it was used with the log-transformed values of the antibody observed at multiple time points. The half-life estimate () was calculated using the equation: where is the estimated slope parameter of the linear mixed-effects model [24–26]. The half-life estimation was performed using data from the first 6 months of life, and provided with 95% confidence intervals (CI), for a set of Abs observed to be decreasing over time. Next, statistical tests were conducted to determine if P. falciparum IgG and/or IgM levels in infants were significantly increased at first P. falciparum infection or if PM status influenced the result. A set of linear mixed effect models were generated considering the responses as log-transformed Ab levels tracked from day 7 of life to the time of first P. falciparum infection or to the last follow-up visit if no infection occurred. First P. falciparum infection was the predictor of primary interest. Adjustments were made for covariates, such as baseline Ab level, length of time from baseline to infection, and for grouping variables such as PM status. Variable associations were determined by testing regression coefficients based on the Wald test. In mixed effect models applied, subject-specific intercepts were used as random components. All statistical analyses were performed using R software version 4.0.2 or GraphPad Prism 9.
Results
Characteristics of the infants
The 70 infants enrolled in the study were healthy newborns, with an average gestational age of 39.2 ± 2.0 weeks and weighing 3,177 ± 484 g (Table 1). Only 7.9% of the babies were low birth weight (LBW), although 46.6% of the mothers were PM-positive (PM +) at delivery. The babies were followed longitudinally through the first year of life, with a mean follow-up time of 341 ± 42 days. Babies were seen an average of 8.5 times (range 6–9) during the first year of life.
Table 1.
Description of infants residing in Ngali II
| Number of infants | 70 |
| Gravidity (median, range) | 3 (range 1–12) |
| Gestational age at birth (weeks)a | 39.2 ± 2.0 |
| Percent full-term births | 89.9% |
| Birth weight (grams)a | 3,177 ± 484 |
| Percent low-birth weight (< 2,500 g) | 7.9% |
| % of women with placental malaria (PM-pos) | 46.6% (27/58) |
| Mean number of visits per infant | 8.5 (range 6–9) |
| Duration of follow-up (days from birth)a | 341 ± 42 |
| Mean number of times malaria-positive by microscopy (range) | 2.7 ± 2.1 (range: 0 to 7) |
| Mean number of times malaria-positive by PCR (range) | 3.9 ± 2.1 (0 to 8) |
aMean ± SD
Plasmodium falciparum infections during the first year of life
At the first time point (mean 9 ± 2 days), only 4% of the newborns were malaria-positive by microscopy and 7% by PCR, suggesting few congenital infections (Fig. 1). The number of infants infected with P. falciparum increased rapidly over the first 4 months of life and then remained relatively constant thereafter. Overall, P. falciparum infections were detected in infants 2.7 ± 2.1 (range 0 to 7) and 3.9 ± 2.1 (range 0 to 8) times by microscopy and PCR, respectively, during the year (Table 1).
Fig. 1.
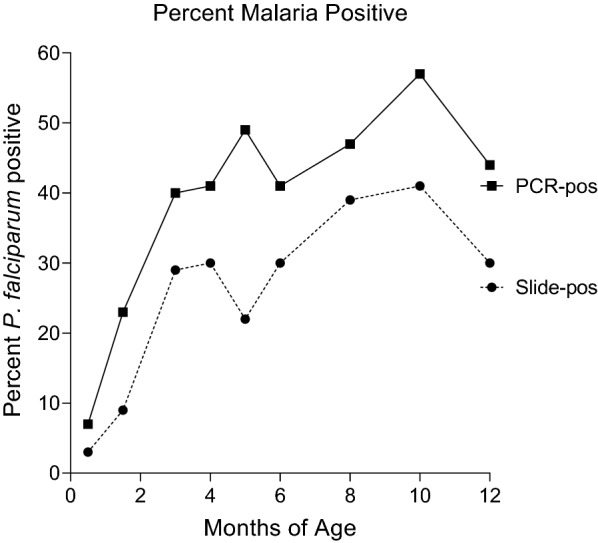
Infants positive for Plasmodium falciparum by PCR and microscopy at different ages (n = 70)
Time until the first infection
Longitudinal data identified when each baby had his/her first P. falciparum infection that was either submicroscopic (i.e., PCR-positive, slide-negative) or detected by microscopy, and symptomatic episode of malaria (Fig. 2). Overall, first infections of 29 babies were sub-microscopic and occurred between the 2nd and 6th month of life. Among these 29 babies, 83% (24/29) were malaria-negative at the next time point (~ 1 month later) by microscopy, showing these babies initial infections were transient and self-resolving with very low parasitaemia. On the other hand, the first microscopically detected infection occurred in 86% (60/70) of the babies, (including some babies who had prior sub-microscopic infections) between 2 to 12 months of life (median of 6 months). Overall, 14% of the babies were malaria-negative by PCR and microscopy throughout the first year of life. Only 26% (18/70) of the babies developed clinical cases of malaria (defined as slide-positive for P. falciparum with axillary temperature ≥ 37.5 °C), that were not severe, with the first episode usually occurring after 5 months of age.
Fig. 2.
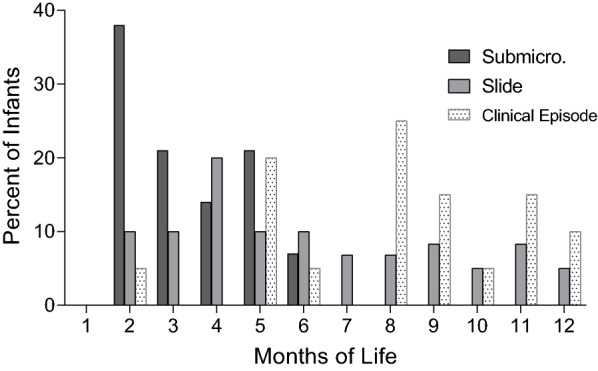
Time of the first Plasmodium falciparum infections and clinical episode. The first infection of 29 babies was a sub-microscopic infection. Among the 70 infants, 60 developed infections detected by microscopy at the time points shown. Only 18 infants had symptomatic clinical episodes of malaria, mainly after 5 months of age
Decline of maternal IgG
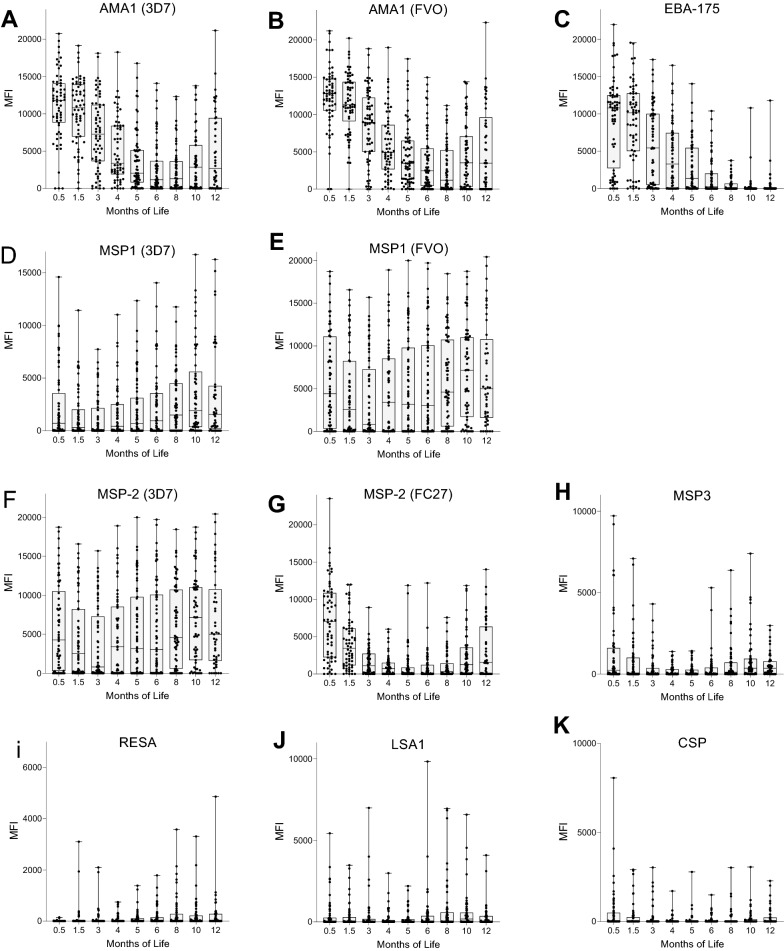
The decline of maternal IgG Ab to 8 malarial antigens (11 recombinant proteins) during the first year of life is shown in Table 2 and Fig. 3. At ~ 1 week of age, babies had high (> 10,000 MFI) levels of maternal IgG Ab to AMA1, EBA-175, (Fig. 3A–C); intermediate (> 1,000 but < 10,000 MFI) Ab levels to MSP1-42 (3D7, FVO) and MSP2 (3D7, FC27) (Fig. 3D-G), and very low levels (< 1000 MFI) to MSP3, RESA, LSA-1 and CSP (Fig. 3H–K). Overall, 80–97% of newborns had maternal IgG Ab to AMA1 (3D7, FVO) and EBA-175 that were detectable for 6 to 8 months, with Ab half-lives ranging from 0.98 to 1.9 months based on data for the first 6 months of life (Table 2). On the other hand, Abs to MSP1-42 and MSP2 declined quickly to background (cut-off) levels by 3 months of age. Thereafter, IgG Ab levels to MSP1-42 and MSP2 quickly increased as the babies produced Abs when they became infected with P. falciparum. Ab half-life estimates for the first 6 months of life was 0.7 months for MSP2, but the half-life to MSP1-42 could not be determined over this period since infants produced IgG to MSP1-42 as early as 3–4 months of age. On the other hand, most infants had very low or no Ab levels to MSP3, RESA, LSA1, and CSP (Fig. 3H–K). As a result, a decrease in maternal IgG over time was not observed, preventing an estimate of Ab half-live.
Table 2.
Characteristics of maternal IgG transplacentally transferred to babies
| Amount of Ab MFI at day 9 ± 2 (median, 1st, 3rd quartile) | Percentage of Ab-positive Babiesa | Ab Half-life, ± 95% CI (months)b | Length of detectable maternal IgG (months) | |
|---|---|---|---|---|
| AMA1 (3D7) | 11,757 (8,765, 14,108) | 96.9 | 1.46 (1.17, 1.94) | 6 |
| AMA1 (FVO) | 12,581 (10,218, 14,870) | 96.9 | 1.88 (1.4, 2.59) | 8 |
| EBA-175 | 10,800 (3,635, 12,460) | 79.7 | 0.98 (0.83,1.21) | 6 |
| MSP1-42 (3D7) | 1,003 (10, 3,868) | 35.9 | NCc | NC |
| MSP1-42 (FVO) | 4,637 (615, 11,207) | 67.2 | NC | 3 |
| MSP2 (3D7) | 6,235 (2,298, 10,163) | 76.6 | 0.78 (0.67, 0.92) | 3 |
| MSP2 (FC27) | 6,935 (2,325, 10,163) | 81.3 | 0.73 (0.64, 0.86) | NC |
| MSP3 | 239 (0, 1,482) | 31.3 | 2.41 (1.58, 5.04) | NC |
| RESA | 0 (0, 0) | 0 | NC | NC |
| LSA-1 | 0 (0, 218) | 23.4 | NC | NC |
| CSP | 0 (0, 352) | 25.0 | 2.39 (1.64, 4.36) | NC |
aPercent of babies at 9 ± 2 days with MFI above cut-off: AMA1(3D7) 1,970 MFI; AMA1 (FVO) 2,050 MFI; EBA-175 (2,020 MFI; MSP1(3D7) 1,500MFI; MSP1(FVO) 1,800 MFI; MSP2(3D7) 1,980 MFI; MSP2(FC27) 1,880 MFI; MSP3 842 MFI; RESA 390 MFI; LSA-1 380 MFI; CSP 490 MFI
bbased on IgG Ab levels between Day 9 ± 2 after birth until 6 months of age
cNot calculatable, because no consistent decline occurred or Ab were produced prior to 6 months
Fig. 3.
Changes in IgG antibody levels during the first year of life IgG. Antibody levels were measured in the 70 infants during the first year of life at 9 time points. A. AMA-1 (3D7), B. AMA1 (FVO); C. EBA-175; D. MSP1-42 (3D7); E. MSP1-42 (FVO); F. MSP2(3D7); G. MSP-2 (FC27); H. MSP3; I. RESA, J. LSA1, and K. CSP. Since MFIs were not normally distributed, scattergrams are presented as Boxplots showing medians and interquartile ranges
Relationship between maternal IgG levels at birth and primary parasitaemia
Ab levels at the first visit were compared between babies whose first infections were sub-microscopic (n = 18), i.e., very low parasitaemia, and those with slide-positive infections (n = 52, range 21–150,000 IE/µl), i.e., higher parasitaemia. No difference in median MFI was found (all p values > 0.05), except for one antigen where MFI were higher in the slide-positive group (Additional file 1: Fig. S1). Thus, the data suggest that high maternal IgG levels to any of the 8 antigens at birth did not help reduce parasitaemia to sub-microscopic levels.
The IgM antibody response of infants during the first year of life
IgM Ab data were available for 47 babies who had ≥ 7 visits (Additional file 2: Fig. S2). The results allowed an answer to the following three questions:
Is the parasitaemia high enough during the first sub-microscopic infection to induce an IgM Ab response? The first infection was sub-microscopic in 38% (18/47) of these babies. At that time, relatively few babies had IgM Ab, with i) 35–44% of babies having IgM Abs to AMA-1 (3D7); ii) 27–33% having Ab to AMA-1 (FVO), MSP-1 (FVO), EBA-175, MSP-2 (3D7, FC27); and, iii) only 11–22% having Abs to RESA, MSP3, LSA-1, and CSP (Table 3). Since the majority of babies were slide-negative for P. falciparum at the next visit (~ 1 month later), these early transient sub-microscopic infections induced an IgM response in only a small proportion of infants. On the other hand, when the first infection was high enough to be detected by microscopy, 68–82% of the babies had IgM to the 8 antigens (Table 3). Thus, sub-microscopic infections could induce IgM in some babies, most babies produced IgM to multiple antigens only when they had parasitaemia detectable by microscopy.
Table 3.
Percentage of infants positive for IgM antibodies at their first sub-microscopic and slide-positive infections, as well as at the first symptomatic clinical episode
| Antigens | Sub-microscopic infectiona (n = 18) | Slide positive infectionsb (n = 39) | First clinical episode (n = 11) |
|---|---|---|---|
| AMA-1 (3D7) | 44.4 | 76.9 | 73 |
| AMA-1 (FV0) | 33.3 | 82.1 | 91 |
| EBA-175 | 27.8 | 66.7 | 73 |
| MSP-1 (3D7) | 22.2 | 76.9 | 91 |
| MSP-1 (FVO) | 33.3 | 79.5 | 91 |
| MSP-2 (3D7) | 27.8 | 79.5 | 91 |
| MSP2 (FC27) | 27.8 | 74.4 | 82 |
| MSP-3 | 11.1 | 59.0 | 91 |
| RESA | 11.1 | 71.8 | 64 |
| LSA-1 | 16.7 | 74.4 | 91 |
| CSP | 22.2 | 69.2 | 82 |
aPCR-positive, but slide negative
bFirst slide-positive infection
Did the infants produce IgM Ab to all 8 malarial antigens or is the response more restricted? As shown in Table 3, babies with primary sub-microscopic infections responded to a restricted repertoire of antigens, showing that some antigens were more immunogenic than others, e.g., 44% of babies with sub-microscopic infections produced IgM to AMA1; whereas, only 11% produced IgM to RESA and MSP3. On the other hand, most babies had IgM Abs to most (i.e., 59 to 82%) of the 8 antigens at the time of their first slide-positive infection (Table 3). Although most babies had Abs to many antigens, variation occurred among the babies, e.g., one baby might have Abs to AMA1, but lack Abs to RESA; whereas, another baby would have Abs to MSP1, but not EBA-175. At the time of the first symptomatic malaria episode, which usually occurred after infants had had several asymptomatic infections, 64 to 91% of infants had IgM Abs to the 8 malarial antigens (Table 3). Thus, following the first sub-microscopic infection the response was quite restricted, but when parasitaemia was high enough to be detected by microscopy most babies produced IgM to most of the antigens studied.
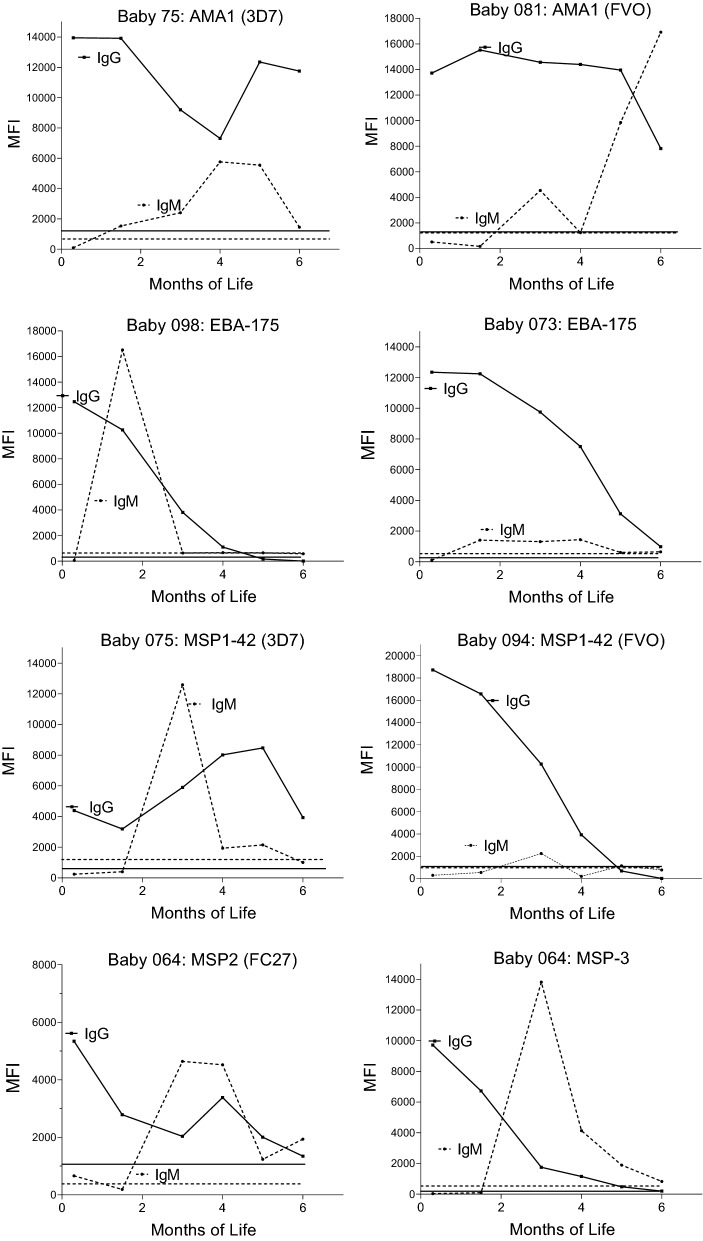
Do infants produce IgM Abs before maternal IgG levels wane, i.e., reach background levels? To answer this question, the primary IgM response (i.e., the first visit when the MFI was above the cut-off) was compared with the corresponding maternal IgG level to determine if the MFI was still positive (above cut-off). Although the first IgM response often occurred in babies who either had not received maternal IgG for the antigen or after maternal Ab had waned (dropped below the IgG cut-off), a significant proportion of infants produced their first IgM response while significant amounts of maternal IgG were still present (Table 4). For example, over half the babies had maternal IgG to MSP1, 60.9% to EBA-175, and > 76% had IgG to AMA1 at the time they first produced IgM to these antigens (Table 4). Examples of the production of infant IgM in the presence of maternal IgG are shown in Fig. 4. Thus, babies can produce a humoral response to antigens even when maternal IgG levels remain positive.
Table 4.
Percentage of infants who produced an IgM response in the presence of maternal IgG
| Antigens | AMA-1 3D7 | AMA-1 FVO | EBA-175 | MSP-1 3D7 | MSP-1 FVO | MSP 2 FC29 | MSP3 |
|---|---|---|---|---|---|---|---|
| 27.3% (12/44) | 34.1% (15/44) | 60.9% (28/46) | 77.8% (35/44) | 76.2% (35/46) | 36.6% (15/41) | 13.3% (6/45) |
Results for RESA, LSA1 and CSP are not included because Ab levels to these antigens were very low and no sustained downward trend in Ab levels was observed over the first few months of life
Fig. 4.
Comparison of declining maternal IgG with the baby’s IgM response. Each graph represents a single baby and one malarial antigen. The horizontal lines at the bottom show the cut-off for positivity: dotted lines (- - - -) for IgM and solid (____) lines for IgG Abs
The IgG antibody response at first post-natal infection
Because of declining maternal IgG Abs in young babies, it is often difficult to determine when a baby first produces IgG Abs after infection (i.e., a rise in IgG Abs). Accordingly, longitudinal regression models were created that took the decline of maternal IgG into consideration when assessing if a baby produced IgG upon primary i) sub-microscopic infection; ii) slide-positive infection; and, iii) when the infant had its first clinical episode of malaria (Table 5). Data included in the analysis were from all 70 infants. Positive regressing results are shown in Table 5, where coefficients with significant p values indicate that an IgG response had occurred. Among the babies with primary sub-microscopic infections, IgG Abs were only produced to AMA1 (3D7, FVO). However, when parasitaemia were adequate to be detected by microscopy, IgG Abs to 5 antigens were produced, including MSP1 (3D7, FVO), MSP2 (FC27), AMA1 (3D7, FVO), RESA and LSA1, but not to MSP2 (3D7), EBA-175, and CSP. A similar pattern was found in the infants who developed clinical episodes of malaria later in life (Table 5). Taken together, data show that upon initial sub-microscopic infections, babies produced IgG Ab to AMA1; however, when parasitaemia reached detectable levels by microcopy IgG Ab to most antigens were detected.
Table 5.
IgG response upon primary Plasmodium falciparum infection (Coefficient, p-value)
| Sub-microscopica | Slide-positive | Clinical episode | |
|---|---|---|---|
| AMA1(3D7) | 0.81; p = 0.036 | 1.17; p = 0.004 | |
| AMA1 (FVO) | 0.97; p = 0.019 | 1.00; p = 0.008 | 1.73; p = 0.026 |
| EBA-175 | |||
| MSP1 (3D7) | 2.30; p < 0.0001 | 2.14; p = 0.033 | |
| MSP1 (FVO) | 2.04; p = 0.001 | ||
| MSP2 (3D7) | 4.01; p < 0.001 | ||
| MSP2 (FC27) | 0.97; p = 0.047 | 3.26; p < 0.001 | |
| MSP3 | 1.23; p = 0.011 | 1.65; p = 0.048 | |
| RESA | 1.31; p < 0.001 | 2.42; p = 0.001 | |
| LSA1 | 1.28; p = 0.003 | 1.76; p = 0.023 | |
| CSP | 1.82; p = 0.007 |
aExcluded all babies whose first infection was microscopically-positive and PCR-negative and microscopic-positive and PCR-positive
BLANK = negative coefficient value or non-significant p value
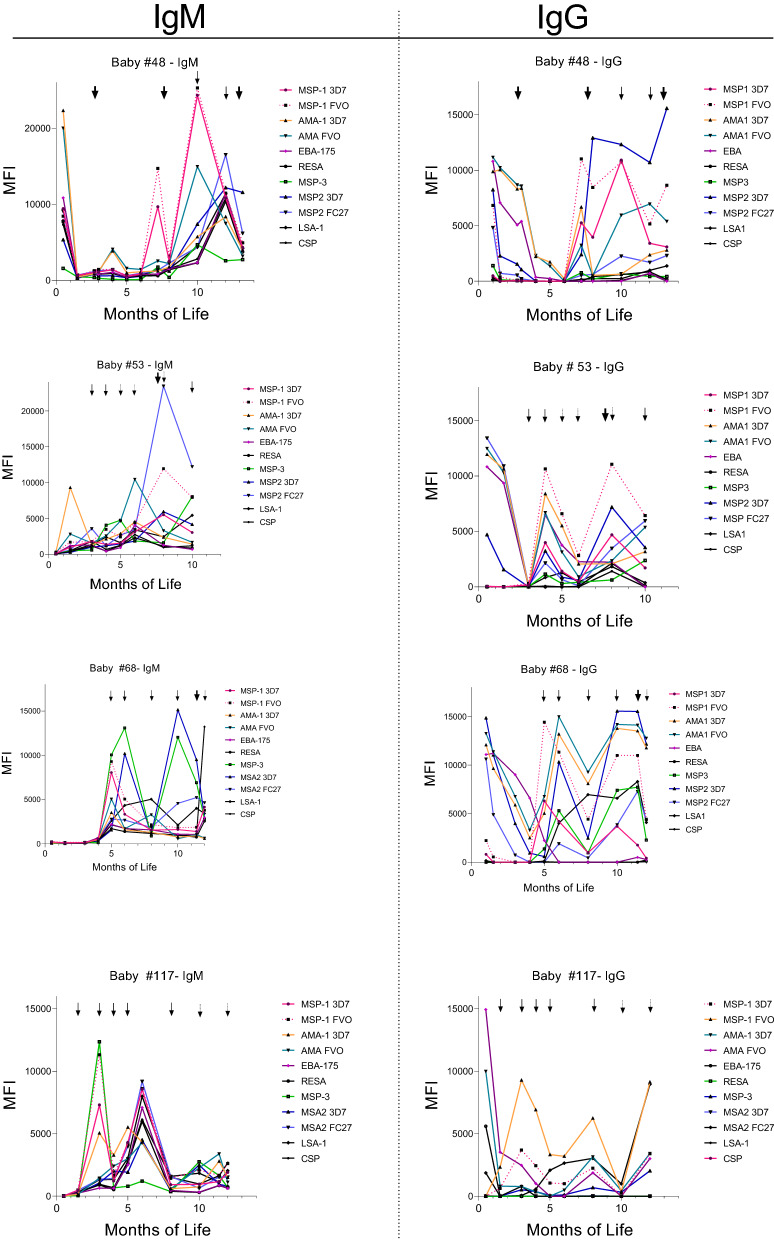
IgM and IgG Ab responses were short-lived
During the first year of life, IgM and corresponding IgG levels increased one or more times and then quickly declined following recent infection (Fig. 5, Additional file 3: Fig. S3). Some babies had second and third peaks of IgM and IgG, which also declined quickly (Fig. 5). In general, primary and secondary IgG responses were similar with minimal or no increase in Ab levels or breadth of the response (Additional file 3: Fig. S3). Overall, a true primary versus secondary response with higher IgG levels and broader antigenic specificity was not observed in infants during the first year of life (Fig. 5, Additional file 1: Fig. S3).
Fig. 5.
Examples of the IgM and IgG levels in four infants during the first year of life. The arrows indicate when malarial parasites were detected by PCR (dotted arrows), slide (solid arrows), and when a clinical episode occurred (bold arrows)
Ab levels at birth were not associated with time to first infection or number of infections
No association was found between Ab levels to any of the antigens at week one of life (i.e., the first time point (mean 9 ± 2 days) and i) time to first infection, ii) number of infections detected by PCR or iii) number of infections detected by microscopy during the first year of life (Additional file 4: Fig. S4, Additional file 5: Fig. S5, additional file 6: Fig. S6).
Discussion
The primary Ab response to P. falciparum is important in establishing the initial repertoire of activated B cells, induction of class-switching, and development of plasma and memory B cells. Previous studies have monitored the primary Ab response in malaria-naïve travelers who contacted malaria abroad and in transmigrants upon moving into a malaria-endemic area [27–34]. Studies show that IgG Abs are rapidly produced during illness to multiple sexual and asexual-stage antigens [27, 29–31, 33–35]; variation in the amount and breadth of the Ab response occurs among individuals; and primary Ab levels are lower than those produced upon secondary infection [28, 30, 33–35]. The primary IgG Ab response to P. falciparum is short-lived [29, 30, 32, 34], with half of individuals becoming Ab-negative in 1.6 to 31 weeks depending on the antigen [30]. The influence of age on the primary Ab response is currently being debated, since similar Ab responses in naïve Mozambican children and European adults were found during their primary infections [27]; however, a study of Javanese transmigrants found that the humoral response was age-related [34]. The current study sought to determine if a similar pattern of immune-responsiveness took place in babies during the first year of life who had circulating maternal IgG, since anti-malaria IgG might complex with antigens and prevent immune activation.
At birth, maternal IgG was detected in all newborns, but substantial variation was observed among the babies, with respect to (i) the antigen-specificity (repertoire) of maternal Abs (Table 2); (ii) the amount of maternal IgG (Fig. 2); and, (iii) the rate of decline (half-life) of maternal IgG for the various antigens (Table 2). In general, at one week of age, newborns were likely to have maternal IgG Abs to AMA1, EBA-175, MSP1 and MSP3 (Table 2), but little or no Abs to MSP3, RESA, LSA-I and CSP (Fig. 3). Thereafter, maternal Abs declined with Ab half-lives ranging from 0.7 to 2.4 months (i.e., mean 21 to 75 days) depending on the antigen (Table 2). Although immunological text books state that the half-life of human IgG is ~ 3 weeks (21 days), a wide-range of Ab half-lives has been reported for passively transferred IgG. The half-life of IgG is dependent on i) the starting Ab concentration (e.g., an inverse relationship between Ab levels and Ab half-lives has been reported) [24]; ii) the total amount of IgG present in circulation; and, iii) relative saturation of Fc neonatal receptors (nFcR). For example, the half-life of passive transfer replacement IgG was found to be 22 to 96 days [36] and the half-lives of maternal IgG in newborns was 29 days to pertussis [37], 40 days to hepatitis A virus [38], 79 days for respiratory syncytial virus [39], and 75 to 147 days (2.5 to 4.9 months) for P. falciparum merozoite antigens in Kenyan infants [24]. Thus, although maternal Ab levels are generally reported to wane in 2–6 months [3, 10–12], the half-life for each antigen is highly variable.
The contribution of maternal IgG to ‘protection’ of newborns is unclear, as some studies have found no relationship between that amount of maternal IgG at birth and time to first infection or risk of infection during the first year of life (reviewed in [15]). In fact, some studies have even reported that higher levels of anti-malarial Ab in newborns at birth were associated with an increased risk of infection [20, 40]. On the other hand, other studies have found a potential protective role for Ab to PfSEA-1 [9], endothelial protein C receptor-binding CIDRα1 [2, 41], and possibly MSP1-19 [18, 42, 43]. It is tempting to hypothesize that maternal IgG Ab helps reduce parasitaemia to low or sub-microscopic levels during the first few months of life [4]; however, data supporting the hypothesis are lacking. A comparison of Ab levels during the first week of life in babies having either i) a sub-microscopic parasitaemia, or, ii) a parasitaemia high enough to be detected by microscopy did not find a relationship between high Ab levels at birth and parasitaemia at first infection (Additional file 2: Fig. S2). The data were also analysed in multiple ways, but no evidence was found that Ab levels to any of the 8 individual antigens studied, including MSP1-42, were associated with fewer infections or shorter time to first infection detected by PCR or microscopy (Additional file 4: Fig. S4, Additional file 5: Fig. S5, additional file 6: Fig. S6). Clearly, immunity is mediated by Abs to a combination of multiple antigens, but there was no evidence in this study that Abs to any of the 8 antigens alone played a role in the control of asymptomatic, self-limiting submicroscopic infections early in life (Table 3).
Throughout the first year, essentially all babies had one or more sub-microscopic and/or slide-positive infections; whereas, only a few infants developed clinical infections (Fig. 1, Table 3). Although the infants were carefully monitored, seroconversion was occasionally detected in the absence of parasitaemia, demonstrating that not all infections had been documented. Thus, the data in Fig. 1 are an underestimate of the true prevalence of malaria in the village. Most sub-microscopic infections were identified during the second and third months of life (Fig. 2). The question became, did these early infections stimulate a humoral response? Results showed that during early sub-microscopic infections only 11 to 44% of the babies had produced IgM Abs to the 8 antigens, and only IgG Abs to AMA-1 (Tables 3 and 5). However, during the first slide-positive infection, more than half of the babies (i.e., 59 to 82% depending on the antigen) produced IgM and modelling revealed a significant increase in IgG to all of the antigens except EBA-175, MSP2 (3D7) and CSP (Tables 5 and 3). Since the lower limit of detection of P. falciparum by thick-blood film is ~ 100 IE per µl and that of the PCR test used in this study was ~ 1 IE/µl; the results suggest that parasitaemia of > 100 IE/µl may be needed to induce an Ab response in most babies, especially to weaker antigens. Currently, information on the amount of antigen or parasitaemia needed to induce an anti-malarial humoral response in infants is unknown.
Although maternal IgG was still detectable in infant circulation, about one-third of babies produced IgM Ab to AMA1, MSP2, and MSP3 and over 50% of babies made IgM to MSP1-42 and EBA-175 during their primary sub-microscopic or slide-positive infections (Table 4, Fig. 4). Previously, it was unclear if passively acquired maternal IgG would complex with malarial antigens and deplete their concentrations to sub-immunogenic levels; therefore, preventing the generation of plasma B cells. However, such does not appear to be the case. Similar results were recently reported by Park et al. showing that maternal Abs in cord blood did not abrogate the development of an infant’s Ab response to the P. falciparum schizont egress antigen-1 (PfSEA-1) [9]. These results suggest that either maternal vaccination or a vaccine given soon after birth may be feasible for protecting young babies from severe disease.
One problem in studying the primary IgG response in babies is that it is not possible to distinguish between IgG made by the babies, which may be produced in small amounts, from maternal IgG, that may be present in relatively high concentrations. Accordingly, a statistical approach was used that took into consideration variables that influence the primary Ab response, including the decline of maternal IgG (Table 5). Among the 70 babies, only IgG Ab to AMA-1 were detected in babies experiencing a primary sub-microscopic infection. This result was not totally unexpected since among the 8 antigens studied, AMA1 was the most immunogenic with respect to Ab prevalence and levels. However, babies experiencing their first infection detected by microscopy made IgG to many of the antigens, including AMA1, MSP1-42, MSP2 (FC27), MSP3, RESA and LSAI (Table 5). The breadth of the response in babies appears to be similar to that reported for malaria-naïve adults who contacted malaria for the first time [27–33]. As expected, infants usually did not have symptomatic cases of P. falciparum until after the first 5 months of life and may have already experienced several previous asymptomatic infections. At the time of a clinical episode, most infants had IgG, and some were still producing IgM, to the 8 antigens.
The initial Ab responses to P. falciparum infections in both infants (Fig. 5, Additional file 1: Fig. S1) and malaria-naïve adults were short-lived [6, 29, 30, 32, 42]. However, the literature reports that secondary infections in adults resemble a secondary-type response that is significantly more robust, in that, higher titers [30, 35], increased number of Ab-secreting B cells [30], and functional Abs were found, e.g., Abs to MSP1 with growth inhibitory activity are produced [28]. In infants, the Ab response throughout the first year of life remains short-lived, with little or no evidence of increased levels of Abs being produced to any of the 8 antigens. Similar results have been reported previously. For example, Branch et al. published longitudinal data for MSP1-19 during the first year of life that was similar to those shown in Fig. 5 [42]. Keenhin et al. compared the Ab response of children and adults who were malaria-naïve prior to migrating into a malaria-endemic area [34]. They reported that the responses in children were slower to develop than those in adults. Overall, the slow maturation of anti-malarial IgG responses in infants is expected because Ab responses are typically of shorter duration, have a delayed onset, and differ in distribution of IgG isotypes than adults (reviewed in [44, 45]). It appears that during the first year of life babies begin to produce a rudimentary Ab IgG response, but the response does not reach maturity until later in life. If this is the case, then vaccinating infants during the first year of life for malaria may be effective, but for only for a short duration. Clearly, additional studies are needed to tease out the details of anti-malarial immunity in infants.
Conclusions
In summary, the first infection in babies residing in malaria-endemic is almost always asymptomatic and self-resolving. Many of the first infections are sub-microscopic and may induce an IgM response to multiple P. falciparum antigens, but class-switching to IgG is restricted to more immunodominant antigens (e.g., AMA1). However, when primary infections were detected by microscopy, babies produced IgG to 6 of the 8 antigens studied. Therefore, both the IgM and IgG responses may occur before maternal IgG Abs wane completely. As reported previously, the primary Ab response is short-lived with both IgM and IgG Ab quickly declining over a few months. Subsequently, babies produce IgG responses upon re-infection, but the response is similar to that after primary infection. Even with repeated infections and clinical episodes, there was little evidence that infants produced a true secondary-type humoral response during the first year of life.
Supplementary Information
Additional file 1: Figure S1. Comparison of antibody levels in babies at 1 week of life whose subsequent primary infections were sub-microscopic (n = 18) or detected by slide-microscopy (n = 39) Horizontal lines = medians. SUB = sub-microscopic infection; Slide = infection detected by microscopy.
Additional file 2: Figure S2. IgM antibody levels during the first year of life. Horizontal lines show the cut-off for antibody positivity. (n = 47 babies). A. AMA-1 (3D7), B. AMA1 (FVO); C. EBA-175; D. MSP2 (3D7); E. MSA2 (FC27) F. MSP1-42 (3D7); G. MSP1-42 (FVO); H. MSP3; I. RESA, J. LSA1, and K. CSP.
Additional file 3: Figure S3. Some infants had several IgG responses during the first year of life Note: MFI were similar between the primary and secondary IgG Ab response. Figure A-H show IgG responses of different infants (Baby#). Babies A-E had only 1 to 2 detected infections; whereas, babies F to H had multiple infections.
Additional file 4: Figsure S4. No correlation between antibody levels at birth and time to first infection. Ab levels (MFI) in plasma collected at the first visit (mean 9 ± 2 days) of each baby were plotted against time (days) to first infection that was detected by PCR or slide. Simple linear regression was used to determine if Ab levels to any of the antigens was associated with a significant delay in time to infection. No association was found (all R2 values were very low < 0.01 indicating no association) and all p values were non-significant (> 0.01), showing the regression lines did not differ significant from zero (horizontal). Babies who remained malaria-negative during the first year were assigned a value of 366 days as the time to first infection.
Additional file 5: Figure S5. No correlation between Ab levels at birth and number of infections detected by PCR. Antibody levels (MFI) for each baby at the first visit (mean 9 ± 2 days) were plotted against the number of times the baby was PCR-positive during the first year of life. No association between Ab levels at birth and number of PCR-detected infections was seen.
Additional file 6: Figure S6. No correlation between Ab levels at birth and number of infections detected by microscopy. Antibody levels (MFI) at the first visit (mean 9 ± 2 days) were plotted against the number of times each baby was slide-positive during the first year of life. No association between high Ab levels at birth and number of slide-positive infections was found.
Additional file 7: Table S1. Recombinant and synthetic antigens used in antibody assays.
Acknowledgements
The authors thank all families, field workers, laboratory personnel, medical students, and clinical staff in Cameroon who participated in the longitudinal follow-up of infants for the first year of life. Without their help, this study would not have been possible. We acknowledge the generous support of colleagues who provided the recombinant proteins: C. Long NIAID, NIH for AMA1, MSP1-42, MSP2; J. Rayner for MSP3-CT, and Science Applications International Corp. (Fredrick, MD) for EBA175. A special thanks goes to N. Bobbili for outstanding technical assistance.
Abbreviations
- Ab
Antibody
- Abs
Antibodies
- Ags
Antigens
- AMA-1
Apical merozoite antigen-1
- CSP
Circum-sporozoite protein
- EBA-175
Erythrocyte binding antigen-175
- IE
Infected erythrocyte
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IVS
Intervillous space
- LBW
Low birthweight
- LSA-1
Liver-stage antigen-1
- MFI
Median fluorescence intensity
- MSP1-42
Merozoite surface protein 1—C-terminal 42 fragment
- MSP2
Merozoite surface protein 2
- MSP3
Merozoite surface protein 3
- Pf
Plasmodium falciparum
- PCR
Polymerase chain-reaction
- PM
Placental malaria
- PM +
Placental malaria-positive
- PM-
Placental malaria-negative
- RESA
Ring-stage erythrocyte surface antigen
- 3D7
Strains or isolates of P. falciparum
- FVO
Strains or isolates of P. falciparum
- FC27
Strains or isolates of P. falciparum
Author contributions
RFGL and DWT designed and obtained funding for the initial study; with GS assistance, RFGL directed the initial field and laboratory studies in Cameroon; NB conducted the PCR studies; STY and GGF preformed antibody studies and analyzed the results; CS and JC performed statistical analyses for the data; the manuscript was prepared by STY and DWT with the advice and input of the other collaborators. All authors read and approved the final manuscript.
Funding
Funding was provided by NIAID, NIH (UO1AI43888 for initial field and laboratory studies; RO1AI071160 for assay development; R21 AI105286 for plasma screening and data analysis) and the Fogarty International Center (D43TW001264 supported GGF, D43TW009074 supported STY). CS and JJC were partially supported by NIH NIMHD U54MD007601.
Availability of data and materials
The dataset used/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The initial study was approved by the IRB at Georgetown University (GU 1998-081) and the Ethics Committee, Cameroon. All women in the study provided written informed consent. The use of de-identified plasma samples, as well as clinical and parasitological information, were demeaned to be exempt from human experimentation by the University of Hawaii (CHS#21752).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samuel Tassi Yunga, Email: tassiyun@ohsu.edu.
Diane Wallace Taylor, Email: dwtaylor@hawaii.edu.
References
- 1.Biggar RJ, Collins WE, Campbell AC. The serological response to primary malaria infection in urban Ghanian infants. Am J Trop Med Hyg. 1980;29:720–724. doi: 10.4269/ajtmh.1980.29.720. [DOI] [PubMed] [Google Scholar]
- 2.Moussiliou A, Turner L, Cottrell G, Doritchamou J, Gbédandé K, Fievet N, et al. Dynamics of PfEMP1 antibody profile from birth to 12 months of age in Beninese infants. J Infect Dis. 2020;221:2010–2017. doi: 10.1093/infdis/jiaa043. [DOI] [PubMed] [Google Scholar]
- 3.Kitua AY, Urassa H, Wechsler M, Smith T, Vounatsou P, Weiss NA, et al. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immun. 1999;21:307–317. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 4.Achidi E, Perlmann H, Salimonu L, Walker O, Asuzu M. Plasmodium falciparum antigens in Nigerian infants during their first year of life. Acta Trop. 1995;59:173–183. doi: 10.1016/0001-706x(95)00076-q. [DOI] [PubMed] [Google Scholar]
- 5.Bejon P, Cook J, Bergmann-Leitner E, Olotu A, Lusingu J, Mwacharo J, et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS, S/AS01E on blood stage immunity in young children. J Infect Dis. 2011;204:9–18. doi: 10.1093/infdis/jir222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Xiao L, Branch OH, Kariuki S, Nahlen BL, Lal AA. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of western Kenya. XIII Asembo Bay Cohort Project. Am J Trop Med Hyg. 2002;66:7–12. doi: 10.4269/ajtmh.2002.66.7. [DOI] [PubMed] [Google Scholar]
- 7.Cham GKK, Turner L, Kurtis JD, Mutabingwa T, Fried M, Jensen ATR, et al. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun. 2010;78:4653–4659. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kangoye DT, Nebie I, Yaro JB, Debe S, Traore S, Ouedraogo O, et al. Plasmodium falciparum malaria in children aged 0–2 Years: the role of foetal haemoglobin and maternal antibodies to two asexual malaria vaccine candidates (MSP3 and GLURP) PLoS ONE. 2014;9:e107965. doi: 10.1371/journal.pone.0107965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Nixon CE, Pond-Tor S, Kabyemela ER, Fried M, Duffy PE, et al. Impact of maternally derived antibodies to Plasmodium falciparum Schizont Egress Antigen-1 on the endogenous production of anti-PfSEA-1 in offspring. Vaccine. 2019;37:5044–5050. doi: 10.1016/j.vaccine.2019.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duah NO, Miles DJC, Whittle HC, Conway DJ. Acquisition of antibody isotypes against Plasmodium falciparum blood stage antigens in a birth cohort. Parasite Immunol. 2010;32:125–134. doi: 10.1111/j.1365-3024.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelimo K, Av Ofulla, Narum DL, Kazura JW, Lanar DE, John CC. Antibodies to Plasmodium falciparum antigens vary by age and antigen in children in a malaria-holoendemic area of Kenya. Pediat Infect Dis J. 2005;24:680–4. doi: 10.1097/01.inf.0000172151.28851.fd. [DOI] [PubMed] [Google Scholar]
- 12.Dent AE, Malhotra I, Wang X, Babineau D, Yeo KT, Anderson T, et al. Contrasting patterns of serologic and functional antibody dynamics to Plasmodium falciparum antigens in a Kenyan birth cohort. Clin Vaccine Immun. 2016;23:104–116. doi: 10.1128/CVI.00452-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natama HM, Rovira-Vallbona E, Somé MA, Zango SH, Sorgho H, Guetens P, et al. Malaria incidence and prevalence during the first year of life in Nanoro, Burkina Faso: a birth-cohort study. Malar J. 2018;17:163. doi: 10.1186/s12936-018-2315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks S, Koram KA, Wagner GE, Tetteh K, Mcguinness D, Wheeler JG, et al. Frequent and persistent, asymptomatic Plasmodium falciparum infections in African infants, characterized by multilocus genotyping. J Infect Dis. 2001;183:796–804. doi: 10.1086/318834. [DOI] [PubMed] [Google Scholar]
- 15.Dobbs KR, Dent AE. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143:129–138. doi: 10.1017/S0031182015001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuinness D, Koramz K, Bennet S, Wagner G, Nkrumah F, Riley E. Clinical case definitions for malaria: clinical malaria associated with very low parasite densities in African infants. Trans R Soc Trop Med Hyg. 1998;92:527–531. doi: 10.1016/s0035-9203(98)90902-6. [DOI] [PubMed] [Google Scholar]
- 17.Snow RW, Nahlen B, Palmer A, Donnelly CA, Gupta S, Marsh K. Risk of severe malaria among African infants: direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177:819–822. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 18.Reynaldi A, Dent AE, Schlub TE, Ogolla S, Rochford R, Davenport MP. Interaction between maternally derived antibodies and heterogeneity in exposure combined to determine time-to-first Plasmodium falciparum infection in Kenyan infants. Malar J. 2019;18:19. doi: 10.1186/s12936-019-2657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassi Yunga S, Fouda GG, Sama G, Ngu JB, Leke RGF, Taylor DW. Increased susceptibility to Plasmodium falciparum in infants is associated with low, not high, placental malaria parasitemia. Sci Rep. 2018;8:169. doi: 10.1038/s41598-017-18574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner G, Koram K, Mcguinness D, Bennett S, Nkrumah F, Riley E. High incidence of asymptomatic malaria infections in a birth cohort of children less than one year of age in Ghana, detected by multicopy gene polymerase chain reaction. Am J Trop Med Hyg. 1998;59:115–123. doi: 10.4269/ajtmh.1998.59.115. [DOI] [PubMed] [Google Scholar]
- 21.Leke R, Bioga JD, Zhou J, Fouda GG, Leke RJ, Tchinda V, et al. Longitudinal Studies of Plasmodium falciparum malaria in pregnant women living in a rural Cameroonian village with high perennial transmission. Am J Trop Med Hyg. 2010;83:996–1004. doi: 10.4269/ajtmh.2010.10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasit. 1993;61:315–320. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 23.Walker-Abbey A, Djokam RR, Eno A, Leke RF, Titanji VP, Fogako J, et al. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg. 2005;72:229–235. [PubMed] [Google Scholar]
- 24.Murungi LM, Sondén K, Odera D, Oduor LB, Guleid F, Nkumama IN, et al. Cord blood IgG and the risk of severe Plasmodium falciparum malaria in the first year of life. Int J Parasit. 2017;47:153–162. doi: 10.1016/j.ijpara.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine natigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 26.Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis. 2012;206:1612–1621. doi: 10.1093/infdis/jis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncunill G, Mayor A, Jiménez A, Nhabomba A, Puyol L, Manaca MN, et al. Cytokine and antibody responses to Plasmodium falciparum in naïve individuals during a first malaria episode: effect of age and malaria exposure. PLoS ONE. 2013;8:e55756. doi: 10.1371/journal.pone.0055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murhandarwati EEH, Black CG, Wang L, Weisman S, de Koning-Ward TF, Baird JK, et al. Acquisition of invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein 1 in a transmigrant population requires multiple infections. J Infect Dis. 2008;198:1212–1218. doi: 10.1086/591943. [DOI] [PubMed] [Google Scholar]
- 29.Eisen DP, Wang L, Jouin H, Murhandarwati EEH, Black CG, Mercereau-Puijalon O, et al. Antibodies elicited in adults by a primary Plasmodium falciparum blood-stage infection recognize different epitopes compared with immune individuals. Malar J. 2007;6:86. doi: 10.1186/1475-2875-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yman V, White MT, Asghar M, Sundling C, Sondén K, Draper SJ, et al. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med. 2019;17:22. doi: 10.1186/s12916-019-1255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott SR, Payne PD, Duffy MF, Byrne TJ, Tham WH, Rogerson SJ, et al. Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am J Trop Med Hyg. 2007;76:860–864. [PubMed] [Google Scholar]
- 32.Bisoffi Z, Bisoffi Z, Bertoldi M, Silva R, Bertoli G, Ursini T, et al. Dynamics of anti-malarial antibodies in non-immune patients during and after a first and unique Plasmodium falciparum malaria episode. Malar J. 2020;19:228. doi: 10.1186/s12936-020-03300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahnmatz P, Sundling C, Yman V, Widman L, Asghar M, Sondén K, et al. Memory B-cell responses against merozoite antigens after acute Plasmodium falciparum malaria, assessed over one year using a novel multiplexed fluoroSpot assay. Front Immun. 2021;11:619398. doi: 10.3389/fimmu.2020.619398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenihan SH, Gramzinksi R, Ratiwayanto S, Hadiputranto H, Riberu W, Soebianto S, et al. Mechanisms of innate and acquired protection against Plasmodium falciparum in Javanese transmigrant adults and children newly resident in malaria-endemic Northwest Papua. Adv Exp Med Biol. 2003;531:83–102. [PubMed] [Google Scholar]
- 35.Fontaine A, Pophillat M, Bourdon S, Villard C, Belghazi M, Fourquet P, et al. Specific antibody responses against membrane proteins of erythrocytes infected by Plasmodium falciparum of individuals briefly exposed to malaria. Malar J. 2010;9:276. doi: 10.1186/1475-2875-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiff R, Rudd C. Alterations in the half-life and clearance of IgG during therapy with intravenous G-globulin in 16 patients with severe primary humoral immunodeficiency. J Clin Immunol. 1986;6:256–264. doi: 10.1007/BF00918706. [DOI] [PubMed] [Google Scholar]
- 37.Healy CM, Rench MA, Swaim LS, Timmins A, Vyas A, Sangi-Haghpeykar H, et al. Kinetics of maternal pertussis-specific antibodies in infants of mothers vaccinated with tetanus, diphtheria and acellular pertussis (Tdap) during pregnancy. Vaccine. 2020;38:5955–5961. doi: 10.1016/j.vaccine.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 38.Brinkhof MWG, Mayorga O, Bock J, Heininger U, Herzog C. Kinetics of maternally acquired anti-hepatitis a antibodies: prediction of waning based on maternal or cord blood antibody levels. Vaccine. 2013;31:1490–1495. doi: 10.1016/j.vaccine.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS ONE. 2009;4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley EM, Wagner GE, Ofori MF, Wheeler JG, Akanmori BD, Tetteh K, et al. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect Immun. 2000;68:5856–5863. doi: 10.1128/iai.68.10.5856-5863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khattab A, Chia YS, May J, le Hesran JY, Deloron P, Klinkert MQ. The impact of IgG antibodies to recombinant Plasmodium falciparum 732var CIDR-1α domain in mothers and their newborn babies. Parasitol Res. 2007;101:767–774. doi: 10.1007/s00436-007-0548-1. [DOI] [PubMed] [Google Scholar]
- 42.Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, Nahlen BL, et al. A Longitudinal investigation of IgG and IgM antibody response to the merozoite surface protein-1 19-Kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg. 1998;58:21–19. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- 43.Høgh B, Marbiah NT, Burghaus PA, Andersen PK. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants in an area of liberia, West Africa, in which malaria is highly endemic. Infect Immun. 1995;63:4034–4038. doi: 10.1128/iai.63.10.4034-4038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 45.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, et al. Challenges in infant immunity implications for responses to infection and vaccines. Nat Immunol. 2011 doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Comparison of antibody levels in babies at 1 week of life whose subsequent primary infections were sub-microscopic (n = 18) or detected by slide-microscopy (n = 39) Horizontal lines = medians. SUB = sub-microscopic infection; Slide = infection detected by microscopy.
Additional file 2: Figure S2. IgM antibody levels during the first year of life. Horizontal lines show the cut-off for antibody positivity. (n = 47 babies). A. AMA-1 (3D7), B. AMA1 (FVO); C. EBA-175; D. MSP2 (3D7); E. MSA2 (FC27) F. MSP1-42 (3D7); G. MSP1-42 (FVO); H. MSP3; I. RESA, J. LSA1, and K. CSP.
Additional file 3: Figure S3. Some infants had several IgG responses during the first year of life Note: MFI were similar between the primary and secondary IgG Ab response. Figure A-H show IgG responses of different infants (Baby#). Babies A-E had only 1 to 2 detected infections; whereas, babies F to H had multiple infections.
Additional file 4: Figsure S4. No correlation between antibody levels at birth and time to first infection. Ab levels (MFI) in plasma collected at the first visit (mean 9 ± 2 days) of each baby were plotted against time (days) to first infection that was detected by PCR or slide. Simple linear regression was used to determine if Ab levels to any of the antigens was associated with a significant delay in time to infection. No association was found (all R2 values were very low < 0.01 indicating no association) and all p values were non-significant (> 0.01), showing the regression lines did not differ significant from zero (horizontal). Babies who remained malaria-negative during the first year were assigned a value of 366 days as the time to first infection.
Additional file 5: Figure S5. No correlation between Ab levels at birth and number of infections detected by PCR. Antibody levels (MFI) for each baby at the first visit (mean 9 ± 2 days) were plotted against the number of times the baby was PCR-positive during the first year of life. No association between Ab levels at birth and number of PCR-detected infections was seen.
Additional file 6: Figure S6. No correlation between Ab levels at birth and number of infections detected by microscopy. Antibody levels (MFI) at the first visit (mean 9 ± 2 days) were plotted against the number of times each baby was slide-positive during the first year of life. No association between high Ab levels at birth and number of slide-positive infections was found.
Additional file 7: Table S1. Recombinant and synthetic antigens used in antibody assays.
Data Availability Statement
The dataset used/or analysed during the current study are available from the corresponding author on reasonable request.