Abstract
Porphyromonas gingivalis, a gram-negative, black-pigmented anaerobe, is among the microorganisms implicated in the etiology of adult periodontal disease. This bacterium possesses a number of factors, including hemagglutinins, of potential importance in virulence. Several hemagglutinin genes have been identified, cloned, and expressed in Escherichia coli. The purpose of this study was to characterize host responses to purified recombinant hemagglutinin B (rHag B), using the conventional Fischer rat as the experimental animal model. The effectiveness of immunization with rHag B on protection against experimental periodontal bone loss following infection with P. gingivalis was also evaluated. Groups of rats were immunized by the subcutaneous route with rHag B in complete Freund’s adjuvant, immunized with rHag B and orally infected with P. gingivalis, nonimmunized and noninfected, or orally infected with P. gingivalis only. Serum and saliva samples were collected throughout the experiment and evaluated for serum immunoglobulin G (IgG) and IgM and salivary IgA antibody activity by enzyme-linked immunosorbent assay. No salivary IgA anti-Hag B activity was detected in the various groups of rats. A slight serum IgM response similar to that seen in preimmune samples was observed. Serum IgG antibody activity to Hag B was detected only in samples from rats immunized with rHag B. This response was primarily of the IgG1 and IgG2a subclasses, followed by IgG2b and low levels of IgG2c. Supernatants from rHag B-stimulated splenic lymphoid cell cultures from immunized rats contained high levels of gamma interferon, followed by interleukin-2 (IL-2), IL-10, and then IL-4. These results are consistent with the induction of T helper type 1 (Th1)- and Th2-like responses. Western blot analysis of sera derived from rHag B-immunized rats reacted with trichloroacetic acid (TCA) precipitates of P. gingivalis 33277, 381, A7A1-28, and W50, revealing a 50-kDa band reflective of Hag B. However, sera derived from rats immunized with P. gingivalis whole cells or from rats infected with P. gingivalis only did not react with rHag B but did react with TCA precipitates of P. gingivalis strains. Finally, radiographic measurements of periodontal bone loss indicated that rats immunized with rHag B had less bone loss than those infected with P. gingivalis only. These results demonstrate the effectiveness of purified rHag B in inducing a protective immune response and support the potential usefulness of this component of P. gingivalis in the development of a vaccine against adult periodontitis.
Human adult periodontitis is an infectious disease initiated and perpetuated by specific gram-negative bacteria. Among these, the oral, black-pigmented anaerobe Porphyromonas gingivalis has been accepted as an etiological factor and thus implicated in the pathogenesis of the disease (10, 38, 39). This microbial pathogen has been commonly isolated from periodontal diseased sites (26, 39, 40), and specific antibodies to this bacterium have been found in patients with periodontitis (29, 30, 45).
P. gingivalis possesses a number of potential virulence factors thought to be important in the disease process (13, 28, 31, 33, 42). Among these are the hemagglutinins, nonfimbrial adhesins expressed on the surface of P. gingivalis. Hemagglutinins have been implicated in virulence due to their ability to agglutinate erythrocytes, which suggests that they may have a role in adhesion to host tissues (12, 34). It is not yet known how many different P. gingivalis hemagglutinins exist, and there is no evidence as to their specific function. Early studies by Inoshita et al. (14) reported the presence of three major proteins in affinity-purified hemagglutinin preparations. Using monospecific polyclonal and monoclonal antihemagglutinin antisera, Mouton et al. (28) detected two major protein antigens in immunoblots of cell surface extracts of P. gingivalis. Extensive studies by Progulske-Fox et al. (34, 35) and Lepine et al. (24, 25) described the cloning of four P. gingivalis genes and their expression in Escherichia coli. These genes encode for the proteins Hag A, B, C, and D. Hag A and D have about 73.8% identity, whereas Hag B and C are 98.6% homologous. However, neither shows significant homology to Hag A. There are several genes encoding for hemagglutinin molecules, which may be an indication of their importance in virulence. Currently, Hag A and B have been more extensively characterized than Hag C or D. Furthermore, there has been a great deal of interest in the potential utilization of Hag B in vaccine development. For instance, Dusek et al. (6, 7) successfully expressed the hagB gene in an avirulent strain of S. typhimurium. With the mouse as the experimental animal model, humoral immune responses were evaluated following the intragastric administration of the attenuated Salmonella strain. Both systemic and mucosal antibody responses to Hag B were induced, thus demonstrating its immunogenicity. In a later study, it was shown that the subsequent administration of the Salmonella strain to mice resulted in a recall response to Hag B in both serum and secretions (23). However, in these studies, the involvement of the immune response in protection from periodontal disease was not determined. Thus, although the utilization of Hag B in vaccine development seems promising, an evaluation of its role in disease protection is essential.
Previous studies in our laboratory have shown that induction of anti-P. gingivalis antibodies was associated with less bone loss in an experimental rat model, suggesting a role for specific antibodies in periodontal disease protection (18, 19). However, the specificity of the protective antibody was not established. The present investigation evaluated the humoral immune response induced following subcutaneous (s.c.) immunization with recombinant Hag B (rHag B) in an experimental rat model. Levels of serum and salivary anti-Hag B antibodies were determined following immunization and/or infection with P. gingivalis. We also assessed the immunoglobulin G (IgG) subclass of the serum anti-Hag B antibodies and the profile of T helper (Th) cytokines induced in rHag B-stimulated splenic lymphoid cell cultures. Last, protection was evaluated by radiographic assessment of periodontal bone loss.
MATERIALS AND METHODS
Animals.
Conventional Fischer CD F(344) rats used in this study were bred and maintained in Trexler isolators and in horizontal laminar flow units at the University of Alabama at Birmingham (UAB). Animals were fed sterile diet L-485 (Harlan Teklad, Madison, Wis.) and were given water ad libitum throughout the experimental period. All experiments were approved by the UAB Institutional Animal Care and Use Committee.
Bacteria.
P. gingivalis ATCC 33277, 381, A7A1-28 (ATCC 53977), and W50 were used in these studies. The bacteria were cultured and maintained on enriched Trypticase soy plates consisting of Trypticase soy agar supplemented with yeast extract (1%), 5% defribinated sheep blood, hemin (5 mg/liter), and menadione (1 mg/liter) at 37°C in an anaerobic atmosphere of 10% H2, 5% CO2, and 85% N2 (43, 44). For the preparation of P. gingivalis for various purposes including infection and for use as whole-cell (WC) coating antigen in the enzyme-linked immunosorbent assay (ELISA), cultures were grown in basal anaerobic broth (44) at 37°C under anaerobic conditions (17, 18). The bacteria were harvested, washed in sterile phosphate-buffered saline (PBS) (6,000 × g for 20 min), and resuspended in PBS. The estimated number of bacteria in the suspension was determined by reading the optical density at 580 nm and extrapolating from a standard curve. For oral challenge, the bacteria was used immediately, whereas the bacteria to be used as coating antigen in ELISA were suspended in sterile PBS containing 0.02% sodium azide (untreated) or 0.1% formalin (where stated) and stored at 4°C until use.
TCA precipitation of bacterial proteins.
Freshly harvested cultures of P. gingivalis 33277, 381, A7A1-28, and W50 were washed twice with sterile PBS and resuspended in PBS to a final optical density at 580 nm of 1.0. An aliquot of the suspensions (2 ml) was centrifuged, and the pellet was resuspended in 0.5 ml 10% trichloroacetic acid (TCA). Following overnight incubation at 4°C, the precipitates were washed three times and then suspended in 0.05 ml of distilled water. Phenylmethylsulfonyl fluoride (100 mM in isopropanol) was added to 1 mM, and the TCA precipitates were stored at 4°C until use.
Hag B purification.
The hagB gene was cloned from P. gingivalis 381 into a pET vector with a lac promoter and histidine tag and expressed in Escherichia coli JM109 (kindly provided by Ann Progulske-Fox and Thomas A. Brown, University of Florida, Gainesville). A culture of E. coli was grown overnight at 30°C in Luria-Bertani (LB) broth containing ampicillin and kanamycin. An aliquot of the overnight culture was transferred to LB broth with antibiotic supplements and incubated for 2 to 3 h at 30°C with vigorous shaking. Isopropyl-β-d-thiogalactoside was added to a final concentration of 1 mM, and the culture was incubated for an additional 3 h. The culture was centrifuged, and the pellet was resuspended in binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]) and stored at −70°C. The cells were thawed, sonicated three times, centrifuged, and passed through an 0.45-μm-pore-size filter. Hag B was purified from the lysate by using a modified pET His · Bind system (Novagen, Madison, Wis.). The fraction containing rHag B was dialyzed against PBS. The purity of rHag B was confirmed by Western blot analysis using a rabbit anti-Hag B antibody (provided by Thomas A. Brown).
SDS-PAGE and Western blotting.
The reactivity of sera from rats s.c.-immunized with rHag B or P. gingivalis 33277 WC or of sera from rats infected with P. gingivalis 33277 against rHag B or TCA precipitates of the various P. gingivalis bacterial strains was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (19, 21). Briefly, samples were separated under dissociating conditions by SDS-PAGE (12.5% polyacrylamide), and the protein(s) was transferred onto a nitrocellulose membrane by using the Mini Trans-Blot electrophoresis system (Bio-Rad, Hercules, Calif.). The membrane was blocked with 2% Tween 20 in wash buffer (50 mM Tris-HCl, 150 mM NaCl [pH 10.2]) and washed three times with incubation buffer (wash buffer containing 0.05% Tween 20). Following incubation for 2 h with rat serum samples or with rabbit anti-Hag B antibody (provided by Thomas A. Brown), the membrane was washed three times and then incubated for 90 min with biotinylated goat anti-rat or anti-rabbit IgG followed by streptavidin-alkaline phosphatase for the last 30 min. After washing, the membrane was developed using 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium tablets dissolved in distilled water.
Experimental design.
Conventional Fischer rats (8 to 10 weeks old; six rats/group) were immunized s.c. once with rHag B (100 μg) in complete Freund’s adjuvant (CFA) (group A), immunized s.c. once with rHag B as described above and orally infected with P. gingivalis (group B), nonimmunized and noninfected (group C), or orally infected with P. gingivalis only (group D). For oral infection, freshly harvested P. gingivalis 33277 (approximately 1010 cells/ml) was mixed with 2% carboxymethylcellulose, and individual rats were given 0.5 ml of the suspension on day 13 following immunization by gastric intubation with the aid of an intubation needle (2, 18, 19, 22). This procedure was repeated twice at 24-h intervals. Sera and saliva samples were collected from individual rats prior to the experimental period and stored at −70°C until assayed by ELISA to determine baseline antibody activity to rHag B and P. gingivalis WC antigens. Starting on day 7 after immunization, sera and saliva were collected at 1- or 2-week intervals until the termination of the experiment (70 to 80 days after immunization). Samples were assessed for specific antibody activity to rHag B and P. gingivalis WC by ELISA. The levels of serum IgM, IgG, and IgG subclasses and of salivary IgA antibody activity were determined. Selected serum samples were further analyzed by Western blot for antibody activity to rHag B and to TCA precipitates of the different P. gingivalis strains. At the end of the experimental period, all rats were sacrificed, and their spleens were removed, processed, and cultured to determine levels of antigen-induced proliferative responses and cytokine production. The mandibles from rats immunized and infected or infected only were then removed for evaluation of periodontal disease by determining the amount of bone loss by radiographic analysis. Control rat anti-P. gingivalis WC serum samples were generated by s.c. immunization of rats once with freshly harvested P. gingivalis 33277 (108) in CFA.
Sample collection.
Prior to the collection of serum and saliva, rats were anesthetized by intramuscular injection (0.05 ml/100 g of body weight) of a solution of ketamine (100 mg/ml; Parke-Davis, Morris Plains, N.J.) and xylazine (Tranquived; 1.5 mg/ml; VedCo, St. Joseph, Mo.). Saliva (∼1 ml) was collected over a 20-min interval with a Pasteur pipette after intraperitoneal injection of carbachol (5 μg in 0.05 ml; Sigma Chemical Co., St. Louis, Mo.) and was clarified by centrifugation (13,000 × g, 10 min, 4°C). Blood was collected from the retro-orbital plexus throughout the experiment and by cardiac puncture at the termination of the experiment. The blood was allowed to clot at 4°C, and the serum was collected after centrifugation. All experimental samples were stored at −70°C until assayed.
Cytokines and proliferative responses.
The procedure used for the preparation of splenic lymphoid cells was similar to that previously described (8). Briefly, at the time of sacrifice, spleens were aseptically removed, and single-cell suspensions were prepared by mechanically dispersing the tissue through a sterile wire mesh into RPMI 1640 (GIBCO Laboratories, Grand Island, N.Y.). Erythrocytes were lysed with ammonium chloride buffer. The cells were washed, suspended in warm (37°C) RPMI 1640 supplemented with 5% fetal calf serum (FCS), and passed through a Sephadex G-10 (Pharmacia, Piscataway, N.J.) column equilibrated with the same warm medium. The eluted cell population was washed twice and suspended in RPMI 1640 supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 10 μg of streptomycin per ml, and 10% FCS (complete medium) for in vitro culture. Lymphoid cells were cultured in 96-well flat-bottom plates (Falcon) in quadruplicate at 4 × 105 cells per well. Cultures were incubated (37°C, 5% CO2) alone or with various concentrations of concanavalin A (ConA; 5.0, 1.0, or 0.1 μg/ml), rHag B (10.0, 5.0, 1.0, or 0.1 μg/ml), or P. gingivalis 33277 WC (8 × 105, 4 × 105, or 2 × 105 WC/ml) for 48 h (ConA) or 96 h (rHag B or WC). Cultures used to evaluate proliferative responses were pulsed with [3H]thymidine ([3H]TdR; 0.5 μCi/well; Amersham Corp., Arlington Heights, Ill.) during the last 18 h of incubation. Cells were harvested onto glass fiber filters with a MASH II cell harvester (Microbiological Associates, Walkersville, Md.), and the amount of [3H]TdR incorporation was measured in a liquid scintillation counter. The proliferative responses are expressed as a stimulation index (SI) calculated as the mean level of [3H]TdR uptake by cultures incubated with stimulant minus the mean level of [3H]TdR uptake by the respective control cultures which contain no stimulant, divided by the control [3H]TdR uptake.
For cytokine analysis, cell cultures (2 × 105/well) were prepared as described above, and the supernatants were harvested at 72 h (ConA) or 96 h (rHag B). Immediately after harvesting, the culture supernatants (CS) were stored at −70°C until assayed. Cytoscreen immunoassay kits (BioSource International, Camarillo, Calif.) were used to determine the concentration of gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-10 in the CS. Appropriate dilutions of CS were placed into 96-well microtiter plates, previously coated with the appropriate anticytokine monoclonal antibody (1 to 10 μg/ml). After incubation and washing, a biotinylated antibody was added, following by streptavidin horseradish peroxidase (HRP) and substrate, according to the manufacturer’s directions. Color development was recorded at 450 nm, using a Vmax microplate reader (Molecular Devices Corp., Menlo Park, Calif.) interfaced with a Macintosh II computer (Apple Computer, Cupertino, Calif.). The final concentrations of cytokines were calculated by interpolation of the standard curves by using a four-parameter logistic algorithm. The lower limits of sensitivity of the ELISAs used were 13 pg/ml for IFN-γ, 10 pg/ml for IL-2, 15 pg/ml for IL-4, and 20 pg/ml for IL-10.
Antibody responses.
Antibody activities to rHag B and P. gingivalis WC were assessed by a previously described ELISA (16, 18, 19, 37). Briefly, individual wells of flat-bottom 96-well plates (Nalge Nunc International, Roshilde, Denmark) were coated with rHag B or with P. gingivalis 33277 WC prepared in bicarbonate-carbonate buffer (pH 9.6; 100 μl; 1 μg of rHag B per ml or 5 × 108 cells/ml) or with anti-rat α, μ, or γ heavy-chain antibody (UAB Immunochemical Core Facility) or affinity purified anti-rat IgG1, IgG2a, IgG2b, or IgG2c (The Binding Site Inc., San Diego, Calif.) in borate-buffered saline (pH 8.2). Nonspecific binding sites were blocked with PBS containing 5% FCS (Gemini Bioproducts, Calabasas, Calif.) and 0.05% Tween 20 (pH 7.4) (Fisher Scientific, Fair Lawn, N.J.) for 2 h at room temperature. From a starting dilution of serum (1:100 for IgM and IgG; 1:50 for IgG subclasses) or saliva (1:5 for IgA) prepared in PBS containing 1% FCS and 0.1% Tween 20, five twofold dilutions were added in duplicate to individual wells. Samples of serum and saliva collected from individual rats throughout the experiment were assayed for antibody activity, and all assays were repeated two or three times. The levels of total salivary IgA and of salivary IgA and serum IgM and IgG anti-Hag B or anti-P. gingivalis antibody activities were determined by using standard curves generated with a calibrated pool of Fischer rat serum with known amounts of the correspondent immunoglobulins and wells coated with anti-rat α, μ, or γ heavy-chain antibody (37) and are shown as geometric means ×/÷ standard deviation (SD). To quantitate IgG subclass antibody activities in the experimental serum samples, a calibrated rat serum (The Binding Site Inc.) with known amounts of the various IgG subclasses was used to generate standard curves. After incubation (2 h at 37°C) and washing of plates, biotin-conjugated anti-rat IgA, IgM, or IgG (UAB Immunochemical Core Facility) or HRP-conjugated anti-rat IgG subclass (The Binding Site Inc.) antibody was added to appropriate wells. After overnight incubation (4°C), plates with biotinylated anti-rat antibodies were washed and streptavidin-HRP (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was added for 30 min at room temperature. All plates were washed, peroxidase substrate [2,2’-azino-bis(3-ethyl-benzthiazoline-6-sulfonic acid)] in citrate buffer (0.3 mg/ml, pH 4.0) containing 0.003% hydrogen peroxide was added, and color development was recorded at 414 nm. The concentrations (nanograms per milliliter) of specific anti-Hag B and anti-P. gingivalis antibody activities were determined by assessing the samples simultaneously with the calibrated pool of Fischer rat sera and interpolating from a standard curve by using a four-parameter logistic algorithm.
Evaluation of periodontal bone destruction.
The amount of periodontal bone loss was assessed by radiographic analysis as previously described (18, 19). At the termination of the experimental period, periodontal bone loss was assessed in rats infected with P. gingivalis or in rats immunized s.c. (rHag B) and infected in order to determine the effect of anti-Hag B antibodies on protection against P. gingivalis infection. The radiographic method assesses vertical bone loss, which is a linear measurement from the cementoenamel junction to the crest of the alveolar bone. Each mandible, with the lingual side down, was placed on top of an intraoral film resting on a styrofoam platform. The radiation source (Trophy model SU47 portable dental unit; Trophy Radiologie, Vincennes, France) was positioned 25 cm from the film platform. A 10-mm stainless steel dimensional reference was incorporated into each radiographic image to ensure consistent magnification and to allow the digital analysis to be expressed in millimeters of alveolar bone loss. The radiographs were taken at 70 kV, 10 mA, and 0.10 s. The film was developed through an automatic film processor. The radiographs were analyzed by digital imaging methods (15). The radiographic results are expressed as the amount of vertical bone loss in millimeters.
Statistics.
The significance of differences in antibody activity, cytokine levels, and amount of bone loss between the experimental and control groups was determined by analysis of variance using StatView 4.0 software (Abacus Concepts, Berkeley, Calif.). A P value of less than 0.05 was considered statistically significant.
RESULTS
Immune response to rHag B.
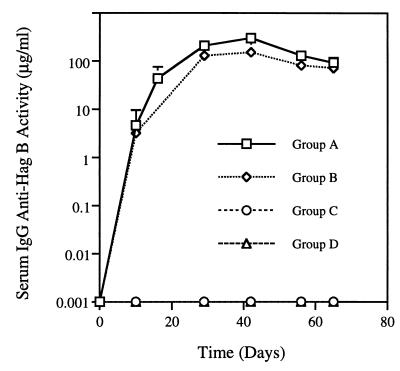
No salivary IgA antibody activity to rHag B was detected in rats immunized by the s.c. route with rHag B and/or infected with P. gingivalis. A slight serum IgM response to rHag B was noted; however, the level of activity was similar to that seen in preimmunization samples (data not shown). A serum IgG anti-Hag B response was seen in the immunized (group A) and the immunized and infected (group B) rats (Fig. 1). The kinetics of the responses were similar in both groups of rats, although rats in group B had levels of antibody activity slightly lower than, but not statistically different from, levels in the group A rats. The responses peaked on day 40 and persisted at a slightly lower magnitude until the termination of the experiment. No serum IgG anti-Hag B antibody activity was detected in the nonimmunized, noninfected (group C) or the nonimmunized, infected (group D) rats. Further analysis of the IgG response to rHag B indicated that the primary subclasses of antibodies induced were IgG1 and IgG2a (Fig. 2A) followed by IgG2b and low levels of IgG2c (Fig. 2B). The IgG1, IgG2a, and IgG2b responses appeared to peak earlier in the group B rats than in the group A rats; however, no significant difference in the IgG subclass responses between the two groups of rats was seen.
FIG. 1.
Serum IgG anti-Hag B activity in rats immunized s.c. with rHag B (group A), in rats immunized s.c. with rHag B and orally challenged with freshly harvested P. gingivalis 33277 (group B), in nonimmunized, noninfected rats (group C), and in rats infected only with P. gingivalis 33277 (group D). Values are the geometric means ×/÷ SD of antibody activity in serum samples from six rats/group. The results are from one experiment but representative of two separate experiments.
FIG. 2.
Serum IgG1 and IgG2a (A) and IgG2b and IgG2c (B) responses to rHag B in rats immunized s.c. with rHag B (group A) and in rats immunized s.c. with rHag B and orally challenged with freshly harvested P. gingivalis 33277 (group B). Values are the geometric means ×/÷ SD of antibody activity in serum samples from six rats/group. The results are from one experiment but representative of two separate experiments.
Antigen-induced proliferative responses and cytokine production.
Proliferative responses to rHag B were seen in splenic lymphoid cell cultures from rats in groups A and B (Fig. 3A). Optimal stimulation to rHag B was seen with a dose of 5 μg/ml in cultures from both groups of rats. Cell cultures derived from rats immunized and infected showed a slightly higher SI to all doses of rHag B tested than those derived from immunized-only rats, although the difference was not significant. Little or no proliferative response was seen when the cells from either group of rats was stimulated with P. gingivalis WC (Fig. 3B). A slight stimulation was seen with 8 × 105 bacterial WC in cell cultures derived from immunized and infected rats. Cell cultures from both groups of rats responded to ConA in a dose-dependent manner (Fig. 3C). The response of cells from immunized rats was higher than (but not significantly different from) that seen with cells from immunized and infected animals.
FIG. 3.
Proliferative responses to rHag B (A), P. gingivalis 33277 WC (B), or ConA (C) of splenic lymphoid cell cultures derived from rats immunized s.c. with rHag B (group A; six rats) or immunized with rHag B and infected with P. gingivalis 33277 (group B; six rats). Culture conditions were done in quadruplicate, and the results are expressed as means of the SI, determined as described in the text. The results are from one experiment but representative of two separate experiments.
Supernatants from lymphoid cells stimulated with rHag B and assayed for the presence and level of cytokines revealed significantly higher (P < 0.05) levels of IFN-γ in the CS of cells derived from immunized and infected rats than in CS from cells derived from immunized-only animals (Fig. 4A). The levels of IL-2 in the CS from the immunized and infected group were also significantly higher (P < 0.05) than those seen in the CS from immunized-only rats (Fig. 4B). IL-4 was detected in CS, but in low amounts (Fig. 4C). The levels of IL-4 in supernatants of rHag B-stimulated cell cultures from immunized rats were slightly higher than (but not significantly different from) those seen in cultures from immunized and infected animals. Finally, the levels of IL-10 detected in the CS of rHag B-stimulated cells derived from immunized and infected rats were higher than but not significantly different from those seen in cultures from the immunized only group (Fig. 4D).
FIG. 4.
Quantitation of IFN-γ (A), IL-2 (B), IL-4 (C), and IL-10 (D) in the supernatant of splenic lymphoid cultures derived from rats immunized with rHag B or immunized with rHag B and orally challenged with P. gingivalis 33277. Cultures were incubated with rHag B (10 μg/ml). Values are the means ± standard errors the means for cytokines in the supernatants of cultures derived from six rats/group. The results are from one experiment but representative of two separate experiments. *, Values are significantly different (P < 0.05).
Immune response to P. gingivalis WC.
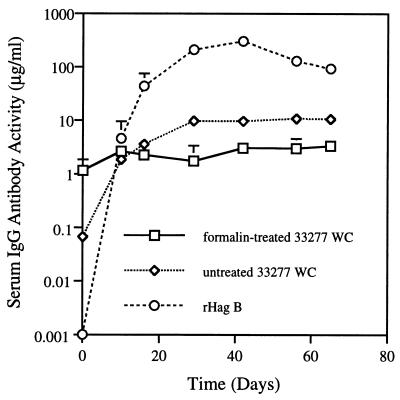
To determine if the anti-Hag B antibodies could react with P. gingivalis, which could help determine the potential protective effect of these antibodies against infection with this pathogen, serum samples from rats immunized with rHag B were tested for reactivity against P. gingivalis WC by ELISA. The reactivity to WC of serum samples from group A rats was 10- to 100-fold lower than that seen to rHag B (Fig. 5). Some antibody activity was seen in serum samples when WC suspended in PBS were used as coating antigen, whereas no increased in activity above baseline (day 0 sample) was seen with WC treated with formalin. Serum samples from rats immunized by the s.c. route with P. gingivalis WC had high levels of antibody activity to formalin-treated P. gingivalis WC and essentially no anti-Hag B antibody activity (Fig. 6). Similar results were obtained when untreated P. gingivalis WC were used as coating antigen (data not shown). Good serum IgG antibody responses to P. gingivalis WC were also seen in sera from rats in group D (data not shown).
FIG. 5.
Serum IgG responses to formalin or untreated P. gingivalis 33277 WC and to rHag B in rats immunized s.c. with rHag B. Values are the geometric means ×/÷ SD of antibody activity in serum samples from six rats/group. The results are from one experiment but representative of two separate experiments.
FIG. 6.
Serum IgG activity to formalin-treated P. gingivalis 33277 WC and to rHag B in rats immunized s.c. with freshly harvested P. gingivalis 33277 in CFA. Values are the geometric means ×/÷ SD of antibody activity in serum samples from six rats/group. The results are from one experiment but representative of two separate experiments.
Western blot analysis of antibody responses.
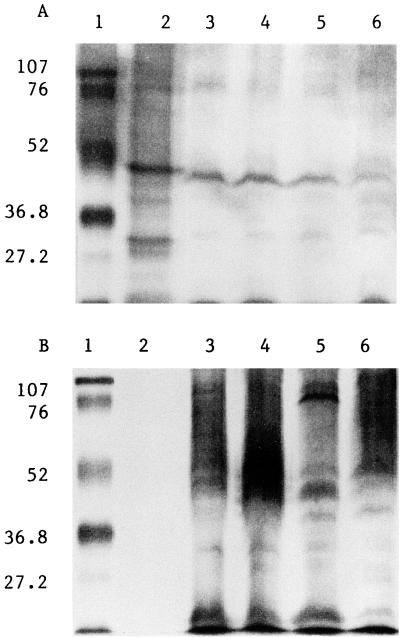
Serum from rats immunized with rHag B primarily reacted with a protein of the approximate size of 50 kDa in the rHag B preparation and with the TCA precipitates of P. gingivalis 33277, 381, A7A1-28, and W50 (Fig. 7A), indicating that Hag B is a membrane component of these P. gingivalis strains. Sera from rats immunized with P. gingivalis WC (Fig. 7B) or infected with P. gingivalis only (data not shown) did not react with rHag B but did react with TCA precipitates of P. gingivalis 33277, 381, A7A1-28, and W50.
FIG. 7.
(A) Immunoblot of serum samples from rats immunized by the s.c. route with rHag B reacted with rHag B (lane 2) and TCA precipitates of P. gingivalis 33277 (lane 3), 381 (lane 4), W50 (lane 5), and A7A1-28 (lane 6). Lane 1, prestained low-molecular-weight markers (in both panels, positions are indicated in kilodaltons on the left). (B) Immunoblot of serum samples from rats immunized by the s.c. route with P. gingivalis 33277 reacted with rHag B (lane 2) and with TCA precipitates of P. gingivalis 33277 (lane 3), 381 (lane 4), W50 (lane 5) and A7A1-28 (lane 6). Lane 1, prestained low-molecular-weight markers.
Assessment of experimental periodontal bone loss.
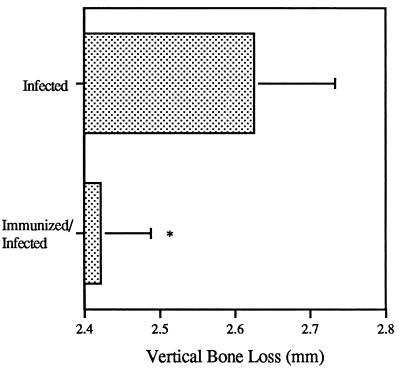
At the end of the experiment (days 70 to 80), the mandibles from individual rats were removed and the amount of bone loss was quantified by the radiographic technique (Fig. 8). Bone loss in rats infected with P. gingivalis was significantly greater (P < 0.05) than that in the animals which were immunized with rHag B and then infected with P. gingivalis.
FIG. 8.
Periodontal bone loss on mandibular molars of rats immunized with rHag B and infected with P. gingivalis 33277 and of rats infected with P. gingivalis 33277 only. Values are the mean total amount of vertical bone loss ± SD as determined by radiographic analyses. Data were collected from mandibles removed from six rats/group between experimental days 70 and 80. The results are from one experiment but representative of two separate experiments. ∗, Values are significantly different (P < 0.05).
DISCUSSION
The purpose of this study was to characterize the immune response induced following systemic immunization with purified rHag B and to determine the effectiveness of the response in protection against periodontal bone loss in an experimental rat model. When serum samples from rats immunized by s.c. administration of rHag B in CFA were assessed for antibody activity, a low level of IgM anti-Hag B activity was detected. The level of activity was similar to that seen in preimmune serum samples and in serum samples from nonimmunized, noninfected animals, suggesting the presence of Hag B determinants which have cross-reactivity with components of the indigenous microorganisms in the rat. This finding was similar to our previous observations on IgM anti-P. gingivalis activity in the rat (19) and to what has been observed in periodontally involved and control individuals (5). In contrast, a good serum IgG anti-Hag B response was seen in rats immunized with the purified protein, demonstrating the immunogenicity of this molecule in the rat model. These results extend the findings of Dusek et al. (6, 7) and Kohler et al. (23), who reported the induction of a systemic and mucosal response to this protein in mice following oral administration of a Salmonella typhimurium strain expressing the cloned Hag B.
Analysis of the IgG subclass responses to rHag B in immunized or immunized and infected rats indicated that IgG1 and IgG2a were the primary IgG subclasses of antibodies induced, followed by IgG2b and a very low level of IgG2c. In the murine system, Th2-like cells produce IL-4, IL-5, and IL-10 and support an IgG1 subclass response, whereas Th1-like cells produce IFN-γ and IL-2 and are involved in isotype switching to IgG2a (9, 27, 41). The correlate of murine IgG1 in the rat is IgG1 and IgG2a, while murine IgG2a is homologous to rat IgG2b (1). Therefore, the IgG subclass response pattern observed in both groups of experimental animals would suggest a mixed Th1 and Th2 response to systemically administered rHag B. The production of IFN-γ, IL-2, IL-10, and some IL-4 by antigen-stimulated splenic lymphoid cells from these rats supports the above observation. At the time of cytokine assessment, cells from immunized and infected rats produced significantly higher levels of IL-2 and IFN-γ than cells from immunized-only rats. This finding suggests that infection with P. gingivalis of immunized rats promoted the Th1-like response pattern. The apparent earlier peak in the IgG2b response in the immunized and infected rats compared to the immunized-only group supports this possibility. Furthermore, previous studies in our laboratory have shown that immunization with P. gingivalis WC induced predominantly IgG2b antibodies, therefore indicating the involvement of Th1-like cells (18). It is unclear why only low levels of IL-4 were detected in the immunized animals since they had high levels of IgG1 and IgG2a anti-Hag B antibodies reflective of a Th2-like response pattern. Others have also reported the induction of a dominant IgG1 antibody response in mice following immunization with an antigen and adjuvant, as well as high IFN-γ and low IL-4 production in vitro (46). It is possible that in our study the cytokine profile induced by rHag B in the splenic lymphoid cell cultures would have revealed a higher level of IL-4 if cells were assessed shortly, instead of 70 to 80 days, after immunization. It is also possible that the amount of antigen induced IL-4 was sufficient to promote a Th2-like response. Nevertheless, the finding of higher levels of IL-4 in the immunized-only group than in the immunized and infected rats supports the notion of a shift toward a Th1-like response upon P. gingivalis infection.
Kohler et al. (23), who analyzed the serum IgG subclass distribution in mice following oral administration of a Salmonella strain expressing the cloned Hag B, reported a predominant systemic IgG2a anti-Hag B response. This predominant Th1-like response pattern could have been influenced by the Salmonella vector, as suggested by other investigators (32, 47) who reported systemic Th1-like responses to cloned antigens expressed by S. typhimurium. Conversely, our laboratory reported a mixed Th1- and Th2-like response pattern to a cloned antigen of Streptococcus mutans when expressed by S. typhimurium (11). In this latter study, responses to the purified S. mutans antigen were primarily Th2-like. This finding suggests that the property of the cloned antigen can influence the host immune response and that the nature of the response is not entirely determined by the Salmonella vector. This possibility gained support from our findings which revealed a mixed Th1- and Th2-like response to purified rHag B even when the antigen was administered in CFA, a condition which would favor a Th1-like response. Further studies will be required to define properties of cloned antigens and immunization regimens important in influencing the nature of the specific host responses.
To determine the relevance of anti-Hag B antibodies in protection against P. gingivalis pathogenesis, we next examined the ability of these antibodies to react with the bacteria. The amount of reactivity to freshly harvested P. gingivalis was more than 10-fold lower than that seen to rHag B. This finding suggests that Hag B antigenic epitopes are minimally expressed on the surface of P. gingivalis cells for induction of immune responses or that differences may exist in the antigenicity of native and rHag B. The former possibility gains support from the finding that serum from rats immunized s.c. with freshly harvested P. gingivalis contained a high level of antibodies reactive with WC but essentially no reactivity to Hag B. These serum samples, as well as those from rats infected with P. gingivalis, also reacted with TCA precipitates of the various P. gingivalis strains, exhibiting a number of bands as has been previously shown (3, 20). Western blot analysis of serum samples from rats immunized with rHag B revealed an antigen reactive band at ∼50 kDa in TCA precipitates of P. gingivalis 33277, 381, W50, and A7A1-28. The band was similar to that seen with purified rHag B, as has been previously reported by Progulske-Fox et al. (35) and Lepine and Progulske-Fox (24), indicating that Hag B is a membrane component of these P. gingivalis strains. Previous studies have shown that purified Hag B inhibits hemagglutination of sheep erythrocytes by WC of P. gingivalis 381 (7), implicating it as an adhesin. The results, taken together, suggest that Hag B is not a dominant surface component although it is sufficiently expressed to mediate hemagglutination and therefore a relevant antigen for vaccine development.
It was of interest to see no increase above baseline in the level of antibody activity to formalin-treated P. gingivalis WC in serum samples from rats immunized with rHag B, suggesting that formalin treatment altered surface components. Formalin treatment has been used to inactivate bacterial toxins or to stabilize bacterial proteins for vaccine preparations (4, 36). However, it has also been suggested that formalin treatment can affect epitopes and influence the nature of the immune response induced. In this regard, Di Tommaso et al. (4) showed that some epitopes of formalin-treated proteins of Bordetella pertussis were presented poorly or not at all to T cells and ascribed this effect to a difference in the ability of the formalin-treated compared to the native proteins to undergo proteolytic processing.
Lastly, radiographic assessment of bone loss was done in rats infected with P. gingivalis or immunized s.c. with rHag B and infected to determine the effect of anti-rHag B antibodies in protection against P. gingivalis infection. Animals orally infected with P. gingivalis had significantly more bone loss than rats that had been immunized with rHag B and then infected. Therefore, these findings suggest that systemic antibodies to rHag B were effective in protecting against the periodontal pathogen P. gingivalis. In a previous study (18), evidence was provided suggesting a role for a systemic Th1-like response and for a salivary IgA response to P. gingivalis WC in protection against experimental periodontal bone loss. In the present study, a salivary IgA anti-Hag B response was not detected, an expected finding since the rats were immunized by a systemic and not a mucosal route. Further studies will be required to establish the contribution of salivary IgA anti-Hag B responses in protection against periodontal disease.
In summary, we have demonstrated that systemic immunization of rats with purified rHag B in CFA results in the induction of a systemic but not a mucosal salivary immune response. A mixed Th1- and Th2-like systemic response was induced, as reflected by the serum IgG subclass distribution of the anti-Hag B response and by antigen-specific proliferative responses and antigen-induced cytokine production by splenic lymphoid cell cultures from immunized animals. Finally, rats immunized with rHag B were protected against experimental periodontal bone loss following infection with P. gingivalis. These results support the potential use of rHag B as a candidate antigen for the development of a vaccine against periodontal disease.
ACKNOWLEDGMENTS
We thank Cecily Harmon for expertise in animal care and radiographic measurements.
This study was supported by Public Health Service grants DE 10607, DE 08228, and DE 08182 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Bazin H, Rousseaux J, Rousseaux-Prevost R, Platteau B, Querinjean P, Malache J M, Delaunay T. Rat immunoglobulins. In: Bazin H, editor. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Fla: CRC Press Inc.; 1990. pp. 5–42. [Google Scholar]
- 2.Chang K M, Ramamurthy N S, McNamara T F, Genco R J, Golub L M. Infection with a gram-negative organism stimulates gingival collagenase production in non-diabetic and diabetic germfree rats. J Periodontal Res. 1988;23:239–244. doi: 10.1111/j.1600-0765.1988.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 3.Curtis M A, Slaney J M, Carman R J, Johnson N W. Identification of the major surface protein antigens of Porphyromonas gingivalis using IgG antibody reactivity of periodontal case-control serum. Oral Microbiol Immunol. 1991;6:321–326. doi: 10.1111/j.1399-302x.1991.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Tommaso A, De Magistris M T, Bugnoli M, Marsili I, Rappuoli I, Abrignani S. Formaldehyde treatment of proteins can constrain a presentation to T cells by limiting antigen processing. Infect Immun. 1994;62:1830–1834. doi: 10.1128/iai.62.5.1830-1834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doty S L, Lopatin D E, Syed S A, Smith F N. Humoral immune response to oral microorganisms in periodontitis. Infect Immun. 1982;37:499–505. doi: 10.1128/iai.37.2.499-505.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dusek D, Progulske-Fox A, Brown T. Systemic and mucosal immune responses in mice orally immunized with avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusek D, Progulske-Fox A, Whitlock J, Brown A. Isolation and characterization of a cloned Porphyromonas gingivalis hemagglutinin from an avirulent strain of Salmonella typhimurium. Infect Immun. 1993;61:940–946. doi: 10.1128/iai.61.3.940-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldridge J H, Kimura S, Morisaki I, Michalek S M, Hamaoka T, Hamada S, McGhee J R. Immunoregulation in the rat: cellular and molecular requirements for B cell responses to types 1, 2, and T-dependent antigens. J Immunol. 1985;134:2236–2246. [PubMed] [Google Scholar]
- 9.Golding B. Cytokine regulation of humoral immune responses. In: Spriggs D, Koff W, editors. Topics in vaccine adjuvant research. Boca Raton, Fla: CRC Press Inc.; 1991. pp. 25–37. [Google Scholar]
- 10.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hechemy K E, Samsonoff W A, Harris H L, McKee M. Adherence and entry of Borrelia burgdorferi in Vero cells. J Med Microbiol. 1992;36:229–238. doi: 10.1099/00222615-36-4-229. [DOI] [PubMed] [Google Scholar]
- 13.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 14.Inoshita E, Amano A, Hanioka T, Tamagawa H, Shizukuishi S, Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis. Infect Immun. 1986;57:421–427. doi: 10.1128/iai.52.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffcoat M K, Reddy M S, Webber R L, Williams R C, Ruttimann U E. Extraoral control of geometry for digital subtraction radiography. J Periodontal Res. 1987;22:396–402. doi: 10.1111/j.1600-0765.1987.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 16.Katz J, Leary R M, Ward D C, Harmon C C, Michalek S M. Humoral response to Porphyromonas (Bacteroides) gingivalis in rats: time course and T-cell dependence. Infect Immun. 1992;60:3579–3585. doi: 10.1128/iai.60.9.3579-3585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz J, Michalek S M. A method for generating antigen-specific rat T helper cell clones. J Immunol Methods. 1991;138:77–86. doi: 10.1016/0022-1759(91)90066-o. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Michalek S M. Effect of immune T cells derived from mucosal or systemic tissue on host responses to Porphyromonas gingivalis. Oral Microbiol Immunol. 1998;13:73–80. doi: 10.1111/j.1399-302x.1998.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 19.Katz J, Ward D C, Michalek S M. Effect of host responses on the pathogenicity of strains of Porphyromonas gingivalis. Oral Microbiol Immunol. 1996;5:309–318. doi: 10.1111/j.1399-302x.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Kennell W, Holt S C. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol Immunol. 1990;5:121–130. doi: 10.1111/j.1399-302x.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 21.Kinder S A, Kornman K S, Holt S C. Characterization of selected gram-negative oral microorganisms by SDS-PAGE. Oral Microbiol Immunol. 1989;4:52–56. doi: 10.1111/j.1399-302x.1989.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 22.Klausen B, Evans R T, Sfintescu C. Two complementary methods of assessing periodontal bone level in rats. Scand J Dent Res. 1989;97:494–499. doi: 10.1111/j.1600-0722.1989.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 23.Kohler J J, Pathangey L B, Brown T A. Oral immunization with recombinant Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin: effect of boosting on mucosal, systemic and immunoglobulin G subclass response. Oral Microbiol Immunol. 1998;13:81–88. doi: 10.1111/j.1399-302x.1998.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 24.Lepine G, Progulske-Fox A. Duplication and differential expression of hemagglutinin genes in Porphyromonas gingivalis. Oral Microbiol Immunol. 1996;11:65–78. doi: 10.1111/j.1399-302x.1996.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 25.Lepine G, Whitlock J A, Han N, Progulske-Fox A. Cloning and characterization of a fourth putative hemagglutinin gene from P. gingivalis, p. In Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 26.Moore W E. Microbiology of periodontal disease. J Periodontal Res. 1987;22:335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 28.Mouton C, Bouchard D, Deslauriers M, Lamonde L. Immunochemical identification and preliminary characterization of a nonfimbrial hemagglutinating adhesin of Bacteroides gingivalis. Infect Immun. 1989;57:566–573. doi: 10.1128/iai.57.2.566-573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouton C, Hammond P G, Slots J, Genco R J. Serum antibodies to Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periodontal disease. Infect Immun. 1981;31:182–92. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naito Y, Okuda K, Takazoe I. Immunoglobulin G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984;45:47–51. doi: 10.1128/iai.45.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa T, Shimauchi H, Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989;57:3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okahashi N, Yamamoto M, VanCott J L, Chatfield S N, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee J R. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuda K, Yamamoto A, Naito Y, Takazoe I, Slots J, Genco R. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986;54:659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Progulske-Fox A, Tumwasorn S, Holt S. The expression and function of a Bacteroides gingivalis hemagglutinin gene in Escherichia coli. Oral Microbiol Immunol. 1989;4:121–131. doi: 10.1111/j.1399-302x.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 35.Progulske-Fox A, Tumwasorn S, Lepine G, Whitlock J, Savett D, Ferretti J, Banas J. The cloning, expression and sequence analysis of a second Porphyromonas gingivalis gene that codes for a protein involved in hemagglutination. Oral Microbiol Immunol. 1995;10:311–318. doi: 10.1111/j.1399-302x.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 36.Rappuoli R. New and improved vaccines against diptheria and tetanus. In: Woodrow G C, Levine M M, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1990. pp. 251–268. [Google Scholar]
- 37.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral responses in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 39.Slots J, Genco R J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 40.Socransky S S, Haffajee A D. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: a critical assessment. J Periodontal Res. 1991;26:195–212. doi: 10.1111/j.1600-0765.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 41.Street N E, Mosmann T R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 42.Sundqvist G, Bloom G D, Enberg K, Johansson E. Phagocytosis of Bacteroides melaninogenicus and Bacteroides gingivalis in vitro by human neutrophils. J Periodontal Res. 1982;17:113–121. doi: 10.1111/j.1600-0765.1982.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 43.Syed S A. Characteristics of Bacteroides asaccharolyticus from dental plaques of beagle dogs. J Clin Microbiol. 1980;11:522–526. doi: 10.1128/jcm.11.5.522-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syed S A, Loesche W J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978;21:821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tew J G, Marshall D R, Burmeister J A, Ranney R R. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun. 1985;49:487–493. doi: 10.1128/iai.49.3.487-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson H S G, Davies M L, Watts M J, Mann A E, Holding F P, O’Neill T, Beech J T, Thompson S J, Leesman G D, Ulrich J T. Enhanced immunogenicity of a recombinant genital warts vaccine adjuvanted with monophosphoryl lipid A. Vaccine. 1998;16:1993–1999. doi: 10.1016/s0264-410x(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 47.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]