Abstract
Epilepsy is a common central nervous system (CNS) disorder that affects 50 million people worldwide. Patients with status epilepticus (SE) suffer from devastating comorbidities and a high incidence of mortalities. Antiepileptic drugs (AEDs) are the mainstream treatment options for the symptomatic relief of epilepsy. The incidence of refractory epilepsy and the dose-dependent neurotoxicity of AEDs such as fatigue, cognitive impairment, dizziness, attention-deficit behavior, and other side effects are the major bottlenecks in epilepsy treatment. In low- and middle-income countries (LMICs), epilepsy patients failed to adhere to the AEDs regimens and consider other options such as complementary and alternative medicines (CAMs) to relieve pain due to status epilepticus (SE). Plant-based CAMs are widely employed for the treatment of epilepsy across the globe including Ethiopia. The current review documented around 96 plant species (PS) that are often used for the treatment of epilepsy in Ethiopia. It also described the in vivo anticonvulsant activities and toxicity profiles of the antiepileptic medicinal plants (MPs). Moreover, the phytochemical constituents of MPs with profound anticonvulsant effects were also assessed. The result reiterated that a lot has to be done to show the association between herbal-based epilepsy treatment and in vivo pharmacological activities of MPs regarding their mechanism of action (MOA), toxicity profiles, and bioactive constituents so that they can advance into the clinics and serve as a treatment option for epilepsy.
Keywords: Epilepsy, Medicinal plants, Anticonvulsant activity, Antiepileptic activity, Ethiopia
Introduction
Epilepsy is a common central nervous system (CNS) disorder and the fourth-largest cause of disease burden worldwide [1]. It is mainly characterized by recurrent, unprovoked seizures, which may trigger anxiety, depression, cognitive decline, schizophrenia, autism that can deteriorate the quality of life (QOL) and increase the incidence of mortality in patients [2, 3]. An imbalance instigated by inhibition of the excitatory γ-aminobutyric acid (GABA)-mediated neurotransmission and activation of inhibitory glutamatergic neurotransmission within the brain including hippocampal, neocortical, cortico-thalamic, and basal ganglia network is often implicated in the pathogenesis of epileptic seizures (ES) [4]. Epilepsy can emanate from a genetic predisposition of the brain to generate seizures or may be caused by brain damage due to tumor, injury, stroke, infection, etc. [5] that can elicit a wide array of abnormalities resulting in seizure generation [6]. According to WHO 2019 factsheet, approximately 50 million people around the globe are suffering from unpleasant symptoms and comorbidities resulting from ES [7]. It is reported that almost 80% of epilepsy cases are found in low—and middle-income countries (LMICs) [4] due to lack of sufficient antiepileptic drugs (AEDs), high cost if any AEDs available, and undesirable outcomes of the existing AEDs [8]. In the case of Ethiopia, epilepsy is one of the 20 leading causes of mortality, and 5.2 out of 1000 people are prone to ES in their lifetime [9, 10]. In general, epilepsy has substantial economic implications, predominantly in Africa, as it triggered a great burden on the underprivileged healthcare system of poor nations [11] as well as on patients owing to the epilepsy-bound poor QOL, stigma, and discrimination in patients and relatives [12] that could ominously increase healthcare expenditure and diminish overall productivity [10].
Modulating the activity of GABAergic, glutamatergic, purinergic neurotransmissions, cholinergic pathways and ATPases is a viable option for the treatment of epilepsy [13]. Attempts have been made to exploit the aforementioned neurotransmission pathways and enzymes implicated in epileptogenesis for the design of novel chemical agents to ameliorate the neurological deficits responsible for the progression of epilepsy. Thus far, more than 30 AEDs have been approved for clinical use [14]. However, the AEDs succeeded only in the symptomatic relief of epilepsy in patients without significantly correcting the underlying biochemical aberrations involved in epileptogenesis [15]. Currently, the treatment of epilepsy has mainly relied on such AEDs which can make patients free of seizures upon proper treatments regimens. Although the existing AEDs are effective in the suppression of seizures in the vast majority of epilepsy patients, 30% of them (15% of children and 34% adults) developed resistance towards AEDs, consequently, nonresponsive towards AEDs [16, 17]. Moreover, the dose-dependent neurotoxicity of AEDs such as fatigue, cognitive impairment, dizziness, attention-deficit behavior, and other side effects are the major bottlenecks in epilepsy treatment [8]. Patients with refractory ES are at increased risk of mortality and morbidity. Adjuvant therapies and AEDs along with ketogenic diet supplements are employed for the treatment of refractory ES [17]. Patients with untreated and/or refractory epilepsy are often desperate to seek nonconventional treatments including but not limited to complementary and alternative medicines (CAMs) [18]. The unaffordable price of newer AEDs and the wider treatment gaps have inspired researchers to focus on plants in the search for safe and effective drugs for the treatment of ES.
Current trends in the treatment of epileptic seizures
AEDs are pretty effective in the treatment of epilepsy if patients properly comply with treatment regimens. However, they are overpriced and seldom possess devastating and inevitable side effects resulting in poor patient compliance [19]. Treatment compliance or adherence is a major factor that can dictate the outcomes of AEDs in controlling the incidence of seizure attacks [20]. There is ample evidence suggesting the presence of a huge treatment gap among epilepsy patients in LMICs ranging from 25 to 100% [21]. In Africa, epilepsy is associated with fear, misunderstanding, witchcraft, discrimination and social stigmatization of patients and their families that can be considered as a driving force for the observed huge treatment gaps due to failure in several intervention mechanisms employed and persistent antiepileptic medications non-adherences (AEMNAs) [22]. Epileptic patients experiencing AEMNAs are more prone to have suboptimal treatment outcomes, recurrent seizure attacks, intermittent hospital admissions, increased healthcare expenditure, lowered level of productivity, and thereby deteriorated QOL [23]. For instance, in Ethiopia, the prevalence of AEMNAs was found to be in the range of 21.8–68%. Poor healthcare system and medical services, lack of medication access, economic constraints, antiepileptic medication side effects, and poor seizure control status are among the factors which significantly contributed to the high burden of AEMNAs in Ethiopia [24]. Moreover, the association of epilepsy with spiritual and predestined fate as well as the presence of different cultural and spiritual beliefs with potential impacts to enforce people to prefer CAMs for the treatment of “spiritual disease” such as epilepsy [25] have significantly contributed to the high incidence of AEMNAs in different parts of Ethiopia. Overall, AEMNAs resulted in treatment failure which in turn triggered devastating social consequences, life-threatening comorbidities, employment restriction, physical injuries, and increased mortality [23]. For instance, in sub-Saharan Africa, untreated ES are the common causes of death with status epilepticus (SE), drowning, falls, burns, and sudden death contributing to epilepsy-associated mortality [26]. A study conducted on 119 patients in Ethiopia revealed that about 58% of epileptic patients who acquired generalized tonic–clonic seizures (GTCS) at a baseline evaluation with a frequency of ≤ 8 times, 23.3% of them died [27]. Another study revealed that among 316 persons with epilepsy, 20 (6.3%) died within 2 year period mostly due to SE and burn [28]. Accordingly, improving the patient compliance towards the existing AEDs through novel intervention approaches and bringing CAMs, especially antiepileptic herbal formulation, into modern pharmacy shelves is an option in the long term to tackle seizure-related morbidity and mortality.
Importance of complementary and alternative medicine in Ethiopia
According to National Center for Complementary and Alternative Medicines (NCAM), CAMs are defined as a traditional healthcare system comprised of biological, spiritual, alternative, physical, and energy therapies [18]. A biological form of CAM that depends on natural products is commonly sought for the treatment of different diseases worldwide [29]. It uses medicinal herbs, medicinal animals, dietary supplements, antioxidants, minerals, vitamins, etc. alone or in combination to diagnose, prevent and treat different ailments [30]. Traditional medicines (TMs) of plant origin have become an integral part of the healthcare system of developed and developing countries [31] where 60% of the population entirely depend on them to relieve different types of ailments. Medicinal plants (MPs) have played a vital role in the treatment of human and livestock ailments since immemorial [32] partly due to the presence of bioactive secondary metabolites. Africa is the home of massive biodiversity rich in different types of animals and PS. The continent is likely to have approximately 45,000 PS of which 5000 species have medicinal importance [33]. Ethiopia is among the most diverse country located in East Africa containing approximately 6500–7000 PS (12% of them are endemic) in its flora [34]. It is also endowed with several languages, diverse cultures, and beliefs which are the driving force for the existence of traditional medical system plurality in the country [35]. Ethiopians have been using MPs and medicinal animals for the prevention, diagnosis, and treatment of different ailments since immemorial [36–40]. The healthcare demand of 80% of the people and 90% livestock in the country largely hinged on different PS [35]. Nearly 800 MPs are constantly employed to treat around 300 physical and mental diseases in the traditional healthcare system of Ethiopia [41]. The economic implication of MPs is noteworthy in Ethiopia. It is estimated that approximately 56,000 tons of wild MPs were collected per annum, which can potentially inject two billion Birr into the economy [42]. Such magnitude of MPs consumption is strongly associated with the accessibility, economic affordability, and cultural acceptability of MPs in different communities of Ethiopia [43].
Data sources and search strategy
The present review describes the ethnobotany of MPs used to treat epilepsy and related symptoms in Ethiopia. It also focuses on the in vivo experimental evidence about the pharmacological efficacy of MPs in attenuating seizures in different animal models and on the type of bioactive compounds with profound anticonvulsant outcomes from the phytochemical investigation of MPs to establish a solid foundation for future research to develop plant-based antiepileptic agents. For this purpose, ethnobotanical data about the antiepileptic MPs found in Ethiopia were searched and downloaded from online research databases (PubMed, Medline, Web of Science, Google Scholar, Science Direct, and other institutional repositories) written in English using specific keywords such as “medicinal plants”, “medicinal herbs”, “ethnobotanical study”, “traditional medicine”, “traditional medication”, “plant remedies”, “herbal remedies”, “traditional healers”, “indigenous knowledge”, “folk medicine”, “traditional healers” + “Ethiopia”. Plant use reports for epilepsy and related symptoms were compiled and examined in terms of the habit of the MPs, parts used, condition of remedy preparation, route of administration, number of use citation (by Districts), target groups, etc. Based on the ethnobotanical information, a combination of keywords such as “scientific name of MPs” + “convulsions”, “anticonvulsant”, “seizure”, “antiseizure”, “epilepsy”, “antiepileptic”, “epileptic seizure”, “phytochemical investigation”, “active compounds”, “phytochemical screening”, “phytoconstituents”, “secondary metabolites”, “toxicity profiles”, etc. were used to search and collect relevant data on MPs with in vivo antiepileptic activities, toxicity profiles and to identify the phytochemicals (with already known anticonvulsant activities) present in the target MPs. The in vivo antiepileptic activities of MPs were analyzed based on the type of seizure-inducing agents, animal model, effective doses, and observed outcomes.
Results and discussion
Ethnobotany of medicinal plants used for the treatment of epilepsy
Plant distribution across families and geography
In this review, a total of 96 PS was found to have traditional healthcare prominence for the treatment of epilepsy and related symptoms in Ethiopia (Table 1). Of which 79 and 8 PS (Agrocharis melanantha, Artemisia abyssinica, Crotalaria spinose, Cucurbita pepo, Erianthemum dregei, Myrica salicifolia, Solanum incanum, and Vigna membrancea) were used to suppress ES in humans and animals, respectively. Arundinaria alpina, Azadirachta indica, Croton macrostachyus, Echinops Kebericho, Embelia schimperi, Nicotiana tabacum, Ocimum lamiifolium, Satureja abyssinica and Vernonia amygdalina were used to treat both human and livestock epilepsy cases. The reported MPs were distributed across 43 families and the highest occurrence belonged to Asteraceae (9, 20.93%), Fabaceae (8, 18.6%), Euphorbiaceae (7, 16.27%), Solanaceae (5, 11.63%), Lamiaceae (4, 9.3%) and Rubiacea (4, 9.3%). Apocynaceae, Celastraceae, and Rutaceae were represented by 3 (6.98%) PS each. In addition, Apiaceae, Cucurbitaceae, Verbenaceae, Malvaceae, Myrsinaceae, Myrtaceae, Oleaceae, Polygonaceae and Vitaceae families possessed 2 (4.65%) PS each. Other 26 families possessed a single PS effective against epilepsy in Ethiopia. Asteraceae, Fabaceae, Euphorbiaceae, and Solanaceae are the dominant families commonly found in the Ethiopian and Eritrean flora [44]. Thus, the mere presence of such PS in a relatively higher number in the antiepileptic MPs list is not a surprise. Overall, the data showed the cultural significance and medicinal importance of Asteraceae, Fabaceae, Euphorbiaceae, and Solanaceae families in the management of ES in Ethiopia. The dominance of Asteraceae, Fabaceae, Euphorbiaceae, and Solanaceae families were also reported in several ethnobotanical surveys conducted to document the MPs and associated indigenous knowledge used to treat different ailments in Ethiopia [45, 46].
Table 1.
Ethnobotanical data of MPs used to treat epilepsy and related symptoms in Ethiopia
| No. | Scientific name | Family | GF | PU | CP | ROA | TGs | Study areas | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Acacia seyal Delile | Fabaceae | T | B | D | N | Hu | Amaro District, SNNPR | [62] |
| 2 | Acalypha fruticosa Forssk | Euphorbiaceae | Sh | L | F | O | Hu | Yalo District, AfR | [63] |
| 3 | Acokanthera schimperi (A. DC.) Benth. & Hook.f. ex Schweinf | Apocynaceae | Sh | R | F/D | – | Hu | Enarso District, AR | [64] |
| 4 | Agrocharis melanantha Hochst | Apiaceae | H | R | F | N | Li | Bale Mountain National Park, OR | [65] |
| 5 | Ajuga integrifolia, Buch.-Hamn | Lamiaceae | H | L | D | O | Hu | Ghimbi District, Selale Mountain Ridges, Jimma Zone, OR | [53, 66, 67] |
| Ap | F | O | Hu | Borecha District, OR | [68] | ||||
| 6 | Ampelocissus bombycina (Baker) Planch | Vitaceae | Cl | R | F | O | Hu | Hawassa Zuria District, SNNPR | [59] |
| 7 | Artemisia abyssinica Sch. Bip. Ex A. Rich | Asteraceae | H | R | F | N | Li | Bale Mountain National Park, OR | [65] |
| 8 | Artemisia afra Jacq. Ex Willd | Asteraceae | H | L, R, SB | F | N | Hu | Bale Mountains National Park, OR | [69] |
| 9 | Arundinaria alpina K. Schum | Poaceae | T | L, Bu | F | O | Hu/Li | Dawuro Zone, SNNPR | [70] |
| 10 | Asparagus africanus Lam | Asparagaceae | Sh | L, R, SB | F/D | N | Hu | Ankober & Enarj Enawga Districts, AR | [71] |
| 11 | Asplenium aethiopicum (Kunth) mett | Aspleniaceae | H | L, R | F | N | Hu | Ankober District, AR | [71] |
| 12 | Azadirachta indica A. Juss | Meliaceae | T | L | F | O | Hu/Li | Adwa District, TR | [72] |
| 13 | Balanites aegyptica (L.) Del | Balantiaceae | T | R | – | N | Hu | Chifra District, AfR | [73] |
| 14 | Biophytum umbraculum Welw | Oxalidaceae | H | R | F | O | Hu | Dawuro Zone, SNNPR | [60] |
| 15 | Brachiaria brizontha (A. Rich.) Stapf | Poaceae | H | R | F | O | Hu | Dawuro Zone, SNNPR | [60] |
| 16 | Brucea antidysenterica J.F.Mill | Simaroubaceae | Sh | L | F | D | Hu | Adwa District, TR | [74] |
| 17 | Breonadia salicina (Vahl) Hepper & Wood | Rubiaceae | T | S | F/D | O | Hu | Berta Ethnic Group, BGR | [75] |
| 18 | Buddleja polystachya | Luganiaceae | T | L, R, B | D | O, N | Hu | Dawuro Zone, SNNPR | [60, 76] |
| 19 | Calpurnia aurea (Ait.) Benth | Fabaceae | Sh | R | F/D | O | Hu | Berta Ethnic Group, BGR | [75] |
| 20 | Capparis tomentosa Lam | Capparidaceae | Cl | R | D | N | Hu | Enarj Enawga District, AR; Asgede Tsimbila District, TR | [77, 78] |
| 21 | Carissa edulis (Forssk). Vahl | Apocynaceae | Sh | R | – | – | Hu | Asgede Tsimbila District, TR | [78] |
| 22 | Caucanthus auriculatus Forssk | Malpighiaceae | Cl | L | F | O | Hu | Gurage, Mareqo, Qebena, & Silti, SNNPR | [79] |
| 23 | Caylusea abyssinica (Fresen.) Fisch. & C.A.Mey | Resedaceae | H | L, R | F | O | Hu | Hamar District, SNNPR | [80] |
| 24 | Chenopodium ambrosioides L | Chenopodiaceae | H | L | F | O, N | Hu | Dawuro Zone, SNNPR | [70] |
| 25 | Cissus petiolata Hook. f | Vitaceae | Cl | S | – | D | Hu | Tahtay Koraro, Medebay Zana & Asgede Tsimbla, TR | [81] |
| 26 | Celosia polystachia (Forssk.) C.C. Towns | Amaranthaceae | H | L | F | O | Hu | Yalo District, AfR | [63] |
| 27 | Clerodendrum myricoides (Hochst.) R.Br. Ex Vatke | Verbenaceae | Sh | L | F | D | Hu | Bale Mountains National Park, OR; Asgede Tsimbila District, TR | [69, 78] |
| 28 | Clutia abyssinica Jaub | Euphorbiaceae | Sh | L | F | D | Hu | Aseko District, OR | [82] |
| 29 | Crotalaria spinosa Hochst. ex Benth | Fabaceae | H | L | F | O | Li | Mana Angetu District, OR | [83] |
| 30 | Croton macrostachyus Del | Euphorbiaceae | T | SB | F/D | O | Hu | Mana Angetu District, OR | [83] |
| L | – | – | Hu | Asgede Tsimbila District, TR | [78] | ||||
| L | F/D | O | Li | Mana Angetu District, OR | [83] | ||||
| 31 | Cucumis ficifolius A. Rich | Solanaceae | H | R, L | F | O | Hu | Asendabo District, OR | [84] |
| 32 | Cucurbita pepo L | Cucurbitaceae | Cl | L | F | O | Li | Mana Angetu District & Jimma Zone, OR | [67, 83] |
| 33 | Desmodium repandum (Vahl) DC | Fabaceae | H | R | F/D | N | Hu | Ankober District, AR | [71] |
| 34 | Dicrocephula integrifolia (L. f.) Kuntaze | Asteraceae | H | L | F | N, D | Hu | Dawuro Zone, SNNPR | [60, 76] |
| 35 | Dregea schimperi (Decne.) Bullock | Apocynaceae | Cl | L | F | O | Hu | Gurage, Mareqo, Qebena & Silti, SNNPR | [79] |
| 36 | Echinops Kebericho Mesfin | Asteraceae | H | R | F | N | Hu | Kembatta Tembaro Zone, SNNPR | [85] |
| R | D | N | Li | Baso Liben & Debre Elias Districts, AR | [86] | ||||
| R, RB | F | O, N | Hu/Li | Dawuro Zone, SNNPR | [70] | ||||
| 37 | Embelia schimperi Vatke | Myrsinaceae | T | Fr | F | O | Hu | Debark Woreda, AR | [87] |
| R | D | O | Li | Baso Liben & Debre Elias Districts, AR | [86] | ||||
| 38 | Erianthemum dregei (Eckl and Zeyh.) V. Tiegh | Loranthaceae | T | L, S, R | F/D | O | Li | Mana Angetu District, OR | [83] |
| 39 | Eucalyptus globulus Labull | Myrtaceae | T | L, Se | F/D | O, N | Hu | Kembatta Tembaro Zone, SNNPR | [85] |
| 40 | Euphorbia tirucalli L | Euphorbiaceae | Sh | R | F/D | O | Hu | Amaro District, SNNPR | [62] |
| 41 | Fagaropsis angolensis (Engl.) Milne-Redh | Rutaceae | T | Se, L | F | O | Hu | Kochere District, SNNPR | [88] |
| 42 | Ficus vasta Forssk | Moraceae | T | B | D | N, D | Hu | Dega Damot District, AR | [52] |
| 43 | Galinirea coffeoides | Rubiaceae | Sh | L, R | F | O | Hu | Dawuro Zone, SNNPR | [60, 76] |
| 44 | Gloriosa superba L | Colchicaceae | Sh | L | F | O | Hu | Harla & Dengego valleys, DDAC | [89] |
| R | F/D | O | Hu | Mana Angetu District, OR | [83] | ||||
| 45 | Guizotia scabra (Vis) Chiov | Compositae | H | R | D | O | Hu | Ada'a District, OR | [90] |
| 46 | Hagenia abyssinica (Bruce) J.F. Gmel | Rosaceae | T | Fl | – | – | Hu | Bale Rural Communities, OR | [91] |
| 47 | Hypericum quartinianum A. Rich | Hypericaceae | Sh | L | D | D | Hu | Around Fiche District, OR | [92] |
| 48 | Indigofera articulata Gouan | Fabaceae | Sh | L, R | F | O | Hu | Yalo District, AfR | [63] |
| 49 | Indigofera coerulea Roxb | Fabaceae | Sh | R | F | O | Hu | Jeldesa Cluster, DDAC | [93] |
| 50 | Inula confertiflora A. Rich | Asteraceae | Sh | L | F | N | Hu | Enarj Enawga District, AR | [77] |
| 51 | Jatropha curcas L | Euphorbiaceae | Sh | Se | F | O | Hu | Gurage, Mareqo, Qebena & Silti, SNNPR | [79] |
| 52 | Jasminum abyssinicum Hochst. Ex DC | Oleaceae | Cl | L | F | N | Hu | Kembatta Tembaro Zone, SNNPR | [85] |
| 53 | Justitia schimperiana Hochst. ex Nees | Acanthaceae | Sh | L | F | O, D | Hu | Dawuro Zone, SNNPR | [70] |
| 54 | Lagenarin abyssinica (Hoof. f) C. Jeffrey | Cucurbitaceae | H | L | F | N | Hu | Asendabo District, OR | [84] |
| 55 | Laggera crispata (Vahl) Hepper & Wood | Asteraceae | Sh | R | F | O | Hu | Yilmana Densa & Quarit Districts, AR | [42] |
| 56 | Lobelia gibberoa Hemsl | Lobeliaceae | T | Se | D | O | Hu | Gubalafto District, AR | [61] |
| 57 | Maytenus gracilipes (Welw.ex Oliv) Exell | Celastraceae | Sh | L | D | O | Hu | Bale Mountains National Park, OR | [69] |
| 58 | Maytenus heterophylla (Eckl. & Zeyh.) Robson | Celastraceae | Sh | L | F | O | Hu | Gurage, Mareqo, Qebena & Silti, SNNPR | [79] |
| 59 | Maytenus senegalensis (Lam.) Excell | Celastraceae | Sh | Se | F/D | O | Hu | Wonago District, SNNPR | [45] |
| 60 | Myrica salicifolia Hochst. ex A. Rich | Myrsinaceae | T | B | D | N | Li | Hulet Eju Enese District, AR | [35] |
| 61 | Nicotiana tabacum L | Solanaceae | Sh | R | D | O, N | Hu | Mana Angetu District, OR; Ankober District, AR | [71, 83] |
| L | F | D, N | Hu | Fadis & Dugda Districts, OR; Ankober District, AR | [71, 94, 95] | ||||
| L | F | O | Li | Mana Angetu District, OR | [83] | ||||
| 62 | Ocimum canum Sims | Lamiaceae | H | L | F | N | Hu | Dawuro Zone, SNNPR | [70] |
| 63 | Ocimum lamiifolium Hochst,ex Benth | Lamiaceae | H | L | F | O, N, D | Hu/Li | Dawuro Zone, SNNPR | [70] |
| 64 | Olea europaea L | Oleaceae | T | L | D | N | Hu | Hulet Eju Enese District, AR | [35] |
| 65 | Olinia rochetiana A. Juss | Oliniaceae | T | R | F/D | N | Hu | Ankober District, AR | [71] |
| 66 | Opuntia ficus-indica (L.) Miller | Cactaceae | H | L | F | D | Hu | Debark District, AR | [87] |
| 67 | Pavetta abyssinica Fresen | Rubiaceae | Sh | Bu, Se | F | N | Hu | Kembatta Tembaro Zone, SNNPR | [85] |
| 68 | Pentas schimperiana (A. Rich) Vatke | Rubiaceae | Sh | RB | F/D | O | Hu | Wonago District, SNNPR | [45] |
| 69 | Plectranthus edulis Vatke | Lamiaceae | H | L, R | - | O | Hu | Abay Chomen District, OR | [96] |
| 70 | Pterolobium stellatum Forsk. Brenan | Fabaceae | Sh | R | F/D | N | Hu | Hulet Eju Enese District, AR | [35] |
| Wh | F | O | Hu | Bahir Dar Zuria District, AR | [97] | ||||
| L, R | F | N | Hu | Hamar District, SNNPR | [80] | ||||
| 71 | Rhamnus staddo A. Rich | Rhamnaceae | Sh | L | F | N | Hu | Enarj Enawga District, AR | [77] |
| 72 | Rhus vulgaris Meikle | Anacardiaceae | Sh | L | F | O, N, D | Hu | Dawuro Zone, SNNPR | [70] |
| 73 | Rumex nepajensis Spreng | Polygonaceae | Sh | R | F | N | Hu | Borecha District, OR | [68] |
| 74 | Ruta chalepensis L | Rutaceae | Sh | L, Se | F | N | Hu | Hulet Eju Enese District, AR | [35] |
| 75 | Satureja abyssinica (Benth.) Briq | Lamiaceae | H | L | F | N | Hu/Li | Dawro Zone, SNNPR | [60, 76] |
| 76 | Securidaca longepedunculata Fres | Polygonaceae | T | R | D | N | Hu | Enemay District, AR | [39] |
| 77 | Solanum incanum L | Solanaceae | Sh | R | F | O | Li | Mana Angetu District, OR | [83] |
| 78 | Sida rhombifolia L | Malvaceae | H | R | – | N | Hu | Tahtay Koraro, Medebay Zana & Asgede Tsimbla, TR | [81] |
| 79 | Sida schimperiana Hochst. Ex A.Rich | Malvaceae | Sh | – | F | O | Hu | Wonago District, SNNPR | [45] |
| 80 | Syzygium guineense (Willd.) DC | Myrtaceae | T | S | D | O, N | Hu | Berta Ethnic Group, BGR | [75] |
| 81 | Tragia cinerea (Pax) Gilbert and Radcl.-Smith | Euphorbiaceae | Cl | R | D | O | Hu | Menz Gera-Midir District, AR | [98] |
| 82 | Tynura pseudochina L | Compositae | Sh | L | F | O | Hu | Borecha District, OR | [68] |
| 83 | Urera hypselodendron (Hochst.) ex A. Rich | Urticaceae | Cl | R | D | O | Hu | Hulet Eju Enese District, AR | [35] |
| 84 | Vangueria volkensii K.Schum | Rubiaceae | Sh | L, R | F | O | Hu | Hamar District, SNNPR | [80] |
| 85 | Verbena bonariensis | Verbenaceae | H | L | D | N | Hu | Mojana District, AR | [99] |
| 86 | Vernonia amygdalina Del | Asteraceae | Sh | L, B | F | O, D | Hu/Li | Dawuro Zone, SNNPR | [70] |
| 87 | Vigna membrancea (L.) A. Rich | Fabaceae | Cl | L, R | F/D | O | Li | Abay Chomen & Kersa Districts, OR | [55, 96] |
| 88 | Withania somnifera (L.) Dun | Solanaceae | Sh | R | F/D | O | Hu | Mana Angetu District, OR | [83] |
| 89 | Xanthium stramonium L | Solanaceae | H | L | F | D | Hu | Fadis District, OR | [95] |
| 90 | Zingiber officinale Roscoe | Zingiberaceae | H | R | F | O | Hu | Amaro District, SNNPR | [62] |
GF growth forms, T Tree, Sh shrub, H herb and Cl climber, Plant PU parts used, L leaf (), S stem, SB stembark, R root, RB rootbark, Bd buds, Ap apex, Se seed, Wh whole plant, Ar aerial part (), Bu bulbs, Lx latex, Fr fruit, Fl flower and Rh rhizome, CP condition of preparation, F fresh, and D dry, ROA routes of administration, O Oral, N nasal, D dermal and Au auricular, TGs target groups, Hu Human and Li livestock, Reginal states of Ethiopia: AR amhara region, AfR Afar region, BGR Benshangul-Gumuz region, DDAC dire dawa administration council, OR oromia region, TR tigray region, SNNPR southern nations, nationalities and peoples and peoples region
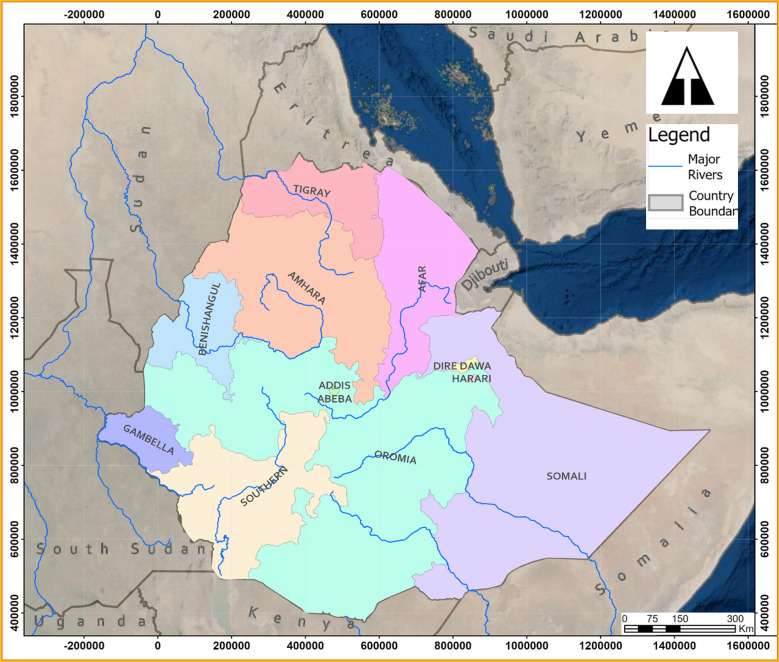
TMs, especially MPs are routinely used for the management of different diseases in the traditional healthcare system of the Regional States of Ethiopia [47–53]. Although these Regional States share some common entities, they have distinct biodiversities, agro-ecology, cultures, livelihood, values, beliefs, etc. which nurture the indigenous knowledge and traditional practices of dwellers. Hence, multifaceted treatment approaches and miscellaneous traditional remedies are prevalent in different cultural groups of Ethiopia [36, 44, 54, 55]. In line with this fact, the present literature review reiterated that the use citations of antiepileptic MPs are widely distributed across the different regional states of Ethiopia (Fig. 1): Oromia (29 PS), Amhara (25 PS), Southern Nations, Nationalities and Peoples (33 PS), Afar (4 PS), Tigray (8 PS), Benshangul-Gumuz (3 PS) and Dire Dawa Administration Council (2 PS). More than 70% of MP species prescribed for the treatment of seizure in Ethiopia belonged to the three most populous and diverse regions, namely Oromia, Amhara, and the SNNP Regional States. This may be attributed to the presence of different biodiversities, cultural pluralities, and thereby rich indigenous MPs knowledge and practice in the regions. Despite the cross-cultural connections and neighborhood manifested by the long common border between Oromia and Amhara regions as well as Oromia and SNNP regions, the consensus of THs on antiepileptic MPs was quite low, only a few MPs were commonly used across the regions.
Fig. 1.
Location map of Ethiopia. The different colored areas represent the regional states in Ethiopia where the use of plant-based medicines are reported
Parts used, condition of preparation, and mode of administration of MPs
Among the reported 95 MPs, shrubs accounted for 35 (36.46%) PS. Herbs 30 (31.25%) and trees 21 (21.88%) were the second and third most abundant growth forms of MPs. On the other hand, 10 (10.42%) MPs were climbers. The relative abundance of shrubs in Ethiopian flora and its accessibility in year-round may have contributed to higher use citation of shrubs in antiepileptic medication preparation [35]. The THs of Ethiopia preferred leaves, (66, 44. 59%) over other plant parts for the preparation of remedies. They also often used roots (52, 34.14%) and seeds (10, 6.76%) for the formulation of medicinal recipes. In addition, bulbs, stembark, rootbark, apex, rhizome, flowers, fruits, the whole plant, and aerial part of MPs were also used for the extraction of effective medicines for seizure. The presence of bioactive compounds, both in therapeutic abundance and variety, in leaves and roots may be associated with the curative effects of such recipes against epilepsy [56, 57]. Fresh organs of plants (81, 64.8%) were often employed for the preparation of antiepileptic medications in Ethiopia. Dry forms of plant parts (23, 18.4%) were also used for the preparation of remedies. Nearly 17% of plant parts were used regardless of the condition they exist (either fresh or dry). As fresh plant parts are rich in bioactive metabolites, they are frequently sought for the formulation of remedies not only for epilepsy but also for other ailments in Ethiopia. In addition, fresh plant parts are convenient to prepare medications using crushing, squeezing, maceration, infusion, decoction, etc., and can be ready for use in a short period as compared to dry plant organs [44].
Diverse approaches and strict procedures are followed by the THs for the preparation of remedies: abstraction of pharmacologically relevant crude extract or essential oils from different plant organs in Ethiopia [47, 58, 59]. Depending on the perceived knowledge of the THs, some may prefer crushing for remedy preparation while others may use tying or burning of the same plant part for the same ailment. The antiepileptic medications in Ethiopia were most commonly prepared using crushing, squeezing, maceration, pounding, grinding, decoction, etc. techniques. Water was the main extraction solvent employed in most preparations to tailor the concentration of the recipe to the supposed level of therapeutic efficacy and to avoid dose-related toxicities in patients [45]. Additives such as milk, “tella” (local beer), “teff injera” (flat bread), sugar, etc. [60–62] were used to improve the taste of the recipe and to enhance patient compliance towards the formulations. Most of the antiepileptic herbal formulations were administered through the oral route (63, 51.64%) by drinking, chewing, etc. followed by nasal (41, 33.61%) in the form of sniffing, smoking, and fumigation. Dermal route of administration (ROA) (18, 14.75%) (through fumigation and washing) was seldom employed for the delivery of antiseizure herbal medications in the Ethiopian context. Oral is described as the primary ROA in several ethnobotanical studies conducted elsewhere [48, 59] due to the fast onset of action and ease of application.
Multiple medicinal plants prescriptions for the treatment of epilepsy
Combinations of two or more PS are seldom used to formulate remedies for epilepsy and related symptoms in Ethiopia and elsewhere [100]. This is based on the fact that the consumption of multiple MPs could have potential synergistic outcomes and thereby enhanced pharmacological activities. For instance, the roots of four MPs including Guizotia scabra, Ajuga integrifolia, Foeniculum vulgare, and Withania somnifera have been used for the preparation of remedy that can be taken through the oral route in Adaꞌa District, Oromia Regional State, Ethiopia [90] that can potentially attenuate convulsions in humans (Table 2). On the other hand, leaves of Artemisia abyssinica, Brucea antidysentrica, and Cucumis ficifolius were employed for the preparation of recipes effective against epilepsy, when taken orally, around Jimma, Oromia Regional State, Ethiopia [101]. Similarly, the leaves of Nicotiana tabacum, Ocimum lamiifolium, and Withania somnifera were also used for the preparation of remedies that can be applied externally (dermal route) to relieve seizure [102]. Herbalists living around Fiche District, Oromia Regional State, Ethiopia prepare a remedy for epilepsy from leaves of Hypericum quartinianum, Podocarpus falactus, and Teclea nobilis for external application through the nasal ROA [92]. The different classes of phytochemicals such as alkaloids, flavonoids, terpenoids, etc. present in these MPs and their combined effect in enhancing the relative abundance/concentration and amplifying the pharmacological efficacy through synergism may be associated with the preparation of efficient antiepileptic recipes from multiple MPs. Ocimum lamiifolium, Nicotiana tabacum, Ruta chalepensis and Withania somnifera were most frequently sought MPs for the preparation of antiseizure medications, each become part of two different formulations [35, 90, 94, 102]. The wide application of Ocimum lamiifolium, Nicotiana tabacum, Ruta chalepensis and Withania somnifera in different formulations might be due to the presence of convulsion-suppressive bioactive compounds in such MPs. For obvious reasons, the use of formulations of multiple MPs is a common practice in the treatment of epilepsy in different parts of the world [103].
Table 2.
Ethnobotanical data of multiple MPs prescriptions used to treat epilepsy and related symptoms in Ethiopia
| No. | Scientific name | Family | GF | PU | CP | ROA | Study area | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Artemisia abyssinica Sch. Bip. Ex A. Rich | Asteraceae | H | L | F | O | Jimma Area District, OR | [101] |
| 2 | Brucea antidysentrica J.F. Mill | Simaroubaceae | Sh | L | F | |||
| 3 | Cucumis ficifolius A. Rich | Solanaceae | Cl | L | F | |||
| 1 | Embelia schimperi Vatke | Myrsinaceae | T | Fr | F | O | Debark District, AR | [87] |
| 2 | Guizotia abyssinica (L. f.) Cass | Asteraceae | H | Se | D | |||
| 1 | Fagaropsis angolensis (Engl.) Milne-Redh | Rutaceae | T | Se | D | O | Kochere District, SNNPR | [88] |
| 2 | Solanum spp. | Solanaceae | H | L | F | |||
| 1 | Guizotia scabra (Vis) Chiov | Compositae | H | R | D | O | Ada'a District, OR | [90] |
| 2 | Ajuga integrifolia, Buch.-Hamn | Lamiaceae | H | R | F/D | |||
| 3 | Foeniculum vulgare Mill | Apiaceae | H | R | F/D | |||
| 4 | Withania somnifera (L.) Dun | Solanaceae | Sh | R | F/D | |||
| 1 | Hypericum quartinianum A. Rich | Hypericaceae | Sh | L | D | D | Around Fiche District, OR | [92] |
| 2 | Podocarpus falactus (Thunb.) R. B. ex Mirb | – | T | L | F | |||
| 3 | Teclea nobilis Del | Rutaceae | T | L | F | |||
| 1 | Nicotiana tabacum L | Solanaceae | H | L | F | D | Dugda District, OR | [94] |
| 2 | Ocimum lamiifolium Hochst | Lamiaceae | H | L | F | |||
| 1 | Nicotiana tabacum L | Solanaceae | H | L | F | D | Seru District, OR | [102] |
| 2 | Ocimum lamiifolium Hochst | Lamiaceae | H | L | F | |||
| 3 | Withania somnifera (L.) Dun | Solanacae | Sh | L | F | |||
| 1 | Pterolobium stellatum Forsk. Brenan | Fabaceae | Cl | R | F | N | Hulet Eju Enese District, AR | [35] |
| 2 | Ruta chalepensis L | Rutaceae | Sh | R | F | |||
| 1 | Ruta chalepensis L | Rutaceae | Sh | L, Se | F | N | Hulet Eju Enese District, AR | [35] |
| 2 | Allium sativum L | Alliaceae | H | Bu | F/D |
GF growth forms, T Tree, Sh shrub, H herb, Cl climber, PU plant parts used, L leaf, R root, Se seed, Ar Aerial part, Bu bulbs and Fr fruit, CP condition of preparation, F Fresh and D dry, ROA routes of administration, O Oral, N nasal and D dermal, Reginal states of Ethiopia AR amhara region, AfR afar region, BGR benshangul-gumuz region, DDAC dire dawa administration council, OR Oromia region, TR Tigray region, SNNPR southern nations, nationalities and peoples and peoples region
Global importance of the medicinal plants in the treatment of Epilepsy
Among the reported MPs for the treatment of epilepsy and related symptoms in Ethiopia, 34 PS were also routinely used for the same indications in different parts of the world including Africa, Asia, the Middle East, and Latin America (Table 3). Among these, Carissa edulis was the most popular (cited in six countries) antiepileptic MP frequently used to control seizure in Ethiopia, Nigeria, South Africa, Uganda, Malawi, and Kenya [104–108]. Similarly, Maytenus senegalensis was another well-known (cited in five countries) anticonvulsant MP in Africa including Ethiopia, Uganda, Zimbabwe, South Africa, and Guinea-Bissau [100, 106, 107, 109]. Withania somnifera was another multipurpose MP (cited in four countries) used to control convulsions in Ethiopia, Lesotho, India, and in East African countries [107, 110, 111]. Moreover, Acacia seyal, Acalypha fruticosa, Allium sativum, Balanites aegyptica, Biophytum umbraculum, Clerodendrum myricoides, Euphorbia tirucalli, Indigofera arrecta, Maytenus heterophylla, Nicotiana tabacum, and Ruta chalepensis were the other MPs reported for their usefulness against convulsions in at least three countries [100, 103, 106, 107, 112–126]. The remaining MPs: Artemisia afra, Asparagus africanus, Azadirachta indica, Capparis tomentosa, Clutia abyssinica, Croton macrostachyus, Cucurbita pepo, Eucalyptus globulus, Indigofera articulata, Indigofera coerulea, Jatropha curcas, Myrica salicifolia, Olea europaea, Opuntia ficus-indica, Sida rhombifolia, Xanthium stramonium, and Zingiber officinale were indicated for epilepsy in Ethiopia and at least one other country [105, 106, 108, 109, 118, 127–137]. The extensive use of MPs across different countries of the globe echoed the existence of shared ethnopharmacological knowledge among the THs, the importance of such MPs in the healthcare system of LMIC, especially in tropical and southern Africa, and more importantly, the pharmacological efficacy of the MPs in the treatment of epilepsy and related symptoms.
Table 3.
List of MPs plants used to treat epilepsy and related symptoms in other parts of the world
| No. | Scientific name | Family | GF | PU | Country/region | Refs. |
|---|---|---|---|---|---|---|
| 1 | Acacia seyal | Fabaceae | T | R | Tanzania and Uganda | [106, 112] |
| 2 | Acalypha fruticosa | Euphorbiaceae | Sh | L, R | Tanzania and Kenya | [113, 114] |
| 3 | Allium sativum | Alliaceae | H | Bu | India and Cameron | [103, 115] |
| 4 | Artemisia afra | Asteraceae | H | L | South Africa | [105] |
| 5 | Arundinaria alpina | Poaceae | T | R | Uganda | [138] |
| 6 | Asparagus africanus | Asparagaceae | Sh | R | Cameron | [127] |
| 7 | Azadirachta indica | Meliaceae | T | L | India | [128] |
| 8 | Balanites aegyptica | Balantiaceae | T | L, B, R | Mali and Saudi Arabia | [116, 117] |
| 9 | Biophytum umbraculum | Oxalidaceae | H | L, Wh | Cameron and Uganda | [115, 118] |
| 10 | Capparis tomentosa | Capparidaceae | Cl | L | Uganda | [106] |
| 11 | Carissa edulis | Apocynaceae | Sh | L,R, RB, Fr | Nigeria, South Africa, Uganda, Malawi and Kenya | [104–108] |
| 12 | Chenopodium ambrosioides | Chenopodiaceae | H | L | Democratic Republic of Congo | [139] |
| 13 | Clerodendrum myricoides | Verbenaceae | Sh | L, R | South Africa and Kenya | [100, 119] |
| 14 | Clutia abyssinica | Euphorbiaceae | Sh | R | Rwanda | [129] |
| 15 | Croton macrostachyus | Euphorbiaceae | T | B | Cameron | [140] |
| 16 | Cucurbita pepo | Cucurbitaceae | Cl | – | Nigeria | [130] |
| 17 | Eucalyptus globulus | Myrtaceae | T | L, B | Kenya | [131] |
| 18 | Euphorbia tirucalli | Euphorbiaceae | S | Lx, Ar | Somalia and East Africa | [120, 121] |
| 19 | Indigofera arrecta | Fabaceae | Sh | L, R | South Africa and Nigeria | [100, 122] |
| 20 | Indigofera articulata | Fabaceae | Sh | Wh | India | [132] |
| 21 | Indigofera coerulea | Fabaceae | Sh | L | India | [133] |
| 22 | Jatropha curcas | Euphorbiaceae | Sh | L | Nigeria | [134] |
| 23 | Maytenus heterophylla | Celastraceae | Sh | R | East Africa | [107] |
| 24 | Maytenus senegalensis | Celastraceae | Sh | L, R, | Uganda, Zimbabwe, South Africa and Guinea-Bissau | [100, 106, 107, 109] |
| 25 | Myrica salicifolia | Myrsinaceae | T | B | Uganda | [118] |
| 26 | Nicotiana tabacum | Solanaceae | H | L | Nigeria and Cameron | [115, 123, 124] |
| 27 | Olea europaea | Oleaceae | T | B, R, Fr | Kenya | [108] |
| 28 | Opuntia ficus-indica | Cactaceae | H | Fl | India | [135] |
| 29 | Ruta chalepensis | Rutaceae | Sh | Ar | Morocco and Mexico | [125, 126] |
| 30 | Sida rhombifolia | Malvaceae | H | Wh | India | [136] |
| 31 | Syzygium guineense | Myrtaceae | T | SB | West Africa | [109] |
| 32 | Withania somnifera | Solanacae | H | S, R | Lesotho, East Africa and India | [107, 110, 111] |
| 33 | Xanthium stramonium | Solanaceae | H | Wh | India | [141] |
| 34 | Zingiber officinale | Zingiberaceae | H | Rh | Japan | [137] |
GF growth forms, T Tree, Sh shrub, H herb, Cl climber, PU plant parts used, L Leaf, S stem, SB stembark, R root, RB rootbark, Wh whole plant, Ar Aerial part, Bu bulbs, Lx latex, Fr fruit, and Rh rhizome
Pharmacological evidence of reported medicinal plants
Animal models for screening of anticonvulsant or antiepileptic agents
The anticonvulsant or antiseizure activity of MPs claimed by THs for the management of epilepsy could be verified by using different in vitro and in vivo experiments. In 1937, electrically-induced convulsions in cats were used to check the bioactivity of phenytoin, the first modern AED [142]. Later, this initiative paved the way for the discovery of other seizure models responsible for the discovery of more safe and efficacious second-generation AEDs such as lamotrigine, levetiracetam, topiramate, lacosamide, pregabalin, etc. [143]. The ability of crude extracts or bioactive compounds to suppress different forms of seizures can be examined by animal models by artificially induced convulsions using maximal electroshock (MES) or drugs such as pentylenetetrazol (PTZ), picrotoxin (PIC), strychnine (STR), pilocarpine (PLC), isonicotinic hydrazide acid (INH), Kainic acid (KA), 4-aminophylline (AMP), bicuculline (BIC), etc. [144]. The similarity in the pattern of seizure triggered by different stimuli in animal models with humans, simplicity upon execution, quick response rate, and most importantly, predictive clinical outcomes in humans [145] make the in vivo seizure models trustworthy in epilepsy research. In general, MES acute seizure tests characterized by tonic extensions of forelimbs in and hind limbs followed by all limb clonus in mice/rat; subcutaneous PTZ acute seizure tests manifested by myoclonic jerks followed by unilateral forelimb and bilateral clonus, vibrissae twitching in mice/rats and a Kindled rodent model of chronic hyperexcitability characterized by unilateral and bilateral forelimbs clonus that progresses to rearing and falling in rats are the most common and “clinically validated” models for early evaluation of AEDs [142]. Albeit, the aforementioned acute seizure models failed to trace bioactive compounds effective against refractory or drug-resistant seizures. Thus, there had been a pressing need for the discovery of alternative seizure models which can embrace the deviations observed in “clinically validated” models. More recently, several non-mammalian seizure models consisting of fruit flies (Drosophila melanogaster), medicinal leeches (Hirudo verbena), planaria, roundworms (Caenorhabditis elegans), tadpoles (Xenopus laevis), zebrafish (Danio rerio), etc. were recognized for their versatility to assess the anticonvulsant activities of synthesized compounds or plant extracts [146, 147]. Of which, the zebrafish larvae were the most frequently used seizure model because of its high fertility rate and development, similar CNS organization with mammals which can be observed in translucent egg and embryo make it ideal to study CNS disorders provoked by external stimuli [148]. PTZ, KA, PLC and electrical stimulation are employed to induce convulsions in in the aforesaid non-mammalian seizure models [147].
In vivo pharmacological activities of antiepileptic medicinal plants
CAMs, especially herbal remedies are extensively used for the treatment of epilepsy across the globe due to their desirable treatment outcomes and tolerable side effects [144]. Moreover, herbal therapies may yield a new horizon for treating patients seeking inexpensive treatments for untreated epilepsy and experiencing refractory seizures. Taking the popularity of the MPs prescribed for treatment and management of epilepsy in different cultural groups across the globe into account, preliminary in vitro and/or in vivo pharmacological evaluation of MPs and phytochemical isolation of bioactive compounds have been conducted to test the validity of the hypothesis made by THs found elsewhere. Researchers employed different animal models to quantify the extent of suppression of different forms of seizures induced via MES, PTZ, PIC. STR, PLC, NIH, and BIC by the crude extracts or solvent fractions of MPs claimed to have potential anticonvulsant activities. This section highlighted the in vivo anticonvulsant activity of MPs (Table 4) whereby ethnobotanical studies conducted in Ethiopia and other parts of the world reiterated their profound pharmacological activities against epilepsy and related symptoms.
Table 4.
Plant crude extracts with in vivo antiepileptic/anticonvulsant activities
| No. | Scientific name | PU | Extract | Seizure-inducing stimuli | Animal models | Doses (mg/kg) | Treatment outcomes | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Acalypha fruticosa | Ar | CH | PTZ, MES & INH | Adult Swiss albino mice (25–30 g) | 30–300 | Protected the mice from PTZ and MES-induced convulsions. Delayed the latency of convulsions triggered by INH | [113] |
| 2 | Ajuga integrifolia | L | HME | PTZ & MES | Swiss albino mice (20–30 g) | 100–400 | HME extract significantly delayed the latency onset of PTZ-induced convulsions at all doses (100, 200 & 400 mg/kg) and decreased the duration of tonic hind limb extension in the MES model. Unlike BU and CH fractions, the AQ fraction didn’t show any effect on latency and duration of convulsions at all doses | [149] |
| 3 | Allium sativum | Bu | AQ | PLC | Male adult Wistar rats (200–250 g) | 100 & 300 | The AQ extract demonstrated neuroprotective potential in PLC-induced neurodegeneration, mitigated the prefrontal cortex (PFC) astrogliosis. However, it didn’t decrease GLU and other neurotransmitter levels | [150] |
| 4 | Artemisia afra | Wh | HET | PTZ | Male BALB/c mice (22–30 g) | 250–1000 | Delay the mean onset of convulsion and decrease the mean duration of convulsions | [151] |
| 5 | Asparagus africanus | R | AQ | PLC | Mus musculus Swiss mice (20–29 g) | 63.5–254 | Decreased the duration and number of clonic and tonic convulsions. Increased the latency time of onset of clonic and tonic convulsions | [127] |
| 6 | Azadirachta indica | – | – | PTZ | Sprague Dawley strain male rats | 100 | Decrease in seizures severity by decreasing the mean onset time of jerks and protecting the brain against anoxic damage and oxidative stress (OS) due to prolonged seizures | [152] |
| R | HET | PTZ & MES | Albino rats of either sex (200–250 g) & albino mice of either sex (30–50 g) | 200–800 | There was no significant increase in the mean duration of hind limb extension in the test groups at all doses (200, 400 & 800 mg/kg). The HET root extract was devoid of any anticonvulsant activity in rodents | [153] | ||
| 7 | Balanites aegyptica | SB | CH & HME | PTZ, MES & PLC | Male Albino Swiss mice (28–38 g) & male Albino Swiss rats (200–225 g) | 200 & 400 | Both solvent extracts significantly suppressed hind limb extension and delayed latency of myoclonic spasm and clonic convulsions of mice at all doses. Similarly, the CH (100 mg) and HME (100 & 200 mg) extracts delayed the latency to rearing with forelimb clonus in rats | [154] |
| 8 | Buddleja polystachya | L | HME | PTZ & MES | S iss albino mice (27–33 gm) | 100–400 | The HME extract elicited a significant anticonvulsant effect in MES (all doses) and PTZ models (200 & 400 mg/kg). The BU fractions showed a significant anticonvulsant effect in both models. In addition, the CH fractions were active against seizure-induced by PTZ (200 & 400 mg/kg). While the AQ fractions were devoid of any anticonvulsant activities in both models | [155] |
| 9 | Carissa edulis | RB | AQ | PTZ, PIC, STR, NMDA, INH & AMP | Swiss Albino mice (18–30 g) & Wistar albino male rats (130–220 g) | 150–600 | The AQ fractions protected PTZ, STR, and NMDA-induced seizures significantly at higher doses. But the AQ fractions and sub-fractions showed no effect on MES-induced seizures | [156] |
| HET | PTZ & MES | Swiss Albino mice of either sex (15–24 g) & White ranger cockerels of either sex (30–41 g) | 5–20 | Delayed the mean onset of convulsions in mice and chicks. It exhibited a dose-dependent inhibition of the convulsion induced by MES (90% protection at 20 mg/kg) | [104] | |||
| 10 | Clerodendrum myricoides | L | HET | PTZ | Male BALB/c mice (22–30 g) | 300–1200 | Unlike the solvent fractions, the crude extract demonstrated a significant delay in the mean latency to onset of seizures and decrease the duration of convulsions in a dose-dependent manner | [157] |
| 11 | Clutia abyssinica | L | HME | PTZ & MES | Male BALB/c mice (20–30 g) | 400 & 800 | Though the crude extract exhibited insignificant dose-dependent delay on the onset of a seizure, it improved the survival of mice | [158] |
| 12 | Croton macrostachyus | SB | AQ | PIC, STR, PTZ, INH & MES | Adult male Mus musculus Swiss mice (19–25 g) | 13–135 | The crude extract prevented the mice from PIC, STR, PTZ, and MES-induced seizures. It also delayed the onset of INH-induced seizures | [140] |
| 13 | Indigofera arrecta | L | ME | PTZ | Zebrafish with an AB or EK strain | 30–300* | The main constituent, idirubin, revealed reduction of epileptiform discharges in PTZ-treated zebrafish larvae | [144] |
| 14 | Jatropha curcas | L | AQ | PTZ & MES | Male albino mice (25–30 g) | 100–400 | Protected the mice against the MES-induced convulsion. While at 400 mg/kg, it significantly protected the mice against PTZ-induced seizures | [134] |
| 15 | Maytenus heterophylla | L, R & SB | ME | PIC | White Swiss albino mice (20–24 g) | 50–200 | The stembark extract significantly suppressed convulsions induced by PIC better than the leaf and root extracts. It also offered up to 62.5% protection against seizure at 200 mg/kg which was significant (p < 0.05) as compared to diazepam | [159] |
| 16 | Nicotiana tabacum | Ar | AQ & HME | PTZ | Random breed albino male mice (18–24 g) | 100 | Both extracts decreased the onset and severity of seizures (but it is statistically insignificant as compared to the negative control group). Both extracts decreased the mortality of PTZ-treated mice | [160] |
| 17 | Olea europaea | – | – | PTZ | Mice weighing (25–30 g) | 20 | The active constituent of Olea europaea leaf, oleuropein (20 mg/kg), caused a significant increase in seizure latency and a significant decrease in the whole body seizure | [161] |
| 18 | Opuntia ficus-indica | Fl | HME | PTZ, MES & STR | Swiss albino mice (20–25 g) | 250 & 500 | Protect the mice against PTZ, MES, and STR-induced seizures | [135] |
| 19 | Pentas schimperiana | RB | HME | PTZ & MES | Swiss albino mice (20–30 g) | 100–400 | The BU and ME fractions significantly inhibited the PTZ and MES-induced seizure at 400 mg/kg | [162] |
| 20 | Pterolobium stellatum | L | AQ & HME | PTZ & MES | Swiss albino mice (25–32 g) | 100–400 | The HME extract exhibited a dose-dependent increase on the latency onset of seizure against PTZ. In addition, both HME and AQ fractions demonstrated a dose-dependent reduction in duration of hind limb tonic extensions in the MES model and myoclonic seizure in the PTZ model at 400 mg/kg | [163] |
| 21 | Ruta chalepensis | Ar | ET | PTZ | Male Swiss albino mice (25–30 g) | 10–1000 | Delayed the onset of seizures and a dose-dependent suppression in the tonic phase and mortality induced by PTZ was noticed | [164] |
| 22 | Securidaca longepedunculata | R | AQ | STR & PIC | Albino mice of either sex (20–25 g) | 100–400 | The extract elicited dose-dependent increase in onset of convulsion and prolongation of the cumulative time spent in the open arms of the elevated plus maze and Y maze compared with the control | [165] |
| SB | AQ | PTZ, MES & AMP | Swiss albino mice of either sex (18–25 g) | 50–200 | The extract afforded significant protection against the mice treated with PTZ (50 & 100 mg/kg) and MES (50 mg/kg). It didn’t attenuate AMP induced seizure though it prolonged the onset of convulasions at 100 and 200 mg/kg | [166] | ||
| 23 | Sida rhombifolia | Wh | ME | PTZ & MES | Swiss albino mice of either sex (25–30 g) | 100–400 | The ME crude extract significantly reduced the duration of seizures at all doses | [136] |
| 24 | Withania somnifera | S & R | ET | PTZ & MES | Albino Wistar rats of either sex (150–200 g) | 100–300 | The extracts significantly suppressed hind limb tonic extension and postictal depression in MES test groups at 300 mg/kg. Moreover, a significant reduction in the mean duration of hind limb tonic flexion, hind limb tonic extension, clonus, and stupor in PTZ test groups | [110] |
| 25 | Xanthium stramonium | Wh | PE | PTZ & MES | Albino Wister albino rats (150–200 g) | 250 & 500 | The crude extract reduced the duration of convulsions. It also delayed the onset of myoclonic spasm and clonic convulsion in albino Wister rats | [167] |
| 26 | Zingiber officinale | Rh | HET | PTZ | Wild type adult zebrafish of the AB strain | 60b | The active constituent of the extract, 6-gingerol (6-GIN), effectively inhibited PTZ-induced seizures | [168] |
| Adult male Swiss mice | 25–200 | It significantly increased the onset time of myoclonic seizures at a dose of 25–100 mg/kg and significantly prevented generalized clonic seizures | [169] |
PU plant parts used, L leaf, S stem, SB stembark, R root, RB rootbark, Wh whole plant, Ar Aerial part, Bu bulbs, Fl flower and Rh rhizome Seizure-inducing agents PIC picrotoxin, STR strychnine, PTZ pentylenetetrazol, INH isonicotinic hydrazide acid and MES maximal electroshock, PLC pilocarpine, AMP 4-aminophylline, and NMDA N-Methyl-D-aspartate. Extraction solvents AQ aqueous, CH chloroform, BU butanol, ET ethanolic, HET hydroalcoholic/hydroethanolic, ME methanolic, HME hydromethanolic, and PE petroleum ether
aand brepresented the plant extract doses given in µM and µg/mL, respectively
In vivo pharmacological activities of crude extracts and solvent fractions
Single stimuli-induced seizure model
PTZ is routinely used as a stimulus to induce convulsions in different animal models by inhibiting the GABAergic neurotransmission [170]. PTZ-induced seizures are characterized by an initial ‘absence-like’ immobility, followed by brief myoclonic jerks, sustained myoclonus, and finally GTCS with a loss of the righting reflex. The subcutaneous administration of PTZ is often used to induce a seizure in mice [171] that can be employed to assess the anticonvulsant activity of MPs. The whole plant and leaf extract of Artemisia afra are traditionally used for the treatment of epilepsy in Ethiopia and South Africa, respectively (Table 3) [105]. Kediso et al. [151] investigated the anticonvulsant effect of the HET and solvent fractions of Artemisia afra whole part against PTZ-induced seizure in mice. Unlike the solvent fractions, the HET crude extract triggered a significant delay in the mean onset of convulsions (504.833 ± 62.835 s, 551.833 ± 74.69 s, and 808.333 ± 64.8 s) and a decrease in the mean duration of convulsions (17.000 ± 1.88 s, 13.000 ± 1.8 s and 7.833 ± 1.07 s) at the respective doses of 250, 500 and 1000 mg/kg. The observed activity of the crude extract might be attributed to the presence of multiple secondary metabolites in the herb. Clerodendrum myricoides is another MP whose leaf extract is traditionally used as an anticonvulsant in Ethiopia, Kenya, and South Africa [100, 119]. Owing this, the anticonvulsant activity of the HET and solvent fractions of the leaf extract was assessed via mice experiencing PTZ-induced seizures [157]. The HET crude extract of Clerodendrum myricoides at 300, 600 and 1200 mg/kg significantly delayed the mean latency in the onset of seizures (299.33 ± 30.129 s, 387.167 ± 27.6 s and 417.833 ± 31.9 s, respectively) and decrease in the duration of convulsions (27.333 ± 1.585 s, 16.833 ± 1.537 s and 10.50 ± 0.671 s, respectively) in a dose dependent manner as compared to the control group. On the other hand, the solvent fractions of Clerodendrum myricoides didn’t show significant anticonvulsant effect in the model.
Ruta chalepnesis is known for its antiepileptic activities in the traditional folklore of Ethiopia, Morocco, and Mexico [125, 126]. The ET extracts of the aerial parts of Ruta chalepnesis were assessed by using PTZ-induced seizure and a dose-dependent suppression in the tonic phase was observed, moreover, it reduced the mortality triggered by PTZ in the experimental animals. Azadirachta indica is employed in the traditional healthcare system of Ethiopia and India to treat epilepsy [128]. Kumar et al. [152] compared the antiseizure activities of Valproic acid (VPA) and Azadirachta indica on PTZ-induced kindling in Sprague Dawley strain male rats at 200 mg/kg and 100 mg/kg, respectively. A decrease in the mean onset time of jerks, clonus, and extensor phases was observed in VPA and Azadirachta indica treated groups. Moreover, an increase in glutathione reductase activity and a decrease in the activity of lipid peroxidation enzymes, glutathione S-transferase activity, catalase, and nitric oxide was observed in the same group, asserting the protective effects of VPA and Azadirachta indica against anoxic damage and OS of the brain due to prolonged seizures. Overall, Azadirachta indica demonstrated better preventive effects than VPA on PTZ-induced chemical kindling in rats. Asparagus africanus is a widely used plant in TM as an anti-inflammatory, antioxidant, for the treatment of CNS disorders including epilepsy. The anticonvulsant activity of the root decoction of Asparagus africanus was evaluated in PLC-induced SE in Mus musculus Swiss mice. It increased the onset time of tonic–clonic convulsions and decreased the duration and number of tonic–clonic convulsions at doses of 63.5, 127, and 254 mg/kg. The anticonvulsant activity of Asparagus africanus emanated from modulation of GABA (increase), GABA-T, TNF-α (decrease) levels, and inhibition of OS in the brain [127].
Dual stimuli-induced seizure models
MES is the second most commonly used seizure-inducing stimuli in different animal models of epilepsy next to PTZ. It is convenient to assess GTCS that can be reproduced with reliable endpoints [172]. The use of two common stimuli, PTZ and MES, in different animal models will help to better understand the pharmacological effects and the MOA of anticonvulsant agents. Carissa edulis is commonly used for the treatment of epilepsy in Africa especially in Ethiopia, Nigeria, South Africa, Uganda, Malawi, and Kenya [104–108]. Owing to this, the anticonvulsant activity of the rootbark of Carissa edulis was investigated using PTZ-induced seizure in mice and the MES test in chicks. It exhibited a suboptimal level of inhibition against seizure as compared to benzodiazepine (BZP) (100%) in the mice model. Moreover, the crude extract elicited 90% protection as compared to phenytoin (100%) at 20 mg/kg in convulsions induced by MES in chicks signifying the beneficial effect of Carissa edulis for the management of epilepsy and related symptoms [104]. Clutia abyssinica is claimed to have antiepileptic activity in traditional herbal medicine folklore of Ethiopia and Rwanda [129]. Although the HET leaf crude extract of Clutia abyssinica improved the mean survival time of epileptic mice, the recorded mean time of hind limb extension was not significant at 400 and 800 mg/kg as compared to the negative control group [158]. Leaves of Jatropha curcas have been used by TH of Ethiopia and Nigeria for the management of epilepsy. Bolanle et al. [134] examined the anticonvulsant activity of AQ leaf extract of Jatropha curcas in PTZ- and MES-induced seizure models. The crude extract delayed the onset of tonic leg extension and the seizure-induced mortality was inhibited in mice. Moreover, it significantly (p < 0.05) protected mice from MES-induced seizure at 100, 200 and 400 mg/kg. at a higher dose, 400 mg/kg, it also significantly inhibited PTZ-induced convulsions.
Pentas schimperiana is a MP used in Ethiopian TM for the treatment of epilepsy. Fisseha et al., [162] assessed the HME rootbark crude extract and CH, BU, and AQ fractions of Pentas schimperiana using PTZ and MES-induced seizure models at doses of 200 and 400 mg/kg. As compared to the control group, the ME and BU fractions, at 400 mg/kg, demonstrated significant (p < 0.001) anticonvulsant activities in both models. In addition, the CH fraction exerted significant (p < 0.001) seizure control in PTZ treated mice whereas the aqueous fraction was devoid of significant antiepileptic activities in both models. In general, the alkaloids, flavonoids, saponins, tannins, phenols, steroids, and terpenoids present in the rootbark may be ascribed to the observed seizure control in mice. Sida rhombifolia is a plant commonly prescribed for the treatment of epilepsy by the THs of Ethiopia and India [136]. The ME crude extract of the whole part of Sida rhombifolia was examined PTZ and MES-induced seizure in mice at 100, 200, and 400 mg/kg. The result reiterated that the ME crude extract of 100, 200, and 400 mg/kg significantly suppressed the duration of seizure as compared to the control group in both models. Xanthium stramonium is a famous MP in China due to its widespread healthcare prominence. It is also used for the treatment of epilepsy in Ethiopia and India [141]. Owing to this, Kumar et al. [167] screened the anticonvulsant activity of the PE whole plant extract of Xanthium stramonium against PTZ and MES-induced seizure models in albino Wistar rats at a dose of 250 and 500 mg/kg. It increased the latency onset of myoclonic spasms and clonic convulsions in PTZ-treated groups. In addition, it also reduced the mean duration of the exterior phase significantly as compared to the control group in the MES test. The root of Azadirachta indica was used in herbal formulations prepared to treat epilepsy in different countries. The in vivo anticonvulsant assessement done on PTZ-induced seizure in mice and MES-induced seizure in Albio rats indicated that the ET root extract has no significant effect on the mean duration of limb extension, mean onset of convulsions and mean number of convulsions at a dose of 800 mg/kg as compared to the control group [153].
Multiple stimuli-induced seizure models
Multiple stimuli-induced seizure models provide better information about the effect of drugs or a plant extract in the target experimental animals. The depth and breadth of data obtained in such multiple seizure models can shed light on the different aspects of the plant extract under consideration: MOA, potential targets for antiepileptic interventions, possible bioactive compounds, etc. In addition to PTZ and MES, one or more of the following stimuli such as INH, PIC, PLC, NMDA, STR, AMP, and BIC are used to induce convulsions (in experimental animals) in epilepsy research. Traditional herbalists of Ethiopia, Tanzania, and Kenya [113, 114] have faith in the curative effect of Acalypha fruticosa for the treatment of epilepsy. Govindu et al., [113] assessed the anticonvulsant activity of the CH crude extracts of the aerial parts of Acalypha fruticosa using PTZ, MES, and INH-induced seizures in Swiss albino mice at doses of 30, 100, and 300 mg/kg. The result confirmed the potential of the crude extract to suppress seizures triggered by MES in a dose-dependent pattern. At 300 mg/kg, as compared to diazepam (4 mg/kg) the extract demonstrated more pronounced anticonvulsant activity. It also inhibited the PTZ-induced seizures better than the positive control, phenobarbitone sodium. While in the INH model, it delayed the onset of convulsions in a dose-dependent manner but failed to protect the mice from seizure-induced mortality. Balanites aegyptiaca is used traditionally in Ethiopia, Mali, Saudi Arabia [116, 117], and India to treat epilepsy. Hence, HMET and CHL extract of stembark of Balanites aegyptiaca were assessed using PTZ, MES-induced convulsions, and PLC-induced SE in rats [154]. Both the HME and CH extract at 200 and 400 mg/kg significantly delayed the onset of myoclonic spasm and clonic convulsions as well as significantly reduced the duration of hind limb extension in PTZ and MES models. In the PLC model, the CH extract (100 mg) and HME extract (100 and 200 mg) delayed the latency to rearing with forelimb clonus significantly.
Carissa edulis is popular in African countries such as Ethiopia, Nigeria, South Africa, Uganda, Malawi, and Kenya [104–108] for its beneficial effect in the management of epilepsy by herbalists or TH. The anticonvulsant activity of the AQ fractions (150, 300, and 600 mg/kg) and sub-fractions (250, 500, 500, and 1000 mg/kg) of the rootbark extract was examined using PTZ, PIC, NMDA, INH, STR, and AMP-induced seizures in mice. The AQ fraction and sub-fractions suppressed 50% and 16.67% of PTZ-induced convulsions. Similarly, the AQ fraction experienced 33.33% and 16.67% protection against strychnine and NMDA seizure models, respectively. Moreover, the AQ fractions elicited 66.67–33.33% protection against AMP-induced seizures at doses of 150 and 600 mg/kg. However, the AQ fractions and sub-fractions did not affect MES-induced seizures. Croton macrostachyus is a common tree used to treat epilepsy in Ethiopia and Cameron [140]. Bum et al. [140] employed MES, STR, PTZ, PIC, and INH-induced seizure models to evaluate the anticonvulsant activity of AQ stembark extract of Croton macrostachyus in Mus musculus Swiss mice. The extract protected 60, 80, 80, and 80% of mice from MES, PTZ, PIC, and STR-induced convulsions, respectively even at an initial dose of 34 mg/kg. It also increased the latency onset of seizures in INH-treated mice. Overall, the result suggested that Croton macrostachyus may have a promising effect in secondary GTCS and primary generalized seizures in humans. Opuntia ficus-indica commonly known as cactus pear is used in the treatment of epilepsy in Ethiopia and India [135]. The in vivo anticonvulsant activity of the flower ME extract was assessed using Swiss Albino mice. The ME extract produced significant inhibition against PTZ, MES, and STR-induced convulsion at 250 and 500 mg/kg. There was an increase in noradrenaline and dopamine level in the mice's brains due to the avoidance of MES-induced convulsions.
In vivo pharmacological activities of isolated compounds/constituents
Indigofera arrecta is a common MP used by the indigenous inhabitants of Ethiopia, Nigeria, Congo, and South Africa [100, 122]. Bioassay-guided fractionation of Indigofera arrecta in zebrafish model results in the identification of indirubin and 6-bromoindirubin-3ꞌ-oxime (BIO-acetoxime), compounds with glycogen synthase kinase (GSK)-3 inhibition activity demonstrated significant anticonvulsant activity in PTZ-induced seizure in zebrafish larvae. Moreover, they also showed significant antiseizure activity in the PLC rat model limbic seizure and the 6-Hz refractory seizure mouse model, demonstrating GSK-3 inhibition as a potential therapeutic target for epilepsy. Olea europaea is among the known MPs used for the management of epilepsy in Ethiopia and Kenya [108]. Oleuropin, a secondary metabolite extracted from the leaves of Olea europaea, elicited a significant increase in seizure latency and a significant decrease in total frequencies of head ticks, head and upper limbs seizures, frequent spinning and jumping, and tonic seizures in PTZ kindling of seizure in mice. Oleuropin treated groups (20 mg/kg) showed downregulation of genes responsible for the expression of IL-1 without change in GLT-1 levels. The significant antepileptic activity of oleuropin may be attributed to its antioxidant and antiinflammatory activities making it an ideal pharmacophore for the synthesis of AEDs. Zingiber officinale is another most frequently used medicinal herb in different parts of the world. For instance, in Ethiopia and Japan Zingiber officinale is used for the management of epilepsy [137]. Its HET extract of rhizome has demonstrated anticonvulsant activity in rodent seizure models [169, 173]. Gawel et al., [168] also proved the anticonvulsant effect of ME crude extract using a PTZ-induced seizure in zebrafish larvae. Inspired by its activity, the group also isolated the major constituent of Zingiber officinale rhizome, 6-gingerol (6-GIN) that exerted dose-dependent antiseizure activity in PTZ-induced hyperlocomotion assay in zebrafish larvae. Rigorous experimental procedures and molecular docking analysis in human NR2B-containing NMDA receptors suggested that the antiepileptic activity of 6-GIN may be partly mediated by restoring the balance between GABA and GLU in the epileptic brains. In general, the in vivo anticonvulsant activity of the aforementioned MPs resonated the potentials of herbal formulations in the healthcare system of different countries. Although most of the antiepileptic MPs claimed by THs were not screened for their anticonvulsant effects through suitable seizure models, this review partly documented the strong association that exist between the indeginous knowledge of THs and pharmacological activities of MPs used to treat epilepsy and related symptoms in Ethiopia and other parts of the world.
Toxicity profiles of antiepileptic or anticonvulsant medicinal plants
Acute toxicity profiles of medicinal plants
Acute toxicity study of plant extracts is performed to the assess the potential inherent toxicity that may be displayed in a short period of time upon a single dose exposure mostly via the oral route as it is considered as a viable route for accidental human exposure for hazardous substances and it allows for hazard classification of test substances [174]. The leaf part of Artemisia afra, Azadirachta indica, Brucea antidysenterica, Buddleja polystachya, Eucalyptus Globulus, Gloriosa superba, Maytenus heterophylla, Nicotiana tabacum, and Ocimum lamiifolium are commonly used for the preparation of remedies used to treat epilepsy and related symptoms in Ethiopia. The acute toxicity studies conducted in the crude extracts, essential oils and bio-oils recapped the absence of gross behavioral, physical changes and signs of overt toxicity such as lacrimation, urination, muscle weakness and convulsions in different animal models [175–181]. As depicted in Table 5, relatively higher LD50 value greater than 5000 mg/kg of body weight were recorded for Artemisia afra, Azadirachta indica, Gloriosa superba, and Nicotiana tabacum extracts. In addition, the EO of Eucalyptus Globulus, and HET extract of Maytenus heterophylla 2.5 mL/kg and > 1200 mg/kg, respectively demonstrating the safety profiles of single dose of the plant extracts. Furthermore, the roots of Asparagus africanus, Biophytum umbraculum, Capparis tomentosa, and Withania somnifera are believed to be rich in bioactive chemicals characterized by attenuating convulsions. Their crude extracts and solvent fractions were devoid of any inherent acute toxicity symptoms at a single dose greater than 2000 mg/kg body weight [182–185]. The AQ and HME stembark extract of Croton macrostachyus (LD50 > 5000) and the ET rootbark crude extract of Carissa edulis, (LD50 ⁓3,808) were found to be safe [186, 187], consequently, the experimental animals manifested neither visible signs of lacrimation, loss of appetite, tremors, hair erection, salivation, diarrhea and convulsion nor mortality in the study period at the estimated doses equivalent to LD50 values. According to Globally Harmonized Classification System (GHCS) for chemical substances and mixtures, synthetic chemicals and plant extracts having an LD50 > 2000 mg/kg of body weight is considered as safe [188]. This reiterated the relative safety profiles of most MPs used to treat epilepsy and related symptoms in Ethiopia.
Table 5.
Acute toxicity profiles of some MPs employed in the treatment of epilepsy and related symptoms
| No. | Scientific name | PU | Extract | Animal models | Acute toxicity studies | Refs. | ||
|---|---|---|---|---|---|---|---|---|
| Doses (mg/kg) | LD50 (mg/kg) | Treatment outcomes | ||||||
| 1 | Ajuga integrifolia | R | HME | Swiss albino male mice (20–30 g) | 2000 | > 2000 | Neither mortality of mice nor any signs of toxicity (behavioral, neurological, autonomic, or physical changes) was observed at 2000 mg/kg of body weight | [189] |
| 2 | Allium sativum | Bu | AQ | Wistar rats (⁓115–126 g) | 100, 1000, 2500 & 5000 | > 5000 | No death was recorded at all doses. The rats treated with 5000 mg/kg of body weight experienced cardiac problem and disorientation | [190] |
| 3 | Artemisia abyssinica | Ar | ET | Swiss albino mice (25–30 g) | 500, 1000 & 3000 | > 3000 | The mice did not show visible toxicity, although at 3000 mg/kg a decreased in locomotor activity was observed | [191] |
| 4 | Artemisia afra | L | AQ | Female adult Swiss albino mice (25–30 g) | 200, 700, 1200, 2200, 3200, 4200 &5000 | > 5000 | Mild toxicities like anxiety and piloerection were observed at higher doses (≥ 3200 mg/kg) that disappear in the wash out periods. No mortality in mice was recorded at all doses | [175] |
| L | ET, DCM & HX | Swiss albino mice (20–22 g) | 1000, 2000 and 2500 | > 2500 | Loss of appetite, hypoactivity, lethargic, dizziness that disappeared in the washout period was noticed in mouse treated with DCM extract at 2500 mg/kg | [192] | ||
| 5 | Asparagus africanus | R | HET & BU | Swiss albino mice (20–25 g) | 1000, 3000 & 5000 | > 5000 | There was no dose-dependent behavioral change, weight change and mortality in mice treated single dose BUT fraction orally | [182] |
| 6 | Azadirachta indica | L | AQ | Female BALB/c mice (average mass of 30 g) | 1250, 2500 & 5000 | > 5000 | The mice treated with the extract were devoid of weight/hair loss, allergy, or other symptoms of discomfort | [176] |
| 7 | Balanites aegyptiaca | SB | AQ | Fishes | 17.5, 20, 22.5 & 25a | ⁓18.99–20.72a | B. nurse, L. intermedius and L. bynni fish species treated with the extract suffered from the debilitating toxic effect | [193] |
| 8 | Biophytum umbraculum | R | AQ, BU & CH | Female Swiss Albion mice (22–30 g) | 2000 | > 2000 | There was no behavioral change, weight change and mortality in mice treated single dose of all fractions | [183] |
| 9 | Brucea antidysenterica | L | AQ, ME & CH | Swiss albino mice (27–36 g) | 500, 1000 & 2000 | – | The extracts lack visible signs of acute toxicity and mice fatality till the dose of 1000 mg/kg. But, at the dose of 2000 mg/kg it caused mortality in all mice with in 24 h | [194] |
| 10 | Buddleja polystachya | L | HME | Female Sprague–Dawley rats (150–200 g) | 2000 | – | There was no visible sign of skin reaction, inflammation, erythema, irritation or redness, and any adverse reaction in rats | [177] |
| 11 | Calpurnia aurea | L | AQ & HME | Female Swiss albino mice | 5000 | > 5000 | The mice were devoid of gross behavioral or physical changes and signs of overt toxicity such as lacrimation, urination, muscle weakness and convulsions | [186] |
| 12 | Capparis tomentosa | R | HME | Male Swiss Albino mice (25–38 g) | 2000, 3000 & 5000 | > 2000 | The mice showed signs of slight rigidity and sleepy activity at higher doses of extract (3000 and 5000 mg/kg). No mortality was recorded at all doses | [184] |
| 13 | Carissa edulis | L | AQ | Wistar albino rats of either sex | 2000 | > 2000 | The rats showed no gross behavioral or physical changes and signs of overt toxicity | [195] |
| RB | ET | Wistar albino rats (124–220 g) & Swiss mice (16–35 g) | 10, 100 & 1000 | ⁓3808 | None of the mice and rats orally treated with the extract manifested signs of toxicity except death at the dose of 5000 mg/kg (in both species) | [187] | ||
| 14 | Caylusea abyssinica | L | HME | Male Swiss albino mice (20–30 g) | 2000 | > 2000 | The mice didn’t experience any behavioral, neurological, autonomic or physical changes | [196] |
| 15 | Clerodendrum myricoides | R | AQ | Swiss albino mice of either sex (25–30 g) | 1134 | – | Behavioral changes such as horripilation, difficulty in breathing, grooming, and asthenia followed by death was noticed in mice treated with 1134 mg/kg | [197] |
| 16 | Croton macrostachyus | R | HME | Female Swiss Albino mice (25–28 g) | 2000 & 5000 | > 5000 | The mice showed no visible signs of lacrimation, loss of appetite, tremors, hair erection, salivation, diarrhea and convulsion | [198] |
| SB | AQ & HME | Female Swiss albino mice | 5000 | > 5000 | The mice were devoid of gross behavioral or physical changes and signs of overt toxicity such as lacrimation, urination, muscle weakness and convulsions | [186] | ||
| SB | HME, AQ & ETAc | Female Swiss albino mice | 2000 | > 2000 | None of the mice treated with crude extract or solvent fractions showed problems in breathing, alertness, motor activity, restlessness, diarrhea and convulsions | [199] | ||
| 17 | Cucumis ficifolius | R | HME & CH | Swiss albino mice (25–30 g) | 125, 250, 500 & 2000 | > 2000 | There were no mortality and signs of overt toxicities at a dose of 2000 mg/kg of body weight | [200] |
| 18 | Echinops kebericho | Tu | EO | Swiss albino mice (18–26 g) | 300 & 2000 | > 2000 | Though the mice showed piloerection, muscle spasm and apathy immediately after administration, there were no significant treatment-related morbidities | [201] |
| Tu | AQ | Wistar albino rats (250–350 g) | 300, 2000 & 5000 | > 5000 | The rats experienced piloerection, muscle twinge, and lethargy after the treatment with the extract (5000 mg/kg) which disappeared after 5 h. But, there were no treatment related morbidity and mortality at 5000 mg/kg | [202] | ||
| 19 | Embelia schimperi | Fr | HET | Female Wistar rats (180–210 g) | 400, 1000, 2000, 3000, 4000 & 5000 | > 5000 | The extract didn’t elicit prominent signs of toxicity and any mortality in rats in the study period | [203] |
| 20 | Eucalyptus Globulus | L | EO | Swiss albino mice of either sex (23–30 g) | 2, 2.5, 3 & 3.5b | 2.5b | The mice treated with the essential oil showed restlessness, debilitation, reduced food and water intake and piloerection which disappeared in the washout period after treatment with ≥ 2.5 mL/kg | [178] |
| 21 | Fagaropsis angolensis | SB | HME, AQ, BU & CH | Adult male Swiss albino mice (25–30 g) | 2000 | ≥ 2000 | Neither mortality nor any signs of toxicity were observed in mice treated with both extracts at 2000 mg/kg body weight | [204] |
| 22 | Foeniculum vulgare | Fr | ET | Swiss labial mice (25–28 g) | 500, 1000 & 3000 | ≥ 3000 | The extract didn’t trigger mortality of mice and overt toxicity except reduced locotmotor activity and piloerection at 3000 mg/kg of body weight | [205] |
| 23 | Gloriosa superba | L | AQ | White male Wistar rats (200–250 g) | ⁓121, 364, 1091 & 3274 | > 1500 | The rats experienced treated with colchicine of standardized Gloriosa superba extract showed no visible sign of overt toxicity | [206] |
| L | HME | Non-pregnant Wistar rats (120–140 g) | 200 & 5000 | > 5000 | There were no visible overt signs of toxicity at 5000 mg/kg. No morbidity or mortality was observed in the rats treated groups at both doses | [179] | ||
| 24 | Justicia schimperiana | L | HME | Swiss albino mice (18–30 g) | 2000 | > 2000 | The extract didn’t trigger signs of overt toxicity. Moreover, no mortality of mice was recorded in the study period | [207] |
| L | HME | Female adult Wistar rats (180–200 g) | 2000 | > 2000 | Rats showed no formation of edema or erythema. No signs of toxicity as well as no mortality were noted during the study period | [208] | ||
| 25 | Maytenus heterophylla | L | HET | Male CD-6 mice (35–40 g) | 1200 | > 1200 | The mice treated with the extract were devoid of physical and behavioral changes at 1200 mg/kg | [180] |
| 26 | Maytenus senegalensis | RB | ET | Swiss albino mice (18–22 g) | 200, 300, 400, 800 & 1600 | > 1600 | The mice treated with the extract were devoid of physical and behavioral changes at 1600 mg/kg | [209] |
| SB | HET | Theiller’s albino mice of either sex | 1000, 2000, 3000, 4000 & 5000 | > 5000 | The mice treated with the extract were devoid of physical and behavioral changes at 5000 mg/kg | [210] | ||
| L & S | HET | Male CD-6 mice (35–40 g) | 1200 | – | The mice treated with leaf extract exhibited some signs of overt toxicity. In addition, the stem extract caused pronounced toxicity at 1200 mg/kg | [180] | ||
| 27 | Myrica salicifolia | R | HME | Non-pregnant female mice | 2000 | > 2000 | There are no visible signs of overt toxicity and mortality in mice treated with the extract at 2000 mg/kg | [211] |
| 28 | Nicotiana tabacum | L | Bio-oil | Female Wistar rats (130–140 g) | 5000 | > 5000 | The rats exhibited no significant change in the body weight and behavior. In addition, there was no mortality of rats in the study period | [181] |
| 29 | Ocimum lamiifolium | L | ME | Swiss albino mice (27–36 g) | 500, 1000 & 2000 | ≥ 2000 | The crude extract didn’t trigger gross visible signs of acute toxicity such as urination, hair erection, lacrimation, and reduction in feeding activity | [194] |
| 30 | Olea europaea | L | ET | Wistar rats of either sex (150–200 g) | 2000 | ≥ 2000 | Oral administration of the extract didn’t cause any mortality or sign of toxicity at 2000 mg/kg of body weight during the study period | [212] |
| 31 | Opuntia ficus-indica | S | HET | White Sprague Dawley rats either sex | 500, 1000 & 2000 | – | The rats exhibited no genotoxicity at all treatments regimens even at the maximum dose of 2000 mg/kg | [213] |
| Se | HX (fixed oil) | Mus musculus mice (20–30 g) | 10, 20, 30, 40, 50, 60 & 70b | 43b | The mice suffered from immediate agitation and behavioral perturbations with temporary writhing, followed by a quiet attitude period and sedation | [214] | ||
| 32 | Pentas schimperiana | L | AQ & HME | Swiss Albino mice of either sex (20–33 g) | 1000, 2000 & 5000 | > 4000 | The mice experienced no visible change in behavior such as restlessness, motor activity, breathing and diarrhea. Moreover, there was no mortality recorded at 5000 mg/kg | [215] |
| 33 | Podocarpus falcactus | Ap | AQ | Female Sprague Dawley rats (260–300 g) | 2000 | > 2000 | The rats showed neither mortality nor gross behavioral changes and mortality at 2000 mg/kg of body weight | [216] |
| 34 | Ruta chalepensis | Ar | ET | Male Swiss albino mice (25–30 g) | 1600, 3000 & 5000 | > 5000 | The extract didn’t trigger mortality nor macroscopic tissue injury or weight loss at 5000 mg/kg per body weight | [164] |
| 35 | Rhus vulgaris | SB | AQ, | Female Swiss albino mice (18–26 g) | 50, 300 & 2000 | > 2000 | The mice were devoid of changes in general appearance and behavioral patterns. In addition, there was no mortality or gross pathology in any organ at necropsy | [217] |
| 36 | Securidaca longepedunculata | L, S & R | AQ & ME/CH (1:1) | Swiss female mice (20–22 g) | 50, 300 & 2000 | > 2000 | The AQ total extracts of leaves and stembark did not show any change in behavior following administration of the crude extracts at 2000 mg/kg of body weight | [218] |
| 37 | Sida rhombifolia | Ar | ET | Adult male Wistar albino rats (180–220 g) | 2000 | > 2000 | There were no visible overt signs of toxicity and mortality in rats treated with 2000 mg/kg of the extract | [219] |
| Ar | HME | Albino Wistar rats (102–134 g) | 4000, 8000, 12000 & 1600 | > 8000 | The rats exhibited slight changes in general behavior such as sow response to external stimuli, stretching and sluggishness | [220] | ||
| R | AQ | Sprague Dawley rats of either sex (130–190 g) | 5000 | > 5000 | The rats experienced neither overt toxicity signs nor mortality at a single dose of 5000 mg/kg | [221] | ||
| 38 |
Syzygium guineense |
L | HME | Wistar rats of either sex (120–140 g) | 2000 & 5000 | > 5000 | In the acute toxicity study, rats treated with 2000 mg/kg and 5000 mg/kg showed no toxicological signs observed on behavior, gross pathology, and body weight of rats | [179] |
| 39 | Teclea nobilis | Wh | HME & AQ | Male Swiss mice (⁓20 g) | 1000, 2000, 3000, 4000 & 5000 | > 5000 | The extract was devoid of any overt toxicities at 5000 mg/kg of body weight. Moreover, there was no mortality recorded in the study period | [222] |
| 40 | Vernonia amygdalina | L | AQ & HME | Female Swiss albino mice | 5000 | > 5000 | The mice exhibited no signs of overt toxicity such as lacrimation, urination, muscle weakness, sedation and convulsions at 5000 mg/kg | [186] |
| L | AQ & ET | Albino Wistar rats (200–250 g) | 2000 | > 2000 | The extracts triggered no significant effect on the biochemical and hematological parameters of treated rats (no lesions were also observed in the liver and kidneys histologically) | [223] | ||
| 41 | Withania somnifera | R | ME | Wistar rats | 5000, 1000 & 2000 | > 2000 | The rats didn’t experience any organ atrophy, hypertrophy, and degenerative or infiltrative lesions even at 2000 mg/kg | [185] |
| 42 | Zingiber officinale | R | HX (fixed oil), EO | Swiss albino mice (23–26 g) and Wistar rats (150–170 g) | 0.02, 0.04, 0.06, 0.08 and 0.1b mL/kg for fixed oil; 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 4, 6, 8 and 10b for EO | – | Observed cardinal signs of toxicity for both oils were decreased motor activity, convulsion and paralysis. In addition, mortality of experimental animals was noticed in both fixed-oil (0.2 mL/kg) and EO treated group | [224] |
PU plant parts used, Ap apex, L leaf, S stem, Se seeds, SB stembark, R root, RB rootbark, Ar Aerial part, Bu bulbs, Tu tuber and Rh rhizome, Extraction solvents, AQ aqueous, CH chloroform, BU butanol, DCM dichloromethane, ET ethanolic, ETAc ethyl acetate, HX hexane, HET hydroalcoholic/hydroethanolic, ME methanolic, HME hydromethanolic, PE petroleum ether and EO essential oil
aand brepresented the plant extract doses and LD50 values are given in mg/L and mL/kg, respectively
Subacute toxicity profiles of medicinal plants
Acute toxicity studies provide preliminary data about the safety profiles of a single dose of chemical agents [225], consequently, it is considered as shallow and sometimes misleading. Better information about the safety of chemicals of synthetic and natural origin can be obtained from the subacute toxicity studies, which involve repeated administration of the chemical agent under consideration. In subacute toxicity assessments, weight loss of experimental animals is an important variable that can be attributed to harmful effects of test substances [179]. A weight loss, that may be attributed to the anti-nutritive and malabsorption effect of chemical agents, that amount to ≥ 10% can be considered a sign of toxicity even in the absence of other changes on target organs, haematological or biochemical effects [226]. The subacute toxicity of plant-based materials including crude extracts, solvent fractions, bio-oils, essential oils, etc. was evaluated through repeated administration a specific dose in different animal models with the intention of assessing its accumulation in the body with gradual effects on tissues and organs [188]. In this regard, Loha et al. [179] assessed the subacute toxicity of HME leaf extract of Syzygium guineense in rats at 500 and 1500 mg/kg of body weight. Herein, the rats were devoid of significant change on behavior, gross pathology, body weight, and hematological and biochemical parameters, asserting the safety profile of the leaf extract at a repeated dose of 1500 mg/kg. In addition, subacute toxicity study was conducted on EO obtained from Echinops kebericho tuber at the doses of 100, 200 and 400 mg/kg [201]. The EO treated groups did not experience significant dose-dependent alterations in body weight, clinical chemistry parameters and relative organ weights. Deyno et al. [202] confirmed that Echinops kebericho decoction was well tolerated up to the dose of 600 mg/kg body weight as food consumption, body weight, organ weight, hematology, clinical chemistry, and histopathology did not show significant alterations between control and treatment groups.
Moreover, subacute toxicity studies conducted on the different extracts of antiepileptic or anticonvulsant MPs such as Allium sativum (AQ bulb extract at 300 mg/kg) [190], Artemisia abyssinica (ET extract of the aerial part at 3000 mg/kg) [191], Artemisia afra (AQ leaf extract at 1800 mg/kg) [175], Asparagus africanus (HET and BU root extracts) [182], Azadirachta indica (AQ leaf extract at 1000 mg/kg) [176], Capparis tomentosa (HME root extract at 1000 mg/kg) [184], Eucalyptus Globulus (EO of leaf at 2 mL/kg) [178], Olea europaea (ET leaf extract at 400 mg/kg) [212], Opuntia ficus-indica (HET stem extract at 2000 mg/kg) [213], Myrica salicifolia (HME root extract at 400 mg/kg) [211], Sida rhombifolia (AQ root extract at 1200 mg/kg) [221], and Withania somnifera (ME root extract at 2000 mg/kg) [185] clearly asserted their safety profiles at the respective maximum doses per body weight as manifested by the absence of significant treatment related variations in clinical observations, ophthalmic examination, body weight gain, feed consumption, clinical pathology evaluation, organ weight, and so on. On the other hand, notable discomforts or mild signs of toxicities were observed on rats treated with some MPs utilized in the management of epilepsy and related symptoms. For instance, Zewdu et al. [203] conducted subacute toxicity study on the HET fruit extract of Embelia Schimperi in Wistar rats at doses of 400 and 1600 mg/kg body weight. The result revealed that chronic administration of the extract (1600 mg/kg) was not significantly associated with body weight loss and organ weights such as liver and kidney. Some haematological and biochemical parameters such as platelets and AST concentration were significantly increased which may be attributed to inflammation of liver and kidney tissue upon repeated dose exposure, stressing the mild toxicity of the fruit extract of Embelia Schimperi at a dose of 1600 mg/kg or higher. In addition, fixed oil of Zingiber officinale root was found to have inherent propensity to trigger a range of toxicities (0.4 mL/kg) including hypertrophy of the liver, kidneys, lungs and spleen, cellular toxicity and oxidative stress following 60-day subchronic toxicity study [224]. Similarly, repeated administration of Clerodendrum myricoides AQ root extracts in mice causes reduction in body weight gain, damage to the liver and kidney and changes in some hematological and biochemical parameters in mice. The research group also reported the significant body weight loss of the AQ leaf extract of Croton macrostachyus at 1000 mg/kg in the treated groups [227].
Developmental toxicity profiles of medicinal plants
Prenatal development is comprised of pre-embryonic, embryonic and fetal stages. The embryonic stage is a critical period where organs of the embryo as well as the placenta can be damaged if exposed to toxic agents directly or indirectly. At times, toxic agents may cross the compromised placental membrane and elicit debilitating effect on the developing embryonic/fetal tissues [228]. The developmental toxicity studies of crude extracts, solvent fractions and/or essential oils has paramount healthcare implications for PS consumed by pregnant women for therapeutic as well as nutritional purpose [229]. In this regard, the effect of some MPs that are frequently employed to relive seizure in patients with epilepsy on prenatal growth (developing embryos and fetuses) are assessed by using different animal models. For instance, the developmental effect HET fruit extract of Embelia schimperi on embryo and fetuses was investigated by using Wistar albino rats and the result echoed that the crude extract was devoid of a significant toxic effect on embryonic and fetal development indices (in the period of organogenesis) at a dose of 1000 mg/kg body weight [230]. Similarly, the HET leaf extract of Syzygium guineense was evaluated at a dose of 250, 500 & 1000 mg/kg in the same animal model and the extract didn’t compromise the number of implantations, fetal resorptions, live births, and stillbirths in the same animal model though there was dose-dependent decrease in the weight of the fetuses and the placentae [228]. Abebe et al., also assessed the teratogenic potentials of the HET leaf extract of Gloriosa superba on Wistar albino rats (220–240 g) at a dose of 250, 500 and 1000 mg/kg of body weight. The crude extract was devoid of any significant teratogenic effects on rat embryos/fetuses up to 500 mg/kg but influenced the growth of embryos at 1000 mg/kg of body weight as manifested by diminished crown-rump length, decreased number of somites and morphological scores [231]. Moreover, the teratogenic effect of the HME leaf extract of Catha edulis was investigated on pregnant Wistar albino rats at a dose of 250, 500 & 750 mg/kg of body weight. The result echoed that khat extract presented dose-dependent toxicity in rat embryo and fetuses such as cytolysis, decidual hypoplasia and atrophy [232]. Overall, the aforementioned acute, subacute and developmental toxicity results witnessed the safety of MPs utilized in the management of epilepsy and related symptoms in Ethiopia.
Phytochemistry of medicinal plants with anticonvulsant activities
MPs have been used as a source of pharmaceutical agents for numerous indications and among small molecule drugs approved between 1981 and 2010, more than half were derived from natural products, mainly plants [233]. Cannabidiol is the first AED of plant origin (extracted from Cannabis sativa) approved by the United States Food and Drug Administration (FDA) in 2018 for the treatment of two rare and severe forms of epilepsy, Dravet syndrome and Lennox-Gastaut syndrome [234]. In LMICs, MPs are consistently used for the treatment of several CNS disorders including epilepsy partly due to their tolerable side effects and impressive efficacy [18]. Most of the MPs prescribed for epilepsy treatment by THs have shown promising anticonvulsant activity against stimuli-induced in vitro and in vivo seizure models [8, 146]. Generally, phytochemical constituents of MPs which belong to the class of alkaloids, flavonoids, terpenoids, glycosides, coumarins, etc. are implicated in the amelioration of convulsions as confirmed by different animal models [235]. They act on different targets such as synapses, receptors, and associated neuronal pathways, ion channels, immune system, inflammatory mediators, glial cells, etc. implicated in the occurrence and progression of epileptogenesis [235]. The antiepileptic activity of MPs discussed before was mostly based on the crude extract or EO rather than isolated active compounds. Consequently, it is difficult to gain full insight into the active constituents, possible targets, effective doses, and MOA of antiepileptic PMs. This section highlights the phytochemical constituents of MPs claimed by THs for their curative effects against epilepsy and proved by in vivo experiments using different stimuli-induced seizure models. It is noteworthy to mention that the bioactive compounds or secondary metabolites of MPs discussed below are obtained from independent phytochemical screening or investigations done elsewhere regardless of their use citations. In this regard, several phytoconstituents with profound anticonvulsant activities were found in different parts including leaf, stem, stembark, root, rootbark, rhizome, flower, aerial and whole part, etc. of the reported antiepileptic MPs (Table 6). Flavonoids and terpenoids (including monoterpenes, sesquiterpenes, diterpenes, triterpenes) are the most frequently encountered phytochemicals in the antiepileptic MPs discussed in previous sections.
Table 6.
Phytoconstituents of MPs with in vivo antiepileptic/anticonvulsant activities
| No. | Scientific name | Active compounds | Refs. |
|---|---|---|---|
| 1 | Ajuga integrifolia | Apigenin and quercetin | [236] |
| 2 | Allium sativum | Quercetin | [237] |
| 3 | Artemisia afra | Borneol, camphor, eucalyptol, eugenol, p-cymene, phytol, α-terpineol, and β-caryophyllene | [238, 239] |
| 4 | Azadirachta indica | Phytol | [240] |
| 5 | Balanites aegyptica | Apigenin, quercetin and rutin | [241] |
| 6 | Buddleja polystachya | Camphor, phytol, rutin, ursolic acid and α-terpineol | [242–244] |
| 7 | Carissa edulis | Lupeol and rutin | [245, 246] |
| 8 | Croton macrostachyus | Lupeol, linalool, p-cymene, α-terpineol, and β-caryophyllene | [247–249] |
| 9 | Jatropha curcas | Lupeol, phytol and rutin | [250–252] |
| 10 | Maytenus heterophylla | Lupeol | [253] |
| 11 | Nicotiana tabacum | Apigenin, lupeol and quercetin | [254, 255] |
| 12 | Olea europaea | Apigenin, oleuropein and quercetin | [256] |
| 13 | Opuntia ficus-indica | Quercetin and rutin | [257] |
| 14 | Ruta chalepensis | Borneol, camphor, carvacrol, linalool, menthol, pulegone, quercetin, rutin and α-terpineol | [258–260] |
| 15 | Sida rhombifolia | Lupeol | [261] |
| 16 | Xanthium stramonium | Borneol, lupeol, p-cymene, quercetin, β-caryophyllene | [262, 263] |
| 17 | Zingiber officinale | 6-gingerol, borneol, camphor, citral, citronellol, linalool, p-cymene, α-terpineol and β-caryophyllene | [168, 264] |
Flavonoids with anticonvulsant activities
Flavonoids, often synthesized by the phenylpropanoid pathway, belong to a class of phenolic compounds with a benzo-γ-pyrone structure that is ubiquitously distributed in plants [265, 266]. They are the first class of phytochemicals involved in the suppression of seizures in different animal models. Apigenin (Fig. 2) is one of the most common flavones found in Ajuga integrifolia, Balanites aegyptica, Nicotiana tabacum, and Olea europaea among others. It elicited pronounced anticonvulsant activity in PTC-induced seizures in SD rats as well as KA-induced seizure model through activation of GABAA receptor and inhibition of glutamatergic neurotransmission. Moreover, apigenin possesses inhibitory activity against hydroxyl radical generation through upregulation of reduced glutathione (GSH), consequently, can inhibit neuronal damage in the hippocampal caused by oxidative glutamate toxicity (involved in neuronal death due to epilepsy) [267]. Rutin is a flavonoid glycoside and a constituent of Balanites aegyptica, Buddleja polystachya, Carissa edulis, Opuntia ficus-indica, Ruta chalepensis among others with profound in vivo antiepileptic activities. Rutin ameliorated PTZ-kindling in KA-induced seizure upon intraperitoneal (IP) administration. It was devoid of significant anticonvulsant activity against PTZ and MES-induced seizure models (at 800 mg/kg) when administered through the IP route. However, intracerebroventricular administration of rutin suppressed clonic and GTCS in the PTZ-induced model. Thus, the effect on GABA, the glutamate pathway, acetylcholine, glycine, serotonin, and adenosine receptors might be implicated for the observed anticonvulsant activity of rutin. Moreover, the antioxidant activity of rutin may also play a crucial role in its antiepileptic outcome [268]. Quercetin is a flavonoid found in Ajuga integrifolia, Allium sativum, Balanites aegyptica, Nicotiana tabacum, Olea europaea, Opuntia ficus-indica, Ruta chalepensis, and Xanthium stramonium that exhibited noticeable anticonvulsant activities in different seizure models. In the KA-induced seizure model involving BALB/c mice, quercetin recorded lower seizure scores as compared to the negative control group [269]. It also elicited significant anticonvulsant outcomes after 30 and 60 min of administration in psychomotor seizures induced by 6-Hz simulation. In addition, it also prolonged the onset of seizures and reduced the generalized seizure duration in PTZ-induced convulsions in the male Albino rat at a dose of 10 mg/kg. Furthermore, at 20 mg/kg, quercetin amplified the latency of PIC-induced seizures [6].
Fig. 2.
Bioactive compounds isolated from plants with anticonvulsant/antiepileptic and convulsive activities
Terpenoids with anticonvulsant activities
Monoterpenes
Terpenoids, also known as terpenes or isoprenoids, are naturally occurring compounds derived from isoprene units and predominantly found in all classes of living organisms [270]. Terpenoids are often classified based on the number of carbon atoms or isoprene units (IPU) they possess: monoterpenes (C10, 2 IPU), sesquiterpenes (C15, 3 IPU), diterpenes (C20, 4 IPU), triterpenes (C30, 6 ITU), etc. [271]. Terpenoids in general and monoterpenes specifically are used for the management of CNS disorders including epilepsy. α-Terpineol is monoterpene alcohol obtained from Artemisia afra, Buddleja polystachya, Croton macrostachyus, Ruta chalepensis, and Zingiber officinale. It has shown significant anticonvulsant activity in PTZ and MES-induced seizure models. Albeit, the exact seizure suppression mechanism of α-terpineol is not known yet [268]. Menthol is a monoterpene found in Ruta chalepensis shown to have profound anticonvulsant effects in different animal models. It elicited its antiseizure activity by delaying the onset of clonic and tonic seizures against PTZ-induced convulsions. Moreover, it also suppressed seizures in hippocampal kindled rats. GABAA receptor activation in the hippocampal neurons and thereby inhibition of neuronal excitation (tonic GABAergic inhibition) is believed for the beneficiary effect of menthol against epileptiform [170]. Camphor is monoterpene predominantly found in PMs such as Artemisia afra, Buddleja polystachya, Ruta chalepensis, and Zingiber officinale among others showed significant anticonvulsant activity in different models. Moreover, it served as a pharmacophore for the synthesis of different anticonvulsant agents. In this regard, benzylidene camphor derivatives containing hydrazone, semicarbazones and thiosemicarbazones exhibited significant antiepileptic activity against MES-induced seizures at 30 mg/kg (comparable to phenytoin) with low neurotoxicity [272]. p-cymene is a constituent of Artemisia afra, Croton macrostachyus, Xanthium stramonium and Zingiber officinale possess anticonvulsant activities. It suppressed convulsions induced by PTZ and MES in mice through modulation of GABAergic neurotransmission via GABAA receptor [273, 274]. Citral is another monoterpene found in Zingiber officinale with biological importance for the treatment of CNS malfunction such as epilepsy. It increased the latency time in PTZ-induced seizure in zebrafish larvae model. Its effect is compromised in flumazenil (FMZ) pretreated groups suggesting the contribution of GABAA receptors. Moreover, downregulation of malondialdehyde (MDA)/NO and upregulation of reduced GSH/catalase (CAT) in brain of citral treated groups reiterated its neuroprotective effect [275]. Pulegone is another monoterpene found in Ruta chalepensis that significantly increased the latency of convulsions in PTZ-induced seizure models [276]. Oleuropein is a glycosylated Seco-iridoids that can be predominantly found in Olea europaea [256]. It unveiled substantial anticonvulsant activity against PTZ-induced seizure through avoidance of neuronal damage via attenuation of generation of reactive oxygen species (ROS) in the epileptic brain [161].
Sesquiterpenes and diterpenes
Sesquiterpenes are the other class of terpenoids with potential anticonvulsant activities. β-caryophyllene is a natural sesquiterpene obtained from Artemisia afra, Croton macrostachyus, Xanthium stramonium, and Zingiber officinale. Contrary to its outcome in PTZ-induced convulsions, β-caryophyllene has reduced seizure severity and OS in the KA-induced seizure model. The result revealed the potential of β-caryophyllene to suppress seizure by inhibiting thiobarbituric acid reactive species and elevating non-protein thiol levels in the KA model [277]. Diterpenes and their derivatives are among the single compounds that demonstrated relevant antiseizure activities in animal models. Phytol is a component of Artemisia afra, Buddleja polystachya, Jatropha curcas, etc. It reduced SE and PLC-induced convulsions by targeting neurotransmitters other than the GABAergic system [268]. 6-GIN, major constituent of Zingiber officinale rhizome, is a diterpenoid with potent anticonvulsant activity. It exerted dose-dependent antiepileptic activity against PTZ-induced hyperlocomotion seizure in the zebrafish larvae model. Its anticonvulsant activity is partly associated with the restoration balance between GABA & GLU neurotransmission in the epileptic brain [168].
Triterpenes
Triterpenoids are a diverse class of phytochemicals with potential CNS effects such as memory enhancement, ameliorating of depression, suppression of epilepsy, etc. Borneol is a triterpenoid found in Artemisia afra, Ruta chalepnesis, Xanthium stramonium, and Zingiber officinale with the ability to alleviate ES in different animal models. It produced an enhanced time of onset of clonic seizures in PTZ-kindled mice. Moreover, the PTZ-kindling was counteracted by borneol as manifested by the decrease in lipid peroxidation (LPO) levels, increased superoxide dismutase (SOD), GSH, CAT levels [278]. Carvacrol, a triterpenoid found in Ruta chalepnesis, suppressed the onset of clonic seizure in the same model at relatively higher doses. These phytoconstituents showed antiepileptic activities after deactivation of GABAA receptor by FMZ, suggesting the involvement of GABAergic neurotransmission in containing seizures through indirect activation of BZP site of GABAA-BZP receptors [279]. Citronellol is also another class of triterpenoid found in different MPs including Zingiber officinale. Inhibition of neuronal excitability through voltage-dependent Na+ channels is the proposed mechanism for the antiepileptic activity of citronellol. Moreover, it also activates the GABAA receptor and thereby foster GABA neurotransmission in the rat brain [280]. Eugenol is a triterpenoid obtained from Artemisia afra. At 100 mg/kg, eugenol suppressed SE and related mortality in PLC-induced SD rats. The involvement of voltage-gated Na+ channel in the anticonvulsant activity of eugenol was proved by its weakened effect upon pre-administration of the Na+ channel antagonist, riluzole [281]. Linalool is found in Croton macrostachyus, Ruta chalepensis and Zingiber officinale. It suppressed quinolic acid (QA)-induced seizure (via NMDA antagonism), delayed NMDA-induced convulsions, increase latency onset and duration of clonic seizures in the PTZ-kindling model. The later seizure model also proved the involvement of a wide array of mechanisms despite glutamate blockage [268]. Ursolic acid is a pentacyclic triterpenoid obtained found in Buddleja polystachya. It has a profound anticonvulsant activity possibly by modulating the non-BZP sites of the GABAA receptor. In addition, it also showed an anticonvulsant effect in MES- and 6 Hz-induced seizure models through activation of the GABAergic pathway [282]. Lupeol is a triterpenoid found in Carissa edulis, Croton macrostachyus, Jatropha curcas, Maytenus heterophylla, Nicotiana tabacum, Sida rhombifolia, Xanthium stramonium, etc. It has shown anticonvulsant activities against PTZ and MES-induced seizure models. Lupeol has increased the mean onset of myoclonic jerks/spasms and differentially protected the mice against mortality [172].
Proconvulsive phytoconstituents of medicinal plants
At this point, it is worthy to mention that some phytoconstituents have convulsive activity (vigorous jerking of the body and loss of consciousness). Crude extracts or essential oils of some MPs can induce seizure upon systemic or topical administration. Phytoconstituents such as eucalyptol and camphor have shown a significant convulsive effect [283]. For instance, one teaspoon of camphor oil taken orally (by a 3 year child) induced GTCS and respiratory depression within 20 min. On the other hand, eucalyptol induced convulsions characterized by the development of long-term SE and showed developmental delay for at least four years following the event [284]. Thus, attention should be given to antiepileptic MPs which contain camphor (Artemisia afra, Buddleja polystachya, Ruta chalepensis, and Zingiber officinale) and eucalyptol (Artemisia afra) when used by THs to manage the convulsive effect and long-term side-effects. Extensive research could be conducted to determine the tolerable dose which can delimit the protective and convulsive outcomes of camphor and eucalyptol. Overall, the anticonvulsant activities of phytoconstituents included in Table 5 signifies the therapeutic potential of the antiepileptic MPs and the importance of evidence-based phytochemical screening to maximize the benefit of MPs and bring about new AEDs of plant origin.
Conclusion
Plants have a central role in the traditional medicinal folklore of Ethiopia. Around 96 PS which belong to 43 families were reported for the treatment of epilepsy and related symptoms in different parts of Ethiopia. A portion of these PS was also used for the same purpose in Africa, the Middle East, Asia, and Latin America. The pharmacological activities of nearly one-third of the MPs claimed by the THs for attenuation of seizure in Ethiopia and other parts of the globe were verified by in vivo experiments using different animal and seizure models. The experimentally proved anticonvulsant activities of MPs have presented the importance of indigenous knowledge and the existing traditional healthcare system in the management of epilepsy in different countries, especially in Ethiopia. A strong association between traditional herbal formulations and pharmacological activities of antiepileptic MPs has been established. Yet, the vast majority of the MPs documented in the present review were not screened for their anticonvulsant activities. In addition, the in vivo experiments conducted elsewhere on the target MPs are shallow and not insightful as far as the MOA of crude extracts, solvent fractions, and EOs are concerned. Furthermore, the in vivo pharmacological experiments (anticonvulsant activities) were not accompanied by isolation and characterization of bioactive phytoconstituents responsible for the antiepileptic MPs. Overall, the majority of the PS documented in this review require additional investigation on pharmacological activities, potential targets and mechanism of seizure attenuation, isolation and characterization of bioactive compounds, and toxicological analysis to validate the significance of MPs to tackle epilepsy-associated comorbidities and mortalities.
Acknowledgements
The author would like to acknowledge Mr. Enawgaw Acham Jemberu for drawing the location map of Ethiopia.
Abbreviations
- AMP
Aminophylline
- AEDs
Antiepileptic drugs
- AEMNAs
Antiepileptic medications non-adherences
- AQ
Aqueous
- BIC
Bicuculline
- BU
Butanol
- CAT
Catalase
- CNS
Central nervous system
- CH
Chloroform
- CAMs
Complementary and alternative medicines
- ET
Ethanolic
- ES
Epileptic seizures
- FMZ
Flumazenil
- GTCS
Generalized tonic clonic seizures
- GLU
Glutamine
- GSK-3
Glycogen synthase kinase-3
- HET
Hydroalcoholic/hydroethanolic
- HME
Hydromethanolic
- IP
Intraperitoneal
- INH
Isonicotinic hydrazide acid
- IPU
Isoprene units
- KA
Kainic acid
- LPO
Lipid peroxidation
- LMICs
Low- and middle-income countries
- MDA
Malondialdehyde
- MES
Maximal electroshock
- MOA
Mechanism of action
- MPs
Medicinal plants
- ME
Methanolic
- NCAM
National center for complementary and alternative medicines
- NMDA
N-methyl-D-aspartate
- OS
Oxidative stress
- PE
Petroleum ether
- PIC
Picrotoxin
- PLC
Pilocarpine
- PS
Plant species
- PTZ
Pentylenetetrazol
- QOL
Quality of life
- QA
Quinolic acid
- ROS
Reactive oxygen species
- GSH
Reduced glutathione
- SNNP
Southern nations nationalities and peoples
- SE
Status epilepticus
- STR
Strychnine
- SOD
Superoxide dismutase
- THS
Traditional healers
- FDA
United States Food and Drug Administration
- TM
Traditional medicine
- VPA
Valproic acid
- WHO
World Health Organization and GABA, γ-aminobutyric acid
Authors’ contributions
YSB collected the relevant works of literature and compiled the review.
Funding
The review did not receive any specific grant from funding agencies.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chipiti T, Viljoen AM, Cordero-Maldonado ML, Veale CGL, Van Heerden FR, Sandasi M, Chen W, Crawford AD, Enslin GM. Anti-seizure activity of African medicinal plants: the identification of bioactive alkaloids from the stem bark of Rauvolfia caffra using an in vivo zebrafish model. J Ethnopharmacol. 2021;279:114282. doi: 10.1016/j.jep.2021.114282. [DOI] [PubMed] [Google Scholar]
- 2.Muhigwa A, Preux P-M, Gérard D, Marin B, Boumediène F, Ntamwira C, Tsai C-H. Comorbidities of epilepsy in low and middle-income countries: systematic review and meta-analysis. Sci Rep. 2020;10(1):9015. doi: 10.1038/s41598-020-65768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao G, Auvrez C, Nightscales R, Barnard S, McCartney L, Malpas CB, Perucca P, Chen Z, Adams S, McIntosh A, et al. Association between psychiatric comorbidities and mortality in epilepsy. Neurol Clin Pract. 2021;11(5):429–437. doi: 10.1212/CPJ.0000000000001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C-H, Hsieh C-L. Chinese herbal medicine for treating epilepsy. Front Neurosci. 2021;15(798):195. doi: 10.3389/fnins.2021.682821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obese E, Biney RP, Henneh IT, Adakudugu EA, Anokwah D, Agyemang LS, Woode E, Ameyaw EO. The anticonvulsant effect of hydroethanolic leaf extract of Calotropis procera (Ait) R Br (Apocynaceae) Neural Plast. 2021;2021:5566890. doi: 10.1155/2021/5566890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akyuz E, Paudel YN, Polat AK, Dundar HE, Angelopoulou E. Enlightening the neuroprotective effect of quercetin in epilepsy: from mechanism to therapeutic opportunities. Epilepsy Behav. 2021;115:107701. doi: 10.1016/j.yebeh.2020.107701. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Arora R, Sarangi SC, Ganeshan NS, Agarwal A, Kaleekal T, Gupta YK. Pharmacodynamic and pharmacokinetic interactions of hydroalcoholic leaf extract of Centella asiatica with valproate and phenytoin in experimental models of epilepsy in rats. J Ethnopharmacol. 2021;270:113784. doi: 10.1016/j.jep.2021.113784. [DOI] [PubMed] [Google Scholar]
- 8.Kaur J, Famta P, Famta M, Mehta M, Satija S, Sharma N, Vyas M, Khatik GL, Chellappan DK, Dua K, et al. Potential anti-epileptic phytoconstituents: an updated review. J Ethnopharmacol. 2021;268:113565. doi: 10.1016/j.jep.2020.113565. [DOI] [PubMed] [Google Scholar]
- 9.Yazie TS, Kefale B, Molla M. Treatment outcome of epileptic patients receiving antiepileptic drugs in Ethiopia: a systematic review and meta-analysis. Behav Neurol. 2021;2021:5586041. doi: 10.1155/2021/5586041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melkamu P, Animut Y, Minyihun A, Atnafu A, Yitayal M. Cost of Illness of epilepsy and associated factors in patients attending adult outpatient department of university of gondar referral hospital Northwest Ethiopia. Risk Manag Healthc Policy. 2021;14:2385. doi: 10.2147/RMHP.S289113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allers K, Essue BM, Hackett ML, Muhunthan J, Anderson CS, Pickles K, Scheibe F, Jan S. The economic impact of epilepsy: a systematic review. BMC Neurol. 2015;15(1):245. doi: 10.1186/s12883-015-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fokoua AR, Ndjenda MK, Kaptué Wuyt A, Tatsinkou Bomba FD, Dongmo AK, Chouna R, Nkeng-Efouet PA, Nguelefack TB. Anticonvulsant effects of the aqueous and methanol extracts from the stem bark of Psychotria camptopus verdc (Rubiacaea) in rats. J Ethnopharmacol. 2021;272:113955. doi: 10.1016/j.jep.2021.113955. [DOI] [PubMed] [Google Scholar]
- 13.Kambli L, Bhatt LK, Oza M, Prabhavalkar K. Novel therapeutic targets for epilepsy intervention. Seizure. 2017;51:27–34. doi: 10.1016/j.seizure.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Perucca E. The pharmacological treatment of epilepsy: recent advances and future perspectives. Acta Epileptol. 2021;3(1):22. [Google Scholar]
- 15.Mishra P, Sinha JK, Rajput SK. Efficacy of cicuta virosa medicinal preparations against pentylenetetrazole-induced seizures. Epilepsy Behav. 2021;115:107653. doi: 10.1016/j.yebeh.2020.107653. [DOI] [PubMed] [Google Scholar]
- 16.Aourz N, Serruys A-SK, Chabwine JN, Balegamire PB, Afrikanova T, Edrada-Ebel R, Grey AI, Kamuhabwa AR, Walrave L, Esguerra CV, et al. Identification of as a potential therapeutic entry point for epilepsy. ACS Chem Neurosci. 2019;10(4):1992–2003. doi: 10.1021/acschemneuro.8b00281. [DOI] [PubMed] [Google Scholar]
- 17.Guery D, Rheims S. Clinical management of drug resistant epilepsy: a review on current strategies. Neuropsychiatr Dis Treat. 2021;17:2229. doi: 10.2147/NDT.S256699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrukh MJ, Makmor-Bakry M, Hatah E, Tan HJ. Use of complementary and alternative medicine and adherence to antiepileptic drug therapy among epilepsy patients: a systematic review. Patient Prefer Adherence. 2018;12:2111. doi: 10.2147/PPA.S179031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faheem M, Ameer S, Khan AW, Haseeb M, Raza Q, Ali Shah F, Khusro A, Aarti C, Umar Khayam Sahibzada M, El-Saber Batiha G, et al. A comprehensive review on antiepileptic properties of medicinal plants. Arabian J Chem. 2022;15(1):103478. [Google Scholar]
- 20.Hasiso TY, Desse TA. Adherence to treatment and factors affecting adherence of epileptic patients at yirgalem general hospital, Southern Ethiopia: a prospective cross-sectional study. PLoS ONE. 2016;11(9):e0163040. doi: 10.1371/journal.pone.0163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalao R, Eshetu T, Tsigebrhan R, Medhin G, Fekadu A, Hanlon C. Implementing integrated services for people with epilepsy in primary care in Ethiopia: a qualitative study. BMC Health Serv Res. 2018;18(1):372–372. doi: 10.1186/s12913-018-3190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boling W, Means M, Fletcher A. Quality of life and stigma in epilepsy, perspectives from selected regions of Asia and Sub-Saharan Africa. Brain Sci. 2018;8(4):59. doi: 10.3390/brainsci8040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilahun M, Habte N, Mekonnen K, Srahbzu M, Ayelegne D. Nonadherence to antiepileptic medications and its determinants among epileptic patients at the university of gondar referral hospital, Gondar, Ethiopia, 2019: an institutional-based cross-sectional study. Neurol Res Int. 2020;2020:8886828–8886828. doi: 10.1155/2020/8886828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belayneh Z, Mekuriaw B. A systematic review and meta-analysis of anti-epileptic medication non-adherence among people with epilepsy in Ethiopia. Arch Pub Health. 2020;78(1):23. doi: 10.1186/s13690-020-00405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwedela MNV, Terai H, Lampiao F, Matsunami K, Aizawa H. Anti-seizure effects of medicinal plants in Malawi on pentylenetetrazole-induced seizures in zebrafish larvae. J Ethnopharmacol. 2022;284:114763. doi: 10.1016/j.jep.2021.114763. [DOI] [PubMed] [Google Scholar]
- 26.Anand P, Othon GC, Sakadi F, Tassiou NR, Hamani ABD, Bah AK, Allaramadji BT, Barry DN, Vogel A, Cisse FA, et al. Epilepsy and traditional healers in the Republic of Guinea: a mixed methods study. Epilepsy Behav. 2019;92:276–282. doi: 10.1016/j.yebeh.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amare A, Zenebe G, Hammack J, Davey G. Status epilepticus: clinical presentation, cause, outcome, and predictors of death in 119 Ethiopian patients. Epilepsia. 2008;49(4):600–607. doi: 10.1111/j.1528-1167.2008.01556.x. [DOI] [PubMed] [Google Scholar]
- 28.Tigistu M, Azale T, Kebebe H, Yihunie T. Frequency of seizure attack and associated factors among patients with epilepsy at University of Gondar referral hospital: a cross-sectional study, Gondar, North West Ethiopia, 2017. BMC Res Notes. 2018;11(1):652. doi: 10.1186/s13104-018-3761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesraoua B, Kissani N, Deleu D, Elsheikh L, Ali M, Melikyan G, Hail HA, Wiebe S, Asadi-Pooya AA. Complementary and alternative medicine (CAM) for epilepsy treatment in the middle east and North Africa (MENA) region. Epilepsy Res. 2021;170:106538. doi: 10.1016/j.eplepsyres.2020.106538. [DOI] [PubMed] [Google Scholar]
- 30.Eshete MA, Molla EL. Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda District, Ethiopia. J Ethnobiol Ethnomed. 2021;17(1):61. doi: 10.1186/s13002-021-00487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Martorell M, Sharopov F, Tumer TB, Kurt B, Lankatillake C, Docea AO, Moreira AC, et al. A Pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem Res. 2021;46(9):2205–2225. doi: 10.1007/s11064-021-03376-0. [DOI] [PubMed] [Google Scholar]
- 32.Tolossa K, Debela E, Athanasiadou S, Tolera A, Ganga G, Houdijk JGM. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):32. doi: 10.1186/1746-4269-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahomoodally MF. Traditional medicines in Africa an appraisal of ten Potent African medicinal plants. Evid.-Based Compl. Altern. Med. 2013;2013:617459. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kefelegn GA, Desta B. Ximenia americana: economic importance, medicinal value, and current status in Ethiopia. Sci World J. 2021;2021:8880021–8880021. doi: 10.1155/2021/8880021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abebe BA, Chane Teferi S. Ethnobotanical Study of medicinal plants used to treat human and livestock ailments in hulet Eju Enese Woreda, East Gojjam Zone of Amhara Region. Ethiopia Evid Based Compl Altern Med. 2021;2021:6668541. doi: 10.1155/2021/6668541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kebebew M, Mohamed E, Meyer-Rochow V. knowledge and use of traditional medicinal animals in the Arba Minch Zuriya District, Gamo Zone Southern Ethiopia. Eur J Therap. 2021;27(2):158–168. [Google Scholar]
- 37.Karunamoorthi K, Hailu T. Insect repellent plants traditional usage practices in the Ethiopian malaria epidemic-prone setting: an ethnobotanical survey. J Ethnobiol Ethnomed. 2014;10(1):22. doi: 10.1186/1746-4269-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birhan YS. Traditional zootherapeutic prescriptions employed in the management of neurological and related disorders in Ethiopia. Acta Ecol. Sinica. 2022 doi: 10.1016/j.chnaes.2022.09.007. [DOI] [Google Scholar]
- 39.Birhan YS, Kitaw SL, Alemayehu YA, Mengesha NM: Medicinal plants with traditional healthcare importance to manage human and livestock ailments in Enemay District, Amhara Region, Ethiopia. Acta Ecol. Sinica In Press. 2022. 10.1016/j.chnaes.2022.05.004.
- 40.Birhan Y, Kitaw S, Alemayehu Y, Mengesha N. Ethnoveterinary medicinal plants and practices in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. Int J Anim Sci. 2018;2(1):1014. [Google Scholar]
- 41.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124(1):69–78. doi: 10.1016/j.jep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Alemneh D. Ethnobotanical study of plants used for human ailments in Yilmana Densa and quarit districts of West Gojjam Zone, Amhara Region Ethiopia. BioMed Res Int. 2021;2021:6615666. doi: 10.1155/2021/6615666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asmerom D, Kalay TH, Araya TY, Desta DM, Wondafrash DZ, Tafere GG. Medicinal plants used for the treatment of erectile dysfunction in Ethiopia: a systematic review. BioMed Res Int. 2021;2021:6656406. doi: 10.1155/2021/6656406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assen Y, Woldearegay M, Haile A. An ethnobotanical study of medicinal plants in Kelala District, south wollo zone of Amhara region, Northeastern Ethiopia. Evid Based Compl. Altern. Med. 2021;2021:6651922. doi: 10.1155/2021/6651922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesfin F, Demissew S, Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2009;5(1):28. doi: 10.1186/1746-4269-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassen A, Muche M, Muasya AM, Tsegay BA. Exploration of traditional plant-based medicines used for livestock ailments in northeastern Ethiopia. S Afr J Bot. 2022;146:230–242. [Google Scholar]
- 47.Kassa Z, Asfaw Z, Demissew S. An ethnobotanical study of medicinal plants in sheka zone of southern nations nationalities and peoples regional state, Ethiopia. J Ethnobiol Ethnomed. 2020;16(1):7. doi: 10.1186/s13002-020-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mengistu M, Kebede D, Atomsa D, Abebe A, Alemnie D. Status and utilization of medicinal and aromatic plants in eastern Hararghe, Ethiopia. Cogent Food Agric. 2019;5(1):1701349. [Google Scholar]
- 49.Kidane L, Gebremedhin G, Beyene T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern zone of tigray, northern Ethiopia. J Ethnobiol Ethnomed. 2018;14(1):64. doi: 10.1186/s13002-018-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kebede E, Mengistu M, Serda B. Ethnobotanical knowledge of pastoral community for treating livestock diseases in Somali regional state, eastern Ethiopia. Trop Anim Health Prod. 2018;50(6):1379–1386. doi: 10.1007/s11250-018-1571-1. [DOI] [PubMed] [Google Scholar]
- 51.Demie G, Negash M, Awas T. Ethnobotanical study of medicinal plants used by indigenous people in and around Dirre Sheikh Hussein heritage site of South-eastern Ethiopia. J Ethnopharmacol. 2018;220:87–93. doi: 10.1016/j.jep.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 52.Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern Ethiopia. BMC Res Notes. 2017;10(1):157. doi: 10.1186/s13104-017-2482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atnafu H, Awas T, Alemu S, Wube S. Ethnobotanical study of medicinal plants in selale mountain ridges, North Shoa. Ethiopia Int J Biodiver. 2018;2(6):567–577. [Google Scholar]
- 54.Abrhaley A, Leta S. Medicinal value of camel milk and meat. J Appl Anim Res. 2018;46(1):552–558. [Google Scholar]
- 55.Gemechu EC. Assessment of indigenous knowledge of medicinal plants used for livestock treatment in five selected kebeles of kersa District, Jimma Zone South Western Ethiopia. J Sci Agri. 2021;5:49–54. [Google Scholar]
- 56.Osman A, Sbhatu DB, Giday M. Medicinal plants used to manage human and livestock ailments in Raya Kobo District of Amhara regional state Ethiopia. Evid Based Compl. Altern. Med. 2020;2020:1329170. doi: 10.1155/2020/1329170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muluye AB, Ayicheh MW. Medicinal plants utilized for hepatic disorders in Ethiopian traditional medical practices: a review. Clin Phytosci. 2020;6(1):52. [Google Scholar]
- 58.Berhanu M, Tintagu T, Fentahun S, Giday M. Ethnoveterinary survey of medicinal plants used for treatment of animal diseases in Ambo district of Oromia regional state of Ethiopia. Evid Based Compl Altern Med. 2020;2020:8816227. doi: 10.1155/2020/8816227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tefera BN, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15(1):25. doi: 10.1186/s13002-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andarge E, Shonga A, Agize M, Tora A. Utilization and conservation of medicinal plants and their associated indigenous knowledge (IK) in Dawuro Zone: An ethnobotanical approach. Int J Med Plant Res. 2015;4:330–337. [Google Scholar]
- 61.Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):55. doi: 10.1186/s13002-017-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mesfin F, Seta T, Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda. Ethiopia Ethnobot Res Appl. 2014;12:341–354. [Google Scholar]
- 63.Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):40. doi: 10.1186/s13002-017-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asfaw A, Lulekal E, Bekele T, Debella A, Abebe A, Degu S. Ethnobotanical investigation on medicinal plants traditionally used against human ailments in Ensaro District, North Shewa Zone, Amhara regional state. Ethiopia. 2021 doi: 10.21203/rs.3.rs-720404/v1. [DOI] [Google Scholar]
- 65.Yineger H, Kelbessa E, Bekele T, Lulekal E. Ethnoveterinary medicinal plants at bale mountains national park. Ethiopia J Ethnopharmacol. 2007;112(1):55–70. doi: 10.1016/j.jep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40. doi: 10.1186/1746-4269-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siraj J, Belew SY, Suleman S. Ethnobotanical assessment and physicochemical properties of commonly used medicinal plants in Jimma zone, Southwest Ethiopia: traditional healers based cross-sectional study. J Exp Pharmacol. 2020;12:665–681. doi: 10.2147/JEP.S267903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tassew G. Ethnobotanical study of medicinal plants in Borecha Woreda, Buno Bedele Zone Southwestern Ethiopia. Int J Sci Res. 2019;8(9):1484–1498. [Google Scholar]
- 69.Yineger H, Kelbessa E, Bekele T, Lulekal E. Plants used in traditional management of human ailments at bale mountains national park Southeastern Ethiopia. J Med Plants Res. 2013;2(6):132–153. [Google Scholar]
- 70.Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of traditional medicinal plants and associated indigenous knowledge in Dawuro Zone of southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18(1):48. doi: 10.1186/s13002-022-00546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara Region, Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):63. doi: 10.1186/1746-4269-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Assefa T, Nigussie N, Mullualem D, Sinshaw G, Adimasu Y. The role of medicinal plants in traditional medicine in Adwa District, tigray northern Ethiopia Asian. J Plant Sci. 2019;3(3–4):1–11. [Google Scholar]
- 73.Seifu T, Asres K, Gebre-Mariam T. Ethnobotanical and ethnopharmaceutical studies on medicinal plants of Chifra District, Afar region north eastern Ethiopia. Ethiop Pharm J. 2006;24:41–58. [Google Scholar]
- 74.Tahir M, Gebremichael L, Beyene T, Van Damme P. Ethnobotanical study of medicinal plants in Adwa District, central zone of tigray regional state, Northern Ethiopia. J Ethnobiol Ethnomed. 2021;17(1):71. doi: 10.1186/s13002-021-00498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flatie T, Gedif T, Asres K, Gebre-Mariam T. Ethnomedical survey of Berta ethnic group Assosa Zone, Benishangul-Gumuz regional state, mid-west Ethiopia. J Ethnobiol Ethnomed. 2009;5(1):14. doi: 10.1186/1746-4269-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan MA, Agize M, Shonga A, Tora A. The utilization and conservation of plants of medicinal value by local traditional medicinal practitioners and the associated indigenous knowledge in dawuro zone of Ethiopia: Northeast Africa—An Ethnobotanical Approach. In: Ozturk M, Hakeem KR, editors. Plant and human health ethnobotany and physiology. Cham: Springer International Publishing; 2018. pp. 267–321. [Google Scholar]
- 77.Birhan YS, Kitaw SL, Alemayehu YA, Mengesha NM. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region Ethiopia SM. J Med Plant Stud. 2017;1(1):1–9. [Google Scholar]
- 78.Zenebe G, Zerihun M, Solomon Z. An ethnobotanical study of medicinal plants in Asgede Tsimbila District, northwestern tigray Northern Ethiopia. Ethnobot Res Appl. 2012;10:305–320. [Google Scholar]
- 79.Teka A, Asfaw Z, Demissew S, Van Damme P. Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south central Ethiopia. J Ethnobiol Ethnomed. 2020;16(1):27. doi: 10.1186/s13002-020-00377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bekele M, Woldeyes F, Lulekal E, Bekele T, Demissew S. Ethnobotanical investigation of medicinal plants in buska mountain range, Hamar district, Southwestern Ethiopia. J Etnobiol Ethnomed. 2022;18(1):60. doi: 10.1186/s13002-022-00558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gebru MG, Lulekal E, Bekele T, Demissew S. Use and management practices of medicinal plants in and around mixed woodland vegetation, tigray regional state Northern Ethiopia. Ethnobot Res Appl. 2021;21:1–26. [Google Scholar]
- 82.Jara JS, Girma Z, Selamo MM. Ethnomedicinal study of plants used against human ailments in Aseko District. South East Ethiopia. 2020 doi: 10.21203/rs.3.rs-23592/v1. [DOI] [Google Scholar]
- 83.Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):10. doi: 10.1186/1746-4269-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parvez N, Yadav S. Ethnopharmacology of single herbal preparations of medicinal plants in Asendabo district, Jimma Ethiopia Indian. J Tradit Knowl. 2010;9(4):724–729. [Google Scholar]
- 85.Maryo M, Nemomissa S, Bekele T. An ethnobotanical study of medicinal plants of the Kembatta ethnic group in Enset-based agricultural landscape of Kembatta Tembaro (KT) Zone, Southern Ethiopia. Asian J Plant Sci Res. 2015;5(7):42–61. [Google Scholar]
- 86.Akele NA: Vascular plant diversity and ethnobotany of medicinal and wild edible plants in Baso Liben and Debre Elias Districts, East Gojjam Zone of Amhara Region, Northwestern, Ethiopia. PhD Dissertation, Addis Ababa University. 2020. http://213.55.95.56/handle/123456789/22266
- 87.Abebe E: Ethnobotanical Study on Medicinal Plants Used by Local Communities in Debark Wereda, North Gondar Zone, Amhara Regional State, Ethiopia. M.Sc. Thesis, Addis Ababa University. 2011. http://etd.aau.edu.et/handle/123456789/4244
- 88.Tamrat S: Study of useful plants in and around GATE UDUMA (Traditional Gedeo Homegardens) in Kochere Wereda of Gedeo Zone, SNNPR, Ethiopia: an Ethnobotanical Approach. M.Sc. Thesis, Addis Ababa University. 2011. http://213.55.95.56/handle/123456789/9329
- 89.Belayneh A, Bussa NF. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):18. doi: 10.1186/1746-4269-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa zone of oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25. doi: 10.1186/s13002-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Assefa B, Glatzel G, Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):20. doi: 10.1186/1746-4269-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. [Google Scholar]
- 93.Ayalew S, Kebede A, Mesfin A, Mulualem G. Ethnobotanical study of medicinal plants used by agro pastoralist Somali people for the management of human ailments in jeldesa cluster, Dire Dawa administration eastern Ethiopia. J Med Plants Res. 2017;11(9):171–187. [Google Scholar]
- 94.Beyi M. Ethnobotanical investigation of traditional medicinal plants in dugda district, Oromia Region. SM J Med Plant Stud. 2018;2(1):1007. [Google Scholar]
- 95.Kindie B, Tamiru C, Abdala T. 2021 Ethnobotanical study of medicinal plants and conservation status used to treat human and livestock ailments in Fadis District, Eastern Ethiopia. Int J Homeopath Nat Med. 2021;7(1):7–17. [Google Scholar]
- 96.Kebebew M. Diversity, knowledge and use of medicinal plants in Abay Chomen district, Horo Guduru Wollega zone, Oromia region of Ethiopia. J Med Plants Res. 2017;11(31):480–500. [Google Scholar]
- 97.Ragunathan M, Abay SM. 2009 Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district northwestern Ethiopia Indian. J Trad Knowl. 2009;8(2):281–284. [Google Scholar]
- 98.Yohannis SW, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local people in menz gera midir district, north shewa zone, amhara regional state Ethiopia. J Med Plants Res. 2018;12(21):296–314. [Google Scholar]
- 99.Abebe M. The ethnomedicinal plants used for human ailments at Mojana Wodera District, central Ethiopia. Biodiversitas. 2021;22(10):4676–4686. [Google Scholar]
- 100.Masondo NA, Stafford GI, Aremu AO, Makunga NP. Acetylcholinesterase inhibitors from southern African plants: An overview of ethnobotanical, pharmacological potential and phytochemical research including and beyond Alzheimer's disease treatment. S Afr J Bot. 2019;120:39–64. [Google Scholar]
- 101.Wubetu M, Sintayehu M, Aeta MA. Ethnobotany of medicinal plants used to treat various mental illnesses in Ethiopia: a systematic review. Asian J Plant Sci Res. 2018;8(1):9–33. [Google Scholar]
- 102.Gebrehiwot M: An Ethnobotanical study of medicinal plants in Seru Wereda, Arsi zone of Oromia Region, Ethiopia. M.Sc. Thesis, Addis Ababa University. 2010 http://213.55.95.56/handle/123456789/6699
- 103.Sharma J, Gairola S, Gaur RD, Painuli RM, Siddiqi TO. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand India. J Ethnopharmacol. 2013;150(1):353–370. doi: 10.1016/j.jep.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 104.Ya’u J, Yaro AH, Abubakar MS, Anuka JA, Hussaini IM. Anticonvulsant activity of Carissa edulis (Vahl) (Apocynaceae) root bark extract. J Ethnopharmacol. 2008;120(2):255–258. doi: 10.1016/j.jep.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 105.Stafford GI, Jäger AK, van Staden J. Activity of traditional South African sedative and potentially CNS-acting plants in the GABA-benzodiazepine receptor assay. J Ethnopharmacol. 2005;100(1):210–215. doi: 10.1016/j.jep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 106.Tabuti JR, Lye KA, Dhillion S. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J Ethnopharmacol. 2003;88(1):19–44. doi: 10.1016/s0378-8741(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 107.Stafford GI, Pedersen ME, van Staden J, Jäger AK. Review on plants with CNS-effects used in traditional south African medicine against mental diseases. J Ethnopharmacol. 2008;119(3):513–537. doi: 10.1016/j.jep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Kigen G, Kipkore W, Wanjohi B, Haruki B, Kemboi J. Medicinal plants used by traditional healers in Sangurur, Elgeyo Marakwet County Kenya. Pharmacognosy Res. 2017;9(4):333–347. doi: 10.4103/pr.pr_42_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maria MR, Maria Cristina D, Bucar I, Luís C. Medicinal plants used to treat neurological disorders in West Africa: a case study with Guinea-Bissau flora. Am J Plant Sci. 2012;3(7):1028–1036. [Google Scholar]
- 110.Raju SK, Basavanna P, Nagesh H, Shanbhag AD. A study on the anticonvulsant activity of Withania somnifera (Dunal) in albino rats. Natl J Physiol Pharm Pharmacol. 2017;7(1):17. [Google Scholar]
- 111.Mabaleha MB, Zietsman PC, Wilhelm A, Bonnet SL. ethnobotanical survey of medicinal plants used to treat mental illnesses in the Berea Leribe and Maseru districts of Lesotho. Nat Prod Commun. 2019;14(7):1934578X19864215. [Google Scholar]
- 112.Augustino S, Gillah PR. Medicinal plants in urban districts of Tanzania: plants, gender roles and sustainable use. Int Forestry Rev. 2005;7(1):44–58. [Google Scholar]
- 113.Govindu S, Adikay S. Evaluation of antiepileptic activity of chloroform extract of Acalypha fruticosa in mice. Pharmacognosy Res. 2014;6(2):108–112. doi: 10.4103/0974-8490.128970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gopalakrishnan S, Saroja K, Elizabeth JD. Chemical investigation of aerial parts of Acalypha fruticosa forssk. Der Pharma Chemica. 2010;2(5):383–389. [Google Scholar]
- 115.Focho D, Ndam W, Fonge B. Medicinal plants of aguambu-bamumbu in the Lebialem highlands, southwest province of Cameroon. Afr J Pharm Pharmacol. 2009;3(1):1–13. [Google Scholar]
- 116.Diallo D, Hveem B, Mahmoud MA, Berge G, Paulsen BS, Maiga A. An ethnobotanical survey of herbal drugs of Gourma district Mali. Pharm Biol. 1999;37(1):80–91. [Google Scholar]
- 117.Tounekti T, Mahdhi M, Khemira H. Ethnobotanical study of indigenous medicinal plants of Jazan region, Saudi Arabia. Evid Based Compl Altern Med. 2019;2019:3190670. doi: 10.1155/2019/3190670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gumisiriza H, Birungi G, Olet EA, Sesaazi CD. Medicinal plant species used by local communities around queen elizabeth national park, maramagambo central forest reserve and ihimbo central forest reserve, south western Uganda. J Ethnopharmacol. 2019;239:111926. doi: 10.1016/j.jep.2019.111926. [DOI] [PubMed] [Google Scholar]
- 119.Ruth L, Manani Solomon D. Ethnobotanical survey and propagation of some endangered medicinal plants from south Nandi district of Kenya. J Anim Plant Sci. 2010;8(3):1016–1043. [Google Scholar]
- 120.Gupta N, Vishnoi G, Wal A, Wal P. Medicinal value of Euphorbia tirucalli. Syst Rev Pharm. 2013;4(1):40. [Google Scholar]
- 121.Auditeau E, Chassagne F, Bourdy G, Bounlu M, Jost J, Luna J, Ratsimbazafy V, Preux P-M, Boumediene F. Herbal medicine for epilepsy seizures in Asia, Africa and Latin America: a systematic review. J Ethnopharmacol. 2019;234:119–153. doi: 10.1016/j.jep.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 122.Ior L, Otimenyin S, Okwori V, Umar D, Azila J. Ethnobotanical survey of plants used in the management of mental illnesses in some selected local government areas of plateau state Nigeria. J Pharmacogn Phytother. 2017;9(10):146–156. [Google Scholar]
- 123.Chauhan AK, Dobhal MP, Joshi BC. A review of medicinal plants showing anticonvulsant activity. J Ethnopharmacol. 1988;22(1):11–23. doi: 10.1016/0378-8741(88)90226-7. [DOI] [PubMed] [Google Scholar]
- 124.Wahab O. Ethnomedicinal antiepileptic plants used in parts of oyo and osun states Nigeria. J Bot Res Int. 2015;8(4):77–81. [Google Scholar]
- 125.Aguilar-Santamaría L, Tortoriello J. Anticonvulsant and sedative effects of crude extracts of Ternstroemia pringlei and Ruta chalepensis. Phytother Res. 1996;10(6):531–533. [Google Scholar]
- 126.Midaoui ME, Maataoui A, Benbella M, Houssa AA, Labazi N. Ethnobotanical study of some aromatic and medicinal plants in the middle Atlas mountains of Morocco. Nat Prod Commun. 2011;6(10):1934578X1100601011. [PubMed] [Google Scholar]
- 127.Elisabeth T, Esther N, Stéphanie NKJ, Gisèle NNC, Vedekoi J, Elisabeth NB. Anticonvulsant effect of asparagus africanus lam root decoction on pilocarpine-induced temporal lobe epilepsy in white mice (Mus musculus Swiss) World J Adv Res Rev. 2020;8(2):296–306. [Google Scholar]
- 128.Henry A, Hosagoudar V, Ravikumar K. Ethno-medicobotany of the Southern Western Ghats of India. Ethnobio. Human Welfare 1996;173–180.
- 129.Ramathal DC, Ngassapa OD. medicinal plants used by rwandese traditional healers in refugee camps in Tanzania. Pharma Biol. 2001;39(2):132–137. [Google Scholar]
- 130.Adesina S. Studies on some plants used as anticonvulsant in Amerindian and African traditional medicine. Fitoterapia. 1982;53:147–162. [Google Scholar]
- 131.Kamau LN, Mbaabu PM, Mbaria JM, Gathumbi PK, Kiama SG. Ethnobotanical survey and threats to medicinal plants traditionally used for the management of human diseases in Nyeri County. Kenya: CELLMED; 2016. [Google Scholar]
- 132.Singh B, Singh S, Kishor A, Singh B. Traditional usage of medicinal plants in humans and animals health care and their chemical constituents from hills and valleys of Jammu province, Western Himalaya Indian. J Nat Prod Resour. 2021;12(1):84–100. [Google Scholar]
- 133.Gerometta E, Grondin I, Smadja J, Frederich M, Gauvin-Bialecki A. A review of traditional uses, phytochemistry and pharmacology of the genus Indigofera. J Ethnopharmacol. 2020;253:112608. doi: 10.1016/j.jep.2020.112608. [DOI] [PubMed] [Google Scholar]
- 134.Bolanle O, Oviasogie O, Owolabi O, Akhigbemen A, Obarisiagbon P, Osaigbovo C. Evaluation of the anti-convulsant activity of aqueous leaf extract of Jatropha curcas (Euphorbiaceae) in mice. Trop J Nat Prod Res. 2018;2(11):489–493. [Google Scholar]
- 135.Kumar K, Shankhdhar PK, Chauhan R. Evaluation of antiepileptic activity of ethanolic extract of populus deltoides leaf in mice. World J Pharma Res. 2017;6(8):923–940. [Google Scholar]
- 136.Sharma V, Sinoriya P, Mehta S. Anticonvulsant and CNS Depressant Activity of Methanolic Extracts of Whole Plant of Sida acuta and Sida rhombifolia in Mice. Curr. Res. Pharm Sci. 2013;03(04):148–153. [Google Scholar]
- 137.Sugaya A, Tsuda T, Sugaya E, Takato M, Takamura K. Effect of Chinese medicine „Saiko–Keishi–To” on the abnormal bursting activity of Snail Neurons. Planta Med. 1978;34(07):294–298. doi: 10.1055/s-0028-1097454. [DOI] [PubMed] [Google Scholar]
- 138.Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, Kalema J. Ethnobotanical survey of medicinal plant species used by communities around Mabira central forest reserve Uganda. J Ethnobiol Ethnomed. 2016;12(1):5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bassoueka DJ, Loufoua BAE, Etou-Ossibi AW, Nsondé-Ntandou GF, Ondelé R, Elion-Itou RDG, Ouamba JM, Abena AA. Plantes anticonvulsivantes du Congo, approche ethnobotanique. Phytothérapie. 2015;13(5):298–305. [Google Scholar]
- 140.Ngo Bum E, Ngah E, Ngo Mune RM, Ze Minkoulou DM, Talla E, Moto FCO, Ngoupaye GT, Taiwe GS, Rakotonirina A, Rakotonirina SV. Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy Behav. 2012;24(3):319–323. doi: 10.1016/j.yebeh.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 141.Fan W, Fan L, Peng C, Zhang Q, Wang L, Li L, Wang J, Zhang D, Peng W, Wu C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L a review. Molecules. 2019;24(2):359. doi: 10.3390/molecules24020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barker-Haliski M, Steve White H. Validated animal models for antiseizure drug (ASD) discovery: advantages and potential pitfalls in ASD screening. Neuropharmacology. 2020;167:107750. doi: 10.1016/j.neuropharm.2019.107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morris G, Rowell R, Cunningham M. Limitations of animal epilepsy research models: can epileptic human tissue provide translational benefit? Altex. 2021;38(3):451–462. doi: 10.14573/altex.2007082. [DOI] [PubMed] [Google Scholar]
- 144.Castañeda R, Cáceres A, Velásquez D, Rodríguez C, Morales D, Castillo A. Medicinal plants used in traditional mayan medicine for the treatment of central nervous system disorders: an overview. J Ethnopharmacol. 2022;283:114746. doi: 10.1016/j.jep.2021.114746. [DOI] [PubMed] [Google Scholar]
- 145.Garba K, Yaro AH. Ya’u J: Anticonvulsant effects of ethanol stem bark extract of Lannea barteri (Anacardiaceae) in mice and chicks. J Ethnopharmacol. 2015;172:227–231. doi: 10.1016/j.jep.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 146.Sucher NJ, Carles MC. A pharmacological basis of herbal medicines for epilepsy. Epilepsy Behav. 2015;52:308–318. doi: 10.1016/j.yebeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 147.Johan Arief MF, Choo BKM, Yap JL, Kumari Y, Shaikh MF. A Systematic review on non-mammalian models in epilepsy research. Front Pharmacol. 2018;9:655–655. doi: 10.3389/fphar.2018.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bertoncello KT, Bonan CD. Zebrafish as a tool for the discovery of anticonvulsant compounds from botanical constituents. Eur J Pharmacol. 2021;908:174342. doi: 10.1016/j.ejphar.2021.174342. [DOI] [PubMed] [Google Scholar]
- 149.Getaneh Y: Anticonvulsant activity of 80% methanol extract and solvent fractions of Ajuga integrifolia Buch Ham (Lamiaceae) leaves in mice MSc Thesis Addis Ababa University 2020. http://etd.aau.edu.et/handle/123456789/26377
- 150.Olatunji SY, Ogunnaike PO, Owolabi JO, Abijo AZ, Alabi A, Adelodun ST, Olanrewaju JA, Adelabi A. Investigating the effects of allium sativum on the prefrontal cortex in lithium chloride pilocarpine-induced epilepsy in wistar rat. Nig J Neurosci. 2021;12(2):56–66. [Google Scholar]
- 151.Kediso TE, Tolessa T, Getachew F, Makonnen E, Seifu D. Effect of 70% ethanol extract and its solvent fractions of Artemisia afra (Jacq. Ex Willd.) against pentylenetetrazole-induced seizure in mice. Evid Based Compl Altern Med. 2021;2021:6690965–6690965. doi: 10.1155/2021/6690965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar G, Rai DV. Effect of valproic acid and azadirachta indica on behavioral alterations and antioxidative stress in pentylenetetrazol-induced kindling in rats. Ann Gen Psychiatry. 2008;7(1):S257. [Google Scholar]
- 153.Dixit R, Patil PR. Alcoholic extract of Azadirachta indica (Neem) root has no anticonvulsant activity in rodents. Natl J Physiol Pharm Pharmacol. 2018;8(8):1171–1174. [Google Scholar]
- 154.Kasture SB, Gupta SC, Kotecha M. Anticonvulsant activity of Balanites aegyptiaca (L) del stem bark. Orient Pharm Exp Med. 2014;14(1):25–29. [Google Scholar]
- 155.Agedew T, Nedi T, Umer S, Shibeshi W. Anticonvulsant activity of 80% methanol leaf extract and solvent fractions of Buddleja polystachya fresen (Buddlejaceae) in mice. Ethiop Pharm J. 2021;36(2):121–130. [Google Scholar]
- 156.Ya’u J, Yaro AH, Malami S, Musa MA, Abubakar A, Yahaya SM, Chindo BA, Anuka JA, Hussaini IM. Anticonvulsant activity of aqueous fraction of Carissa edulis root bark. Pharma Biol. 2015;53(9):1329–1338. doi: 10.3109/13880209.2014.981280. [DOI] [PubMed] [Google Scholar]
- 157.Kediso TE, Tolessa T, Getachew F, Makonnen E, Seifu D. Evaluation of hydro-alcoholic extract of Clerodendrum myricoides (hochst Vatke) leaves and its solvent fractionsin pentylenetetrazole-induced convulsion in mice. J Compl Altern Med Res. 2020;10(3):1–10. [Google Scholar]
- 158.Salile SS, Abula T. In vitro and in vivo anticonvulsant effect of hydroalcoholic extracts of Clutia abyssinica in mice model. J Compl Altern Med Res. 2021;13(4):8–14. [Google Scholar]
- 159.Mureka EW: Evaluation of anticonvulsant activity of extracts and isolates from Maytenus Heterophylla M.Sc. Thesis, Maseno University 2017. https://repository.maseno.ac.ke/handle/123456789/1250
- 160.Tsyvunin V, Shtrygol S, Prokopenko Y, Georgiyants V, Blyznyuk N. Influence of dry herbal extracts on pentylenetetrazole-induced seizures in mice screening results and relationship “chemical composition pharmacological effect. ScienceRise Pharma Sci. 2016;1:18–28. [Google Scholar]
- 161.Asgharzade S, Rabiei Z, Rabiei S, Bijad E, Rafieian-Kopaei M. Therapeutic effects of oleuropein in improving seizure, oxidative stress and cognitive disorder in pentylenetetrazole kindling model of epilepsy in mice. Iran J Pharm Res. 2020;19(1):98–110. doi: 10.22037/ijpr.2019.14212.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fisseha N, Shibeshi W, Bisrat D. Evaluation of anticonvulsant activity of 80% methanolic root bark extract and solvent fractions of Pentas schimperiana (A Rich) Vatke (Rubiaceae) in swiss albino mice. Adv Pharmacol Pharmaceut Sci. 2021;2021:6689879. doi: 10.1155/2021/6689879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hussien Y: Evaluation of anticonvulsant activity of aqueous and 80% methanol extract of Pterolobium stellatum (Forssk) in Swiss albino mice. MSc Thesis, Addis Ababa University, 2020. http://213.55.95.56/handle/123456789/24641?show=full
- 164.Gonzalez-Trujano ME, Carrera D, Ventura-Martinez R, Cedillo-Portugal E, Navarrete A. Neuropharmacological profile of an ethanol extract of Ruta chalepensis L in mice. J Ethnopharmacol. 2006;106(1):129–135. doi: 10.1016/j.jep.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 165.Adeyemi OO, Akindele AJ, Yemitan OK, Aigbe FR, Fagbo FI. Anticonvulsant, anxiolytic and sedative activities of the aqueous root extract of Securidaca longepedunculata Fresen. J Ethnopharmacol. 2010;130(2):191–195. doi: 10.1016/j.jep.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 166.Abubakar U, Danmallam U, Ibrahim H, Maiha B. Anticonvulsant activity of aqueous stem bark extract of securi dacalong ipedunculata Fresen (Polygalaceae) Dutse J Pure Appl Sci. 2020;6(2):277–286. [Google Scholar]
- 167.Kumar KS, Rajkapoor B. Evaluation of anti-epileptic activity of Xanthium strumarium L. Pharmacologyonline. 2010;2:850–855. [Google Scholar]
- 168.Gawel K, Kukula-Koch W, Banono NS, Nieoczym D, Targowska-Duda KM, Czernicka L, Parada-Turska J, Esguerra CV. 6-Gingerol, a major constituent of zingiber officinale rhizoma, exerts anticonvulsant activity in the Pentylenetetrazole-induced seizure model in larval zebrafish. Int J Mol Sci. 2021;22(14):7745. doi: 10.3390/ijms22147745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hosseini A, Mirazi N. Acute administration of ginger (Zingiber officinale rhizomes) extract on timed intravenous pentylenetetrazol infusion seizure model in mice. Epilepsy Res. 2014;108(3):411–419. doi: 10.1016/j.eplepsyres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 170.Zhang X-B, Jiang P, Gong N, Hu X-L, Fei D, Xiong Z-Q, Xu L, Xu T-L. A-type GABA receptor as a central target of TRPM8 agonist menthol. PLoS ONE. 2008;3(10):e3386. doi: 10.1371/journal.pone.0003386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sills GJ, Brodie MJ. Antiepileptic drugs | preclinical drug development in epilepsy. In: Schwartzkroin PA, editor. Encyclopedia of basic epilepsy research. Oxford: Academic Press; 2009. pp. 97–103. [Google Scholar]
- 172.Muhammad KJ, Jamil S, Basar N, Garba M. Anticonvulsant studies on the isolated compounds from the leaves of Scurrula parasitica L (Loranthaceae) Mal J Fund Appl Sci. 2019;15(6):806–810. [Google Scholar]
- 173.Hosseini A, Mirazi N. Alteration of pentylenetetrazole-induced seizure threshold by chronic administration of ginger (Zingiber officinale) extract in male mice. Pharma Biol. 2015;53(5):752–757. doi: 10.3109/13880209.2014.942789. [DOI] [PubMed] [Google Scholar]
- 174.Ng’uni T, Klaasen JA, Fielding BC. Acute toxicity studies of the South African medicinal plant Galenia africana. Toxicol. Rep. 2018;5:813–818. doi: 10.1016/j.toxrep.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mekonen K, Afework M, Makonnen E, Debela A, Ergete W, Tolessa T. Evaluation of acute and sub-acute toxicity of aqueous extracts of artemisia afra leaves on brain, heart and suprarenal glands in swiss albino mice. Ethiop J Health Sci. 2020;30(6):981–990. doi: 10.4314/ejhs.v30i6.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Braga TM, Rocha L, Chung TY, Oliveira RF, Pinho C, Oliveira AI, Morgado J, Cruz A. Azadirachta indica a juss in vivo toxicity-an updated review. Molecules. 2021;26(2):252. doi: 10.3390/molecules26020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Getahun A, Kifle ZD, Ambikar D, Atnafie SA. In vivo evaluation of 80% methanolic leaves crude extract and solvent fractions of buddleja polystachya fresen (buddlejaceae) for wound healing activity in normal and diabetic mice. Metab Open. 2021;11:100110. doi: 10.1016/j.metop.2021.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu Z, Feng R, Xiang F, Song X, Yin Z, Zhang C, Zhao X, Jia R, Chen Z, Li L. Acute and subchronic toxicity as well as evaluation of safety pharmacology of eucalyptus oil-water emulsions. Int J Clin Exp Med. 2014;7(12):4835. [PMC free article] [PubMed] [Google Scholar]
- 179.Loha M, Mulu A, Abay SM, Ergete W, Geleta B. Acute and subacute toxicity of methanol extract of Syzygium guineense Leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid Based Compl Altern Med. 2019;2019:5702159. doi: 10.1155/2019/5702159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.da Silva G, Taniça M, Rocha J, Serrano R, Gomes E, Sepodes B, Silva O. In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Hum Exp Toxicol. 2011;30(7):693–700. doi: 10.1177/0960327110379242. [DOI] [PubMed] [Google Scholar]
- 181.Andjani HN, Sentosa Y, Yati K, Fauzantoro A, Gozan M, Yoo YJ. Acute oral toxicity test of Nicotiana tabacum L bio-oil against female winstar rats. IOP Conf Ser Earth Environ Sci. 2019;353(1):012047. [Google Scholar]
- 182.Kebede S, Afework M, Debella A, Ergete W, Makonnen E. Toxicological study of the butanol fractionated root extract of Asparagus africanus Lam on some blood parameter and histopathology of liver and kidney in mice. BMC Res Notes. 2016;9(1):49. doi: 10.1186/s13104-016-1861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fisseha N, Hammeso WW, Nureye D. Anticonvulsant activity of hydro alcoholic extract and solvent fractions of biophytum umbraculum welw syn (Oxalidaceae) root in mice. J Exp Pharmacol. 2022;14:291–299. doi: 10.2147/JEP.S374890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gebrehiwot S. Evaluation of acute and sub-acute toxicity of hydro-alcoholic extract of Capparis tomentosa Lam in swiss albino mice. J Sci Innov Res. 2018;7(3):60–63. [Google Scholar]
- 185.Patel SB, Rao NJ, Hingorani LL. Safety assessment of Withania somnifera extract standardized for withaferin a: acute and sub-acute toxicity study. J Ayurveda Integr Med. 2016;7(1):30–37. doi: 10.1016/j.jaim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Muluye RA, Berihun AM, Gelagle AA, Lemmi WG, Assamo FT, Gemeda HB, Fekadu N, Getnet SD, woldekidanHirpasa S, TesfayeAyele K. Evaluation of in vivo antiplasmodial and toxicological effect of Calpurnia aurea, Aloe debrana, Vernonia amygdalina and Croton macrostachyus extracts in mice. Med Chem. 2021;11:534. [Google Scholar]
- 187.Ngulde SI, Sandabe UK, Tijjani MB, Barkindo AA, Hussaini IM. Phytochemical constituents, antimicrobial screening and acute toxicity studies of the ethanol extract of Carissa edulis vahl root bark in rats and mice. Am J Res Commun. 2013;1(9):99–110. [Google Scholar]
- 188.Ugwah-Oguejiofor CJ, Okoli CO, Ugwah MO, Umaru ML, Ogbulie CS, Mshelia HE, Umar M, Njan AA. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N E brown in mice and rats. Heliyon. 2019;5(1):e01179. doi: 10.1016/j.heliyon.2019.e01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alene M, Abdelwuhab M, Belay A, Yazie TS. Evaluation of antidiabetic activity of ajuga integrifolia (Lamiaceae) root extract and solvent fractions in mice. Evid Based Compl Altern Med. 2020;2020:6642588. doi: 10.1155/2020/6642588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lawal B, Shittu OK, Oibiokpa FI, Mohammed H, Umar SI, Haruna GM. Antimicrobial evaluation, acute and sub-acute toxicity studies of allium sativum. J Acute Dis. 2016;5(4):296–301. [Google Scholar]
- 191.Qureshi S, Ageel AM, Al-Yahya MA, Tariq M, Mossa JS, Shah AH. Preliminary toxicity studies on ethanol extracts of the aerial parts of Artemisia abyssinica and a inculta in mice. J. Ethnopharmacol. 1990;28(2):157–162. doi: 10.1016/0378-8741(90)90025-o. [DOI] [PubMed] [Google Scholar]
- 192.Kane NF, Kyama MC, Nganga JK, Hassanali A, Diallo M, Kimani FT. Acute toxicity effect of artemisia afra plant extracts on the liver, kidney, spleen and in vivo antimalarial assay on swiss albino mice. Adv Biosci Bioeng. 2019;7(4):64. [Google Scholar]
- 193.Alamrew E, Abebe G. Acute toxicity evaluation of water extract stem barks of Balanites aegyptiaca on adults of three different fish species. J Toxicol Environ Health Sci. 2019;11(2):9–15. [Google Scholar]
- 194.Kefe A, Giday M, Mamo H, Erko B. Antimalarial properties of crude extracts of seeds of Brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Compl Altern Med. 2016;16(1):118. doi: 10.1186/s12906-016-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Osseni R, Akoha S, Adjagba M, Azonbakin S, Lagnika L, Awede B, Bigot A, Diouf A, Darboux R, Lalèyè A. In vivo toxicological assessment of the aqueous extracts of the leaves of Carissa edulis (Apocynaceae) in wistar rats. Eur J Med Plants. 2016;15(1):1–10. [Google Scholar]
- 196.Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen) fisch & mey on glucose handling in normal glucose loaded and diabetic rodents. BMC Compl Altern Med. 2012;12(1):151. doi: 10.1186/1472-6882-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kebede H, Afework M, Makonnen E, Ergete W, Urga K. The effect of Clerodendrum myricoides aqueous extract on blood, liver and kidney tissues of mice. Momona Ethiop J Sci. 2011;3(2):48–63. [Google Scholar]
- 198.Bantie L, Gebeyehu E. Antidiabetic activity of hydroalcoholic extract of the root of Croton macrostachys in streptozotocin induced diabetic mice. World J Pharm Sci. 2015;3(2):185–191. [Google Scholar]
- 199.Ayza MA, Rajkapoor B, Wondafrash DZ, Berhe AH. Protective effect of Croton macrostachyus (euphorbiaceae) stem bark on cyclophosphamide-induced nephrotoxicity in rats. J Exp Pharmacol. 2020;12:275. doi: 10.2147/JEP.S260731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Araya EM, Adamu BA, Periasamy G, Sintayehu B, Gebrelibanos Hiben M. In vivo hepatoprotective and In vitro radical scavenging activities of Cucumis ficifolius a rich root extract. J. Ethnopharmacol. 2019;242:112031. doi: 10.1016/j.jep.2019.112031. [DOI] [PubMed] [Google Scholar]
- 201.Deyno S, Tola MA, Bazira J, Makonnen E, Alele PE. Acute and repeated-dose toxicity of Echinops kebericho Mesfin essential oil. Toxicol Rep. 2021;8:131–138. doi: 10.1016/j.toxrep.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Deyno S, Abebe A, Tola MA, Hymete A, Bazira J, Makonnen E, Alele PE. Acute and sub-acute toxicity of Echinops kebericho decoction in rats. BMC Compl Med Therapies. 2020;20(1):2. doi: 10.1186/s12906-019-2794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zewdu M, Seyoum G, Makonnen E. Effect of acute and chronic treatment of the 80% Ethanolic fruit extract of Embelia schimperi on blood, liver and kidney of rats. Ethiop Pharm J. 2016;32(2):101–116. [Google Scholar]
- 204.Alemu BK, Misganaw D. Antimalarial activity of Fagaropsis angolensis (Rutaceae) crude extracts and solvent fractions of its stem bark against Plasmodium berghei in mice. J Exp Pharmacol. 2020;12:683. doi: 10.2147/JEP.S289478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tanira MOM, Shah AH, Mohsin A, Ageel AM, Qureshi S. Pharmacological and toxicological investigations on Foeniculum vulgare dried fruit extract in experimental animals. Phytother Res. 1996;10(1):33–36. [Google Scholar]
- 206.Widiastuti H, Primaharinastiti P, Prihatiningtyas S. Toxicity test from gloriosa superba leaves extract in rats (rattus novegicus) Int J Pharm Pharm Sci. 2014;6(5):183–187. [Google Scholar]
- 207.Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal activity of 80% methanolic leaf extract of Justicia schimperiana. Evid Based Compl Altern. Med. 2018;2018:3037120. doi: 10.1155/2018/3037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ambikar D, Tsegaw A, Belayneh YM. Wound healing activity of 80% methanolic crude extract and solvent fractions of the leaves of Justicia schimperiana (Hochst ex Nees) T Anderson (Acanthaceae) in mice. J. Exp. Pharmacol. 2022;14:167. doi: 10.2147/JEP.S340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Malebo HM, Wiketye V, Katani SJ, Kitufe NA, Nyigo VA, Imeda CP, Ogondiek JW, Sunguruma R, Mhame PP, Massaga JJ, et al. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malaria J. 2015;14(1):79. doi: 10.1186/s12936-014-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Haule EE, Moshi MJ, Nondo RSO, Mwangomo DT, Mahunnah RLA. A study of antimicrobial activity, acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCl gastric ulcer model. BMC Res Notes. 2012;5(1):546. doi: 10.1186/1756-0500-5-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goshu BT. Evaluation of toxic effect of hydro-methanolic root extract of Myrica salicifolia A. Rich (Myrica-ceae) on brain, heart, spleen and blood parameters in swiss albino mice. EC Pharmacol. Toxicol. 2020;8:01–11. [Google Scholar]
- 212.Guex CG, Reginato FZ, Figueredo KC, da Silva ARHd, Pires FB, Jesus RdS, Lhamas CL, Lopes GHH, Bauermann LdF. Safety assessment of ethanolic extract of Olea europaea L. leaves after acute and subacute administration to Wistar rats. Regul. Toxicol. Pharmacol. 2018;95:395–399. doi: 10.1016/j.yrtph.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 213.Han EH, Lim MK, Lee SH, Rahman MM, Lim Y-H. An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var saboten. BMC Compl Altern Med. 2019;19(1):31. doi: 10.1186/s12906-019-2442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Boukeloua A, Belkhiri A, Djerrou Z, Bahri L, Boulebda N, Pacha YH. Acute toxicity of Opuntia ficus indica and Pistacia lentiscus seed oils in mice. Afr J Tradit Comple Altern Med. 2012;9(4):607–611. [PMC free article] [PubMed] [Google Scholar]
- 215.Dinku T, Tadesse S, Asres K. Antidiabetic activity of the leaf extracts of Pentas schimperiana subsp schimperiana (A Rich) Vatke on alloxan induced diabetic mice. Ethiop Pharm J. 2010 doi: 10.4314/epj.v28i1.2. [DOI] [Google Scholar]
- 216.Meharie BG, Tunta TA. Evaluation of diuretic activity and phytochemical contents of aqueous extract of the shoot apex of podocarpus falcactus. J Exp Pharmacol. 2020;12:629. doi: 10.2147/JEP.S287277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mutuku A, Mwamburi L, Keter L, Ondicho J, Korir R, Kuria J, Chemweno T, Mwitari P. Evaluation of the antimicrobial activity and safety of Rhus vulgaris (Anacardiaceae) extracts. BMC Compl Med Therap. 2020;20(1):272. doi: 10.1186/s12906-020-03063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nguta JM. In vivo antimalarial activity, toxicity, and phytochemical composition of total extracts from securidaca longepedunculata Fresen (polygalaceae) Biomed Biotechnol Res J. 2019;3(3):196. [Google Scholar]
- 219.Luciana DSNR, Gabriela TD, Edla JRCEG, Micaelly DSO, Andressa BL, Kardilndia MDO, Otemberg SC, Josu DAR, Alexandre RDP, Janine AP. Acute toxicity evaluation of ethanolic extract of the air parts of Sida rhombifolia L in wistar rats Afr. J Pharm Pharmacol. 2019;13(14):181–187. [Google Scholar]
- 220.Assam Jp A, Dzoyem JP, Pieme CA, Penlap VB. In vitro antibacterial activity and acute toxicity studies of aqueous-methanol extract of Sida rhombifolia Linn (Malvaceae) BMC Compl. Altern. Med. 2010;10(1):40. doi: 10.1186/1472-6882-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sireeratawong S, Lertprasertsuke N, Srisawat U, Thuppia A, Ngamjariyawat A, Suwanlikhid N, Jaijoy K. Acute and subchronic toxicity study of the water extract from root of Sida rhombifolia Linn in rats Songklanakarin J. Sci. Technol. 2008;30(6):729-737.
- 222.Nyangacha R, Gathirwa J, Muthaura C, Mungai G, Mwikwabe N, Ondicho J, Moindi E, Omar S, Rukunga G, Maranga R. Antimalarial activity and toxicity evaluation of Kenyan Hugonia castaneifolia Engl Teclea nobilis Del and Turraea mombassana C DC. Afr J Health Sci. 2012;23(4):305–315. [Google Scholar]
- 223.Legba B, Dougnon V, Deguenon E, Agbankpe J, Senou M, Aniambossou A, Gbaguidi C, Sintondji K, Baba-Moussa L, Dougnon J. Toxicological characterization of six plants of the beninese pharmacopoeia used in the treatment of Salmonellosis. J Toxicol. 2019;2019:3530659. doi: 10.1155/2019/3530659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Idang EO, Yemitan OK, Mbagwu HO, Udom GJ, Ogbuagu EO, Udobang JA. Toxicological assessment of Zingiber officinale Roscoe (Ginger) root oil extracts in Albino rats. Toxicol Digest. 2019;4(1):108–119. [Google Scholar]
- 225.Bhide RM, Bethapudi B, Chalichem NSS, Nithyanantham M, Murugan SK, Mundkinajeddu D. Acute and subchronic toxicity study of flavonoid rich extract of <i>Glycyrrhiza glabra</i> (GutGard®) in sprague dawley rats. J Toxicol. 2022;2022:8517603. doi: 10.1155/2022/8517603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Manekeng HT, Mbaveng AT, Ntyam Mendo SA, Agokeng A-JD, Kuete V. Evaluation of acute and subacute toxicities of psidium guajava methanolic bark extract: a botanical with in vitro antiproliferative potential. Evid Based Compl Altern Med. 2019;2019:8306986. doi: 10.1155/2019/8306986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Abera T, Ashebir R, Basha H, Debebe E, Abebe A, Meresa A, Woldekidan S. Phytochemical-constituents, safety and efficacy of commonly used medicinal plants for the treatment of malaria in Ethiopia-a review. Pharm Pharmacol Int J. 2019;7(6):284–295. [Google Scholar]
- 228.Abebe MS, Asres K, Bekuretsion Y, Woldekidan S, Sisay B, Seyoum G. Prenatal developmental toxicity and histopathological changes of the placenta induced by Syzygium guineense leaf extract in rats. J Toxicol. 2022;2022:5209136. doi: 10.1155/2022/5209136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Adane F, Asres K, Ergete W, Woldekidan S, Seyoum G. The developmental toxicity of Thymus schimperi essential oil in rat embryos and fetuses. J Toxicol. 2022;2022:4091839. doi: 10.1155/2022/4091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Animaw Z, Asres K, Tadesse S, Basha H, Taye S, Abebe A, Debebe E, Seyoum G. Teratogenic evaluation of 80% ethanol extract of Embelia schimperi vatke fruits on rat embryo and fetuses. J Toxicol. 2022;2022:4310521. doi: 10.1155/2022/4310521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Abebe M, Asres K, Bekuretsion Y, Woldkidan S, Debebe E, Seyoum G. Teratogenic effect of high dose of Syzygium guineense (Myrtaceae) leaves on wistar albino rat embryos and fetuses. Evid Based Compl Altern. Med. 2021;2021:6677395. doi: 10.1155/2021/6677395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Belete S, Asres K, Bekuretsion Y, Ashebir R, Abebe MS, Seyoum G. Toxic effect of Khat in rat embryos and fetuses. BioMed Res Int. 2021;2021:9933389. doi: 10.1155/2021/9933389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pferschy-Wenzig E-M, Bauer R. The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav. 2015;52:344–362. doi: 10.1016/j.yebeh.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 234.Ryan M. Cannabidiol in epilepsy: the indications and beyond. Ment Health Clin. 2020;10(6):317–325. doi: 10.9740/mhc.2020.11.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He L-Y, Hu M-B, Li R-L, Zhao R, Fan L-H, He L, Lu F, Ye X, Huang Y-l, Wu C-J. Natural medicines for the treatment of epilepsy: bioactive components Pharmacology and Mechanism. Front Pharmacol. 2021;12(28):604040. doi: 10.3389/fphar.2021.604040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ullah MA, Gul FZ, Khan T, Bajwa MN, Drouet S, Tungmunnithum D, Giglioli-Guivarc’h N, Liu C, Hano C, Abbasi BH. Differential induction of antioxidant and anti-inflammatory phytochemicals in agitated micro-shoot cultures of Ajuga integrifolia Buch Ham ex D Don with biotic elicitors. AMB Express. 2021;11(1):137. doi: 10.1186/s13568-021-01297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.El-Saber Batiha G, Magdy Beshbishy A, Wasef G, Elewa L, YHA A, Al-Sagan A, Abd El-Hack ME, Taha Abd-Elhakim AEM, Prasad Devkota YH. Chemical constituents and pharmacological activities of garlic (Allium sativum L) A review. Nutrients. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.More G, Lall N, Hussein A, Tshikalange TE. Antimicrobial constituents of Artemisia afra Jacq ex willd against periodontal pathogens. Evid Based Compl Altern. Med. 2012;2012:252758. doi: 10.1155/2012/252758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Falowo AB, Mukumbo FE, Muchenje V. Phytochemical constituents and antioxidant activity of Artemisia Afra and Bidens pilosa essential oil in ground pork. J Essent Oil-Bear Plants. 2019;22(1):176–186. [Google Scholar]
- 240.Pandey IP, Ahmed S, Chhimwal SL, Pandey S. Chemical composition and wound healing activity of volatile oil of leaves of Azadirachta indica a.juss. adv. pure appl. chem. 2012;1:2167–0854. [Google Scholar]
- 241.Khamis G, Saleh AM, Habeeb TH, Hozzein WN, Wadaan MAM, Papenbrock J, AbdElgawad H. Provenance effect on bioactive phytochemicals and nutritional and health benefits of the desert date Balanites aegyptiaca. J Food Biochem. 2020;44(6):e13229. doi: 10.1111/jfbc.13229. [DOI] [PubMed] [Google Scholar]
- 242.Getahun A, Kifle ZD, Ambikar D, Atnafie SA. In vivo evaluation of 80% methanolic leaves crude extract and solvent fractions of buddleja polystachya fresen (buddlejaceae) for wound healing activity in normal and diabetic mice. Metab Open. 2021;11:100110–100110. doi: 10.1016/j.metop.2021.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.El-Gamal A, Al-Massarani S, Fawzy G, Ati H, Al-Rehaily A, Basudan O, Abdel-Kader M, Tabanca N, Becnel J. Chemical composition of Buddleja polystachya aerial parts and its bioactivity against Aedes aegyptica. Nat Prod Res. 2018;32(23):2775–2782. doi: 10.1080/14786419.2017.1378213. [DOI] [PubMed] [Google Scholar]
- 244.Ati HYA, Gamal AAE, Fawzy GA. Chemical composition, in vitro antimicrobial and cytotoxic activities of Buddleja polystachya essential oils. J Essent Oil-Bear Plants. 2014;17(6):1112–1119. [Google Scholar]
- 245.Al-youssef H, Hassan WH. Phytochemical and biological studies of the aerial parts of Carissa edulis growing in Saudi Arabia. Biosci Biotechnol Res Asia. 2016;7(2):635–646. [Google Scholar]
- 246.Kaunda JS, Zhang Y-J. The genus Carissa: an ethnopharmacological, phytochemical and pharmacological review. Nat Prod Bioprospect. 2017;7(2):181–199. doi: 10.1007/s13659-017-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Meresa A, Ashebir R, Gemechu W, Teka F, Basha H, Abebe A, Debebe E, Samuel W. Kidan Tadele A Ethno medicinal uses, phytochemistry and anti-malarial effect of Croton macrostachyus (Bisana): a review. J Med Plants Stud. 2019;7:79–88. [Google Scholar]
- 248.Tariku Y, Hymete A, Hailu A, Rohloff J. Constituents, antileishmanial activity and toxicity profile of volatile oil from berries of Croton macrostachyus. Nat Prod Commun. 2010;5(6):975–980. [PubMed] [Google Scholar]
- 249.Gebrehiwot H, Zelelew D, Gebremariam A. Chemical Analysis and Medicinal Activities of Volatile Components from the Seeds of Croton Macrostachyus. Int J Sci Basic Appl Res. 2018;37:316–330. [Google Scholar]
- 250.Adeosun TE, Ogunwande IA, Avoseh ON, Raji I, Lawal OA. Composition and anti-inflammatory activity of essential oil of Jatropha curcas. Nat Prod Commun. 2017;12(3):439–440. [PubMed] [Google Scholar]
- 251.Bastos-Cavalcante N, Rodrigues dos Santos-Barbosa C, Silva-Pereira RL, Feitosa-Muniz D, de Melo-Coutinho HD, Araújo-Rolim L, da Silva Almeida JRG. Phytochemical analysis, antibacterial activity and antibiotic modifying action of Jatropha mollissima (Pohl.) Baill. (Euphorbiaceae) Anales de Biología. 2020;22:85–94. [Google Scholar]
- 252.Vega-Ruiz YC, Hayano-Kanashiro C, Gámez-Meza N, Medina-Juárez LÁ. Determination of chemical constituents and antioxidant activities of leaves and stems from Jatropha cinerea (Ortega) Müll Arg and Jatropha cordata (Ortega) Müll. Arg Plants. 2021;10:212. doi: 10.3390/plants10020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Murayama T, Eizuru Y, Yamada R, Sadanari H, Matsubara K, Rukung G, Tolo FM, Mungai GM, Kofi-Tsekpo M. Anticytomegalovirus activity of pristimerin, a triterpenoid quinone methide isolated from Maytenus heterophylla (Eckl & Zeyh) Antiviral Chem Chemother. 2007;18(3):133–139. doi: 10.1177/095632020701800303. [DOI] [PubMed] [Google Scholar]
- 254.Yang C, Xie S-N, Ni L, Du Y-M, Liu S, Li M-Y, Xu K. Chemical Constituents from Nicotiana tabacum L and their antifungal activity. Nat Prod Commun. 2021;16(11):11059578. [Google Scholar]
- 255.Zou X, Bk A, Rauf A, Saeed M, Al-Awthan YS, Al-Duais A, Bahattab M, Hamayoon O, Khan M, Suleria HAR. Screening of polyphenols in Tobacco (Nicotiana tabacum) and determination of their antioxidant activity in different Tobacco varieties. ACS Omega. 2021;6(39):25361–25371. doi: 10.1021/acsomega.1c03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zaïri A, Nouir S, Zarrouk A, Haddad H, Khélifa A, Achour L. Phytochemical profile cytotoxic antioxidant and allelopathic potentials of aqueous leaf extracts of Olea europaea. Food Sci Nutr. 2020;8(9):4805–4813. doi: 10.1002/fsn3.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Slimen IB, Najar T, Abderrabba M. Opuntia ficus-indica as a source of bioactive and nutritional phytochemicals. J Food Nutr Sci. 2016;4(6):162–169. [Google Scholar]
- 258.Alotaibi SM, Saleem MS, Al-Humaidi JG. Phytochemical contents and biological evaluation of Ruta chalepennsis L growing in Saudi Arabia. Saudi Pharm J. 2018;26(4):504–508. doi: 10.1016/j.jsps.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jaradat N, Adwan L, K’aibni S, Zaid AN, Shtaya MJY, Shraim N, Assali M. Variability of chemical compositions and antimicrobial and antioxidant activities ofRuta chalepensis leaf essential oils from three palestinian regions. BioMed Res Int. 2017;2017:2672689. doi: 10.1155/2017/2672689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nahar L, El-Seedi HR, Khalifa SAM, Mohammadhosseini M, Sarker SD. Ruta essential oils: composition and bioactivities. Molecules. 2021;26(16):4766. doi: 10.3390/molecules26164766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mah SH, Teh SS, Ee GCL. Anti-inflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharma Biol. 2017;55(1):920–928. doi: 10.1080/13880209.2017.1285322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Khan Y, Shah S, Ullah S. Ethnomedicinal, pharmacological and phytochemical evaluation of Xanthium strumarium L. Int J Biol Sci. 2020;11(7):587. [Google Scholar]
- 263.Kamboj A, Saluja AK. Phytopharmacological review of Xanthium strumarium L (Cocklebur) Int J Green Pharm. 2010;4(3):129–139. [Google Scholar]
- 264.Liu Y, Liu J, Zhang Y. Research progress on chemical constituents of zingiber officinale roscoe. BioMed Res Int. 2019;2019:5370823. doi: 10.1155/2019/5370823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47–e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Diniz TC, Silva JC, Lima-Saraiva SRGd, Ribeiro FPRdA, Pacheco AGM, de Freitas RM, Quintans-Júnior LJ, Quintans JDSS, Mendes RL, Almeida JRGdS. The role of flavonoids on oxidative stress in Epilepsy. Oxid Med Cell Longev. 2015;2015:171756. doi: 10.1155/2015/171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhu H-L, Wan J-B, Wang Y-T, Li B-C, Xiang C, He J, Li P. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55(1):3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]
- 269.Carmona-Aparicio L, Cárdenas-Rodríguez N, Delgado-Lamas G, Pedraza-Chaverri J, Montesinos-Correa H, Rivera-Espinosa L, Torres-Espíndola LM, Hernández ME, López-Aceves T, Pérez-Lozano DL, et al. Dose-dependent behavioral and antioxidant effects of quercetin and methanolic and acetonic extracts from heterotheca inuloides on several rat tissues following kainic acid-induced status epilepticus. Oxid Med Cell Longev. 2019;2019:5287507. doi: 10.1155/2019/5287507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds? New Phytol. 2018;220(3):692–702. doi: 10.1111/nph.14178. [DOI] [PubMed] [Google Scholar]
- 271.Reyes BAS, Dufourt EC, Ross J, Warner MJ, Tanquilut NC, Leung AB. Chapter 4—selected phyto and marine bioactive compounds alternatives for the treatment of type 2 diabetes. In: Atta R, editor. Studies: Natural Products Chemistry. Amsterdam: Elsevier; 2018. [Google Scholar]
- 272.Angelova V, Karabeliov V, Andreeva-Gateva PA, Tchekalarova J. recent developments of hydrazide/hydrazone derivatives and their analogs as anticonvulsant agents in animal models. Drug Dev Res. 2016;77(7):379–392. doi: 10.1002/ddr.21329. [DOI] [PubMed] [Google Scholar]
- 273.Raza M, Alghasham AA, Alorainy MS, El-Hadiyah TM. Potentiation of valproate-induced anticonvulsant response by nigella sativa seed constituents: the role of gaba receptors. Int J Health Sci. 2008;2(1):15–25. [PMC free article] [PubMed] [Google Scholar]
- 274.Wang Z-J, Heinbockel T. Essential oils and their constituents targeting the GABAergic system and sodium channels as treatment of neurological diseases. Molecules. 2018;23(5):1061. doi: 10.3390/molecules23051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hacke ACM, Miyoshi E, Marques JA, Pereira RP. Cymbopogon citratus (DC) Stapf, citral and geraniol exhibit anticonvulsant and neuroprotective effects in pentylenetetrazole-induced seizures in zebrafish. J Ethnopharmacol. 2021;275:114142. doi: 10.1016/j.jep.2021.114142. [DOI] [PubMed] [Google Scholar]
- 276.Sousa DPD, Nóbrega FFF, Lima MRVd, Almeida RND. Pharmacological activity of (R)-(+)-pulegone, a chemical constituent of essential oils. Z Naturforsch C. 2011;66(7–8):353–359. doi: 10.1515/znc-2011-7-806. [DOI] [PubMed] [Google Scholar]
- 277.Oliveira CCD, Oliveira CVD, Grigoletto J, Ribeiro LR, Funck VR, Grauncke ACB, Souza TLD, Souto NS, Furian AF, Menezes IRA, et al. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016;56:26–31. doi: 10.1016/j.yebeh.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 278.Tambe R, Jain P, Patil S, Ghumatkar P, Sathaye S. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice Naunyn-Schmiedeberg's. Arch Pharmacol. 2016;389(5):467–475. doi: 10.1007/s00210-016-1220-z. [DOI] [PubMed] [Google Scholar]
- 279.Quintans-Júnior LJ, Guimarães AG, Araújo BE, Oliveira GF, Santana MT, Moreira FV, Santos MR, Cavalcanti SC, Júnior WL, Botelho MA. Carvacrol, (-)-borneol and citral reduce convulsant activity in rodents. Afr J Biotechnol. 2010;9(39):6566–6572. [Google Scholar]
- 280.de Sousa DP, Gonçalves JCR, Quintans-Júnior L, Cruz JS, Araújo DAM, de Almeida RN. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett. 2006;401(3):231–235. doi: 10.1016/j.neulet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 281.Jeong KH, Lee D-S, Kim SR. Effects of eugenol on granule cell dispersion in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2015;115:73–76. doi: 10.1016/j.eplepsyres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 282.Khan I, Karim N, Ahmad W, Abdelhalim A, Chebib M. GABA-A RECEPTOR modulation and anticonvulsant, anxiolytic, and antidepressant activities of constituents from. Evid Based Compl. Altern. Med. 2016;2016:1215393. doi: 10.1155/2016/1215393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mathew T, John SK, Kamath V, Kumar RS, Jadav R, Shaji A, Nadig R, Sarma GRK, Parry GJ. Essential oil-related status epilepticus: a small case series study. J Am Coll Emerg Physicians Open. 2020;1(5):918–921. doi: 10.1002/emp2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bahr TA, Rodriguez D, Beaumont C, Allred K. The effects of various essential oils on epilepsy and acute seizure: a systematic review. Evid based compl Med Altern. 2019 doi: 10.1155/2019/6216745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.